Abstract
Background
Data are lacking on the relative incidence of thrombotic thrombocytopenic purpura (TTP), haemolytic uraemic syndrome (HUS) caused by Shiga toxin–producing Escherichia coli (STEC) and atypical HUS (aHUS) in patients presenting with thrombotic microangiopathies (TMAs).
Methods
This was a prospective, cross-sectional, multicentre and non-interventional epidemiological study. Patients fulfilling criteria for TMAs (platelet consumption, microangiopathic haemolytic anaemia and organ dysfunction) were included in the study. The primary objective was to assess the relative incidence of TTP, STEC-HUS, aHUS and ‘other’ physician-defined diagnoses. The secondary objective was to develop an algorithm to predict a severe deficiency in ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) activity (≤10%) using routine laboratory parameters. A post hoc classification using the recent Kidney Disease: Improving Global Outcomes diagnostic criteria was then undertaken to further classify patient groups.
Results
aHUS was diagnosed with a relative incidence of 61%, whereas TTP, STEC-HUS and ‘other’ were diagnosed in 13, 6 and 20% of patients, respectively. In the post hoc analysis, 27% of patients with a TMA were classified as ‘primary aHUS’ and 53% as ‘secondary aHUS’. Multivariate analysis revealed that severe deficiency in ADAMTS13 activity (≤10%) was unlikely to underlie TMA if platelet and serum creatinine were above threshold values of 30 × 109/L and 1.8 mg/dL, respectively (negative predictive value of 92.3 and 98.1, respectively, if one or both values were above the threshold).
Conclusions
In this study, aHUS was the most common single diagnosis among patients presenting with a TMA. In the absence of an ADAMTS13 activity result, platelet count and serum creatinine may aid in the differential diagnosis.
Keywords: ADAMTS13, atypical haemolytic uraemic syndrome, haemolytic uraemic syndrome, thrombotic microangiopathy, thrombotic thrombocytopenic purpura
INTRODUCTION
Thrombotic thrombocytopenic purpura (TTP), haemolytic uraemic syndrome (HUS) caused by Shiga toxin–producing Escherichia coli (STEC) and atypical HUS (aHUS) are rare but serious clinical conditions. They belong to a group of entities known as thrombotic microangiopathies (TMAs), which present with platelet consumption, microangiopathic haemolytic anaemia (MAHA) and organ dysfunction resulting from endothelial damage and microvascular thrombosis [1, 2]. Although these conditions have similar clinical presentations, the underlying pathophysiology is different, requiring specific management. TTP results from a severe deficiency of the von Willebrand factor (VWF)-cleaving protease ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13), which causes platelet aggregation in the microvasculature due to accumulation of ultralarge VWF multimers [3]. The deficiency of ADAMTS13 activity can be hereditary (Upshaw–Schulman syndrome) or acquired, as a result of anti-ADAMTS13 autoantibodies [3]. Definitive diagnosis requires an assay to evaluate plasma ADAMTS13 activity, where a result of ≤5–10% denotes TTP [1, 3–8]. Treatment consists of plasmapheresis combined with immunosuppression [9]. In addition, recently, caplacizumab, an anti-VWF humanized single-variable domain immunoglobulin, has been found to be of benefit in the initial treatment of TTP [10].
STEC-HUS results from a STEC infection and is diagnosed by detection of Shiga toxin in patient stool samples via a STEC stool culture and polymerase chain reaction (PCR) assay [2]. Management of STEC-HUS is supportive; currently, plasmapheresis and eculizumab are not recommended and there is debate over the efficacy of antibiotics [9, 11, 12].
The term ‘aHUS’ historically defines all patients not diagnosed with TTP or STEC-HUS. Within this group, complement-mediated forms (‘primary’) and secondary forms with defined triggering conditions causing TMA are summarized with clinical and pathophysiological overlap [13]. Primary aHUS is caused by dysregulation of the complement system, usually from a genetic predisposition [2]. However, a genetic mutation in or autoantibodies against parts of the complement system are identified in only ∼60–70% of patients with aHUS tested for complement abnormalities after exclusion of secondary causes [13–15]. Thus aHUS is a diagnosis based on clinical and laboratory features, with a positive genetic test supporting the diagnosis but a negative genetic test not excluding it. Of note, 70% of patients with an underlying complement abnormality present with triggering conditions [14]. In the context of an endogenous predisposition, triggering conditions may lead to clinical manifestation of TMA [16]. The recommended treatment for primary aHUS is eculizumab [1, 9, 13, 17]; however, its role in cases where the disease seems to be driven by an external trigger is controversial. There is currently no definitive test for aHUS, therefore diagnosis is made by exclusion of TTP and STEC-HUS via ADAMTS13 and STEC assays, respectively, and consideration of other potential causes of TMA, such as malignancy, certain drugs and solid-organ and bone marrow transplant (BMT) [2]. These conditions might either be the root cause of the TMA manifestation or a triggering condition in the context of an underlying primary aHUS [1]. The treating physician must carefully consider laboratory parameters and patient presentation to determine appropriate diagnosis and management.
Because of the overlap in clinical presentation of the different TMAs, differential diagnosis can be challenging. Knowing the relative probability of these conditions is of interest in this situation. Therefore we conducted this study to evaluate the relative incidence of TTP, STEC-HUS and aHUS in patients presenting with TMA in Germany.
MATERIALS AND METHODS
The Cross-sectional Evaluation of clinical Symptoms And epidemiologic paRameters in patients with TMA, differentiated by laboratory parameters (CESAR) study was a prospective, cross-sectional, multicentre and non-interventional epidemiological investigation of the relative incidence of TTP, STEC-HUS and aHUS in clinically suspected cases of TMA. The study was approved by the ethics committees of the Bayerische Landesärztekammer and participating centres and followed the principles of the Declaration of Helsinki. Recruiting centres included 10 adult centres in university hospitals, 10 adult centres in non-university hospitals and 2 paediatric centres in university hospitals.
