Abstract
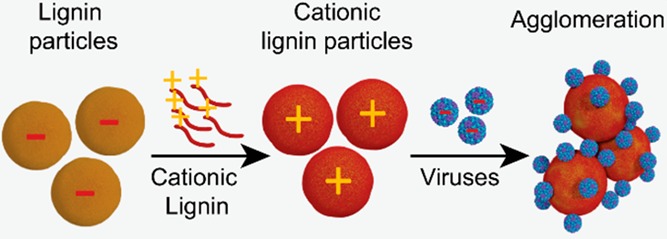
Virus contamination of water is a threat to human health in many countries. Current solutions for inactivation of viruses mainly rely on environmentally burdensome chemical oxidation or energy-intensive ultraviolet irradiation, which may create toxic secondary products. Here, we show that renewable plant biomass-sourced colloidal lignin particles (CLPs) can be used as agglomeration agents to facilitate removal of viruses from water. We used dynamic light scattering (DLS), electrophoretic mobility shift assay (EMSA), atomic force microscopy and transmission electron microscopy (AFM, TEM), and UV spectrophotometry to quantify and visualize adherence of cowpea chlorotic mottle viruses (CCMVs) on CLPs. Our results show that CCMVs form agglomerated complexes with CLPs that, unlike pristine virus particles, can be easily removed from water either by filtration or centrifugation. Additionally, cationic particles formed by adsorption of quaternary amine-modified softwood kraft lignin on the CLPs were also evaluated to improve the binding interactions with these anionic viruses. We foresee that due to their moderate production cost, and high availability of lignin as a side-stream from biorefineries, CLPs could be an alternative water pretreatment material in a large variety of systems such as filters, packed columns, or flocculants.
Keywords: Colloidal lignin particles, Lignin nanoparticles, Virus interaction, Water treatment
Short abstract
Water purification is facilitated by agglomeration of viruses in the presence of charged lignin particles.
Introduction
Limited access to clean water is a human health concern, which is intensifying due to fast population growth and climate change.1−3 Today, contamination of natural water resources with chemical or biological pollutants from human activities induces sickness and death in countries with limited access to potable water.4 Infections are caused by parasites, bacteria, and viruses.4 In addition to contamination of fresh water, treatment of wastewaters and ensuring safe storage conditions are also crucial for securing clean water supply.5
Current solutions for advanced water purification involve several disadvantages. Microorganisms can be partially inactivated by utilizing ozone or chlorine-based disinfectants. However, their use releases toxic components such as aldehydes, ketones, and chlorite/chlorate ions into the treated water.1,6−10 Alternative physical methods such as UV irradiation require high energy input from mixing of water due to the short penetration depth of the UV light, while pasteurization is not feasible in large-scale water treatment due to the high energy input needed for heating the water to the boiling point.1 Photovoltaic systems coupled to solar disinfection is a promising water purification technique, but large-scale utilization is limited by meteorological conditions and the high-cost of photovoltaic technologies.11−13 The low efficiency of many water purification methods is also due to the high resistance of some microorganisms such as thermophilic and sporulating bacteria. This issue becomes even more significant when viruses are targeted.14 Thus, antimicrobial nanomaterials15 such as gold nanoparticles,16 zerovalent iron,17 carbon nanotubes,18 or copper nanofibers containing titanium dioxide,19 or nanoparticles (NPs) made of silver or iron/nickel,6,20−22 have been studied for virus inactivation. However, the presence of these nanoscaled substances in treated water poses a new concern of nanotoxicity.16,21,23,24
Among chromatographic techniques, ion exchange chromatography25 and size exclusion membranes26 are not sufficiently scalable, robust, or efficient for large-scale treatment of wastewaters from natural or industrial sources. Filtration and sedimentation would be favorable low-cost and scalable techniques, but the nanoscaled size of viruses hampers their removal by these methods. Flocculants or agglomeration agents facilitate removal of microbial contaminants, but most of the commercial substances are either inorganic compounds or synthetic polyelectrolytes27,28 that are costly and poorly biodegradable, such as aluminum-based coagulants,29−31 which release toxic metal hydroxides in water.32,33 Inorganic materials, such as metals, melt and cause corrosion problems during combustion of resulting wastes and consequently there is a need for combustible water purification agents made of organic material.
Among the natural polymers of renewable plant biomass, cellulose and lignin are the most abundant ones. While cellulose has been widely used in many shapes for antiviral activity34−36 and virus removal from water,37−39 interest in lignin, an untapped byproduct from biorefineries and the pulp and paper industry, is growing due to its multifunctional adsorption properties.40,41 A total of 50 million tons of lignin is produced every year by the pulp and paper industries, but only 2% of it is used for industrial applications,42 while the bulk of it is used as a solid biofuel. Thus, lignin is a low-cost material which is a suitable and potentially sustainable candidate for large-scale wastewater treatment. Indeed, various types of lignins have been studied mainly for the removal of heavy metal ions or dyes in wastewaters. To cite the most recent examples, raw and demineralized lignins were studied as biosorbents of nickel(II) ions.43 Cationic lignin was evaluated as an adsorbent for removing sulfates and anionic dyes from water,40,44 and in a similar way, anionic lignin with sulfomethylated groups was used for adsorbing cationic dyes.45 In an effort to combine positive and negative charges, zwitterionic lignin was prepared to remove dye pigments.46,47 More recently, a composite made of chitosan, acrylamide, and lignin was synthesized as a flocculant for dye removal.48 Chitosan is another natural polymer that was also studied in substitution of inorganic flocculants.28 Despite the benefits of lignin, its use for wastewater treatment is mainly limited to demonstration with dyes and mainly in the form of composite materials. For example, the field of hybrid magnetic NPs is emerging using either lignin49 or chitosan.50
The development of lignin-based products has been limited by its structural heterogeneity due to the differences in botanical origin and extraction processes and by its varying solubility in water.51−53 Various techniques have been developed in recent years to overcome all these issues by transforming this polymeric material into colloidal lignin particles (CLPs, also termed lignin nanoparticles or LNPs).54−58 Furthermore, preparation of CLPs has been found possible via a low-cost process on a large scale.57−59 Despite the large surface area offered by the submicrometer diameter of CLPs,60,61 polymers are used more often than NPs as agglomeration agents in water purification. In particular, it has recently been concluded that there are no works on interactions of viruses with colloidal lignins.62 However, previous studies during the past two decades on antiviral activity of lignin-carbohydrate complexes (LCCs)63 and lignin derivatives64 encouraged us to explore CLPs to facilitate virus removal from water.
In the present work, we prepared spherical CLPs following the nanoprecipitation approach61,65 in which lignin is first solubilized with an organic solvent and the particles are then formed by fast addition of the lignin solution into water. To demonstrate that CLPs can be used as agglomeration agents for water purification, we used negatively charged cowpea chlorotic mottle viruses (CCMVs) as a model viral contaminant.66 To improve the virus affinity of the particles, the anionic CLPs were coated with cationized lignin resulting in cationic lignin particles (c-CLPs)67 with a net positive charge that can adsorb negatively charged biomolecules. Binding of regular anionic CLPs, spherical c-CLPs, and soluble cationic lignin with CCMVs was systematically compared in an electrophoretic mobility shift assay (EMSA); dynamic light scattering (DLS), transmission electron microscopy (TEM), and atomic force microscopy (AFM) were used to characterize the formed agglomeration complexes that enabled separation of CCMVs from water either by centrifugation or filtration.
Experimental Section
Materials
Softwood kraft lignin (SKL, BioPiva 100 from UPM) used for the preparation of CLPs and cationic lignin were already characterized in a previous study41 by 31P nuclear magnetic resonance (NMR) spectroscopy.68 Briefly, the lignin contained 1.89 mmol g–1 of aliphatic groups, 0.57 mmol g–1 of carboxylic acids, 4.05 mmol g–1 of phenolic groups, and a total amount of 6.32 mmol g–1 of hydroxyl groups. CCMV particles were grown and isolated from California black-eye beans as reported previously.66 Agarose (sulfate ≤0.08%) was ordered from Biotop and used as received. Ethidium bromide (∼95%), acetic acid (HOAc, ≥99%), sodium acetate (NaOAc, ≥99%), and ethylenediaminetetraacetic acid (EDTA, >99%) were ordered from Sigma-Aldrich and used as received. Acetone (≥99.8%, VWR) was used as received. Deionized water was used in all experiments.
Preparation of Cationic Lignin
The same batch of pH 7 water-soluble cationic lignin was used as described earlier.67 Briefly, glycidyltrimethylammonium chloride (GTMAC) was used for cationization of SKL in 0.2 M NaOH solution at 70 °C for 1 h. The final product was characterized by 31P NMR spectroscopy.67 Data are presented in Table S1.
Preparation of Colloidal Lignin Particles (CLPs and c-CLPs)
The CLPs used in this work were produced following the same procedure as described earlier with a few modifications.61 Briefly, SKL was dissolved in acetone/water (mass ratio, 3:1), insoluble impurities were removed by filtration, and CLPs were formed by rapid pouring of lignin solution into water. CLPs were purified by dialysis against water. The cationic lignin particles (c-CLPs) were prepared by adding the CLP dispersion into the water-soluble fraction of cationic lignin under vigorous stirring.67 The ratio of cationic lignin to CLPs was 200 mg g–1. Two batches of each type of particles were prepared. Characterization of both batches can be found in Table S2.
Particle Size and Zeta Potential
Particle size and zeta potential of CLPs, c-CLPs, and CCMVs were measured using a Zetasizer Nano-ZS90 instrument (Malvern, UK). The zeta potential was determined with a dip cell probe and calculated from the electrophoretic mobility data using a Smoluchowski model. Three measurements for each sample were conducted to evaluate the reproducibility of the measurement. A volume of 1 mL was collected for all measurements with a concentration of 0.2 mg mL–1 for CLPs, c-CLPs, and of 0.05 mg mL–1 for CCMVs. For the c-CLP/CCMV mixture, a volume ratio of 1:1 of the above dispersion and solution was used.
