Abstract
Objective:
The aim of the study was to evaluate the effect of a single-capsule 17β-estradiol/progesterone (E2/P4), TX-001HR, on endometrial safety, to report on amenorrhea and bleeding patterns of users, and to identify predictors of amenorrhea.
Methods:
The REPLENISH trial (NCT01942668) evaluated use of TX-001HR in menopausal women (40-65 y) with vasomotor symptoms (VMS) and a uterus. Women were randomized to daily E2/P4 (mg/mg: 1/100, 0.5/100, 0.5/50, or 0.25/50), or placebo for 12 months. Incidence rate of endometrial hyperplasia was calculated from endometrial biopsies conducted at screening and study completion. Women reported bleeding and spotting in daily diaries. The number of bleeding and/or spotting days and the proportion of women with no bleeding or amenorrhea were compared between treatment and placebo using the Fisher exact test. Predictors of cumulative amenorrhea were assessed by univariate analyses.
Results:
Women (n = 1,835) who took at least one study dose comprised the safety population; 1,255 had baseline and 12-month biopsies and comprised the endometrial safety population. Incidence of endometrial hyperplasia was ≤0.36% with any dose of TX-001HR after 1 year of use (one-sided upper 95% confidence interval ≤4%). Cumulative amenorrhea (no bleeding/spotting) rates increased over time and were relatively high from cycle 1 to 13 with TX-001HR (56%-73%; placebo 79%; P < 0.05 except with 0.25/50 dose). Few vaginal bleeding adverse events (1.0%-4.6% TX-001HR vs 0.7% placebo) were reported and discontinuations due to bleeding were low (0.4%-1.4% vs 0%). Cumulative amenorrhea was significantly more frequent in older women, those further from their last menstrual period, and those with lower baseline E2 concentrations (all; P < 0.01).
Conclusions:
All doses of TX-001HR provided endometrial protection and were associated with an improved bleeding profile over time; older age, further last menstrual period, or lower baseline E2 may predict amenorrhea with TX-001HR.
Keywords: Amenorrhea, Bleeding, Endometrial hyperplasia, Estradiol, Progesterone
Estrogens are the pharmacologic treatment of choice for most postmenopausal women with moderate to severe vasomotor symptoms (VMS). Unopposed estrogen therapy is, however, well known to be associated with an increased incidence of endometrial cancer in postmenopausal women with a uterus.1 This effect is mitigated by adding a progestogen to estrogen therapy, with progesterone (P4) as one of the more common progestogens used. The REPLENISH trial evaluated TX-001HR (TherapeuticsMD, Boca Raton, FL), a once-daily, oral capsule containing bioidentical E2 and P4 as active ingredients, biochemically identical to their endogenous counterparts. This is the first time that E2 and P4 have been studied together and combined in a single, oral capsule for the treatment of moderate to severe VMS in postmenopausal women with a uterus. One of the primary objectives of the REPLENISH trial was to determine whether TX-001HR would protect the endometrium by having a yearly hyperplasia incidence rate of less than 1%, as required by the US Food and Drug Administration (FDA) guidance and similar to an untreated population.2
The efficacy and overall safety of TX-001HR for the treatment of moderate to severe VMS in postmenopausal women with an intact uterus were recently reported,3 and the 1 mg E2/100 mg P4 dose (Bijuva) was approved by the FDA in October 2018 for the treatment of moderate to severe VMS in postmenopausal women.4 We report here the effect of TX-001HR used daily for 1 year on endometrial safety (ES), review amenorrhea and bleeding patterns of users, and identify predictors of amenorrhea with its use.
METHODS
Study design
REPLENISH (NCT01942668) was a phase 3, randomized, double-blind, placebo-controlled, multicenter trial that evaluated TX-001HR in postmenopausal women with a uterus. The study was conducted in accordance with Good Clinical Practice and the protocol was approved by an institutional review board; all women provided written informed consent before participation.
