Abstract
Background
YKL-40 is a glycoprotein associated with inflammatory conditions, including atherosclerosis and endothelial dysfunction. The objective was to analyse serum YKL-40 levels in a haemodialysis population and explore their association with dialysis dosing measures, inflammation, body composition and development of cardiovascular (CV) events.
Methods
We performed a prospective study of 78 chronic haemodialysis patients enrolled in 2013 and followed up until 2018. At baseline, serum YKL-40, inflammatory and nutrition markers and body composition were assessed. During a median follow-up of 43 (interquartile range 24–66) months, CV events were recorded.
Results
The mean age of patients was 62 ± 16 years and 66% were men. The mean YKL-40 was 207 ± 106 ng/dL. Higher YKL-40 levels were associated with lower Kt/Vurea, convective volume, serum albumin and prealbumin and with higher troponin T. During follow-up, 50% developed CV events. Cox analysis showed an association between CV events and YKL-40, diabetes, hypertension, C-reactive protein, lower prealbumin, β2-microglobulin, glycosylated haemoglobin and troponin T values. The multivariate Cox analysis confirmed an independent association between CV events and YKL-40 {hazard ratio [HR] 1.067 [95% confidence interval (CI) 1.009–1.211]; P: 0.042}, troponin T [HR 1.037 (95% CI 1.009–1.683); P: 0.007], lower prealbumin [HR 0.827 (95% CI 0.224–0.988); P: 0.009] and diabetes [HR 2.103 (95% CI 1.554–3.172); P: 0.008]. Kaplan–Meier confirmed the association between CV events and YKL-40 (log rank 7.28; P = 0.007).
Conclusions
YKL-40 is associated with CV events in haemodialysis patients. Higher dialysis dose and convective volume are associated with lower serum YKL-40 levels.
Keywords: cardiovascular events, haemodialysis, inflammation, online haemodiafiltration, YKL-40
INTRODUCTION
Haemodialysis patients have higher morbidity and mortality than the general population. Cardiovascular (CV) events are the main cause of mortality in this group of patients. Nephrologists have strived to better understand the pathogenesis, drivers and predictive factors for CV disease in patients with chronic kidney disease (CKD). Both traditional and new risk factors have been associated with CV disease in this population, but outcomes are still dismal and further research is needed [1, 2].
YKL-40 (chitinase-3-like protein 1, or human cartilage glycoprotein-39) is a 40-kDa glycoprotein, a member of the mammalian chitinase-like protein family [3, 4]. YKL-40 is produced by macrophages, neutrophils and cancer cells and regulates vascular endothelial growth factor (VEGF), being involved in inflammation and angiogenesis, remodelling of the extracellular matrix and fibrosis [5]. Thus YKL-40 has been associated with inflammation disorders, arteriosclerosis and endothelial dysfunction [6]. Both in the general population and in patients with chronic diseases, serum YKL-40 levels have been associated with increased mortality risk [7, 8]. Recently a link between YKL-40 and mortality was reported in haemodialysis patients [9]. However, studies are necessary to evaluate a potential link between YKL-40 and CV events in haemodialysis patients and to identify additional factors potentially associated with serum YKL-40 levels.
The objective was to study serum YKL-40 in haemodialysis patients and its association with dialysis dose, inflammation, body composition and development of CV events.
MATERIALS AND METHODS
Design of the study
This is a prospective observational study that started in March 2013 and patients where followed up until March 2018. Variables were collected at the beginning of the study and CV events were collected at the end.
Study population
Eighty-four prevalent haemodialysis patients from a single dialysis unit in Madrid, Spain were enrolled. Inclusion criteria were clinical stability with no recent hospitalization (in the past 3 months), age >18 years and informed consent. Exclusion criteria were an inability to understand the study, recent hospitalization and contraindication of bioimpedance spectroscopy (BIS) (patients with pacemakers or limb amputation), resulting in the exclusion of six patients. The procedures were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975 and its latest revision.
