Abstract
Background
Steroid-dependent nephrotic syndrome (SDNS) patients experience frequent relapse or adverse effects on long-term treatment with steroids or cyclophosphamide. This study assessed the efficacy and side effect profile of mycophenolate mofetil (MMF) therapy in children with nephrotic syndrome in our population.
Methods
A retrospective study was performed on children with SDNS who were on MMF therapy for a minimum period of 1 year, and were on regular follow-up in the Department of Nephrology at the Institute of Child Health and hospital for children attached to Madras Medical College.
Results
The study included 87 patients, with a male:female ratio of 2:1. The median age at diagnosis of nephrotic syndrome was 3 years [95% confidence interval (CI): 1–8 years], which was found to be a statistically significant risk factor for MMF failure. The median duration of follow-up after initiation of MMF therapy was 3 years and 3 months (95% CI: 1 year and 3 months to 6 years and 6 months). At initial evaluation, 31 (36%) patients presented with SDNS while the remaining had frequently relapsing nephrotic syndrome progressing to SDNS. Intravenous cyclophosphamide was used as first-line therapy in 82 patients, of whom 24 patients had persistent proteinuria while the remaining 58 had attained remission for a median duration of 6 months. The median duration of treatment with MMF was 2 years and 6 months (95% CI: 1 year and 3 months to 4 years and 6 months). MMF was used at a mean dose of 28.5 mg/kg. Seventy-two (83%) patients were MMF-sensitive, and these patients had a reduction in mean prednisolone dose from 1.28 to 0.35 mg/kg (P < 0.05). Among the MMF-sensitive patients, 31 had stopped MMF after a minimum period of 2 years, following which they had a median remission period of 5 months (95% CI: 1–8 months). MMF failure occurred in 15 (17%) patients. Adverse events were documented in 19 (22%) patients.
Conclusions
Continuous MMF therapy achieved remission in 83% of patients. MMF was well tolerated in the study population and discontinuation of MMF resulted in 100% relapse.
Keywords: MMF, nephrotic syndrome, steroid-dependent
INTRODUCTION
The nephrotic syndromes that occur during childhood predominantly have minimal change disease patterns on biopsy and are usually 90–95% steroid-sensitive [1]. Among the steroid-sensitive nephrotic syndrome patients who attain remission, 80–90% have relapse of nephrotic syndrome [2]. These patients become infrequent or frequent relapsers, or they develop steroid-dependent nephrotic syndrome (SDNS). Of the total number of children who present with idiopathic nephrotic syndrome, 30% have SDNS [3].
Steroid toxicity features tend to occur in patients who are exposed to corticosteroids for longer periods of time. Other immunosuppressive medications have been tried as steroid-sparing agents in order to maintain remission in SDNS patients. A meta-analysis has revealed that a significant number of children do not respond to cyclophosphamide- and levamisole-based therapies [4]. Risk of calcineurin inhibitor toxicity limits their prolonged usage, though they do tend to decrease relapse rates [5].
Mycophenolate mofetil (MMF) is an antimetabolite drug that acts by inhibiting the inosine monophosphate dehydrogenase enzyme in a selective and reversible manner, thereby retarding the de novo synthesis of purines in cells. It selectively exerts its effect on lymphocytes because they are not equipped to use salvage pathways to produce purines [6].
MMF has been used in SDNS patients along with steroids, and their utility has been demonstrated in various studies worldwide, but there are limited data from the Indian subpopulation regarding the efficacy and safety of MMF.
The objective of this study was to assess the efficacy and safety of MMF therapy in SDNS patients, and to assess the relapse rate after cessation of MMF therapy.
MATERIALS AND METHODS
This retrospective single-centre study included patients presenting with SDNS who received treatment with MMF in the Department of Nephrology at the Institute of Child Health attached to Madras Medical College, Chennai.
The cases were defined by Kidney Disease Improving Global Outcome (KDIGO) definitions of nephrotic syndrome and also the remission episodes [7].
