Abstract
Objective:
To examine the relationship between menopausal status and mental well-being, and whether this relationship varies as a function of physical activity (PA).
Methods:
Based on a hormonal analysis and bleeding diary, women aged 47 to 55 were categorized as pre (n = 304), early peri (n = 198), late peri (n = 209), or postmenopausal (n = 387). Mental well-being was assessed using the Centre for Epidemiologic Studies Depression Scale, the International Positive and Negative Affect Schedule Short Form, and the Satisfaction with Life Scale. PA was self-reported and categorized as low, medium, and high. Associations between variables were analyzed using multivariate linear regression adjusted for age, marital and employment status, parity, self-reported mental disorder, use of psycholeptics and psychoanaleptics, and menopausal symptoms.
Results:
Depressive symptoms were lower amongst the pre than postmenopausal women (B = 0.07, confidence interval 0.01-0.13). Menopausal symptoms attenuated these associations. Menopausal status showed no associations with life satisfaction, or with positive or negative affectivity.
Women with high PA scored higher on positive affectivity, and the pre, early peri, and postmenopausal women scored higher on life satisfaction (B = 0.79, P < 0.001; B = 0.63, P = 0.009; B = 0.42, P = 0.009, respectively) and scored lower on depressive symptoms (B = −0.13, P = 0.039; B = −0.18, P = 0.034; and B = −0.20, P < 0.001, respectively) than their low PA counterparts. The pre and postmenopausal women with medium PA scored higher on life satisfaction (B = 0.54, P = 0.001; B = 0.038, P = 0.004, respectively) than those with low PA.
Conclusions:
Postmenopausal women reported marginally higher depressive symptoms scores compared with premenopausal women, but menopause was not associated with positive mental well-being. However, this association varies with the level of PA.
Video Summary:
Keywords: Depressive symptoms, Life satisfaction, Menopausal status, Negative affectivity, Physical activity, Positive affectivity
The menopause, defined as the final menstruation, occurs between the ages of 46 and 52, and signifies aging of a woman's reproductive system.1 Menopause-related hormonal changes begin several years before the menopause and are characterized by a gradual increase in follicle-stimulating hormone (FSH) and more rapid decline in systemic female sex steroids (estradiol and estrone) within 6 months around the menopause.2 Reproductive aging among women has a far-reaching effect on the function of different body systems, and also on the psychological functioning and well-being among middle-aged and older women. Maintaining sufficient mental well-being is important for functioning in later life, as it is associated with, for example, better health outcomes and survival.3,4
Menopause is an important life transition phase and has been suggested to be a time of increased vulnerability in well-being.5 Although some cross-sectional studies have found no relationship between menopausal status and mental well-being,6,7 other longitudinal studies have identified an increased risk for elevated depressive symptoms and negative mood in the transition phase of menopause.8-10
As midlife has traditionally been considered to induce psychological stress due to several competing social roles, such as care giving and work roles, and a cumulative number of losses,11 the menopause literature has operationalized the measurement of mental well-being by focusing on its negative aspects. However, more recent studies indicate that, psychologically, midlife may be a rather satisfying stage of life,12 characterized by a high level of mental well-being.13 Thus, as a specific age-related transition in mid-adulthood, menopause may differentially influence both negative, and also positive dimensions of mental well-being.14 Study of the relationship between menopausal status and mental well-being has been challenged by numerous methodological issues. These include a precise definition of menopausal status, the measurement of well-being, and the confounding influence of a prior level of mental distress and social circumstances.15,16
Despite the seeming changes in mental well-being associated with menopause and social circumstances, physical activity may attenuate the negative influence of both these factors. A longitudinal study in which middle-aged women participated in a 4-month exercise program showed, at 2-year follow-up, that physical activity positively influenced satisfaction with life through improvement in affect and self-worth.17 Another cross-sectional study found that physically active middle-aged women reported better menopausal and global quality of life.18 Moreover, physical activity alleviates some menopausal symptoms19 and subsequently may weaken the negative impact of menopause on mental well-being. However, a decline in physical activity and a shift towards increased sedentary behavior, both of which have been observed during the menopause transition,20,21 may be among the reasons for the adverse influence of menopause on mental well-being among low physically active women.
Although earlier studies consistently support a positive influence of physical activity on well-being and its dimensions in a variety of populations,22-24 few of them have examined the simultaneous contribution to mental well-being of menopausal status and physical activity.
The aim of this study was to examine the relationship between menopausal status, and positive and negative dimensions of mental well-being, and whether these relationships vary as a function of physical activity.
METHODS
Study design and participants
This study utilized data from the ongoing Estrogenic Regulation of Muscle Apoptosis (ERMA) study.25 In this study, women aged 47 to 55 years, living in the city of Jyväskylä and its neighboring municipalities, were randomly selected from the Finnish National Registry administered by the Population Register Centre (Fig. 1). A postal invitation was sent to 6,878 potential participants. Exclusion criteria were conditions or use of medications affecting ovarian function, including bilateral ovariectomy, estrogen-containing hormonal preparations or other medications affecting ovarian function, a self-reported body mass index (BMI) >35 kg/m2, being pregnant or lactating, diagnosis of a mental illness, and conditions or use of continuous medications affecting daily mental or physical function. Eligible participants (n = 1,627) were invited to the laboratory, where 1,393 women filled in the health screen questionnaire and gave fasting blood samples. After dropout of 36 participants and exclusion of 443 participants for health-related reasons preventing them from participating in the further physiological measurements, 1,098 eligible participants remained in the study and completed the well-being measurements.
FIG. 1.
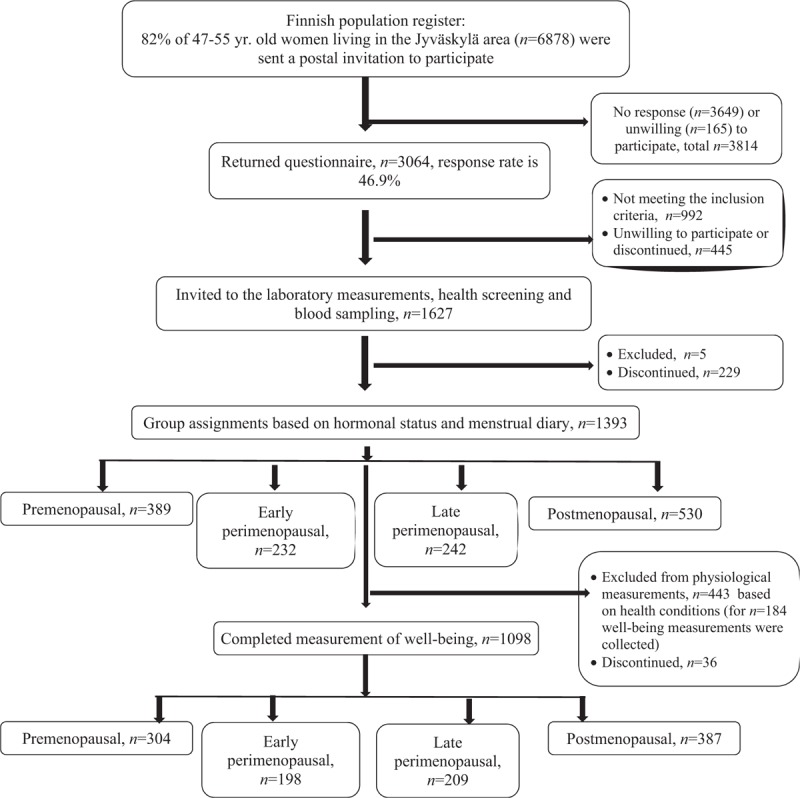
Enrollment of study participants.
All participants provided a written informed consent. The study protocol followed good clinical and scientific practice, and the Declaration of Helsinki, and was approved by the Ethics Committee of the Central Finland Health Care District (K-SSHP Dnro 8U/2014).