Baseline variables
At baseline, demographic and clinical data were collected, including age, sex, CKD aetiology, dialysis vintage, presence of diabetes mellitus, hypertension (defined using the Eighth Joint National Committee report [10]), dyslipidemia (defined using Adult Treatment Panel III guidelines [11]) and a history of prior CV events, including congestive heart failure (CHF), myocardial infarction, peripheral vascular disease (PVD) and stroke.
Fluid status and body composition were assessed at baseline using BIS (Body Composition Monitor, Fresenius Medical Care, Bad Homburg, Germany). Measurements were taken pre-dialysis, after a 10-min resting period in the supine position. Hydration parameters were total body water (TBW), extracellular water (ECW), intracellular water (ICW) and fluid overload/overhydration (OH), defined as water not included in extracellular and intracellular spaces and considered as excess water. We estimated relative OH as OH/ECW [12]. We also determined the lean tissue index (LTI) and fat tissue index (FTI).
Dialysis features were obtained by checking medical records of dialysis sessions. Kt/Vurea and convective volume per session were obtained from haemodialysis monitors. Serum beta-2-microglobulin and β2-microglobulin (B2M) reduction ratio were assessed.
Serum laboratory variables recorded included cardiac markers such as N-terminal prohormone of brain natriuretic peptide (NT-proBNP) and troponin T, nutritional and inflammatory parameters [prealbumin, albumin and C-reactive protein (CRP)], glycosylated haemoglobin (HbA1c), serum glucose, calcium, phosphorus, parathyroid hormone, 25-hydroxyvitamin D and anaemia parameters [haemoglobin and transferrin saturation index (TSI)].
YKL-40 concentrations were measured using a commercially available enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s protocol.
Follow-up
During follow-up {median 43 [interquartile range (IQR) 24–66 months]}, new CV events were recorded. CV events were defined as ischaemic or haemorrhagic stroke (diagnosed by computed tomography), myocardial infarction (diagnosed by cardiac injury marker elevation and electrocardiography, and confirmed by coronary angiography), CHF [diagnosed by the presence of acute pulmonary oedema and an echocardiogram with ventricular systolic dysfunction; left ventricular ejection fraction (LVEF) <45%] or peripheral vascular events (diagnosed by stenosis of major arteries or lower limbs confirmed by arteriography and/or the need for amputation).
Statistical analysis
All variables were analysed using a Kolmogorov–Smirnov test to classify them as normally or non-normally distributed. Values are given as mean [standard deviation (SD)] or median (IQR). Variables were compared using Student’s t-test. Non-parametric variables were compared using the Mann–Whitney U test. Univariate analysis was performed using Cox regression. A multivariate Cox regression analysis was performed to establish an independent association with CV events. The models included factors that showed a significant association or those considered confounding factors. Outcomes were analysed using Kaplan–Meier plots and survival curves were compared using a log-rank test. All statistical analyses were performed with the SPSS 18.0 statistical package (SPSS, Chicago, IL, USA). A P-value <0.05 was considered statistically significant.
RESULTS
Baseline characteristics of the study population
We included 78 patients. The mean age was 62 ± 16 years, 66% were males and 45% had a history of previous CV events before enrolment. About 82% were receiving convective transport with post-dilution online haemodiafiltration (OL-HDF). Baseline characteristics are shown in Table 1. The mean YKL-40 level was 207 ± 106 ng/mL.
Table 1.
