Abstract
Chronic kidney disease (CKD) is one of the fastest growing causes of death worldwide. Only early diagnosis will allow prevention of both CKD progression and the negative impact of CKD on all-cause and cardiovascular mortality. Klotho is a protein produced by the kidneys that has anti-ageing and phosphaturic properties, preventing excess positive phosphate balance. There is evidence that Klotho downregulation is one of the earliest consequences of kidney injury. Thus the development of reliable assays to monitor Klotho levels may allow an early diagnosis of CKD and monitoring the impact of therapies aimed at preserving Klotho expression or at preventing CKD progression. However, the performance of Klotho assays has been suboptimal so far. In this issue of Clinical Kidney Journal, Neyra et al. explore methods to improve the reliability of Klotho assays.
Keywords: blind spot, chronic kidney disease, Klotho, proteomics
In 1997, Kuro-O et al. [1] described Klotho as a novel protein mainly synthetized by the kidneys, which had anti-ageing properties. It was later shown that protection from excess dietary phosphate intake was a key element in the anti-ageing effects of Klotho [2]. This was related to both direct effects on proximal tubular phosphate transporters, promoting phosphaturia [3], and on its role as a co-receptor for fibroblast growth factor 23 (FGF23), also in proximal tubular cells [4]. Further studies disclosed that both acute kidney injury (AKI) and chronic kidney disease (CKD) are conditions of acquired Klotho deficiency [5]. Indeed, several of the features of genetic Klotho deficiency, such as bone disease, vascular calcification, left ventricular hypertrophy, premature mortality, increased FGF23 levels and hyperphosphataemia, among others, are also features of CKD, a syndrome with a negative impact on multiple organs and systems that has been characterized as a syndrome of accelerated ageing [6, 7]. The widespread consequences of Klotho deficiency have led to the proposal that Klotho acts as an integrator of organ systems [8]. Given this background, it was widely expected that assessment of Klotho levels in biological fluids might provide useful information for risk stratification or patient categorization. However, this has not been the case and the literature has often been contradictory. In contrast, circulating FGF23 is convincingly associated with adverse outcomes [9], and preclinical studies have shown that kidney-specific Klotho deficiency reproduces the phenotype of Klotho-deficient animals, including very low circulating Klotho levels and extremely high circulating FGF23 levels [10].
KLOTHO DEFICIENCY: THE FIRST MANIFESTATION OF KIDNEY DYSFUNCTION AND A DRIVER OF DISEASE PROGRESSION?
The issue acquires further relevance as there is evidence that Klotho deficiency develops in very early stages of CKD as a response to pathological albuminuria, kidney inflammation or even systemic inflammation at a distant organ site that influences the biology of a healthy kidney [11–13]. Thus a reliable assessment of Klotho levels and the identification of biological fluid (plasma/serum versus urine) more reliable for assessing kidney Klotho production could potentially be a game-changing development that influences the way we define or categorize CKD. This would be part of a wider trend towards earlier diagnosis of CKD, including the development of a urine peptidomics classifier such as CKD273 or modifications of it that allow the risk stratification for progression of CKD even in patients who do not fulfil the current criteria to diagnose CKD (i.e. pathological albuminuria or decreased glomerular filtration rate) [14, 15].
Indeed, some of the most reliable evidence of an early decrease in kidney Klotho is derived from the analysis of urine Klotho by either immunoprecipitation–immunoblotting (IP–IB) or immunoblotting [11, 16]. While in the specific patients studied, the driver of decreased urinary Klotho levels in early CKD (A2–A3, G1–G2) is open to question, preclinical evidence indicates that decreased urinary Klotho indeed reflects low kidney Klotho gene and protein expression [11].
HOW DO FINDINGS BY NEYRA ET AL. FIT IN?
