Abstract
Background
Doppler ultrasound (DU) monitoring early after arteriovenous fistula (AVF) creation allows the identification of low blood flow (Qa) requiring prompt revision, but it is costly (needs skilled operators and technical instruments) and is not available in all dialysis units. Therefore alternative first-line methods to measure Qa would be welcomed. We reasoned that once an AVF is created, an increment in central venous oxygen saturation (ScvO2) is predictable and proportional to Qa.
Methods
Accordingly, in patients receiving dialysis through a central venous catheter (CVC) in whom an AVF was created, we measured, by means of blood gas analysis, the ScvO2 increment before and after manual compression of the arteriovenous shunt and verified its correlation with DU-measured Qa.
Results
We sampled blood gas in 18 patients with CVC and AVF before and after 30 s manual compression of the AVF. ScvO2 averaged 70.5 ± 3% before and 65.2 ± 3% after AVF closure, with an average drop of 5.1 ± 3% (range 1–12). AVF Qa, which was measured within 24 h by means of DU, averaged 635 ± 349 mL/min (range 50–1300) and was strictly and positively correlated with ΔScvO2 (r = 0.954, P < 0.0001).
Conclusions
Therefore we suggest that in patients with CVC and a newly created AVF, it is possible to monitor AVF Qa without DU by simply measuring blood gas and ΔScvO2. This technique is simple, cheap, repeatable, non-invasive and operator independent and represents a new useful screening test to detect delayed AVF access maturation deserving prompt DU measurement and surgical revision. It helps to quickly identify patients in urgent need of DU verification and possible surgical revision. Regrettably, it is applicable only in patients with CVC.
Keywords: arteriovenous fistula, chronic haemodialysis, CKD, haemodialysis, vascular access
INTRODUCTION
Primary arteriovenous fistula (AVF) is the best vascular access for haemodialysis (HD) patients. Despite technical progress, the early failure rate of AVFs (within 3 months of creation) still averages 20–30% [1], with higher percentages (50–60%) in fragile patients [2]. Currently there are no unique parameters that can predict AVF failure risk, but recent papers highlight AVF blood flow (Qa) as a key parameter to identify a high risk for new AVFs deserving urgent revision to avoid thrombosis [3–5]. Since early detection and pre-emptive repair improve long-term AVF outcome, close monitoring of Qa is mandatory, in particular, in patients with predictably slow and difficult AVF maturation (e.g. late referrals, elderly or diabetics) [6, 7]. However, Doppler ultrasound (DU), the gold standard technique to monitor AVF Qa, although non-invasive, reliable and applicable in any patient at any stage of maturation, is costly, requires a skilled operator with adequate equipment and is not commonly available in all dialysis units. Therefore, alternative first-line methods to measure Qa would be welcomed.
We describe here a new method to estimate Qa that relies on the evaluation of blood oxygen saturation and thus requires only a blood gas analyser, a device much more commonly available in nephrology and dialysis units. This low-cost, non-invasive technique could benefit patients dialysed through a central venous catheter (CVC) before AVF creation and during its maturation.
MATERIALS AND METHODS
Rationale
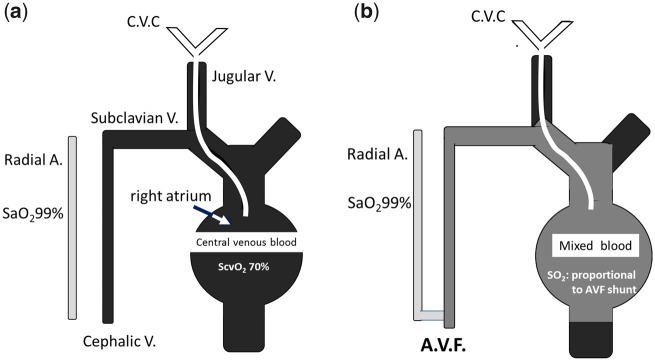
We reasoned that once an AVF is created, some increase in central venous oxygen saturation (ScvO2) would be observed, likely proportional to the Qa (Figure 1). We thus believed that in patients receiving dialysis through a CVC, we could detect changes in ScvO2. Moreover, manual compression of the AV shunt is expected to produce quick changes in blood oxygenation such that the differences in ScvO2 could be used to infer Qa.
