Abstract
Background
Elderly patients with chronic kidney disease (CKD) are often excluded from clinical trials; this may affect their use of essential drugs for cardiovascular complications. We sought to assess the impact of age on cardiovascular drug use in elderly patients with CKD.
Methods
We used baseline data from the Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD-REIN) cohort including 3033 adult patients with CKD Stages 3 and 4. We studied the use of recommended drugs for coronary artery disease (CAD), stroke and atrial fibrillation by age, after adjusting for socio-demographic and clinical conditions.
Results
The patients’ mean age was 66.8 years (mean estimated glomerular filtration rate 32.9 mL/min/1.73 m2). The prevalence of CAD was 24.5% [81.3% receiving antiplatelet agents, 75.6% renin–angiotensin system (RAS) blockers, 65.4% β-blockers and 81.3% lipid-lowering therapy], that of stroke 10.0% (88.8% receiving antithrombotic drugs) and that of atrial fibrillation 11.1% (69.5% receiving oral anticoagulants). Compared with patients aged <65 years, older age (≥65 years) was associated with greater use of antithrombotic drugs in stroke [adjusted odds ratio (aOR) (95% confidence interval) = 2.83 (1.04–7.73) for patients aged (75–84 years)] and less use of RAS blockers [aOR = 0.39 (0.16–0.89) for patients aged ≥85 years], β-blockers [aOR = 0.31 (0.19–0.53) for patients aged 75–84 years] and lipid-lowering therapy [aOR = 0.39 (0.15–1.02) for patients aged ≥85 years, P for trend = 0.01] in CAD. Older age was not associated with less use of antiplatelet agents in CAD or oral anticoagulants in atrial fibrillation.
Conclusions
In patients with CKD, older age per se was not associated with the underuse of antithrombotic drugs but was for other major drugs, with a potential impact on cardiovascular outcomes.
Keywords: atrial fibrillation, chronic kidney disease, coronary artery disease, elderly, stroke, underuse
INTRODUCTION
Older adults account for a growing proportion of patients with chronic kidney disease (CKD) [1]. Although the relative risk of end-stage renal disease remains constant across age groups with similar glomerular filtration rates (GFRs), the absolute risk of death is higher than the absolute risk of reaching CKD Stage 5 for elderly patients with CKD [2]. Cardiovascular disease (CVD) is the leading cause of death in patients with CKD [3]. Although CVD and CKD share several risk factors (such as diabetes and hypertension), CKD itself is a major risk factor for CVD [3]. The incidences of various types of CVD are higher in patients with CKD than in populations without CKD; these diseases include both atheromatous CVD [such as coronary artery disease (CAD) and stroke] and non-atheromatous CVD (such as atrial fibrillation and heart failure), which often accumulate with age [3, 4].
The current guidelines for CVD management are based on high-level evidence [5–7]. Most of these guidelines state that non-dialysed patients with CKD should be treated with the same drug classes as patients without CKD. Even though elderly patients with CKD experience a high CVD burden, they are often excluded from clinical trials assessing the efficiency of drugs used to treat CVD. It has also been reported that patients with CKD do not receive recommended treatment for various CVDs, relative to patients with normal kidney function [8–10]. Moreover, age has been associated with the underuse of cardiovascular drugs in non-CKD populations [9, 11–14]. However, the impact of age on CVD drug use by patients with CKD has not been extensively investigated.
By examining data from a large cohort of patients with CKD, we sought to accurately determine the impact of age and GFR level on the patients’ use of drugs recommended for several CVDs. We hypothesized that elderly patients with CKD and CVD might be less frequently treated than younger patients with CKD and CVD.
MATERIALS AND METHODS
Population
The present analysis drew on baseline data collected from the Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD-REIN) cohort (ClinicalTrials.gov identifier: NCT03381950), a prospective cohort comprising 3033 CKD Stages 3–4 patients receiving nephrologist-led care in France. These patients were recruited from July 2013 to April 2016 by 40 nephrology clinics that were representative in terms of their geographical location in France and their legal status (i.e. public or private ownership) [15].
Study eligibility required two measures of estimated GFR (eGFR) between 15 and 60 mL/min/1.73 m2 at least 1 month apart, with no prior chronic dialysis or kidney transplantation. Patients aged <18 years or unable to give informed consent were excluded, as were those who planned to move or who refused to participate. The CKD-REIN patients will be followed up once a year for at least 5 years. This study is a cross-sectional analysis of the entire cohort population at inclusion. The study protocol was approved by the Institut National de la Santé Et de la Recherche Médicale (INSERM) institutional review board (reference: IRB00003888), and all patients gave their informed consent to participation.
Information
The CKD-REIN database includes information on medical variables (clinical variables, a set of routine laboratory values, the CKD’s history, comorbidities and medications taken) and socio-demographic variables. The information was collected from patient interviews and from medical records by trained clinical research associates. For the data on medications, patients were asked to bring their prescriptions for the previous 3 months to each follow-up visit. Clinical research associates recorded the prescription drugs.
