Abstract
Background
A novel data-driven cluster analysis identified distinct pathogenic patterns in C3-glomerulopathies and immune complex-mediated membranoproliferative glomerulonephritis. Our aim was to replicate these observations in an independent cohort and elucidate disease pathophysiology with detailed analysis of functional complement markers.
Methods
A total of 92 patients with clinical, histological, complement and genetic data were involved in the study, and hierarchical cluster analysis was done by Ward method, where four clusters were generated.
Results
High levels of sC5b-9 (soluble membrane attack complex), low serum C3 levels and young age at onset (13 years) were characteristic for Cluster 1 with a high prevalence of likely pathogenic variations (LPVs) and C3 nephritic factor, whereas for Cluster 2—which is not reliable because of the small number of cases—strong immunoglobulin G staining, low C3 levels and high prevalence of nephritic syndrome at disease onset were observed. Low plasma sC5b-9 levels, decreased C3 levels and high prevalence of LPV and sclerotic glomeruli were present in Cluster 3, and patients with late onset of the disease (median: 39.5 years) and near-normal C3 levels in Cluster 4. A significant difference was observed in the incidence of end-stage renal disease during follow-up between the different clusters. Patients in Clusters 3–4 had worse renal survival than patients in Clusters 1–2.
Conclusions
Our results confirm the main findings of the original cluster analysis and indicate that the observed, distinct pathogenic patterns are replicated in our cohort. Further investigations are necessary to analyse the distinct biological and pathogenic processes in these patient groups.
Keywords: C3-glomerulopathy; C3-glomerulonephritis; complement; dense deposit disease, membranoproliferative glomerulonephritis
INTRODUCTION
Membranoproliferative glomerulonephritis (MPGN), characterized by mesangial hypercellularity, endocapillary proliferation and capillary wall thickening with double contour formation, is a pathological pattern detected by light microscopy on kidney biopsies [1]. Recent improvements in our understanding of the disease pathogenesis, with the recognition of the pivotal role of complement alternative pathway (AP) [2], led to the description of complement-mediated (C3-glomerulopathy, C3G) and immune complex-mediated MPGN (IC-MPGN) forms [3]. C3G presents with microscopic haematuria, proteinuria and renal failure, with dominant (minimum two-order magnitude stronger C3 staining than any other immunoreactant) C3 stain on immunofluorescence microscopy, and is caused by the dysregulation of the AP due to the presence of variations of complement genes [Complement Factor H (CFH), Complement Factor I (CFI), Membrane cofactor protein (CD46), Complement Factor B (CFB), Thrombomodulin (THBD)] [1, 4–6], or by autoantibodies [C3 nephritic factor (C3NeF), anti-Factor H, anti-C3, anti-Factor B and C4 nephritic factor (C4NeF)] against complement proteins [7–11]. Despite thorough analysis of potential risk factors, complement-related predisposing factors in several patients with C3G remain unidentified. C3G is divided according to electron microscopy, where C3-glomerulonephritis (C3GNs) usually has less dense mesangial, subendothelial and subepithelial deposits, whereas dense deposit disease (DDD) is characterized by electron-dense intramembranous deposits.
Despite improved understanding of disease pathogenesis, several questions still remain unanswered, including deviation between pathological and clinical presentations, hampered differentiation between C3GN and DDD (sometimes with IC-MPGN) or even changes in the characteristic patterns if repeated biopsies are done [3, 12, 13]. Even more importantly, the assumption that AP dysregulation is the most characteristic differentiating feature between immunoglobulin (Ig)-negative and Ig-positive, MPGN does not seem to be powerful enough since a large portion of patients with Ig-positive MPGN may be positive for C3NeF, or show decreased C3 levels with normal C4 [1, 14]. Taking all these limitations and considerations into account, Iatropoulos et al. [15] recently explored the potential utility of unsupervised cluster analysis to generate clinically meaningful subgroups of C3G/IC-MPGN patients. In their study, four distinct clusters were generated based on the clinical, histological, complement and genetic parameters. Patients in Clusters 1 and 2 had elevated terminal pathway activation marker (sC5b-9) levels with low C3 concentrations, whereas patients with dense deposits, decreased C3 but only moderately elevated sC5b-9 fell into Cluster 3. Patients in Clusters 1–3 had a similarly high prevalence of C3NeF and/or presence of likely pathogenic variation (LPVs) in complement genes, which was in contrast to Cluster 4, where C3 activation in glomeruli (not in circulation), presence of sclerotic glomeruli and absence of LPVs or C3NeF were characteristic. Interestingly, each cluster included patients with IC-MPGN, DDD or C3GN, strengthening the overlap between these entities. Notably, reclassification of C3GN, DDD and IC-MPGN patients by cluster analysis proved to be better at predicting renal survival, a result pointing to the feasibility of the ultimate goal of efficient patient stratification for complement-modulating therapies. However, as such unsupervised mathematical approaches are completely dependent on the studied cohort and on the variable set, validation in independent cohorts is essential.
Therefore, our aim was to validate the observations of Iatropoulos et al. [15] in an independent cohort. We enrolled 92 patients with pathologically diagnosed IC-MPGN/C3G, repeated the cluster analysis by analysing the same variable set (Supplementary data, Table S1), with the generation of four clusters, and observed very similar patterns to that of the original study. In addition, we tested further complement parameters to better characterize ongoing pathophysiology in the different clusters.
MATERIALS AND METHODS
A total of 92 patients with histologically proven C3G or IC-MPGN were enrolled in the study from 34 centres in Central and Eastern Europe (Supplementary data, Figure S1). Clinical and histological data were collected from clinicians, and genetic analysis (CFH, CFI, CD46, C3, CFB and THBD) was performed by Sanger sequencing (Supplementary data, Table S2). Serum C3, C4 concentration was determined by turbidimetry. Classical pathway activity was measured by haemolytic test [16]. Other complement parameters [AP activity, sC5b-9, anti-Factor H, anti-Factor B, anti-C3, anti-C1q, C4d, Bb, Factor D (FD)] were measured using home-made or commercially available ELISA kits. C3NeF activity was detected using haemolytic test [17].
Detailed methods are presented in Supplementary materials.