Baseline characteristics of the study population (N = 78)
| General characteristics | |
| Sex (male), % | 66 |
| Age (years) | 62 ± 16 |
| Charlson index | 7.2 ± 2.7 |
| Diabetes, % | 30 |
| Hypertension, % | 90 |
| Dyslipidaemia, % | 60 |
| CKD aetiology, % | |
| Glomerular | 40 |
| Diabetes | 24 |
| Vascular | 17 |
| Interstitial | 9 |
| Polycystic kidney disease | 5 |
| Others | 5 |
| Dialysis features | |
| Dialysis vintage (months) | 27 (9–52) |
| Kt/Vurea | 2.0 ± 0.4 |
| Serum B2M (mg/dL) | 25.1 ± 7.5 |
| B2M reduction ratio, % | 79.2 ± 14.1 |
| OL-HDF, % | 82 ± 23 |
| Convective volume (L) | 28.7 ± 6.5 |
| Convective volume adjusted to ECW | 1.7 ± 0.5 |
| AV fistula, % | 66 ± 12 |
| Laboratory parameters | |
| YKL-40 (ng/mL) | 207 ± 106 |
| Haemoglobin (g/L) | 11.4 ± 1.1 |
| TSI (%) | 29.4 ± 10.1 |
| Albumin (g/dL) | 3.7 ± 0.3 |
| Prealbumin (mg/dL) | 23.5 ± 6.2 |
| CRP (mg/dL) | 0.4 (0.1–1.1) |
| Total cholesterol (mg/dL) | 142.5 ± 37.5 |
| Triglycerides (mg/dL) | 120.0 ± 64.2 |
| HbA1c (%) | 5.7 ± 1.0 |
| BNP (pg/mL) | 188 (90–408) |
| NT-proBNP (ng/dL) | 3450 (1350–8410) |
| Troponin T (ng/mL) | 53.1 (31.7–76.2) |
| Calcium (mg/dL) | 8.5 ± 0.6 |
| Phosphorus (mg/dL) | 4.1 ± 1.3 |
| PTH (pg/mL) | 420 (177–631) |
| Vitamin D (µg/L) | 10.3 ± 4.4 |
| Hydration status and body composition | |
| BMI (kg/m²) | 25.9 ± 4.9 |
| LTI (kg/m²) | 15.6 ± 4.0 |
| FTI (kg/m²) | 12.0 ± 9.3 |
| OH (L) | 1.3 (−0.02–2.2) |
| ECW (L) | 17.0 ± 3.5 |
| ICW (L) | 19.7 ± 4.7 |
| ECW/ICW | 0.8 ± 0.1 |
| OH/ECW (%) | 2.3 ± 0.8 |
| Medications | |
| Patients with antihypertensive treatment, % | 50 |
| Number of antihypertensive | 1.5 (0–3) |
| Patients with erythropoietin treatment, % | 89 |
| Doses erythropoietin per week (IU/week) | 4067 ± 1043 |
| Echocardiographic data | |
| LVEF (%) | 50 ± 10 |
| LVMI (g/m2) | 132 ± 45 |
Variables with this symbol were expressed as median IQR or mean ± SD.
OL HDF, Online hemodiafiltration; TSI, transferrin saturation index; BNP, brain natriuretic protein; BMI, body mass index; LTI, lean tissue index; FTI, fat tissue index; OH, overhydration; ECW, extracellular water; ICW, intracellular water; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index.
Serum YKL-40 levels and dialysis dose
Patients were divided into two groups according to baseline YKL-40 as ≤207 ng/mL or >207 ng/mL. There was a significant association between lower serum YKL-40 levels and higher dialysis dose as assessed by the Kt/Vurea, litres of convective volume and B2M reduction ratio (Table 2). We did not find differences between the groups regarding dialysis complications (i.e. number of cramps and intradialytic hypotension).
Table 2.
Relationship between dialysis dosing measures and serum YKL-40 levels
| Higher YKL-40 group | Lower YKL-40 group | P-value | |
|---|---|---|---|
| (n = 47) | (n = 31) | ||
| Kt/Vurea | 1.92 ± 0.37 | 2.28 ± 0.44 | 0.04 |
| Convective volume in OL-HDF (L) | 27.53 ± 3.99 | 31.12 ± 5.89 | 0.03 |
| B2M reduction ratio (%) | 77.1 ± 11 | 81.2 ± 14 | 0.03 |
| Convective volume adjusted to ECW (L) | 2.0 ± 0.3 | 1.6 ± 0.5 | 0.035 |
| Serum B2M (mg/dL)) | 22.7 ± 5.9 | 24.1 ± 5.4 | 0.883 |
| Dialysis vintage (months) | 123 ± 117 | 107 ± 100 | 0.597 |
Variables are significant when P-value is less than 0.05.