Measurements
Menopausal status
Menopausal status was determined based on bleeding diaries and serum concentrations of follicle-stimulating hormone (FSH) following the Stages of Reproductive Aging Workshop (STRAW) criteria.26 FSH assessments were performed from fasting serum samples which were collected between 8:00 and 10:00 am. If the women had a menstrual cycle, then collection was performed during cycle days 1 to 5. Serum was separated by centrifugation for 10 minutes at 2.200×g. Systemic FSH was immunoassayed using IMMULITE 2000 XPi (Siemens Healthcare Diagnostics, UK).
A complete description of the grouping by menopausal status has been reported earlier.25 In short, participants were assigned to the premenopausal group if they reported a regular menstrual cycle and had FSH <17 IU/L (M = 7.72, SD = 3.50), to the early perimenopausal group if they had FSH 17 to 25 IU/L, or, if they reported an irregular menstrual cycle, FSH >9.5 IU/L (M = 16.76, SD = 4.77), to the late perimenopausal group if they had FSH 25 to 30 IU/L, or, if they reported occasional menstrual bleeding during the past 3 months, FSH >30 IU/L (M = 44.90, SD = 19.96), and to the postmenopausal group if they reported no menstrual bleeding during the past 6 months and had FSH >30 IU/L, or reported no menstrual bleeding during the past 3 months and had FSH >39 IU/L (M = 82.48, SD = 28.82). For women with nonreported menstrual cycle information (eg, users of progesterone-containing medications), the categorization was based solely on FSH level, and stricter cut-off values were applied (premenopausal: FSH <15 IU/L, perimenopausal: FSH 15-39 IU/L, and postmenopausal: FSH >39 IU/L).
Menopausal symptoms
Menopausal symptoms were collected using a structured questionnaire which asked participants to indicate whether they had any of the following symptoms: sweating, hot flashes, sleeping problems, headache, joint pain, tiredness, mood swings, vaginal symptoms, urinary track problems, or sexual problems, or if they had some other symptoms. Based on the responses, four categories were formed: vasomotor symptoms; somatic or pain symptoms; psychological symptoms; and urogenital symptoms.27 For the main analysis, we aggregated these responses into a dichotomous variable (has symptoms/no symptoms) to indicate if a woman was experiencing any of the menopausal symptoms.
Mental well-being
Depressive symptoms were assessed with the 20-item Center for Epidemiological Studies Depression Scale (CES-D28). The participant rated the frequency of each symptom during the previous week. Each item was scored from 0 to 3, and a mean score for the 20 items was calculated. Higher scores indicate more depressive symptoms. This mean score was used in the main analyses. In addition, for descriptive purposes, a sum score ranging from 0 to 60 was calculated, and a score of 16 or above was used to indicate a sign of potential clinical depression.29
Positive and negative affectivity was assessed with the International Positive and Negative Affect Schedule Short Form (I-PANAS-SF30) with five positive affect adjectives and five negative affect adjectives. Participants were asked to rate each of these adjectives on a 5-point scale (from “does not describe me” to “describes me very well”) according to the extent to which each describes the way they usually feel. Mean scores ranging from 1 to 5 were calculated for positive and negative affectivity. For both positive and negative effects, a higher mean score indicates a higher tendency to experience a positive and negative affectivity.
Global cognitive judgments of one's life satisfaction was measured using the 5-item Satisfaction with Life Scale.31 Participants indicate the extent to which they agree or disagree with each of the five items on a 7-point scale ranging from 1 (strongly disagree) to 7 (strongly agree). The mean score for the five items, ranging from 1 to 7, was calculated, with higher scores indicating more satisfaction with life.
Physical activity
Current level of leisure physical activity was assessed on a 7-point scale32 ranging from household chores to competitive sports, and with questions assessing leisure-time and physical activity patterns. The response options were: I do not move more than is necessary in my daily routines; I go for casual walks and engage in light outdoor recreation 1 to 2 times a week; I go for casual walks and engage in light outdoor recreation several times a week; Once or twice a week, I engage in brisk physical activity (eg, yard work, walking, cycling) that causes some shortness of breath and sweating; Several times a week (3-5), I engage in brisk physical activity (eg, yard work, walking, cycling) that causes some shortness of breath and sweating; I exercise several times a week in a way that causes rather strong shortness of breath and sweating during the activity; I do competitive sports and maintain my fitness through regular training. For the analysis, the response categories were combined to low level of physical activity (categories 1 to 3), medium level of physical activity (4 and 5), and a high level of physical activity (6 to 7).
Socioeconomic, lifestyle variables, and use of prescribed medications
Level of education was assessed by a question, and categorized as primary, secondary, and tertiary (applied science degree, bachelor's degree, nurse training, master's degree, and PhD). Marital status was categorized as single, married, or living with a partner, and divorced, separated, or widowed. Parity was categorized as nulliparous, one or two children, and three or more children. Smoking status defined participants as never, former, or current smoker. Participants’ alcohol consumption was categorized as never or rarely (less than 1 unit/wk), weekly (1-5 units/wk), and often (more than 5 units/wk). Employment status was classified as either employed (paid or self-employed) or not regularly employed (studying, unemployed, working occasionally, retired, taking care of the home). Response options for self-rated health were poor, average, good, and very good. The latter two responses were merged into one category, labeled “good and very good.” For self-reported mental disorder, women were asked to indicate if they have (or have had) a mental disorder diagnosed by a physician as yes/no. Information on use of medications was collected and coded according to the Anatomical Therapeutic Chemical Classification System.33 For this article, we report on use of N05 (psycholeptics) and N06 (psychoanaleptics) as medications that may influence mental well-being.
Data analysis
Participant characteristics are shown as means and standard deviations (SDs), or as percentages. To test differences in the studied variables between the groups, analysis of variance (ANOVA) was used with the post hoc Tukey test. For categorical variables, differences were tested with a chi-square test.
Multiple regression analyses were conducted to examine the association of menopausal status and different dimensions of mental well-being. To test if any of the socioeconomic and lifestyle variables were potential confounders in the relationship between menopausal status and mental well-being, we first ran a correlation analysis (Spearman's rho, rs). We observed that marital status, parity, and employment status showed a significant association with the dimensions of mental well-being (rs ranges from 0.070 to 0.185; P ranges from <0.001 to 0.003). Use of psycholeptics (N05), use of psychoanaleptics (N06), self-reported mental disorders, and menopausal symptoms showed a positive association with depressive symptoms (rs = 0.109, P < 0.001; rs = 0.159, P < 0.001; rs = 0.212, P < 0.001; rs = 0.164, P < 0.001, respectively) and a negative association with life satisfaction (rs = −0.094, P = 0.002; rs = −0.067, P = 0.027; rs = −0.148, P < 0.001; rs = −0.006, P < 0.05). Positive affectivity showed a negative association with the use of psychoanaleptics (rs = −0.108, P < 0.001), self-reported mental disorder (rs = −0.172, P < 0.001), and menopausal symptoms (rs = −0.122, P < 0.001), whereas negative affectivity showed a positive association with these variables (rs = 0.115, P < 0.001; rs = 0.172, P < 0.001; rs = 0.110, P < 0.001, respectively). Age, education, smoking, and alcohol consumption showed no significant associations with any of the mental well-being components. However, as the compared groups differed by age, we also included age as a confounder. The final model was thus adjusted for age, marital status, parity, employment status, self-reported mental disorder, use of psycholeptics (N05), use of psychoanaleptics (N06), and menopausal symptoms.
To investigate the role of physical activity in the relationship between menopausal status and mental well-being, we analyzed marginal means differences, that is, the means of categories across menopausal status and physical activity level adjusted for all the previously mentioned confounders. Analysis of marginal means differences gives additional information beyond the full model with inclusion of interaction terms, especially in the case of categorical variables with more than two categories.34 In these analyses, in each menopausal status group, the women with medium and high physical activity were compared with those with low physical activity. All analyses were performed with R, version 3.3.3.35,36
RESULTS
The socioeconomic and lifestyle characteristics of the participants and their level of physical activity across the four menopausal stages are presented in Table 1. As expected, the postmenopausal women were significantly older than the late perimenopausal women followed by early perimenopausal women. The premenopausal women were the youngest.
TABLE 1.