Nephrotic syndrome: oedema, urine protein creatinine ratio (uPCR) ≥2000 mg/g (≥200 mg/mmol) or ≥300 mg/dL, or 3+ protein on urine dipstick, hypoalbuminaemia ≤ 2.5 g/dL.
Complete remission: uPCR ≤200 mg/g (≤20 mg/mmol) or ≤1+ of protein on urine dipstick for 3 consecutive days.
Partial remission: proteinuria reduction of ≥50% from the presenting value, and absolute uPCR between 200 and 2000 mg/g.
Relapse: uPCR ≥2000 mg/g (≥200 mg/mmol) or ≥3+ protein on urine dipstick for 3 consecutive days.
Frequent relapse: two or more relapses within 6 months of initial response, or four or more relapses in any 12-month period.
Steroid dependence: two consecutive relapses during corticosteroid therapy or within 14 days of ceasing therapy.
Being a referral centre, children who were in different states of the disease and who had undergone various treatment protocols were referred to us. These included both newly identified nephrotic syndrome patients and previously treated steroid-sensitive nephrotic syndrome who were presenting with frequently relapsing nephrotic syndrome or SDNS.
The patients were started on oral prednisone as a single daily dose starting at 60 mg/m2/day, or 2 mg/kg/day to a maximum of 60 mg/day was given for 4–6 weeks followed by alternate-day medication as a single daily dose starting at 40 mg/m2 or 1.5 mg/kg (maximum 40 mg on alternate days), and continued for 2–5 months with tapering of the dose.
SDNS patients were started on full-dose steroids that were continued until they attained remission, beyond which they were started on second-line immunosuppressive drugs along with tapering of the steroid dose.
According to the department protocol, cyclophosphamide was preferred as the initial second-line immunosuppressive drug, which was given as monthly intravenous doses of 500 mg for a period of 6 months and regularly monitored. Patients who did not achieve remission or who developed relapse after therapy were started on MMF therapy. The dose of MMF on initiation of therapy was 30 mg/kg in two divided doses. The patients were monitored for adverse effects and the steroid dose was reduced to the minimal level that maintained remission.
The following clinical data were recorded: age of disease onset, gender, median age of commencement of MMF therapy, median years of follow-up on MMF therapy, prior immunosuppressant used, renal biopsy details, steroid dose needed for remission before and after starting MMF therapy, relapses while on MMF therapy and adverse effects.
MMF was stopped when the duration of therapy was >2 years, and children were in prolonged remission or they had features of MMF resistance, which were considered when the patient had frequent relapses while on MMF therapy without any precipitating events, or the patient had any major adverse effects. Patients who developed relapses while MMF treatment was paused were restarted on MMF.
Patients’ haemograms and renal function were monitored monthly, or according to symptoms; urine protein dipsticks were monitored fortnightly and the steroid doses changed accordingly.
The inclusion criteria were children <12 years who had SDNS and had been on MMF therapy for a minimum period of 1 year with normal renal function, and who had provided informed written consent in English or Tamil.
Patients with congenital nephrotic syndrome and biopsy-proven secondary glomerular disorders were excluded from the study.
The steroid doses required to maintain remission while on MMF therapy at 3, 6, 12, 18 and 24 months of follow-up and their averages were taken for statistical comparison.
Statistics
The statistical data were documented as medians along with interquartile ranges. The Student’s paired t-test was used to compare steroid dose requirements before and after the initiation of MMF therapy. The Chi-square test was used for categorical variables, while the Student’s t-test with independent variables was used for continuous variables to assess the risk factors for MMF therapy failure. P < 0.05 was considered significant. SPSS software version 18 was used for analysis.
RESULTS
The total number of SDNS patients studied was 87. The gender distribution showed predominantly male patients [58 (67%)], with a male:female ratio of 2:1.
Course of disease
The median age at diagnosis of nephrotic syndrome was 3 years [95% confidence interval (CI): 1–8 years] at initial evaluation: 31 (36%) patients presented with SDNS while the remaining had frequently relapsing nephrotic syndrome progressing to SDNS. Among the frequently relapsing nephrotic syndrome patients, the median period at which they developed SDNS was 2 years. The majority of patients [80 (92%)] had intravenous cyclophosphamide as the initial second-line immunosuppressive agent: four patients (4%) had MMF, two patients (2%) had levamisole, while one patient (1%) had tacrolimus as the initial second-line immunosuppressive agent.