Age was divided into four categories: <65, 65–74, 75–84 and ≥85 years. The eGFR values [calculated according to the Chronic Kidney Disease - Epidemiology Collaboration (CKD-EPI) creatinine equation] were divided into two categories: ≥30 and <30 mL/min/1.73 m2. The CKD-EPI creatinine value was corrected for ethnicity in patients originating from the French West Indies or sub-Saharan Africa. Albuminuria (or proteinuria) was collected either from a 24-h urine sample or a single urine sample and then expressed as a Kidney Disease: Improving Global Outcomes (KDIGO) grade (A1, normal; A2, moderately increased; A3, severely increased) [16]. Participants were classed as having diabetes if (i) diabetes was reported in their medical records, (ii) they were being treated with a glucose-lowering medication or (iii) the blood HbA1c level was ≥7%. Dyslipidaemia was defined as a history of dyslipidaemia or ongoing treatment with lipid-lowering medication. Data were available for five activities of daily living (ADL) from the Katz Index (bathing, dressing, going to the toilet, transference and feeding) and four instrumental ADL (IADL) from the IADL scale (ability to use the telephone, handle finances, take medication and travel). Personal autonomy was defined in two ways: lack of impairment in the five ADLs or lack of impairment in the four IADLs. The educational level was defined as the number of years in full-time education, in two categories: <12 versus ≥12 years. The CHA2DS2-VASc score was calculated according to the current guidelines [6].
CVDs studied and the corresponding recommended drugs
We studied the use of recommended drug classes whose several medications do not have contraindications for age and GFR: antiplatelet agents, renin–angiotensin system (RAS) blockers, β-blockers, and statins or ezetimibe in patients with CAD; oral anticoagulants in patients with atrial fibrillation and a CHA2DS2-VASc score ≥2 in men and ≥3 in women; antithrombotic drugs in patients with a history of stroke or transient ischaemic attack [5–7]. Appropriate drugs for CAD included antithrombotic drugs, RAS blockers, β-blockers, and statins or ezetimibe. The RAS blockers included angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Oral anticoagulants included vitamin K antagonists and direct oral anticoagulants. Antithrombotic drugs included antiplatelet agents and oral anticoagulants.
Statistical analysis
We first described the use of the drugs recommended for each CVD by age class. Next, we evaluated the number of appropriate drugs for CAD per patient, by age and by eGFR. The number of appropriate drugs in CAD was computed by ordinal logistic regression, and included an age × eGFR interaction term. Finally, we analysed the use of recommended drugs in a logistic regression for each drug separately in the three disease subgroups. For each drug, the multivariate analysis included age and eGFR. The other variables included in the multivariate models were those with a P < 0.1 in a univariate analysis. The following variables were tested for each drug: sex, albuminuria, diabetes, marital status, ADL, IADL, educational level, total number of drugs and a cardiology consultation in the previous year. The other tested variables were potential confounding factors for each drug: oral anticoagulant use, liver cirrhosis and a history of gastrointestinal bleeding for the analysis of antiplatelet agents; systolic blood pressure, a history of acute kidney injury (AKI) and heart failure for the analysis of RAS blockers; asthma or chronic obstructive pulmonary disease (COPD), systolic blood pressure, atrial fibrillation and heart failure for the analysis of β-blockers; body mass index and serum albumin for the analysis of statins or ezetimibe; liver cirrhosis, a history of gastrointestinal bleeding and a history of stroke or transient ischaemic attack for the analysis of oral anticoagulants in atrial fibrillation; liver cirrhosis, a history of gastrointestinal bleeding and a history of atrial fibrillation for the analysis of antithrombotic drugs in stroke or transient ischaemic attack. Since a small number of patients had experienced brain haemorrhages, this variable was not included in the analysis of antithrombotic drugs. The interaction between age and eGFR was tested for each drug.
Missing data were taken into account by performing multiple imputations. For each missing value, 20 imputations were computed, leading to 20 different databases. The results of the 20 databases were pooled according to Rubin’s rules [17].
