Abstract
Background
The 2019 novel coronavirus (2019-nCoV) causing an outbreak of pneumonia in Wuhan, Hubei province of China was isolated in January 2020. This study aims to investigate its epidemiologic history, and analyze the clinical characteristics, treatment regimens, and prognosis of patients infected with 2019-nCoV during this outbreak.
Methods
Clinical data from 137 2019-nCoV-infected patients admitted to the respiratory departments of nine tertiary hospitals in Hubei province from December 30, 2019 to January 24, 2020 were retrospectively collected, including general status, clinical manifestations, laboratory test results, imaging characteristics, and treatment regimens.
Results
None of the 137 patients (61 males, 76 females, aged 20–83 years, median age 57 years) had a definite history of exposure to Huanan Seafood Wholesale Market. Major initial symptoms included fever (112/137, 81.8%), coughing (66/137, 48.2%), and muscle pain or fatigue (44/137, 32.1%), with other, less typical initial symptoms observed at low frequency, including heart palpitations, diarrhea, and headache. Nearly 80% of the patients had normal or decreased white blood cell counts, and 72.3% (99/137) had lymphocytopenia. Lung involvement was present in all cases, with most chest computed tomography scans showing lesions in multiple lung lobes, some of which were dense; ground-glass opacity co-existed with consolidation shadows or cord-like shadows. Given the lack of effective drugs, treatment focused on symptomatic and respiratory support. Immunoglobulin G was delivered to some critically ill patients according to their conditions. Systemic corticosteroid treatment did not show significant benefits. Notably, early respiratory support facilitated disease recovery and improved prognosis. The risk of death was primarily associated with age, underlying chronic diseases, and median interval from the appearance of initial symptoms to dyspnea.
Conclusions
The majority of patients with 2019-nCoV pneumonia present with fever as the first symptom, and most of them still showed typical manifestations of viral pneumonia on chest imaging. Middle-aged and elderly patients with underlying comorbidities are susceptible to respiratory failure and may have a poorer prognosis.
Keywords: Coronavirus, 2019 Novel coronavirus (2019-nCoV), Clinical characteristics, Treatment, Hubei province
Introduction
In late 2019, Wuhan in Hubei province, China became the focus of the world owing to an outbreak of pneumonia with unknown etiology. A pathogen was successfully isolated in January, 2020 and named the 2019 novel coronavirus (2019-nCoV).[1] Here, we report the clinical manifestations, laboratory test results, imaging characteristics, and treatment regimen of 2019-nCoV-infected patients admitted to the respiratory departments of several tertiary hospitals in Hubei to provide a basis for further specification of the diagnosis and treatment protocol for this disease.
Methods
Ethics
This study was conducted in accordance with the Declaration of Helsinki and was approved by the medical ethics committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology (No. TJ-IRB20200419). Informed consent was waived given the context of emerging infectious diseases.
Patients and inclusion criteria
Data were analyzed from 137 patients admitted to the respiratory departments identified to be nucleic acid-positive for 2019-nCoV in nine tertiary hospitals in Hubei province from December 30, 2019 to January 24, 2020. Of these patients, 61 were males and 76 were females, with an age range of 20 to 83 years and a median age of 57 years (mean age: 55 ± 16 years). The inclusion procedures and criteria were as follows. Suspected cases were screened out according to the diagnosis and treatment protocol for novel coronavirus pneumonia (NCP) (Trial Version 3).[2] Once selected, respiratory tract secretions and other samples were acquired for real-time fluorescence reverse transcription-polymerase chain reaction (RT-PCR). Patients tested positive for the nucleic acids of this CoV were identified as confirmed cases and enrolled in the study.