Neyra et al. [17] have addressed the discrepancies in the reported relationship between circulating Klotho and kidney disease in this issue of Clinical Kidney Journal. They found that IP–IB appears to provide a reliable assessment of Klotho levels in fresh or only once-thawed serum. In contrast, one of the most widely used enzyme-linked immunosorbent assays (ELISAs) was unreliable in detecting exogenously added recombinant Klotho, and serum Klotho levels assessed by ELISA did not correlate with glomerular filtration rate (GFR) (r = 0.18, P = NS), as opposed to the significant correlation found between Klotho IP–IB and estimated GFR (eGFR) values (Klotho: r = 0.80, P < 0.001). An intriguing finding was that the recovery of exogenous Klotho was lower in uraemic serums, suggesting that uraemia-specific factors may decrease Klotho protein levels or antigenicity. No modification of the sample processing protocol improved the ELISA reliability. A significant correlation between ELISA Klotho and IP–IB Klotho was observed, although the strength of the association was suboptimal (r = 0.28, P = 0.01). This is bad news for the advance of the Klotho field since IP–IB is a time-consuming technique, not appropriate for large studies. Furthermore, it was found that the quality of specific antibody batches may also influence the results. While other authors have found an association of pathological albuminuria with decreased urinary Klotho, as assessed by IB in fresh urine [11], Neyra et al. [17] did not assess the impact of albuminuria on serum Klotho or the combined impact of eGFR and albuminuria on circulatory Klotho levels. Indeed, most albuminuria values in their samples were within the physiological range and just 25% of the values within the one cohort that collected these values were >41 mg/g urinary creatinine.
CONCLUSIONS
Clearly, more reliable, high-throughput ways to assess kidney Klotho production or Klotho levels in biological fluids should be developed, including testing the feasibility of proteomics techniques, such as multiple reaction monitoring/selective reaction monitoring. The development of assays and subsequent routine monitoring of Klotho levels or production may address the so-called blind spot in CKD [18] (Figure 1). This is an early stage in the natural history of CKD when the kidneys are already damaged but eGFR and albuminuria do not yet meet the thresholds to diagnose CKD and no other CKD diagnostic criteria is yet met. The proof-of-concept for the existence of the blind spot in CKD is autosomal dominant polycystic kidney disease in which, in the absence of imaging, CKD cannot be diagnosed for decades. The search is on for ‘imaging-like’ techniques that shed light on early stages of CKD in non-polycystic CKD and reliable assessment of Klotho levels may be one such technique.
FIGURE 1.
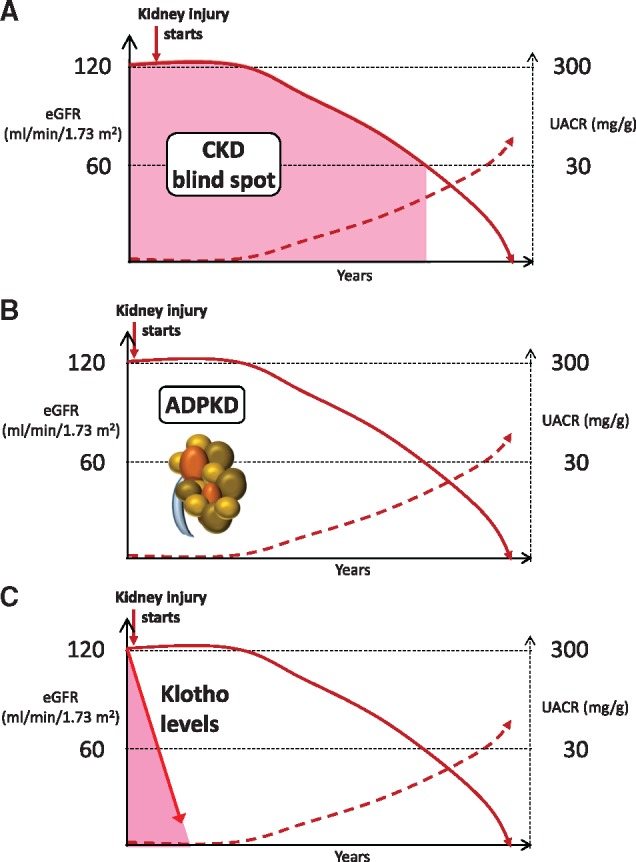
The CKD blind spot. (A) CKD blind spot. The most frequently used diagnostic and risk categorization criteria for CKD are GFR and urinary albumin:creatinine ratio (UACR). However, they represent late events, allowing for a blind spot in the diagnosis of CKD, which may last for years. This blind spot is characterized by the progression of undiagnosed kidney injury, that is, by progressive loss of GFR or progressive increase in albuminuria, but yet within limits that are not diagnostic of CKD. As it is the case with driving, a blind spot may be fatal since specific therapy may be initiated too late to prevent the need for renal replacement therapy or premature death. The CKD blind spot is coloured pink in the image. (B) Imaging addresses the blind spot in autosomal dominant polycystic kidney disease, allowing the identification of multiple bilateral cysts and the diagnosis of CKD decades before GFR or UACR become pathological. (C) If reliable biological fluid processing and Klotho assessment methods are developed, and suggestions of an early decrease of Klotho following kidney injury are confirmed, assessment of Klotho levels may potentially provide an earlier diagnosis of CKD than either GFR or UACR. (A) and (B) reproduced by Sanchez-Niño et al. [18].
