Abstract
Precis:
At 1-year postoperative follow-up, concurrent placement of a dexamethasone intravitreal implant and glaucoma drainage device effectively controlled intraocular pressure (IOP) and inflammation in eyes with uveitic glaucoma with no changes in systemic immunomodulatory therapy.
Purpose:
The purpose of this study was to assess 1-year postoperative outcomes in eyes with uncontrolled uveitic glaucoma following concurrent placement of a dexamethasone intravitreal implant and glaucoma drainage device.
Materials and Methods:
This is a retrospective, observational case series of patients with chronic, noninfectious uveitis and uveitic glaucoma uncontrolled on maximal tolerated medical therapy with at least 1-year postoperative follow-up. The main outcomes were visual acuity, IOP, number of glaucoma medications, recurrent inflammation, frequency of topical steroids, systemic immunomodulatory therapy, and adverse events. Success was defined as IOP <21 mm Hg and IOP reduced by >20% from baseline on at least 2 consecutive visits after 3 months either with or without glaucoma medications (ie, partial or complete success, respectively).
Results:
Eight eyes in 6 patients met the inclusion criteria. The average age was 44.1±19.7 years (range: 10 to 68 y) and 50% were female. At 1-year, there was no significant change in visual acuity. No eyes lost ≥3 lines of vision. The majority of eyes (87.5%) achieved complete (n=2) or partial success (n=5) with a decrease in average IOP from 36.5 to 11.8 mm Hg (P=0.002). Glaucoma medication use decreased from 3.0 to 1.3 medications (P=0.04). There was a significant decrease in the number of episodes of recurrent inflammation in the 6 months following surgery compared with the 6 months before surgery (P=0.004).
Conclusion:
In this small case series, dexamethasone intravitreal implant combined with Ahmed glaucoma drainage device appears to be an effective approach for the management of uncontrolled uveitic glaucoma.
Key Words: dexamethasone intravitreal implant, uveitic glaucoma, glaucoma drainage device, uveitis, postoperative outcomes
Uveitis is responsible for 10% of all blindness in the United States.1 Corticosteroids are first-line therapy for noninfectious uveitis to control ocular inflammation and decrease the risk of vision loss. However, many patients with uveitis may require systemic immunomodulatory medications and long-term use of ocular corticosteroids to control their intraocular inflammation. A major complication from uveitis is glaucoma secondary to either chronic corticosteroids or chronic ocular inflammation. Secondary glaucoma occurs in 10% to 20% of patients with uveitis.2 The cumulative incidence of one or more intraocular pressure (IOP) measurements ≥24 and ≥30 mm Hg is 34% and 15%, respectively, among patients treated with periocular corticosteroids at 1-year.3
Glaucoma surgery is indicated when IOP is uncontrolled and/or there is evidence of progressive glaucomatous optic neuropathy despite maximum tolerated medical therapy. Glaucoma drainage implants have been shown to be effective for long-term success in uveitic glaucoma.4,5 Perioperative inflammation control can be challenging in uveitic eyes after surgery with postoperative inflammation and reactivation of uveitis has been reported to occur in 5.2% to 31.1% of cases.6 In addition, patients with uveitis are more susceptible to postoperative complications such as severe inflammation or elevated IOP. Postoperative hypotony may occur due to subsequent ciliary body inflammation and shutdown.
Careful management of perioperative immunosuppressive agents is critical for the successful management of uveitic eyes undergoing intraocular surgery, especially in eyes with a history of inflammation that has been difficult to control.7 Ozurdex (dexamethasone intravitreal implant; Allergan Inc., Irvine, CA) delivers bioerodible, sustained release of 0.7 mg dexamethasone to the posterior segment.8 It is Food and Drug Administration (FDA)-approved for the management of adults with noninfectious posterior uveitis and macular edema secondary to diabetes, branch or central retinal vein occlusion. Previous small case series and retrospective case-control studies have described favorable outcomes from the concurrent placement of a glaucoma drainage device and the fluocinolone acetonide intravitreal implant (Retisert; Bausch & Lomb Inc., Rochester, NY).9,10 The fluocinolone acetonide intravitreal implant typically requires the assistance of a fellowship-trained retina or uveitis surgeon (to perform a pars plana vitrectomy, sclerotomy, and suture the implant into position). An advantage of the dexamethasone intravitreal implant is that it can be injected directly into the posterior segment through the pars plana by a surgeon without retina or uveitis fellowship training.