RESULTS
Generation of clinical patterns by cluster analysis
Clinical characteristics (Tables 1 and 2) of the patients and comparison of the two cohorts (Supplementary data, Figure S2) are summarized in Supplementary results. Similar to the study of Iatropoulos et al. [15], four distinct clusters were generated (Supplementary data, Figure S3). Forty-five patients were regrouped into Cluster 1, 4 patients into Cluster 2, 17 subjects into Cluster 3 and 26 patients into Cluster 4 (Tables 3 and 4). In our analysis Cluster 3, including mainly young adults with lower sC5b-9 levels and higher prevalence of sclerotic glomeruli, was completely separated from the remaining clusters. Thereafter, Cluster 4 was separated from Clusters 1 and 2, which includes adult patients with later disease onset and higher C3 levels (Supplementary data, Figure S3).
Table 1.
Clinical characteristics of patients with histologically proven diagnosis of C3G or IC-MPGN
| Variables | C3GN, n = 37 | DDD, n = 11 | IC-MPGN, n = 44 | All, n = 92 | P-valueb |
|---|---|---|---|---|---|
| Sex, % men | 17 (46) | 3 (27.3) | 26 (59.1) | 46 (50) | 0.14 |
| Age at diagnosis, years | 23.5 (13–39) | 17 (13.5–27.5) | 15 (9–33) | 16 (10–33) | 0.1 |
| Microhaematuria, present | 24 (64.8) | 8 (72.7) | 24 (54.5) | 56 (60.9) | 0.44 |
| Gross haematuria, present | 7 (19) | 2 (18.2) | 11 (25) | 20 (21.7) | 0.76 |
| Non-nephrotic proteinuria, present | 18 (48.6) | 4 (36.4) | 22 (50) | 44 (48.5) | 0.71 |
| Nephrotic syndrome, present | 15 (40.5) | 7 (63.6) | 21 (47.7) | 43 (47.3) | 0.39 |
| Renal impairment, present | 13 (35.1) | 4 (36.4) | 15 (34.1) | 32 (35.2) | 0.98 |
| Renal failure, present | 4 (10.8) | 1 (9) | 6 (13.6) | 11 (12.1) | 0.88 |
| Creatinine, μmol/L | 86 (53–240) | 79 (52–121) | 82 (55–168) | 83 (54–196) | 0.89 |
| Trigger, present | 5 (13.5) | 3 (27.3) | 7 (16) | 15 (16.5) | 0.55 |
| Familiarity, present | 5 (13.2) | 0 (0) | 5 (11.4) | 10 (10.9) | 0.44 |
| Serum C3, g/L | 0.68 (0.34–1.05) | 0.5 (0.25–0.86) | 0.69 (0.31–0.96) | 0.67 (0.33–0.99) | 0.93 |
| Serum C4, g/L | 0.28 (0.19–0.41) | 0.21 (0.16–0.36) | 0.2 (0.13–0.25)a | 0.23 (0.17–0.32) | 0.02 |
| Low serum C3 with normal serum C4 | 19 (51.3) | 7 (63.6) | 18 (41) | 44 (47.8) | 0.34 |
| sC5b-9, ng/mL | 421 (271–661) | 509 (327–1069) | 375 (243–731) | 406 (261–690) | 0.7 |
| LPV carriers | 7 (20) | 2 (18.2) | 9 (21.4) | 18 (20.5) | 0.96 |
| C3NeF positivity, present | 7 (19.4) | 5 (45.4) | 11 (25) | 23 (25.3) | 0.2 |
| LPV carriers and/or C3NeF positivity, present | 11 (29.7) | 5 (45.4) | 17 (38.6) | 33 (35.8) | 0.18 |
The data are given as median and interquartile range or number and percentages. Reference range: C3, 0.9–1.8 g/L; C4, 0.15–0.55 g/L; sC5b-9, 110–252 ng/mL.
Significantly different from C3GN.
P-values are given as the results of Chi-square or Kruskal–Wallis tests of the patients with IC-MPGN, C3GN and DDD.
Table 2.
Histological characteristics of patients with histologically proven diagnosis of C3G or IC-MPGN
| Variables | C3GN, n = 37 | DDD, n = 11 | IC-MPGN, n = 44 | All, n = 92 | P-valuee |
|---|---|---|---|---|---|
| Time onset to biopsy, month | 0 (0–2.03) | 0 (0–4) | 0 (0–0.55) | 0 (0–0.88) | 0.59 |
| Light microscopy | |||||
| Sclerotic glomeruli, % | 6.8 (0–39) | 0 (0–4) | 0 (0–20) | 4 (0–23) | 0.16 |
| Crescent, % | 0 (0–9) | 0 (0–7) | 0 (0–9) | 0 (0–8) | 0.65 |
| Degree of mesangial proliferation | |||||
| 0/1/2/3a | 7/9/11/10 | 1/4/5/1 | 6/13/19/6 | 14/26/35/17 | 0.56 |
| Median + 25–75th range | 2 (1–3) | 2 (1–2) | 2 (1–3) | 2 (1–2) | |
| Degree of endocapillary proliferation | |||||
| 0/1/2/3a | 7/9/15/6 | 5/3/2/1 | 21/7/10/6 | 33/19/27/13 | 0.17 |
| Median + 25–75th range | 2 (1–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) | |
| Degree of interstitial inflammation | |||||
| 0/1/2/3a | 14/13/7/3 | 2/5/4/0 | 14/15/6/3 | 38/33/17/1 | 0.67 |
| Median + 25–75th range | 1 (0–2) | 1 (1–2) | 0.5 (0–1) | 1 (0–1) | |
| Degree of interstitial fibrosis | |||||
| 0/1/2/3a | 13/13/8/3 | 2/6/2/1 | 24/8/12/0 | 39/27/22/4 | 0.055 |
| Median + 25–75th range | 1 (0–2) | 1 (1–2) | 0 (0–2) | 1 (0–2) | |
| Arteriolar sclerosis, present | 13 (35) | 3 (27.3) | 10 (22.7) | 26 (28.3) | 0.46 |
| Immunofluorescence microscopy | |||||
| C3 | |||||
| 0/1/2/3a | 0/0/8/29 | 0/0/1/10 | 2/3/17/22 | 2/3/26/61 | 0.047 |
| Median + 25–75th range | 3 (3–3)b | 3 (3–3) | 2.5 (2–3) | 3 (2–3) | |
| IgA | |||||
| 0/1/2/3a | 32/4/0/0 | 7/4/0/0 | 22/9/4/9 | 61/17/4/9 | 0.002 |
| Median + 25–75th range | 0 (0–0) | 0 (0–1) | 0.5 (0–2)c,d | 0 (0–1) | |
| IgG | |||||
| 0/1/2/3a | 28/9/0/0 | 5/6/0/0 | 14/9/9/12 | 47/24/9/12 | <0.0001 |
| Median + 25–75th range | 0 (0–0) | 1 (0–1) | 1 (0–3)c,d | 0 (0–1) | |
| IgM | |||||
| 0/1/2/3a | 24/12/0/0 | 5/6/0/0 | 5/14/18/7 | 34/32/18/7 | <0.0001 |
| Median + 25–75th range | 0 (0–1)b,c | 1 (0–1) | 2 (1–2) | 1 (0–2) | |
| C1q | |||||
| 0/1/2/3a | 27/8/0/0 | 3/7/0/0 | 11/9/13/8 | 41/24/13/9 | <0.0001 |
| Median + 25–75th range | 2 (0–2)b,c | 1 (0–1) | 2 (0–2) | 1 (0–1.5) | |
| Electron microscopy | |||||
| Mesangial deposit, present | 28 (75.7) | 8 (72.7) | 25 (56.8) | 61 (66.3) | 0.18 |
| Subepithelial deposit, present | 15 (40.5) | 5 (45.4) | 21 (47.7) | 41 (44.6) | 0.8 |
| Subendothelial deposit, present | 22 (57.4) | 5 (45.4) | 30 (68.2) | 57 (62) | 0.35 |
| Intramembranous deposit, present | 15 (40.5) | 11 (100)b,d | 18 (40.9) | 44 (47.8) | 0.001 |
The data are given as median and interquartile range or number and percentages if not otherwise stated. Some patients have missing values in the following data: IgA, IgM, C1q immunofluorescence staining.