All statistical analyses were performed with R software (R Foundation, Vienna, Austria, version 3.3.0; library mice). The threshold for statistical significance was set to P < 0.05.
RESULTS
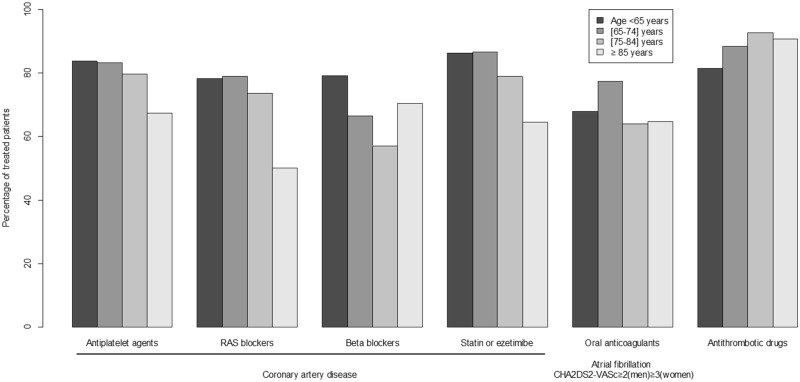
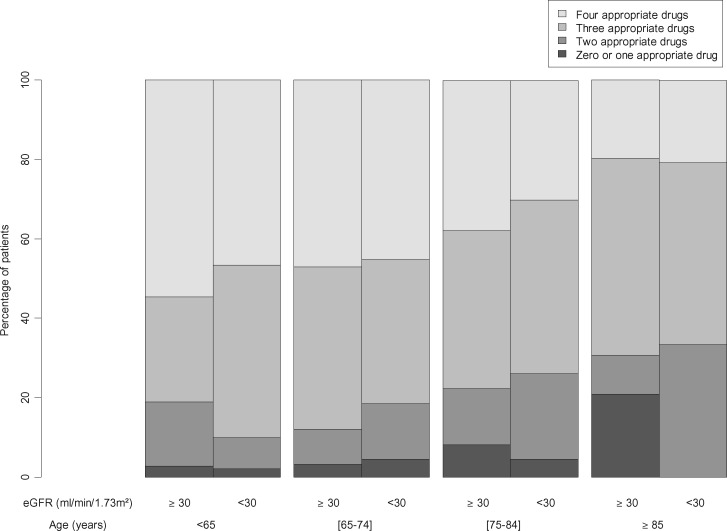
All the 3033 patients in the CKD-REIN cohort were included in the present analysis. The characteristics of the study population are summarized in Table 1. The mean age was 66.8 years, 65% of the patients were male and 41.3% had diabetes. A total of 25% of the patients had a history of CAD, 11.1% had a history of atrial fibrillation with a CHA2DS2-VASc score ≥2 (men) or ≥3 (women) (the prevalence regardless of the CHA2DS2-VASc score was 11.5%) and 10.0% had a history of stroke or transient ischaemic attack. Among the patients with CAD, 81.3% were being treated with antiplatelet agents, 75.6% with RAS blockers, 65.4% with β-blockers and 82.7% with statins or ezetimibe. Oral anticoagulants were used by 69.5% of the patients with atrial fibrillation and a CHA2DS2-VASc score ≥2 (men) or ≥3 (women); of these, 3.7% were being treated with a direct oral anticoagulant. Antithrombotic drugs were used by 88.8% of the patients with a history of stroke or transient ischaemic attack. The percentages of patients using drugs recommended in CVD are shown by age class in Figure 1. Results by sex are shown in Supplementary data. Older patients tended to use CAD drugs less than younger patients (univariate P for trend = 0.05 for antiplatelet agents, 0.01 for RAS blockers, <0.001 for β-blockers and 0.001 for statins or ezetimibe). The numbers of appropriate drugs for CAD per patient are shown in Figure 2. Older age (but not lower eGFR) was significantly associated with a lower total number of appropriate drugs for CAD. The interaction term between age and eGFR was not significant (P = 0.47). In contrast, there was no age trend for the use of oral anticoagulants in patients with atrial fibrillation (P for trend = 0.11). There was a trend towards the more frequent use of antithrombotic drugs in older patients with stroke or transient ischaemic attack (P for trend = 0.04). The numbers of appropriate drugs for CAD per patient by sex are shown in Supplementary data.
Table 1.
Characteristics of the study population by age category
| Variable | Age (years) |
CKD-REIN population | Imputed values (%) | |||||
|---|---|---|---|---|---|---|---|---|
| <65 | 65–74 | 75–84 | ≥85 | |||||
| Sample size (% of the study population) | 1059 (34.9) | 1057 (34.8) | 800 (26.4) | 117 (3.9) | 3033 (100.0) | |||
| Socio-demographic parameters and functional status | ||||||||
| Age (years)a | 52.8 ± 10.0 | 69.3 ± 2.8 | 79.0 ± 2.7 | 87.2 ± 2.1 | 66.8 ± 12.9 | 0.0 | ||
| Male sexb | 59.2 | 69.3 | 68.6 | 65.0 | 65.4 | 0.0 | ||
| At least one impairment in ADLb | 6.8 | 9.3 | 11.1 | 9.9 | 8.9 | 11.7 | ||
| At least one impairment in IADLb | 18.1 | 25.2 | 35.6 | 47.4 | 26.3 | 11.7 | ||
| Marital statusb | ||||||||
| Married or living together | 61.3 | 70.6 | 66.6 | 52.8 | 65.6 | 12.7 | ||
| Single | 21.2 | 6.7 | 4.7 | 2.3 | 11.1 | |||
| Widow(er) or divorced | 17.5 | 22.7 | 28.7 | 45.0 | 23.3 | |||
| Did not complete high schoolb | 55.5 | 65.0 | 70.5 | 75.2 | 63.5 | 1.1 | ||
| Comorbidities | ||||||||
| Diabetesb | 29.8 | 49.0 | 47.9 | 30.2 | 41.3 | 0.7 | ||
| Hypertensionb | 85.0 | 92.9 | 94.2 | 99.1 | 90.7 | 0.3 | ||
| Systolic blood pressure (mmHg)a | 137.0 ± 19.0 | 144.1 ± 20.5 | 146.3 ± 20.3 | 149.1 ± 22.4 | 142.4 ± 20.4 | 2.4 | ||
| Heart failureb | 6.6 | 12.4 | 14.5 | 23.1 | 13.1 | 0.3 | ||
| CADb | 12.7 | 28.0 | 34.9 | 29.1 | 24.5 | 2.1 | ||
| Atrial fibrillationb | 4.0 | 11.9 | 18.2 | 29.1 | 11.5 | 0.3 | ||
| Stroke or transient ischaemic attackb | 5.8 | 10.4 | 14.5 | 13.2 | 10.0 | 2.5 | ||
| Asthma/COPDb | 10.5 | 15.0 | 14.8 | 10.3 | 13.2 | 2.2 | ||
| Liver cirrhosisb | 2.3 | 1.9 | 1.0 | 0.9 | 1.8 | 5.7 | ||
| History of gastrointestinal bleedingb | 3.0 | 3.4 | 6.2 | 4.5 | 4.0 | 5.8 | ||
| Cardiology consultation during the previous yearb | 52.1 | 73.5 | 81.0 | 78.2 | 68.2 | 17.3 | ||
| Nutritional and nephrological parameters | ||||||||
| Dyslipidaemiab | 63.1 | 81.3 | 79.4 | 73.2 | 74.1 | 2.5 | ||
| LDL cholesterol (mmol/L)a | 2.9 ± 1.1 | 2.6 ± 1.0 | 2.5 ± 1.0 | 2.9 ± 1.0 | 2.7 ± 1.1 | 16.0 | ||
| HDL cholesterol (mmol/L)a | 1.4 ± 0.5 | 1.3 ± 0.5 | 1.3 ± 0.4 | 1.4 ± 0.4 | 1.3 ± 0.5 | 14.0 | ||
| Body mass index (kg/m²)a | 27.9 ± 6.2 | 29.8 ± 6.0 | 28.6 ± 5.1 | 27.3 ± 4.1 | 28.7 ± 5.9 | 2.1 | ||
| Serum albumin (g/L)a | 40.3 ± 4.8 | 40.1 ± 4.0 | 39.9 ± 3.9 | 39.9 ± 4.2 | 40.1 ± 4.3 | 18.9 | ||
