Abstract
Background
Since early December 2019, the 2019 novel coronavirus disease (COVID-19) has caused pneumonia epidemic in Wuhan, Hubei province of China. This study aimed to investigate the factors affecting the progression of pneumonia in COVID-19 patients. Associated results will be used to evaluate the prognosis and to find the optimal treatment regimens for COVID-19 pneumonia.
Methods
Patients tested positive for the COVID-19 based on nucleic acid detection were included in this study. Patients were admitted to 3 tertiary hospitals in Wuhan between December 30, 2019, and January 15, 2020. Individual data, laboratory indices, imaging characteristics, and clinical data were collected, and statistical analysis was performed. Based on clinical typing results, the patients were divided into a progression group or an improvement/stabilization group. Continuous variables were analyzed using independent samples t-test or Mann-Whitney U test. Categorical variables were analyzed using Chi-squared test or Fisher's exact test. Logistic regression analysis was performed to explore the risk factors for disease progression.
Results
Seventy-eight patients with COVID-19-induced pneumonia met the inclusion criteria and were included in this study. Efficacy evaluation at 2 weeks after hospitalization indicated that 11 patients (14.1%) had deteriorated, and 67 patients (85.9%) had improved/stabilized. The patients in the progression group were significantly older than those in the disease improvement/stabilization group (66 [51, 70] vs. 37 [32, 41] years, U = 4.932, P = 0.001). The progression group had a significantly higher proportion of patients with a history of smoking than the improvement/stabilization group (27.3% vs. 3.0%, χ2 = 9.291, P = 0.018). For all the 78 patients, fever was the most common initial symptom, and the maximum body temperature at admission was significantly higher in the progression group than in the improvement/stabilization group (38.2 [37.8, 38.6] vs. 37.5 [37.0, 38.4]°C, U = 2.057, P = 0.027). Moreover, the proportion of patients with respiratory failure (54.5% vs. 20.9%, χ2 = 5.611, P = 0.028) and respiratory rate (34 [18, 48] vs. 24 [16, 60] breaths/min, U = 4.030, P = 0.004) were significantly higher in the progression group than in the improvement/stabilization group. C-reactive protein was significantly elevated in the progression group compared to the improvement/stabilization group (38.9 [14.3, 64.8] vs. 10.6 [1.9, 33.1] mg/L, U = 1.315, P = 0.024). Albumin was significantly lower in the progression group than in the improvement/stabilization group (36.62 ± 6.60 vs. 41.27 ± 4.55 g/L, U = 2.843, P = 0.006). Patients in the progression group were more likely to receive high-level respiratory support than in the improvement/stabilization group (χ2 = 16.01, P = 0.001). Multivariate logistic analysis indicated that age (odds ratio [OR], 8.546; 95% confidence interval [CI]: 1.628–44.864; P = 0.011), history of smoking (OR, 14.285; 95% CI: 1.577–25.000; P = 0.018), maximum body temperature at admission (OR, 8.999; 95% CI: 1.036–78.147, P = 0.046), respiratory failure (OR, 8.772, 95% CI: 1.942–40.000; P = 0.016), albumin (OR, 7.353, 95% CI: 1.098–50.000; P = 0.003), and C-reactive protein (OR, 10.530; 95% CI: 1.224−34.701, P = 0.028) were risk factors for disease progression.
Conclusions
Several factors that led to the progression of COVID-19 pneumonia were identified, including age, history of smoking, maximum body temperature at admission, respiratory failure, albumin, and C-reactive protein. These results can be used to further enhance the ability of management of COVID-19 pneumonia.