OL-HDF, Online hemodiafiltration; ECW, extracellular water.
Serum YKL-40 levels and laboratory parameters
There was an association between higher serum YKL-40 levels and lower serum albumin and prealbumin levels and with higher troponin T levels, CRP and TSI but not with other biochemical, inflammatory or cardiac parameters (Table 3).
Table 3.
Relationship between serum biochemistry, inflammatory and cardiac parameters and serum YKL-40 levels
| Higher YKL-40 group | Lower YKL-40 group | P-value | |
|---|---|---|---|
| (n = 47) | (n = 31) | ||
| Albumin (g/dL) | 3.57 ± 0.29 | 3.82 ± 0.38 | 0.04 |
| Prealbumin (ng/dL) | 27.53 ± 3.99 | 31.12 ± 5.89 | 0.02 |
| Troponin T (ng/mL)a | 80.4 ± 50.5 | 50.35 ± 20.3 | 0.028 |
| CRP (mg/dL)a | 0.29 ± 0.34 | 1.01 ± 1.33 | 0.032 |
| TSI (%) | 34.27 ± 9.4 | 29.1 ± 9.7 | 0.011 |
| Haemoglobin (g/L) | 11.6 ± 0.8 | 11.6 ± 0.9 | 0.43 |
| Total cholesterol (mg/dL) | 138 ± 29 | 143 ± 43 | 0.978 |
| Triglycerides (mg/dL) | 118 ± 64 | 110 ± 62 | 0.878 |
| HbA1c (%) | 5.3 ± 0.9 | 5.9 ± 0.8 | 0.125 |
| BNP (pg/mL)a | 186 ± 124 | 358 ± 198 | 0.099 |
| NT-proBNP (ng/dL)a | 2878 ± 1966 | 5116 ± 3134 | 0.122 |
| Calcium (mg/dL) | 8.6 ± 0.5 | 8.5 ± 0.5 | 0.968 |
| Phosphorus (mg/dL) | 4.8 ± 1.6 | 4.0 ± 1.1 | 0.677 |
| PTH (pg/mL)a | 430 ± 232 | 517 ± 313 | 0.452 |
| 25-hydroxyvitamin D (ng/mL) | 11.7 ± 4.9 | 10.3 ± 3.9 | 0.835 |
| Number of antihypertensive | 1.45 (0–3) | 1.51 (0–3) | 0.656 |
| Doses erythropoietin per week (IU/week) | 4312 ± 1219 | 3864 ± 1533 | 0.153 |
| LVEF (%) | 50 ± 10 | 50 ± 10 | 0.979 |
| LVMI (g/m2) | 148 ± 57 | 116 ± 41 | 0.540 |
Variables are significant when P-value is less than 0.05.
This variables are not normally distributed. CRP: U Mann Whitney test = 422.5, P: 0.007; BNP: Mann Whitney test = 433.5, P: 0.180; NT-proBNP: Mann Whitney test = 417, P: 0.106; troponin T: Mann Whitney test = 462, P: 0.01; PTH: Mann Whitney test = 540.5, P: 0.141.
C-RP, C reactive protein; TSI, transferrin saturation index; BNP, Brain natriuretic protein; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; PTH, parathyroid hormone.
YKL-40 levels and body composition
There was an association between YKL-40 levels and hydration parameters such as TBW, ECW and ICW but not between YKL-40 levels and overhydration (OH or OH/ECW) or other parameters of body composition measures assessed by body mass index (BMI) (lean tissue and fat tissue) (Table 4).
Table 4.
