Abstract
Biomaterials regulate macrophages and promote regeneration function, which is a new hot pot in tissue engineering and regenerative medicine. The research based on macrophage materials biology has appeared happy future, but related research on regulating macrophages and promoting tissue regeneration is still in its infancy. The surface roughness of biomaterials is one of the important factors affecting macrophage behavior. Previous study also found that the surface roughness of many biomaterials regulating macrophage polarization, but not including mineralized collagen (MC). In this study, we designed and fabricated MC with different roughness and investigated the influence of MC with different roughness on macrophages. In the study, we found that on the rough surface of MC, macrophages exhibited M1 phenotype-amoeboid morphology and high-level secretory of inflammatory factor (tumor necrosis factor-α and interleukin-6), while smoother surface exhibited M2 phenotype. These data will be beneficial to understand the mechanism deeply and enrich biomaterials tissue regeneration theory, provide a new train of thought biomaterials inducing tissue regeneration and repair and guide the optimum design of new biomaterials, development and reasonable applications.
Keywords: inflammatory response, mineralized collagen, roughness, macrophage, polarization
Introduction
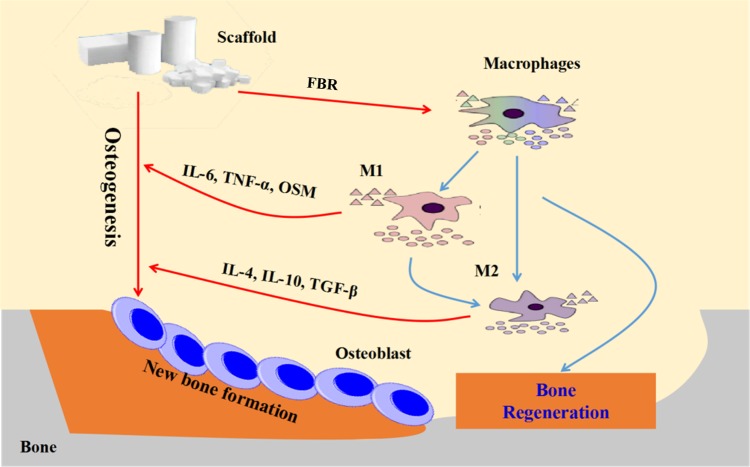
The immune system plays an important role in the success of tissue engineering strategies. In the recent, more and more experts and scholars have become increasingly recognized the importance of local environment surrounding regenerating tissue, and turned much attention to how the immune system can be manipulated to achieve the purpose of implanted scaffolds, especially in inhibiting foreign body rejection and strengthening the binding of scaffolds to natural tissue [1]. When implanted into human body, all biomaterials may trigger a series of host responses. Macrophages are the main cell types in acute and chronic inflammation, as well as the following wound healing or fibrosis reactions [2]. Under different conditions, macrophages can be differently polarized, exhibit M1-(proinflammation) and M2-(tissue repair) phenotypes and secrete different cytokines and small molecules [3]. The schematic diagram of macrophages interactions in bone regeneration was shown in Fig. 1. It is known that the transition and balance between M1- and M2-phenotype are essential for tissue repair. The disorder of macrophage polarization and failure to restore M1 and M2 phenotypes to a normal balance may lead to the formation of foreign body giant cells, which is related to the failure of implant materials [4]. After implantation, a series of events caused by the interaction between immune cells and biomaterials surface properties determine the biomaterials fate [5–7].
Figure 1.
Schematic diagram of macrophages interactions in bone regeneration
Previous study also found that the influence of the surface roughness of biomaterial implantations on bone resorption. Rae and his colleagues [8] found that PTFE, epoxy resin and HDP materials with rough surfaces to which macrophages adheration had been shown to influence the amount of more bone resorption that they stimulated than did those with a smooth surface (PMMA). Olivares-Navarrete and his colleagues [9] found that the activation of inflammatory macrophages (M1-like) induced by smooth titanium and the activation of macrophages induced by hydrophilic rough titanium were similar to those induced by anti-inflammatory M2-like state. But till now, no literature was reported about different surface roughness of mineralized collagen (MC)-regulated macrophage polarization to influence bone regeneration.