Subjects
All patients provided informed consent prior to inclusion. Patients were included in the study based on the presence of platelet consumption, MAHA and organ dysfunction. Platelet consumption was defined as a platelet count <150 × 109/L or a decrease in platelet count >25% within 1 week. MAHA could be demonstrated by the presence of schistocytes, increased lactate dehydrogenase, decreased haemoglobin and decreased haptoglobin. If schistocytes were absent, then the patient could still be included if the treating physician had a high clinical suspicion of TMA. In addition, patients were required to present with at least one of the following signs or symptoms of organ dysfunction: neurological symptoms, such as confusion, cerebral changes, convulsions or spasms, dysarthria, dysphasia, aphasia, impaired consciousness or other neurological symptoms revealed during clinical assessment; renal impairment, such as increased creatinine, decreased estimated glomerular filtration rate, increased blood pressure or abnormal urinalysis; or gastrointestinal symptoms, such as diarrhoea with or without blood, nausea or vomiting, abdominal pain or gastroenteritis. Cardiovascular (any sign possibly indicating heart symptoms according to clinical assessment), pulmonary and other organ symptoms did not qualify for inclusion but were recorded. Plasma therapy prior to blood sampling for ADAMTS13 analysis was also an exclusion criterion.
STEC testing
STEC testing in stool samples was performed centrally at the Institute of Hygiene, University Hospital Münster, Münster, Germany. PCR assays were performed on all available stool samples following an 18- to 24-h incubation period in an enrichment broth. Samples were analysed for Shiga toxin 1–, Shiga toxin 2–, intimin– and E. coli O157-antigen–encoding genes using in-house protocols, as described previously [18, 19]. PCR results were verified by immunomagnetic separation using paramagnetic beads coated with anti-E. coli O157, O26, O103, O111 and O145 antibodies added to the enrichment broth to detect the appropriate strains or by next-generation sequencing analysis [20, 21].
ADAMTS13 analysis
Blood samples were collected in a citrate buffer prior to any plasma therapy. ADAMTS13 activity assays were performed centrally at Medilys Laborgesellschaft, Hamburg, Germany. Plasma ADAMTS13 activity was assessed using the commercially available Technozyme ELISA (Technoclone, Vienna, Austria), a chromogenic test, which measures residual recombinant VWF, using a monoclonal anti-N10 antibody, after patient plasma is added to immobilized VWF fragments [22]. The reference range and sensitivity of the ADAMTS13 assay were evaluated in the laboratory; the reference range of ADAMTS13 activity was 50–110% of normal and the assay sensitivity for ADAMTS13 activity in plasma was 0.5% [1, 3].
Diagnoses
Treating physicians diagnosed patients as TTP, STEC-HUS, aHUS or ‘other’ based on the results of ADAMTS13 activity, STEC test, clinical presentation and laboratory parameters. Diagnosis could be revised until the end of the study if additional information became available. Within the aHUS group, details of potential triggering conditions were also recorded. For patients with a diagnosis of ‘other’, the relevant diagnostic information was entered into the database as free text.
Data analysis and endpoints
Analyses were carried out on an intention-to-diagnose basis. The primary objective of this study was to evaluate the relative incidence of TTP, STEC-HUS and aHUS in patients presenting with TMA. Demographic and baseline characteristics were analysed descriptively. Continuous parameters including mean [standard deviation (SD)] and median [interquartile range (IQR)] were calculated. Qualitative variables were expressed as proportions. Data were stratified by age at enrolment (paediatric <18 years, adult ≥18 years).
A secondary exploratory objective was to assess whether routine laboratory parameters at clinical presentation could be used to predict or exclude a diagnosis of TTP. To address this endpoint we developed an algorithm to predict a severe deficiency in ADAMTS13 activity (≤10%) in adult patients at clinical presentation. All available variables were analysed for correlation with the final diagnosis. Threshold values were identified by maximizing the Youden Index, a measure of the performance of diagnostic tests accounting for the proportions of correctly diagnosed individuals with and without disease (sensitivity + specificity − 1), [23] and then rounding to the nearest easy-to-recall value.
Post hoc analysis
After completion of this study, the Kidney Disease: Improving Global Outcomes (KDIGO) published the results from a controversies conference on aHUS in which they suggested a classification of TMAs [13]. To increase objectivity and limit potential bias, we conducted a post hoc analysis to reclassify adult patients from aHUS and ‘other’ groups to the KDIGO classification [13]. To classify all patients, groups for those presenting with malignant hypertension (MHT), renal disease and unspecified TMAs were added to this classification system. Because many patients presented with more than one trigger, a hierarchical algorithm was developed to categorize each patient to only one group (Supplementary Methodology).
RESULTS
Patient characteristics
From 25 April 2014 to 31 March 2017, 232 patients were enrolled in 22 German centres, including 19 adult nephrology centres, 2 paediatric nephrology centres and 1 adult haematology centre. Demographic and clinical data according to differential diagnoses are shown in Table 1 (physician-made diagnoses split by age group), Supplementary data, Table S1 (physician-made diagnoses, all patients) and Table 2 (post hoc classification, adult patients). Overall, the mean age at enrolment was 53.3 years, 219/232 (94%) patients were adults and 125/232 (54%) were female.
Table 1.
