Abstract
Background
Peritoneal dialysis (PD) is an underutilized modality for hospitalized patients with an urgent need to start renal replacement therapy in the USA. Most patients begin hemodialysis (HD) with a tunneled central venous catheter (CVC).
Methods
We examined the long-term burden of dialysis modality-related access procedures with urgent-start PD and urgent-start HD in a retrospective cohort of 73 adults. The number of access-related (mechanical and infection-related) procedures for each modality was compared in the first 30 days and cumulatively through the duration of follow-up.
Results
Fifty patients underwent CVC placement for HD and 23 patients underwent PD catheter placement for urgent-start dialysis. Patients were followed on average >1 year. The PD group was significantly younger, with less diabetes, with a higher pre-dialysis serum creatinine and more likely to have a planned dialysis access. The mean number of access-related procedures per patient in the two groups was not different at 30 days; however, when compared over the duration of follow-up, the number of access-related procedures was significantly higher in the HD group compared with the PD group (4.6 ± 3.9 versus 0.61 ± 0.84, P < 0.0001). This difference persisted when standardized to procedures per patient-month (0.37 ± 0.57 versus 0.081 ± 0.18, P = 0.019). Infection-related procedures were similar between groups. Findings were the same even after case-matching was performed for age and diabetes mellitus with 18 patients in each group.
Conclusions
Urgent-start PD results in fewer invasive access procedures compared with urgent-start HD long term, and should be considered for urgent-start dialysis.
Keywords: access-related procedures, end-stage renal disease, urgent-start hemodialysis, urgent-start peritoneal dialysis
INTRODUCTION
The last decade has seen the emergence of urgent-start peritoneal dialysis (PD) for late-referred end-stage renal disease (ESRD) patients in North America [1–6]. Urgent-start PD refers to the practice of initiating dialysis when required urgently but non-emergently before the traditional 2-week period after PD catheter insertion using low-volume, supine exchanges, primarily to avoid peri-catheter leak [2, 7]. Recent studies compared outcomes between urgent-start PD and hemodialysis (HD) cohorts and reported similar survival rates between the two. Patient survival at 1 year was reported as 92.1 and 93% for PD and HD groups, respectively, in one study [8], and 82.9 and 78.9%, respectively, in another [9]. Koch et al. [10] demonstrated that urgent-start HD patients had a higher half-year overall mortality rate than PD patients (42.1% versus 30%), but the difference did not reach significance (P = 0.191).
Utilization of PD as the initial dialysis modality allows for avoidance of central venous catheters (CVCs) and preservation of residual renal function and vascular access [11–13]. CVC use for dialysis access is associated with a higher risk of mortality, infection and hospitalizations compared with other types of dialysis access, including PD catheters [2, 14–16]. Yet, according to the US Renal Data System, 93% of patients who required dialysis in 2014 were initiated on HD, where the majority (80.3%) had a CVC as their initial access. This practice has changed minimally since 2005 [16].
Although the precise rate of CVC use in urgent-start HD is not known, incident use of acute dialysis catheters (non-tunneled) has been described to be ∼14% in Europe and 34% in the USA according to the Dialysis Outcomes and Practice Patterns Study [17]. In chronic HD, 40–60% of arteriovenous access required procedures for maturation or had primary non-function and up to 65% of fistulas required ongoing treatment to maintain patency [18]. In stable PD patients, only 5–13% of catheters have been reported to require revisions [19, 20]. Direct comparisons of procedures between the chronic dialysis modalities further substantiated higher rates of invasive procedures with HD compared with PD [20].
At our center, a teaching hospital where 20% of chronic dialysis patients receive PD in a hospital-based dialysis clinic, we introduced an urgent-start PD program in 2015. We arranged for <48-h catheter placement and secured the infrastructure [21] necessary for initiation of low-volume, supine PD as soon as the day of catheter placement as modeled on previous studies of urgent-start PD [1, 3, 6].
The purpose of this study was to measure the long-term procedure rates related to dialysis modality selection, comparing patients who started dialysis urgently on HD with tunneled CVCs and PD during the same period before and after case-matching for age and diabetes mellitus (DM). We examined the burden of mechanical procedures and infection-related procedures associated with each modality.