Relationship between body composition and serum YKL-40 levels
| Higher YKL-40 group | Lower YKL-40 group | P-value | |
|---|---|---|---|
| (n = 47) | (n = 31) | ||
| TBW (L) | 39.2 ± 8.5 | 34.8 ± 6.4 | 0.012 |
| ECW (L) | 18.0 ± 3.5 | 16.1 ± 2.7 | 0.010 |
| ICW (L) | 21.2 ± 5.4 | 18.6 ± 4.2 | 0.032 |
| BMI (kg/m²) | 24.0 ± 3.4 | 27.9 ± 5.5 | 0.011 |
| LTI (kg/m²) | 17.9 ± 4.5 | 15.2 ± 3.6 | 0.291 |
| FTI (kg/m²) | 6.6 ± 3.6 | 8.6 ± 3.8 | 0.126 |
| OH (L) | 1.0 ± 1.3 | 1.3 ± 1.4 | 0.624 |
| ECW/ICW | 0.8 ± 0.1 | 0.9 ± 0.3 | 0.07 |
| OH/ECW (%) | 6.7 ± 3.5 | 7.2 ± 3.9 | 0.545 |
Variables are significant when P-value is less than 0.05.
TBW, total body water; ECW, extracellular water; ICW, intracellular water; BMI, body mass index; LTI, lean tissue index; FTI, fat tissue index; OH, overhydration.
Follow-up
During a median of 43 (IQR 21–49) months of follow-up, 39/78 (50%) patients experienced a CV event. The most common CV event was myocardial infarction (50%), followed by PVD (21%), stroke (20%) and heart failure (9%). At the end of the study, 28/78 (36%) patients died, 19/78 (24%) received a renal transplant and 31/78 (40%) remained in haemodialysis.
Univariate analysis showed that previous CV events, diabetes, hypertension, serum glucose levels, HbA1c, CRP, troponin T, B2M, YKL-40 and low levels of prealbumin were associated with CV events.
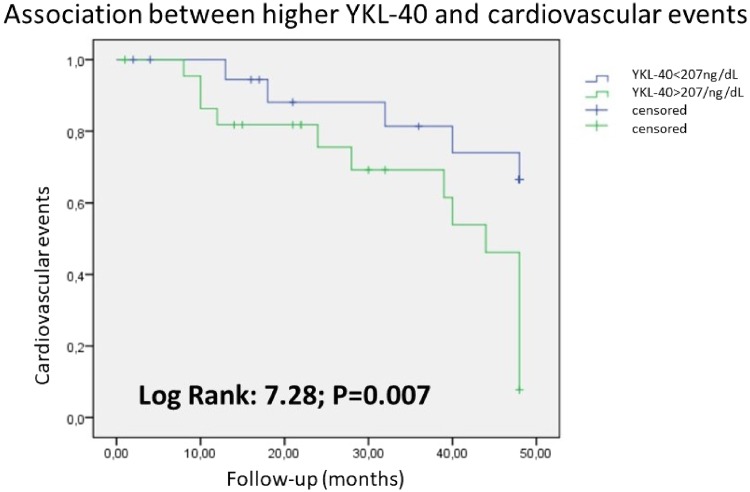
Multivariate analysis confirmed an independent association between diabetes {hazard ratio [HR] 2.103 [95% confidence interval (CI) 1.554–3.172]; P + 0.008}, troponin T [HR 1.037 (95% CI 1.009–1.683); P = 0.007], YKL-40 [HR 1.067 (95% CI 1.009–1.211); P + 0.042] and low prealbumin levels [HR 0.827 (95% CI 0.224–0.988); P + 0.009] with CV events (Table 5). Kaplan–Meier analysis confirmed a higher risk of CV events in patients with YKL-40 values above the mean (log rank 7.28; P = 0.007) (Figure 1).
Table 5.