Keywords: 2019 Novel coronavirus disease, Disease outcome, Predictors
Introduction
Since December 2019, unexplained pneumonia has been successively identified in several patients with a history of exposure to the Huanan Seafood Market, in multiple hospitals in the city of Wuhan, Hubei Province, China. These patients have now been confirmed as acute respiratory infection (i.e., pneumonia) caused by a novel coronavirus.[1,2] Clinical investigation of confirmed cases and cases under observation has shown that the number of patients with no history of exposure to the Huanan Seafood Market has been rapidly increasing. As of February 1, 2020, there were 14,380 confirmed cases of 2019 novel coronavirus disease (COVID-19) in the mainland of China.[3]
A previous study found that the highest temperature, dyspnea, respiratory rate (RR), white blood cell count, neutrophil count, lymphocyte count, D-dimer, albumin, procalcitonin were risk factors for intensive care unit (ICU) care in patients with COVID-19.[4] Therefore, it is absolutely necessary to evaluate the possible factors affecting the progression of disease in COVID-19 patients. We investigated factors affecting the outcomes of COVID-19 patients to evaluate the prognosis and further improve the treatment of patients with COVID-19 associated pneumonia with the hope of reducing mortality.
Methods
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Central Hospital of Wuhan (No. 2020 [11]). The requirement for written informed consent was waived given the context of emerging infectious diseases.
Subjects
Patients included in the study had been diagnosed with COVID-19 associated pneumonia between December 30, 2019, and January 15, 2020, and hospitalized at one of the three tertiary hospitals in Wuhan for over 2 weeks. Specific inclusion criteria were: (1) patients with confirmed diagnosis from a positive test result for COVID-19 nucleic acids by real-time fluorescence reverse transcription-polymerase chain reaction (RT-PCR) according to the “Diagnosis and Treatment Protocol for Novel Coronavirus Infection-Induced Pneumonia Version 4 (trial)” [5]; (2) patients who had been hospitalized for over 2 weeks when preparing the manuscript, died while hospitalized, or had recovered and been discharged.
Evaluation of conditions
All patients were evaluated and clinically typed upon admission, according to the “Diagnosis and Treatment Protocol for Novel Coronavirus Infection-Induced Pneumonia Version 4 (trial).”[5] Specific clinical types included: (1) common: fever, respiratory tract infection symptoms, and so on, with imaging indicating pneumonia; (2) severe (any of the following conditions): I, respiratory distress, RR ≥30 breaths/min; II, oxygen saturation ≤93% at rest; III, partial pressure of oxygen (PaO2)/fraction of inspired oxygen ≤300 mmHg (1 mmHg = 0.133 kPa); (3) critical (any of the following conditions): I, respiratory failure and a requirement for mechanical ventilation; II, shock; III, concomitant failure of other organs and requirement for ICU monitoring and treatment.
In addition to clinical typing, laboratory indices of all patients were measured, and the details of which are listed in the biochemical test section.
After 2 weeks of hospitalization, disease evaluation and clinical typing were performed on all patients according to the “Diagnosis and Treatment Protocol for Novel Coronavirus Infection-Induced Pneumonia Version 4 (trial).”[5] All patients were divided into a progression group or an improvement/stabilization group based on clinical typing results. Specific criteria were as follows: (1) progression group: common-type changed to severe- or critical-type, or death; severe-type changed to critical-type or death; critical-type progressed to death. (2) improvement/stabilization group: common-, severe-, and critical-types remained unchanged; common-type recovered; severe-type changed to common-type; critical-type changed to severe- or common-type.
Specimen collection, etiology, and biochemical tests
COVID-19 was detected by real-time fluorescence RT-PCR of samples collected by using nasopharyngeal swabs. Influenza A virus, influenza B virus, respiratory syncytial virus, adenovirus, parainfluenza virus, chlamydia, and mycoplasma were detected by collecting body fluid (nasopharyngeal swabs and sputum) samples. Relevant laboratory indicators were tested with conventional methods, including routine blood tests (white blood cell [WBC], lymphocytes, neutrophils, platelets), liver and kidney function (alanine aminotransferase [ALT], aspartate aminotransferase [AST], creatinine, and albumin), infection indices (procalcitonin and C-reactive protein), D-dimer, and PaO2.
Data collection
The personal data and clinical data of patients included in the study were collected. Personal data included sex, age, epidemiological history, history of smoking, and comorbidities (e.g., chronic obstructive pulmonary disease [COPD], cancer, hypertension, and/or diabetes). Clinical data included initial symptoms, clinical presentation, vital signs, therapeutic drug-use, respiratory support, and disease outcomes.