Sample collection and pathogen identification
After admission to the hospital, respiratory tract samples including sputum and nasopharyngeal swabs were collected from the patients, which were tested for influenza, avian influenza, respiratory syncytial virus, adenovirus, parainfluenza virus, severe acute respiratory syndrome (SARS)-CoV, and Middle East respiratory syndrome (MERS)-CoV, along with routine bacterial, fungal, and pathogenic microorganism tests. Respiratory samples were employed for real-time fluorescence-based RT-PCR to detect the presence of 2019-nCoV. Total RNA was extracted using TRIzol according to the manufacturer's instructions. RT-PCR was performed according to the manufacturer's instructions by using the 2019-nCoV (ORF1ab/N) nucleic acid detection kit (Bio-germ, Shanghai, China). The following primers and probe for CoV envelope genes were used: forward primer 5′-TCAGAATGCCAATCTCCCCAAC-3′, reverse primer 5′-AAAGGTCCACCCGATACATTGA-3′, and probe 5′-CY5-CTAGTTACACTAGCCATCCTTACTGC-3′ BHQ1. The amplification conditions were 50°C for 15 min and 95°C for 3 min, followed by 45 cycles of 95°C for 15 s and 60°C for 30 s.
Data collection
Basic information (age, gender, smoking history, and comorbidities) was collected for each patient. In addition, epidemiologic histories were taken, including (1) long-time vendors, employees, and workers at Huanan Seafood Wholesale Market as of December 1, 2019; (2) staff who conducted 3 h or more work in processing, sale, slaughter, treatment, and transportation activities at Huanan Seafood Wholesale Market 2 weeks prior to disease onset as of December 1, 2019; (3) definite history of contact with birds or wildlife at Huanan Seafood Wholesale Market 2 weeks prior to disease onset as of December 1, 2019; (4) close contact with individuals suspected or confirmed to be infected by the virus, such as common transport or staying in the same room. Clinical manifestations were recorded (fever, coughing/expectoration/myalgia or fatigue/hemoptysis/headache/heart palpitations/diarrhea/dyspnea, etc), along with disease condition changes. Laboratory test results were compiled, including standard blood counts (absolute white blood cells and lymphocytes), blood biochemistry (alanine transaminase, aspartate transaminase, creatine kinase, creatinine), coagulation function, procalcitonin, C-reactive protein, erythrocyte sedimentation rate, and myocardial enzyme spectrum. Additional data collected included medical imaging, treatment regimens (antiviral, antibacterial, systemic corticosteroid, immunoglobulin G, respiratory support), and prognosis (recovered and discharged, inpatient treatment, or death).
Data analysis
Continuous data were expressed as medians and ranges since they were non-normally distributed (the data were checked for normality of distribution by Kolmogorov-Smirnov test), and categorical data were presented as counts and percentages.
Results
General information
More than half of the confirmed cases included in this study were from Wuhan (69/137, 50.4%). According to epidemiologic histories, most of the patients admitted to hospitals in other cities of Hubei province had a history of travel to or residence in Wuhan [Figure 1].
Figure 1.
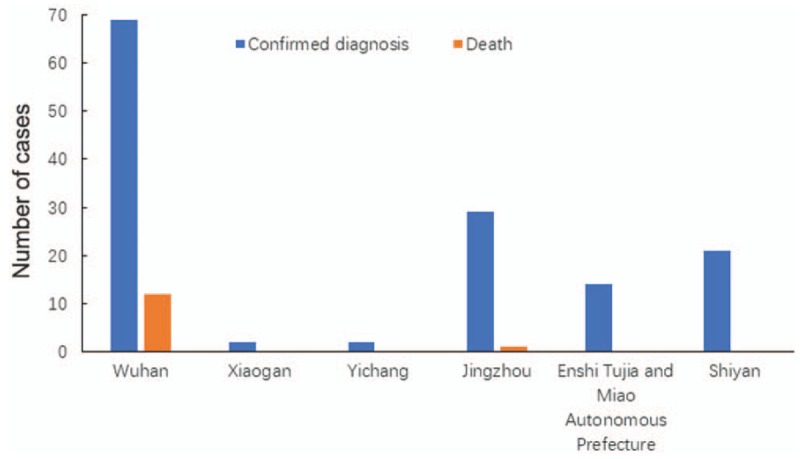
Regional distribution of 2019-nCoV-infected patients included in the study. 2019-nCoV: 2019 Novel coronavirus.