FIGURE 1.
(A) With jugular CVC, we have an easy access to ScvO2. (B) When AVF is created, we have a change of ScvO2 proportional to AVF shunt.
Patient selection
Eligibility criteria for this study were age ≥18 years, chronic HD treatment by means of a permanent jugular CVC, the presence of an AVF and no evidence of acute underlying illness. Exclusion criteria were evidence of a displaced or malfunctioning CVC, chronic obstructive pulmonary disease or arterial oxygen saturation (SaO2) < 90% in resting condition and severe refractory anaemia (haemoglobin <9 g/dL despite adequate erythropoietin and iron supplement therapy).
From January to December 2017 we enrolled all patients in our unit who had both a CVC and AVF that fulfilled the eligibility criteria and, in particular, who had capillary SaO2 >90% as measured from any finger of the non-AVF arm [peripheral pulse oximeter (Max Puls Two; EuroSanitas, Montecatini-Terme Pistoia, Italy); accuracy 2%].
In all patients before each of the weekly dialysis sessions, we measured blood oxygen (GEM 4000 Premier; Instrumentation Laboratory, Milan, Italy; SO2 accuracy 2%) from the CVC ‘arterial’ branch both before and after 30 s of AVF closure. AVF flow interruption was obtained soon after heparin administration by manually compressing the AVF 1 cm distal to the anastomosis. Effective flow interruption was confirmed by verifying the disappearance of the bruit with a phonendoscope.
The change in ScvO2 (ΔScvO2) was the difference between ScvO2 with the AVF open and ScvO2 with the AVF closed. In each patient the Qa was then measured by DU within a maximum of 24 h, with the DU operator blinded to the ScvO2 results. In three patients the same measurements were repeated in five consecutive dialysis sessions, by different nurses, to verify the repeatability of the method. In one patient, measurements were repeated after 1 week and after 1, 2 and 3 months of AVF creation to verify Qa changes along with AVF maturation.
The present study is a subanalysis of the ‘Studio No. 107055/2017’ approved by the Ethics Committee of the ASL Rm2 and was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants.
RESULTS
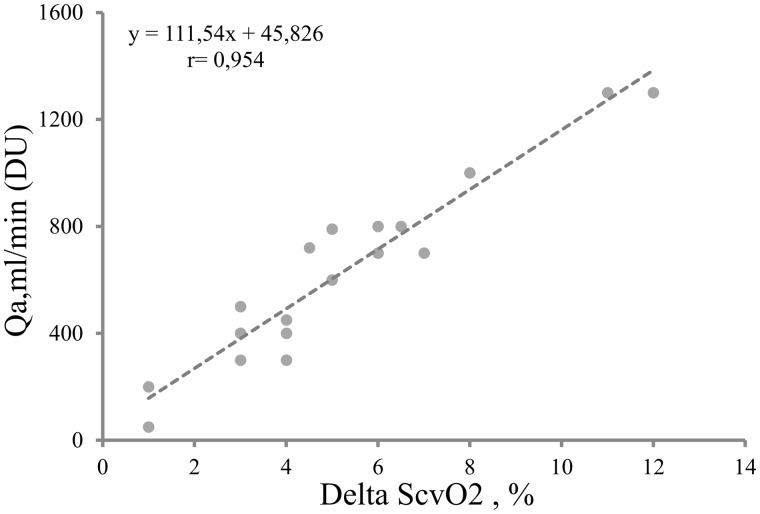
We included 18 CVC–AVF patients (10 male and 8 female, age 63.2 ± 15 years, HD vintage 19.8 ± 15 months, SaO2 range 93–99%; Table 1). ScvO2 before AVF closure averaged 70.5 ± 3% and ScvO2 after AVF closure averaged 65.2 ± 3%. This last value is indeed lower than normal (ScvO2 normal value 70%), but is in agreement with those observed in HD patients [8]. As a result, ΔScvO2 averaged 5.1 ± 3% (range 1–12). AVF Qa measured with DU averaged 635 ± 349 mL/min (range 50–1300) and was strictly and positively correlated with ΔScvO2 (r = 0.954, P < 0.0001). We thus considered that we could estimate AVF flow (eQa) as a function of ΔScvO2 with the following linear regression model equation (Figure 2):
Table 1.