MC is the basic building block of hierarchically organized structures of natural bone [10]. MC was prepared in vitro biomimetic mineralization and had been shown definite advantage in degradation rapid, stiffness and promoting osteogenic differentiation of human mesenchymal stem cells [11, 12]. Cui and his colleagues [13] had shown that the MC group was more likely to induce macrophage differentiation into M2 than the HA group. Whether the surface topography of MC will affect the polarization and function of macrophages has not been reported.
In this study, we induced THP-1 cells with PMA into macrophages and examined the morphology and cytokine secretion of macrophages cultured on MC with different surface roughness.
Materials and methods
Preparation and characterization of MC with different surface roughness
Preparation of MC with different surface roughness
The MC membrane was produced by Beijing Allgens Medical Science and Technology Co., Ltd. MC membrane with different surface roughness was prepared by following two main steps.
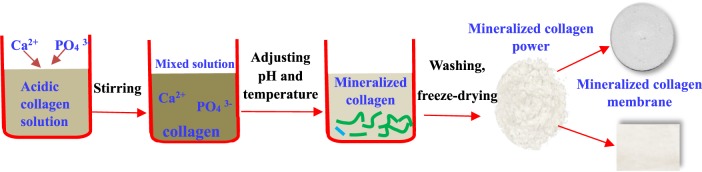
In the first step, the biomimetic MC was prepared via an in vitro biomimetic mineralization process [14]. Water solutions containing Ca2+ or were added into an acidic collagen solution, respectively; then the pH value and temperature of the liquid mixture were adjusted to form MC deposition. This step was similar to the biomineralization process of the natural bone. During such process, the nucleation and growth of HA crystals were directed by the collagen fibrils. The deposition was washed three times, and then freeze-dried to obtain porous MC with different surface roughness according to the amount of water used for washing.
In the second step, after thorough freeze-drying, the MC membrane was finally molded by rolling. The final MC membrane of different surface roughness was sterilized by 60Co radiation. They were divided into four groups: MC-A, MC-B, MC-C and MC-D. The MC used in this study was hierarchical self-assembled nano-hydroxyapatite/collagen composites, which was synthesized via biomimetic mineralization process in vitro as previous reported (as shown in Fig. 2).
Figure 2.
The product process of MC membrane
Characterization of MC membrane with different surface roughness
The surface roughness was examined using a field emission scanning electron microscope (FESEM; SU8000) at 6 kV and a China Roughness Measuring Instrument (Model 2206).
Cell culture on MC membrane with different surface roughness
THP-1 cell line was presented by Liaocheng People’s Hospital Oral Biomaterial Laboratory and cultured in 1640 RPMI (Gibco by Life Technologies) supplemented with 10% fetal bovine serum (Gibco), penicillin (100 μg/ml) and streptomycin (100 μg/ml) (Sangon Biotech) at 37°C in a humidified atmosphere of 5% CO2. Cells were scraped, counted and diluted to 1 × 105 cells/ml when they achieved ∼80–90% confluence. Before cultured on the materials, 100 ng/ml PMA (Sigma) was used to induce them into macrophages. As CD14 is a macrophage surface marker, we used CD14 antibody (APC anti-human CD14, Biolegend) to detect whether it successfully differentiated into macrophages. Then macrophages were cultured on the surface of different MC membrane. The MC membrane was placed in 24-well plate, incubated in phosphate-buffered saline (PBS; Sangon Biotech) medium for each 4 h prior to macrophage seeding. 1 ml cell suspension was added on each substrate.
Morphology and micromorphology of macrophage cultured on different MC membrane
The 1 × 106 monocytes were seeded on the surface of each well, cultured for 1 and 3 days, respectively, and the macrophages were rinsed with PBS and fixed with 2.5% glutaraldehyde (Sangon Biotech) aqueous solution for 20 min. After rinsing for three times, they were dehydrated by 30, 50, 70, 90 and 100% gradient ethanol (Sangon Biotech) solution, each concentration was 10 min ×2 times, then placed in a freeze dryer, lyophilized 1 h and slowly sealed back to temperature. Then gold (platinum) was sprayed on the surface of the sample for 5 min using an ion sputtering apparatus to improve surface conductivity. After the sample preparation was completed, using a FESEM (SU8000) to observe the population morphology at a low magnification, and the individual morphology at a high magnification.