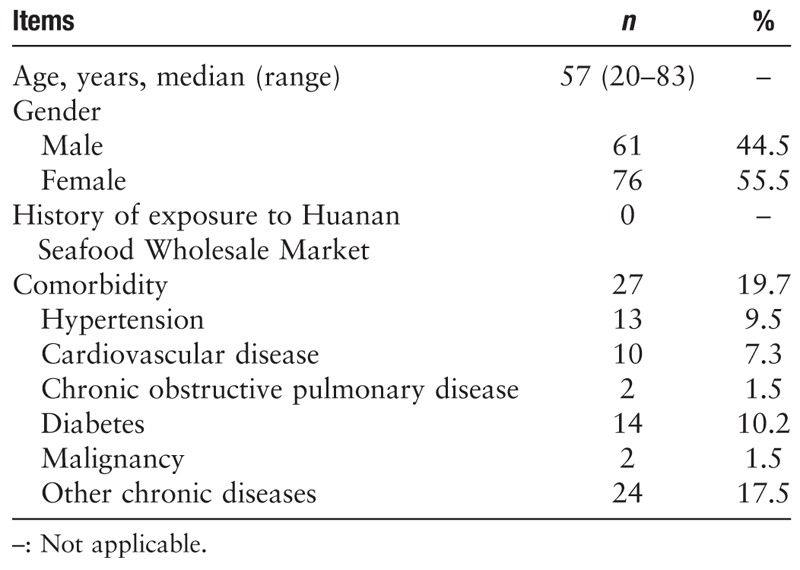
Basic characteristics of the study population are summarized in Table 1. The median and mean ages of the patients were 57 and 55 years, respectively, suggesting that middle-aged and elderly people were more susceptible to infection, whereas healthy, young adult patients were less susceptible. In addition, none of the 137 patients included in this study had a clear history of exposure to Wuhan Huanan Seafood Wholesale Market, suggesting that the virus is capable of secondary or tertiary transmission. This highlights the extreme likelihood of human-human transmission, which aligns with the current national epidemic situation. Moreover, critically ill patients more frequently had an underlying comorbid systemic disease, resulting in a poor prognosis.
Table 1.
Demographic and general characteristics of 2019-novel coronavirus-infected patients included in the study (N = 137).
Clinical manifestations
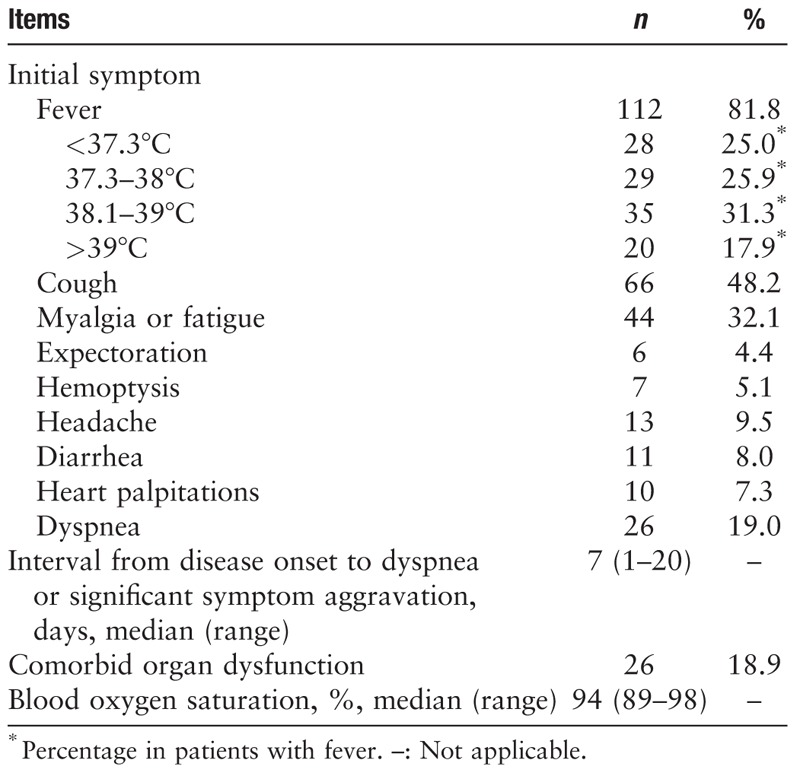
As shown in Table 2, the majority of the patients showed an initial symptom of fever, nearly half of whom developed a high fever. By contrast, one fifth of the patients did not develop fever, alerting to the need for caution of atypical cases. In addition to fever, the major symptoms were coughing and myalgia or fatigue. Some patients developed initial symptoms in the cardiovascular system, digestive system, and nervous system, which increased the difficulty of diagnosis. The median interval from the onset of initial symptoms to dyspnea or significant symptom aggravation in 26 patients was 7 days, ranging between 1 day (i.e., appearance of acute respiratory distress syndrome) up to 20 days, which was consistent with previous report.[3] Nearly 20% of the patients showed comorbidities with respect to dysfunction of other organs, primarily renal impairment. Patients with underlying cardiovascular diseases often demonstrated comorbid heart failure. Since respiratory support was administered to most of the patients upon admission, blood oxygen saturation could be maintained at above 90% as indicated by pulse oximetry monitoring.