Statistical analysis
Categorical variables were presented as numbers (percentages) and analyzed using Chi-squared test or Fisher's exact test. Continuous variables with normal distribution were expressed as mean ± standard deviation and analyzed using independent samples t-test, while those with skewed distribution were shown as median (Q1, Q3) and analyzed using Mann-Whitney U test. Univariate and multivariate logistic regression analysis were adopted to identify risk factors of disease progression. All variables from the univariate analysis with a P value <0.1 were entered into a forward-stepwise multivariate logistic regression analysis. SPSS software version 25.0 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. A two-sided P < 0.05 was considered statistically significant.
Results
General characteristics and clinical presentations
In the present study, 78 patients with COVID-19 associated pneumonia included 39 males and 39 females. No patient had a history of exposure to the Huanan Seafood Market. The median age (Q1, Q3) was 38 (33, 57) years, and only 15 patients were aged ≥60 years (19.2%). Among the 78 patients, there were 70 patients with the common-type (89.7%) and eight patients with the severe-type (10.3%). Re-examination after 2 weeks of hospitalization showed that among the 70 patients with the common-type symptoms, there were eight patients with progression and 62 patients with improvement/stabilization. Among the eight patients with the severe-type symptoms, three patients showed progression (including two deaths) and five showed improvement/stabilization. A total of 11 patients (14.1%) were included in the progression group, and 67 patients (85.9%) were included in the improvement/stabilization group.
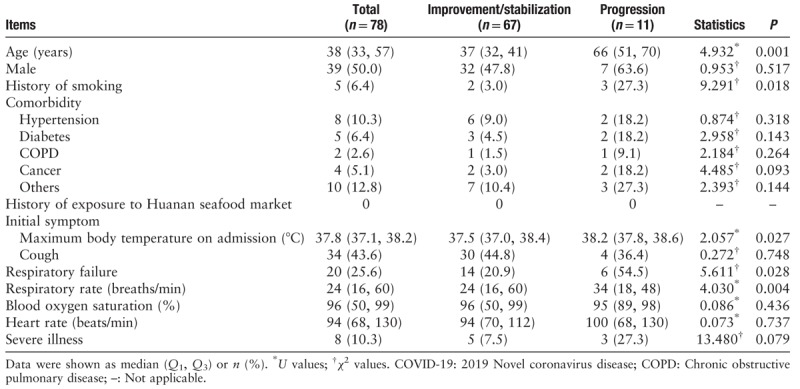
The patients in the progression group were significantly older than those in the improvement/stabilization group (66 [51, 70] vs. 37 [32, 41] years, U = 4.932, P = 0.001). This study suggested that the progression group had a significantly higher proportion of patients with a history of smoking than the improvement/stabilization group (27.3% vs. 3.0%, χ2 = 9.291, P = 0.018). Twenty patients (25.6%) had comorbidities, of which hypertension was the most common. There was no significant difference in sex between the two groups (P > 0.05). There was no significant difference in any comorbidity including hypertension, diabetes, COPD, cancer, and others between the two groups (all P > 0.05). Fever was the primary initial symptom. Fifty-seven patients (73.1%) sought treatment for fever, and 37.3 to 38.0°C was the most commonly observed maximum body temperature in 31 patients (39.7%). The maximum body temperature at admission was significantly higher in the progression group than in the improvement/stabilization group (38.2 [37.8, 38.6] vs. 37.5 [37.0, 38.4]°C, U = 2.057, P = 0.027). A total of 20 of the 78 patients (25.6%) developed respiratory failure, with the proportion of respiratory failure being significantly higher in the progression group than in the improvement/stabilization group (54.5% vs. 20.9%, χ2 = 5.611, P = 0.028). The median RR of the 78 patients with COVID-19 was 24 breaths/min, and the RR in the progression group was significantly higher than in the improvement/stabilization group (34 [18, 48] vs. 24 [16, 60] breaths/min, U = 4.030, P = 0.004). There were no significant differences in blood oxygen saturation, or heart rate between the two groups (both P > 0.05). Eight (10.3%) of the 78 patients with COVID-19 were severely ill, and the proportions of severely ill patients were not significantly different between the two groups (27.3% vs. 7.5%, χ2 = 13.480, P = 0.079) [Table 1].