Acridine orange/propidium iodide double staining morphological analysis
Approximately 2 × 105 cells/ml of macrophages were seeded on the surface of MC membrane and incubated for 24 h at 37°C in a humidified, 5% CO2 atmosphere. After the cells were incubated, the cells were washed with PBS. The cell suspension (10 μl) was placed on the glass slide and mixed with 10h μl of acridine orange (AO; 50 μg/ml) and propidium iodide (PI; 50 μg/ml). The cells were observed under a fluorescence microscope (Leica, Germany).
Cytokine determinations
M1-cytokine (tumor necrosis factor-α [TNF-α] and interleukin [IL]-6) and M2-cytokine (IL-10 and IL-4) levels were measured with sandwich enzyme-linked immunosorbent assays as prescribed by the manufacturer (Abcam).
Statistical analysis
All the analyses were performed using the software SPSS 22.0 (IBM SPSS, Armonk, NY, USA). All the data were expressed as means ± SD and analyzed using one-way ANOVA followed by least significant difference post hoc test. A level of significance was set at P < 0.05.
Results
Characterization of different MC membrane
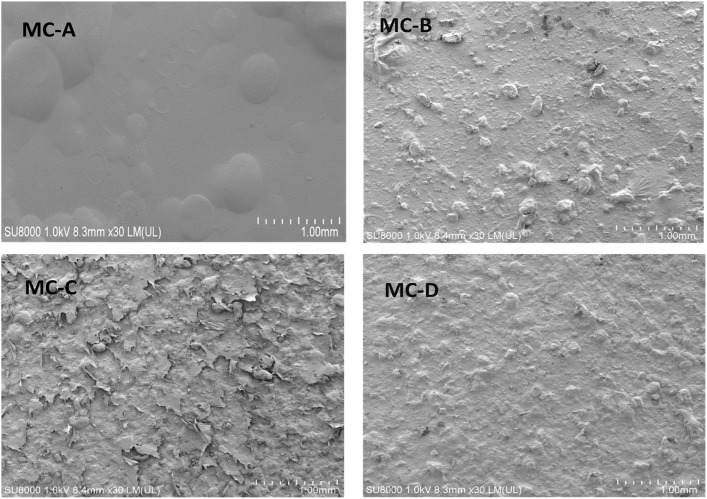
The Ra, average roughness and value of the replica surfaces of MC membrane were shown in Table 1. The surface roughness of different MC membrane had been extensively characterized. FESEM micrographs of the samples were shown in Fig. 3.
Table 1.
The difference roughness of different materials
| Materials | MC-A | MC-B | MC-C | MC-D |
|---|---|---|---|---|
| Roughness (µm) | 0.92 ± 0.05 | 6.41 ± 0.15 | 8.31 ± 0.21 | 12 ± 0.36 |
Figure 3.
FESEM micrographs of MC-A, MC-B, MC-C and MC-D
Identification of macrophages
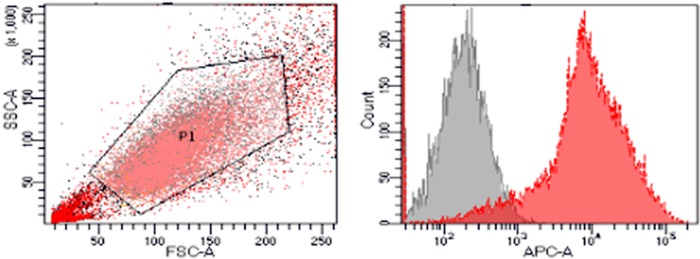
Due to the high expression of CD14 on the surface of macrophages, CD14 was selected as a macrophage surface marker and its purity was detected by flow cytometry. The results of flow cytometry were shown in Fig. 4. The purity of macrophages was >97%.
Figure 4.