Study design details have been published elsewhere.3 Women with moderate to severe hot flushes (≥7/day or ≥50/wk) were included in a VMS substudy and were randomized 1:1:1:1:1 to daily E2/P4 (mg/mg) of 1/100, 0.5/100, 0.5/50, or 0.25/50, or placebo for 12 months. Women not meeting VMS substudy eligibility were randomized 1:1:1:1 to the same active E2/P4 doses only. Women randomized either way could be eligible to be part of the primary safety endpoint analysis of endometrial hyperplasia, as described below.3 Per study protocol, treatments were taken orally at bedtime with food as it has been shown that concomitant food ingestion increases the bioavailability of progesterone.4 Randomization was performed at each site using a reproducible, computer-generated, block randomization schedule, and all study investigators and participants were blinded to treatment.
Study participants
Complete eligibility criteria for study participation were described previously.3 Women were required to be between the ages of 40 and 65 years, postmenopausal (defined as ≥12 mo of spontaneous amenorrhea, or at least 6 mo of spontaneous amenorrhea with screening serum follicle-stimulating hormone level of >40 mIU/mL, or ≥6 wk postsurgical bilateral oophorectomy), and seeking treatment or relief for VMS associated with menopause.
Of relevance to ES, women were excluded if they reported a history of endometrial hyperplasia, uterine or endometrial cancer, or undiagnosed vaginal bleeding. Women with an abnormal endometrial biopsy at baseline (hyperplasia or atypia or uterine polyps with atypia) were excluded. An abnormal Pap smear at the time of screening was also a basis for exclusion.
Women could not have recently used any estrogen pellets or progestational-injected drugs (within 6 mos); intrauterine device (within 12 wks); any oral, transdermal, or vaginal estrogen (with or without progestin), selective estrogen receptor modulator, or androgen preparation (within 8 wks); any CYP3A4 enzyme inducer or inhibitor (within 4 wks); or any preparation (including over-the-counter) that could alter estrogen or P4 activity or VMS symptoms (within 4 wks). CYP3A4 inducers included phenobarbital, carbamazepine, and rifampin and CYP3A4 inhibitors included erythromycin, clarithromycin, ketoconazole, itraconazole, ritonavir, pomegranate, and grapefruit juice. Throughout the trial, women were prohibited from using any estrogen, progestin, P4, or selective estrogen receptor modulator other than the study medications; any CYP3A4 inducers or inhibitors; or any medications (including herbal or nutritional preparations) that may interact with hormone therapy (HT).
Endometrial histology
The primary safety endpoint of the REPLENISH trial was the incidence of endometrial hyperplasia at 12 months in the ES population. All participants were included in the ES study population if they (1) took at least one capsule of study treatment; (2) had no major protocol violations; (3) had an acceptable biopsy at baseline (ie, at least one biopsy with evaluable tissue and no read of endometrial hyperplasia or cancer or endometrial polyp with hyperplasia, glandular atypia of any degree, or cancer); and (4) had a biopsy at month 12 or a diagnosis of endometrial hyperplasia before month 12.
All endometrial biopsies were performed using a Pipelle de Cornier Mark II by board certified gynecologists, and were sent to a central laboratory for slide preparation. Screening endometrial biopsies were centrally evaluated by two pathologists. Acceptable reports at screening included: proliferative endometrium; weakly proliferative endometrium; disordered proliferative pattern; secretory endometrium; endometrial tissue other (including benign, inactive, or atrophic fragments of endometrial epithelium, glands, stoma, etc); endometrial tissue insufficient for diagnosis; no endometrium identified; or no tissue identified.
End of treatment endometrial biopsies were read centrally by three pathologists, two of whom had been designated a priori as the primary pathologists. Each pathologist's report was classified in one of the following categories: category 1: nonendometrial malignancy/nonhyperplasia; category 2: endometrial hyperplasia with or without atypia; category 3: endometrial malignancy. Final diagnosis was based on the consensus reads of two pathologists. If the two primary pathologists disagreed, the result of the third pathologist was incorporated and the diagnosis was based on the majority. If each of the three pathologists’ reads were different, the most severe read was chosen as the final diagnosis.
Each postbaseline endometrial biopsy had to specifically note the presence or absence of endometrial polyps. If identified, polyps were categorized as a benign polyp, polyp with hyperplasia without atypia, polyp with hyperplasia with atypia, carcinomatous polyp, or polyp other. The method for histologic diagnosis of endometrial polyps was the same as for endometrial hyperplasia.