Table 2.
Clinical manifestations and physical signs of 2019-novel coronavirus-infected patients (N = 137).
Laboratory tests and imaging examinations
A definitive diagnosis of 2019-nCoV was acquired by real-time fluorescence-based RT-PCR. As shown in Table 3, about 80% of the patients had normal or decreased white blood cell counts, and 72.3% (99/137) of the patients developed lymphocytopenia, consistent with the main characteristic of viral infection. The lung images of most patients showed abnormal characteristics that involved both of the lungs in most cases (116/137, 84.7%). Figure 2 shows representative lung images of a patient in which lesions developed in multiple lobes, most of which were dense, and ground-glass opacity co-existing with consolidation or cord-like shadows.
Table 3.
Laboratory test and imaging data for 2019-novel coronavirus-infected patients (N = 137).
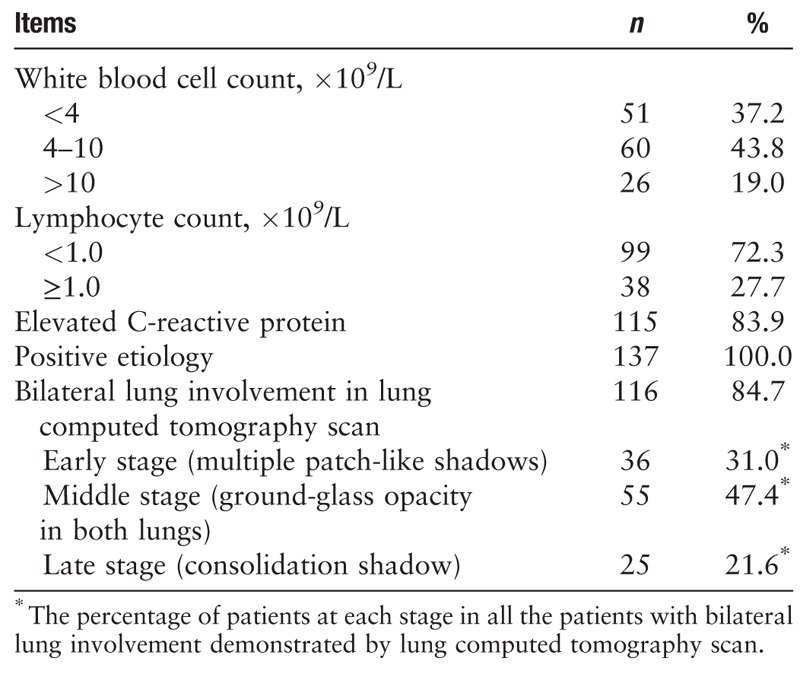
Figure 2.