Table 1.
Demographic data and clinical presentations of COVID-19 patients in the improvement/stabilization group and progression group.
Laboratory indices and imaging characteristics
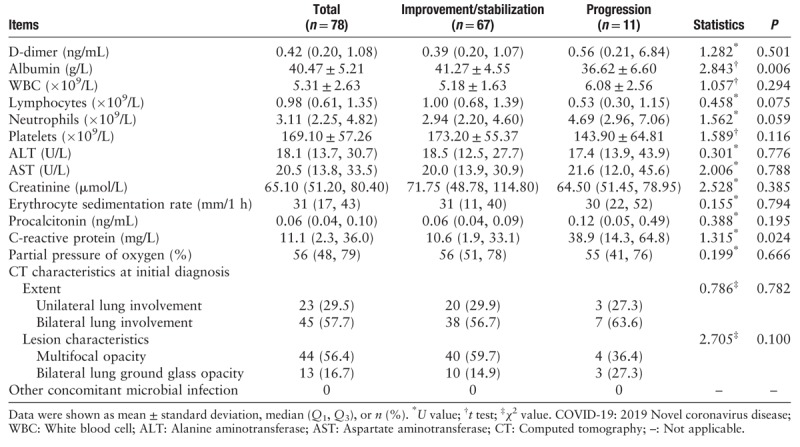
In this study, relevant laboratory indices of the 78 patients with COVID-19 were determined at the time of admission. These results showed that C-reactive protein was significantly elevated in the progression group compared to the improvement/stabilization group (38.9 [14.3, 64.8] vs. 10.6 [1.9, 33.1] mg/L, U = 1.315, P = 0.024). Albumin was significantly decreased in the progression group compared to the improvement/stabilization group (36.62 ± 6.60 vs. 41.27 ± 4.55 g/L, U = 2.843, P = 0.006). There were no significant differences in D-dimer, WBC, lymphocytes, neutrophils, platelets, ALT, AST, creatinine, erythrocyte sedimentation rate, procalcitonin, PaO2, and extent and characteristics of lesions on computer tomography (CT) scan between the two groups (all P > 0.05) [Table 2]. No patients had other concomitant microbial infection.
Table 2.
Laboratory indices and imaging characteristics of COVID-19 patients in the improvement/stabilization group and progression group.
Treatment
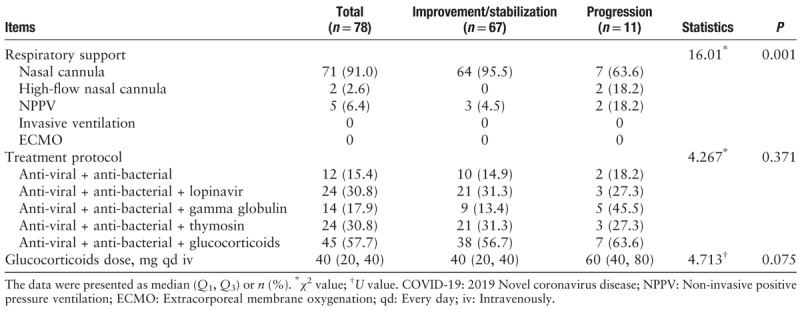
Among the 78 hospitalized patients, the most common treatment was a combination of anti-virals + anti-bacterials with glucocorticoids (45/78, 57.7%). The most commonly used anti-viral drug was ribavirin, and the most commonly used anti-bacterial drugs were cephalosporins or quinolone antibiotics. The median glucocorticoids dose was 40 (20, 40) mg intravenously (iv) every day (qd) and there was no significant difference between the two groups (60 [40, 80] vs. 40 [20, 40] mg qd iv, U = 4.713, P = 0.075). The proportions of patients using different drug protocols including anti-virals + anti-bacterials, anti-virals + anti-bacterials + glucocorticoids, anti-virals + anti-bacterials + gamma globulin, anti-virals + anti-bacterial + thymosins, and anti-virals + anti-bacterials + lopinavir between improvement/stabilization group and progression group were not significantly different (P > 0.05). All hospitalized patients had some degree of hypoxia. Nasal cannula was the most common form of respiratory support (71/78, 91.0%), followed by continuous non-invasive positive pressure ventilation. The progression group typically had more severe hypoxia and was significantly more likely to receive higher levels of respiratory support compared to the improvement/stabilization group (χ2 = 16.01, P = 0.001) [Table 3].