Demographic and clinical characteristics of adult (n = 219) and paediatric patients (n = 13) with TMA of different underlying causes
| Characteristic | Adult patients |
Paediatric patients |
||||
|---|---|---|---|---|---|---|
| aHUS (n = 138) | STEC-HUS (n = 6) | TTP (n = 31) | Other (n = 44) | aHUS (n = 4) | STEC-HUS (n = 9) | |
| Age at enrolment (years), mean (SD) | 57.5 (18.7) | 55.8 (22.4) | 50.5 (16.7) | 55.9 (20.6) | 4.0 (2.0) | 5.3 (5.8) |
| Gender (female), n (%) | 80 (58) | 4 (67) | 21 (68) | 15 (34) | 0 (0) | 5 (56) |
| Presenting organ manifestations, n (%) | ||||||
| Renal | 128 (93) | 6 (100) | 22 (71) | 41 (93) | 4 (100) | 9 (100) |
| Gastrointestinal | 68 (49) | 5 (83) | 11 (35) | 21 (48) | 4 (100) | 8 (89) |
| Neurological | 54 (39) | 3 (50) | 18 (58) | 11 (25) | 1 (25) | 2 (22) |
| Cardiovascular | 50 (36) | 3 (50) | 7 (23) | 4 (9) | 0 (0) | 0 (0) |
| Pulmonary | 46 (33) | 1 (17) | 4 (13) | 6 (14) | 0 (0) | 0 (0) |
| Other | 46 (33) | 2 (33) | 10 (32) | 19 (43) | 0 (0) | 0 (0) |
| Platelets (×109/L), median (IQR) | 98.0a (47.0–123.0) | 51.5 (34.8–99.0) | 25.0 (10.5–55.0) | 65.0 (28.3–83.5) | 54.5 (17.8–95.3) | 58.0 (40.0–88.0) |
| Haemoglobin reduced below LLN, n (%) | 130 (94) | 6 (100) | 26 (84) | 40 (91) | 4 (100) | 8 (89) |
| Serum creatinine (mg/dL), median (IQR) | 2.1a (1.3–3.7) | 3.1 (2.6–4.8) | 1.1 (0.9–1.4) | 2.5b (1.3–4.2) | 1.5 (0.9–4.2) | 5.0 (3.4–5.3) |
| Bilirubin (mg/dL), median (IQR) | 1.0c (0.6–2.2) | 1.4 (0.6–2.3) | 2. 1d (1.9–2.8) | 1.1e (0.7–2.2) | – | – |
Data presented from an = 137.
n = 43.
n = 104.
n = 21.
n = 32 adult patients. There were no cases of TTP or ‘other’ diagnoses in paediatric patients.
aHUS, atypical haemolytic uraemic syndrome; IQR, interquartile range; LLN, lower limit of normal; SD, standard deviation; STEC-HUS, Shiga toxin-producing Escherichia coli HUS; TTP, thrombotic thrombocytopenic purpura.
Table 2.
Demographic and clinical characteristics of adult (n = 219) patients with clinical suspicion of TMA: post hoc classification
| Characteristic | Primary aHUS (n = 51) | Trigger-associated aHUS (n = 98) | STEC-HUS (n = 6) | TTP (n = 31) | No TMA (n = 33) |
|---|---|---|---|---|---|
| Age at enrolment (years), mean (SD) | 57.9 (12.6) | 56.3 (17.5) | 55.8 (22.4) | 50.5 (16.7) | 58.3 (19.6) |
| Gender (female), n (%) | 27 (53) | 57 (58) | 4 (67) | 21 (68) | 11 (33) |
| Presenting organ manifestations, n (%) | |||||
| Renal | 43 (84) | 96 (98) | 6 (100) | 22 (71) | 30 (100) |
| Gastrointestinal | 30 (59) | 43 (44) | 5 (83) | 11 (35) | 16 (48) |
| Neurological | 22 (43) | 35 (36) | 3 (50) | 18 (58) | 8 (24) |
| Cardiovascular | 25 (49) | 25 (26) | 3 (50) | 7 (23) | 4 (12) |
| Pulmonary | 21 (41) | 25 (26) | 1 (17) | 4 (13) | 6 (18) |
| Other | 8 (16) | 44 (45) | 2 (33) | 10 (32) | 13 (39) |
| Platelets (×109/L), median (IQR) | 108.0 (71.0–136.0) | 74.0 (38.0–121.0) | 51.5 (34.8–99.0) | 25.0 (10.5–55.0) | 66.0 (22.0–87.0) |
| Haemoglobin reduced below LLN, n (%) | 49 (96) | 92 (94) | 6 (100) | 26 (84) | 29 (88) |
| Serum creatinine (mg/dL), median (IQR) | 1.67 (1.3–3.7) | 2.3 (1.7–3.7) | 3.1 (2.6–4.8) | 1.1 (0.9–1.4) | 2.10a (0.9–4.2) |
| Bilirubin (mg/dL), median (IQR) | 1.0b (0.6–4.3) | 1.0c (0.7–5.6) | 1.4 (0.6–2.3) | 2.1d(1.9–2.8) | 1.1e (0.7–2.2) |
Data presented from an = 23.
n = 35.
n = 78.
n = 21.
n = 23 adult patients.
aHUS, atypical haemolytic uraemic syndrome; IQR, interquartile range; LLN, lower limit of normal; SD, standard deviation; STEC-HUS, Shiga toxin-producing Escherichia coli HUS; TTP, thrombotic thrombocytopenic purpura.