Outcomes from combined dexamethasone intravitreal implant and glaucoma drainage device surgery for uncontrolled uveitic glaucoma have not been previously reported. We hypothesized that this approach would be effective in achieving IOP control and minimizing recurrent inflammation. The purpose of this study was to assess the 1-year postoperative outcomes of concurrent placement of a dexamethasone intravitreal implant and Ahmed glaucoma drainage device among patients with uveitic glaucoma and IOPs inadequately controlled on maximal tolerated medical therapy.
METHODS
Study Population, Design, and Outcome Measures
Patient eligibility criteria included adults and children with a history of chronic noninfectious anterior, intermediate, posterior uveitis or panuveitis and uncontrolled IOP despite maximal tolerated medical therapy who underwent concurrent placement of a dexamethasone (0.7 mg) intravitreal implant (Ozurdex; Allergan Inc.) and Ahmed glaucoma drainage device (FP7; New World Medical Inc., Rancho Cucamonga, CA) in a single surgical session. Patients with both implants placed sequentially were excluded. All patients underwent surgery between January 2013 and April 2018 at the University of Wisconsin-Madison under the supervision of 2 glaucoma surgeons (Y.L. and A.C.M.). Glaucoma and anti-inflammatory medical therapy were adjusted in accordance with the observed clinical response at each postoperative visit. The dexamethasone intravitreal implant is an off-label use of an FDA-approved medication in this patient population.
We reviewed medical records from the preoperative clinic visit (baseline), postoperative day 1, week 1, month 1, month 3, month 6, month 9, and month 12. Data collected included best-corrected visual acuity (BCVA), applanation tonometry (Tono-Pen; Reichert Inc., Depew, NY or Goldmann applanator; Haag Streit USA Inc., Mason, OH), as well as anterior and posterior segment examination findings from slit-lamp biomicroscopy and indirect ophthalmoscopy. Intraoperative complications and postoperative adverse events such as hypotony (IOP≤5 mm Hg), suprachoroidal hemorrhage, endophthalmitis, retinal detachment, tube erosion, and reoperation were recorded.
Surgical Procedure
After obtaining informed patient consent, all surgeries were performed either under general anesthesia, retrobulbar block or peribulbar block (0.5% bupivacaine and 2% lidocaine in a 1:1 mixture). The operative eye was prepped and draped in a sterile manner and a Lieberman speculum was inserted into the eye. The glaucoma drainage implant (Ahmed Model FP7; New World Medical Inc.) was placed in the superotemporal quadrant ∼8 mm posterior to the limbus and secured to the sclera with 8-0 nylon. A long-term donor cornea patch graft was used to cover the tube portion of the glaucoma drainage device and sutured to the sclera with 8-0 polyglactin suture. Following closure of the conjunctiva over the glaucoma drainage device, the dexamethasone intravitreal implant (Ozurdex; Allergan Inc.) was injected in the inferotemporal quadrant 3.5 to 4.0 mm posterior to the limbus through a beveled scleral path using the 22 G applicator needle and the sclerotomy site was secured with a single, interrupted 8-0 polyglactin suture. At the conclusion of the procedure, cefazolin (100 mg) and dexamethasone (5 mg) were injected subconjunctivally in the inferior fornix.