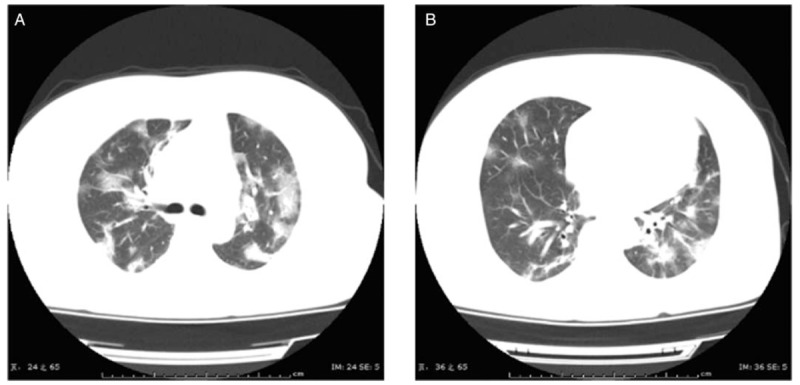
Chest CT images of a 47-year-old female patient upon admission, who had symptoms of fever for 7 days and post-exertional shortness of breath for 2 days (A and B). Transverse chest CT images showed the bilateral multiple lobular and sub-segmental areas of consolidation. CT: Computed tomography.
Treatment regimen and prognosis
At present, there are no drugs available that can target CoVs. Therefore, treatment was focused on symptomatic and respiratory support [Table 4]. For patients with rapid progression (median interval from onset of initial symptoms to dyspnea less than 3 days), it was recommended that respiratory support (119/137, 86.9%) should be administered as soon as possible, and that γ-immunoglobulin should be administered based on the patient's condition. Although clear medical evidence was not available, intravenous methylprednisolone (30–80 mg/day) was still provided to some patients (40/137, 29.2%) who either suffered from persistent high fever that did not subside or showed significant short-term disease progression determined by imaging results. Based on the mechanism of action of the drug, it was expected that systemic corticosteroid treatment could inhibit a cytokine storm and promote the absorption of exudative lesions. However, this treatment neither significantly shortened the disease course nor improved the prognosis.
Table 4.
Treatment regimen and prognosis of 2019-novel coronavirus-infected patients (N = 137).
For example, the images in Figure 3 are from a patient who was treated with 40 mg methylprednisolone intravenously (iv) every day (qd) after admission. On day 6 of treatment, review of the lung computed tomography (CT) scan showed significant lesion progression and the patient ultimately died, indicating that lung changes caused by the 2019-nCoV were not inhibited by corticosteroid as was expected.
Figure 3.
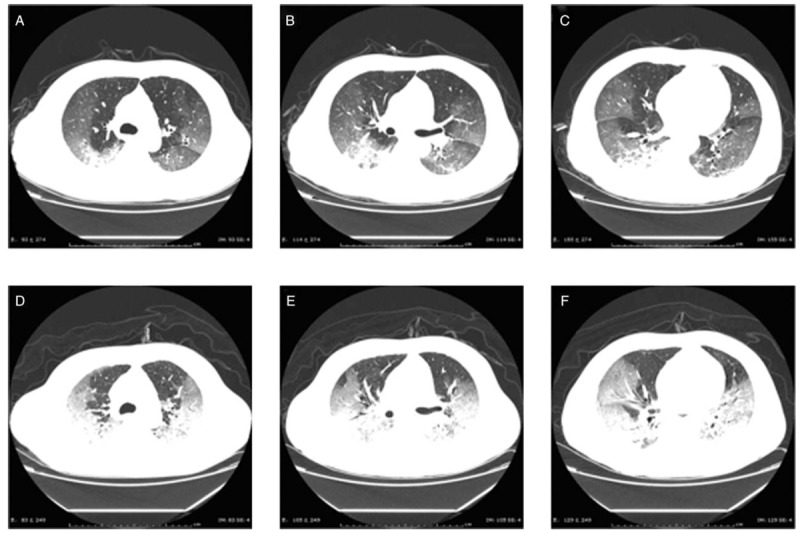
Lung computed tomography (CT) images of a 66-year-old male patient admitted for symptoms of high fever for 2 days and dyspnea for 1 day (A–C). After admission on January 8, 2020, 40 mg intravenously (iv) every day (qd) methylprednisolone was administered, but the subsequent CT review 6 days later showed significant disease progression (D–F).