Renal impairment was the most common organ manifestation across all groups. Patients frequently presented with one or more extrarenal organ involved. Gastrointestinal symptoms were the most prevalent extrarenal complication in patients with STEC-HUS (87%), patients with aHUS (51%) and patients with ‘other’ diagnoses (48%). However, 35% of TTP patients also developed gastrointestinal symptoms. Of interest, one paediatric and one adult patient with confirmed STEC-HUS did not present with gastrointestinal symptoms. Two of the four children diagnosed with aHUS presented with infectious diarrhoea (non-STEC), considered to be a triggering condition. Neurological symptoms were not only the most common extrarenal complication for patients with TTP (58%), but were also common in aHUS (39%) and STEC-HUS (50%). Pulmonary symptoms occurred less frequently than in other organ systems across each TMA entity, affecting 32% of patients with aHUS, 13% of patients with TTP and 7% of patients with STEC-HUS. Patients with TTP had a lower platelet count (median platelet count 25.0 × 109/L) than patients with STEC-HUS (58.0 × 109/L) or aHUS (97.0 × 109/L).
A total of 182 triggering conditions were reported by the treating physicians in 104 (73%) patients diagnosed with aHUS (Table 3). The most common triggering conditions reported were solid tumours (22%), use of calcineurin inhibitors (21%), prior solid-organ transplant (18%), airway infection (12%) and BMT (8%).
Table 3.
Triggering conditions identified among 142 patients diagnosed with aHUS (according to physician’s assessment)
| Triggering condition | n (%) |
|---|---|
| Solid tumour | 31 (22) |
| Calcineurin inhibitors | 30 (21) |
| Solid-organ transplant | 25 (18) |
| Airway infections | 17 (12) |
| Bone marrow transplantation | 12 (8) |
| Infection, other | 10 (7) |
| Malignant arterial hypertension | 7 (5) |
| Pregnancy | 7 (5) |
| Gemcitabine, mitomycin | 7 (5) |
| Organ rejection | 4 (3) |
| Vasculitides | 4 (3) |
| IgA nephropathy | 3 (2) |
| Infectious diarrhoea (other than STEC) | 3 (2) |
| Systemic lupus erythematosus | 3 (2) |
| Vaccination | 3 (2) |
| C3 glomerulopathy | 2 (1) |
| Hepatitis C or D | 2 (1) |
| Inflammatory bowel disease | 2 (1) |
| mTOR inhibitors | 2 (1) |
| VEGF inhibitors | 2 (1) |
| Radiotherapy | 2 (1) |
| Interferon | 1 (1) |
| Oral contraceptives | 1 (1) |
| Varicella-zoster virus | 1 (1) |
| BK virus | 1 (1) |
| HIV | 1 (1) |
| No triggering condition recorded | 38 (27) |
Patients may present with one or more triggering condition.
HIV, human immunodeficiency virus; IgA, immunoglobulin A; mTOR, mechanistic target of rapamycin; VEGF, vascular endothelial growth factor.
Differential diagnosis
aHUS was the most commonly diagnosed TMA entity (Supplementary data, Figure S1). The relative incidence of aHUS was 61% overall (n = 142): 63% (n = 138) for adult patients and 31% (n = 4) for paediatric patients. STEC-HUS was infrequently observed in adult patients [n = 6 (3%)] but accounted for 69% (n = 9) of paediatric patients in this cohort. Paediatric patients were only diagnosed with aHUS or STEC-HUS; there were no TTP or ‘other’ diagnoses recorded for this group. TTP was diagnosed in 14% (n = 31) of adults. Overall, 20% (n = 44) of adult patients were diagnosed as ‘other’ (Table 4). Septicaemia was the most common single ‘other’ diagnosis (n = 11). Eight patients were classified as ‘secondary TMA’ by the investigator, of which solid tumour (n = 2), drugs (n = 2) and infection (n = 2) were the most common causes.
Table 4.
All ‘other’ investigator-made diagnoses (N = 44)
| Other diagnoses | n (%) |
|---|---|
| Septicaemia/infection | 11 (25) |
| Secondary TMA | 8 (18) |
| Drugs | 2 (5) |
| Infection | 2 (5) |
| Solid tumour | 2 (5) |
| Malignant arterial hypertension | 1 (2) |
| Other | 1 (2) |
| Haematological disease/bone marrow failure | 4 (9) |
| Liver failure | 4 (9) |
| Unclear TMA | 3 (7) |
| Haemolysis resulting from a mechanical valve | 2 (5) |
| Idiopathic thrombocytopenic purpura | 2 (5) |
| Renal disease | 2 (5) |
| Allergy to dialysis filter | 1 (2) |
| Autoimmune haemolytic anaemia | 1 (2) |
| Haemolysis, elevated liver enzymes, low platelet count | 1 (2) |
| Intermittent leucopenia, anaemia and thrombocytopenia | 1 (2) |
| Malignant nephrosclerosis with Coombs-negative haemolytic anaemia and schistocytes | 1 (2) |
| Thromboembolic disease (secondary to dilated carotid sinus) | 1 (2) |
| Unclear haemolysis and thrombocytopenia | 1 (2) |
| Unclear pain syndrome | 1 (2) |
Patients in this table were classified by the investigator as ‘other’. Diagnosis was entered as free text.
The investigators diagnosed TTP only in patients with severe deficiency in ADAMTS13 activity (≤10%; Supplementary data, Figure S2). These patients had a median ADAMTS13 activity of 0.4% (IQR 0.3–1.1). Patients with STEC-HUS, aHUS and ‘other’ diagnoses had median ADAMTS13 activity levels >65%. A total of 25 (18%) patients with aHUS, 1 (7%) patient with STEC-HUS and 13 (30%) patients with an ‘other’ diagnosis had ADAMTS13 activity levels below the lower reference range boundary of 50%, but in each of these cases, ADAMTS13 activity was >10%.