Clinical and biochemical characteristics of the population
| Patients | n = 18 |
|---|---|
| Male/female, n | 10/8 |
| Age (years) | 63.2 ± 15 |
| HD vintage (months) | 19.8 ± 15 |
| AVF vintage (months) | 2.5 ± 2.2 |
| BMI (kg/m2) | 25.1 ± 1.2 |
| ABI | 0.7 ± 0.1 |
| Haemoglobin (g/dL) | 10.1 ± 1.1 |
| K t/V | 1.48 ± 0.0 |
| nPCR (g/kg/day) | 1.03 ± 0.0 |
| SaO2 (%), mean ± SD (range) | 96.8 ± 0.1 (99–93) |
| ScvO2 AVF closed (%) | 65.2 ± 3.2 |
| ScvO2 AVF opened (%) | 70.5 ± 3.3 |
| ΔScvO2 (%), mean ± SD (range) | 5.1 ± 3.1 (1–12) |
| Qa (mL/min via DU), mean ± SD (range) | 635 ± 349 (50–1300) |
Values are presented as mean ± SD unless stated otherwise. BMI, body mass index; ABI, ankle brachial index; Kt/V, urea clearance (K) multiplied by dialysis time (t) divided by the volume of water in the body; nPCR, normalized protein catabolic rate; Qa, vascular access flow.
FIGURE 2.
Correlation test of ΔScvO2 and Qa evaluated with DU.
eQa = 45.82 + 111.54·ΔScvO2
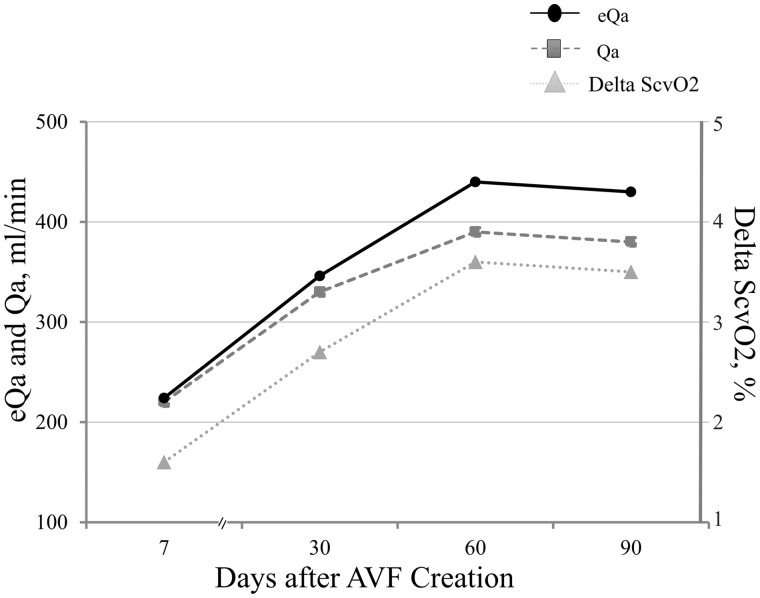
No side effect was recorded secondary to the procedure. In the three patients who had ΔScvO2 measured five consecutive times, the maximum calculated difference was <6.6%, pointing to substantial stability and repeatability of the parameter. In the patient who was sampled after 1 week and 1, 2 and 3 months from AVF creation, ΔScvO2 values were 1.6% (eQa 224 mL/min), 2.7% (eQa 346 mL/min), 3.6% (eQa 440 mL/min) and 3.5% (eQa 430 mL/min), respectively, consistent with the corresponding DU Qa values of 220, 330, 390 and 380 mL/min, respectively (Figure 3).