None of the patients is currently on invasive respiratory support and extracorporeal membrane oxygenation (ECMO). This is likely because the patients included in this study were predominantly admitted to the respiratory department and therefore relevant data from the intensive care unit and other departments are insufficient, which could lead to a biased conclusion. Sixteen of the 137 (11.7%) patients eventually died from complications of this infection. This is a higher mortality rate than previously reported (24/549),[4] which might be attributed to the fact that these were all inpatients with more critical conditions than most other patients infected in this outbreak.
Discussion
CoV, as an enveloped RNA virus that is ubiquitous in humans, other mammals, and birds, can cause respiratory, digestive, liver, and nervous system disorders.[5,6] To date, six CoVs have been known to cause human infection.[7] Among them, two zoonotic viruses, SARS-CoV and MERS-CoV, were responsible for serious outbreaks: in China in 2002 to 2003[8,9] and in the Middle East in 2012,[10] respectively. In addition, cross-species infection and occasional spillover events may lead to the cyclical emergence of new CoVs.[11] In December 2019, a “pneumonia of unknown etiology” appeared in Wuhan in Hubei province, the cause of which has since been confirmed as the 2019-nCoV. Of particular concern, our observations found that 137 patients had no clear history of exposure to the Wuhan Huanan Seafood Wholesale Market, suggesting a strong likelihood of significant human-to-human transmission of the disease. At the meantime, the rapid increase in cases across the country so far confirms the virus's human-to-human transmission.
The rapid spread of 2019-nCoV is reminiscent of the SARS outbreak in China in 2003. Although more widespread transmission of the SARS virus was successfully prevented, owing to inadequate experience with outbreaks of this nature at the time, SARS-CoV still caused more than 8000 infections leading to 774 deaths.[12] The typical clinical manifestations of SARS included sudden fever (usually >38°C) accompanied by chills or headache, myalgia, fatigue, and coughing. Some patients also developed significant respiratory distress within a short period. Laboratory tests showed either a normal or decreased peripheral white blood cell count. Imaging showed focal or patch-like shadows or reticular exudates, although this did rapidly progress to diffuse consolidations in some patients.[13] In the present cohort of patients, the typical initial symptoms of NCP were primarily fevers, most of which were high fevers that occurred within several days and were not alleviated by routine anti-infective drugs. However, some middle-aged and elderly patients with underlying comorbidities only developed a moderate, low, or no fever during the disease course. In addition, some patients suffered from fatigue and dry cough on disease onset.
Nevertheless, these atypical initial symptoms deserve similar attention with the more common symptoms. For example, there have been reports of patients with NCP presenting with diarrhea as the initial symptom of disease onset.[14] Some patients presented with elevated troponin levels and myocarditis.[15] Others developed headache, myalgia, and other symptoms similar to those of influenza.[14] It should be emphasized that the authors observed that some patients in the outpatient clinic even had no obvious symptoms, or came to see the doctor only with “discomfort.” Moreover, a previous study reported that one asymptomatic patient was diagnosed with 2019-nCoV infection[16]; therefore, the presence of asymptomatic carriers requires due attention, and relevant contacts should be tracked and isolated as soon as possible. In this study, routine peripheral blood tests showed either normal or decreased white blood cell counts and lymphocytopenia, as well as elevated C-reactive protein, all of which are generally consistent with previous reports of patients with NCP.[3] In these patients, early-stage lung CT scans mostly showed multiple, small patch-like shadows, and interstitial changes, which were more obvious in the extrapulmonary region. These shadows subsequently progressed to multiple ground-glass opacities in both lungs, along with infiltration shadows with a “large white lung” observed in more severe cases. The median interval from the onset of initial symptoms to dyspnea was 7 days. In some severe cases, the disease rapidly progressed to acute respiratory distress syndrome, septic shock, refractory metabolic acidosis, and coagulation disorder, eventually leading to death.