Post hoc analysis
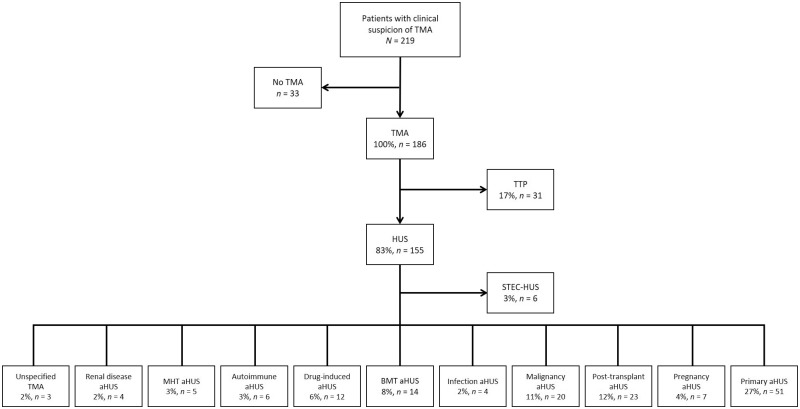
Results from the post hoc analysis, conducted in adult patients with TMA, are shown in Figure 1 and Supplementary data, Figure S3. Primary aHUS was the most common single diagnosis, with 27% of patients presenting with a TMA categorized to this group, followed by TTP (17%). Trigger-associated aHUS accounted for 53% of all diagnoses. Of these, post-transplant aHUS and solid tumour aHUS were the most common, with 12 and 11% of patients, respectively, categorized to these groups.
FIGURE 1.
Post hoc analysis of adult patients presenting with TMA according to the KDIGO classification [13]. Note that groups for MHT, renal disease and unspecified TMA have been added to this classification.
Prediction of TTP from routine laboratory parameters
All available variables were analysed for correlation with TTP diagnosis. Platelet count and serum creatinine concentration both significantly correlated with TTP diagnosis (P < 0.01) and were selected for the prediction algorithm. Using the Youden Index as a reference, threshold values were identified (platelet count 30 × 109/L, serum creatinine 1.8 mg/dL; Table 5). Using these values, a multivariate analysis (Table 6 and Supplementary data, Table S2) revealed that if platelet and serum creatinine levels were above threshold values, severe deficiency in ADAMTS13 activity (≤10%) was almost certainly not the cause of the TMA, with a negative predictive value of 98.1 and 92.3 for one or both values above threshold, respectively. Of note, the maximum platelet count recorded in TTP was 55 × 109/L.
Table 5.
Calculation of threshold platelet and serum creatinine values using the Youden Index
| Parameter | AUROC | Youden Index | Threshold |
|---|---|---|---|
| Platelet count (× 109/L) | 0.83 | 34.5 | 30.0 |
| Serum creatinine (mg/dL) | 0.79 | 1.82 | 1.8 |
AUROC, area under the receiver operator characteristic curve.
Table 6.
Multivariate analysis to predict a severe deficiency in ADAMTS13 activity (≤10%)
| Parameter | Adjusted odds ratio | 95% CI | P-value | Specificity | Sensitivity | Positive predictive value | Negative predictive value |
|---|---|---|---|---|---|---|---|
| Platelet count ≤30 × 109/L | 5.9 | 2.51–14.47 | <0.01 | 84.0 | 61.3 | 38.8 | 92.9 |
| Serum creatinine ≤1.8 mg/dL | 7.6 | 2.71–27.17 | <0.01 | 54.8 | 90.3 | 25.0 | 97.1 |
|
– | – | – | 90.8 | 54.8 | 50.0 | 92.3 |
|
– | – | – | 54.6 | 93.5 | 25.7 | 98.1 |
DISCUSSION
TMA is a rare medical condition with various causes. However, the relative incidence of TTP, STEC-HUS and aHUS remains unclear. This is the first study to prospectively evaluate the relative frequency of TTP, STEC-HUS and aHUS in Germany. We found that aHUS was the most common physician-made diagnosis in patients presenting with clinically suspected TMA defined by platelet consumption, MAHA and organ dysfunction, accounting for 61% of cases. In children, STEC-HUS was more common than aHUS, accounting for 69% of TMA cases, which is less frequent than in previous works, which report an 81–89% relative incidence of STEC-HUS within paediatric HUS diagnoses [24–26]. None of the children in our study were diagnosed with TTP, but this should be interpreted with caution due to the small cohort (n = 13).
Previous studies used the presence of diarrhoea as the discriminating factor between aHUS and STEC-HUS [24, 26]. However, it has become clear that diarrhoea is not unique to STEC-HUS, as 30–40% of aHUS patients also present with gastrointestinal symptoms [27–29]. Misclassification of aHUS patients as STEC-HUS due to the presence of diarrhoea could have previously led to an overestimation of STEC-HUS. A recent Japanese study of 258 paediatric patients with TMA, which did not rely on the presence of diarrhoea for a diagnosis, found a relative incidence of STEC-HUS of 64%, remarkably close to our result [30]. In our small paediatric sample, 100% of patients with aHUS and 89% of patients with STEC-HUS suffered gastrointestinal symptoms, highlighting the limitations of symptomatology in differential diagnosis. Furthermore, there are strong regional differences in STEC-HUS incidence [2]. Our paediatric patients all came from two large city-based university hospitals in northern Germany with catchment areas including large rural areas with cattle agriculture. Conversely, the low relative incidence of STEC-HUS of 3% in adult patients in our study was expected given that, excluding outbreaks, STEC-HUS is rare in adults [2].