Statistical Analysis and Institutional Review Board Approval
The main outcomes were logMAR BCVA, IOP, number of glaucoma medications, recurrent inflammation, frequency of topical steroids, and adverse events within the 1-year postoperative period. Success was defined as IOP <21 mm Hg and IOP reduced by >20% from baseline on at least 2 consecutive visits after 3 months either with or without medications (partial or complete success, respectively). Main outcome measures were analyzed using paired t tests and Wilcoxon signed-rank tests with Minitab version 18 (Minitab LLC, State College, PA). P-values <0.05 were considered statistically significant. This study was approved by the University of Wisconsin-Madison Health Sciences Institutional Review Board. All research activities were conducted in accordance with the Declaration of Helsinki and all federal or state laws.
RESULTS
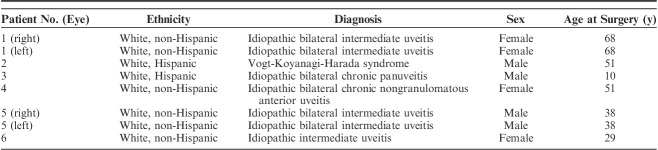
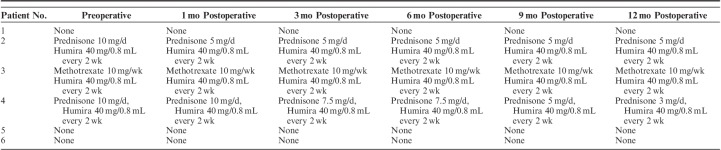
Eight eyes of 6 patients were included with a mean postoperative follow-up of 32.5±17.5 months (range: 13.3 to 51.7 mo). The average age at the time of surgery was 44.1±19.7 years (range: 10 to 68 y) and 50% of patients were female (Table 1). Mean preoperative logMAR BCVA was 0.55±0.40 and the mean preoperative IOP was 36.5±9.9 mm Hg. Before surgery, patients took an average of 3.0±0.7 glaucoma medications. In the 6 months before surgery, the average number of episodes of recurrent inflammation was 1.6±0.8 (range: 0 to 3) and the average number of prednisolone-equivalent topical corticosteroid drops daily was 1.5±2.5 (range: 0 to 6). Three eyes (37.5%) had active inflammation at their last clinic visit before surgery. Among the 5 eyes (62.5%) that were quiet at the time of surgery, the average duration of quiescence before surgery was 4.9±5.4 months (range: 0.6 to 15.2 mo).
TABLE 1.
Patient Demographics
Postoperative BCVA was not significantly different at any time point within the 1-year postoperative period compared with preoperative BCVA (Fig. 1). No eyes lost ≥3 lines of vision. Mean IOP significantly improved to 11.8±2.8 mm Hg at 12 months (P=0.002, Fig. 2). At 12-month follow-up, 25% of eyes achieved complete success (n=2) and 62.5% of eyes achieved partial success (n=5). One eye (12.5%) achieved partial success at 3-year follow-up. This eye had the lowest preoperative IOP (<21 mm Hg) compared with the other 7 eyes in this study (preoperative IOP>35 mm Hg). There was a significant reduction in the average number of glaucoma medications used postoperatively (1.3±1.0, P=0.04, Fig. 3).
FIGURE 1.
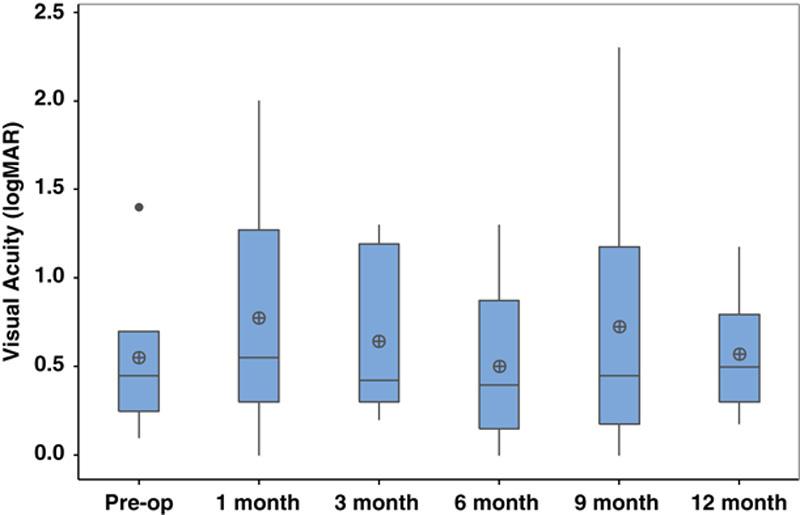
Visual Acuity (logMAR) preoperatively and postoperatively after combined intravitreal dexamethasone implant and glaucoma drainage device placement.