Electrophoretic Mobility Shift Assays
The effectiveness of binding between various lignin samples and CCMVs was studied by observing the electrophoretic mobility of CCMVs (50 mg L–1) in an agarose gel with varying lignin concentration (from 0 to 200 mg L–1). All measurements were conducted in agarose gels stained with ethidium bromide (625 μg L–1 in the final gel solution) and using 10 mM sodium acetate buffer with 1 mM EDTA (pH 4.7) as a running buffer. The gels were run at 100 V for 35 min and imaged using Bio-Rad Gel DocTM EZ imaging system. The quantification of the migration bands was completed using the gel analysis plugin on ImageJ software. Total area of all bands in the lane was used to calculate the percentage of diminution relative to the pure CCMV migration band.
Quantification of CCMVs Retained by CLPs with Centrifugation Method
Prior to the experiments, the c-CLPs and CLPs were washed at least three times by centrifugation (relative centrifugal force (rcf) = 21 130, 20 min) in 10 mM sodium acetate buffer at pH 5 (Table S3) to remove the free cationic lignin, which was not associated with the CLPs. CLP and c-CLP dispersions at different concentrations (0.034, 0.102, 0.204, 0.340, and 0.680 mg mL–1) and a stock dispersion of CCMV at 0.17 mg mL–1 with an absorbance of 1 at 260 nm were prepared. Two microliters of CLP or c-CLP dispersions (or buffer for reference) was mixed with 2 μL of CCMV dispersion from stock solution and centrifuged at rcf = 21 130 for 10 min. Two microliters of the supernatant was collected, and its absorbance was measured using a NanoDrop Lite spectrophotometer (Thermo Scientific, USA) with a light path length of 0.05 cm. The experiments were repeated at least three times. A second set of experiments was carried out as above but using aqueous solutions of three different salts: sodium chloride (NaCl), calcium chloride (CaCl2), and sodium sulfate (Na2SO4). The concentration of acetate buffer was 10 mM and pH 5. The salt concentration was fixed at 1 mM, while the weight ratio of lignin particles to CCMVs was fixed at 2:1. Three stock solutions of CCMVs were prepared in buffer and each containing a different salt. Stock solutions of CLPs and c-CLPs were prepared only in buffer. A two-way ANOVA statistical study was completed via Origin software, evaluating the type of salt and the charge of the lignin particles as factors. Mean values and variances were compared with a Tukey test.
Quantification of CCMVs Retained by c-CLPs with Filtration Method
The filtration experiments were completed in two different scales. In the first setup, three samples were prepared in water (total volume, 2 mL) with known concentrations: c-CLPs, CCMVs, and c-CLP/CCMV (4:1, w/w). Initial virus concentration in the mixture was 5 mg L–1. UV–vis spectra were collected (200–800 nm) using a Shimadzu (Japan) UV–vis spectrophotometer (UV-2550) equipped with a Shimadzu temperature controller (TCC controller) equilibrated at 25 °C. The samples were diluted in water to get an absorbance close to 1. Then, the dispersions were passed through a syringe filter with a 0.45 μm PTFE membrane, and the absorbance of the filtrate was measured again.
In the second setup, the filtration experiments were repeated twice using a smaller volume (200 μL) and a weight ratio of 2:1 (c-CLP/CCMV) with the same concentrations as previously described in the centrifugation setup and using a 0.45 μm GHP Acrodisc 13 (PALL) syringe filter. The UV-absorbance of the permeate was measured at 280 and 350 nm with a BioTek Eon microplate spectrophotometer using a type Take 3 plate.
Quantification of the amount of viruses removed by filtration using both setups was performed by calculation of the mass extinction coefficient of each sample (c-CLPs, CCMVs, and c-CLP/CCMV) at two wavelengths: 280 and 350 nm. At 280 nm, the absorbance is influenced by lignin and virus contents, while absorbance at 350 nm was mainly due to lignin. The concentration of all CCMVs after filtration was calculated from the difference in absorbance at 280 and 350 nm.
Atomic Force Microscopy (AFM)
Dispersions of CLPs, c-CLPs, and CCMVs were diluted in 10 mM NaOAc buffer (pH 5) in order to reach a concentration of 0.17 mg mL–1. A volume ratio of 1:1 was used for preparation of the mixtures, c-CLP/CCMV and CLP/CCMV. A minimum of 20 μL of each sample was spin-coated on freshly cleaved mica plates at 2000 rpm for 2 min using a WS-650X-6NPP/Lite spin coater (Laurell Technologies Corporation, North Wales, USA). High-resolution AFM images were recorded with a MultiMode 8 atomic force microscope equipped with a NanoScope V controller (Bruker Corporation, Billerica, MA). The images were obtained in air in tapping mode using NCHV-A probes (Bruker) with a tip radius below 10 nm. Research NanoScope 8.15 software (Bruker) was used for image analysis, processing, and correction.
Transmission Electron Microscopy (TEM)
CLP, c-CLP, and CCMV dispersions were imaged by transmission electron microscopy (TEM). The samples were deposited on carbon film in copper square mesh grids and dried under ambient conditions. Negative staining of CCMVs and agglomeration complexes with CLPs and c-CLPs was made with 3 wt % uranyl acetate. TEM images were acquired in bright-field mode on an FEI Tecnai 12 (USA) operating at 120 kV.
Results and Discussion
The objective of this work was to understand the nature of the interactions between the colloidal lignin particles and the viruses in order to evaluate the feasibility of using CLPs for virus removal. To reach this goal, we studied how the size, shape, and surface charge of the lignin particles affect the adsorption of viruses. Thereafter, we used a combination of techniques to study the complex formation in the course of agglomeration to elucidate how the different lignin samples interact with virus particles. Finally, we considered how these CLPs could be integrated into a water purification process. Characterization of the various lignin materials used in this work was the first important task to understand their interactions with viruses.
Characterization of Lignin Materials
Two cationic lignin materials and one anionic lignin were prepared. The anionic colloidal lignin particles (CLPs) were prepared from softwood kraft lignin following the previously reported nanoprecipitation method by rapidly pouring the lignin solution in aqueous acetone into water.61 The cationic lignin solution was a water-soluble fraction resulting from the cationization of kraft lignin, and c-CLPs were prepared by adsorption of water-soluble cationic lignin onto CLPs.67 These lignins mainly differ in their surface charge (zeta potential) and particle diameter (Table 1). The fresh batches of CLPs and c-CLPs prepared herein were found to have similar zeta potential values compared to our prior reports.65,67 The hydrodynamic diameters (Dh) of CLPs and c-CLPs were 109 and 122 nm, respectively. The slightly larger size of c-CLPs is due to the coating of CLPs with cationic lignin molecules. The diameter of the CCMVs was 30 nm, which is close to the literature value of 28 nm.66 The isoelectric point (pI = 3.8)66 of CCMVs indicates negative charge at pH 5 used in the experiments.
Table 1. Main Characteristics of Lignin Samples Tested for Virus Adsorption Experimentsa.
| lignin format | hydrodynamic diameter (nm) | PDI | zeta potential (mV) |
|---|---|---|---|
| CLPsb | 108.6 ± 1.7 | 0.065 ± 0.020 | –35.9 ± 1.6 |
| cationic ligninc | N/A | N/A | +21.7 ± 1.6 |
| c-CLPsb | 122.4 ± 0.5 | 0.056 ± 0.005 | +24.1 ± 1.4 |
At least three measurements were completed for each parameter. Error ranges are standard deviations. N/A: not analyzed
Values measured at pH 5 in 10 mM NaOAC buffer.
Value measured at pH 5 in water, sample diluted in acetic acid (pH 4.7).
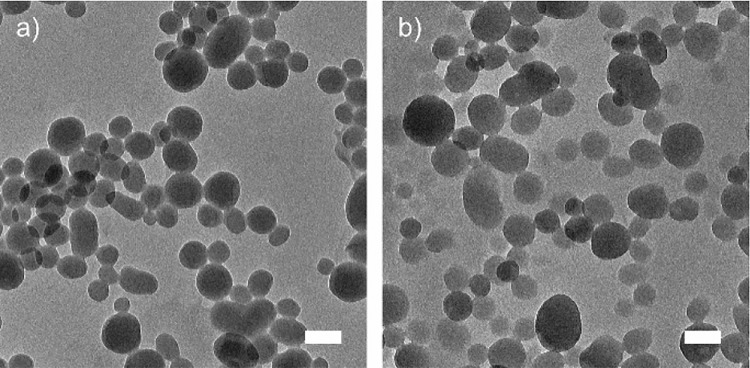
To evaluate differences in particle shape and morphology, the anionic and cationic CLPs were imaged using TEM (Figure 1). For both materials, individual particles were identified. The CLPs and c-CLPs that were prepared from aqueous acetone solution were smaller (Dh ∼ 100 nm) than the CLPs previously obtained from aqueous THF or THF/ethanol mixture (Dh ∼ 200–300 nm).56−58 This observation is commonly made when acetone is used as a solvent for dissolution of lignin instead of THF.67 Both acetone and THF are polar aprotic solvents, thus the slight difference in the particle diameters might be due to the lower polarity difference between water (relative polarity of 1) and acetone (relative polarity of 0.355) compared to the one between water and THF (relative polarity of 0.207).69 Additionally, due to its smaller size in comparison to THF, acetone can be more rapidly exchanged by water from the solvated lignin molecules, causing more rapid aggregation and thus smaller particles. One more parameter that could affect the particle size is the drying effect; however this was not observed with the CLPs and c-CLPs. The performance of these characterized lignin materials for virus binding will be shown in the next section.