Cox proportional hazards regression analysis for CV events
| Univariate | P-value | Multivariate | P-value | |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Previous CV events | 1.005 (1.002–1.217) | 0.03 | ||
| Diabetes (%) | 1.007 (1.002–1.198) | 0.01 | 2.103 (1.554–3.172) | 0.008 |
| Hypertension (%) | 1.002 (1.001–1.005) | 0.035 | ||
| Glucose (mg/dL) | 1.012 (1.001–1.097) | 0.006 | ||
| Prealbumin (mg/dL) | 0.885 (0.250–0.920) | 0.009 | 0.827(0.224–0.988) | 0.009 |
| CRP(log) (mg/dL) | 1.534 (1.006–1.990) | 0.048 | ||
| B2M (mg/dL) | 1.122 (1.008–1.310) | 0.001 | ||
| HbA1c (%) | 2.494 (1.389–4.290) | 0.002 | ||
| Troponin T (ng/mL) | 1.024 (1.091–1.300) | 0.001 | 1.037 (1.009–1.683) | 0.005 |
| YKL-40 (ng/mL) | 1.005 (1.001–1.099) | 0.03 | 1.067 (1.009–1.211) | 0.042 |
FIGURE 1.
Kaplan–Meier analysis for CV events and YKL-40. Log rank 7.28; P = 0.007.
DISCUSSION
The main finding is an association between higher serum YKL-40 and the risk of CV events and in stable haemodialysis patients during a median of 43 months of follow-up. This is one of the first reports on the biomarker potential of YKL-40 for CV events in haemodialysis. In addition, an inverse relationship was found between serum YKL-40 levels and parameters of dialysis dosing.
Baseline serum YKL-40 in haemodialysis patients (mean 207 ng/mL) were higher than in other populations, as described in previous studies [13, 14]. The normal range has been established as 65–100 ng/mL in healthy populations [15]. Baseline chronic inflammation and loss of renal function could be involved. In this regard, this study shows an interesting association between lower serum YKL-40 values and dialysis efficacy. This dialysis population was receiving high dialysis doses, as there was a high percentage of OL-HDF with 28.7 L of mean convective volume per session. There was an association between lower serum YKL-40 levels and small molecules removal (assessed by Kt/Vurea), medium-size molecules removal (assessed by the B2M reduction ratio) and litres of convective volume in OL-HDF. As YKL-40 is a 40-kDa protein, convective therapies could theoretically increase YKL-40 removal during dialysis sessions. Few reports have studied YKL-40 removal during dialysis; Nielsen et al. [16] described a 15% reduction in serum YKL-40 during dialysis sessions in patients receiving both haemodialysis and OL-HDF. The same group found that patients with higher serum YKL-40 levels after a dialysis session had a two-fold increased risk of all-cause mortality [13]. Convective therapies are associated with better CV survival in dialysis patients, mainly due to a greater removal of medium-size uremic toxins [17]. Moreover, new therapies such as expanded haemodialysis also provide better removal of toxins of this size [18]. If YKL-40 is increased by inflammation and is associated with CV events, convective therapies could contribute to increase its removal and lower YKL-40 levels. However, there were interindividual differences in predialysis serum YKL-40 levels in patients with similar dialysis features that might be explained by different YKL-40 production rates. Further studies should address this question [19]. In any case, the observation that higher dialysis doses are related with lower serum YKL-40 levels and the association of YKL-40 to outcomes make it an interesting marker to explore novel dialysis dosing methods, especially with regard to convective therapies.
YKL-40 is a glycoprotein expressed by different cell types, such as macrophages and tumour cells, and is involved in inflammation and atherosclerosis [20–22]. Given the association of YKL-40 with inflammation, an association with body composition as fat tissue or lean tissue may have also been expected [23–26]. Indeed, an association between low lean tissue and CV events has been observed in patients with advanced CKD not in dialysis [27]. However, YKL-40 was not associated with body composition parameters. In addition, YKL-40 was not associated with OH parameters, another predictor of adverse outcomes [12, 28].
Among risk factors analysed, only YKL-40, troponin T, diabetes and lower prealbumin values were independently associated with CV events during the follow-up. Of these, YKL-40 represents a relatively new biomarker and further studies should explore its place among biomarkers associated with adverse outcomes in haemodialysis patients.