FIGURE 2.
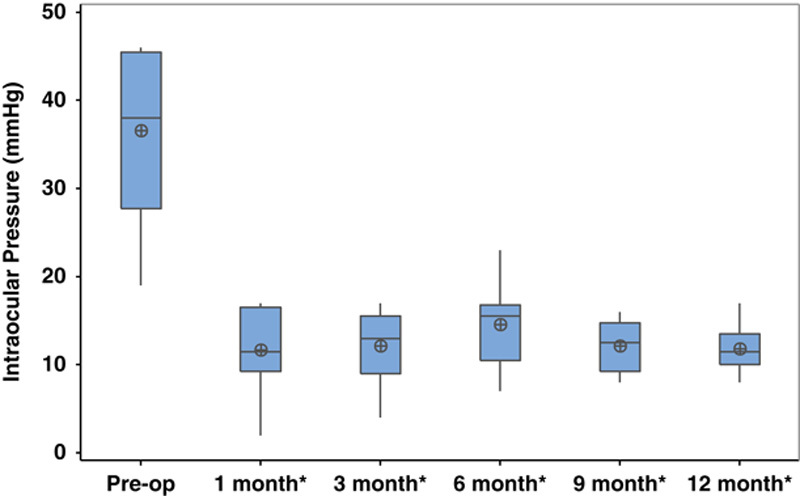
Intraocular pressure (mm Hg) preoperatively and postoperatively after combined intravitreal dexamethasone implant and glaucoma drainage device placement. *P≤0.05.
FIGURE 3.
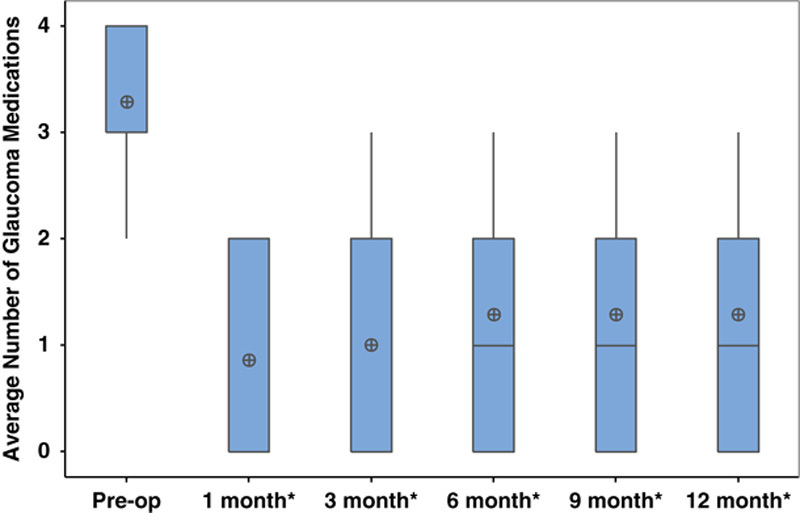
Average number of glaucoma medications preoperatively and postoperatively after combined intravitreal dexamethasone implant and glaucoma drainage device placement. *P≤0.05.
At 12-month follow-up, 7 of 8 (86%) eyes were quiet with an average of 0.63±0.5 episodes of recurrent inflammation (range: 0 to 2). There was a significant reduction in episodes of recurrent inflammation in the 6 months following surgery compared with the 6 months before surgery (P=0.004). No eyes required escalation of systemic immunomodulatory therapy postoperatively (Table 2). Of the 3 patients who used systemic immunomodulatory therapy preoperatively, 2 patients had a modest reduction in their oral prednisone use that was not considered clinically significant and 1 patient maintained the same systemic regimen. There was no difference in topical corticosteroid use (number prednisolone-equivalent drops) at any time point within the 1-year postoperative period compared with preoperatively (Fig. 4).