Sample size for the ES population was calculated assuming that active treatment would achieve a ≤1% incidence rate of endometrial hyperplasia at 12 months, with an upper bound of the 95% confidence interval (CI) of ≤4%.2 Therefore, with 250 women in each active treatment arm completing 12 months of treatment (and having an end-of-study biopsy readable for endometrial hyperplasia), 2 or fewer cases of endometrial hyperplasia would result in an annual incidence of ≤1%, with an upper bound of the 1-sided 95% CI of ≤2.5%. CIs were used to determine whether the rate of endometrial hyperplasia was acceptable. An incidence rate of ≤1% with an upper limit of the one-sided 95% CI being ≤4% was considered acceptably low and in accordance with FDA guidance.2
A Spearman correlation coefficient was calculated to evaluate the relationship between baseline E2 level and baseline endometrial histology.
Bleeding
All women were required to complete a daily bleeding/spotting diary. Spotting was defined as not requiring sanitary protection, while bleeding required sanitary protection. Amenorrhea was defined as the absence of bleeding or spotting. Data for bleeding/spotting and amenorrhea were based on participants in the safety population who had at least one post-baseline bleeding/spotting diary entry. The number of days with bleeding and/or spotting was summarized by cycle and treatment group. Percentages of women with no bleeding or amenorrhea were calculated by cycle, consecutive cycles, and trimester for all treatment groups. Fisher exact tests were used to compare each E2/P4 group with the placebo group.
Incidence of treatment emergent adverse events (AEs) were identified in the overall safety population (all women who took at least one capsule of treatment) using the AE case report forms. Treatment emergent AEs were summarized by System Organ Class and preferred term using MedDRA version 18.0 to identify bleeding-related AEs: metrorrhagia, postmenopausal hemorrhage, uterine hemorrhage, and vaginal hemorrhage.
Predictors of amenorrhea
The impact of age, race, body mass index (BMI), smoking, time since last menstrual period (LMP), age at LMP, tubal ligation, parity, baseline E2 concentration, and baseline frequency and severity of VMS (mild = 1 to severe = 3) was assessed on the cumulative incidence of amenorrhea after three cycles in participants of the safety population who provided bleeding/spotting diary data for more than three cycles. Logistic regressions were used to calculate odds ratio (OR) estimates.
Risks of bleeding with hypertensive medications
Although high blood pressure [SBP (systolic blood pressure) ≥140 mm Hg or DBP (diastolic blood pressure) ≥90 mm Hg] at baseline was an exclusion criterion, women who used antihypertensive medications (up to two) were allowed to enroll. The associations between the use of any antihypertensive medications, as well as angiotensin II receptor antagonists, and bleeding were evaluated. Logistic regressions were used to calculate the OR estimates.
RESULTS
Disposition and demographics
A total of 1,845 women were randomized and 1,835 took at least one capsule of study drug (safety population). Overall discontinuation rates from the study at 12 months were lower with TX-001HR (28.3%) than with placebo (38.4%); 1,275 (69.5%) women completed the entire 52-week study. Of the 1,835 participants in the safety population, 1,255 women comprised the ES population. The disposition of all study participants is shown in Figure 1.
FIG. 1.
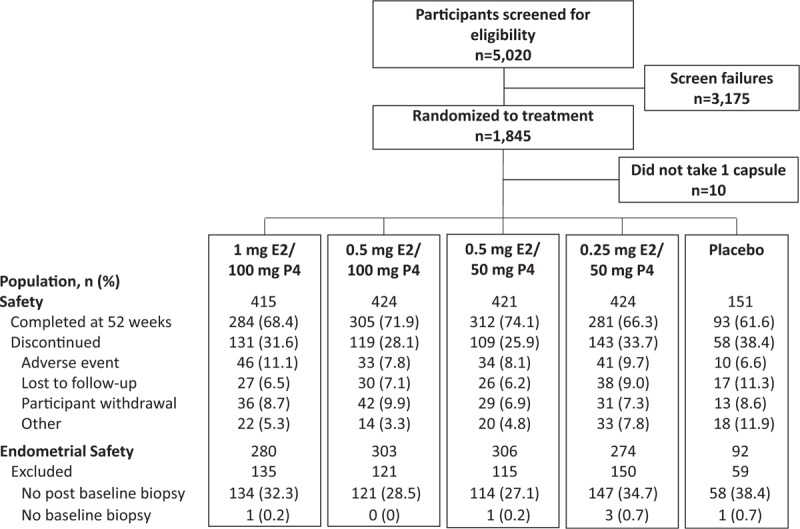
Disposition of study participants in safety and endometrial safety (ES) populations. E2, 17β-estradiol; P4, progesterone.