Participants’ socioeconomic and lifestyle characteristics and information relevant to mental health by menopausal stages
| Variables | Premenopausal (n = 304) | Early perimenopausal (n = 198) | Late perimenopausal (n = 209) | Postmenopausal (n = 387) | P |
| Age, y | 50.3 (1.67) | 50.7 (1.81) | 51.6 (1.90) | 52.3 (1.98) | <0.001 |
| Pre-E.Peri 0.018Pre-L.Peri < 0.001Pre-Post < 0.001E.Peri-L.Peri <0.001E.Peri-Post <0.001L.Peri-Post <0.001 | |||||
| Education | (n = 304) | (n = 198) | (n = 209) | (n = 387) | 0.117 |
| Primary | 5 (1) | 3 (1) | 7 (3) | 9 (2) | |
| Secondary | 165 (55) | 109 (55) | 106 (51) | 239 (62) | |
| Tertiary | 134 (44) | 86 (44) | 96 (46) | 139 (36) | |
| Marital status | (n = 303) | (n = 198) | (n = 209) | (n = 385) | 0.560 |
| Single | 21 (7) | 19 (10) | 16 (7) | 37 (10) | |
| Married or registered partnership | 242 (80) | 148 (75) | 164 (79) | 278 (72) | |
| Divorced, separated or widowed | 40 (13) | 30 (15) | 29 (14) | 70 (18) | |
| Parity: number of children born | (n = 302) | (n = 196) | (n = 208) | (n = 386) | 0.926 |
| Nulliparous | 35 (12) | 25 (13) | 26 (12) | 53 (13) | 0.894 |
| One or two | 178 (59) | 114 (58) | 113 (55) | 219 (58) | |
| Three or more | 89 (29) | 57 (29) | 69 (33) | 114 (29) | |
| Smoking | (n = 300) | (n = 196) | (n = 207) | (n = 385) | 0.333 |
| Never | 194 (65) | 136 (70) | 131 (63) | 260 (68) | |
| Former | 90 (30) | 47 (24) | 55 (27) | 98 (25) | |
| Current | 16 (5) | 13 (6) | 21 (10) | 27 (7) | |
| Menopausal symptom (yes) | 168 (55) | 148 (74) | 172 (82) | 353 (91) | <0.001 |
| Pre-E.Peri < 0.001Pre-L.Peri < 0.001Pre-Post < 0.001E.Peri-L.Peri 0.070E.Peri-Post <0.001L.Peri-Post 0.025 | |||||
| Alcohol consumption | (n = 304) | (n = 198) | (n = 209) | (n = 387) | 0.7447 |
| Never or rarely (<1 units/wk) | 100 (34) | 90 (45) | 88 (42) | 129 (34) | |
| Weekly (1 to 5 units/wk) | 145 (48) | 94 (46) | 104 (50) | 193 (50) | |
| Often (>5 units/wk) | 55 (18) | 14 (9) | 16 (8) | 65 (16) | |
| Employment status | (n = 304) | (n = 198) | (n = 209) | (n = 387) | 0.258 |
| Employed | 275 (90) | 168 (85) | 182 (87) | 335 (87) | |
| Not regularly employed | 29 (10) | 30 (15) | 27 (13) | 52 (13) | |
| Self-rated health | (n = 304) | (n = 198) | (n = 209) | (n = 387) | |
| Good or very good | 244 (80) | 145 (73) | 163 (78) | 294 (76) | 0.252 |
| Average | 57 (19) | 52 (26) | 43 (21) | 85 (22) | |
| Poor | 3 (1) | 1 (1) | 3 (1) | 9 (1) | |
| Physical Activity level: | (n = 304) | (n = 198) | (n = 209) | (n = 387) | 0.019 |
| Pre-E.Peri 0.279Pre-L.Peri 0.032Pre-Post 0.338E.Peri-L.Peri 0.338E.Peri-Post 0.338L.Peri-Post 0.032 | |||||
| Low | 50 (17) | 37 (19) | 30 (16) | 81 (21) | |
| Medium | 174 (57) | 124 (62) | 145 (66) | 218 (56) | |
| High | 80 (26) | 37 (19) | 34 (22) | 88 (23) | |
| CES-D ≥16 | (n = 304) | (n = 198) | (n = 209) | (n = 387) | 0.207 |
| 30 (10) | 22 (11) | 18 (10) | 52 (13) | ||
| Self-reported mental disorders | (n = 303) | (n = 194) | (n = 209) | (n = 385) | 0.260 |
| 29 (9) | 21 (11) | 16 (8) | 25 (6) | ||
| Users of psycholeptics (N05) | (n = 304) | (n = 197) | (n = 209) | (n = 387) | 0.397 |
| 9 (3) | 7 (3) | 3 (1) | 7 (2) | ||
| Users of psychoanaleptics (N06) | (n = 304) | (n = 197) | (n = 209) | (n = 387) | 0.620 |
| 26 (8) | 19 (10) | 13 (6) | 30 (8) |
Values in bold indicate statistically significant results.
Values given are n (%) for variables without units of measurement and mean (SD) for variables with units of measurement.
CES-D, Center for Epidemiologic Studies Depression Scale.
No significant group differences were observed in the proportions of education, marital status, parity, alcohol consumption, smoking, employment status, and self-rated health. The postmenopausal women reported having menopausal symptoms significantly more often than the late, followed by the early peri and premenopausal women. The PA distribution differed significantly between groups, indicating that the late perimenopausal group included proportionally more medium physically active women. The distribution of signs of clinically relevant depressive symptoms, self-reported mental disorder, and the use of psycholeptics and psychoanaleptics was similar across the menopausal status groups.
The depressive symptoms score was significantly lower among the premenopausal than among early peri and postmenopausal women (Table 2). No statistically significant differences in life satisfaction or positive or negative affectivity were observed between the study groups.
TABLE 2.
Mental well-being dimensions by menopausal stages
| Variables | Premenopausal (n = 302) | Early perimenopausal (n = 196) | Late perimenopausal (n = 208) | Postmenopausal (n = 387) | P for trend |
| Depressive symptoms, mean | 0.42 (0.33) | 0.51 (0.39) | 0.46 (0.35) | 0.49 (0.40) | 0.019Pre-E.Peri 0.038Pre-L.Peri 0.534Pre-Post 0.035E.Peri-L.Peri 0.597E.Peri-Post 0.974L.Peri-Post 0.745 |
| Life satisfaction, mean | 5.20 (1.12) | 5.15 (1.05) | 5.34 (0.98) | 5.16 (1.17) | 0.264 |
| Positive effect, mean | 3.80 (0.61) | 3.82 (0.64) | 3.83 (0.63) | 3.73 (0.63) | 0.156 |
| Negative effect, mean | 1.51 (0.46) | 1.56 (0.50) | 1.55 (0.51) | 1.56 (0.54) | 0.499 |
Values in bold indicate statistically significant results.
Values given are mean (SD).
A significant association between menopausal status and depressive symptoms was observed after controlling for age, marital status, parity, employment status, self-reported mental disorders, and use of psycholeptics and psychoanaleptics (Table 3). In the adjusted model for depressive symptoms, the early peri and postmenopausal women showed higher values than the premenopausal women. When menopausal symptoms were included in this regression analysis, the association between menopausal status and depressive symptoms remained, but was of a lower magnitude. No significant association of menopausal status with negative affectivity (Table 4), life satisfaction (Table 5), or positive affectivity (Table 6) was observed.
TABLE 3.