Flow cytometry detection of macrophage surface marker CD14
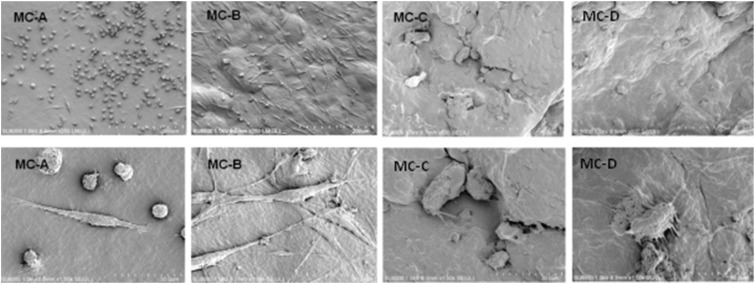
Population and individual morphology of macrophages grown on different MC membrane
The results of the number and morphology of macrophages on different materials after cultured on the surface of each group for 1 day were shown in Fig. 5. From Fig. 5, we found that the macrophages displayed dramatically different shapes and quantities on the different surface roughness of MC membrane. When compared with MC-C and MC-D groups, there were more macropahges on MC-A and MC-B groups. Most macrophages on the MC-A group had a round shape and MC-B groups exhibited fusiform or spindle shape, while the macrophages on groups MC-C and MC-D protruded many protrusions.
Figure 5.
The population and individual morphology of macrophages cultured on different groups of 1 day. The upper row showed the population morphology and the lower row showed the individual morphology
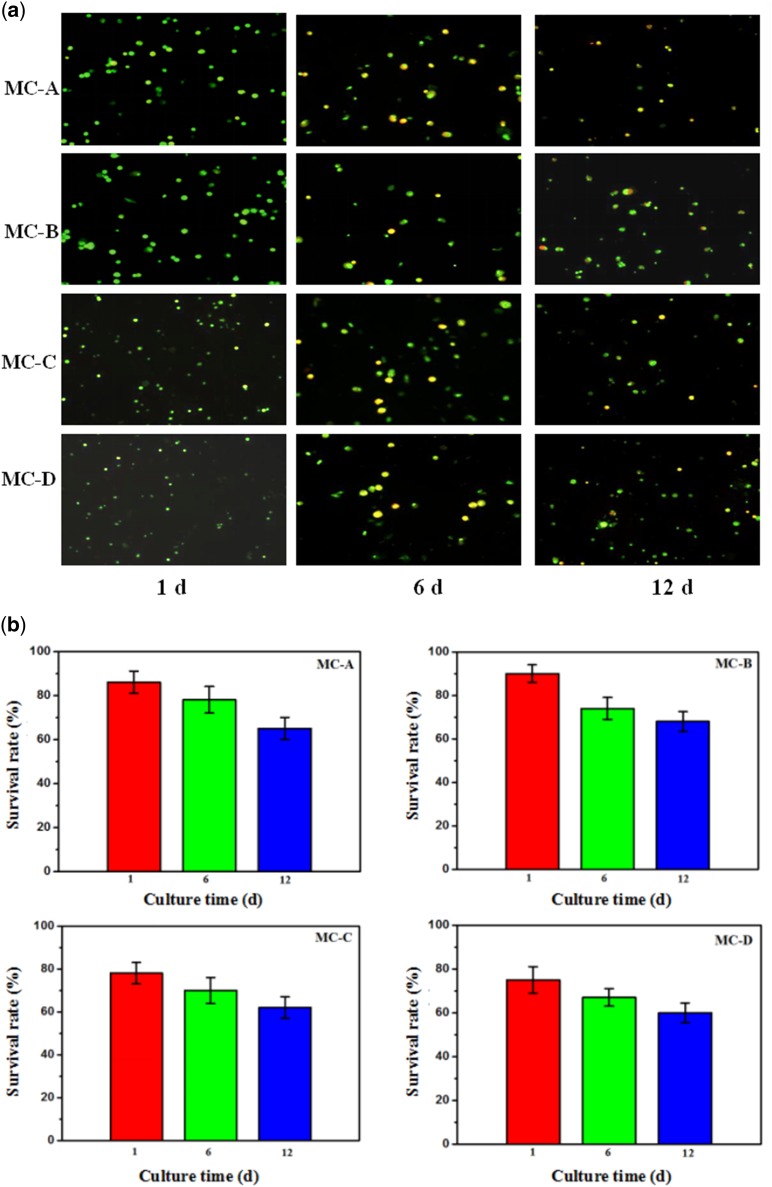
Cell activity cultured on different MC membrane in vitro
When cells were stained with AO/PI, the dead cells produced a classical orange to red fluorescence while live cells showed a green glow, the results were shown in Fig. 6a. These results indicated that the survival rates of MC-C and MC-D groups were lower than MC-A and MC-B groups after 1 day; however, there was no significant difference between the four groups after 12 days. From Fig. 6b, the results showed the survival rate of cells can still remain above 60%.