MATERIALS AND METHODS
Study design
In a single-center retrospective cohort of 73 adults at an academic teaching hospital, we examined the burden of invasive access procedures over a 3-year period between those initiating dialysis with urgent-start PD versus urgent-start HD. Institutional review board approval was obtained through Lifespan at Rhode Island Hospital.
Patients ≥18 years of age who underwent tunneled CVC placement or tunneled PD catheter placement for urgent-start dialysis initiation between 2015 and 2018 were included for comparison. Urgent-start dialysis was deemed necessary for those who either presented with late stage chronic kidney disease (CKD) or had unexpected acute decline in kidney function requiring dialysis urgently in HD patients without a mature vascular access, or in PD patients <2 weeks after PD catheter placement prior to complete healing of the PD catheter cuff. A total of 432 patients who underwent urgent-start HD with a tunneled CVC were screened for PD eligibility. All patients who started dialysis with a non-tunneled CVC eventually received a tunneled CVC. Patients were excluded if the tunneled CVC was placed for purposes other than dialysis, or if the patients were already receiving chronic dialysis prior to catheter placement. Those who had psychosocial, medical and/or surgical contraindications to urgent-start PD were also excluded. Based on the previous literature and our own experience, we reasoned that severe hyperkalemia refractory to medical management, hemodynamic and/or respiratory instability (pressor requirement, intubation or need for intensive care unit level of respiratory support), active abdominal pathology (recent abdominal surgery, colitis or colonic ischemia) and psychosocial barriers (unstable psychiatric illness, cognitive impairment or severe physical handicap) would preclude safe placement of a PD catheter and/or safe transition onto home PD (Figure 1).
FIGURE 1.
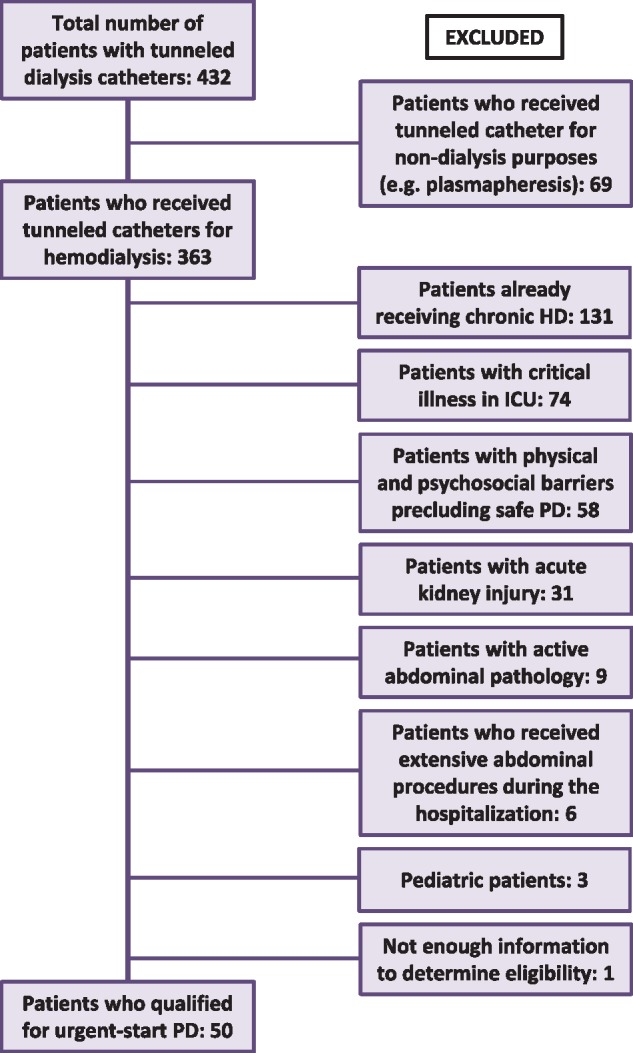
Flow chart of patients who received a tunneled dialysis catheter for HD and met inclusion or exclusion criteria for eligibility for PD. ICU, intensive care unit.