In this study we found a strong overlap in the clinical presentation of patients with renal, neurological and gastrointestinal symptoms presenting at a high frequency in each of the three TMA entities. Thus clinical signs and symptoms of TMA are not sufficient in isolation to diagnose underlying disease. This reinforces the importance of additional tests such as STEC PCR and ADAMTS13 activity assays for correct diagnosis.
Classification of aHUS is currently a matter of debate and is continually evolving. Of note, during the course of this study, from inception (2013), recruitment (2014–17), analysis (2017) and even writing of this article (2018), various suggestions for terminology were made by different authors [1, 2, 13, 31, 32]. Over the course of the past 5 years or so there has been increasing consensus that, in the majority of patients, a combination of endogenous predisposition (genetic mutation, complement factor H autoantibodies, risk polymorphisms or other unknown or unidentified factors) and a triggering condition (e.g. autoimmune disease, transplant, pregnancy, infections, drugs, trauma, vaccinations and MHT) is required [15, 16, 31, 32]. Mutations in complement regulatory genes have been found in patients with MHT [33], pregnancy-related TMA [34], systemic lupus erythematosus [35], STEC-HUS [36] and kidney transplant–associated TMA [37]. Variants in complement regulatory genes were found in patients with BMT-associated TMA [38]. Conversely, only 60–70% of patients with aHUS have identified complement abnormalities [14, 15]. Recently it has been suggested that there is a continuum between ‘complement-mediated’ and ‘secondary’ aHUS [32].
During this study we did not predefine diagnostic criteria but left the diagnosis to the investigators’ clinical judgement after assessment of STEC and ADAMTS13-activity and consideration of clinical presentation, including evaluation of possible secondary causes. It is possible that based on the clinical presentation of patients, some physicians might have differing opinions on diagnosis; for example, some physicians may diagnose a patient presenting with MHT as ‘secondary TMA’, whereas others may classify the same patient as ‘aHUS with a triggering condition’.
After completion of data collection for our study, the KDIGO published the results from a controversies conference on aHUS in which they suggest a classification of TMAs based on disease triggers [13]. We adopted this classification in adults with physician-made diagnoses of aHUS and ‘other’ as a post hoc analysis for further objectivity and limitation of bias. Primary aHUS was the most common single diagnosis in this analysis, affecting 27% (n = 51) of patients considered to have a TMA (n = 186), followed by TTP [17% (n = 31)]. The majority of patients (53%) fell into the category of aHUS presenting with a triggering condition, the most common of which was post-transplant aHUS [11% (n = 23)]. The post hoc analysis sheds further light on how patients could have been diagnosed and classified based on presentation, and we would consider this classification more appropriate than the pre-KDIGO classification system used as our primary endpoint.
As noted in the KDIGO Controversies Conference on aHUS, ‘additional work is required to define the impact of complement risk factors in these subgroups’ [13]. When there is a robust consensus, further studies are warranted to clearly define the relative incidence of the various subtypes of TMAs. Until then this study reflects current clinical practice in Germany.
TTP is a syndrome of a severe deficiency in ADAMTS13 activity, with a level of <5–10% firmly establishing the diagnosis [1, 3, 13]. However, as the assay has a considerable turnaround time, previous studies have reported methods of using laboratory and clinical characteristics to predict a diagnosis of TTP [39, 40]. We confirmed that a platelet count ≤30 × 109/L and a serum creatinine ≤1.8 mg/dL correlate with the diagnosis of TTP in adults, in agreement with others [39, 40]. If both criteria are fulfilled, then the specificity for TTP is >90%. However, due to its rarity, the positive predictive value is still only 50%, limiting the utility to confirm TTP based on these criteria. Conversely, if one or both of these criteria are not fulfilled, severe deficiency in ADAMTS13 activity (<5–10%) is highly unlikely, with a negative predictive value of 92 and 98%, respectively. In any case, confirmation of diagnosis through ADAMTS13 testing is warranted.
Our study has several limitations. Paediatric patients were underrepresented (n = 13), limiting conclusions that can be drawn from this group. A Japanese study found a relative incidence of 6% for TTP [30], indicating that our sample may have been too small to expect any cases of paediatric TTP. Also, the underrepresentation of paediatric centres in our study could have led to underestimation of STEC-HUS. As the hallmark features of TMA—platelet consumption, MAHA and organ dysfunction—were inclusion criteria for this study, patients with incomplete presentations of TMA, such as aHUS with mild haematological changes [41], could have been overlooked. Finally, as 21/22 recruiting centres were nephrology departments, some degree of selection bias could have occurred. A higher proportion of haematology centres may have resulted in a higher proportion of diagnosed TTP cases. The influence of this imbalance is unknown. However, in contrast to other countries, in Germany most nephrology departments and some haematology departments offer plasmapheresis and thus manage TTP patients. Similarly, inclusion of obstetrics centres may have increased the number of patients with pregnancy-associated aHUS.
While this study was not designed to assess the absolute incidence of TMAs in relation to the general population, we specifically evaluated the relative incidence of TTP, STEC-HUS and aHUS within the context of patients presenting with TMA and present a post hoc classification of patients based on the KDIGO classification [13]. The relative incidence is relevant for treating physicians as it informs them of the pretest probability of the respective clinical entities when managing a patient. The post hoc classification system further shows how patients could potentially be classified, based on presentation, and may provide further information on how patients with aHUS are likely to present, that is, the majority present with concomitant triggering conditions, which could be useful in clinical practice.