Based on present data, the mortality rate of 2019-nCoV is lower than that of SARS.[12,17] Moreover, there are some other notable epidemiologic differences between the two outbreaks. For example, most of the critically ill SARS patients were young adults who required invasive ventilation for treatment, whereas most of the critically ill patients included in this study were middle-aged and elderly people. This age difference may have contributed to the poor prognosis in some cases. Thus, the proportion of young adult patients with SARS with moderate and severe disease was substantially higher than that of young adult patients with the 2019-nCoV. However, this is not a reason to not take this new threat seriously. Although the mortality caused by the 2019-nCoV is seemingly lower than that of SARS, its incubation period may be longer, producing a much larger number of potential asymptomatic carriers. This possibility imposes further pressure on securing a definitive diagnosis of the disease and containment of its transmission.
Based on the experience and lessons learned from the SARS and MERS outbreaks, the treatment site and protocol for confirmed cases of NCP are decided by disease severity: patients with mild symptoms (i.e., coughing, low-grade fever, runny nose, and slight sore throat) are quarantined at home, whereas patients with moderate or severe disease are hospitalized for treatment. Our study cohort included only patients who were already critically ill. As there is currently no effective drug against the 2019-nCoV, symptomatic treatment and respiratory support were provided. Since the large-dose glucocorticoids used in the treatment of SARS resulted in serious adverse reactions[18,19] but did not effectively decrease the mortality rate of CoV infection,[20–22] we treated patients with low-dose (30–80 mg/day) and short-term (3–5 days) methylpredisolone to alleviate the pulmonary exudates and inhibit a systemic cytokine storm. Unfortunately, this treatment did not provide significant benefits. As an alternative, intravenous injection with γ-immunoglobulin can be offered; however, more clinical data are required to determine the efficacy of this treatment. In the middle and late stages of the disease, patients often develop additional bacterial or even fungal infections; therefore, careful attention must be paid to the rational use of antibiotics. In addition to the aforementioned treatments, respiratory support should be provided as early as possible. In this cohort, different types of oxygen therapy were administered according to each patient's pulse oximetry oxygen saturation and oxygenation index. For most patients, early non-invasive ventilation could promote positive outcomes. Alternatively, for critically ill patients, invasive ventilation or even ECMO should be considered. To date, ECMO has been successfully used to resuscitate one critically ill patient infected with this new CoV. Several drugs have already been tested against the 2019-nCoV and relevant clinical observational studies have been initiated with encouraging preliminary results. In particular, research on anti-coronaviral drugs and vaccines should be a continuous priority. None of these currently tested treatments was used in our cohort of patients, and thus their efficacy remains unknown.
Our study has some limitations. For example, all of the patients included in this study were inpatients and our sample size was relatively small. We did not collect and analyze data from outpatients with mild symptoms or from suspected patients under home observation and awaiting a definitive diagnosis. This may result in some bias in our general understanding of the disease. In addition, since most of the patients in this study were admitted to the respiratory departments of some tertiary hospitals in Hubei province, data from the intensive care units and other departments are insufficient, which can similarly lead to a biased understanding of the disease. On this basis, a broader and larger study is necessary in the immediate future.
In conclusion, by analyzing 137 confirmed cases of infection with the 2019-nCoV in some tertiary hospitals in Hubei province, we preliminarily identified major clinical characteristics and corresponding treatment principles for the disease. However, there is still a large gap in our understanding of the origin, epidemiology, and persistence of human transmission of this disease. Therefore, continuous monitoring and tracing is required to secure an in-depth understanding of the disease, thereby providing an improved evidentiary basis for standardizing the diagnosis and treatment of 2019-nCoV.
Conflicts of interest
None.