Table 3.
Treatment for COVID-19 patients in the improvement/stabilization group and progression group.
Risk factors for disease progression in COVID-19 patients
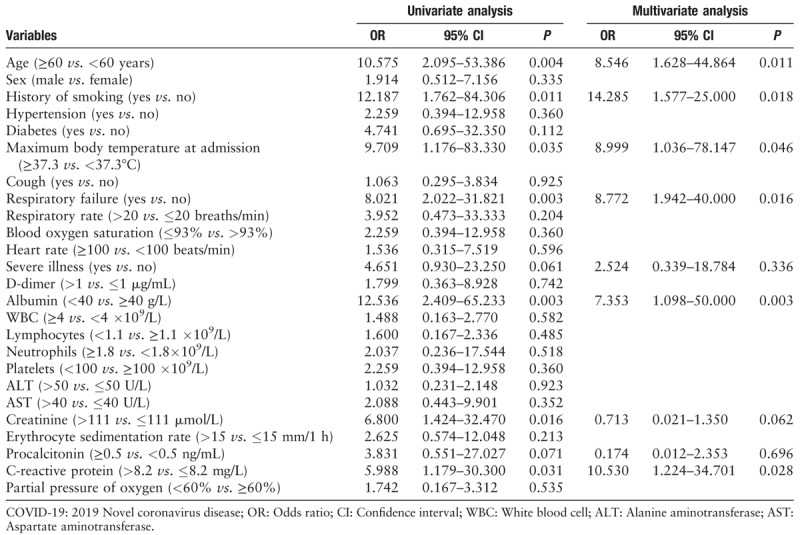
The results of univariate logistic analysis found that age (OR, 10.575; 95% CI: 2.095–53.386; P = 0.004), history of smoking (OR, 12.187; 95% CI: 1.762–84.306; P = 0.011), maximum body temperature at admission (OR, 9.709; 95% CI: 1.176–83.330; P = 0.035), respiratory failure (OR, 8.021; 95% CI: 2.022–31.821; P = 0.003), severe illness (OR, 4.651; 95% CI: 0.930–23.250; P = 0.061), albumin (OR, 12.536; 95% CI: 2.409–65.233; P = 0.003), creatinine (OR, 6.800; 95% CI: 1.424–32.470; P = 0.016), procalcitonin (OR, 3.831; 95% CI: 0.551–27.027; P = 0.071), C-reactive protein (OR, 5.988; 95% CI: 1.179–30.300; P = 0.031) were significantly associated with the disease progression. Furthermore, the multivariate logistic analysis indicated that age (OR, 8.546; 95% CI: 1.628–44.864; P = 0.011), history of smoking (OR, 14.285; 95% CI: 1.577–25.000; P = 0.018), maximum body temperature at admission (OR, 8.999; 95% CI: 1.036–78.147, P = 0.046), respiratory failure (OR, 8.772, 95% CI: 1.942–40.000; P = 0.016), albumin (OR, 7.353, 95% CI: 1.098–50.000; P = 0.003) and C-reactive protein (OR, 10.530; 95% CI: 1.224–34.701, P = 0.028) were risk factors for disease progression [Table 4].
Table 4.
Logistic analysis results of risk factors for disease progression in COVID-19 patients (n = 78).
Discussion
Coronavirus is a highly contagious pathogen found in several domestic animals, pets, and humans, causing a variety of acute and chronic diseases.[6] Currently, six coronaviruses are known to infect humans, including 229E and NLR6 in the α-genus. The β-genus comprises OC43, HKU1, Middle East respiratory syndrome (MERS)-related coronavirus (MERSr-CoV),[7] and severe acute respiratory syndrome-related coronavirus (SARSr-CoV).[8] Coronavirus has gradually become a popular topic of research in the field of virology because of the outbreak of SARSr-CoV in 2003 and MERSr-CoV in 2012.[9] The current outbreak is due to a novel coronavirus in the β-genus, which was isolated from the lower respiratory tract in patients with unexplained pneumonia, in Wuhan, China.[10,11] Currently, the source and pathogenesis of the COVID-19 remain unclear, and there are no uniform diagnostic and treatment standards. Unfortunately, in certain patients, the disease progresses rapidly, and respiratory failure can occur within a short time, even leading to death. Therefore, we investigated the disease outcomes and factors affecting the outcomes of patients with COVID-19 pneumonia at three tertiary hospitals in Wuhan to provide a basis for improving hospitals’ efforts to effectively treat patients with COVID-19 pneumonia.