| eGFR (mL/min/1.73 m²)a | 34.5 ± 13.1 | 33.4 ± 12.0 | 30.9 ± 10.9 | 27.7 ± 9.9 | 32.9 ± 12.2 | 0.0 | ||
| CKD stage | ||||||||
| 3 | 58.4 | 57.4 | 49.8 | 40.2 | 55.1 | 0.0 | ||
| 4 | 41.6 | 42.6 | 50.2 | 59.8 | 44.9 | |||
| Albuminuriab | ||||||||
| A1 | 22.1 | 29.2 | 33.3 | 28.6 | 27.8 | 11.2 | ||
| A2 | 28.2 | 31.7 | 34.9 | 38.3 | 31.6 | |||
| A3 | 49.7 | 39.1 | 31.8 | 33.1 | 40.6 | |||
| History of AKIb | 23.8 | 23.4 | 23.8 | 21.4 | 23.6 | 8.0 | ||
| Drugs | ||||||||
| Antiplatelet agentsb | 23.9 | 47.5 | 53.3 | 47.9 | 40.8 | 0.0 | ||
| Oral anticoagulantsb | 7.1 | 14.8 | 21.8 | 29.1 | 14.5 | 0.0 | ||
| Antithrombotic drugs | 29.7 | 58.5 | 69.2 | 76.1 | 52.0 | |||
| RAS blockersb | 76.8 | 77.3 | 71.6 | 54.7 | 74.7 | 0.0 | ||
| β-blockersb | 35.8 | 44.6 | 44.9 | 42.7 | 41.4 | 0.0 | ||
| Statins or ezetimibeb | 49.2 | 68.1 | 63.3 | 53.8 | 59.7 | 0.0 | ||
| Total number of drugsa | 6.8 ± 4.0 | 8.6 ± 4.0 | 8.8 ± 3.5 | 8.8 ± 3.7 | 8.0 ± 3.9 | 0.0 | ||
Expressed as the mean ± standard deviation.
Expressed as the proportion of the age category.
ADL, activities of daily living; IADL, instrumental activities of daily living; eGFR, estimated glomerular filtration rate, according to the CKD-EPI equation; albuminuria or proteinuria was expressed as the KDIGO grade (A1: normal; A2: moderately increased; A3: severely increased); COPD, chronic obstructive pulmonary disease; antithrombotic drugs included antiplatelet agents and oral anticoagulants; RAS, reninangiotensin system; LDL, low-density lipoprotein; HDL, high-density lipoprotein; AKI, acute kidney injury; CKD, chronic kidney disease.
FIGURE 1.
Percentage of CVD drug use by age class in patients with CKD. RAS: reninangiotensin system; the antithrombotic drugs included antiplatelet agents and oral anticoagulants.
FIGURE 2.
Percentages of patients according to the number of recommended drugs used to treat CAD: comparison by age class and estimated glomerular filtration rate (eGFR) class. The appropriate drugs for CAD included antithrombotic drugs (antiplatelet agents or oral anticoagulants), renin–angiotensin blockers, β-blockers, and statins or ezetimibe.
Antithrombotic drug use
In a multivariate analysis, age was not associated with the use of antiplatelet agents in CAD after adjustment for oral anticoagulant use (Table 2). A higher number of prescribed drugs were associated with a greater probability of treatment with antiplatelet agents in CAD. The latter association was found to be statistically significant for all drugs analysed in this study (Tables 2 and 3). Similarly, age was not associated with the use of oral anticoagulants in patients with atrial fibrillation but was associated with elevated use of antithrombotic agents in patients with CKD and a history of stroke or transient ischaemic attack (P-value for trend = 0.05). A low eGFR was not significantly associated with the use of antithrombotic drugs studied here. A significant, positive interaction between age and eGFR was found only for the use of antithrombotic agents in patients with a history of stroke or transient ischaemic attack.
Table 2.
Percentages and adjusted odds ratios (95% CI) for antithrombotic drug use in patients with CKD and CAD, atrial fibrillation or a history of stroke or transient ischaemic attack
| Antiplatelet agent use in patients with CAD | Oral anticoagulant use in patients with atrial fibrillation with CHA2DS2-VASc ≥2 score (men) or ≥3 (women) | Antithrombotic drug use in patients with stroke or transient ischaemic attack | ||||
|---|---|---|---|---|---|---|
| Variable |
n/N = 604/744 (81.3%) |
n/N = 233/336 (69.5%) |
n/N = 269/297 (88.8%) |
|||
| % use | aOR (95% CI) | % use | aOR (95% CI) | % use | aOR (95% CI) | |
| Age (years) | ||||||
| <65 (ref.) | 83.7 | 1 | 67.9 | 1 | 81.4 | 1 |
| 65–74 | 83.3 | 1.46 (0.77–2.76) | 77.4 | 1.73 (0.69–4.32) | 88.5 | 1.92 (0.76–4.86) |
| 75–84 | 79.7 | 1.45 (0.76–2.76) | 64.0 | 0.94 (0.39–2.26) | 92.8* | 2.83 (1.04–7.73)** |
| ≥85 | 67.4* | 0.76 (0.26–2.19) | 64.7 | 1.05 (0.35–3.10) | 90.9 | 3.09 (0.38–25.12) |
| P-value for trend | 0.81 | 0.22 | 0.05 | |||
| eGFR ≥30 mL/min/1.73 m² (ref.) | 82.5 | 1 | 72.0 | 1 | 89.8 | 1 |
| eGFR <30 mL/min/1.73 m² | 80.0 | 0.73 (0.47–1.14) | 65.8 | 0.72 (0.44–1.18) | 87.9 | 0.52 (0.23–1.16) |
| No oral anticoagulant (ref.) | 90.5 | 1 | ||||
| Oral anticoagulant use | 48.6* | 0.06 (0.04–0.10)** | ||||
| Total number of drugs (per 1 drug increment) | 1.23 (1.14–1.32)** | 1.09 (1.02–1.17)** | 1.30 (1.13–1.50)** | |||
P < 0.05 versus the reference (ref.) in a univariate analysis.