Degree of light microscopy, immunofluorescence findings were defined using scales 0–3.
Significantly different from IC-MPGN.
Significantly different from DDD.
Significantly different from C3GN.
P-values are given as the results of Chi-square or Kruskal–Wallis tests of the patients with IC-MPGN, C3GN and DDD.
Table 3.
Clinical characteristics of patients according to the classification by cluster analysis
| Variables | Cluster 1, n = 45 | Cluster 2, n = 4 | Cluster 3, n = 17 | Cluster 4, n = 26 | P-value |
|---|---|---|---|---|---|
| N | 45 | 4 | 17 | 26 | |
| Sex, % (men) | 20 (44.4) | 3 (75) | 12 (70.6) | 11 (42.3) | 0.16 |
| Age at diagnosis, years | 13 (7–16) | 9.5 (8–20) | 25 (10–33) | 39.5 (33–50)a,b | <0.0001 |
| Microhaematuria, present | 27 (60) | 2 (50) | 11 (64.7) | 16 (61.5) | 0.94 |
| Gross haematuria, present | 9 (20) | 2 (50) | 4 (23.5) | 5 (19.2) | 0.93 |
| Non-nephrotic proteinuria, present | 24 (53.3) | 1 (25) | 7 (41.2) | 12 (48) | 0.68 |
| Nephrotic syndrome, present | 17 (37.7) | 3 (75) | 10 (58.8) | 13 (52) | 0.25 |
| Renal impairment, present | 9 (20)b | 2 (50) | 11 (64.7) | 10 (40) | 0.003 |
| Renal failure, present | 4 (8.9) | 1 (25) | 1 (5.9) | 5 (20) | 0.27 |
| Creatinine, μmol/L | 61 (49–79)b | 215 (50–569) | 170 (63–229) | 166 (92–285) | 0.005 |
| Trigger, present | 12 (26.7)c | 1 (25) | 1 (5.9) | 1 (4) | 0.02 |
| Familiarity present (%) | 4 (8.9) | 0 (0) | 4 (23.5) | 2 (7.7) | 0.21 |
| Serum C3, g/L | 0.5 (0.23–0.84)c | 0.64 (0.29–0.93) | 0.77 (0.4–0.92) | 0.93 (0.66–1.17) | 0.02 |
| Serum C4, g/L | 0.19 (0.12–0.3)b | 0.35 (0.18–0.6) | 0.25 (0.21–0.4) | 0.24 (0.2–0.3) | 0.01 |
| Low serum C3 with normal serum C4, present | 20 (44.4) | 3 (75) | 11 (64.7) | 10 (38.5) | 0.22 |
| sC5b-9, ng/mL | 540 (332–1136)b | 438 (275–724) | 250 (189–404) | 407 (261–500) | 0.003 |
| LPV carriers | 10 (23.2) | 0 (0) | 3 (17.6) | 5 (20.8) | 0.89 |
| C3NeF positivity, present | 17 (38.6)c | 1 (25) | 2 (11.8) | 3 (11.5) | 0.02 |
| LPV carriers and/or C3NeF positivity, present | 21 (46.7) | 1 (25) | 4 (23.5) | 7 (26.9) | 0.21 |
The data are given as median and interquartile range or number and percentages. P-values are given as the results of Chi-square or Kruskal–Wallis tests of patients in Clusters 1, 3 and 4. Reference range: C3 0.9–1.8 g/L; C4 0.15–0.55 g/L; sC5b-9 1110–252 ng/mL. Some patients have missing values in the following data: proteinuria, renal impairment/failure, sC5b-9, LPV and C3NeF.
Significantly different from Cluster 1.
Significantly different from Cluster 3.
Significantly different from Cluster 4.
Table 4.