We found that primary aHUS was the most common single diagnosis, most patients presented with renal impairment, clinical signs and symptoms are not useful in determining the underlying cause of TMA, triggering conditions are common in patients with aHUS and platelet count and serum creatinine profiles can aid in ruling out TTP.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank all the patients and their families for their contribution. They also thank Zrinjka Hardenberg from Alexion Pharma Germany for her dedicated project management. The following investigators also contributed patients to this study: Wolfram Jabs, Klinikum Berlin-Friedrichshain; Thorsten Feldkamp, Universitätsklinikum Kiel; Sylvia Stracke, Universitätsmedizin Greifswald; Horst Weihprecht, Klinikum Augsburg; Jun Oh, Universitätsklinikum Hamburg-Eppendorf; Angelika Köhler, Klinikum Sindelfingen; Arne Gäfgen, Klinikum Region Hannover; Stefan Büttner, Uniklinik Frankfurt; Mark Dominik Alscher, Robert-Bosch-Krankenhaus, Stuttgart; Jens Gaedeke, Charité Berlin; Adrian Schreiber, Campus Virchow Klinikum 1, Charité Berlin; Mathias Steinker, Klinikum Fulda.
Medical writing support (funded by Alexion Pharmaceuticals) was provided by Dr Ciaran Wright PhD of Bioscript, Macclesfield, UK.
Qualified academic investigators may request participant-level, de-identified clinical data and supporting documents (statistical analysis plan and protocol) pertaining to this study. Further details regarding data availability, instructions for requesting information and our data disclosure policy are available on the Alexion.com website (http://alexion.com/responsibility).
FUNDING
The CESAR study was supported by Alexion Pharma Germany.
AUTHORS’ CONTRIBUTIONS
All authors discussed and agreed on the content, which derived from previous posters presented at scientific congresses. The first draft of this article was developed by the medical writer under guidance from U.S. and M.J. and agreed on by the authors. Statistical analyses were undertaken by D.S. All authors reviewed and provided comprehensive feedback of all manuscript drafts and approved the final version for journal submission.
CONFLICT OF INTEREST STATEMENT
B.S. received consultancy fees from Alexion. C.S.H. received consultancy fees from Ablynx; consultancy fees, speaker fees, travel support and study fees from Alexion and consultancy fees, speaker fees and travel support from Swedish Orphan Biovitrum. D.S. received study fees from Alexion. L.P. received travel grants and consultancy fees from Alexion. M.J. is an employee and stockholder of Alexion. M.S. received consultancy and study documentation fees from Alexion. V.B. received travel grants from Alexion. U.S. received study fees, travel support and consultancy fees from Alexion and study fees and consultancy fees from Ablynx. N.B., R.D., A.M., S.M. and W.R. declare no conflicts of interest.
REFERENCES
- 1. Campistol JM, Arias M, Ariceta G. et al. An update for atypical haemolytic uraemic syndrome: diagnosis and treatment. A consensus document. Nefrologia 2015; 35: 421–447 [DOI] [PubMed] [Google Scholar]
- 2. Fakhouri F, Zuber J, Fremeaux-Bacchi V. et al. Haemolytic uraemic syndrome. Lancet 2017; 390: 681–696 [DOI] [PubMed] [Google Scholar]
- 3. Scully M, Hunt BJ, Benjamin S. et al. Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol 2012; 158: 323–335 [DOI] [PubMed] [Google Scholar]
- 4. Furlan M, Robles R, Solenthaler M. et al. Deficient activity of von Willebrand factor-cleaving protease in chronic relapsing thrombotic thrombocytopenic purpura. Blood 1997; 89: 3097–3103 [PubMed] [Google Scholar]
- 5. Furlan M, Lammle B.. Aetiology and pathogenesis of thrombotic thrombocytopenic purpura and haemolytic uraemic syndrome: the role of von Willebrand factor-cleaving protease. Best Pract Res Clin Haematol 2001; 14: 437–454 [DOI] [PubMed] [Google Scholar]
- 6. Furlan M, Robles R, Galbusera M. et al. Von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome. N Engl J Med 1998; 339: 1578–1584 [DOI] [PubMed] [Google Scholar]
- 7. Tsai HM, Lian EC.. Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura. N Engl J Med 1998; 339: 1585–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moake JL. Thrombotic microangiopathies. N Engl J Med 2002; 347: 589–600 [DOI] [PubMed] [Google Scholar]
- 9. Schwartz J, Padmanabhan A, Aqui N. et al. Guidelines on the use of therapeutic apheresis in clinical practice-evidence-based approach from the writing committee of the American society for apheresis: the seventh special issue. J Clin Apher 2016; 31: 149–162 [DOI] [PubMed] [Google Scholar]
- 10. Peyvandi F, Scully M, Kremer Hovinga JA. et al. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med 2016; 374: 511–522 [DOI] [PubMed] [Google Scholar]
- 11. Menne J, Nitschke M, Stingele R. et al. Validation of treatment strategies for enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome: case-control study. BMJ 2012; 345: e4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Freedman SB, Xie J, Neufeld MS. et al. Shiga toxin-producing Escherichia coli infection, antibiotics, and risk of developing hemolytic uremic syndrome: a meta-analysis. Clin Infect Dis 2016; 62: 1251–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goodship TH, Cook HT, Fakhouri F. et al. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 2017; 91: 539–551 [DOI] [PubMed] [Google Scholar]
- 14. Noris M, Caprioli J, Bresin E. et al. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol 2010; 5: 1844–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fremeaux-Bacchi V, Fakhouri F, Garnier A. et al. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol 2013; 8: 554–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riedl M, Fakhouri F, Le Quintrec M. et al. Spectrum of complement-mediated thrombotic microangiopathies: pathogenetic insights identifying novel treatment approaches. Semin Thromb Hemost 2014; 40: 444–464 [DOI] [PubMed] [Google Scholar]
- 17. Zuber J, Fakhouri F, Roumenina LT. et al. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol 2012; 8: 643–657 [DOI] [PubMed] [Google Scholar]
- 18. Mellmann A, Bielaszewska M, Zimmerhackl LB. et al. Enterohemorrhagic Escherichia coli in human infection: in vivo evolution of a bacterial pathogen. Clin Infect Dis 2005; 41: 785–792 [DOI] [PubMed] [Google Scholar]
- 19. Friedrich AW, Nierhoff KV, Bielaszewska M. et al. Phylogeny, clinical associations, and diagnostic utility of the pilin subunit gene (sfpA) of sorbitol-fermenting, enterohemorrhagic Escherichia coli O157:H−. J Clin Microbiol 2004; 42: 4697–4701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Osek J. Rapid and specific identification of Shiga toxin-producing Escherichia coli in faeces by multiplex PCR. Lett Appl Microbiol 2002; 34: 304–310 [DOI] [PubMed] [Google Scholar]
- 21. Bielaszewska M, Mellmann A, Bletz S. et al. Enterohemorrhagic Escherichia coli O26:H11/H−: a new virulent clone emerges in Europe. Clin Infect Dis 2013; 56: 1373–1381 [DOI] [PubMed] [Google Scholar]
- 22.Technoclone. Technozym ADAMTS-13 Activity Vienna, Austria: Technoclone Herstellung von Diagnostika und Arzneimitteln, 2016.