Women in the ES population had a mean age of 54.7 years (range, 40-65) and a mean BMI of 26.9 kg/m2 at study entry (Table 1). Approximately two-thirds of the women were white (67.3%) and one-third were African American (30.4%). The demographics for the ES population were similar to those reported for the safety population.3
TABLE 1.
Participant demographics and baseline characteristics of the endometrial safety population
| Estradiol/progesterone | |||||
| Characteristic | 1 mg/100 mg | 0.5 mg/100 mg | 0.5 mg/50 mg | 0.25 mg/50 mg | Placebo |
| n | 280 | 303 | 306 | 274 | 92 |
| Age, y | 54.8 ± 4.3 | 54.7 ± 4.5 | 54.8 ± 4.1 | 54.5 ± 4.0 | 54.3 ± 4.4 |
| Race, n (%) | |||||
| White | 191 (68.2) | 207 (68.3) | 205 (67.0) | 179 (65.3) | 62 (67.4) |
| African American | 83 (29.6) | 90 (29.7) | 92 (30.1) | 87 (31.8) | 29 (31.5) |
| Othera | 6 (2.1) | 6 (2.0) | 9 (2.9) | 8 (2.9) | 1 (1.1) |
| BMI, kg/m2 | 26.7 ± 4.2 | 26.7 ± 4.4 | 26.8 ± 3.9 | 26.7 ± 4.0 | 26.6 ± 4.0 |
| Time since menopause, y | 5.7 ± 4.7 | 6.2 ± 5.2 | 5.4 ± 436 | 5.5 ± 4.5 | 5.6 ± 4.4 |
Data presented as mean ± SD, unless stated otherwise.
BMI, body mass index; SD, standard deviation.
aOther includes: other (n = 13), Asian (n = 9), American Indian or Alaska Native (n = 4), Native Hawaiian or Pacific Islander (n = 3), and unknown (n = 1).
Endometrial histology
No cases of consensual reads (two out of three pathologists’ readings) of endometrial hyperplasia were observed during the trial. FDA assessment of the new drug application, was, assigned a single case of endometrial hyperplasia (single pathologist read) to the 1 mg E2/100 mg P4 group, resulting in an incidence rate of 0.36% with a one-sided upper 95% confidence limit of 1.97 (Table 2). No endometrial malignancies were reported during the study. The percentage of women with proliferative endometrium at month 12 ranged from 0.3% (0.5 mg E2/50 mg P4) to 2.9% (1 mg E2/100 mg P4), with no cases of proliferative endometrium in the placebo group. The incidence of endometrial polyps ranged from 1.6% to 2.3% with TX-001HR at screening and 1.4% to 3.3% at month 12; the placebo group had no polyps at screening or month 12 (Table 2). All polyps diagnosed at screening and month 12 were generally asymptomatic and pathologically benign.
TABLE 2.
Endometrial safety endpoints in endometrial safety population
| Estradiol/progesterone | |||||
| 1 mg/100 mg | 0.5 mg/100 mg | 0.5 mg/50 mg | 0.25 mg/50 mg | Placebo | |
| Treatment, n (%) | (n = 281) | (n = 303) | (n = 306) | (n = 274) | (n = 92) |
| Hyperplasia at 12 mosa | |||||
| Incidence rate | 1 (0.36) | 0 | 0 | 0 | 0 |
| One-sided upper 95% CI | 1.97% | 0.98% | 0.97% | 1.09% | 3.93% |
| Proliferative endometriumb | |||||
| Screening | 2 (0.7) | 5 (1.7) | 2 (0.7) | 1 (0.4) | 0 |
| Month 12 | 8 (2.9) | 5 (1.7) | 1 (0.3) | 3 (1.1) | 0 |
| Weakly proliferative | |||||
| Screening | 0 | 0 | 0 | 0 | 0 |
| Month 12 | 1 (0.4) | 2 (0.7) | 2 (0.7) | 2 (0.7) | 0 |
| Endometrial polyps | |||||
| Screening | 5 (1.8) | 7 (2.3) | 5 (1.6) | 5 (1.8) | 0 |
| Month 12 | 4 (1.4) | 6 (2.0) | 10 (3.3) | 7 (2.6) | 0 |
CI, confidence interval.