Intravenous cyclophosphamide was used as first-line therapy in 80 patients, of whom 23 patients had persistent proteinuria while the remaining 57 had attained remission within a period of 6 months. These patients remained in remission for a median duration of 6 months, after which they relapsed.
MMF was initiated as the initial therapy in only four patients as per department protocol, which preferred intravenous cyclophosphamide as the initial drug of choice. Other patients who were treated with other immunosuppressants were started on MMF if they continued to have persistent proteinuria or relapsed after stopping the initial therapy. The median age of commencement of MMF therapy was 7 years (95% CI: 2–12 years), while the median duration of follow-up after initiation of MMF therapy was 3 years and 3 months (95% CI: 1 year and 3 months to 6 years and 6 months), and the median duration of treatment with MMF was 2 years and 6 months (95% CI: 1 year and 3 months to 4 years and 6 months).
Efficacy
MMF was considered to be efficacious if it was able to maintain proteinuric remission in patients with a minimal dose of steroids along with a reduction in relapse rate. MMF was used at a mean dose of 28.5 mg/kg. Seventy-two (83%) patients were MMF-sensitive; these patients had a reduction in mean prednisolone dose from 1.28 to 0.35 mg/kg, which was found to be statistically significant by the paired Student’s t-test (P < 0.001). Relapses in MMF-sensitive patients were associated with non-compliance, infection and rapid steroid tapering.
Relapses usually responded to an increased steroid dose; the protocol followed was an increase in steroid dose to 0.5 mg/kg or twice the dose of the minimal dose of steroid being maintained (the lowest dose among the above two options for an individual patient was maintained for a minimum period of 2 weeks, followed by tapering to the lowest dose). Sixty-three patients relapsed while on MMF, of which 32 had a single relapse, 16 had two relapses and 15 had three or more relapses.
MMF failure
MMF failure, defined as frequent relapse while on MMF, occurred in 15 (17%) patients without any of the precipitating factors mentioned earlier. The varying time periods during which they developed MMF failure are depicted in Figure 1.
FIGURE 1.
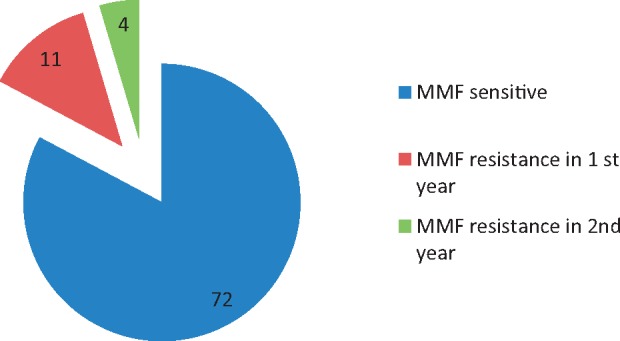
Response to MMF therapy data expressed as numbers of patients.
Among the patients who had MMF therapy failure, the drug was stopped after a minimum period of 1 year and they were switched over to other alternative immunosuppressants. The risk factor analysis for MMF failure was assessed with regard to the various clinical and treatment-related factors, of which age at diagnosis of nephrotic syndrome was found to be statistically significant (P = 0.034), with later age of onset of nephrotic syndrome predicting a higher risk of MMF failure. The other risk factors analysed are depicted in Table 1.
Table 1.
Risk factor analysis
| Variable | MMF-sensitive | MMF-resistant | P-value |
|---|---|---|---|
| Sex, n (%) | |||
| Male | 51 (70.8) | 7 (46.7) | 0.071 |
| Female | 21 (29.2) | 8 (53.3) | |
| Clinical presentation, n (%) | |||
| FRNS | 46 (63.9) | 11 (73.3 ) | 0.484 |
| SDNS | 26 (36.1) | 4 (26.7) | |
| Cyclophosphamide response, n (%) | |||
| Remission | 50 (74.6) | 7 (46.7) | 0.141 |
| Persistent proteinuria | 17 (25.4) | 8 (53.3) | |
| Age at diagnosis of nephrotic syndrome, mean ± SD (years) | 2.95 ± 1.64 | 3.40 ± 2.52 | 0.034 |
| Age at starting MMF, mean ± SD (years) | 7.25 ± 2.64 | 7.16 ± 2.51 | 0.883 |
FRNS, frequently relapsing nephrotic syndrome.