Figure 1.
TEM images of (a) CLPs and (b) c-CLPs. Scale bars: 100 nm.
Affinity of Viruses to Lignin Materials
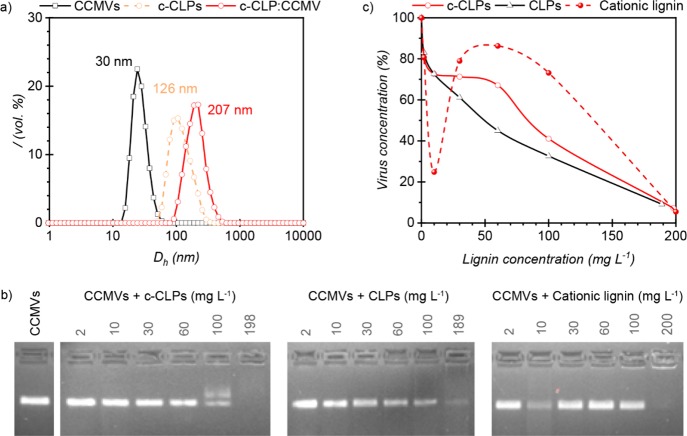
Dynamic Light Scattering (DLS) experiments were performed to preliminarily evaluate the possible interactions between the c-CLPs and anionic virus particles. The measured hydrodynamic diameters of CCMVs and c-CLPs were 30 nm (±0.1 nm) and 126 nm (±0.9 nm), respectively, with a low polydispersity index (PDI) value for both (0.08 ± 0.02 and 0.10 ± 0.04, respectively; Figure 2a). When c-CLPs were mixed with CCMVs at a 4:1 weight ratio, the average diameter of the particles increased to 207 nm (±3.3 nm) with a low PDI of 0.12 ± 0.01. This is more than what would be expected if a monolayer of viruses adsorbed on CLPs and suggests that c-CLPs could act as a flocculating agent. Furthermore, no second peak around 30 nm was detected during the DLS measurement of the mixture, thus no free viruses were detected, which in turn suggests a complete association of CCMVs with c-CLPs, or, at least, a reduction of free-virus concentration below the minimum threshold of detection. In addition, intensity distribution and autocorrelation function curves of the materials indicated monodisperse distributions (Figure S1). In previous research, a multiangle light scattering technique was used to determine removal of anionic polystyrene latex particles from water by supplemented cationic polyelectrolytes.70 In that case, several peaks were observed by light scattering. The appearance of these peaks was ascribed to a kinetic formation of large flocculates. Commonly, bulk polymers are used for flocculation, and few reports exist on virus removal by nano- or microscale particles.66 In the case of cationic polymers, several mechanisms have been proposed such as bridging, charge neutralization, or formation of electrostatic patches.28 Bridging flocculation usually happens with high molecular weight and low charged polyelectrolytes.71 In our case, we have spherical particles with low molecular weight cationic lignin adsorbed onto their surface that interact with very small virus particles of opposite charge, so typical bridging flocculation is not expected. The kraft lignin utilized in this study has a low molecular weight (weight average molecular weight 5250 g mol–1 based on gel permeation chromatography of acetylated lignin),41 compared to other flocculants such as polyacrylamide, polysaccharide, and chitosan derivatives (104 to 106 g mol–1).71 Patch flocculation is not probable in this case. Charge neutralization is undoubtedly important for the association of viruses and c-CLPs, but as will be discussed later, it is not the only driving force for the association.
Figure 2.
(a) Hydrodynamic diameter of CCMV (black line with squares), c-CLPs (dashed orange line with circles), and c-CLP:CCMV complex (4:1 w/w; red line with circles). Measurements were performed in water. (b) EMSA gel images of CCMVs (50 mg L–1) in the presence of colloidal and soluble lignin materials. Measurements were performed in 10 mM sodium acetate buffer (pH 4.7). (c) Quantification of the EMSA results using ImageJ. Lines in c are only to guide the eye. Data point at 10 mg L–1 of cationic lignin may be an outlier as indicated by the diffusion of the band at lower and higher fields compared to the main band.
To further assess association of virus particles with c-CLPs, a gel electrophoretic mobility shift assay (EMSA) was carried out. The objective was to quantify and compare the binding affinity of viruses to cationic and anionic lignin particles by following the migration of viruses in an agarose gel. Figure 2b presents the EMSA results for the anionic and cationic CLPs in comparison to water-soluble cationic lignin. The CCMV concentration was 50 mg L–1, and the first column indicates the extent of migration of CCMVs alone. When an increasing concentration of c-CLPs and soluble cationic lignin was added, we observed that the virus migration was completely prevented at the highest concentration tested (c.a. 200 mg L–1 of lignin). However, the virus migration appeared to be hindered already at a lower lignin concentration with the c-CLPs (100 mg L–1) as compared to 200 mg L–1 for soluble cationic lignin. Thus, the insoluble cationic lignin particles appeared more capable of associating with CCMVs than the soluble cationic lignin molecules.
Interestingly, it appears that not only cationic particles but also the negatively charged CLPs can partially interact with the viruses. In this case, the behavior was different, since the intensity of the virus band decreased already at a lower concentration (10 mg L–1) than observed with cationic samples but did not disappear completely even at the highest lignin concentration (189 mg L–1). These observations can be confirmed by quantification of migration bands. Figure 2c shows that there is a reduction of 67% in virus concentration when the concentration of CLPs was twice as high as the concentration of viruses (at 100 mg L–1). At the same lignin/virus ratio, the c-CLPs reduced 59% of the virus concentration, while it was only of 27% for cationic lignin. The reduction in the mobility of CCMVs could even reach 90–95% with all the tested lignin materials when the lignin amount was four times as high as that of the viruses. At the highest lignin concentration, c-CLPs were the most efficient ones. Their efficiency is probably even higher than it is indicated by the quantification since no spots are actually visible. Thus, we can consider that the CCMVs are completely stopped at 198 mg L–1 of c-CLPs.
Clearly, the lignin particles interacted with viruses not only electrostatically, but also by other noncovalent interactions. There may be hydrophobic interactions present. Indeed, prior literature has indicated aggregation of viruses in the presence of nonfunctionalized gold nanoparticles due to hydrophobic interactions,72 while porous activated carbon modified with silver and copper oxide nanoparticles showed high virus inactivation.73 Nonetheless, in the second case, the antiviral effect was due to the well-known antiviral effect of metal NPs. In another study, cationic membranes prepared by layer-by-layer assembly of polyethyleneimine/terephthalaldehyde and containing silver and copper NPs exhibited both inactivation and virus removal.74 Additionally, amine-modified silica NPs showed inactivation of anionic viruses by charge neutralization.75 However, clearance efficiency is mostly described in the literature by reduction of the log10 value and not the percentage reduction of the virus concentration in water.6,8,13,73,74 It is expressed as log(N0/Nt), where N0 is the initial concentration of virus and Nt the virus concentration after the removal or disinfection. A 90% reduction of the virus concentration corresponds to a reduction of 1 log10. By conversion, we can observe than our results are lower than the above cited systems.
To give a few examples, chlorine disinfection8 can reach a 4–5 log10 viral deactivation, while it is higher than 6 for the photovoltaic system.13 For systems based on metallic NPs (silver and copper oxide), the log10 removal can be higher than 3 when associated to activated carbon73 and even higher than 4 when the NPs are integrated in a layer-by-layer cationic membrane.74 Bacteriophages can be removed by 4.8 log10 within 1 h by iron/nickel nanoparticles.6 Magnetic nanoparticles75 reached 95% of removal of both viruses and bacteria in water. However, some of these results are obtained under idealized conditions (pH, salt, operating parameters), and their efficiency can be reduced under industrial conditions.76
Nonetheless, compared to these earlier studies, our materials consisting of renewable lignin hold many benefits in terms of a lower carbon footprint and minimization of waste generated in the material synthesis.58,59 Furthermore, water purification is not a one step process. Indeed, viruses are not the only pollutants present in water. There are also many biological (parasites, bacteria) and chemical (heavy metals, plastics) contaminants. For instance, industrial and municipal wastewaters are treated in a multistage process combining several technologies.76 Thus, the CLPs could be a substitute for primary treatments involving coagulation/flocculation and sedimentation.
In theory, the smaller the particles, the higher the hindering effect on virus migration, due to an increase in surface area. However, here we do not observe this. Instead, the rather large c-CLPs were more efficient binders than the soluble cationic lignin at similar concentration ratios relative to CCMV. Combining this observation with the effect of negatively charged CLPs, we suggest that the affinity of viruses to c-CLPs is a combination of electrostatic affinity and other types of interactions with lignin. CCMVs are protein cages and have amino acids on their surfaces that can participate in electric interactions with the phenolic rings (π–π and charge-π interactions) and electrostatic interactions with the carboxylic acid groups of lignin.66 Nonelectrostatic interactions such as hydrophobic and π–π interactions as well as hydrogen bonding were also previously discussed between lignin and enzymes and lignin and noncatalytic proteins.77,78 These nonelectrostatic interactions could contribute to the binding between CCMVs and the anionic CLPs, while the additional charge interactions induce a stronger complexation between the viruses and the c-CLPs. Although a large variety of lignin-based materials, including commercial lignosulfonic acid,79 sulfated lignin,80 or lignin-carbohydrate complexes, have been tested,81 cationic lignin has not been studied for viral inhibition. These prior works suggest that lignin either directly inhibits the viral RNA transcriptase or physically prevents the access of viruses into mammalian cells. Our results are in accordance with the latter mechanisms. The fact that c-CLPs form relatively large aggregates together with the CCMV facilitates removal of the complexes by centrifugation or filtration, and the feasibility of these two methods for virus removal is demonstrated in the section below.