The present study included 78 patients diagnosed with the COVID-19. All patients were evaluated for therapeutic efficacy after at least 2 weeks of hospitalization. These results indicated progression in 11 patients (14.1%) and improvement/stabilization in 67 patients (85.9%). The median age of the patients was 38 (33, 57) years, which suggest that middle-aged people are susceptible to COVID-19. Also, the age of patients in the progression group was significantly higher than that in the improvement/stabilization group, and multivariate logistic analysis indicated that higher age was a risk factor for disease progression. Elderly individuals are physically frail and are likely to have several comorbidities, which not only increases the risk of pneumonia,[12] but also affects their prognosis.[13] The assessment of comorbidities is an essential component in determining the prognosis of several diseases, especially pneumonia.[14] Probably because of the small sample size, there was no significant difference in any comorbidity including hypertension, diabetes, COPD, cancer, and others between the two groups. The potential impact of comorbidities on the disease outcomes of patients with COVID-19 pneumonia requires further observation and research. The proportion of patients with a history of smoking was significantly higher in the progression group compared to the improvement/stabilization group, suggesting that smoking is associated with disease progression.
A significant symptom of SARS is a body temperature above 38°C for over 2 weeks. Additionally, 60% of patients diagnosed with MERS presented with fever.[15] In the present study, 73.1% of patients with COVID-19 sought treatment for the fever. The results showed that the maximum body temperature at admission in the progression group was significantly higher than in the improvement/stabilization group, and multivariate logistic models indicated that higher temperature was a risk factor for disease progression. Therefore, patients presenting with a high fever, long fever duration, and rapid fever progression should be monitored more closely during clinical diagnosis and treatment in order to avoid complications associated with high fevers, which lead to poor prognosis.
Vital signs are essential indicators for assessing the current symptoms of patients. Respiratory system indices, such as RR and whether respiratory failure occurred, are particularly crucial for assessing the condition severity in patients with COVID-19. The present study found that the median RR of 78 patients with COVID-19 pneumonia was 24 breaths/min, which was higher than the normal RR (12–20 breaths/min). The RR and proportion of patients with respiratory failure in the progression group were significantly higher than in the improvement/stabilization group. Abnormal respiratory indices can directly reflect the extent of lung invasion and multivariate logistic models revealed that respiratory failure was a risk factor for disease progression. Therefore, respiratory indices should be one of the top priorities in the efficacy evaluation.
The present study suggests that elevated C-reactive protein, and decreased albumin are factors associated with poor prognosis of COVID-19 infection. Albumin is the most intuitive index of the nutritional status of the body. When albumin decreases, the body loses resistance to the virus, leading to disease progression.[16] Elevated C-reactive protein is an important inflammatory index in addition to abnormal blood coagulation function. Close monitoring of dynamic changes in these indices has a significant proactive effect on understanding changes in the patient's condition. In addition, studies have shown that lymphocytes are the main target cells of viral infections.[17] Viral infections in the human body primarily involve damage to the immune system, which presents as decrease in the absolute number of lymphocytes.[18] The present study did not find these indices significant for assessing the outcome of COVID-19 patients, and their correlation requires further investigation. This study included CT scan characteristics of patients with COVID-19 pneumonia for analysis and suggested that the extent and characteristics of the lesion had no statistical significance on disease outcomes. However, the use of CT scans at earlier stages for disease assessment is still of great significance for early detection, early diagnosis, and improved prognosis.