The cohort was additionally case-matched by age and DM resulting in each group having 18 patients available for comparison.
Baseline characteristics, including demographics (age, sex and race), laboratory data (potassium, bicarbonate, blood urea nitrogen, creatinine and albumin) and comorbid disease (coronary artery disease, congestive heart failure, hypertension, DM, active malignancy and obesity) were compared between the two cohorts.
Clinical outcomes
Outcomes were assessed after the placement of the PD catheter and included: (i) the number of access-related procedures at 30 days per patient; (ii) the cumulative number of access-related procedures over the duration of follow-up per patient; and (iii) the number of access-related procedures per patient-months. The first dialysis initiation procedure was excluded, and any ‘additional’ access-related (mechanical and infection-related) procedures were assessed.
For the HD cohort, access-related procedural burden was labeled ‘mechanical’ if there was catheter malfunction, stenosis of dialysis access or central veins, access bleeding, access recirculation or clotting requiring procedures including CVC removal or replacement, and also included additional procedures required for access [arteriovenous fistula/arteriovenous graft (AVF/AVG)] creation beyond the initial CVC and any complications related to maintaining AVF/AVG patency (such as angioplasty or thrombectomy). In the PD cohort, ‘mechanical’ procedures were related to primary nonfunction primarily due to catheter migration or entrapment. For both groups, ‘infection-related procedures’ included access-related procedure for bacteremia and peritonitis and for any infection (such as exit site or tunnel infection) requiring removal or revision of the catheter.
Statistical analysis
Baseline patient characteristics were compared using Chi-squared tests for categorical variables and Mann–Whitney test for continuous variables, and paired t-tests for analysis of the case-matched cohort. The average (and median) number of procedures per patient as well as the number of procedures per patient-months were compared using the Mann–Whitney test given the primarily non-normal distribution of the data.
RESULTS
Patient characteristics
A total of 363 patients received tunneled CVCs between 1 January 2014 and 1 January 2018. Fifty of these patients met inclusion criteria and were considered eligible for urgent-start PD (Figure 1). During the same period, 58 patients initiated PD and 23 of them received urgent-start PD. Therefore, a total of 73 patients were included in this retrospective study (50 HD and 23 PD patients). The mean (±SD) follow-up duration for the HD group and urgent-start PD group was 16.7 ± 11.1 months and 14.7 ± 10.3 months, respectively (P = 0.47). Compared with the HD group, the urgent-start PD group was significantly younger, with less heart failure and DM, with higher pre-dialysis serum creatinine and were more likely to have a planned dialysis access (but not mature or completely healed for use) prior to dialysis initiation (Table 1, All patients). There was no significant difference in baseline patient characteristics between groups after case-matching (Table 1 case-matched).
Table 1.
Baseline characteristics of urgent-start HD and PD patients
| Baseline characteristics | All |
Case-matched |
||||
|---|---|---|---|---|---|---|
| HD (n = 50) | PD (n = 23) | P-value | HD (n = 18) | PD (n = 18) | P-value | |
| Age (years) | 60 ± 16 | 47 ± 16 | 0.002 | 52 ± 14 | 52 ± 14 | 0.99 |
| Gender (male %) | 66 | 78 | 0.29 | 72 | 72 | 1.0 |
| Race (Caucasian %) | 38 | 52 | 0.26 | 33 | 55 | 0.18 |
| Follow-up duration (months) | 16.7 ± 11.1 | 14.7 ± 10.3 | 0.47 | 18.2 ± 11.8 | 15.1 ± 10.8 | 0.30 |
| Potassium (mEq/L) | 4.6 ± 0.7 | 4.3 ± 0.7 | 0.05 | 4.5 ± 0.7 | 4.3 ± 0.6 | 0.23 |
| HCO3 (mEq/L) | 19 ± 5 | 19 ± 5 | 0.92 | 19 ± 3 | 19 ± 4 | 0.70 |
| BUN (mg/dL) | 94 ± 40 | 110 ± 44 | 0.14 | 94 ± 43 | 104 ± 38 | 0.37 |
| Creatinine (mg/dL) | 8.1 ± 4.3 | 11.8 ± 5.7 | 0.0031 | 9.9 ± 3.3 | 9.7 ± 3.4 | 0.82 |
| Albumin (g/dL) | 3.3 ± 0.5 | 3.3 ± 0.6 | 0.41 | 3.2 ± 0.5 | 3.3 ± 0.6 | 0.68 |
| Coronary artery disease (%) | 38 | 17 | 0.08 | 33 | 22 | 0.46 |
| Congestive heart failure (%) | 44 | 48 | 0.76 | 33 | 50 | 0.31 |
| Hypertension (%) | 92 | 96 | 0.56 | 89 | 94 | 0.55 |
| DM (%) | 58 | 30 | 0.029 | 33 | 33 | 1.0 |
| Malignancy (%) | 12 | 4 | 0.30 | 6 | 6 | 1.0 |
| Obesity (BMI >30) (%) | 24 | 35 | 0.34 | 33 | 33 | 1.0 |
| Dialysis accessa (%) | 10 | 30 | 0.029 | 11 | 33 | 0.10 |
Dialysis access planned (but not mature or healed at the time of urgent-start dialysis).