Histological characteristics of patients according to the classification by cluster analysis
| Variables | Cluster 1, n = 45 | Cluster 2, n = 4 | Cluster 3, n = 17 | Cluster 4, n = 26 | P-valuee |
|---|---|---|---|---|---|
| Time onset to biopsy (months) | 0 (0–0.69) | 1.85 (0.15–4.91) | 0 (0–0.6) | 0 (0.46–2.1) | 0.22 |
| Light microscopy | |||||
| Sclerotic glomeruli, % | 0 (0–5) | 0 (0–0) | 60 (47–70)a,b | 6 (0–19) | <0.0001 |
| Crescent, % | 0 (0–4) | 55 (46–62)a,b,c | 0 (0–6) | 0 (0–9) | 0.06 |
| Degree of mesangial proliferation | |||||
| 0/1/2/3d | 5/11/18/11 | 1/1/2/0 | 2/3/6/4 | 4/11/9/2 | 0.34 |
| Median + 25–75th range | 2 (1–2) | 1.5 (0.5–2) | 2 (1–2) | 1 (1–2) | |
| Degree of endocapillary proliferation | |||||
| 0/1/2/3d | 13/8/18/6 | 2/0/1/0 | 7/4/3/3 | 11/7/5/3 | 0.48 |
| Median + 25–75th range | 2 (0–2) | 1 (0–2.5) | 1 (0–2) | 1 (0–2) | |
| Degree of interstitial inflammation | |||||
| 0/1/2/3d | 24/18/3/0 | 2/1/1/0 | 2/6/6/3 | 10/8/7/1 | 0.001 |
| Median + 25–75th range | 0 (0–1)b,c | 0.5 (0–1.5) | 2 (1–2)b | 1 (0–2) | |
| Degree of interstitial fibrosis | |||||
| 0/1/2/3d | 27/15/3/0 | 2/1/1/0 | 1/3/10/3 | 9/8/8/1 | <0.0001 |
| Median + 25–75th range | 0 (0–1)b,c | 0.5 (0–1.5) | 2 (2–2)b | 1 (0–2) | |
| Arteriolar sclerosis, present | 3 (6.7)b,c | 1 (25) | 10 (58.8) | 12 (46.2) | <0.0001 |
| Immunofluorescence microscopy | |||||
| C3 | |||||
| 0/1/2/3d | 1/1/12/31 | 0/1/0/3 | 0/1/4/12 | 1/0/10/15 | 0.72 |
| Median + 25–75th range | 3 (2–3) | 3 (2–3) | 3 (2–3) | 3 (2–3) | |
| IgA | |||||
| 0/1/2/3d | 28/9/3/5 | 2/1/0/1 | 12/3/0/1 | 19/4/1/2 | 0.89 |
| Median + 25–75th range | 0 (0–1) | 0.5 (0–2) | 0 (0–0.5) | 0 (0–1) | |
| IgG | |||||
| 0/1/2/3d | 22/8/7/8 | 0/2/0/2 | 9/7/0/1 | 16/7/2/1 | 0.13 |
| Median + 25–75th range | 1 (0–2) | 2 (1–3) | 0 (0–1) | 0 (0–1) | |
| IgM | |||||
| 0/1/2/3d | 14/16/10/5 | 1/3/0/0 | 7/5/2/2 | 12/8/6/0 | 0.5 |
| Median + 25–75th range | 1 (0–2) | 1 (0.5–1) | 1 (0–1) | 1 (0–1.5) | |
| C1q | |||||
| 0/1/2/3d | 18/11/9/7 | 1/1/1/1 | 10/5/0/1 | 12/7/3/0 | 0.16 |
| Median + 25–75th range | 1 (0–2) | 1.5 (0.5–2.5) | 0 (0–1) | 0 (0–1) | |
| Electron microscopy | |||||
| Mesangial deposit, present | 30 (66.7) | 2 (50) | 12 (70.6) | 17 (65.4) | 0.93 |
| Subepithelial deposit, present | 24 (53.3) | 3 (75) | 7 (41.2) | 7 (26.9) | 0.09 |
| Subendothelial deposit, present | 31 (68.9) | 3 (75) | 10 (58.8) | 13 (50) | 0.28 |
| Intramembranous deposit, present | 24 (53.3) | 2 (50) | 11 (64.7) | 7 (26.9)a | 0.07 |
The data are given as median and interquartile range or number and percentages if not otherwise stated. Some patients have missing values in the following data: IgA, IgM, C1q immunofluorescence staining.
Significantly different from Cluster 1.
Significantly different from Cluster 4.
Significantly different from Cluster 3.
Degree of light microscopy, immunofluorescence findings were defined using scales 0–3.
P-values are given as the results of Chi-square or Kruskal–Wallis tests of patients in Clusters 1, 3 and 4.
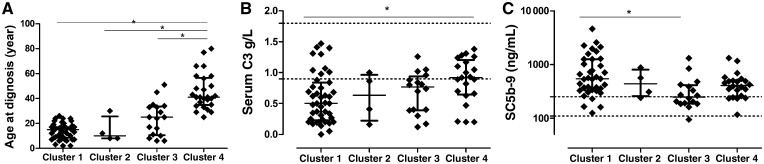
Cluster 1 was characterized by low C3 levels (median 0.5 g/L), very high sC5b-9 (median 540 ng/mL) levels and high prevalence of LPVs and C3NeF. The median age of onset was 13 years. Renal impairment and creatinine levels were low in that cluster at the time of onset. On light microscopy, no sclerotic glomeruli or crescents were seen, the strong C3 staining was not isolated and Ig staining was also seen in Cluster 1 on immunofluorescence microscopy, in concordance with the original study of Iatropoulos et al. [15].
The four patients in Cluster 2 had the strongest glomerular staining for IgA, IgG and C1q, with substantial crescent formation, three of them had nephrotic-range proteinuria; hence, the descriptive characteristics are very similar to those published by Iatropoulos et al. However, due to the low case number, we excluded these patients from further analysis.
In Cluster 3, the lowest sC5b-9 levels (median 250 ng/mL) were observed, compared with other clusters, along with moderately decreased C3 concentration (median 0.77 g/L) and with high prevalence of LPVs (Figure 1). On light microscopy, the highest degree of interstitial fibrosis, interstitial inflammation and the highest prevalence of sclerotic glomeruli were seen with low prevalence of crescents, compared with the other clusters.
FIGURE 1.
Scatterplots of age at diagnosis (A), serum C3 levels (B) and plasma sC5b-9 levels (C) across the different clusters. Horizontal lines show medians, whiskers interquartile ranges of the groups. Horizontal dotted lines indicate reference range limits. For the comparison of Clusters 1, 3 and 4, Kruskal–Wallis analysis of variance was used (panel A, P = 0.018; panel B, P = 0.003; panel C, P < 0.0001) with Dunn’s post hoc test. *P < 0.05.