Multiple linear regression analysis for depressive symptoms as dependent variable
| Depressive symptoms | |||||||||
| Predictors | Estimates | CI | P | Estimates | CI | P | Estimates | CI | P |
| (Intercept) | 1.10 | 0.50 to 1.71 | <0.001 | 1.03 | 0.44 to 1.62 | 0.001 | 1.04 | 0.46 to 1.63 | <0.001 |
| Early perimenopausala | 0.09 | 0.02 to 0.16 | 0.009 | 0.08 | 0.02 to 0.15 | 0.016 | 0.06 | −0.01 to 0.12 | 0.084 |
| Late perimenopausala | 0.06 | −0.01 to 0.13 | 0.082 | 0.07 | 0.01 to 0.14 | 0.032 | 0.04 | −0.02 to 0.11 | 0.222 |
| Postmenopausala | 0.09 | 0.03 to 0.15 | 0.003 | 0.11 | 0.05 to 0.17 | <0.001 | 0.07 | 0.01 to 0.13 | 0.034 |
| Age | −0.01 | −0.02 to −0.00 | 0.045 | −0.01 | −0.02 to 0.00 | 0.042 | −0.01 | −0.02 to −0.00 | 0.022 |
| Married/partnershipb | −0.04 | −0.13 to 0.04 | 0.321 | −0.02 | −0.11 to 0.06 | 0.597 | −0.03 | −0.12 to 0.05 | 0.480 |
| Divorcedb | 0.04 | −0.06 to 0.14 | 0.459 | 0.05 | −0.05 to 0.15 | 0.349 | 0.04 | −0.05 to 0.14 | 0.379 |
| Parity (1 or 2)c | −0.04 | −0.11 to 0.04 | 0.307 | −0.04 | −0.11 to 0.04 | 0.340 | −0.04 | −0.11 to 0.04 | 0.338 |
| Parity (3 or >)c | −0.10 | −0.18 to −0.02 | 0.020 | −0.08 | −0.16 to 0.00 | 0.055 | −0.07 | −0.15 to 0.01 | 0.077 |
| Not employedd | 0.15 | 0.08 to 0.21 | <0.001 | 0.12 | 0.05 to 0.18 | 0.001 | 0.12 | 0.05 to 0.18 | <0.001 |
| Self-reported mental disorders (yes)e | 0.22 | 0.13 to 0.31 | <0.001 | 0.22 | 0.13 to 0.30 | <0.001 | |||
| User of psycholeptics (N05)f | 0.14 | −0.01 to 0.28 | 0.064 | 0.13 | −0.01 to 0.28 | 0.068 | |||
| User of psychoanaleptics (N06)f | 0.15 | 0.06 to 0.23 | 0.001 | 0.14 | 0.05 to 0.23 | 0.002 | |||
| Menopausal symptoms (yes)g | 0.12 | 0.06 to 0.17 | <0.001 | ||||||
| Observations | 1,087 | 1,079 | 1,077 | ||||||
Values in bold indicate statistically significant results.
aReference category is premenopausal.
bReference category is single.
cReference category is nulliparious.
dReference category is employed.
eReference category is no mental disorder.
fReference category is no user.
gReference category is no symptoms.
TABLE 4.
Multiple linear regression analysis for negative affectivity as dependent variable
| Negative affectivity | |||||||||
| Predictors | Estimates | CI | P | Estimates | CI | P | Estimates | CI | P |
| (Intercept) | 1.45 | 0.62 to 2.28 | 0.001 | 1.37 | 0.55 to 2.19 | 0.001 | 1.39 | 0.57 to 2.20 | 0.001 |
| Early perimenopausala | 0.05 | −0.04 to 0.14 | 0.316 | 0.04 | −0.05 to 0.13 | 0.414 | 0.02 | −0.07 to 0.11 | 0.681 |
| Late perimenopausala | 0.04 | −0.05 to 0.13 | 0.367 | 0.05 | −0.04 to 0.14 | 0.295 | 0.02 | −0.07 to 0.12 | 0.624 |
| Postmenopausala | 0.05 | −0.03 to 0.14 | 0.217 | 0.06 | −0.02 to 0.14 | 0.145 | 0.03 | −0.06 to 0.11 | 0.536 |
| Age | −0.00 | −0.02 to 0.02 | 0.981 | 0.00 | −0.02 to 0.02 | 0.953 | −0.00 | −0.02 to 0.02 | 0.921 |
| Married/Partnershipb | 0.11 | −0.02 to 0.23 | 0.086 | 0.12 | −0.00 to 0.24 | 0.051 | 0.11 | −0.01 to 0.23 | 0.066 |
| Divorcedb | 0.10 | −0.04 to 0.24 | 0.171 | 0.10 | −0.04 to 0.24 | 0.147 | 0.10 | −0.04 to 0.24 | 0.164 |
| Parity (1 or 2)c | −0.01 | −0.11 to 0.09 | 0.834 | −0.02 | −0.12 to 0.08 | 0.718 | −0.02 | −0.12 to 0.08 | 0.714 |
| Parity (3 or >)c | −0.09 | −0.20 to 0.02 | 0.125 | −0.08 | −0.19 to 0.03 | 0.147 | −0.08 | −0.19 to 0.03 | 0.178 |
| Not employedd | 0.14 | 0.05 to 0.23 | 0.003 | 0.11 | 0.02 to 0.20 | 0.021 | 0.11 | 0.02 to 0.20 | 0.017 |
| Self-reported mental disorders (yes)e | 0.30 | 0.17 to 0.42 | <0.001 | 0.29 | 0.16 to 0.41 | <0.001 | |||
| User of psycholeptics (N05)f | −0.14 | −0.35 to 0.06 | 0.166 | −0.14 | −0.35 to 0.06 | 0.162 | |||
| User of psychoanaleptics (N06)f | 0.11 | −0.01 to 0.23 | 0.080 | 0.11 | −0.02 to 0.23 | 0.092 | |||
| Menopausal symptoms (yes)g | 0.10 | 0.03 to 0.18 | 0.008 | ||||||
| Observations | 1,086 | 1,078 | 1,076 | ||||||
Values in bold indicate statistically significant results.
aReference category is premenopausal.
bReference category is single.
cReference category is nulliparious.
dReference category is employed.
eReference category is no mental disorder.
fReference category is no user.
gReference category is no symptoms.
TABLE 5.
Multiple linear regression analysis for life satisfaction as dependent variable
| Life satisfaction | |||||||||
| Predictors | Estimates | CI | P | Estimates | CI | P | Estimates | CI | P |
| (Intercept) | 3.96 | 2.24 to 5.67 | <0.001 | 4.04 | 2.33 to 5.75 | <0.001 | 4.06 | 2.36 to 5.77 | <0.001 |
| Early perimenopausala | 0.02 | −0.17 to 0.21 | 0.805 | 0.04 | −0.14 to 0.23 | 0.644 | 0.08 | −0.11 to 0.27 | 0.412 |
| Late perimenopausala | 0.14 | −0.05 to 0.33 | 0.155 | 0.12 | −0.07 to 0.31 | 0.209 | 0.17 | −0.02 to 0.36 | 0.088 |
| Postmenopausala | −0.00 | −0.18 to 0.17 | 0.973 | −0.03 | −0.20 to 0.14 | 0.717 | 0.03 | −0.15 to 0.21 | 0.749 |
| Age | 0.02 | −0.02 to 0.05 | 0.375 | 0.02 | −0.02 to 0.05 | 0.364 | 0.02 | −0.02 to 0.05 | 0.333 |
| Married/partnershipb | 0.48 | 0.23 to 0.73 | <0.001 | 0.44 | 0.19 to 0.69 | 0.001 | 0.45 | 0.20 to 0.70 | <0.001 |
| Divorcedb | −0.17 | −0.46 to 0.12 | 0.250 | −0.20 | −0.48 to 0.09 | 0.185 | −0.19 | −0.48 to 0.10 | 0.196 |
| Parity (1 or 2)c | 0.16 | −0.06 to 0.37 | 0.149 | 0.16 | −0.05 to 0.37 | 0.143 | 0.16 | −0.06 to 0.37 | 0.149 |
| Parity (3 or >)c | 0.29 | 0.06 to 0.52 | 0.015 | 0.25 | 0.03 to 0.48 | 0.030 | 0.25 | 0.02 to 0.48 | 0.035 |
| Not employedd | −0.60 | −0.79 to −0.41 | <0.001 | −0.54 | −0.73 to −0.35 | <0.001 | −0.54 | −0.73 to −0.35 | <0.001 |
| Self-reported mental disorders (yes)e | −0.50 | −0.76 to −0.24 | <0.001 | −0.49 | −0.75 to −0.23 | <0.001 | |||
| User of psycholeptics (N05)f | −0.51 | −0.93 to −0.09 | 0.018 | −0.50 | −0.93 to −0.08 | 0.019 | |||
| User of psychoanaleptics (N06)f | 0.00 | −0.25 to 0.26 | 0.970 | 0.02 | −0.24 to 0.27 | 0.903 | |||
| Menopausal symptoms (yes)g | −0.16 | −0.31 to 0.00 | 0.052 | ||||||
| Observations | 1,087 | 1,079 | 1,077 | ||||||
Values in bold indicate statistically significant results.
aReference category is premenopausal.
bReference category is single.
cReference category is nulliparious.
dReference category is employed.
eReference category is no mental disorder.
fReference category is no user.
gReference category is no symptoms.