Data are presented as mean ± SD or %.
BUN, blood urea nitrogen; BMI, body mass index.
Modality change from PD to HD occurred in 6 of the 23 (26%) PD patients, and modality change from HD to PD was observed in 3 of 50 (6%) HD patients. Reasons for modality switch varied and included inability to train, catheter malfunction, poor dialysis compliance and patient choice for the PD cohort, and primarily patient choice for the HD cohort.
Mechanical and infection-related procedures
The predominant HD mechanical procedures were required due to catheter malfunction, stenosis of dialysis access or central veins, access bleeding, access recirculation or clotting requiring procedures including CVC removal or replacement, AVF/AVG creation, angioplasty or thrombectomy. Mean number of days to AVF/AVG creation was 94 days (range −80 to 331 days) with respect to CVC placement. For the PD group, catheter malfunction due to primary non-function (catheter migration or entrapment) was the most common reason for a procedure, which were repositioned surgically. Peri-catheter leak occurred in only one patient with nephrotic syndrome. Infection-related procedures in the HD group were required primarily due to CVC-related bacteremia and in the PD group due to non-resolving tunnel infection. Peritonitis did not lead to PD catheter removal and bacteremia did not occur in the PD group.
Most HD patients (88%) required a procedure during the follow-up period compared with less than half (43%) of the PD group. In the HD group (n = 50), the number of access-related procedures (mean) due to mechanical and infection-related reasons did not significantly differ from the PD group (0.38 ± 0.73 versus 0.21 ± 0.42, P = 0.32) at 30 days (Table 2, All patients). However, the HD group received a significantly higher cumulative number of access-related procedures through the duration of follow-up (4.6 ± 3.9) when compared with the PD group (0.61 ± 0.84, P < 0.0001). The number of access-related procedures per patient-month in the HD group (0.37 ± 0.27) was significantly greater than in the PD group (0.081 ± 0.18, P = 0019).
Table 2.