In Cluster 4, the median age of onset (39.5 years) was the highest compared with the other clusters, and this cluster was also characterized by near-normal C3 levels (median 0.93 g/L; Table 3 and Figure 1) and with lower prevalence of intramembranous deposits (P = 0.046). The prevalence of thrombotic microangiopathy (TMA) was the highest in this cluster in Italian cluster [15]. We have only two patients with data for TMA, but they were excluded because of missing data, which is why we could not examine this aspect of Cluster 4.
The key pathogenic features of the different clusters in the two cohorts are shown in comparative manner in Supplementary data, Figure S4.
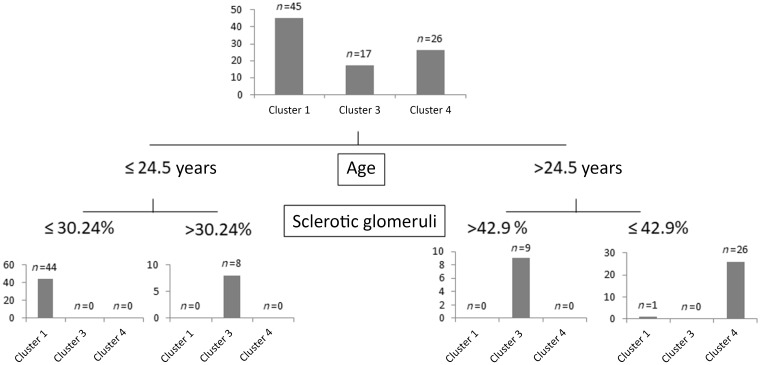
Decision tree analysis was done to select the minimum set of features to predict membership of patients in the clusters (Figure 2), and predictor variables were ranked according to their classification importance (Supplementary data, Table S3). The top two classifiers were the presence and extent of sclerotic glomeruli and age at diagnosis. Using three cut-points of these variables, all but one patient were correctly classified into Clusters 1, 3 and 4. Variable ranking indicated that besides age at diagnosis and extent of glomerular sclerosis, the most important predictor variables for cluster membership were the presence of interstitial fibrosis, interstitial inflammation, arteriolar sclerosis and sC5b-9 level.
FIGURE 2.
Decision tree to assign patients into the different clusters. Analysis based on classification and regression model built to predict independent clusters as categorical variable, and on the same predictor variable set that was used for the cluster analysis (for details see Materials and methods section). Four patients in Cluster 2 were excluded from this analysis.
No difference was observed with regard to the occurrence or distribution of LPVs between the different clusters, but LPVs affecting C3 and CFH were identified only in Cluster 1. The generated clusters did not show significant connection with the original histology-based diagnosis (Supplementary data, Table S4) although patients with C3GN and IC-MPGN fall mainly into Clusters 1 and 4.
Prognostic significance of the clusters
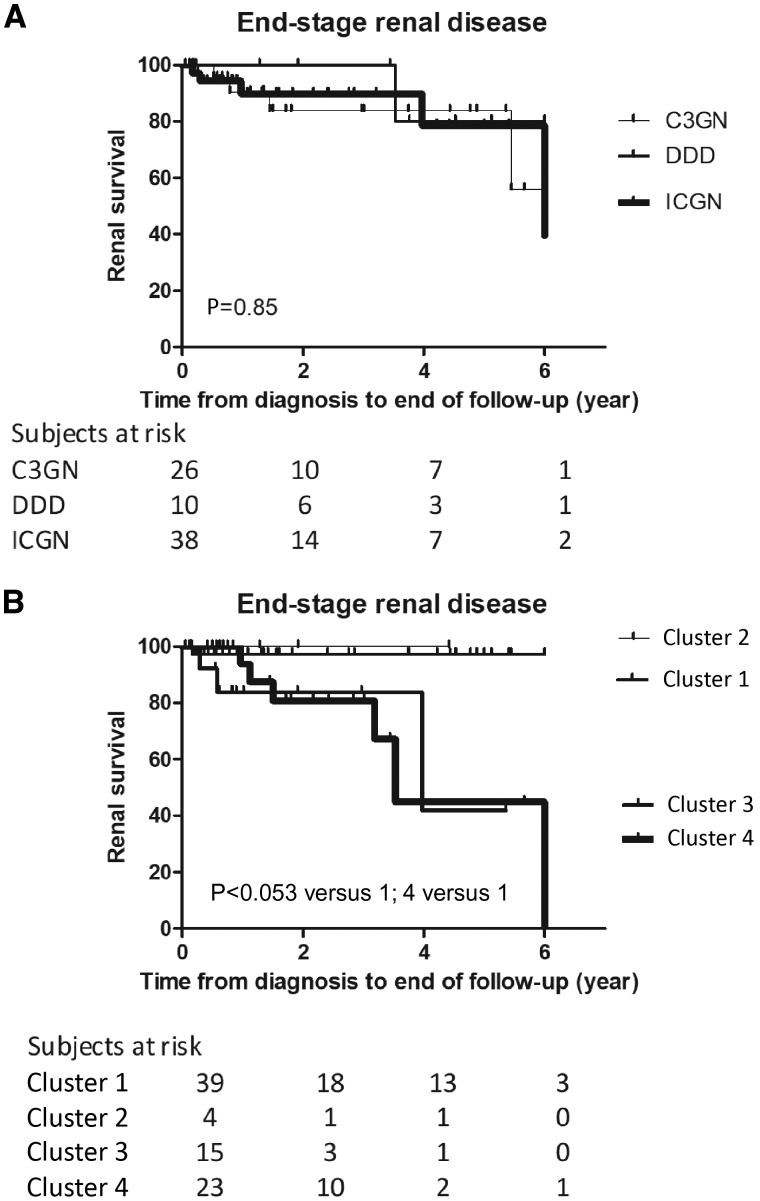
Clinical relevance of the clusters was evaluated by analysis of their association with renal outcome. During the follow-up period, we managed to collect data on the development of end-stage renal disease (ESRD), defined as estimated glomerular filtration rate decrease to <15 mL/min/1.73 m2 with or without renal replacement therapy (such as haemodialysis, peritoneal dialysis or kidney transplantation). Ultimately, 10 out of the 79 successfully followed patients progressed to ESRD including 5 patients with acute kidney injury at the time of diagnosis but later temporary improvement of kidney function. Renal outcome was found to be worse in Clusters 3 and 4 compared with Cluster 1 with Kaplan–Meier analysis (P < 0.05 for Clusters 3 versus 1 and Clusters 4 versus 1), indicating that patients with higher age at diagnosis, more prevalent glomerular sclerosis at diagnosis and lower prevalence of C3NeF and/or LPV have inferior renal outcome. There was no difference in renal survival according to the histology groups (Figure 3). We have data of patients’ treatment strategy at the time of diagnosis, which may affect renal survival. Going through the data, most of the patients received antihypertensive drugs [angiotensin-converting enzyme-I and angiotensin-receptor blockers (ARB) and immunosuppressants (steroid, cyclophosphamide and mycophenolate mofetil). In some cases, plasmatherapy and renal replacement therapy were indicated at the time of diagnosis. No relevant differences were seen between patients’ initial therapy in different clusters (Supplementary data, Table S5). The various treatment modifications during follow-up period were not collected.