In total, 31 MMF-sensitive patients had been on the drug for a minimum period of 2 years and had maintained remission for sustained period; these patients had their MMF doses tapered slowly and eventually stopped, following which they were monitored periodically for relapse. All of these patients subsequently had relapses on follow-up with a median remission period of 5 months (95% CI: 1–8 months). These patients were reinitiated on MMF.
Renal biopsy
Renal biopsy was performed only in a small subset of patients (18 patients, which constituted only 21% of the total study population). All of these patients underwent renal biopsy a mean (±SD) of 1.35 ± 0.65 years before initiation of MMF therapy. Eight had minimal change pattern, seven had minimal change patterns with immunoglobulin M (IgM) deposits, while three had diffuse mesangial proliferation with IgM deposits. When the biopsy patients were analysed, there were no statistically significant differences in responses to MMF therapy with regard to the various biopsy patterns (P = 0.199).
Safety and tolerability
The majority of patients (88%) had no adverse effects. Adverse events were documented in 19 (22%) patients, of which the most frequently encountered were urinary infection, diarrhoea and leucopenia, as depicted in Figure 2.
FIGURE 2.

Adverse effects in patients receiving MMF therapy. UTI, urinary tract infection.
Patients who had adverse events had their MMF treatment stopped temporarily until they recovered completely and later restarted the drug without any further adverse events. There were no deaths in the patients who had been on MMF.
DISCUSSION
This study, which included 87 patients, is the largest series on paediatric nephrotic syndrome patients who were evaluated for the safety and efficacy of MMF in steroid-dependent patients.
The majority of our patients had presented with frequently relapsing nephrotic syndrome that later progressed to a steroid-dependent state. These patients, who had been on steroid treatment for several years, had received intravenous cyclophosphamide as per department protocol as the initial second-line immunosuppressant drug therapy. Patients who had persistent proteinuria or relapses were started on MMF.
The patients who were steroid-dependent and had undergone prior multiple immunosuppressant therapy were considered to be ‘difficult to treat’ individuals; despite this, the majority of patients had prolonged remission with MMF and low-dose steroids in more than two-thirds of cases.
The median treatment duration with MMF was 2 years. Six months has been the longest duration described in previous similar studies [8, 9]. Similarly, the follow-up period after the initiation of MMF was 3 years. Three months is the longest previously reported follow-up period, emphasizing the long-term efficacy and safety of MMF.
A limitation of the study was the absence of cohorts who did not receive MMF treatment for comparison to analyse the response rates and factors influencing MMF responsiveness. Biopsies were performed in a minimal number of patients, so the correlation of histopathological patterns to MMF responses could not be properly assessed. The retrospective nature of the study may also have underestimated the adverse effects. We could not collect information on patients treated with MMF for <1 year. Thus, we cannot exclude that MMF was stopped in such patients due to a severe adverse effect.
When comparing our study to similar studies that have shown MMF to have beneficial effects in childhood SDNS patients, our study had the largest numbers. Afzal et al. [10] investigated 42 SDNS patients, Baudouin et al. [11] published on 23 SDNS patients, Banerjee et al. [12] reported the outcomes of 46 SDNS patients, while the study by Hasan et al. [13] reported on 73 patients.
The dosage used in our study was comparable to that of Afzal et al. [10], where the mean daily dose was 26.5 mg/kg compared with our mean dose of 28.5 mg/kg. Efficacy data were also similar to those in our study: Baudouin et al. [11] previously showed that MMF prescribed along with low-dose steroids was effective in maintaining remission in the long-term, as well as enabling a reduction in steroid dose to 25% at 6 months in relation to the pre-MMF dose of steroids. Our study also showed a similar reduction of 25% of the pre-MMF steroid dose. Similar to our study, other studies have shown mild reversible profiles of side effects.