Comparison of the mean number of access procedures performed per patient cumulatively over follow-up duration, and per patient-month between urgent start HD and PD patients (all and case-matched for age, sex and DM)
| All patients | HD (n = 50) | PD (n = 23) | P-value |
|---|---|---|---|
| Number of all access-related procedures, mean ± SD (median) | |||
| 30 days | 0.38 ± 0.73 (0) | 0.21 ± 0.42 (0) | 0.32 |
| Cumulative events | 4.6 ± 3.9 (4) | 0.61 ± 0.84 (0) | <0.0001 |
| Per patient-months | 0.37 ± 0.57 (0.23) | 0.081 ± 0.18 (0) | 0.019 |
| Mechanical procedures mean ± SD (median) | |||
| 30 days | 0.34 ± 0.63 (0) | 0.22 ± 0.42 (0) | 0.40 |
| Cumulative events | 4.4 ± 3.9 (3.5) | 0.52 ± 0.79 (0) | <0.0001 |
| Per patient-months | 0.30 ± 0.27 (0.21) | 0.078 ± 0.18 (0) | 0.0007 |
| Infection-related procedures mean ± SD (median) | |||
| 30 days | 0.040 ± 0.28 (0) | 0.0 ± 0.0 (0) | 0.32 |
| Cumulative events | 0.22 ± 0.68 (0) | 0.087 ± 0.42 (0) | 0.39 |
|
Per patient-months |
0.073 ± 0.38 (0) |
0.0028 ± 0.014 (0) |
0.38 |
|
Case-matched |
HD (n = 18) |
PD (n = 18) |
P-value |
| Number of all access-related procedures mean ± SD (median) | |||
| 30 days | 0.56 ± 0.86 (0) | 0.22 ± 0.43 (0) | 0.16 |
| Cumulative events | 5.7 ± 4.4 (5) | 0.61 ± 0.85 (0) | 0.0002 |
| Per patient-months | 0.39 ± 0.29 (0.29) | 0.094 ± 0.20 (0) | 0.0026 |
| Mechanical procedures mean ± SD (median) | |||
| 30 days | 0.56 ± 0.86 (0) | 0.22 ± 0.43 (0) | 0.16 |
| Cumulative events | 5.4 ± 4.4 (5) | 0.61 ± 0.85 (0) | 0.0004 |
| Per patient-months | 0.34 ± 0.26 (0.26) | 0.094 ± 0.20 (0) | 0.0096 |
| Infection-related procedures mean ± SD (median) | |||
| 30 days | 0.0 ± 0.0 (0) | 0.0 ± 0.0 (0) | – |
| Cumulative events | 0.33 ± 0.97 (0) | 0.0 ± 0.0 (0) | 0.16 |
| Per patient-months | 0.073 ± 0.17 (0) | 0.0 ± 0.0 (0) | 0.28 |
Even when accounting for additional procedures needed for modality change, the HD group still required more procedures than the PD group cumulatively (HD: 4.7 ± 3.8 versus PD: 1.4 ± 1.5, P < 0.0001) and per patient-month (HD: 0.42 ± 0.60 versus PD: 0.21 ± 0.33, P = 0.0032).
Mechanical procedures
At 30 days, the HD and PD groups did not require a significantly different number of mechanical procedures (HD: 0.34 ± 0.63 versus PD: 0.22 ± 0.42, P = 0.40) (Table 2, All patients). The cumulative incidence of mechanical procedures through the follow-up duration was 4.4 ± 3.9 with HD versus 0.52 ± 0.79 with PD (P < 0.0001). The mechanical procedure burden per patient-month (mean) was also significantly higher in the HD group (0.30 ± 0.27) compared with the PD group (0.078 ± 0.18, P = 0.0007).
Infection-related procedures
Infection-related procedures were not significantly different between groups: 26% with peritonitis or exit site infection in the PD group compared with 12% with bacteremia (exit site infection data not available) in the HD group (P = 0.14). However, only one of the infectious complications (refractory tunnel infection) resulted in a procedure for the PD group. The mean procedure requirement was 0.0 ± 0.0 in the PD group compared with 0.040 ± 0.28 in the HD group in the first 30-day period post catheter placement (P = 0.32) as shown in Table 2, All patients.
Similarly, the cumulative incidence of infection-related procedures over the follow-up duration was not significantly different between the HD and PD groups either (0.22 ± 0.68 versus 0.087 ± 0.42, respectively, P = 0.39). Although the PD group had fewer infection-related procedures per patient-month (0.0028 ± 0.014) when compared with the HD group (0.073 ± 0.38), this was not statistically different (P = 0.38).
Case-matched urgent-start outcomes
Outcomes comparing the case-matched HD and PD groups were similar (Table 2, Case-matched). By 30 days after catheter placement, the number of access-related procedures was slightly greater in the HD group but not statistically different between groups. However, the cumulative incidence of mechanical procedures through the follow-up duration was nine times greater for the HD group compared with the PD group. The number of access-related procedures per patient-month in the HD group was also significantly (4-fold) greater than in the PD group. There was no difference between groups for infection-related procedures.