Figure 6.
(a) The AO/PI staining results about cell activity in vitro, (b) cell survival rates under fluorescence microscope (no significant difference among the four groups after 12 days, P < 0.01)
Cytokine release
The release of cytokines was influenced by the surface roughness of MC membrane on which macrophages were cultured.
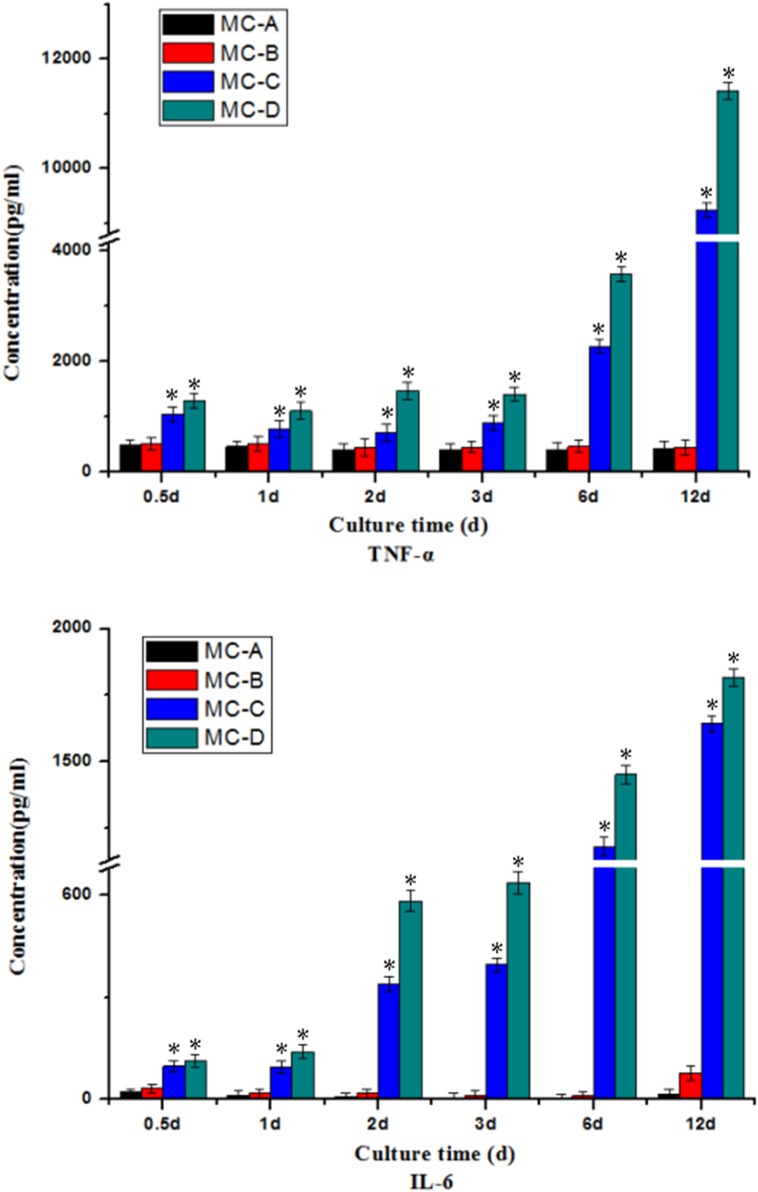
Secretion of M1-cytokines
The secretion level of proinflammation cytokines was different in each group with the prolongation of time (as shown in Fig. 7). After 3 days, the secretion of TNF-α and IL-6 by macrophages cultured on the MC-C and MC-D groups increased significantly compared with MC-A and MC-B.
Figure 7.