P<0.05 versus the reference (ref.) in multivariate analysis.
The odds ratios were calculated using multivariable logistic regression; the antithrombotic drugs include antiplatelet agents and oral anticoagulants; eGFR, estimated glomerular filtration rate, according to the CKD-EPI equation; the variables included were age, eGFR and variables with a P < 0.1 in the univariate analysis.
aOR, adjusted odds ratio.
Table 3.
Percentages and adjusted odds ratios (95% CI) for drug use in patients with CKD and CAD
| RAS blocker use | β-blocker use | Statin or ezetimibe use | ||||
|---|---|---|---|---|---|---|
| Variable |
n/N = 562/744 (75.6%) |
n/N = 486/744 (65.4%) |
n/N = 615/744 (82.7%) |
|||
| % use | aOR (95% CI) | % use | aOR (95% CI) | % use | aOR (95% CI) | |
| Age (years) | ||||||
| <65 (ref.) | 78.3 | 1 | 79.2 | 1 | 86.2 | 1 |
| 65–74 | 79.1 | 1.07 (0.63–1.80) | 66.5* | 0.47 (0.28–0.78)*** | 86.7 | 0.96 (0.51–1.82) |
| 75–84 | 73.7 | 0.92 (0.54–1.56) | 57.0* | 0.31 (0.19–0.53)*** | 78.9** | 0.59 (0.32–1.10) |
| ≥85 | 50.0* | 0.38 (0.16–0.89)*** | 70.4 | 0.62 (0.25–1.54) | 64.5* | 0.39 (0.15–1.02) |
| P-value for trend | 0.11 | <0.001 | 0.01 | |||
| Female sex (ref.) | 69.4 | 1 | 75.7 | 1 | ||
| Male sex | 77.2* | 1.41 (0.91–2.19) | 84.5* | 2.25 (1.36–3.72)*** | ||
| No diabetes (ref.) | 71.6 | 1 | ||||
| Diabetes | 78.3* | 1.30 (0.88–1.92) | ||||
| No heart failure (ref.) | 79.4 | 1 | 63.2 | 1 | ||
| Heart failure | 65.0* | 0.48 (0.32–0.70)*** | 74.6* | 1.47 (1.01–2.14)*** | ||
| No history of AKI | 79.9 | 1 | ||||
| History of AKI | 64.2* | 0.44 (0.30–0.66)*** | ||||
| No asthma or COPD (ref.) | 67.8 | 1 | ||||
| Asthma/COPD | 53.1* | 0.44 (0.29–0.68)*** | ||||
| Body mass index (per 1 kg/m² increment) | 1.02 (0.98–1.07) | |||||
| eGFR ≥30 mL/min/1.73 m² (ref.) | 80.7 | 1 | 64.9 | 1 | 82.9 | 1 |
| eGFR <30 mL/min/1.73 m² | 70.5* | 0.60 (0.42–0.88)*** | 66.6 | 1.08 (0.80–1.46) | 82.4 | 0.91 (0.60–1.39) |
| Total number of drugs (per 1 drug increase) | 1.13 (1.06–1.19)*** | 1.09 (1.04–1.14)*** | 1.23 (1.15–1.32)*** | |||
| Autonomy for ADL (ref.) | 76.8 | 1 | ||||
| At least one impairment | 66.7* | 0.57 (0.32–1.02) | ||||
| Autonomy for IADL (ref.) | 79.3 | 1 | ||||
| At least one impairment | 69.2* | 0.68 (0.45–1.04) | ||||
| Married or concubine (ref.) | 67.6 | 1 | 85.4 | 1 | ||
| Single | 64.9 | 0.62 (0.31–1.22) | 85.6 | 0.87 (0.34–2.19) | ||
| Widow(er) or divorced | 59.9** | 0.66 (0.44–0.98)*** | 74.2* | 0.58 (0.36–0.93)*** | ||
| Completed high school (ref.) | 80.1 | 1 | ||||
| Did not complete high school | 73.6** | 0.75 (0.49–1.14) | ||||
P < 0.05 versus the reference (ref.) in a univariate analysis.
P < 0.10 the ref. in a univariate analysis.
P < 0.05 versus the reference (ref.) in multivariate analysis.
The odds ratios were calculated using multivariable logistic regression; eGFR, estimated glomerular filtration rate, according to the CKD-EPI equation; albuminuria or proteinuria was expressed as the KDIGO grade (A1: normal; A2: moderately increased; A3: severely increased); AKI, acute kidney injury; COPD, chronic obstructive pulmonary disease; RAS, reninangiotensin system; ADL, activities of daily living; IADL, instrumental activities of daily living; the variables included were age, eGFR and variables with P < 0.1 in the univariate analysis.
aOR, adjusted odds ratio.