Appropriate antibiotic treatment can be administered to prevent secondary infection in critical-type viral pneumonia.[19] We analyzed the diagnosis and treatment protocols of patients with COVID-19 pneumonia, and results suggested that some patients undergoing anti-viral treatment were also proactively undergoing anti-bacterial treatment. Whether viral pneumonia should be treated with glucocorticoids has been controversial. Some researchers believe that the use of glucocorticoids in viral pneumonia can easily aggravate the disease and increase the risk of secondary infections, leading to an increase in mortality, thus advocating against the use of glucocorticoids.[20] Other studies have suggested that the use of an appropriate dose of glucocorticoids at early stages could inhibit the elevated secretion of inflammatory cytokines due to excessive activation of immune cells because of the viral infection, thereby preventing continued exacerbation of lung injury.[21] We found that the combination of anti-virals, anti-bacterials, and glucocorticoids had the highest use rate in the treatment of COVID-19 pneumonia. Moreover, other researchers have suggested using thymosin and gamma globulin during the early stages of infection to improve patient immunity. In addition, relevant ongoing studies suggest that COVID-19 and HIV have structural similarities. Thus, certain researchers have proposed that the anti-HIV drug, lopinavir, may play a role in inhibiting COVID-19. In this study, a comparison of efficacy of anti-virals + anti-bacterials, anti-virals + anti-bacterials + glucocorticoids, anti-virals + anti-bacterials + gamma globulin, anti-virals + anti-bacterial + thymosins, and anti-virals + anti-bacterials + lopinavir was performed. The results did not suggest that drug protocols affected disease outcomes. Therefore, further studies should include more drugs for the treatment of COVID-19. COVID-19 pneumonia is characterized by an acute onset and rapid progression. Therefore, the early use of glucocorticoids with proactive anti-viral and anti-bacterial treatment after comprehensive evaluation may block the inflammatory cascade caused by severe viral infections and prevent acute inflammation. The lung damage caused by such infections can further progress to acute respiratory distress syndrome. Respiratory support is an essential treatment for patients with severe viral infections. The present study revealed that all patients with COVID-19 were treated with respiratory support, and the majority of patients were administered nasal cannula oxygen and continuous positive airway pressure. The progression group was significantly more likely to receive higher levels of respiratory support. No patients in this study were treated with invasive ventilation and extracorporeal membrane oxygenation due to the refusal of the patients’ family. When treating patients with severe viral pneumonia, timely application of glucocorticoids and respiratory support therapy is essential, in combination with personalized treatment specific to each patient.
There were a few limitations for this observational study. CT scan imaging has delayed scanning time, which may introduce bias in our results. In addition, a relatively small sample size was included in this study, which may lead to biased results. Thus, a multi-center large-scale study with additional researchers is required. Currently, the best diagnostic and treatment protocols for COVID-19 are still being investigated. Early diagnosis and dynamic monitoring of prognostic factors are essential for improving the ability to treat the COVID-19.
Conflicts of interest
None.
Footnotes
How to cite this article: Liu W, Tao ZW, Wang L, Yuan ML, Liu K, Zhou L, Wei S, Deng Y, Liu J, Liu HG, Ming Y, Hu Y. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J 2020;133:1032–1038. doi: 10.1097/CM9.0000000000000775
Wei Liu and Zhao-Wu Tao contributed equally to this work.