Secretion of M1-(proinflammation) cytokines: TNF-α and IL-6. *Significant difference (P < 0.05) compared with the MC-A group
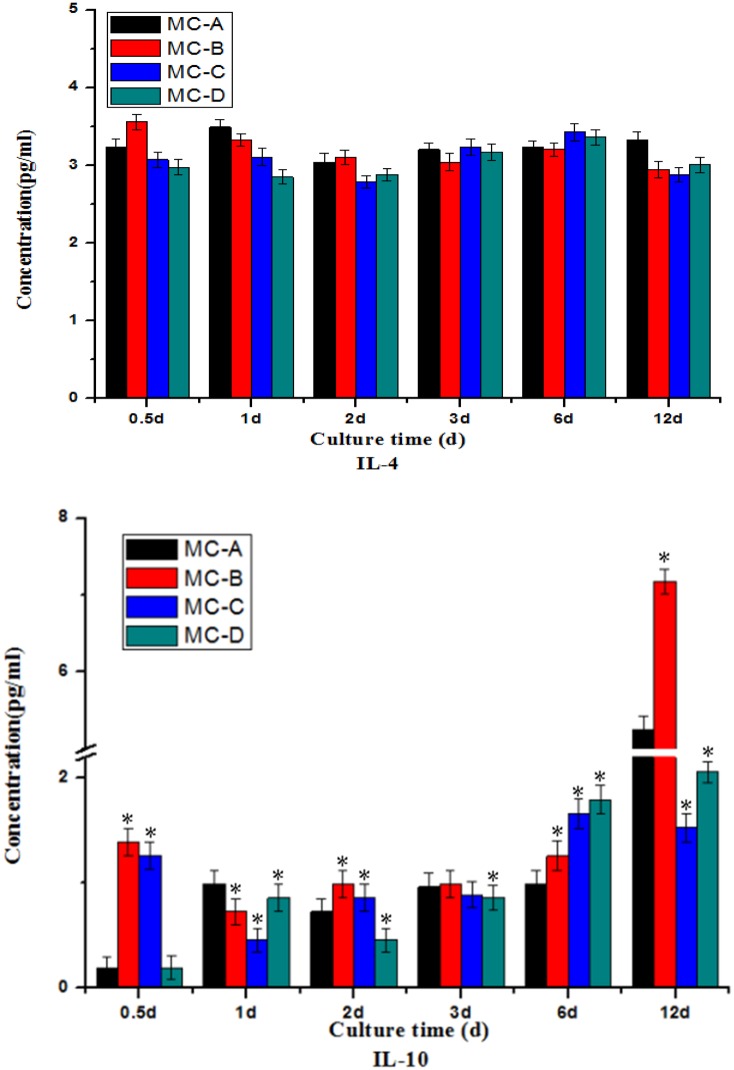
Secretion of M2-cytokines
In terms of the amount of secretion, the IL-4 concentration in the MC-A group was higher, but there was no statistically significant difference (as shown in Fig. 8). After 6 days, the secretion of IL-10 increased in MC-A and MC-B groups, whereas MC-C and MC-D showed no significant changes.
Figure 8.
Secretion of M2-(tissue repair) cytokines: IL-4 and IL-10. *Significant difference (P < 0.05) compared with the MC-A group
Discussion
As multifunctional immune cells, macrophages play a key role in tissue regeneration and inflammation because of their various phenotypes and plasticity [15]. Macrophages respond to biomaterials by ‘re-educated’ and are polarized into M1 or M2 phenotype. The M1 macrophages are characterized by production of proinflammatory cytokines including TNF-α, IL-1β and IL-6, and at the beginning of the inflammatory response, the host immune status plays a role, but also leads to inflammatory lesions in the normal tissues of the body. Whereas M2 macrophages are commonly associated with secretion of IL-4, IL-10 and IL-13 and play an important part in the late stage of inflammation [16, 17]. Therefore, macrophages play a key role in bone development and regeneration and the different ratios of M1 and M2 can indicate the direction of inflammation, healing and reconstruction [18].
The morphological changes of biomaterials caused by surface topography are closely related to macrophage function and polarization [19]. Roughness can directly affect cell adhesion and spreading through its effects on protein adsorption [20, 21]. It is still controversial about the influence of surface roughness on macrophage polarization. McWhorter et al. [22] have shown that M1 macrophages exhibited round, pancake-like shape, which have bigger cell surface area than unstimulated M0 macrophages, whereas M2 macrophages have elongated morphology. Barth et al. [23] found that the secretion of cytokines on roughness surface was closer to the activity of M2-like phenotype. Although others found that unstimulated macrophages increased the secretion of the proinflammatory cytokine (TNF-α) when surfaces became rough [24]. This study indicated that surface roughness of MC affected the adhesion and diffusion of macrophages, and can also regulate the release of inflammatory cytokines and chemokines from macrophages. The shape changes of macrophages under the stimulation of different surface roughness strongly imply a possible modulation of the immune environment.