TABLE 6.
Multiple linear regression analysis for positive affectivity as dependent variable
| Positive affectivity | |||||||||
| Predictors | Estimates | CI | P | Estimates | CI | P | Estimates | CI | P |
| (Intercept) | 4.57 | 3.54 to 5.60 | <0.001 | 4.72 | 3.71 to 5.73 | <0.001 | 4.71 | 3.69 to 5.72 | <0.001 |
| Early perimenopausala | 0.05 | −0.07 to 0.16 | 0.403 | 0.05 | −0.07 to 0.16 | 0.422 | 0.07 | −0.04 to 0.19 | 0.202 |
| Late perimenopausala | 0.06 | −0.06 to 0.17 | 0.331 | 0.05 | −0.07 to 0.16 | 0.414 | 0.08 | −0.03 to 0.20 | 0.151 |
| Postmenopausala | −0.03 | −0.13 to 0.07 | 0.545 | −0.05 | −0.15 to 0.05 | 0.353 | 0.00 | −0.10 to 0.11 | 0.980 |
| Age | −0.02 | −0.04 to 0.00 | 0.121 | −0.02 | −0.04 to 0.00 | 0.083 | −0.02 | −0.04 to 0.00 | 0.118 |
| Married/partnershipb | 0.02 | −0.13 to 0.17 | 0.813 | −0.01 | −0.15 to 0.14 | 0.927 | 0.00 | −0.14 to 0.15 | 0.975 |
| Divorcedb | 0.04 | −0.13 to 0.22 | 0.635 | 0.02 | −0.15 to 0.19 | 0.785 | 0.03 | −0.14 to 0.20 | 0.760 |
| Parity (1 or 2)c | 0.02 | −0.10 to 0.15 | 0.712 | 0.02 | −0.10 to 0.15 | 0.746 | 0.02 | −0.10 to 0.15 | 0.747 |
| Parity (3 or >)c | 0.08 | −0.06 to 0.22 | 0.245 | 0.06 | −0.07 to 0.20 | 0.376 | 0.05 | −0.08 to 0.19 | 0.437 |
| Not employedd | −0.18 | −0.29 to −0.06 | 0.003 | −0.13 | −0.24 to −0.02 | 0.024 | −0.13 | −0.25 to −0.02 | 0.020 |
| Self-reported mental disorders (yes)e | −0.38 | −0.53 to −0.23 | <0.001 | −0.38 | −0.53 to −0.22 | <0.001 | |||
| User of psycholeptics (N05)f | 0.21 | −0.04 to 0.46 | 0.102 | 0.21 | −0.04 to 0.46 | 0.094 | |||
| User of psychoanaleptics (N06)f | −0.09 | −0.24 to 0.06 | 0.249 | −0.08 | −0.23 to 0.07 | 0.304 | |||
| Menopausal symptoms (yes)g | −0.14 | −0.24 to −0.05 | 0.003 | ||||||
| Observations | 1,087 | 1,079 | 1,077 | ||||||
Values in bold indicate statistically significant results.
aReference category is premenopausal.
bReference category is single.
cReference category is nulliparious.
dReference category is employed.
eReference category is no mental disorder.
fReference category is no user.
gReference category is no symptoms.
With respect to menopausal symptoms, we also performed sensitivity analyses in which we separately studied if different menopausal symptom classes would yield similar results to a model which included an aggregated menopausal symptoms variable. We observed that the results were very similar to those we obtained when using an aggregated variable for menopausal symptoms, although the strength of the association varied between different symptom classes. Therefore, we assume that the aggregated variable for menopausal symptoms was sufficient to consider in our analysis.
As shown in Table 7, in the pre and early perimenopausal women, the mean score for depressive symptoms was lower among those with high than those with medium or low physical activity, after adjusting for age, marital status, parity, employment status, self-reported mental disorder, use of psycholeptics and psychoanaleptics, and menopausal symptoms. Among the postmenopausal women, a medium or high physical activity level associated with lower scores on depressive symptoms scores than a low physical activity level. The differences in negative affectivity by menopausal status and level of physical activity were not statistically significant.
TABLE 7.
Marginal mean difference by menopausal stages and physical activity levels for negative well-being
| Depressive symptoms | Negative affectivity | |||||
| Ba | CI | P | Ba | CI | P | |
| Premenopausal | ||||||
| Low physical activity | 1.39 | 0.72 to 2.05 | 1.12 | 0.17 to 2.06 | ||
| Medium physical activity | −0.09 | −0.20 to 0.02 | 0.125 | −0.00 | −0.16 to 0.16 | 0.970 |
| High physical activity | −0.13 | −0.26 to −0.01 | 0.039 | 0.04 | −0.14 to 0.22 | 0.686 |
| Early perimenopausal | ||||||
| Low physical activity | 1.36 | 0.68 to 2.04 | 1.14 | 0.18 to 2.11 | ||
| Medium physical activity | 0.05 | −0.08 to 0.18 | 0.470 | 0.02 | −0.17 to 0.21 | 0.858 |
| High physical activity | −0.18 | −0.34 to −0.01 | 0.034 | −0.00 | −0.24 to 0.23 | 0.967 |
| Late perimenopausal | ||||||
| Low physical activity | 1.41 | 0.72 to 2.10 | 1.20 | 0.22 to 2.18 | ||
| Medium physical activity | −0.08 | −0.22 to 0.06 | 0.266 | −0.06 | −0.25 to 0.14 | 0.590 |
| High physical activity | −0.15 | −0.32 to 0.03 | 0.102 | −0.14 | −0.39 to 0.11 | 0.281 |
| Postmenopausal | ||||||
| Low physical activity | 1.52 | 0.83 to 2.21 | 1.16 | 0.18 to 2.14 | ||
| Medium physical activity | −0.21 | −0.30 to −0.12 | <0.001 | −0.05 | −0.18 to 0.08 | 0.446 |
| High physical activity | −0.20 | −0.31 to −0.10 | <0.001 | 0.02 | −0.14 to 0.17 | 0.816 |
Values in bold indicate statistically significant results.
aB coefficients for women with medium and high physical activity (PA) show the differences from the corresponding values of the women with low PA. All values are adjusted for age, marital status, parity, employment status, self-reported mental disorders, use of psycholeptics, use of psychoanaleptics, and menopausal symptoms.
For positive mental well-being, particularly positive affectivity, women with high physical activity scored significantly higher than women with low physical activity in all four menopausal status groups (Table 8). The same was observed for life satisfaction, where women with low physical activity scored significantly lower than women with medium or high physical activity in the pre, early peri, and postmenopausal groups.
TABLE 8.