TABLE 2.
Systemic Anti-Inflammatory Medication Type and Dosage Preoperatively and Postoperatively After Combined Intravitreal Dexamethasone Implant and Glaucoma Drainage Device Placement
FIGURE 4.
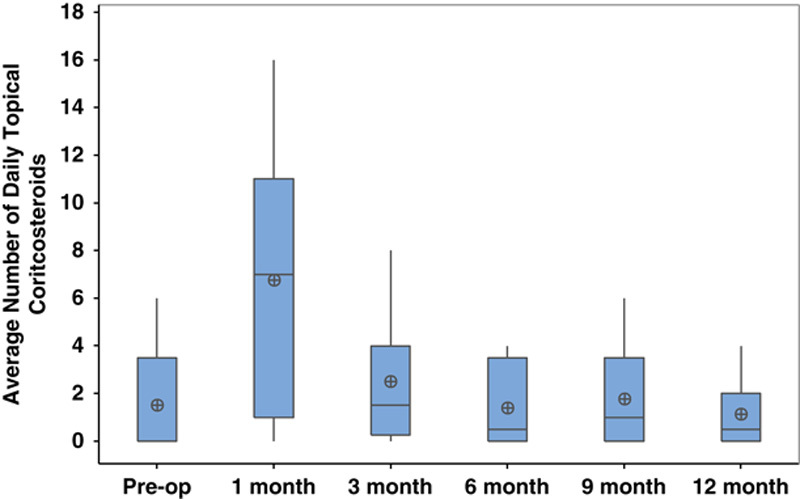
Average number of daily topical corticosteroids preoperatively and postoperatively after combined intravitreal dexamethasone implant and glaucoma drainage device placement.
There were no intraoperative complications. Postoperatively, 1 eye required to return to the operating room within 1 week for tube revision due to cornea-tube tip touch, which was attributed to misjudgment of tube position at the time of surgery. One eye in another patient had a tube erosion 35 months following surgery. Another eye had hypotony at postoperative-month #1 and postoperative-month #3 that resolved by postoperative month #6. There were no episodes of hypotony (IOP<5 mm Hg) after the 3-month postoperative period. No eyes developed endophthalmitis, vitreous hemorrhage, retinal detachment or required reoperation for management of IOP.
DISCUSSION
Our study found successful control of IOP, reduced use of glaucoma medications, and a reduction in recurrent inflammation at 1-year postoperative follow-up in the majority of eyes with uveitic glaucoma following combined placement of a dexamethasone intravitreal implant and glaucoma drainage device in a single surgical session. There was no change in BCVA and no patient lost ≥3 lines of vision. Prior studies have reported similar outcomes from the combined placement of a sustained-release fluocinolone acetonide intravitreal implant with glaucoma drainage device among patients with uveitic glaucoma.9,11–16 These results were consistent among a variety studies that included patients with single-session combined surgery (with or without concurrent cataract extraction) and/or consecutive glaucoma drainage device placement following fluocinolone acetonide intravitreal implantation.