- 23. Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3: 32–35 [DOI] [PubMed] [Google Scholar]
- 24. Jenssen GR, Hovland E, Bjerre A. et al. Incidence and etiology of hemolytic-uremic syndrome in children in Norway, 1999–2008 a retrospective study of hospital records to assess the sensitivity of surveillance. BMC Infect Dis 2014; 14: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ardissino G, Salardi S, Colombo E. et al. Epidemiology of haemolytic uremic syndrome in children. Data from the North Italian HUS network. Eur J Pediatr 2016; 175: 465–473 [DOI] [PubMed] [Google Scholar]
- 26. Constantinescu AR, Bitzan M, Weiss LS. et al. Non-enteropathic hemolytic uremic syndrome: causes and short-term course. Am J Kidney Dis 2004; 43: 976–982 [DOI] [PubMed] [Google Scholar]
- 27. Langman C. Systemic multi-organ complications in atypical hemolytic uremic syndrome (aHUS): retrospective study in a medical practice setting. Haematologica 2012; 97(Suppl 1): 195–196 [Google Scholar]
- 28. Geerdink LM, Westra D, van Wijk JAE. et al. Atypical hemolytic uremic syndrome in children: complement mutations and clinical characteristics. Pediatr Nephrol 2012; 27: 1283–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schaefer F, Ardissino G, Ariceta G. et al. Clinical and genetic predictors of atypical hemolytic uremic syndrome phenotype and outcome. Kidney Int 2018; 94: 408–418 [DOI] [PubMed] [Google Scholar]
- 30. Ashida A, Matsumura H, Sawai T. et al. Clinical features in a series of 258 Japanese pediatric patients with thrombotic microangiopathy. Clin Exp Nephrol 2018; 22: 924–930 [DOI] [PubMed] [Google Scholar]
- 31. Knoop M, Haller H, Menne J.. Humangenetik beim atypischen hämolytisch-urämischen syndrom – rolle in diagnostik und therapie. Der Internist 2018; 59: 799–804 [DOI] [PubMed] [Google Scholar]
- 32. Asif A, Nayer A, Haas CS.. Atypical hemolytic uremic syndrome in the setting of complement-amplifying conditions: case reports and a review of the evidence for treatment with eculizumab. J Nephrol 2017; 30: 347–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Timmermans S, Abdul-Hamid MA, Vanderlocht J. et al. Patients with hypertension-associated thrombotic microangiopathy may present with complement abnormalities. Kidney Int 2017; 91: 1420–1425 [DOI] [PubMed] [Google Scholar]
- 34. Bruel A, Kavanagh D, Noris M. et al. Hemolytic uremic syndrome in pregnancy and postpartum. Clin J Am Soc Nephrol 2017; 12: 1237–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Park MH, Caselman N, Ulmer S. et al. Complement-mediated thrombotic microangiopathy associated with lupus nephritis. Blood Adv 2018; 2: 2090–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alberti M, Valoti E, Piras R. et al. Two patients with history of STEC-HUS, posttransplant recurrence and complement gene mutations. Am J Transplant 2013; 13: 2201–2206 [DOI] [PubMed] [Google Scholar]
- 37. Le Quintrec M, Lionet A, Kamar N. et al. Complement mutation-associated de novo thrombotic microangiopathy following kidney transplantation. Am J Transplant 2008; 8: 1694–1701 [DOI] [PubMed] [Google Scholar]
- 38. Jodele S, Zhang K, Zou F. et al. The genetic fingerprint of susceptibility for transplant-associated thrombotic microangiopathy. Blood 2016; 127: 989–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bendapudi PK, Hurwitz S, Fry A. et al. Derivation and external validation of the plasmic score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. Lancet Haematol 2017; 4: e157–e164 [DOI] [PubMed] [Google Scholar]
- 40. Coppo P, Schwarzinger M, Buffet M. et al. Predictive features of severe acquired ADAMTS13 deficiency in idiopathic thrombotic microangiopathies: the French TMA reference center experience. PLoS One 2010; 5: e10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sellier-Leclerc AL, Fremeaux-Bacchi V, Dragon-Durey MA. et al. Differential impact of complement mutations on clinical characteristics in atypical hemolytic uremic syndrome. J Am Soc Nephrol 2007; 18: 2392–2400 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.