Our study has several limitations. We did not study YKL-40 after dialysis sessions or the YKL-40 reduction ratio, and the design does not allow us to address cause-and-effect relationships. The sample size is relatively small, although the number of events was large. This point is the main limitation of our study. We included all our prevalent patients from our dialysis unit that fulfilled the criteria. Even with this reduced number (78 patients), the association between CV events and high levels of YKL40 is significant. So with this study we can only show an association between CV events and YKL-40, but this is a starting point for further studies. Nevertheless, our study contributes to a better comprehension of the synthesis of YKL-40, dialysis doses and CV events. An external validation could confirm this association.
In conclusion, serum YKL-40 levels are associated with CV morbidity in haemodialysis patients. Since an association was found between lower serum YKL-40 levels and higher dialysis doses and convective volume, future studies should address whether using YKL-40 as a marker of dialysis adequacy may improve outcomes and experimental studies should address whether YKL-40 may be causally related to CV disease.
ACKNOWLEDGEMENTS
The authors would like to thank the nursing staff for their help.
AUTHORS’ CONTRIBUTIONS
A.V. designed the study, wrote the article, collected data and performed the statistical analysis. M.D.S.-N. determined the serum YKL-40 levels. A.O. contributed to the design and revised the manuscript. S.A., I.A., N.M., A.G.-P. and A.S. collected data and performed the statistical analysis. E.T., E.H., A.H., L.S.-C. and L.V.-R collected data. J.L. designed the study.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Okamoto T, Tsutaya C, Hatakeyama S. et al. Low serum butyrylcholinesterase is independently related to low fetuin-A in patients on hemodialysis: a cross-sectional study. Int Urol Nephrol 2018; 50: 1713–1720 [DOI] [PubMed] [Google Scholar]
- 2. See EJ, Hedley J, Agar JWM.. Patient survival on haemodiafiltration and haemodialysis: a cohort study using the Australia and New Zealand Dialysis and Transplant Registry. Nephrol Dial Transplant 2019; 34: 326–338 [DOI] [PubMed] [Google Scholar]
- 3. Lee CG, Da Silva CA, Dela Cruz CS. et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling and injury. Annu Rev Physiol 2011; 73: 479–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rathcke CN, Vestergaard H.. YKL-40, a new inflammatory marker with relation to insulin resistance and with a role in endothelial dysfunction and atherosclerosis. Inflamm Res 2006; 55: 221–227 [DOI] [PubMed] [Google Scholar]
- 5. Volck B, Price PA, Johansen JS.. YKL-40, a mammalian member of the chitinase family, is a matrix protein of specific granules in human neutrophils. Proc Assoc Am Physicians 1998; 110: 351–360 [PubMed] [Google Scholar]
- 6. Prakash M, Bodas M, Prakash D. et al. Diverse pathological implications of YKL-40: answers may lie in “outside-in” signaling. Cell Signal 2013; 25: 1567–1573 [DOI] [PubMed] [Google Scholar]
- 7. Bojesen SE, Johansen JS, Nordestgaard BG.. Plasma YKL-40 levels in healthy subjects from the general population. Clin Chim Acta 2011; 412: 709–712 [DOI] [PubMed] [Google Scholar]
- 8. Kastrup J, Johansen JS, Winkel P. et al. High serum YKL-40 concentration is associated with cardiovascular and all-cause mortality in patients with stable coronary artery disease. Eur Heart J 2009; 30: 1066–1072 [DOI] [PubMed] [Google Scholar]
- 9. Lorenz G, Schmalenberg M, Kemmner S. et al. Mortality prediction in stable hemodialysis patients is refined by YKL-40, a 40 kDa glycoprotein associated with inflammation. Kidney Int 2018; 93: 221–230 [DOI] [PubMed] [Google Scholar]
- 10. James PA, Oparil S, Carter BL. et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311: 507–520 [DOI] [PubMed] [Google Scholar]