Use of other specific drugs for CAD
In the multivariate analysis, patients with CKD and CAD aged >85 years were significantly less likely to be treated with RAS blockers than patients aged <65 years (Table 3). Patients aged between 65 and 85 years were less likely to be treated with β-blockers than patients aged <65 years. The P-values for trend according to age were statistically significant for β-blockers and for statins or ezetimibe, indicating lower rates of use of these drugs in older patients with CKD and CAD.
Surprisingly, RAS blockers were significantly less used in patients with heart failure and an eGFR of <30 mL/min/1.73 m2. A history of AKI was also associated with underuse of this drug class. β-blockers were less used in patients with asthma or COPD but more used in patients with heart failure. Statins or ezetimibe were used more frequently used in men (Table 3). The interactions between age and eGFR were not significant for these three drug classes.
DISCUSSION
We found that the recommended drugs for CVD were being used in a large majority of patients with CKD receiving nephrology care. In the CKD-REIN cohort, age per se was not associated with the use of antiplatelet agents in CAD or the use of oral anticoagulants in atrial fibrillation. Interestingly, we observed the greater use of antithrombotic drugs in older patients with a history of stroke or transient ischaemic attack. In contrast, RAS blockers, β-blockers and lipid-lowering therapies were used less in older patients with CAD than in younger ones, despite a higher overall number of prescribed drugs in the elderly patients. Furthermore, a low eGFR (<30 mL/min/1.73 m2) was not associated with underuse of any studied drug classes, with the exception of RAS blockers in CAD.
In this study, the percentages of patients using a particular drug were in line with previous reports on patients with chronic CAD; these literature values range from 75% to 88% for antiplatelet agents, 52% to 86% for RAS blockers, 57% to 75% for β-blockers and 38% to 96% for lipid-lowering therapies [18–23]. Recent studies have reported that between 56% and 75% of patient with atrial fibrillation use oral anticoagulants, which is in line with the findings of our study [13, 14]. In the literature, the percentage of patients taking an antithrombotic drug on discharge after hospitalization for stroke is higher than that recorded in this study, although data on the chronic use of antithrombotic drugs in patients with a history of stroke are scarce [12, 24].
CAD is frequent in patients with CKD [3]. The CAD drugs studied here have been shown to be effective in non-dialysed patients with CKD (although the evidence for β-blockers is unclear) [25] and are therefore recommended in the current guidelines [5, 7]. However, previous studies have observed lower use of the recommended CAD drugs in patients with lower eGFRs [8, 9]. In fact, higher CKD stages have been associated with the less frequent initiation of these drugs and worse long-term adherence [8, 9, 19]. We did not observe this relationship in this study, although the range of eGFRs was narrower than in most of the literature publications. However, we did observe underuse of RAS blockers in patients with lower eGFRs and in patients with heart failure. The underuse of antiplatelet agents in patients treated with oral anticoagulants for another indication was observed elsewhere [22].
Older age has already been found to be associated with underuse of recommended drugs in non-CKD patients with CAD [9, 11]. There are also data on these recommended drugs in elderly patients with CAD [9, 21, 26, 27]. The current guidelines for CAD strongly recommend secondary prevention with these drugs in elderly patients [5, 7]. Our study distinguishes the different drug classes and found different use patterns according to age. We did not find any association between age and use of antiplatelet agents, but RAS blockers, β-blockers and lipid-lowering agents were all underused in the oldest patients studied here. As is the case for low GFR, the literature data show that older age is associated with less frequent initiation of these drugs after a coronary event and with worse long-term adherence [9, 11, 19].
Atrial fibrillation and CKD are strongly associated [3]. Whereas patients with CKD have a higher risk of thromboembolism than non-CKD patients, the risk of anticoagulant-related bleeding is also higher. This has prompted debate on the benefit–risk ratio of these drugs in patients with CKD, although most of the data come from observational studies [10, 28, 29]. Although the association between low GFR and underuse of oral anticoagulants in atrial fibrillation has not been thoroughly assessed, our present data did not highlight such a relationship. Age has previously been found to be associated with the underuse of oral anticoagulants, despite the fact that the latter drugs are clearly effective in the elderly [13, 14, 30]. An earlier study found an association between loss of autonomy and underuse of oral anticoagulants [13]. In contrast to these literature studies, we did not observe underuse of oral anticoagulants in elderly patients with CKD. This disparity might be due to differences in medical practice between CKD and non-CKD patients, or between France and other countries.
We found that antithrombotic drugs were more frequently used in older patients with a history of stroke or transient ischaemic attack. Previous studies have found conflicting results for the association between age and the use of oral anticoagulants on discharge after hospitalization for a stroke due to atrial fibrillation [12, 24]. However, in a study of the use of antithrombotic drugs in patients with recurrent stroke, older age was not associated with drug underuse [23]. Moreover, a study of drug discontinuation 1 year after a stroke found that age was associated with greater use of drugs for secondary prevention of stroke [31]. Another study found that older age was associated with better long-term adherence to antiplatelet agents and worse adherence to oral anticoagulants after stroke [32].
Our analysis had several strengths. First, we used data from a large cohort of patients with CKD: a high proportion of the elderly patients had a history of CVD. Secondly, the database included a large number of variables, which made it possible to take account of potential confounding factors (e.g. personal autonomy and educational). However, our study also had some limitations. First, the cross-sectional design prevented us from establishing whether the studied drugs were never taken at all or were discontinued due to side effects, since elderly patients are particularly exposed to iatrogenic risks. Secondly, the ethical requirement for informed consent might mean that patients with dementia at baseline were not included; this might have led to selection bias. Lastly, our patients were recruited by nephrology departments and so might not reflect exactly prescription patterns in patients with CKD as a whole.