It comes to light that the balance of M1/M2 macrophages should influence tissue regeneration and wound healing after biomaterials implanted into the body [13]. Many efforts need to determine the appropriate early immune environments to activate osteogenesis-enhancing M2 macrophages and enhance new bone regeneration. Prolonged M1 polarization could increase the release of fibrosis cytokines from M2 macrophages, and it resulted in fibrocapsule formed. As a comparison, a timely and effective transformation of M1 macrophage phenotype could lead to the enhanced cytokine release pattern of M2 macrophage osteogenesis, and then formed a new bone tissue [25]. Our study suggested that the increase on surface roughness of MC enhanced the secretion of proinflammatory cytokines from macrophages and the increase on surface roughness of MC could induce macrophages polarization toward M1 than M2 evidently. Yang et al. [19] found that the knockout of IL-6 delayed the maturity, mineralization, and remodeling of callus, indicating that IL-6 played the essential role in the early stage of fracture healing. Xiao and his colleagues [26, 27] also had shown that the generated osteoimmune environment subsequently affected the osteogenic differentiation of BMSCs and the new bone formation. It can be beneficial that in the early stages of fracture repair, inflammation can also lead to new bone formation. This research indicated that the osteoimmunomodulation could be manipulated through biomaterials physicochemical properties, which implied a valuable strategy to develop advanced bone biomaterials with favorable osteoimmunomodulatory properties.
Therefore, the induction of macrophage polarization by biomaterials to promote bone regeneration has become a new direction in the design of bone repair biomaterials. Much research should be required a better and deeper understanding of macrophage switching patterns and the mechanism of that affected the bone healing process. This understanding will lead to the rapid development of ‘smart' bone substitute biomaterials with immunomodulatory function.
Conclusion
This study demonstrated that MC surface topography, particularly the surface roughness, modulated the population and individual morphology as well as the production of cytokines such as TNF-α, IL-6, IL-4 and IL-10 from the macrophage cell line extracted in a time-dependent manner. These findings provide a basis for modulating macrophage polarization by MC surface optimal design to regulate host response. And we need further research to obtain more details on the biomaterial-dependent reaction of macrophages at the molecular level, as well as the role of immunomodulatory properties of bone repair materials in bone regeneration and repair.
Acknowledgements
This work was in part supported by Medicine and Health Science Technology Development plan of Shandong Province of China (Grant no: 2018WSA15023) and Science Foundation of Shandong Province of China (Grant nos: ZR2017PH037 and ZR2017PH056).
Conflict of interest statement. None declared.