Timing of access-related procedures after dialysis initiation
Access-related procedures in the PD group occurred soon after catheter placement, where 35% of procedures were performed within 30 days and 50% within 45 days. In contrast, only 6% of access-related procedures took place within 30 days of access placement in the HD group. Sixty-six percent of procedures occurred later for the HD group, by 1 year after dialysis initiation.
PD education prior to dialysis initiation
In the HD cohort (n = 50), we found documentation of dialysis modality discussion including PD in only 16% of patient’s chart prior to the placement of CVC for initiation of dialysis. All patients in the PD group received both HD and PD education as documented in the medical record.
DISCUSSION
In the USA, PD continues to be an underutilized dialysis modality for late-referred patients with ESRD [2]. While other studies have demonstrated the feasibility of PD for urgent-start dialysis in ESRD and have shown similar mortality and complication rates to HD, ours is the first study to compare the number of modality-related invasive access procedures between HD and PD long term.
We found a significantly reduced number of procedures associated with PD compared with HD in the urgent-start dialysis population long term. Our cohort had a mean follow-up duration extending beyond 1 year and as long as 2 years for some patients, reflecting complications that were occurring beyond the immediate dialysis initiation period. Other publications on urgent-start dialysis have focused more on short-term complications [8].
In our urgent-start dialysis cohort, the majority of HD patients (88%) required an access procedure, a number which far exceeded the PD group (43%), similar to the findings in chronic dialysis populations (32% HD versus 4.6% PD) but at much higher rates [20]. Furthermore, the overall median number of procedures per patient was four for the HD group compared with none in the PD group, not including the initial access procedure. Mechanical procedures rather than infection-related procedures primarily accounted for this difference.
HD patients typically required an access revision or replacement in addition to the usual requirement of two procedures, an arteriovenous access creation and removal of the initial tunneled CVC. Even if we were to disregard these two ‘requisite’ procedures for the HD patients, the HD patients still on average needed at least two more procedures than the PD patients. Our findings are consistent with published chronic dialysis data by Oliver et al. [20], who found that HD patients required 2.3 procedures per patient-year when compared with 1.9 procedures per patient-year for PD patients (P = 0.04).
The PD cohort was younger, with lower prevalence of DM, and more likely to have a planned dialysis access than the HD cohort, which likely reflects physician selection bias. This may explain the lower number of invasive procedures in the PD patients. Diabetes-related peripheral vascular disease has been associated with difficult arteriovenous fistula creation [22]. However, upon case-matching for age and comorbidity of DM (Table 1, Case-matched), the procedure burden remained high in the HD group compared with the PD group (Table 2, Case-matched).
Although the infection rates did not differ between the two groups, no PD patients developed bloodstream infections. The number of procedures resulting from infections was higher in the HD group, though not statistically significant, possibly due to small event numbers. In the HD group, infection-related CVC removal was all due to bacteremia, which is well known to be associated with increased mortality [14, 23]. Similar to our findings, Jin et al. [8] and Koch et al. [10] reported higher rates of bacteremia in urgent-start HD cohorts when compared with PD cohorts, but they did not find a significant difference in the infection rates between the two.
Access procedures occurred soon after catheter placement for the PD patients but tended to take place later for HD. Half of the procedures occurred within 45 days of catheter placement for the PD group. For the HD group, procedure requirement occurred later in the first year (80%). This may explain the higher rate of modality change from PD to HD. Initial frustration related to primary non-function could lead to early abandonment of PD. Most HD patients had no access issues early on, but often had repeated need for procedures even after arteriovenous maturation. This delay in complications may allow for greater retention of patients in the HD modality in contrast to the PD patients.
Most hospitalized patients were referred for tunneled CVCs for HD, and we found little evidence in the medical record of dialysis options discussion by the inpatient nephrologist. Meanwhile, the patients experienced a significant number of procedures in the ensuing months, including exchange of non-functional tunneled CVCs, creation of new arteriovenous fistulas/grafts, revision of fistulas/grafts and removal of tunneled CVCs when they were no longer required. These procedures create extra costs for the healthcare system [24, 25]. In 2014, Liu et al. [25] compared dialysis-related costs during the first 90 days between urgent-start PD and urgent-start HD patients in the USA. The investigators showed that costs were significantly lower for urgent-start PD ($16 398) compared with urgent-start HD ($19 352). Cost difference stemmed from the extra expense of HD-related vascular access.