An MMF failure rate of 17% was reported in our study, which was also comparable to those of other studies such as that by Hassan et al. [13], which reported a failure rate of 18% in their population. Later age of onset was found to be significant risk factor for failure of MMF therapy in our study.
CONCLUSION
This study substantiates that MMF is a relatively safe and efficacious second-line drug for SDNS patients in an Indian population, even though they had previously been exposed to other second-line agents. Despite the drawback that all of the patients relapsed within a few months of stopping the drug, in view of its relatively safe profile, MMF can be used for prolonged periods compared with other second-line immunosuppressants.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Hodson EM, Knight JF, Willis NS. et al. Corticosteroid therapy in nephrotic syndrome: a meta-analysis of randomized controlled trials. Arch Dis Child 2000; 83: 45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hodson EM, Willis NS, Craig JC.. Non-corticosteroid treatment for nephrotic syndrome in children. Cochrane Database Syst Rev 2008; 1: CD002290. [DOI] [PubMed] [Google Scholar]
- 3. Srivastava RN, Bagga A.. Nephrotic syndrome In: Srivastava RN, Bagga A (eds), Pediatric Nephrology, 3rd edn. New Delhi, India: Jaypee, 2001, 128–157 [Google Scholar]
- 4. Latta K, von Schnakenburg C, Ehrich JH.. A metaanalysis of cytotoxic treatment for frequently relapsing nephrotic syndrome in children. Pediatr Nephrol 2001; 16: 271–282 [DOI] [PubMed] [Google Scholar]
- 5. Durkan AM, Hodson EM, Willis NS. et al. Immunosuppressive agents in childhood nephrotic syndrome: a meta-analysis of randomized controlled trials. Kidney Int 2001; 59: 1919–1927 [DOI] [PubMed] [Google Scholar]
- 6. Allison AC, Eugui E.. Immunosuppressive and other effects of mycophenolic acid and an ester prodrug, mycophenolate mofetil. Immunol Rev 1993; 125: 5–28 [DOI] [PubMed] [Google Scholar]
- 7. Chapter 3: steroid-sensitive nephrotic syndrome in children. Kidney Int Suppl 2012; 2: 163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Novak I, Frank R, Vento S. et al. Efficacy of mycophenolate mofetil in pediatric patients with steroid-dependent nephrotic syndrome. Pediatr Nephrol 2005; 20: 1265–1268 [DOI] [PubMed] [Google Scholar]
- 9. Hogg RJ, Fitzgibbons L, Bruick J. et al. Mycophenolate mofetil in children with frequently relapsing nephrotic syndrome: a report from the Southwest Pediatric Nephrology Study Group. Clin J Am Soc Nephrol 2006; 1: 1173–1178 [DOI] [PubMed] [Google Scholar]
- 10. Afzal K, Bagga A, Menon S. et al. Treatment with mycophenolate mofetil and prednisolone for steroid-dependent nephrotic syndrome. Pediatr Nephrol 2007; 22: 2059–2065 [DOI] [PubMed] [Google Scholar]
- 11. Baudouin V, Alberti C, Lapeyraque A-L. et al. Mycophenolate mofetil for steroid-dependent nephrotic syndrome: a phase II Bayesian trial. Pediatr Nephrol 2012; 27: 389–396 [DOI] [PubMed] [Google Scholar]
- 12. Banerjee S, Pahari A, Sengupta J. et al. Outcome of severe steroid-dependent nephrotic syndrome treated with mycophenolate mofetil. Pediatr Nephrol 2013; 28: 93–97 [DOI] [PubMed] [Google Scholar]
- 13. Hassan AV, Sinha MD, Waller S.. A single-centre retrospective study of the safety and efficacy of mycophenolate mofetil in children and adolescents with nephrotic syndrome. Clin Kidney J 2013; 6: 384–3893 [DOI] [PMC free article] [PubMed] [Google Scholar]