FIGURE 3.
Kaplan–Meier renal survival curves for groups of MPGN patients based on the histological diagnosis (A) or cluster (B). P-values of log-rank tests are indicated. Cluster 2 was excluded from Kaplan–Meier analysis. To better visualize the renal survival of the patients, we stopped the curves at 6 years because of a shorter follow-up in the majority of the cases. ICGN, immune complex-mediated MPGN.
Complement activation and regulation in relation to clinical patterns
The findings of the cluster analysis motivated us to examine an extended set of complement parameters. Due to the association of high sC5b-9 levels and C3 consumption with clusters (Table 3), we measured additional complement proteins, pathway activities and activation markers (Table 5). Significantly lower classical pathway (CP) (P = 0.001) and alternative pathway (AP) (P = 0.02) activity, along with decreased FD concentrations (P = 0.006) were observed in Cluster 1, where C1q levels were also the lowest. These results together with decreased C3 and C4 levels in Cluster 1 indicating overactivation and consumption of CP/AP compared with the Clusters 3 and 4. Concentrations of the complement activation split products C4d, Bb and C3a were low and did not show any significant difference among the different clusters.
Table 5.
Additional complement factors, activation markers and autoantibodies in the different clusters
| Variables | Cluster 1 | Cluster 2 | Cluster 3 | Cluster 4 | P-value |
|---|---|---|---|---|---|
| Classical pathway activity, CH50/mL | 30.5 (6.5–47)a | 50 (10.75–69.75) | 60 (32.5–62) | 54 (36.5–54) | 0.001 |
| AP activity, % | 7 (1–63)a | 82.5 (18–103.5) | 65 (16.5–84.5) | 75 (19–97) | 0.02 |
| C1q antigen, mg/L | 95 (73–123) | 94 (75.75–131.8) | 112.5 (96.75–136) | 105 (79–117) | 0.09 |
| Anti-C1q positivity, present | 11 (24.4)a | 0 (0) | 1 (5.9) | 1 (3.8) | 0.018 |
| Anti-Factor H positivity, present | 3 (7.1) | 0 (0) | 2 (11.8) | 0 (0) | 0.24 |
| Anti-C3 positivity, present | 4 (9.3) | 1 (25) | 0 (0) | 0 (0) | 0.14 |
| Anti-Factor B positivity, present | 2 (4.7) | 1 (25) | 2 (12.5) | 1 (4.2) | 0.47 |
| Positivity for >1 complement autoantibodyb | 5 (11.1)a | 1 (25) | 1 (5.8) | 0 (0) | 0.04 |
| FD, µg/mL | 1.4 (0.65–2.49)a,c | 2.65 (0.76–5.19) | 3.16 (2.37–4.4) | 2.48 (1.28–4.66) | 0.006 |
| C4d, µg/mL | 4.18 (3.05–7.51) | 9.37 (7.32–10.83) | 3.42 (2.73–9.04) | 5.76 (3.31–10.06) | 0.32 |
| Bb, µg/mL | 1.34 (0.85–2.09) | 1.34 (0.69–3.58) | 1.6 (1.19–2.08) | 1.49 (1.03–2.84) | 0.38 |
| C3a, ng/mL | 176 (98.5–229.1) | 102 (77.46–223) | 123 (68–174.7) | 172 (105.5–244.5) | 0.2 |
The data are given as median and interquartile range or number and percentages. P-values are given as the results of Chi-square or Kruskal–Wallis tests of patients in Clusters 1, 3 and 4. Reference range: sC5b-9 110–252 ng/mL; C3a 70–270 ng/mL; C4d 0.7–6.3 µg/mL; Bb 0.49–1.42 µg/mL; FD 0.51–1.59 µg/mL. Some patients have missing values in the following data: C1q antigen, anti-C1q, anti-FH, anti-C3 and anti-FB, FD, C4d, Bb, C3.
Significantly different from Cluster 4.
Out of C3NeF, anti-C1q, anti-FH, anti-C3 and anti-FB .
Significantly different from Cluster 3.
C3NeF positivity was more prevalent in Cluster 1; therefore, additional complement autoantibodies including anti-C1q, anti-Factor H, anti-Factor B and anti-C3 were also determined. Cluster 1 captured 11 out of 13 anti-C1q-positive patients (P = 0.018), whereas no difference in the prevalence of anti-Factor H, anti-C3 and anti-Factor B among the four clusters was observed. Multiple positivity for the examined autoantibodies was observed more frequently in Cluster 1. We did not find any association between the studied parameters and the histology-based groups (Supplementary data, Table S6).
DISCUSSION
Current classification of MPGN is based on immunofluorescence microscopy and classifies patients as C3G/IC-MPGN [3]. Recently, an unsupervised, data-driven method was applied by Iatropoulos and associates for a cohort of MPGN patients to see whether clinically meaningful groups can be identified based on clinical, biochemical, histological or genetic data [15]. This new statistical approach generated clusters with small within-cluster, as opposed to large between-cluster, differences. The clusters showed clear associations with AP abnormalities, additional pathogenic features and risk of progression to ESRD. As was pointed out in an editorial [18] commenting the results of Iatropoulos et al., repetition of these observations in an independent cohort is essential to validate if such clusters exist or not.