References
- 1.Hui DS, I Azhar E, Madani TA, Ntoumi F, Kock R, Dar O, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health - the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis 2020; 91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang N, Wang L, Deng X, Liang R, Su M, He C, et al. Recent advances in the detection of respiratory virus infection in humans. J Med Virol 2020; 92:408–417. doi: 10.1002/jmv.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cumulative Number of Reported Cases of 2019 Novel Coronavirus Pneumonia. Beijing: National Health Commission of the People's Republic of China, 2020. Available from: http://www.nhc.gov.cn/xcs/yqtb/202002/d5c495da742f4739b7f99339c3bd032f.shtml. [Accessed February 2, 2020]. [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Health Commission of the People's Republic of China, National Administration of Traditional Chinese Medicine of the People's Republic of China. Diagnosis and Treatment Protocol for Novel Coronavirus Infection-Induced Pneumonia Version 4 (trial). Available from: http://www.nhc.gov.cn/yzygj/s7653p/202001/4294563ed35b43209b31739bd0785e67/files/7a9309111267475a99d4306962c8bf78.pdf. [Accessed January 27, 2020]. [Google Scholar]
- 6.Chen Y, Liu Q, Guo D. Coronaviruses: genome structure, replication, and pathogenesis. J Med Virol 2020; 92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Enserink M. Infectious diseases. Clues to the animal origins of SARS. Science 2003; 300:1351.doi: 10.1126/science.300.5624.1351a. [DOI] [PubMed] [Google Scholar]
- 8.Hui DSC, Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect Dis Clin North Am 2019; 33:869–889. doi: 10.1016/j.idc.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C, Chen Z, Hu Y, Ji H, Yu D, Shen W, et al. Complemented palindromic small RNAs first discovered from SARS coronavirus. Genes (Basel) 2018; 9:E442.doi: 10.3390/genes9090442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020; 20:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren LL, Wang YM, Wu ZQ, Xiang ZC, Guo L, Xu T, et al. Identification of a novel coronavirus causing severe pneumonia in human: a descriptive study. Chin Med J 2020; 133:1015–1024. doi: 10.1097/CM9.0000000000000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ticinesi A, Nouvenne A, Folesani G, Prati B, Morelli I, Guida L, et al. An investigation of multimorbidity measures as risk factors for pneumonia in elderly frail patients admitted to hospital. Eur J Intern Med 2016; 28:102–106. doi: 10.1016/j.ejim.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 13.Cilloniz C, Polverino E, Ewig S, Aliberti S, Gabarrus A, Menendez R, et al. Impact of age and comorbidity on cause and outcome in community-acquired pneumonia. Chest 2013; 144:999–1007. doi: 10.1378/chest.13-0062. [DOI] [PubMed] [Google Scholar]
- 14.Ladha KS, Zhao K, Quraishi SA, Kurth T, Eikermann M, Kaafarani HM, et al. The Deyo-Charlson and Elixhauser-van Walraven comorbidity indices as predictors of mortality in critically ill patients. BMJ Open 2015; 5:e008990.doi: 10.1136/bmjopen-2015-008990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohamed R, Aleanizy FS, Alqahtani FY, Alanazi MS, Mohamed N. Common co-morbidities are challenging in the diagnosis of middle east respiratory syndrome (MERS-CoV) in Saudi Arabia. Pak J Biol Sci 2020; 23:119–125. doi: 10.3923/pjbs.2020.119.125. [DOI] [PubMed] [Google Scholar]
- 16.Duc S, Rainfray M, Soubeyran P, Fonck M, Blanc JF, Ceccaldi J, et al. Predictive factors of depressive symptoms of elderly patients with cancer receiving first-line chemotherapy. Psychooncology 2017; 26:15–21. doi: 10.1002/pon.4090. [DOI] [PubMed] [Google Scholar]
- 17.Ho TS, Wang SM, Liu CC. Historical review of pandemic influenza A in Taiwan, 2009. Pediatr Neonatol 2010; 51:83–88. doi: 10.1016/S1875-9572(10)60016-2. [DOI] [PubMed] [Google Scholar]
- 18.Wu CT, Hsia SH, Huang JL. Influenza B-associated rhabdomyolysis in Taiwanese children. Acta Paediatr 2010; 99:1701–1704. doi: 10.1111/j.1651-2227.2009.01595.x. [DOI] [PubMed] [Google Scholar]
- 19.Kato S, Fujisawa T, Enomoto N, Inui N, Nakamura Y, Suda T. Severe respiratory failure associated with influenza B virus infection. Respirol Case Rep 2015; 3:61–63. doi: 10.1002/rcr2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA 2015; 313:677–686. doi: 10.1001/jama.2015.88. [DOI] [PubMed] [Google Scholar]
- 21.Kil HR, Lee JH, Lee KY, Rhim JW, Youn YS, Kang JH. Early corticosteroid treatment for severe pneumonia caused by 2009 H1N1 influenza virus. Crit Care 2011; 15:413.doi: 10.1186/cc10082. [DOI] [PMC free article] [PubMed] [Google Scholar]