In a French cohort of patients with CKD receiving nephrology care, older age was not associated with the underuse of antithrombotic drugs for CAD, atrial fibrillation and stroke. However, in contrast to the current guidelines, elderly patients with CKD and CAD were less likely to be treated with RAS blockers, β-blockers and lipid-lowering agents than younger patients were. Robust evidence on the effectiveness of CVD drugs is available for elderly patients and for patients with CKD but not for elderly patients with CKD. The latter population has a high cardiovascular mortality rate; further randomized controlled trials and longitudinal studies are therefore needed to assess the benefits and risks of CVD drugs and the reasons for their underuse.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank the CKD-REIN study coordination staff for their efforts in setting up the CKD-REIN cohort: M.M., Elodie Speyer, Céline Lange, Sophie Renault, Reine Ketchemin and all the clinical research associates.
The CKD-REIN Study Group. Steering committee and coordinators include: Carole Ayav, Vanessa Besson-Dubourg, S.B., Dorothée Cannet, C.C., D.F., L.F., Yves-Edouard Herpe, C.J., M.L., Z.A.M., Christophe Pascal, Bruce M. Robinson, B.S., Céline Lange, Karine Legrand, S.L., M.M. and Elodie Speyer.
CKD-REIN investigators/collaborators include: Thierry Hannedouche, Bruno Moulin, Sébastien Mailliez, Gaétan Lebrun, Eric Magnant, Gabriel Choukroun, Benjamin Deroure, Adeline Lacraz, Guy Lambrey, Jean Philippe Bourdenx, Marie Essig, Thierry Lobbedez, Raymond Azar, Hacène Sekhri, Mustafa Smati, Mohamed Jamali, Alexandre Klein, Michel Delahousse, C.C., Séverine Martin, Isabelle Landru, Eric Thervet, Z.A.M., Philippe Lang, Xavier Belenfant, Pablo Urena, Carlos Vela, L.F., Dominique Chauveau, Viktor Panescu, Christian Noel, François Glowacki, Maxime Hoffmann, Maryvonne Hourmant, Dominique Besnier, Angelo Testa, François Kuentz, Philippe Zaoui, Charles Chazot, Laurent Juillard, Stéphane Burtey, Adrien Keller, Nassim Kamar, D.F. and M.L.
FUNDING
CKD-REIN is supported by the Agence Nationale de la Recherche through the 2010 Cohortes-Investissements d’Avenir programme and the 2010 national Programme Hospitalier de Recherche Clinique programme. CKD-REIN is also supported by a public–private partnership with Amgen, Fresenius Medical Care and GlaxoSmithKline (GSK) since 2012, Lilly France since 2013, Otsuka Pharmaceutical since 2015, Baxter and Merck Sharp & Dohme-Chibret (MSD France) from 2012 to 2017, Sanofi-Genzyme from 2012 to 2015 and Vifor Fresenius since 2018. Inserm Transfert set up the collaboration in 2011 and has managed it since then.
AUTHORS’ CONTRIBUTIONS
C.V., S.L., M.M., N.M., B.S. and Z.A.M. contributed to the study concept and design. S.L., M.M., C.C., D.F., L.F., C.J., M.L., S.B., R.L.P., B.S. and Z.A.M. performed the acquisition of data. C.V., S.L., M.M., B.S. and Z.A.M. performed the analysis and interpretation of data. C.V., B.S. and Z.A.M. contributed to drafting the article. S.L., M.M., C.C., D.F., L.F., C.J., M.L., S.B., R.L.P. and N.M. contributed to the critical revision of the manuscript for important intellectual content. All authors approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
C.V., S.L., M.M., C.C., D.F., L.F., C.J., M.L., S.B., R.L.P. and N.M. have no disclosures relative to this study. B.S. declares being member of a scientific advisory board on a project funded by MSD. Z.A.M. has received grants for CKD-REIN and other research projects from Amgen, Baxter, Fresenius Medical Care, GlaxoSmithKline, Merck Sharp and Dohme-Chibret, Sanofi-Genzyme, Lilly, Otsuka and the French government, as well as fees and grants to charities from Amgen, Bayer and Sanofi-Genzyme.