The aim of our study was to replicate the unsupervised hierarchical cluster analysis made by Iatropoulos et al. [15] in an independent C3G/IC-MPGN cohort and to validate the existence of clinically meaningful clusters. We recruited 92 patients and determined the same clinical, biochemical, histological and genetic parameters as in the original study [15]. Our independent cohort seemed to be appropriate for validation study since the most important characteristics were similar to the original cohort (Supplementary data, Figure S2). With unsupervised, data-driven clustering method, four clusters were generated yielding Clusters 1, 3 and 4 with sufficiently high patient numbers, whereas Cluster 2 with only four patients was excluded from further analysis. Clusters 1, 3 and 4 showed clear associations with clinical, biochemical or histological variables with a very similar pattern to that of Iatropoulos et al. (Supplementary data, Figure S4). Clinical relevance of the clusters was demonstrated by the significant association of Clusters 3 and 4 with development of ESRD during follow-up (Figure 3).
In the study of Iatropoulos et al. [15], a three-step algorithm was created to assign patients into different clusters. Unfortunately, we have no data to differentiate between highly electron-dense ribbon-like and granular deposits in basement membrane. That is why the minimum data set necessary to predict membership in the clusters was determined by decision tree analysis. Based on the presence and extent of sclerotic glomeruli, and age at onset, we could divide the patients into four clusters (Figure 2). Patients with higher percentages of sclerotic glomeruli and older age fall into Clusters 3 and 4, and have inferior renal survival. These findings are consistent with the well-known fact that renal survival is connected with the chronicity of the glomerular lesions, but the pathomechanism of the resulting pathological image is not known. It is noteworthy and would be important to explore why the pattern of some important clinical and complement parameters show association with these clusters and with outcome, too.
The patterns of clinical and pathogenetic factors of the different clusters were very similar in our study to those in Iatropoulos et al. Cluster 1 identified patients with very high sC5b-9 and decreased C3 levels, along with the high prevalence of LPVs and/or C3NeF in younger patients. Patients in Cluster 3 were typically young adults characterized also by complement consumption (decreased C3) but lower sC5b-9 levels, whereas the histological picture was more severe as indicated by higher degree of interstitial inflammation, fibrosis and presence of sclerotic glomeruli. Based on these results, we could not unequivocally match the current Cluster 3 to the Italian Cluster 3. The importance of the difference is also supported by the different renal survival of Cluster 3. Cluster 4 included adults with normal levels of C3, lack of severe complement activation and inferior renal survival.
Beyond the similarities, some differences were also noted between our study and that of Iatropoulos et al. [15], the most important being some discrepancies in histology. We enrolled patients from several centres, without the option of centralized second review of biopsy. Rather, aggregate evaluation of biopsy descriptions was done by a structured questionnaire. Furthermore, the mean ages in Clusters 3 and 4 are higher by 10 years in our study, and this may explain why the prevalence of sclerotic glomeruli is also higher in Cluster 3. Cluster 3 differs in our study from the original study in additional aspects too, including the lack of crescents and lower C3NeF prevalence. The difference of C3NeF prevalence between the two studies could be explained by the different method of the detection of C3NeF activity. In contrast to the Italian study where purified IgG was used for the detection, we used patients’ sera following the protocol of Rother [17]. This fact can make this slight difference between the prevalence of C3NeF.
Higher age, and lack of crescents but presence of sclerosis, indicating advanced disease stage may explain the inferior renal survival of patients in Cluster 3. Cluster 4 is best characterized by lack of systemic signs of complement activation and consumption, but with intense glomerular C3 stain and inferior survival. The answer to why patients in Clusters 3 and 4 have advanced glomerular lesions and higher age at presentation with inferior renal survival, and whether these features are related to the molecular pathophysiology, remains elusive today. The presence of complement consumption and a high sC5b-9 level in Cluster 1 prompted us to explore additional complement biomarkers and autoantibodies to understand the underlying pathophysiology. We found that Cluster 1 encompassed most of the patients with anti-C1q autoantibodies. Remarkably, 2/11 and 5/11 anti-C1q-positive patients in Cluster 1 were double or triple positive for additional complement autoantibodies listed in Table 5. The observation that different complement autoantibodies occur together in Cluster 1 (P = 0.043 when compared with Clusters 3 and 4) also raises the possibility of strong polyclonal response and epitope spreading towards complement proteins. In addition to the autoantibodies presented in this study (Table 5), presence of C4NeF also seems to be more prevalent in Cluster 1 (N. Garam et al., unpublished work).
We acknowledge the potential limitations of our study. Some of our observations and conclusions stem from underpowered analysis and may suffer from Type I error. However, since this is an independent replication study, and most of the key observations and differences were similar in the two studies, the appearance of false-positive decisions twice is highly unlikely. In addition, our study lacked centralized second look-up of biopsy results, potentially leading to higher variance in histology-based scores. However, the most important cluster-defining histological marker was presence and extent of sclerotic glomeruli, a feature not prone to suffer from large inter-observer differences. We could not distinguish between granular and ribbon-like highly electron-dense intramembranous deposits in our study because only intramembranous highly electron-dense deposits were mentioned in most of the pathology reports.
Considering the extremely low prevalence of C3G (2–3/1 000 000 people), large multicentre studies are essentials to confirm the original studies of Iatropoulos et al. [15]. Although we could not collect some data in exactly the same way as it was described in the Italian study, this study is the first that managed to collect enough patients to run this analysis again in a very similar manner and this international collaboration allowed us to validate the main findings of the original analysis in this rare disorder. With the ultimate goal to support treatment decisions for patients with these rare diseases, our results confirmed the existence of Clusters 1 and 4. Patients in Cluster 1 are best characterized by younger age, lack of glomerular sclerosis, high prevalence of LPVs and autoantibodies, and signs of systemic complement activation and consumption, hence, intense C5-convertase activity. These signs support the presence of fluid-phase dysregulation; hence, patients falling into Cluster 1 are potential candidates for C5-targeted therapies. In contrast, patients in Cluster 4 had no signs of systemic complement activation or consumption despite intense C3 deposition in the kidney, indicating the presence of surface-limited complement dysregulation. The fact that four out of five LPV carriers in Cluster 4 carried LPVs in the genes of surface expressed cofactors CD46 and THBD seems to support this idea. Therefore, the proposition of Iatropoulos et al. to consider complement inhibitors, targeting C3 activation products on cell surfaces (like TT30) [19], seems to be rational and is also supported by our observations. Finally, despite independent validation of the original observations [15], it is still a question whether this new, data-driven, pathogenesis-connected classification can change the histology-based classification in the long term, with the final goal of better classification to support appropriate treatment decisions.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank Edina Szabó, Beáta Takács, Zsuzsanna Szendrei, Márta Kókai and Erika Kertészné for expert technical assistance. They also thank Nóra Veszeli, Blanka Mező and Luca Laszip for their supporting work in this study.