Marginal mean difference by menopausal stages and physical activity levels for positive well-being
| Positive affectivity | Life satisfaction | |||||
| Ba | CI | P | Ba | CI | P | |
| Premenopausal | ||||||
| Low physical activity | 3.98 | 2.83 to 5.12 | 2.76 | 0.82 to 4.70 | ||
| Medium physical activity | 0.04 | −0.15 to 0.23 | 0.695 | 0.54 | 0.21 to 0.87 | 0.001 |
| High physical activity | 0.34 | 0.13 to 0.56 | 0.002 | 0.79 | 0.43 to 1.16 | <0.001 |
| Early perimenopausal | ||||||
| Low physical activity | 3.91 | 2.75 to 5.08 | 3.13 | 1.16 to 5.11 | ||
| Medium physical activity | 0.20 | −0.02 to 0.43 | 0.080 | 0.12 | −0.26 to 0.50 | 0.541 |
| High physical activity | 0.63 | 0.35 to 0.91 | <0.001 | 0.63 | 0.16 to 1.11 | 0.009 |
| Late perimenopausal | ||||||
| Low physical activity | 4.04 | 2.86 to 5.22 | 3.09 | 1.09 to 5.09 | ||
| Medium physical activity | 0.11 | −0.14 to 0.35 | 0.393 | 0.41 | −0.00 to 0.82 | 0.052 |
| High physical activity | 0.37 | 0.07 to 0.67 | 0.017 | 0.42 | −0.09 to 0.94 | 0.104 |
| Postmenopausal | ||||||
| Low physical activity | 3.86 | 2.68 to 5.05 | 3.01 | 1.00 to 5.01 | ||
| Medium physical activity | 0.26 | 0.11 to 0.42 | 0.001 | 0.38 | 0.12 to 0.65 | 0.004 |
| High physical activity | 0.35 | 0.16 to 0.53 | <0.001 | 0.42 | 0.10 to 0.73 | 0.009 |
Values in bold indicate statistically significant results.
aB coefficients for women with medium and high physical activity (PA) show the differences from the corresponding values of the women with low PA. All values are adjusted for age, marital status, parity, employment status, self-reported mental disorders, use of psycholeptics, use of psychoanaleptics, and menopausal symptoms.
DISCUSSION
This study examined the association between menopausal status and mental well-being, and the role of physical activity in this association, among 47 to 55-year-old women. Our data suggest that menopausal status is associated with negative mental well-being (ie, depressive symptoms), but not with negative affectivity or positive mental well-being (ie, life satisfaction and positive affectivity). The postmenopausal women reported more depressive symptoms than the premenopausal women. Furthermore, the pre, early peri, and postmenopausal women with high physical activity experienced depressive symptoms less often, were more satisfied with life, and had higher positive affectivity scores than their low physically active counterparts. Pre and postmenopausal women reporting medium physical activity were more satisfied with life than those reporting low physical activity. At the same time, the postmenopausal women with a medium physical activity level experienced depressive symptoms less often and had higher positive affectivity scores than those with a low level of physical activity.
Previous studies measuring positive well-being or quality of life have suggested that mental well-being may be more affected by different social, psychological, and health factors than by menopausal status per se.7,37 Studies on the negative dimensions of mental well-being have found an association between menopause and an elevated level of ill-being. For example, a longitudinal study with 405 participants followed for 4 years reported an increase in negative affect and decrease in positive affect in the first 2 years after the final menstruation.38 When the follow-up was extended to 9 years with 226 of the same participants, the results showed a decline in negative, but not in positive mood. Consequently, well-being increased after the menopause transition.39 In another cross-sectional comparison, the early perimenopausal women showed the highest rate of psychological distress, even after controlling for vasomotor symptoms and sleep difficulties.40 Interestingly, a longitudinal follow-up study of the same cohort over 5 years reported an increase in depressive symptoms when women entered the early perimenopausal stage.8
Some studies highlight that women are more susceptible to the development of depression in the late perimenopausal stage than in the pre or postmenopausal stages.40-42 However, when menopausal status was closely monitored employing FSH measures, the late peri and early postmenopausal women showed a similar risk for developing depression relative to the premenopausal level.43 The fact that the postmenopausal status of our participants was established over a 6-month period may explain the peak observed in depressive symptoms in the postmenopausal group, as these women were in a relatively early postmenopausal stage. The nonsignificant difference between the pre and late postmenopausal women in depressive symptoms is, however, somewhat unexpected. It may be due to high variability in depressive symptoms in this group and/or to difficulties in establishing temporal continuity of a rate of depressive symptoms and changes in reproductive function due to the use of a cross-sectional design.
When we added menopausal symptoms to the regression models, the association between menopausal status and depressive symptoms remained only in the postmenopausal group. A similar observation has been made previously.16 Closer scrutiny of a specific group of symptoms (eg, vasomotor symptoms, somatic or pain symptoms, psychological symptoms, and urogenital symptoms) yielded similar results. This suggests that the association between depressive symptoms and menopausal status may partially be explained by menopausal symptoms: that is, experienced menopausal symptoms may act as a mediator between a fall in estrogen levels and depressive symptoms. However, given that the fall in estrogen extends beyond menopause, it remains unclear whether estrogen deficiency is a primary cause of the elevation in depressive symptoms, as longitudinal studies have reported that depressive symptoms and negative mood return to premenopausal levels in postmenopause.7,16
In contrast to negative mental well-being (eg, depressive symptoms), which fluctuated across the menopausal status groups, positive mental well-being was maintained across the studied groups. Positive well-being is related to multiple outcomes such as employment and family life, and also health and longevity.4,44 Thus, positive mental well-being can be viewed as a resource that compensates for an increase in negative well-being.14
Physical activity promotes higher quality of life both in middle-aged women18 and during menopause.45 Studies have reported a positive role of physical activity in decreasing anxiety and depression among menopausal women.46,47 Our previous study with the present cohort48 suggests further that physical activity attenuates the menopause-related decline in physical fitness, which, theoretically, may be linked to improvements in mental well-being.49
While much research effort has been expended on the direct link between menopausal status and mental well-being, less attention has been paid to the possible role of physical activity in this link. Slaven and Lee50 assessed acute psychological response to exercise among menopausal women. They concluded that regularly exercising women reported a lower level of negative mood in comparison to non-exercising women irrespective of menopausal status. While this result may be explained by a transient reduction in anxiety following an exercise bout, our study, which assessed leisure physical activity, extends it to long-term differences in habitual physical activity. The present results showed that irrespective of menopausal status high physically active women showed better positive well-being. For negative well-being, however, we observed that the difference in depressive symptoms as a function of physical activity was more pronounced in the postmenopausal than pre- or perimenopausal women. Negative affectivity was the only dimension of mental well-being included in this study that was not linked to physical activity.
The same beneficial role of physical activity had been reported previously19 showing that among the postmenopausal group only, highly physically active women had a lower level of depressive symptoms, perceived stress, and anxiety than the less physically active women. Moreover, in the “late-transition group” (late perimenopausal women), the level of depressive symptoms was highest among the “moderately,” but not highly active women. We observed a similar beneficial effect of physical activity with respect to depressive symptoms. Particularly, the postmenopausal women with a medium and high physical activity level had a lower level of depressive symptoms than their low physically active counterparts, whereas among the pre and perimenopausal women, such a difference was less clear. These results suggest that low physically active women form a subgroup potentially vulnerable to an increased level of depressive symptoms during menopause. However, the reverse relationship is also possible. That is, women with a higher level of depressive symptoms may be less physically active.51 A longitudinal analysis, based on middle-aged women and men, showed that high mental well-being contributed to physical activity rather than vice versa.52 It may also be the case here that low physical activity during early perimenopause20 may partially be explained by a higher rate of depressive symptoms.
Positive mental well-being is not merely the opposite of negative mood, but has a specific independent and positive relation to health.53,54 Our study suggests that, particularly during the early postmenopausal stage, a medium level of physical activity may be enough to maintain positive mental well-being. More focused research on physical activity intensity as a way of alleviating the menopause-related decline in mental well-being is needed.
The link between physical activity and mental well-being during the menopause transition is complex and may involve several mechanisms, ranging from positive social interaction and improving personal resources to alleviating menopausal symptoms that contribute to enhancing affective state. Our results suggest that physical activity may independently of menopausal symptoms help maintain positive mental well-being and counteract the possible negative influence of menopause on depressive symptoms. However, due to the cross-sectional study design, we cannot exclude the opposite association. That is, it is equally possible that physically active women experience a lower level of menopausal symptoms; if so, having a lower level of menopausal symptoms may explain the greater mental well-being found in the high physically active women. Consequently, the present results should be interpreted as demonstrating that while menopausal status, mental well-being, and physical activity are linked to each other, longitudinal data are required to reveal their specific causal and possibly cumulative associations.