The benefits of using sustained-release corticosteroids at the time of glaucoma drainage device implantation may include longer IOP control, fewer glaucoma medications, and improved inflammation control in patients with uveitic glaucoma. Moore et al11 found longer surgical success in IOP-lowering in 22 eyes with uveitic glaucoma who underwent combined glaucoma drainage device placement with fluocinolone acetonide intravitreal implant compared with 16 eyes with uveitic glaucoma that underwent glaucoma drainage device placement alone (629 vs. 361 d, respectively). The authors postulated that sustained corticosteroid release may help slow the development of the inflammation-mediated fibrous capsule surrounding the glaucoma drainage device plate that is implicated in accelerated failure to control IOP. In addition, Sevgi et al16 reported that fewer glaucoma medications were required in uveitic eyes that received combined surgery with the fluocinolone intravitreal implant and that this group had better inflammation control than the group that received glaucoma drainage device alone. Conversely, a study by Zivney et al13 suggested no additional benefit from the fluocinolone acetonide intravitreal implant among patients with uveitic glaucoma undergoing glaucoma drainage device placement. However, this retrospective study had a shorter follow-up timeframe of 6 months, a higher proportion of patients with posterior uveitis in the fluocinolone acetonide group, and 15 of 17 eyes (82.4%) had sequential (rather than concurrent) glaucoma drainage device placement, with an average time interval between surgeries of 1.1±1.3 years.
The adverse events in our study were similar to those found in prior studies of uveitic glaucoma patients who underwent combined fluocinolone intravitreal implant with glaucoma drainage device placement. Malone et al9 examined outcomes in 7 eyes of 5 patients. Their study found 2 eyes that underwent reoperation, including one within the first 30 days following surgery for tube repositioning.11 Sevgi et al16 also noted 2 tube erosions in the same patient among 7 eyes that underwent combined surgery. Moore et al11 reported adverse events in 2 of 22 eyes, including 1 eye that lost light perception vision, and 1 eye that required an additional IOP-lowering procedure. Zivney et al13 described hypotony in 3 of 17 eyes, all of which resolved following the first postoperative week. Adverse events were similar in the comparator groups of eyes with uveitic glaucoma that underwent glaucoma drainage device alone.11,13 These outcomes reflect the challenging nature of managing uveitic glaucoma postoperatively following glaucoma drainage device implantation.17
When comparing these sustained-release corticosteroids, the fluocinolone acetonide intravitreal implant may offer a longer duration and greater potency of immunosuppression, but the dexamethasone intravitreal implant is accessible to more surgeons. The fluocinolone implant can reduce inflammation for a longer period of time than the dexamethasone implant (up to 36 vs. 6 mo, respectively).18–20 Among patients with idiopathic panuveitis, Arcinue et al21 showed no difference in uveitis recurrence at 12 and 24 months of follow-up between the fluocinolone acetonide and dexamethasone intravitreal implant. However, studies evaluating the efficacy of the fluocinolone acetonide implant in controlling intraocular inflammation reported that the majority of eyes were able to discontinue systemic therapy.18,21 In our study, 2 of 3 patients who used systemic immunomodulatory therapy preoperatively were able to modestly decrease their dosage postoperatively, but no patients were able to discontinue systemic therapy. Although topical steroids are not the mainstay of therapy for patients with intermediate or panuveitis, we did note a significant reduction in topical steroid use in the 12 months following surgery. Our findings were more consistent with those from Malone et al9 in which 3 of 5 patients were able to gradually reduce their systemic therapy following concurrent fluocinolone acetonide implantation and glaucoma drainage device placement. In contrast, Chang et al14 found that 7 of 10 patients were able to discontinue systemic therapy following concurrent surgery.
However, the ease of delivery and repeatability of the dexamethasone intravitreal implant represents a significant advantage over the fluocinolone acetonide implant.18,21,22 The fluocinolone implant typically requires the assistance of a vitreoretinal or uveitis fellowship-trained surgeon to perform a vitrectomy through a 3.5 mm pars plana sclerotomy and suture the fluocinolone implant to the sclera. The coordination and cost associated with scheduling 2 surgeons (eg, glaucoma and vitreoretinal) can be challenging and limits the widespread adoption of this combined procedure. In contrast, the dexamethasone implant can be injected via a pars plana approach without vitrectomy. Thus, the dexamethasone implant is more time-efficient and accessible to glaucoma surgeons for the management of uveitic glaucoma patients, particularly in patients whose intraocular inflammation has been difficult to control.