Footnotes
How to cite this article: Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, Xiao W, Wang YN, Zhong MH, Li CH, Li GC, Liu HG. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744
References
- 1.Novel Coronavirus (2019-nCoV). Geneva: World Health Organization, 2019. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. [Accessed January 26, 2020] [Google Scholar]
- 2. Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 3). Beijing: National Health Commission of the People's Republic of China. Available from: http://www.nhc.gov.cn/xcs/zhengcwj/202001/f492c9153ea9437bb587ce2ffcbee1fa/files/39e7578d85964dbe81117736dd789d8f.pdf. [Accessed on January 26, 2020] [Google Scholar]
- 3.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.A cumulative number of 549 novel coronavirus cases was reported in Hubei province. Wuhan: Hubei Provincial People's Government. Available from: http://www.hubei.gov.cn/zhuanti/2020/gzxxgzbd/zxtb/202001/t20200124_2014817.shtml. [Accessed on January 24, 2020] [Google Scholar]
- 5.Weiss SR, Leibowitz JL. Coronavirus pathogenesis. Adv Virus Res 2011; 81:85–164. doi: 10.1016/b978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou P, Fan H, Lan T, Yang XL, Shi WF, Zhang W, et al. Fatal swine acute diarrhoea syndrome caused by an HKU2-related coronavirus of bat origin. Nature 2018; 556:255–258. doi: 10.1038/s41586-018-0010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol 2016; 24:490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Normile D. Infectious diseases. Battling SARS on the frontlines. Science 2003; 300:714–715. doi: 10.1126/science.300.5620.714. [DOI] [PubMed] [Google Scholar]
- 9.Zhong NS, Zheng BJ, Li YM, Poon, Xie ZH, Chan KH, et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet 2003; 362:1353–1358. doi: 10.1016/s0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaki AM, Van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med 2012; 367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 11.Wong G, Liu W, Liu Y, Zhou B, Bi Y, Gao GF. MERS, SARS, and Ebola: The role of super-spreaders in infectious disease. Cell Host Microbe 2015; 18:398–401. doi: 10.1016/j.chom.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Summary of probable SARS cases with onset of illness from November 1, 2002 to July 31, 2003. Geneva: World Health Organization. Available from: https://www.who.int/csr/sars/country/table2003_09_23/en/. [Accessed on January 26, 2020] [Google Scholar]
- 13.Hui DS, Wong PC, Wang C. SARS: clinical features and diagnosis. Respirology 2003; 8 Suppl:S20–S24. doi: 10.1046/j.1440-1843.2003.00520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fever and coughing are not the only first symptoms in patients with novel coronavirus pneumonia (2019-nCoV). Wuhan: Renmin Hospital of Wuhan University. Available from: http://www.xinhuanet.com/2020-01/24/c_1125500055.htm [Accessed on January 30, 2020] [Google Scholar]
- 15.Wuhan Municipal Health Commission. Latest report on the novel coronavirus pneumonia from Wuhan Municipal Health Commission. Available from: http://wjw.wuhan.gov.cn/front/web/showDetail/2020011609057. [Accessed on January 26, 2020] [Google Scholar]
- 16.Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020; 395:514–523. doi: 10.1016/s0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Daily broadcast: New coronavirus (2020.01.24). DXY public number. Available from: http://infect.dxy.cn/article/676158?keywords=%E6%AF%8F%E6%97%A5%E7%96%AB%E6%83%85. [Accessed January 30, 2020] [Google Scholar]
- 18.Griffith JF, Antonio GE, Kumta SM, Hui DS, Wong JK, Joynt GM, et al. Osteonecrosis of hip and knee in patients with severe acute respiratory syndrome treated with steroids. Radiology 2005; 235:168–175. doi: 10.1148/radiol.2351040100. [DOI] [PubMed] [Google Scholar]
- 19.Xu N, Zhou L, Xu M. Analysis of glucocorticoid treatment in 453 cases of infectious atypical pneumonia (in Chinese). Chin J Hospital Pharmacy 2005; 2:58–59. doi: 10.3321/j.issn:1001-5213.2005.02.027. [Google Scholar]
- 20.Ho W. Guideline on management of severe acute respiratory syndrome (SARS). Lancet 2003; 361:1313–1315. doi: 10.1016/s0140-6736(03)13085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy MM, Baylor MS, Bernard GR, Fowler R, Franks TJ, Hayden FG, et al. Clinical issues and research in respiratory failure from severe acute respiratory syndrome. Am J Respir Crit Care Med 2005; 171:518–526. doi: 10.1164/rccm.200405-621WS. [DOI] [PubMed] [Google Scholar]
- 22.Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 2003; 361:1767–1772. doi: 10.1016/s0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