- 11.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106: 3143–3421 [PubMed] [Google Scholar]
- 12. Wizemann V, Wabel P, Chamney P. et al. The mortality risk of overhydration in haemodialysis patients. Nephrol Dial Transplant 2009; 24: 1574–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Okyay GU, Er RE, Tekbudak MY. et al. Novel inflammatory marker in dialysis patients: YKL-40. Ther Apher Dial 2013; 17: 193–201 [DOI] [PubMed] [Google Scholar]
- 14. Pawlak K, Rozkiewicz D, Mysliwiec M. et al. YKL-40 in hemodialyzed patients with and without cardiovascular complications – the enhancement by the coexistence of the seropositivity against hepatitis C virus infection. Cytokine 2013; 62: 75–80 [DOI] [PubMed] [Google Scholar]
- 15. Xu T, Zhong C, Wang A. et al. YKL-40 is a novel biomarker for predicting hypertension incidence among prehypertensive subjects: a population-based nested case-control study in China. Clin Chim Acta 2017; 472: 146–150 [DOI] [PubMed] [Google Scholar]
- 16. Nielsen TL, Plesner LL, Warming PE. et al. YKL-40 in patients with end-stage renal disease receiving haemodialysis. Biomarkers 2018; 23: 357–363 [DOI] [PubMed] [Google Scholar]
- 17. Maduell F, Moreso F, Pons M. et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol 2013; 24: 487–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirsch AH, Lyko R, Nilsson L-G. et al. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol Dial Transplant 2017; 32: 165–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Persson F, Borg R.. YKL-40 in dialysis patients: another candidate in the quest for useful biomarkers in nephrology. Kidney Int 2018; 93: 21–22 [DOI] [PubMed] [Google Scholar]
- 20. Gao MZ, Wei YY, Xu QW. et al. Elevated serum YKL-40 correlates with clinical characteristics in patients with polymyositis or dermatomyositis. Ann Clin Biochem 2018; 56: 95–99 [DOI] [PubMed] [Google Scholar]
- 21. Höbaus C, Tscharre M, Herz CT. et al. YKL-40 levels increase with declining ankle-brachial index and are associated with long-term cardiovascular mortality in peripheral arterial disease patients. Atherosclerosis 2018; 274: 152–156 [DOI] [PubMed] [Google Scholar]
- 22. Keskin GS, Helvacı Ö, Yayla Ç.. Relationship between plasma YKL-40 levels and endothelial dysfunction in chronic kidney disease. Turk J Med Sci 2019; 49: 139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farhangi MA, Mesgari-Abbasi M, Hajiluian G. et al. Adipose tissue inflammation and oxidative stress: the ameliorative effects of vitamin D. Inflammation 2017; 40: 1688–1697 [DOI] [PubMed] [Google Scholar]
- 24. Szczepankiewicz D, Skrzypski M, Pruszyńska-Oszmałek E. et al. Interleukin 4 affects lipid metabolism and the expression of pro-inflammatory factors in mature rat adipocytes. Immunobiology 2018; 223: 677–683 [DOI] [PubMed] [Google Scholar]
- 25. Nguyen-Tu MS, Nivoit P, Oréa V. et al. Inflammation-linked adaptations in dermal microvascular reactivity accompany the development of obesity and type 2 diabetes. Int J Obes 2019; 43: 556–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rong YD,, Bian AL, Hu HY. et al. Study on relationship between elderly sarcopenia and inflammatory cytokine Il-6, anti-inflammatory cytokine IL-10. BMC Geriatr 2018; 18: 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vega A, Abad S, Macías N. et al. Low lean tissue index is an independent factor for mortality in patients with stage 4 and 5 non-dialysis chronic kidney disease. Clin Kidney J 2017; 10: 170–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vega A, Abad S, Macías N. et al. Any grade of relative overhydration is associated with long-term mortality in patients with stage 5 and 5 non-dialysis chronic kidney disease. Clin Kidney J 2018; 11: 372–376 [DOI] [PMC free article] [PubMed] [Google Scholar]