Water Purification by Centrifugation and Filtration of Agglomerated Complexes
The materials presenting the highest virus binding were evaluated for water purification separetely by the following two methods: sedimentation assisted by centrifugation and filtration of the formed complexes (Scheme 1).
Scheme 1. Concept of Virus Removal by Cationic Lignin Particles.
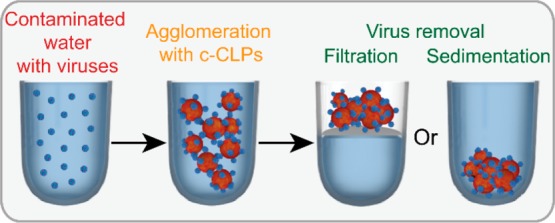
One of the main advantages anticipated from using colloidal lignin particles for virus removal is the possibility to form flocs that easily sediment or can be filtered from the water and combusted. The simplicity of the approach, the availability of lignin in large quantities to a relatively low price, and the techno-economical feasibility of the CLP manufacturing57−59 could make this a suitable method for the countries where the scarceness of clean water is the most acute. In accordance with the EMSA results, we observed upon centrifugation that both CLPs and c-CLPs flocculated viruses with a similar trend (Figure 3a). The absorbance of the supernatant decreased exponentially with increasing lignin content, leveling off when the ratio of lignin to CCMV was higher than 2:1 w/w. While the experimental uncertainty of the results with the anionic CLPs was rather high, the supernatant samples could be more reliably separated from the sediment in the case of cationic c-CLPs. This observation indicates that the c-CLP/CCMV complex was stronger than the one with CLP/CCMV, indicating that electrostatic interaction is the main driving force for association of viruses and the c-CLPs. This is in accordance with previous research in wastewater treatment with lignin-based materials.45,71 Additionally, the adsorption isotherm of CCMV on c-CLPs did not fit with the Langmuir model, confirming the aforementioned DLS results suggesting that instead of monolayer adsorption, viruses and lignin particles formed agglomeration complexes (Figure S2). These results are promising, since virus concentrations are typically low in the contaminated water, but there is a scarcity of accurate concentration data in the published reports. Indeed, there is a wide research area focused on virus detection.82−86 In turn, due to this low concentration in water, a high efficiency of virus removal could be achieved with a moderate supplementation of c-CLPs.
Figure 3.
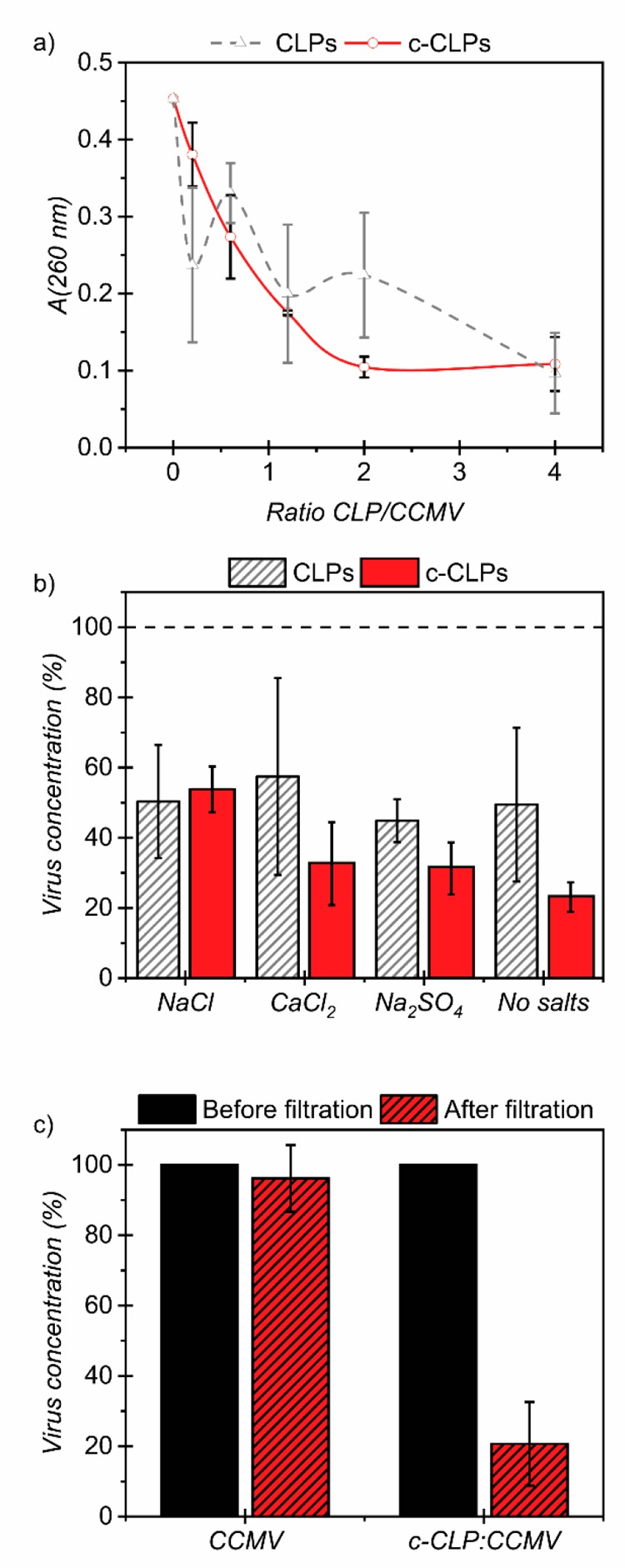
(a) Absorbance of the supernatant after centrifugation of CCMVs mixed with increasing concentration of CLPs or c-CLPs. (b) Remaining CCMV concentration in the supernatant obtained from centrifugation of c-CLPs or CLPs in the presence of different salts (1 mM) at a weight ratio of 2:1 (lignin/virus). (c) Remaining CCMV concentration before and after filtration through a 0.45 μm membrane. Error bars indicate one standard deviation relative to the mean values.
Actual industrial or municipal wastewaters contain salt, and with the purpose to upscale this process, it is important to know what would be the impact of the salt on the efficiency of the virus removal. For this reason, additional centrifugation experiments in the presence of different salts, NaCl, CaCl2, and Na2SO4, were completed. A salt concentration of 1 mM was chosen based on previous literature.87−89 Some studies have used higher salt concentrations,90,91 but rather to improve their systems, which is not relevant for our experiments. In our case, we wanted to see if the salt ions, which are naturally present in wastewaters, influence the removal of the viruses. A fixed weight ratio of 2:1 c-CLP/CCMV (dry basis) was selected since it was shown to be efficient by titration. Figure 3b shows the concentration of viruses measured in the supernatant after centrifugation. It was observed that the final CCMV concentrations were higher for all salts with the c-CLPs compared to the results obtained without added salts. However, the experimental variance was also higher. In case of the anionic CLPs, no significant differences were observed. In order to draw conclusions, a statistical study was performed, and overall ANOVA results are presented in Table S4. The study indicates that the presence of salts did not affect the efficiency of the virus removal. On the contrary, and in accordance with the discussion above, the most impactful factor was the charge of the CLPs.
Filtration is another common unit operation to separate particulate matter in water purification processes.39,74 For the filtration method, two samples were prepared at a ratio of 2:1 c-CLP/CCMV, and then agglomerated viruses were separated by filtration. The full UV–vis spectra were recorded before and after filtration and compared to those measured from c-CLPs and CCMVs alone in order to determine the percentage of virus removal. By filtration, we observed a similar effect as with centrifugation. CCMV concentration was reduced by 79% when viruses were mixed with c-CLPs (Figure 3c). Furthermore, CCMVs were not removed with the same filtration using a 0.45 μm Acrodisc 13 syringe filter in the absence of lignin particles, illustrating why filtration alone may not be an efficient way to remove nanosized viruses from water. Characteristic UV spectra of c-CLPs and CCMVs can be seen in the Supporting Information (Figure S3) using a ratio of 4:1 c-CLPs and CCMVs. A mass ratio of 2:1 of c-CLPs to CCMVs was required to reach 80% virus removal both using filtration or centrifugation. The closest work found for comparison describes lignin particles in complex with gelatin as flocculants for the bacteria Staphylococcus aureus and Escherichia coli.92 Another study presents a lignin-based nanotrap for metallic ion pollutants and an antimicrobial effect against E. coli.93 However, we are not aware of any previous reports of lignin-based flocculants or filtration membranes for the virus removal.
Morphology of Agglomerated Complexes of CCMVs and Colloidal Lignins
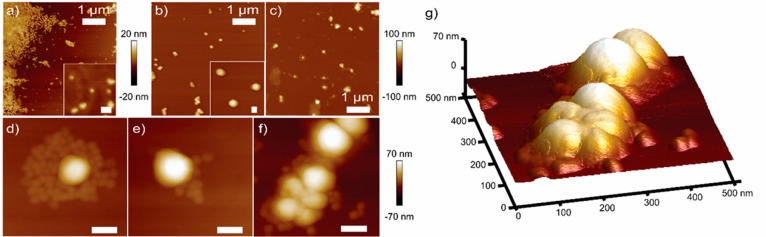
Understanding of the morphology of the agglomeration complex is important to improve the virus removal concept. In Figure 4, AFM height images of c-CLPs, CCMVs, and the c-CLP/CCMV complexes are presented. Particles of two distinct sizes were present in the mixture of c-CLPs and viruses. The small particles around 44 nm in width and 10 nm in height correspond to viruses and the larger 142 nm sized particles to lignin particles (see Table S5). The imaged material was dried prior to analysis, inducing aggregation of the particles into patches as seen in Figure 4c. The c-CLPs and the CCMVs were found in different shapes of clusters, suggesting that they agglomerated already in water. In Figure 4d and e, viruses surround one c-CLP, while in Figure 4f, a cluster of several c-CLPs and viruses has formed. Furthermore, in the absence of viruses (Figure 4b), similar clusters were not seen. The peaks detected at 207 nm by DLS could correspond to complexes observed in Figure 4d and e. Extended contact time could have favored formation of bigger complexes as those observed in Figure 4f, while drying during spin-coating could have removed some viruses from the surface of the c-CLPs. Thus, there might be an opportunity to separate CLPs from the agglomerated virus complexes by utilizing the anticipated difference in the specific gravity between these two materials.