References
- 1. Molina ER, Smith BT, Shah SR. et al. Immunomodulatory properties of stem cells and bioactive molecules for tissue engineering. J Control Release 2015;219:107–18. [DOI] [PubMed] [Google Scholar]
- 2. Zhou G, Groth T.. Host responses to biomaterials and anti-inflammatory design-a brief review. Macromol Biosci 2018;18:1800112.. [DOI] [PubMed] [Google Scholar]
- 3. Dong L, Wang C.. Harnessing the power of macrophages/monocytes for enhanced bone tissue engineering. Trends Biotechnol 2013;31:342–6. [DOI] [PubMed] [Google Scholar]
- 4. Fearing BV, Van Dyke ME.. In vitro response of macrophage polarization to a keratin biomaterial. Acta Biomater 2014;10:3136–44. [DOI] [PubMed] [Google Scholar]
- 5. Mavrogenis AF, Dimitriou R, Parvizi J. et al. Biology of implant osseointegration. J Musculoskelet Neuronal Interact 2009;9:61–71. [PubMed] [Google Scholar]
- 6. Anderson JM, Rodriguez A, Chang DT.. Foreign body reaction to biomaterials. Semin Immunol 2008;20:86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Novak ML, Koh TJ.. Macrophage phenotypes during tissue repair. J Leukoc Biol 2013;93:875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Murray DW, Rae T, Rushton N.. The influence of the surface energy and roughness of implants on bone resorption. J Bone Joint Surg 1989;71:632–7. [DOI] [PubMed] [Google Scholar]
- 9. Hotchkiss KM, Reddy GB, Hyzy SL. et al. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater 2016;31:425–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Quade M, Schumacher M, Bernhardt A. et al. Strontium-modification of porous scaffolds from mineralized collagen for potential use in bone defect therapy. Mater Sci Eng 2018;84:159–67. [DOI] [PubMed] [Google Scholar]
- 11. Wang Y, Hua Y, Zhang Q. et al. Using biomimetically mineralized collagen membranes with different surface stiffness to guide regeneration of bone defects. J Tissue Eng Regen Med 2018;12:1545–55. [DOI] [PubMed] [Google Scholar]
- 12. Liu Y, Liu S, Luo D. et al. Hierarchically staggered nanostructure of mineralized collagen as a bone-grafting scaffold. Adv Mater 2016;28:8740–8. [DOI] [PubMed] [Google Scholar]
- 13. Shi XD, Chen LW, Li SW. et al. The observed difference of RAW264.7 macrophage phenotype on mineralized collagen and hydroxyapatite. Biomed Mater 2018;13:041001.. [DOI] [PubMed] [Google Scholar]
- 14. Liao SS, Cui FZ, Zhang W. et al. Hierarchically biomimetic bone scaffold materials: nano-HA/collagen/PLA composite. J Biomed Mater Res 2004;69:158–65. [DOI] [PubMed] [Google Scholar]
- 15. Quan H, Kim Y, Chul Park H. et al. Effects of phosphatidylserine-containing supported lipid bilayers on the polarization of macrophages. J Biomed Mater Res 2018;106:2625–33. [DOI] [PubMed] [Google Scholar]
- 16. Ben-Mordechai T, Holbova R, Landa-Rouben N. et al. Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J Am Coll Cardiol 2013;62:1890–901. [DOI] [PubMed] [Google Scholar]
- 17. Wynn TA, Vannella KM.. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016;44:450–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vi L, Baht GS, Whetstone H. et al. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. J Bone Miner Res 2015;30:1090–102. [DOI] [PubMed] [Google Scholar]
- 19. Yang X, Ricciardi BF, Hernandez-Soria A. et al. Callus mineralization and maturation are delayed during fracture healing in interleukin-6 knockout mice. Bone 2007;41:928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rostam HM, Singh S, Vrana NE. et al. Impact of surface chemistry and topography on the function of antigen presenting cells. Biomater Sci 2015;3:424–41. [DOI] [PubMed] [Google Scholar]
- 21. Ma QL, Zhao LZ, Liu RR. et al. Improved implant osseointegration of a nanostructured titanium surface via mediation of macrophage polarization. Biomaterials 2014;35:9853–67. [DOI] [PubMed] [Google Scholar]
- 22. McWhorter FY, Wang T, Nguyen P. et al. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci USA 2013;110:17253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Barth KA, Waterfield JD, Brunette DM.. The effect of surface roughness on RAW 264.7 macrophage phenotype. J Biomed Mater Res 2013;101:2679–88. [DOI] [PubMed] [Google Scholar]
- 24. Refai AK, Textor M, Brunette DM. et al. Effect of titanium surface topography on macrophage activation and secretion of proinflammatory cytokines and chemokines. J Biomed Mater Res 2004;70:194–205. [DOI] [PubMed] [Google Scholar]
- 25. Chen Z, Klein T, Murray RZ. et al. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater Today 2016;19:304–21. [Google Scholar]
- 26. Chen Z, Bachhuka A, Han S. et al. Tuning chemistry and topography of nanoengineered surfaces to manipulate immune response for bone regeneration applications. ACS Nano 2017;11:4494–506. [DOI] [PubMed] [Google Scholar]
- 27. Chen Z, Wu C, Gu W. et al. Osteogenic differentiation of bone marrow MSCs by b -tricalcium phosphate stimulating macrophages via BMP2 signalling pathway. Biomaterials 2014;35:1507–18. [DOI] [PubMed] [Google Scholar]