No published studies have previously described outcomes from combined dexamethasone intravitreal implantation and glaucoma drainage device placement. However, there are few published studies on outcomes from dexamethasone intravitreal implants performed in conjunction with cataract surgery in uveitic eyes. Ragam et al22 found that concurrent dexamethasone intravitreal implant successfully controlled intraoperative inflammation in patients with chronic recurrent uveitis undergoing cataract extraction and intraocular lens implantation. Gupta and colleagues compared concurrent dexamethasone intravitreal implantation to the use of systemic corticosteroid in uveitic eyes undergoing cataract extraction. The authors concluded that dexamethasone intravitreal implant may be a reasonable alternative to systemic corticosteroid for achieving perioperative inflammation control.23
Limitations of our study include the small sample size and being a retrospective case series. As a result of being a retrospective study, we included measures of IOP using both Tono-Pen and Goldmann applanation, which may have introduced additional variability in our results. Four eyes of 2 patients were included in this study, which may have resulted in more similar outcomes than had the 4 eyes been from 4 different patients. More detailed data on the duration of preoperative systemic therapy was not available but would have been helpful in clarifying whether any of the modest reductions in oral prednisone were related to planned tapering in concert with the institution of steroid-sparing immunosuppressants. Although regression to the mean may have contributed to our finding of decreased episodes of recurrent inflammation postoperatively, our results are consistent with prior studies of cataract surgery combined with dexamethasone intravitreal implant.22,23 Future prospective studies randomizing patients to either the combined placement of a dexamethasone intravitreal implant with glaucoma drainage device, fluocinolone intravitreal implant with glaucoma drainage device or glaucoma drainage device alone would provide greater evidence as to whether the concurrent use of sustained-release corticosteroids results in improved postoperative outcomes in patients with uncontrolled uveitic glaucoma. In addition, comparing outcomes from patients who had sequential versus concurrent surgery would be helpful in determining whether either method (and which sequence order) may lead to superior outcomes and/or reduced postoperative risks.
In our study, there was a significant reduction of IOP and topical glaucoma medication use following combined dexamethasone intravitreal implant with Ahmed glaucoma drainage device placement. No patient lost ≥3 lines of vision and adequate postoperative inflammation control was achieved. The use of the dexamethasone intravitreal implant combined with Ahmed glaucoma drainage device appears to be an effective approach in the management of uncontrolled uveitic glaucoma.
Footnotes
Supported in part by an institutional grant from Research to Prevent Blindness to the University of Wisconsin Department of Ophthalmology and Visual Sciences.
Disclosure: The authors declare no conflict of interest.
REFERENCES
- 1.Archarya NR, Tham VM, Esterberg E, et al. Incidence and prevalence of uveitis: results from the Pacific Ocular Inflammation Study. JAMA Ophthalmol. 2013;131:1405–1412. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi T, Ohtani S, Miyata K, et al. A clinical evaluation of uveitis-associated secondary glaucoma. Jpn J Ophthalmol. 2002;46:556–562. [DOI] [PubMed] [Google Scholar]
- 3.Sen HN, Vitale S, Gangaputra SS, et al. Periocular corticosteroid injections in uveitis: effects and complications. Ophthalmology. 2014;121:2275–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bettis D, Morshedi R, Chaya C, et al. Trabeculectomy with mitomycin C or Ahmed valve implanation in eyes with uveitic glaucoma. J Glaucoma. 2015;24:591–599. [DOI] [PubMed] [Google Scholar]