Figure 4.
AFM height images of (a) CCMVs, (b) c-CLPs, and (c–f) c-CLP/CCMV (2:1 w/w) complex. (g) 3D image of f. If no indications, scale bar of 100 nm.
When the viruses were mixed with anionic CLPs, the formation of larger agglomerates was suppressed (AFM images in Figure S4). Thus, the anionic CLPs do not agglomerate fully with the anionic CCMVs due to the charge repulsion. This result confirms the observation of weaker interaction between CLPs and CCMVs from the centrifugation experiments. Therefore, among the lignin materials tested, c-CLPs are the best candidates for virus removal.
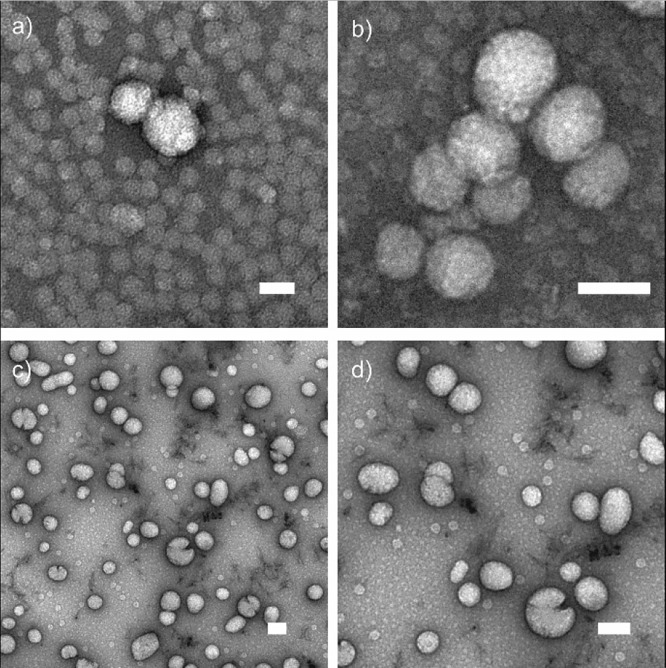
TEM images (Figure 5a and b) support the observations made above from the AFM investigation. The c-CLPs are overlapping with the virus particles, although free viruses are also observed. In Figure 5b, a c-CLP network is observed when viruses are added, but not when the c-CLPs are alone in solution, proving the virus-agglomerating effect of lignin particles and explaining the efficient virus removal observed combining flocculation using lignin particles and centrifugation or filtration. We found that some of the CLPs cracked in the presence of CCMVs (Figure 5c and d and Figures S5 and S6), and such distorted particles were observed with nonstained CLPs, but not with c-CLPs (Figure 5a and b). Although these features may be artifacts from the sample preparation, we have not previously detected such disintegration of lignin particles under the electron beam.
Figure 5.
TEM images of c-CLP/CCMV complexes (panels a and b) and CLP/CCMV complexes (panels c and d). All ratios: (2:1 w/w). All scale bars: 100 nm.
In a previous study using modified CNCs,36 the adsorption of CCMVs was facilitated by grafting of long cationic polymer chains that extended away from the particle surface. However, CNCs have a similar size to the viruses, and hence both particles were easier to fix at the focus area of TEM in comparison to here, where the CLPs are 5 to 10 times larger than the viruses. While these CNCs were very efficient in adsorbing viruses,36 they did not form agglomerates—a necessary feature to enable simple removal of the viruses in practice. Although AFM and TEM imaging does not give information on the nature of the interactions, the observations support the conclusions made based on the DLS results and virus removal experiments. Interestingly, at a mass ratio of 2:1 of CLPs to CCMVs, centrifugation or filtration is more efficient compared to EMSA. However, when the lignin concentration increases, the virus concentration was not reduced, while in EMSA, a 4 to 1 ratio allowed a reduction of 90–100% of the initial virus concentration. These observations indicate that the technique could be improved by inserting the lignin particles into physical matrices such as hydrogels,94 membranes,65 or columns. Furthermore, integration of these systems within a closed-water cycle could ensure that either the CLPs are saturated or the viruses are fully attached to the CLPs before replacement.
Our results presented in this study outline a particle/particle agglomeration system. These results could have a marked environmental impact and find application in the biomedical field since the lignin particles are nanoscaled and their agglomeration effect suggests an interaction with the viruses. Expanding the utilization of CLPs for advanced water purification processes or as a low-cost pretreatment of water purification would be a great advance regarding the lignocellulosic biorefinery concept. The production cost of lignin is estimated to be below 0.5 €/kg and production of CLPs below 1 €/kg,58 while the added value in the virus removal application is expectedly significantly higher. In comparison, the majority of chemical coagulants and flocculants were sold between 0.130 and 2.980 €/kg in 2005.28 Furthermore, association of these CLPs with existing membranes could help to increase the lifetime of the membranes. Although some points need to be clarified, such as the biocompatibility of cationic particles, commercialization and upscaling of the process with real wastewater can be done with anionic CLPs or cationic CLPs based on chitosan-coated CLPs.61 However, the priority for further investigations could be given to the anionic CLPs, since the preparation has been already found feasible on a semi-industrial scale, and less synthetic steps are needed compared to the use of cationic CLPs.
Conclusion
The applicability of colloidal lignin particles as water purification agents for virus removal was demonstrated. Cationic lignin particles agglomerated viruses, enabling removal of these nanoscale contaminants from water by scalable filtration and centrifugation methods. We showed that renewable plant biomass-sourced CLPs act as agglomeration agents to facilitate virus removal—perhaps the most challenging biological species due to their virulence and small size—that paves the way for further exploration of CLPs in water purification. Overall, our results showed that CLPs are promising low-cost water purification agents that hold potential for large-scale applications. Since CLPs are readily tailorable by simple adsorption approaches, colloidal lignins hold potential for the treatment of a broad spectrum of industrial wastewater containing organic and microbial contaminants. Several systems can be engineered for up-scaled utilization of lignin particles, such as immobilization of CLPs into packed columns or filtration membranes, or as flocculation and sedimentation agents in water purification processes. We expect that successful demonstration of CLPs in water purification applications will support commercialization of lignocellulosic biorefineries that need to find lucrative applications for their lignin side-streams.
Acknowledgments
This publication is a part of the Zelcor project and has received funding from the Bio Based Industry Joint Undertaking under the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 720303. M.H.S. acknowledges Academy of Finland for funding (Grant 296547). We are grateful for the support by the FinnCERES Materials Bioeconomy Ecosystem. This work made use of the Aalto University Bioeconomy facilities.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acssuschemeng.9b06915.