Issues of causality aside, habitual physical activity may help the accumulation over time of positive affective states, which have been highlighted as an important psychological resource55 and which may be beneficial for a woman's ability to cope well and to feel in control of her menopausal symptoms.56 Thus, physical activity programs can be viewed as an alternative to hormonal therapy as they help to alleviate various psychological symptoms.19 While hormonal therapy remains an effective treatment option in managing symptoms,57 its use has declined substantially due to concerns and uncertainty about the possible link between hormonal therapy and risk for coronary heart disease, stroke, and breast cancer.58 In addition, a majority of menopausal women have reported a desire for life-style change programs (eg, physical activity).59 The present data extend previous findings on the importance of a medium or higher level of physical activity for maintaining physical fitness48 and mental well-being during menopause.
Several limitations of this study should be noted. First, although we controlled for some important confounders (age, marital status, parity, employment status, self-reported mental disorder, use of psycholeptics and psychoanaleptics, and menopausal symptoms) related to well-being, we did not include other life events that may influence mental well-being. Second, the cross-sectional nature of the research design did not allow us to control for the initial levels of depressive symptoms or for the duration of the menopause transition, or for exposure to menopausal symptoms. All these factors may contribute to the level of the measured mental well-being variables.10,16 In addition, menopausal symptoms were studied broadly as an aggregated entity. Although we also ran analyses in which different symptoms were included separately to check the robustness of the results, different menopausal symptoms (eg, vasomotor symptoms vs psychological symptoms) may differ in their influence on mental well-being, a possibility that was not fully captured by the present analysis. We assumed that physical activity contributes to improved well-being; however, the reverse or a bidirectional association is also possible. Therefore, further studies with a prospective design controlling for potential confounders are needed before these findings can be translated into effective interventions. Third, physical activity was assessed with a self-report questionnaire, which may cause underestimation of the number of low and overestimation of the number of high physically active individuals.60 However, the questionnaire used captures diverse forms of leisure physical activity and has shown good outcomes when compared with accelerometer-based physical activity and mobility measures among middle-aged women who participated in this study.61 In future studies, however, it would be useful to complement the present self-reported measurements of physical activity with accelerometer-based data to see if the observed benefits of physical activity for mental well-being are replicated.
The strength of this study is the careful categorization of menopausal status through the use of menstrual diaries and hormonal analysis following the STRAW+10 criteria. Many previous studies have assessed menopausal status using self-report questionnaires without hormone measurements and thus may have misclassified some participants, making it difficult to establish the role of menopause in well-being. In the present study, we consider the possibility of misclassification to be minimal.
Previous cross-sectional studies have reported on the relationship between mental well-being and menopausal status without controlling for self-reported mental disorders or use of medications that can influence mental well-being. As suggested by earlier longitudinal studies, a prior level of depressive symptoms may increase the rate of depressive symptoms during menopause10,16; our study contributes information about these important confounders to the existing models. Moreover, this study recruited a large homogeneous cohort of relatively healthy, nonseverely obese women, thereby reducing the possible influence of unobservable variables (such as ethnicity, income or access to health care).
CONCLUSIONS
This study showed that while menopausal status is associated with elevated depressive symptomatology, it does not compromise positive mental well-being (ie, life satisfaction or positive affectivity). A high level of physical activity, irrespective of menopausal status, was related to better mental well-being, particularly to a lower level of depressive symptoms and higher levels of satisfaction with life and positive affect. A medium level of physical activity was associated with higher satisfaction with life in the pre and postmenopausal women and with lower depressive symptoms and higher positive affectivity among the postmenopausal women. Thus physical activity might alleviate the potential negative influence of menopause on mental well-being. It would therefore be important to ascertain the causal links between menopausal symptoms, physical activity, and mental well-being.
Supplementary Material
Footnotes
Funding/support: This study was supported by funding from the European Union's Horizon 2020 research and innovation Programme under the Marie Sklodowska-Curie grant agreement No 675003, and by the Academy of Finland (ERMA study grant agreement 275323, Vuokko Kovanen).
Financial disclosure/conflicts of interest: None reported.
REFERENCES
- 1.Schoenaker DA, Jackson CA, Rowlands JV, Mishra GD. Socioeconomic position, lifestyle factors and age at natural menopause: a systematic review and meta-analyses of studies across six continents. Int J Epidemiol 2014; 43:1542–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rannevik G, Jeppsson S, Johnell O, Bjerre B, Laurell-Borulf Y, Svanberg L. “Reprint of” A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas 2008; 61:67–77. [DOI] [PubMed] [Google Scholar]
- 3.Friedman HS, Kern ML. Personality, well-being, and health. Annu Rev Psychol 2014; 65:719–742. [DOI] [PubMed] [Google Scholar]
- 4.Steptoe A, Wardle J. Enjoying Life and Living Longer. Arch Intern Med 2012; 172:273–275. [DOI] [PubMed] [Google Scholar]
- 5.Soares CN, Zitek B. Reproductive hormone sensitivity and risk for depression across the female life cycle: a continuum of vulnerability? J Psychiatry Neurosci JPN 2008; 33:331. [PMC free article] [PubMed] [Google Scholar]
- 6.Moilanen JM, Aalto A-M, Raitanen J, Hemminki E, Aro AR, Luoto R. Physical activity and change in quality of life during menopause-an 8-year follow-up study. Health Qual Life Outcomes 2012; 10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith-DiJulio K, Woods NF, Mitchell ES. Well-being during the menopausal transition and early postmenopause: a longitudinal analysis. Menopause 2008; 15:1095–1102. [DOI] [PubMed] [Google Scholar]
- 8.Bromberger JT, Matthews KA, Schott LL, et al. Depressive symptoms during the menopausal transition: the Study of Women's Health Across the Nation (SWAN). J Affect Disord 2007; 103:267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry 2006; 63:385–390. [DOI] [PubMed] [Google Scholar]
- 10.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry 2006; 63:375–382. [DOI] [PubMed] [Google Scholar]
- 11.Lachman ME. Development in Midlife. Annu Rev Psychol 2004; 55:305–331. [DOI] [PubMed] [Google Scholar]
- 12.Lachman ME, Teshale S, Agrigoroaei S. Midlife as a pivotal period in the life course: balancing growth and decline at the crossroads of youth and old age. Int J Behav Dev 2015; 39:20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kokko K, Feldt T. Longitudinal profiles of mental well-being as correlates of successful aging in middle age. Int J Behav Dev 2017; 42:485–495. [Google Scholar]
- 14.Brown L, Bryant C, Judd FK. Positive well-being during the menopausal transition: a systematic review. Climacteric 2015; 18:456–469. [DOI] [PubMed] [Google Scholar]
- 15.Sternfeld B, Dugan S. Physical activity and health during the menopausal transition. Obstet Gynecol Clin North Am 2011; 38:537–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avis NE, Brambilla D, McKinlay SM, Vass K. A longitudinal analysis of the association between menopause and depression Results from the Massachusetts women's health study. Ann Epidemiol 1994; 4:214–220. [DOI] [PubMed] [Google Scholar]
- 17.Elavsky S. Physical activity, menopause, and quality of life: the role of affect and self-worth across time. Menopause 2009; 16:265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansikkamäki K, Raitanen J, Malila N, et al. Physical activity and menopause-related quality of life: a population-based cross-sectional study. Maturitas 2015; 80:69–74. [DOI] [PubMed] [Google Scholar]