FIGURE 3.
AVF maturation over time for one patient. DU Qa, eQa and ΔScvO2 were evaluated at 7, 30, 60 and 90 days after AVF creation with a strictly concordant trend.
DISCUSSION
Our study shows that in patients with a CVC and AVF, it is possible to estimate AVF Qa by means of ΔScvO2 measurement, which could thus represent a new tool in monitoring vascular access maturation. Since AVF Qa assessment is required to identify an AVF at risk of failure, having a first-line screening method to identify an AVF with a reduced Qa deserving urgent DU seems useful. Indeed, as shown in Figure 2, ΔScvO2 values of ∼3% indicate flows of ∼300 mL/min, which is the threshold value for an AVF at increased risk of thrombosis. Furthermore, unlike other methods for measuring Qa during an HD session, this method can be used during AVF maturation without positioning fistula needles, before the AVF is ready for venipuncture. For this reason, our simple, cheap, repeatable, non-invasive and operator-independent approach seems to be a useful screening tool to monitor AVF flow and maturation without dedicated instruments and expert operators. It helps to quickly identify patients in urgent need of DU verification and possible surgical revision. Regrettably, and as a limitation, it is applicable only in patients with a CVC.
ACKNOWLEDGEMENTS
This was a spontaneous clinical study where the involved personnel did not receive grants. Assays are based on routinely available parameters. We acknowledge the nurses and patients at Polo Pontino – ICOT Hospital – Dialysis Unit for their willingness to contribute to this work.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Kim HS, Park JW, Chang JH.. Early vascular access blood flow as a predictor of long-term vascular access patency in incident hemodialysis patients. J Korean Med Sci 2010; 5: 728–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baris A, Rengin E.. The primary arteriovenous fistula failure—a comparison between diabetic and non-diabetic patients: glycemic control matters. Int Urol Nephrol 2012; 44: 575–581 [DOI] [PubMed] [Google Scholar]
- 3. Benaragama KS, Barwell J, Lord C. et al. Post-operative arteriovenous fistula blood flow influences primary and secondary patency following access surgery. J Ren Care 2018; 9 [DOI] [PubMed] [Google Scholar]
- 4. Seong C, Yu-Ji L, Sung-Rok K.. Value of Doppler evaluation of physically abnormal fistula: hemodynamic guidelines and access outcomes. Korean J Intern Med 2017; 18: 137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robbin ML, Greene T, Allon M. et al. Prediction of arteriovenous fistula clinical maturation from postoperative ultrasound measurements: findings from the Hemodialysis Fistula Maturation Study. J Am Soc Nephrol 2018; 29: 2735–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pillado E, Behdad M, Williams R. et al. Flow rates at thirty days after construction of radiocephalic arteriovenous fistula predict hemodalysis function. Ann Vasc Surg 2018; 49: 268–272 [DOI] [PubMed] [Google Scholar]
- 7. Schwab SJ, Oliver M, McCann S.. Hemodialysis arteriovenous access: detection of stenosis and response to treatment by vascular access blood flow. Kidney Int 2001; 1: 358–362 [DOI] [PubMed] [Google Scholar]
- 8. Rotondi S, Tartaglione L, Muci ML. et al. Oxygen extraction ratio (OER) as a measurement of hemodialysis (HD) induced tissue hypoxia: a pilot study. Sci Rep 2018; 8: 5655. [DOI] [PMC free article] [PubMed] [Google Scholar]