31P NMR data of cationic lignin; characterization of the different CLP and c-CLP batches; absorbance at 260 nm and zeta potential of c-CLP dispersions before and after centrifugation and washing; additional dynamic light scattering data; fitting of CCMV-lignin complexation to Langmuir equation; overall ANOVA study from virus removal experiments by centrifugation; full UV–vis spectra conducted for the virus removal by filtration; height and width of particles by AFM sections; additional AFM and TEM images of CLP:CCMV complexes (PDF)
Author Contributions
G.N.R. designed the experiments with contributions from all authors. T.Z. prepared the lignin particles with input from G.N.R. and M.H.S. A.K. carried out the EMSA tests and assisted G.N.R. in the analysis of the data. M.H.S. collected the TEM images with G.N.R. The manuscript was written by G.N.R. with contributions from all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Pichel N.; Vivar M.; Fuentes M. The Problem of Drinking Water Access: A Review of Disinfection Technologies with an Emphasis on Solar Treatment Methods. Chemosphere 2019, 218, 1014–1030. 10.1016/j.chemosphere.2018.11.205. [DOI] [PubMed] [Google Scholar]
- Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baselines; World Health Organization; United Nations Children’s Fund: Geneva, 2017. [Google Scholar]
- Field C. B.; Barros V. R.; Dokken D. J.; Mach K. J.; Mastrandrea M. D.; Bilir T. E.; Chatterjee M.; Ebi K. L.; Estrada Y. O.; Genova R. C.; et al. Summary for Policymakers. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, U.K., 2014. [Google Scholar]
- Benetti A. D.; Prüss-Üstün A.; Corvalán C. Preventing Disease through Healthy Environments: A Global Assessment of the Burden of Disease. Eng. Sanit. Ambient. 2007, 12, 115. 10.1590/S1413-41522007000200001. [DOI] [Google Scholar]
- Progress on Safe Treatment and Use of Wastewater: Piloting the Monitoring Methodology and Initial Findings for SDG Indicator 6.3.1. World Health Organization; UN-HABITAT: Geneva, 2018, 10.1128/MCB.24.8.3430. [DOI] [Google Scholar]
- Cheng R.; Kang M.; Zhuang S.; Wang S.; Zheng X.; Pan X.; Shi L.; Wang J. Removal of Bacteriophage F2 in Water by Fe/Ni Nanoparticles: Optimization of Fe/Ni Ratio and Influencing Factors. Sci. Total Environ. 2019, 649, 995–1003. 10.1016/j.scitotenv.2018.08.380. [DOI] [PubMed] [Google Scholar]
- Keswick B. H.; Satterwhite T. K.; Johnson P. C.; DuPont H. L.; Secor S. L.; Bitsura J. A.; Gary G. W.; Hoff J. C. Inactivation of Norwalk Virus in Drinking Water by Chlorine. Appl. Environ. Microbiol. 1985, 50 (2), 261–264. 10.1128/AEM.50.2.261-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin G.-A.; Sobsey M. D. Inactivation of Norovirus by Chlorine Disinfection of Water. Water Res. 2008, 42 (17), 4562–4568. 10.1016/j.watres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Camel V.; Bermond A. The Use of Ozone and Associated Oxidation Processes in Drinking Water Treatment. Water Res. 1998, 32 (11), 3208–3222. 10.1016/S0043-1354(98)00130-4. [DOI] [Google Scholar]
- Shin G.-A.; Sobsey M. D. Reduction of Norwalk Virus, Poliovirus 1 and Bacteriophage MS2 by Ozone Disinfection of Water. Appl. Environ. Microbiol. 2003, 69 (7), 3975–3978. 10.1128/AEM.69.7.3975-3978.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichel N.; Vivar M.; Fuentes M. Performance Analysis of a Solar Photovoltaic Hybrid System for Electricity Generation and Simultaneous Water Disinfection of Wild Bacteria Strains. Appl. Energy 2016, 171, 103–112. 10.1016/j.apenergy.2016.03.050. [DOI] [Google Scholar]
- Pichel N.; Vivar M.; Fuentes M. Performance Study of a Hybrid Photovoltaic and Solar Water Disinfection System Considering Climatic Variations over a Year. Energy Convers. Manage. 2017, 144, 312–321. 10.1016/j.enconman.2017.04.080. [DOI] [Google Scholar]
- Wang Y.; Jin Y.; Huang Q.; Zhu L.; Vivar M.; Qin L.; Sun Y.; Cui Y.; Cui L. Photovoltaic and Disinfection Performance Study of a Hybrid Photovoltaic-Solar Water Disinfection System. Energy 2016, 106, 757–764. 10.1016/j.energy.2016.03.112. [DOI] [Google Scholar]
- Pinon A.; Vialette M. Survival of Viruses in Water. Intervirology 2019, 61 (5), 214–222. 10.1159/000484899. [DOI] [PubMed] [Google Scholar]
- Qu X.; Alvarez P. J. J.; Li Q. Applications of Nanotechnology in Water and Wastewater Treatment. Water Res. 2013, 47 (12), 3931–3946. 10.1016/j.watres.2012.09.058. [DOI] [PubMed] [Google Scholar]
- Boisselier E.; Astruc D. Gold Nanoparticles in Nanomedicine: Preparations, Imaging, Diagnostics, Therapies and Toxicity. Chem. Soc. Rev. 2009, 38 (6), 1759–1782. 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- Mueller N. C.; Braun J.; Bruns J.; Černík M.; Rissing P.; Rickerby D.; Nowack B. Application of Nanoscale Zero Valent Iron (NZVI) for Groundwater Remediation in Europe. Environ. Sci. Pollut. Res. 2012, 19 (2), 550–558. 10.1007/s11356-011-0576-3. [DOI] [PubMed] [Google Scholar]
- Upadhyayula V. K. K.; Deng S.; Mitchell M. C.; Smith G. B. Application of Carbon Nanotube Technology for Removal of Contaminants in Drinking Water: A Review. Sci. Total Environ. 2009, 408 (1), 1–13. 10.1016/j.scitotenv.2009.09.027. [DOI] [PubMed] [Google Scholar]
- Zheng X.; Shen Z.-P.; Cheng C.; Shi L.; Cheng R.; Yuan D.-H. Photocatalytic Disinfection Performance in Virus and Virus/Bacteria System by Cu-TiO2 Nanofibers under Visible Light. Environ. Pollut. 2018, 237, 452–459. 10.1016/j.envpol.2018.02.074. [DOI] [PubMed] [Google Scholar]
- Fan M.; Gong L.; Huang Y.; Wang D.; Gong Z. Facile Preparation of Silver Nanoparticle Decorated Chitosan Cryogels for Point-of-Use Water Disinfection. Sci. Total Environ. 2018, 613–614, 1317–1323. 10.1016/j.scitotenv.2017.09.256. [DOI] [PubMed] [Google Scholar]
- Dos Santos C. A.; Seckler M. M.; Ingle A. P.; Gupta I.; Galdiero S.; Galdiero M.; Gade A.; Rai M. Silver Nanoparticles: Therapeutical Uses, Toxicity, and Safety Issues. J. Pharm. Sci. 2014, 103 (7), 1931–1944. 10.1002/jps.24001. [DOI] [PubMed] [Google Scholar]
- Liga M. V.; Bryant E. L.; Colvin V. L.; Li Q. Virus Inactivation by Silver Doped Titanium Dioxide Nanoparticles for Drinking Water Treatment. Water Res. 2011, 45 (2), 535–544. 10.1016/j.watres.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Soenen S. J. H.; Himmelreich U.; Nuytten N.; De Cuyper M. Cytotoxic Effects of Iron Oxide Nanoparticles and Implications for Safety in Cell Labelling. Biomaterials 2011, 32 (1), 195–205. 10.1016/j.biomaterials.2010.08.075. [DOI] [PubMed] [Google Scholar]
- Auffan M.; Rose J.; Bottero J.-Y.; Lowry G. V.; Jolivet J.-P.; Wiesner M. R. Towards a Definition of Inorganic Nanoparticles from an Environmental, Health and Safety Perspective. Nat. Nanotechnol. 2009, 4 (10), 634–641. 10.1038/nnano.2009.242. [DOI] [PubMed] [Google Scholar]
- Pan C.; Becerra-Arteaga A.; Tran B.; Chinn M.; Wang H.; Chen Q.; Lutz H.; Zhang M. Characterizing and Enhancing Virus Removal by Protein A Chromatography. Biotechnol. Bioeng. 2019, 116, 846–856. 10.1002/bit.26866. [DOI] [PubMed] [Google Scholar]
- Kosiol P.; Kahrs C.; Thom V.; Ulbricht M.; Hansmann B. Investigation of Virus Retention by Size Exclusion Membranes under Different Flow Regimes. Biotechnol. Prog. 2019, 35 (2), e2747. 10.1002/btpr.2747. [DOI] [PubMed] [Google Scholar]
- Bolto B.; Gregory J. Organic Polyelectrolytes in Water Treatment. Water Res. 2007, 41 (11), 2301–2324. 10.1016/j.watres.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Lee C. S.; Robinson J.; Chong M. F. A Review on Application of Flocculants in Wastewater Treatment. Process Saf. Environ. Prot. 2014, 92 (6), 489–508. 10.1016/j.psep.2014.04.010. [DOI] [Google Scholar]
- McCurdy K.; Carlson K.; Gregory D. Floc Morphology and Cyclic Shearing Recovery: Comparison of Alum and Polyaluminum Chloride Coagulants. Water Res. 2004, 38 (2), 486–494. 10.1016/j.watres.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Ghafari S.; Aziz H. A.; Isa M. H.; Zinatizadeh A. A. Application of Response Surface Methodology (RSM) to Optimize Coagulation-Flocculation Treatment of Leachate Using Poly-Aluminum Chloride (PAC) and Alum. J. Hazard. Mater. 2009, 163 (2–3), 650–656. 10.1016/j.jhazmat.2008.07.090. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Gao B.; Yue Q. Coagulation Performance and Residual Aluminum Speciation of Al2(SO4)3 and Polyaluminum Chloride (PAC) in Yellow River Water Treatment. Chem. Eng. J. 2010, 165 (1), 122–132. 10.1016/j.cej.2010.08.076. [DOI] [Google Scholar]