FUNDING
The authors were supported by FIS PI16/02057, PI18/01366, PI19/00588, PI19/00815, ERA-PerMed-JTC2018 (KIDNEY ATTACK AC18/00064 and PERSTIGAN AC18/00071), DTS18/00032, ISCIII-RETIC REDinREN RD016/0009 Fondos FEDER, Sociedad Española de Nefrología, Comunidad de Madrid B2017/BMD-3686 CIFRA2-CM and Miguel Servet MS14/00133 to MDSN.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Kuro-O M, Matsumura Y, Aizawa H. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997; 390: 45–51 [DOI] [PubMed] [Google Scholar]
- 2. Kurosu H, Yamamoto M, Clark JD. et al. Suppression of aging in mice by the hormone Klotho. Science 2005; 309: 1829–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hu MC, Shi M, Zhang J. et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 2010; 24: 3438–3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuro-O M. Overview of the FGF23-Klotho axis. Pediatr Nephrol 2010; 25: 583–590 [DOI] [PubMed] [Google Scholar]
- 5. Izquierdo MC, Perez-Gomez MV, Sanchez-Niño MD. et al. Klotho, phosphate and inflammation/ageing in chronic kidney disease. Nephrol Dial Transplant 2012; 27(Suppl 4): iv6–iv10 [DOI] [PubMed] [Google Scholar]
- 6. Zoccali C, Vanholder R, Massy ZA. et al. The systemic nature of CKD. Nat Rev Nephrol 2017; 13: 344–358 [DOI] [PubMed] [Google Scholar]
- 7. Kooman JP, Kotanko P, Schols A. et al. Chronic kidney disease and premature ageing. Nat Rev Nephrol 2014; 10: 732–742 [DOI] [PubMed] [Google Scholar]
- 8. Cheikhi A, Barchowsky A, Sahu A. et al. Klotho: an elephant in aging research. J Gerontol A Biol Sci Med Sci 2019; 74: 1031–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gutiérrez OM, Mannstadt M, Isakova T. et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 2008; 359: 584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lindberg K, Amin R, Moe OW. et al. The kidney is the principal organ mediating klotho effects. J Am Soc Nephrol 2014; 25: 2169–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fernandez-Fernandez B, Izquierdo MC, Valiño-Rivas L. et al. Albumin downregulates Klotho in tubular cells. Nephrol Dial Transplant 2018; 33: 1712–1722 [DOI] [PubMed] [Google Scholar]
- 12. Moreno JA, Izquierdo MC, Sanchez-Niño MD. et al. The inflammatory cytokines TWEAK and TNFα reduce renal klotho expression through NFκB. J Am Soc Nephrol 2011; 22: 1315–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thurston RD, Larmonier CB, Majewski PM. et al. Tumor necrosis factor and interferon-gamma down-regulate Klotho in mice with colitis. Gastroenterology 2010; 138: 1384–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pontillo C, Mischak H.. Urinary peptide-based classifier CKD273: towards clinical application in chronic kidney disease. Clin Kidney J 2017; 10: 192–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rodríguez-Ortiz ME, Pontillo C, Rodríguez M. et al. Novel urinary biomarkers for improved prediction of progressive eGFR loss in early chronic kidney disease stages and in high risk individuals without chronic kidney disease. Sci Rep 2018; 8: 15940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hu MC, Shi M, Zhang J. et al. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 2011; 22: 124–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neyra JA, Moe OW, Pastor J. et al. Performance of soluble Klotho assays in clinical samples of kidney disease. Clin Kidney J 2020; 13: 235--244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanchez-Niño MD, Sanz AB, Ramos AM. et al. Clinical proteomics in kidney disease as an exponential technology: heading towards the disruptive phase. Clin Kidney J 2017; 10: 188–191 [DOI] [PMC free article] [PubMed] [Google Scholar]