- 19.Nelson DB, Sammel MD, Freeman EW, Lin H, Gracia CR, Schmitz KH. Effect of physical activity on menopausal symptoms among urban women. Med Sci Sports Exerc 2008; 40:50–58. [DOI] [PubMed] [Google Scholar]
- 20.Lovejoy J, Champagne C, de Jonge L, Xie H, Smith S. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes 20052008; 32:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karine D, Denis P, Rémi R-L, et al. Effects of the menopausal transition on factors related to energy balance. A MONET group study: I. Energy expenditure. Eur J Clin Nutr 2013; 67:407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Netz Y, Wu M-J, Becker BJ, Tenenbaum G. Physical activity and psychological well-being in advanced age: a meta-analysis of intervention studies. Psychol Aging 2005; 20:272–284. [DOI] [PubMed] [Google Scholar]
- 23.Reed J, Buck S. The effect of regular aerobic exercise on positive-activated affect: a meta-analysis. Psychol Sport Exerc 2009; 10:581–594. [Google Scholar]
- 24.Chekroud SR, Gueorguieva R, Zheutlin AB, et al. Association between physical exercise and mental health in 1·2 million individuals in the USA between 2011 and 2015: a cross-sectional study. Lancet Psychiatry 2018; 5:739–746. [DOI] [PubMed] [Google Scholar]
- 25.Kovanen V, Aukee P, Kokko K, et al. Design and protocol of Estrogenic Regulation of Muscle Apoptosis (ERMA) study with 47 to 55-year-old women's cohort: novel results show menopause-related differences in blood count. Menopause 2018; 25:1020–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harlow SD, Gass M, Hall JE, et al. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause 2012; 19:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laakkonen EK, Kulmala J, Aukee P, et al. Female reproductive factors are associated with objectively measured physical activity in middle-aged women. PLoS One 2017; 12:e0172054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas 1977; 1:385–401. [Google Scholar]
- 29.Boyd, Weissman MM, Thompson W, Myers JK. Screening for depression in a community sample: understanding the discrepancies between depression symptom and diagnostic scales. Arch Gen Psychiatry 1982; 39:1195–1200. [DOI] [PubMed] [Google Scholar]
- 30.Thompson ER. Development and validation of an internationally reliable short-form of the Positive and Negative Affect Schedule (PANAS). J Cross-Cult Psychol 2007; 38:227–242. [Google Scholar]
- 31.Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. J Pers Assess 1985; 49:71–75. [DOI] [PubMed] [Google Scholar]
- 32.Hirvensalo M, Lampinen P, Rantanen T. Physical exercise in old age: an eight-year follow-up study on involvement, motives, and obstacles among persons age 65-84. J Aging Phys Act 1998; 6:157–168. [Google Scholar]
- 33.World Health Organization: Collaborating Center for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment. 2017. Available at: https://www.whocc.no/atc_ddd_index Accessed August 12, 2019. [Google Scholar]
- 34.Robinson CD, Tomek S, Schumacker R. Tests of moderation effects: difference in simple slopes versus the interaction term. Mult Linear Regres Viewp 2013; 39:16–25. [Google Scholar]
- 35.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. 2016. Available at: http://www.r-project.org/ Accessed August 10, 2016. [Google Scholar]
- 36.R Package, Fife D. Fifer: A Biostatisticians Toolbox for Various Activities, Including Plotting, Data Cleanup, and Data Analysis. 2017. [Google Scholar]
- 37.Smith-DiJulio K, Woods NF, Mitchell ES. Well-being during the menopausal transition and early postmenopause: a within-stage analysis. Womens Health Issues 2008; 18:310–318. [DOI] [PubMed] [Google Scholar]
- 38.Dennerstein L, Dudley E, Burger H. Well-being and the menopausal transition. J Psychosom Obstet Gynecol 1997; 18:95–101. [DOI] [PubMed] [Google Scholar]
- 39.Dennerstein L, Lehert P, Guthrie J. The effects of the menopausal transition and biopsychosocial factors on well-being. Arch Womens Ment Health 2002; 5:15–22. [DOI] [PubMed] [Google Scholar]
- 40.Bromberger JT, Meyer PM, Kravitz HM, et al. Psychologic distress and natural menopause: a multiethnic community study. Am J Public Health 2001; 91:1435–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hay AG, Bancroft J, Johnstone EC. Affective symptoms in women attending a menopause clinic. Br J Psychiatry 1994; 164:513–516. [DOI] [PubMed] [Google Scholar]
- 42.Stewart DE, Boydell K, Derzko C, Marshall V. Psychologic distress during the menopausal years in women attending a menopause clinic. Int J Psychiatry Med 1992; 22:213–220. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt PJ, Haq N, Rubinow DR. A longitudinal evaluation of the relationship between reproductive status and mood in perimenopausal women. Am J Psychiatry 2004; 161:2238–2244. [DOI] [PubMed] [Google Scholar]
- 44.Lyubomirsky S, King L, Diener E. The benefits of frequent positive affect: does happiness lead to success? Psychol Bull 2005; 131:803–855. [DOI] [PubMed] [Google Scholar]
- 45.Elavsky S, McAuley E. Physical activity, symptoms, esteem, and life satisfaction during menopause. Maturitas 2005; 52:374–385. [DOI] [PubMed] [Google Scholar]
- 46.Asbury EA, Chandrruangphen P, Collins P. The importance of continued exercise participation in quality of life and psychological well-being in previously inactive postmenopausal women: a pilot study. Menopause 2006; 13:561–567. [DOI] [PubMed] [Google Scholar]
- 47.Mirzaiinjmabadi K, Anderson D, Barnes M. The relationship between exercise, body mass index and menopausal symptoms in midlife Australian women. Int J Nurs Pract 2006; 12:28–34. [DOI] [PubMed] [Google Scholar]
- 48.Bondarev D, Laakkonen EK, Finni T, et al. Physical performance in relation to menopause status and physical activity. Menopause 2018; 25:1432–1441. [DOI] [PubMed] [Google Scholar]
- 49.Baumeister SE, Leitzmann MF, Bahls M, et al. Associations of leisure-time and occupational physical activity and cardiorespiratory fitness with incident and recurrent major depressive disorder, depressive symptoms, and incident anxiety in a general population. J Clin Psychiatry 2017; 78:e41–e47. [DOI] [PubMed] [Google Scholar]
- 50.Slaven L, Lee C. Psychological effects of exercise among adult women: the impact of menopausal status. Psychol Health 1994; 9:297–303. [DOI] [PubMed] [Google Scholar]
- 51.van Gool CH, Kempen GIJM, Penninx BWJH, Deeg DJH, Beekman ATF, van Eijk JTM. Relationship between changes in depressive symptoms and unhealthy lifestyles in late middle aged and older persons: results from the Longitudinal Aging Study Amsterdam. Age Ageing 2003; 32:81–87. [DOI] [PubMed] [Google Scholar]
- 52.Kekäläinen T, Freund AM, Sipilä S, Kokko K. Cross-sectional and longitudinal associations between leisure time physical activity, mental well-being and subjective health in middle adulthood. Appl Res Qual Life 2019; 1–18. [Google Scholar]
- 53.Diener E, Chan MY. Happy people live longer: subjective well-being contributes to health and longevity. Appl Psychol Health Well-Being 2011; 3:1–43. [Google Scholar]
- 54.Pressman SD, Cohen S. Does positive affect influence health? Psychol Bull 2005; 131:925. [DOI] [PubMed] [Google Scholar]
- 55.Hobfoll SE. Conservation of resources: a new attempt at conceptualizing stress. Am Psychol 1989; 44:513–524. [DOI] [PubMed] [Google Scholar]
- 56.Kishida M, Elavsky S. Daily physical activity enhances resilient resources for symptom management in middle-aged women. Health Psychol 2015; 34:756–764. [DOI] [PubMed] [Google Scholar]
- 57.The NAMS 2017 Hormone Therapy Position Statement Advisory Panel The 2017 hormone therapy position statement of The North American Menopause Society. Menopause 2017; 24:728–753. [DOI] [PubMed] [Google Scholar]
- 58.Gartlehner G, Patel SV, Feltner C, et al. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: Evidence report and systematic review for the US Preventive Services Task Force. JAMA 2017; 318:2234–2249. [DOI] [PubMed] [Google Scholar]
- 59.Marlatt KL, Beyl RA, Redman LM. A qualitative assessment of health behaviors and experiences during menopause: a cross-sectional, observational study. Maturitas 2018; 116:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cerin E, Cain KL, Oyeyemi AL, et al. Correlates of agreement between accelerometry and self-reported physical activity. Med Sci Sports Exerc 2016; 48:1075–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hyvärinen M, Sipilä S, Kulmala J, et al. Validity and reliability of a single question for leisure time physical activity assessment in middle-aged women. J Aging Phys Act 2019; 1–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