- Flaten T. P. Aluminium as a Risk Factor in Alzheimer’s Disease, with Emphasis on Drinking Water. Brain Res. Bull. 2001, 55 (2), 187–196. 10.1016/S0361-9230(01)00459-2. [DOI] [PubMed] [Google Scholar]
- Ward R. J. S.; McCrohan C. R.; White K. N. Influence of Aqueous Aluminium on the Immune System of the Freshwater Crayfish Pacifasticus Leniusculus. Aquat. Toxicol. 2006, 77 (2), 222–228. 10.1016/j.aquatox.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Serizawa T.; Sawada T.; Okura H.; Wada M. Hydrolytic Activities of Crystalline Cellulose Nanofibers. Biomacromolecules 2013, 14 (3), 613–617. 10.1021/bm4000822. [DOI] [PubMed] [Google Scholar]
- Zoppe J. O.; Ruottinen V.; Ruotsalainen J.; Rönkkö S.; Johansson L.-S.; Hinkkanen A.; Järvinen K.; Seppälä J. Synthesis of Cellulose Nanocrystals Carrying Tyrosine Sulfate Mimetic Ligands and Inhibition of Alphavirus Infection. Biomacromolecules 2014, 15 (4), 1534–1542. 10.1021/bm500229d. [DOI] [PubMed] [Google Scholar]
- Rosilo H.; McKee J. R.; Kontturi E.; Koho T.; Hytönen V. P.; Ikkala O.; Kostiainen M. A. Cationic Polymer Brush-Modified Cellulose Nanocrystals for High-Affinity Virus Binding. Nanoscale 2014, 6 (20), 11871–11881. 10.1039/C4NR03584D. [DOI] [PubMed] [Google Scholar]
- Gustafsson O.; Manukyan L.; Mihranyan A. High-Performance Virus Removal Filter Paper for Drinking Water Purification. Glob. Challenges 2018, 2 (7), 1800031. 10.1002/gch2.201800031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson S.; Westermann F.; Hanrieder T.; Jung L.; Ruppach H.; Mihranyan A. Comparative Analysis of Dry and Wet Porometry Methods for Characterization of Regular and Cross-Linked Virus Removal Filter Papers. Membranes 2019, 9 (1), 1–13. 10.3390/membranes9010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manukyan L.; Li P.; Gustafsson S.; Mihranyan A. Growth Media Filtration Using Nanocellulose-Based Virus Removal Filter for Upstream Biopharmaceutical Processing. J. Membr. Sci. 2019, 572, 464–474. 10.1016/j.memsci.2018.11.002. [DOI] [Google Scholar]
- Wahlström R.; Kalliola A.; Heikkinen J.; Kyllönen H.; Tamminen T. Lignin Cationization with Glycidyltrimethylammonium Chloride Aiming at Water Purification Applications. Ind. Crops Prod. 2017, 104, 188–194. 10.1016/j.indcrop.2017.04.026. [DOI] [Google Scholar]
- Sipponen M. H.; Farooq M.; Koivisto J.; Pellis A.; Seitsonen J.; Österberg M. Spatially Confined Lignin Nanospheres for Biocatalytic Ester Synthesis in Aqueous Media. Nat. Commun. 2018, 9 (1), 1–7. 10.1038/s41467-018-04715-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurichesse S.; Avérous L. Chemical Modification of Lignins: Towards Biobased Polymers. Prog. Polym. Sci. 2014, 39 (7), 1266–1290. 10.1016/j.progpolymsci.2013.11.004. [DOI] [Google Scholar]
- Betancur M.; Bonelli P. R.; Velásquez J. A.; Cukierman A. L. Potentiality of Lignin from the Kraft Pulping Process for Removal of Trace Nickel from Wastewater: Effect of Demineralisation. Bioresour. Technol. 2009, 100 (3), 1130–1137. 10.1016/j.biortech.2008.08.023. [DOI] [PubMed] [Google Scholar]
- Kong F.; Parhiala K.; Wang S.; Fatehi P. Preparation of Cationic Softwood Kraft Lignin and Its Application in Dye Removal. Eur. Polym. J. 2015, 67, 335–345. 10.1016/j.eurpolymj.2015.04.004. [DOI] [Google Scholar]
- He W.; Zhang Y.; Fatehi P. Sulfomethylated Kraft Lignin as a Flocculant for Cationic Dye. Colloids Surf., A 2016, 503, 19–27. 10.1016/j.colsurfa.2016.05.009. [DOI] [Google Scholar]
- Guo Y.; Gao W.; Kong F.; Fatehi P. One-Pot Preparation of Zwitterion-Type Lignin Polymers. Int. J. Biol. Macromol. 2019, 140, 429–440. 10.1016/j.ijbiomac.2019.08.135. [DOI] [PubMed] [Google Scholar]
- Sipponen M. H.; Österberg M. Aqueous Ammonia Pre-Treatment of Wheat Straw: Process Optimization and Broad Spectrum Dye Adsorption on Nitrogen-Containing Lignin. Front. Chem. 2019, 7, 1–14. 10.3389/fchem.2019.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou T.; Cui G.; Xun J.; Wang X.; Feng N.; Zhang J. Synthesis of a Terpolymer Based on Chitosan and Lignin as an Effective Flocculant for Dye Removal. Colloids Surf., A 2018, 537, 149–154. 10.1016/j.colsurfa.2017.10.012. [DOI] [Google Scholar]
- Zhang Y.; Ni S.; Wang X.; Zhang W.; Lagerquist L.; Qin M.; Willför S.; Xu C.; Fatehi P. Ultrafast Adsorption of Heavy Metal Ions onto Functionalized Lignin-Based Hybrid Magnetic Nanoparticles. Chem. Eng. J. 2019, 372, 82–91. 10.1016/j.cej.2019.04.111. [DOI] [Google Scholar]
- Fan H. L.; Zhou S. F.; Jiao W. Z.; Qi G. S.; Liu Y. Z. Removal of Heavy Metal Ions by Magnetic Chitosan Nanoparticles Prepared Continuously via High-Gravity Reactive Precipitation Method. Carbohydr. Polym. 2017, 174, 1192–1200. 10.1016/j.carbpol.2017.07.050. [DOI] [PubMed] [Google Scholar]
- Baumberger S.; Lapierre C.; Monties B. Utilization of Pine Kraft Lignin in Starch Composites: Impact of Structural Heterogeneity. J. Agric. Food Chem. 1998, 46 (6), 2234–2240. 10.1021/jf971067h. [DOI] [Google Scholar]
- Stewart D. Lignin as a Base Material for Materials Applications: Chemistry, Application and Economics. Ind. Crops Prod. 2008, 27 (2), 202–207. 10.1016/j.indcrop.2007.07.008. [DOI] [Google Scholar]
- Gellerstedt G. Softwood Kraft Lignin: Raw Material for the Future. Ind. Crops Prod. 2015, 77, 845–854. 10.1016/j.indcrop.2015.09.040. [DOI] [Google Scholar]
- Ago M.; Huan S.; Borghei M.; Raula J.; Kauppinen E. I.; Rojas O. J. High-Throughput Synthesis of Lignin Particles (from 30 Nm to 2 Mm) via Aerosol Flow Reactor: Size Fractionation and Utilization in Pickering Emulsions. ACS Appl. Mater. Interfaces 2016, 8 (35), 23302–23310. 10.1021/acsami.6b07900. [DOI] [PubMed] [Google Scholar]
- Ago M.; Tardy B. L.; Wang L.; Guo J.; Khakalo A.; Rojas O. J. Supramolecular Assemblies of Lignin into Nano- and Microparticles. MRS Bull. 2017, 42 (5), 371–378. 10.1557/mrs.2017.88. [DOI] [Google Scholar]
- Lievonen M.; Valle-Delgado J. J.; Mattinen M.-L.; Hult E.-L.; Lintinen K.; Kostiainen M. A.; Paananen A.; Szilvay G. R.; Setälä H.; Österberg M. Simple Process for Lignin Nanoparticle Preparation. Green Chem. 2016, 18 (5), 1416–1422. 10.1039/C5GC01436K. [DOI] [Google Scholar]
- Leskinen T.; Smyth M.; Xiao Y.; Lintinen K.; Mattinen M.-L.; Kostiainen M. A.; Oinas P.; Österberg M. Scaling Up Production of Colloidal Lignin Particles. Nord. Pulp Pap. Res. J. 2017, 32 (04), 586–596. 10.3183/NPPRJ-2017-32-04-p586-596. [DOI] [Google Scholar]
- Lintinen K.; Xiao Y.; Bangalore Ashok R.; Leskinen T.; Sakarinen E.; Sipponen M.; Muhammad F.; Oinas P.; Österberg M.; Kostiainen M. Closed Cycle Production of Concentrated and Dry Redispersible Colloidal Lignin Particles with a Three Solvent Polarity Exchange Method. Green Chem. 2018, 20 (4), 843–850. 10.1039/C7GC03465B. [DOI] [Google Scholar]
- Bangalore Ashok R. P.; Oinas P.; Lintinen K.; Sarwar G.; Kostiainen M. A.; Österberg M. Techno-Economic Assessment for the Large-Scale Production of Colloidal Lignin Particles. Green Chem. 2018, 20 (21), 4911–4919. 10.1039/C8GC02805B. [DOI] [Google Scholar]
- Qian Y.; Zhong X.; Li Y.; Qiu X. Fabrication of Uniform Lignin Colloidal Spheres for Developing Natural Broad-Spectrum Sunscreens with High Sun Protection Factor. Ind. Crops Prod. 2017, 101, 54–60. 10.1016/j.indcrop.2017.03.001. [DOI] [Google Scholar]
- Zou T.; Sipponen M. H.; Österberg M. Natural Shape-Retaining Microcapsules With Shells Made of Chitosan-Coated Colloidal Lignin Particles. Front. Chem. 2019, 7, 370. 10.3389/fchem.2019.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipponen M. H.; Lange H.; Crestini C.; Henn A.; Österberg M. Lignin for Nano- and Microscaled Carrier Systems: Applications, Trends, and Challenges. ChemSusChem 2019, 12 (10), 2039–2054. 10.1002/cssc.201900480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu N.; Naoe T.; Kawazoe Y.; Sakagami H.; Nakashima H.; Murakami T.; Yamamoto N. Lignified Materials as Medicinal Resources. VI. Anti-HIV Activity of Dehydrogenation Polymer of p-Coumaric Acid, a Synthetic Lignin, in a Quasi-In-Vivo Assay System as an Intermediary Step to Clinical Trials. Biol. Pharm. Bull. 1993, 16 (4), 434–436. 10.1248/bpb.16.434. [DOI] [PubMed] [Google Scholar]