- 5.Iverson SM, Bhardwaj N, Shi W, et al. Surgical outcomes of inflammation glaucoma: a comparison of trabeculectomy and glaucoma drainage device implantation. Jpn J Ophthalmol. 2015;59:179–186. [DOI] [PubMed] [Google Scholar]
- 6.Prata JJ, Neves R, Minckler D, et al. Trabeculectomy with mitomycin C in glaucoma associated with uveitis. Ophthalmic Surg. 1994;25:616–620. [PubMed] [Google Scholar]
- 7.Barton K, Hall AJH, Rosen PH, et al. Systemic steroid prophylaxis for cataract surgery in patients with posterior uveitis. Ocul Immunol Inflamm. 1994;2:207–216. [DOI] [PubMed] [Google Scholar]
- 8.Allergan, Inc. Ozurdex: Highlights of Prescribing Information. Madison, NJ: Allergan; 2018. [Google Scholar]
- 9.Malone P, Herndon LW, Muir KW, et al. Combined fluocinole acetonide intravitreal insertion and glaucoma drainage device for chronic uveitis and glaucoma. Am J Ophthalmol. 2010;149:800–806. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad ZM, Hughes BA, Abrams GW, et al. Combined posterior chamber intraocular lens, vitrectomy, retisert and pars plana tube in noninfectious uveitis. Arch Ophthalmol. 2012;130:908–913. [DOI] [PubMed] [Google Scholar]
- 11.Moore DB, Stinnett S, Jaffee GJ, et al. Improved surgical success of combine glaucoma tube shunt and retisert implantation in uveitic eyes: a retrospective study. Ophthalmol Ther. 2015;4:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hennein L, Hou J, Stewart J, et al. Comparison of surgical outcome after ahmed valve implantation for patients with and without fluocinolone intravitreal implant (Retisert). J Glaucoma. 2016;25:772–776. [DOI] [PubMed] [Google Scholar]
- 13.Zivney M, Lin P, Edmunds B, et al. Combined glaucoma tube shunt (Ahmed) and fluocinolone acetonide (Retisert) implantation compared to Ahmed alone in uveitic glaucoma. Ophthalmol Ther. 2016;5:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang IT, Gupta D, Slabaugh MA, et al. Combined Ahmed glaucoma valve placement, intravitreal fluocinolone acetonide implantation and cataract extraction for chronic uveitis. J Glaucoma. 2016;25:842–846. [DOI] [PubMed] [Google Scholar]
- 15.Kubaisi B, Maleki A, Ahmed A, et al. Ahmed glaucoma valve in uveitic patients with fluocinolone acetonide implant-induced glaucoma: 3-year follow-up. Clin Ophthalmol. 2018;12:799–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sevgi DD, Davoudi S, Talcott KE, et al. A retrospective study on the outcomes of Ahmed valve versus Ahmed valve combined with fluocinolone implant in uveitic glaucoma. Digit J Ophthalmol. 2017;23:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozdal PC, Vianna R, Deschenes J. Ahmed valve implantation in glaucoma secondary to chronic uveitis. Eye. 2006;20:178–183. [DOI] [PubMed] [Google Scholar]
- 18.Callanan D, Jaffe G, Martin D, et al. Treatment of posterior uveitis with a fluocinolone acetonide implant. Arch Ophthalmol. 2008;126:1191–1201. [DOI] [PubMed] [Google Scholar]
- 19.Pacella F, Ferraresi AF, Turchetti P, et al. Intravitreal injection of Ozurdex implant in patients with persistent diabetic macular edema, with six-month follow-up. Ophthalmol Eye Dis. 2016;8:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bausch and Lomb, Inc Retisert: Highlights of Prescribing Information. Bridgewater, NJ: Bausch and Lomb; 2017. [Google Scholar]
- 21.Arcinue CA, Ceron OM, Foster CS. A comparison between the fluocinolone acetonide (Retisert) and dexamethasone (Ozurdex) intravitreal implants in uveitis. J Ocul Pharmacol Ther. 2013;29:501–507. [DOI] [PubMed] [Google Scholar]
- 22.Ragam AP, Kolomeyer AM, Nayak NV, et al. The use of Ozurdex (dexamethasone intravitreal implant) during anterior segment surgery in patients with chronic recurrent uveitis. J Ocul Pharmacol Ther. 2015;31:344–349. [DOI] [PubMed] [Google Scholar]
- 23.Gupta A, Ram J, Gupta A, et al. Intraoperative dexamethasone implant in uveitis patients with cataract undergoing phacoemulsification. Ocul Immunol Inflamm. 2013;21:462–467. [DOI] [PubMed] [Google Scholar]