Lack of dialysis modality education is considered one of the potential causes for underutilization of PD [26]. A survey found that patients with ESRD did not receive uniform education on dialysis modalities—69.7% of ESRD patients received in-center HD education, but fewer patients (58.2%) were informed about PD [27]. Another study surveyed patients with CKD Stages 3–5 and found that >50% of patients had no knowledge of PD [28]. In our study, we sought to determine if HD patients received modality education before their unplanned dialysis start by seeking such documentation in medical records. Among the urgent-start HD patients, only 16% of patient’s medical record revealed discussion of PD prior to placement of a CVC for dialysis. Although we cannot with certainty conclude that the absence of medical record documentation equates to the absence of dialysis options dialog, the very low number of documented PD discussions in our cohort suggests that dialysis modality education remains inadequate, particularly for hospitalized patients.
Limitations of our data include the retrospective nature of this cohort study at a single center with a relatively small sample size. Even with case-matching, the HD cohort was more racially diverse and had less evidence of access planning (though neither statistically different), which may reflect lower socioeconomic status or less access to healthcare resources contributing to worse outcomes. All PD patients were followed regularly in our PD clinic and dialysis center, and thus we were able to record with certainty all the events. On the other hand, after the initial hospitalization for tunneled CVC placement and dialysis initiation, HD patients were referred to one of several outpatient dialysis centers in the community, and we were not able to record all subsequent complications and procedures. It is possible therefore that events were missed in the HD cohort, which would have resulted in underreporting of complications and procedures over the duration of follow-up.
Despite this, our study showed that urgent-start PD was associated with fewer long-term mechanical procedures and all access-related procedures than HD, and that PD did not significantly differ from HD in number or rate of infection-related procedures. Greater consideration should be made for urgent-start PD in the late-referred ESRD patient.
ACKNOWLEDGEMENTS
We appreciate Dr J. Gary Abuelo for his editorial review. The study protocol has been approved by Lifespan, the research institute’s committee on human research.
FUNDING
No funding disclosures.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Alkatheeri AM, Blake PG, Gray D. et al. Success of urgent-start peritoneal dialysis in a large Canadian renal program. Perit Dial Int 2016; 36: 171–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arramreddy R, Zheng S, Saxena AB. et al. Urgent-start peritoneal dialysis: a chance for a new beginning. Am J Kidney Dis 2014; 63: 390–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Casaretto A, Rosario R, Kotzker WR. et al. Urgent-start peritoneal dialysis: report from a U.S. private nephrology practice. Adv Perit Dial 2012; 28: 102–105 [PubMed] [Google Scholar]
- 4. Ghaffari A, Kalantar-Zadeh K, Lee J. et al. PD first: peritoneal dialysis as the default transition to dialysis therapy. Semin Dial 2013; 26: 706–713 [DOI] [PubMed] [Google Scholar]
- 5. Povlsen JV, Sorensen AB, Ivarsen P.. Unplanned start on peritoneal dialysis right after PD catheter implantation for older people with end-stage renal disease. Perit Dial Int 2015; 35: 622–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Masseur A, Guest S, Kumar V.. Early technique success after initiation of treatment with urgent-start peritoneal dialysis. Adv Perit Dial 2014; 30: 36–39 [PubMed] [Google Scholar]
- 7. Ponce D, Brabo AM, Balbi AL.. Urgent start peritoneal dialysis. Curr Opin Nephrol Hypertens 2018; 27: 478–486 [DOI] [PubMed] [Google Scholar]