REFERENCES
- 1. Bowling CB, Sharma P, Fox CS. et al. Change in prevalence of reduced estimated glomerular filtration rate among the oldest-old US adults from 1988–1994 through 2005–2010. JAMA 2013; 310: 1284–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hallan SI, Matsushita K, Sang Y. et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA 2012; 308: 2349–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gansevoort RT, Correa-Rotter R, Hemmelgarn BR. et al. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet 2013; 382: 339–352 [DOI] [PubMed] [Google Scholar]
- 4. Villain C, Metzger M, Combe C. et al . Prevalence of atheromatous and non-atheromatous cardiovascular disease by age in chronic kidney disease. Nephrol Dial Transplant 2018; doi: 10.1093/ndt/gfy277 [DOI] [PubMed] [Google Scholar]
- 5. Amsterdam EA, Wenger NK, Brindis RG. et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014; 64: e139–e228 [DOI] [PubMed] [Google Scholar]
- 6. Kirchhof P, Benussi S, Kotecha D. et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 2016; 37: 2893–2962 [DOI] [PubMed] [Google Scholar]
- 7. O’Gara PT, Kushner FG, Ascheim DD. et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127: e362–e425 [DOI] [PubMed] [Google Scholar]
- 8. Fox CS, Muntner P, Chen AY. et al. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation 2010; 121: 357–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Natsuaki M, Morimoto T, Furukawa Y. et al. Effect of statin therapy on cardiovascular outcomes after coronary revascularization in patients ≥ 80 years of age: observations from the CREDO-Kyoto Registry Cohort-2. Atherosclerosis 2014; 237: 821–828 [DOI] [PubMed] [Google Scholar]
- 10. Olesen JB, Lip GYH, Kamper A-L. et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med 2012; 367: 625–635 [DOI] [PubMed] [Google Scholar]
- 11. Ali RC, Melloni C, Ou F-S. et al. Age and persistent use of cardiovascular medication after acute coronary syndrome: results from medication applied and sustained over time. J Am Geriat Soc 2009; 57: 1990–1996 [DOI] [PubMed] [Google Scholar]
- 12. Asberg S, Henriksson KM, Farahmand B. et al. Ischemic stroke and secondary prevention in clinical practice: a cohort study of 14,529 patients in the Swedish Stroke Register. Stroke 2010; 41: 1338–1342 [DOI] [PubMed] [Google Scholar]
- 13. Biteker M, Başaran Ö, Doğan V. et al. Real-world clinical characteristics and treatment patterns of individuals aged 80 and older with nonvalvular atrial fibrillation: results from the ReAl-life Multicenter Survey Evaluating Stroke Study. J Am Geriat Soc 2017; 65: 1684–1690 [DOI] [PubMed] [Google Scholar]
- 14. Mazzone A, Bo M, Lucenti A. et al. The role of comprehensive geriatric assessment and functional status in evaluating the patterns of antithrombotic use among older people with atrial fibrillation. Arch Gerontol Geriat 2016; 65: 248–254 [DOI] [PubMed] [Google Scholar]
- 15. Stengel B, Combe C, Jacquelinet C. et al. The French Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD-REIN) cohort study. Nephrol Dial Transplant 2014; 29: 1500–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Levin A, Stevens PE, Bilous RW. et al. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [Google Scholar]
- 17. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons, 2004 [Google Scholar]
- 18. Brown TM, Voeks JH, Bittner V. et al. Achievement of optimal medical therapy goals for U.S. adults with coronary artery disease: results from the REGARDS Study (REasons for Geographic And Racial Differences in Stroke). J Am Coll Cardiol 2014; 63: 1626–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang TI, Desai M, Solomon DH. et al. Kidney function and long-term medication adherence after myocardial infarction in the elderly. Clin J Am Soc Nephrol 2011; 6: 864–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gandhi S, Dorian P, Greenlaw N. et al. Characteristics and evidence-based management of stable coronary artery disease patients in Canada compared with the rest of the world: insights from the CLARIFY registry. Can J Cardiol 2014; 30: 132–137 [DOI] [PubMed] [Google Scholar]
- 21. Pilotto A, Gallina P, Panza F. et al. Relation of statin use and mortality in community-dwelling frail older patients with coronary artery disease. Am J Cardiol 2016; 118: 1624–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Curl K, LeBude B, Ruggiero N. et al. Frequency of use of statins and aspirin in patients with previous coronary artery bypass grafting. Am J Cardiol 2016; 118: 40–43 [DOI] [PubMed] [Google Scholar]
- 23. Béjot Y, Zeller M, Lorgis L. et al. Secondary prevention in patients with vascular disease. A population based study on the underuse of recommended medications. J Neurol Neurosurg Psychiatry 2013; 84: 348–353 [DOI] [PubMed] [Google Scholar]
- 24. Saposnik G, Black SE, Hakim A. et al. Age disparities in stroke quality of care and delivery of health services. Stroke 2009; 40: 3328–3335 [DOI] [PubMed] [Google Scholar]
- 25. Agrawal H, Aggarwal K, Littrell R. et al. Pharmacological and non pharmacological strategies in the management of coronary artery disease and chronic kidney disease. Curr Cardiol Rev 2015; 11: 261–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Afilalo J, Duque G, Steele R. et al. Statins for secondary prevention in elderly patients: a hierarchical Bayesian meta-analysis. J Am Coll Cardiol 2008; 51: 37–45 [DOI] [PubMed] [Google Scholar]
- 27. Shepherd J, Blauw GJ, Murphy MB. et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360: 1623–1630 [DOI] [PubMed] [Google Scholar]
- 28. Bonde AN, Lip GYH, Kamper A-L. et al. Net clinical benefit of antithrombotic therapy in patients with atrial fibrillation and chronic kidney disease: a nationwide observational cohort study . J Am Coll Cardiol 2014; 64: 2471–2482 [DOI] [PubMed] [Google Scholar]
- 29. Keskar V, McArthur E, Wald R. et al. The association of anticoagulation, ischemic stroke, and hemorrhage in elderly adults with chronic kidney disease and atrial fibrillation. Kidney Int 2017; 91: 928–936 [DOI] [PubMed] [Google Scholar]
- 30. Desai Y, El-Chami MF, Leon AR. et al. Management of atrial fibrillation in elderly adults. J Am Geriat Soc 2017; 65: 185–193 [DOI] [PubMed] [Google Scholar]
- 31. Bushnell CD, Olson DM, Zhao X. et al. Secondary preventive medication persistence and adherence 1 year after stroke. Neurology 2011; 77: 1182–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Glader E-L, Sjölander M, Eriksson M. et al. Persistent use of secondary preventive drugs declines rapidly during the first 2 years after stroke. Stroke 2010; 41: 397–401 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.