- Vinardell M. P.; Mitjans M. Lignins and Their Derivatives with Beneficial Effects on Human Health. Int. J. Mol. Sci. 2017, 18 (6), 1219. 10.3390/ijms18061219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq M.; Zou T.; Riviere G.; Sipponen M. H.; Österberg M. Strong, Ductile, and Waterproof Cellulose Nanofibril Composite Films with Colloidal Lignin Particles. Biomacromolecules 2019, 20 (2), 693–704. 10.1021/acs.biomac.8b01364. [DOI] [PubMed] [Google Scholar]
- Korpi A.; Ma C.; Liu K.; Nonappa; Herrmann A.; Ikkala O.; Kostiainen M. A. Self-Assembly of Electrostatic Cocrystals from Supercharged Fusion Peptides and Protein Cages. ACS Macro Lett. 2018, 7 (3), 318–323. 10.1021/acsmacrolett.8b00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipponen M. H.; Smyth M.; Leskinen T.; Johansson L. S.; Österberg M. All-Lignin Approach to Prepare Cationic Colloidal Lignin Particles: Stabilization of Durable Pickering Emulsions. Green Chem. 2017, 19 (24), 5831–5840. 10.1039/C7GC02900D. [DOI] [Google Scholar]
- Granata A.; Argyropoulos D. S. 2-Chloro-4,4,5,5-Tetramethyl-1,3,2-Dioxaphospholane, a Reagent for the Accurate Determination of the Uncondensed and Condensed Phenolic Moieties in Lignins. J. Agric. Food Chem. 1995, 43 (6), 1538–1544. 10.1021/jf00054a023. [DOI] [Google Scholar]
- Reichardt C.; Welton T.. Solvents and Solvent Effects in Organic Chemistry; Wiley-VCH; Weinheim, 2006. [Google Scholar]
- Biggs S.; Habgood M.; Jameson G. J.; Yan Y. Aggregate Structures Formed via a Bridging Flocculation Mechanism. Chem. Eng. J. 2000, 80 (1–3), 13–22. 10.1016/S1383-5866(00)00072-1. [DOI] [Google Scholar]
- Fang R.; Cheng X.; Xu X. Synthesis of Lignin-Base Cationic Flocculant and Its Application in Removing Anionic Azo-Dyes from Simulated Wastewater. Bioresour. Technol. 2010, 101 (19), 7323–7329. 10.1016/j.biortech.2010.04.094. [DOI] [PubMed] [Google Scholar]
- Palomino-Vizcaino G.; Valencia Reséndiz D. G.; Benítez-Hess M. L.; Martínez-Acuña N.; Tapia-Vieyra J. V.; Bahena D.; Díaz-Sánchez M.; García-González O. P.; Alvarez-Sandoval B. A.; Alvarez-Salas L. M. Effect of HPV16 L1 Virus-like Particles on the Aggregation of Non-Functionalized Gold Nanoparticles. Biosens. Bioelectron. 2018, 100, 176–183. 10.1016/j.bios.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Shimabuku Q. L.; Arakawa F. S.; Fernandes Silva M.; Ferri Coldebella P.; Ueda-Nakamura T.; Fagundes-Klen M. R.; Bergamasco R. Water Treatment with Exceptional Virus Inactivation Using Activated Carbon Modified with Silver (Ag) and Copper Oxide (CuO) Nanoparticles. Environ. Technol. 2017, 38 (16), 2058–2069. 10.1080/09593330.2016.1245361. [DOI] [PubMed] [Google Scholar]
- Sinclair T. R.; Patil A.; Raza B. G.; Reurink D.; van den Hengel S. K.; Rutjes S. A.; de Roda Husman A. M.; Roesink H. D. W.; de Vos W. M. Cationically Modified Membranes Using Covalent Layer-by-Layer Assembly for Antiviral Applications in Drinking Water. J. Membr. Sci. 2019, 570–571, 494–503. 10.1016/j.memsci.2018.10.081. [DOI] [Google Scholar]
- Zhan S.; Yang Y.; Shen Z.; Shan J.; Li Y.; Yang S.; Zhu D. Efficient Removal of Pathogenic Bacteria and Viruses by Multifunctional Amine-Modified Magnetic Nanoparticles. J. Hazard. Mater. 2014, 274, 115–123. 10.1016/j.jhazmat.2014.03.067. [DOI] [PubMed] [Google Scholar]
- Bolisetty S.; Peydayesh M.; Mezzenga R. Sustainable Technologies for Water Purification from Heavy Metals: Review and Analysis. Chem. Soc. Rev. 2019, 48 (2), 463–487. 10.1039/C8CS00493E. [DOI] [PubMed] [Google Scholar]
- Sipponen M. H.; Rahikainen J.; Leskinen T.; Pihlajaniemi V.; Mattinen M.-L.; Lange H.; Crestini C.; Österberg M. Structural Changes of Lignin in Biorefinery Pretreatments and Consequences to Enzyme-Lignin Interactions. Nord. Pulp Pap. Res. J. 2017, 32 (04), 550–571. 10.3183/NPPRJ-2017-32-04-p550-571. [DOI] [Google Scholar]
- Leskinen T.; Valle-Delgado J. J.; Lintinen K.; Witos J.; Österberg M.; Kostiainen M.; Mattinen M.-L.; Wiedmer S. K. Adsorption of Proteins on Colloidal Lignin Particles for Advanced Biomaterials. Biomacromolecules 2017, 18 (9), 2767–2776. 10.1021/acs.biomac.7b00676. [DOI] [PubMed] [Google Scholar]
- Gordts S. C.; Férir G.; D’Huys T.; Petrova M. I.; Lebeer S.; Snoeck R.; Andrei G.; Schols D. The Low-Cost Compound Lignosulfonic Acid (LA) Exhibits Broad-Spectrum Anti-HIV and Anti-HSV Activity and Has Potential for Microbicidal Applications. PLoS One 2015, 10 (7), e0131219. 10.1371/journal.pone.0131219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuraman A.; Tiwari V.; Zhao Q.; Shukla D.; Debnath A. K.; Desai U. R. Viral Inhibition Studies on Sulfated Lignin, a Chemically Modified Biopolymer and a Potential Mimic Heparan Sulfate. Biomacromolecules 2007, 8 (5), 1759–1763. 10.1021/bm0701651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar J. N.; Tiwari V.; Desai U. R. Nonsulfated, Cinnamic Acid-Based Lignins Are Potent Antagonists of HSV-1 Entry into Cells. Biomacromolecules 2010, 11 (5), 1412–1416. 10.1021/bm100161u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim M. R.; Pontius F. W.; LeChevallier M. W. Detection of Noroviruses in Water—Summary of an International Workshop. J. Infect. Dis. 2004, 189 (1), 21–28. 10.1086/380132. [DOI] [PubMed] [Google Scholar]
- Gall A. M.; Mariñas B. J.; Lu Y.; Shisler J. L. Waterborne Viruses: A Barrier to Safe Drinking Water. PLoS Pathog. 2015, 11 (6), e1004867. 10.1371/journal.ppat.1004867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hryniszyn A.; Skonieczna M.; Wiszniowski J.ła. Methods for Detection of Viruses in Water and Wastewater. Adv. Microbiol. 2013, 3, 442–449. 10.4236/aim.2013.35060. [DOI] [Google Scholar]
- Varughese E. A.; Brinkman N. E.; Anneken E. M.; Cashdollar J. L.; Fout G. S.; Furlong E. T.; Kolpin D. W.; Glassmeyer S. T.; Keely S. P. Estimating Virus Occurrence Using Bayesian Modeling in Multiple Drinking Water Systems of the United States. Sci. Total Environ. 2018, 619–620, 1330–1339. 10.1016/j.scitotenv.2017.10.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E.; Kitajima M.; Hata A.; Torrey J. R.; Masago Y.; Sano D.; Katayama H. A Review on Recent Progress in the Detection Methods and Prevalence of Human Enteric Viruses in Water. Water Res. 2018, 135, 168–186. 10.1016/j.watres.2018.02.004. [DOI] [PubMed] [Google Scholar]
- Suzuki K.; Tanaka Y.; Osada T.; Waki M. Removal of Phosphate, Magnesium and Calcium from Swine Wastewater through Crystallization Enhanced by Aeration. Water Res. 2002, 36, 2991–2998. 10.1016/S0043-1354(01)00536-X. [DOI] [PubMed] [Google Scholar]
- Blaney L. M.; Cinar S.; Sengupta A. K. Hybrid Anion Exchanger for Trace Phosphate Removal from Water and Wastewater. Water Res. 2007, 41, 1603–1613. 10.1016/j.watres.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Acelas N. Y.; Martin B. D.; López D.; Jefferson B. Selective Removal of Phosphate from Wastewater Using Hydrated Metal Oxides Dispersed within Anionic Exchange Media. Chemosphere 2015, 119, 1353–1360. 10.1016/j.chemosphere.2014.02.024. [DOI] [PubMed] [Google Scholar]
- van Voorthuizen E. M.; Ashbolt N. J.; Schäfer A. I. Role of Hydrophobic and Electrostatic Interactions for Initial Enteric Virus Retention by MF Membranes. J. Membr. Sci. 2001, 194, 69–79. 10.1016/S0376-7388(01)00522-1. [DOI] [Google Scholar]
- Bartels J.; Hildebrand N.; Nawrocki M.; Kroll S.; Maas M.; Colombi Ciacchi L.; Rezwan K. Effect of Divalent versus Monovalent Cations on the MS2 Retention Capacity of Amino- Functionalized Ceramic Filters. Phys. Chem. Chem. Phys. 2018, 20 (16), 11215–11223. 10.1039/C8CP01607K. [DOI] [PubMed] [Google Scholar]
- Yin H.; Liu L.; Wang X.; Wang T.; Zhou Y.; Liu B.; Shan Y.; Wang L.; Lü X. A Novel Flocculant Prepared by Lignin Nanoparticles-Gelatin Complex from Switchgrass for the Capture of Staphylococcus Aureus and Escherichia Coli. Colloids Surf., A 2018, 545, 51–59. 10.1016/j.colsurfa.2018.02.033. [DOI] [Google Scholar]
- Xiao D.; Ding W.; Zhang J.; Ge Y.; Wu Z.; Li Z. Fabrication of a Versatile Lignin-Based Nano-Trap for Heavy Metal Ion Capture and Bacterial Inhibition. Chem. Eng. J. 2019, 358, 310–320. 10.1016/j.cej.2018.10.037. [DOI] [Google Scholar]
- Thakur S.; Govender P. P.; Mamo M. A.; Tamulevicius S.; Mishra Y. K.; Thakur V. K. Progress in Lignin Hydrogels and Nanocomposites for Water Purification: Future Perspectives. Vacuum 2017, 146, 342–355. 10.1016/j.vacuum.2017.08.011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.