Special thanks to all of the pathologists for their work to evaluate the biopsy: Marijana Ćorić, Merica Glavina Durdov, Prof. Dusan Ferluga, Cosmin Florescu, Danica Galesic, Jaromir Hacek, Prof. Eva Honsova, Béla Iványi, Magdolna Kardos, Ilona Kaszás, Arvydas Laurinavicius, Prof. Violina Minkova, Dr Oleksiy Tsybrovskyy, Nicoleta Petre, Kristýna Pivovarčíková, Thomas Tichy and Prof. Alenka Vizjak.
FUNDING
This work was supported by the grant of the National Research Fund (National Research, Development and Innovation Office) of Hungary, PD116119 and by the Bolyai János Research Fellowship (2015–18) to D.Csuka. The research was financed by the Higher Education Institutional Excellence Program of the Ministry of Human Capacities in Hungary, within the framework of the molecular biology thematic programme of Semmelweis University. N.G. received financial support from the EFOP-3.6.3-VEKOP-16-2017-00009 grant.
AUTHORS’ CONTRIBUTIONS
Study designation was performed by Z.P., D.Csuka, Á.Szilágyi and N.G. Experiments are conducted by D.Csuka and N.G. Data analysis was done by Z.P., D.Csuka, Á.Szilágyi and N.G. Data collection was performed by C.A., A.Schmidt, M.G., G.S.-P., D.B., J.B., A.D., D.Cejka, S.F., H.F., Á.Haris, Á.Hartmann, A.Heilos, T.M., K.R., K.A., J.H., D.J., M.Sinko, E.Szigeti, C.B., V.J., K.K., G.S.R., A.J.S., N.Klenk, K.K, N.Kojc, M.K., M.L., A.C.L., A.M., R.R., T.K.-L., E.M., M.M., A.P., T.Stompór, L.P., M.R., G.M., R.R., J.R., M.Saraga, T.Seeman, J.Z., E.Sládková, T.Szabó, A.C., S.S., M.T., K.G., A.T., I.V., M.W., T.Z., G.Z. Z.P., D.Csuka, Á.Szilágyi and N.G. All authors revised the article and approved the final version of the manuscript.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Iatropoulos P, Noris M, Mele C. et al. Complement gene variants determine the risk of immunoglobulin-associated MPGN and C3 glomerulopathy and predict long-term renal outcome. Mol Immunol 2016; 71: 131–142 [DOI] [PubMed] [Google Scholar]
- 2. Bu F, Borsa NG, Jones MB. et al. High-throughput genetic testing for thrombotic microangiopathies and C3 glomerulopathies. J Am Soc Nephrol 2016; 27: 1245–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pickering MC, D'Agati VD, Nester CM. et al. C3 glomerulopathy: consensus report. Kidney Int 2013; 84: 1079–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Licht C, Heinen S, Józsi M. et al. Deletion of Lys224 in regulatory domain 4 of Factor H reveals a novel pathomechanism for dense deposit disease (MPGN II). Kidney Int 2006; 70: 42–50 [DOI] [PubMed] [Google Scholar]
- 5. Abrera-Abeleda MA, Nishimura C, Frees K. et al. Allelic variants of complement genes associated with dense deposit disease. J Am Soc Nephrol 2011; 22: 1551–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong EKS, Anderson HE, Herbert AP. et al. Characterization of a factor H mutation that perturbs the alternative pathway of complement in a family with membranoproliferative GN. J Am Soc Nephrol 2014; 25: 2425–2433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marinozzi MC, Roumenina LT, Chauvet S. et al. Anti-factor B and Anti-C3b Autoantibodies in C3 glomerulopathy and Ig-associated membranoproliferative GN. J Am Soc Nephrol 2017; 28: 1603–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang Y, Meyer NC, Fervenza FC. et al. C4 nephritic factors in C3 glomerulopathy: a case series. Am J Kidney Dis 2017; 70: 834–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blom AM, Corvillo F, Magda M. et al. Testing the activity of complement convertases in serum/plasma for diagnosis of C4NeF-mediated C3 glomerulonephritis. J Clin Immunol 2016; 36: 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sethi S, Fervenza FC, Zhang Y. et al. C3 glomerulonephritis: clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int 2012; 82: 465–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang Y, Meyer NC, Wang K. et al. Causes of alternative pathway dysregulation in dense deposit disease. Clin J Am Soc Nephrol 2012; 7: 265–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hou J, Markowitz GS, Bomback AS. et al. Toward a working definition of C3 glomerulopathy by immunofluorescence. Kidney Int 2014; 85: 450–456 [DOI] [PubMed] [Google Scholar]
- 13. Figuères M-L, Frémeaux-Bacchi V, Rabant M. et al. Heterogeneous histologic and clinical evolution in 3 cases of dense deposit disease with long-term follow-up. Hum Pathol 2014; 45: 2326–2333 [DOI] [PubMed] [Google Scholar]
- 14. Servais A, Noël L-H, Roumenina LT. et al. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int 2012; 82: 454–464 [DOI] [PubMed] [Google Scholar]
- 15. Iatropoulos P, Daina E, Curreri M. et al. Cluster analysis identifies distinct pathogenetic patterns in C3 glomerulopathies/immune complex-mediated membranoproliferative GN. J Am Soc Nephrol 2018; 29: 283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fetterhoff TJ, McCarthy RC.. A micromodification of the CH50 test for the classical pathway of complement. J Clin Lab Immunol 1984; 14: 205–208 [PubMed] [Google Scholar]
- 17. Rother U. A new screening test for C3 nephritis factor based on a stable cell bound convertase on sheep erythrocytes. J Immunol Methods 1982; 51: 101–107 [DOI] [PubMed] [Google Scholar]
- 18. Cook HT, Pickering MC.. Clusters not classifications: making sense of complement-mediated kidney injury. J Am Soc Nephrol 2018; 29: 9–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fridkis-Hareli M, Storek M, Mazsaroff I. et al. Design and development of TT30, a novel C3d-targeted C3/C5 convertase inhibitor for treatment of human complement alternative pathway-mediated diseases. Blood 2011; 118: 4705–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.