- 8. Jin H, Fang W, Zhu M. et al. Urgent-start peritoneal dialysis and hemodialysis in ESRD patients: complications and outcomes. PLoS ONE 2016; 11: e0166181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lobbedez T, Lecouf A, Ficheux M. et al. Is rapid initiation of peritoneal dialysis feasible in unplanned dialysis patients? A single-centre experience. Nephrol Dial Transplant 2008; 23: 3290–3294 [DOI] [PubMed] [Google Scholar]
- 10. Koch M, Kohnle M, Trapp R. et al. Comparable outcome of acute unplanned peritoneal dialysis and haemodialysis. Nephrol Dial Transplant 2012; 27: 375–380 [DOI] [PubMed] [Google Scholar]
- 11. Abdel-Aal AK, Dybbro P, Hathaway P. et al. Best practices consensus protocol for peritoneal dialysis catheter placement by interventional radiologists. Perit Dial Int 2014; 34: 481–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jansen MA, Hart AA, Korevaar JC. et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int 2002; 62: 1046–1053 [DOI] [PubMed] [Google Scholar]
- 13. Moist LM, Port FK, Orzol SM. et al. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol 2000; 11: 556–564 [DOI] [PubMed] [Google Scholar]
- 14. Ravani P, Palmer SC, Oliver MJ. et al. Associations between hemodialysis access type and clinical outcomes: a systematic review. J Am Soc Nephrol 2013; 24: 465–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coentrao L, Santos-Araujo C, Dias C. et al. Effects of starting hemodialysis with an arteriovenous fistula or central venous catheter compared with peritoneal dialysis: a retrospective cohort study. BMC Nephrol 2012; 13: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saran R, Li Y, Robinson B. et al. US Renal Data System 2015Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2016; 67: A7–A8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oliver MJ. Acute dialysis catheters. Semin Dial 2001; 14: 432–435 [DOI] [PubMed] [Google Scholar]
- 18. Falk A. Maintenance and salvage of arteriovenous fistulas. J Vasc Interv Radiol 2006; 17: 807–813 [DOI] [PubMed] [Google Scholar]
- 19. Haskins IN, Schreiber M, Prabhu AS. et al. Peritoneal dialysis catheter placement as a mode of renal replacement therapy: long-term results from a tertiary academic institution. Surgery 2017; 162: 1112–1120 [DOI] [PubMed] [Google Scholar]
- 20. Oliver MJ, Verrelli M, Zacharias JM. et al. Choosing peritoneal dialysis reduces the risk of invasive access interventions. Nephrol Dial Transplant 2012; 27: 810–816 [DOI] [PubMed] [Google Scholar]
- 21. Ghaffari A, Kumar V, Guest S.. Infrastructure requirements for an urgent-start peritoneal dialysis program. Perit Dial Int 2013; 33: 611–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Beks PJ, Mackaay AJ, de Neeling JN. et al. Peripheral arterial disease in relation to glycaemic level in an elderly Caucasian population: the Hoorn study. Diabetologia 1995; 38: 86–96 [DOI] [PubMed] [Google Scholar]
- 23. Hung AM, Alp Ikizler T.. Hemodialysis central venous catheters as a source of inflammation and its implications. Semin Dial 2008; 21: 401–404 [DOI] [PubMed] [Google Scholar]
- 24. Coentrao LA, Araujo CS, Ribeiro CA. et al. Cost analysis of hemodialysis and peritoneal dialysis access in incident dialysis patients. Perit Dial Int 2013; 33: 662–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu FX, Ghaffari A, Dhatt H. et al. Economic evaluation of urgent-start peritoneal dialysis versus urgent-start hemodialysis in the United States. Medicine (Baltimore) 2014; 93: e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chaudhary K, Sangha H, Khanna R.. Peritoneal dialysis first: rationale. Clin J Am Soc Nephrol 2011; 6: 447–456 [DOI] [PubMed] [Google Scholar]
- 27. Fadem SZ, Walker DR, Abbott G. et al. Satisfaction with renal replacement therapy and education: the American Association of Kidney Patients survey. Clin J Am Soc Nephrol 2011; 6: 605–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Finkelstein FO, Story K, Firanek C. et al. Perceived knowledge among patients cared for by nephrologists about chronic kidney disease and end-stage renal disease therapies. Kidney Int 2008; 74: 1178–1184 [DOI] [PubMed] [Google Scholar]