aAn incidence rate of ≤1% with an upper limit of the one-sided 95% CI being ≤4% was considered acceptably low.2
bIncludes proliferative and disordered proliferative endometrium.
A positive correlation was observed between baseline E2 level and baseline endometrial histology in the ES population (r = 0.167, P < 0.0001). Women with baseline E2 levels of 10 pg/mL or higher were more likely to have baseline readings of proliferative endometrium, than women with baseline E2 less than 5 pg/mL or in the 5 to <10 pg/mL range.
Bleeding
Overall, breakthrough bleeding and spotting decreased over time. Cumulative amenorrhea (consecutive cycles of no bleeding or spotting) from cycles 1 to 13 was reported by 56.1% of women in the 1 mg E2/100 mg P4 group (P < 0.001), 67.6% in the 0.5 mg E2/100 mg P4 group (P = 0.048), 68.1% in the 0.5 mg E2/50 mg P4 group (P = 0.049), and 73.1% in the 0.25 mg E2/50 mg P4 group versus 78.9% in the placebo group (Fig. 2A). Amenorrhea rates in cycle 13 ranged from 90.2% with the 1 mg E2/100 mg P4 dose to 96.2% with the lowest E2/P4 dose compared with 97.8% for placebo. Cumulative no bleeding (consecutive cycles of no bleeding) from cycle 1 to 13 was reported by 73.4% of women with 1 mg E2/100 mg P4 (P < 0.001 vs placebo), and 83.9% to 89.2% for the other E2/P4 doses versus 91.1% with placebo (Fig. 2B). At cycle 13, rates of no bleeding were more than 97% in all treatment groups and not statistically different from placebo. Percentages of women with amenorrhea were 69.9% to 79.8% with E2/P4 doses versus 89.3% with placebo during trimester 1 and increased to 82.6% to 92.8% with E2/P4 doses versus 94.6% with placebo during trimester 4 (Fig. 3).
FIG. 2.
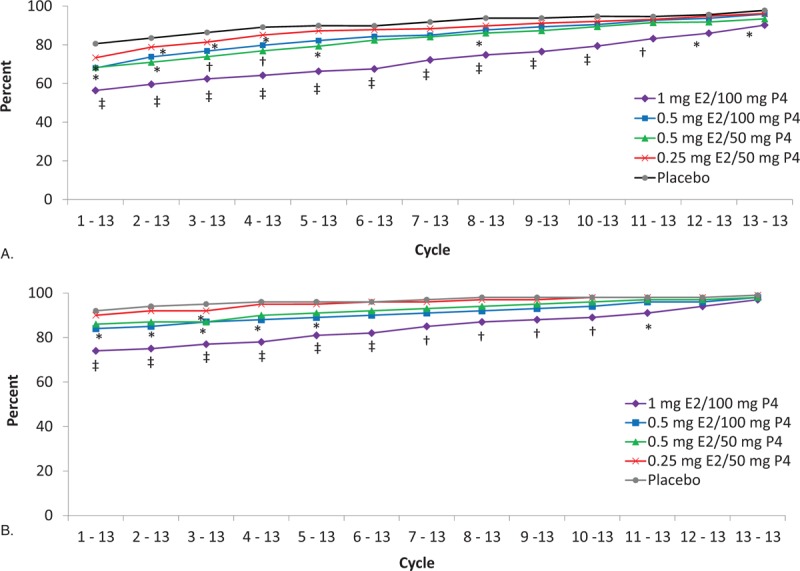
Proportion of cumulative (A) amenorrhea (no bleeding or spotting) and (B) no bleeding only from cycle 1 to 13 with TX-001HR in the safety population. ∗P < 0.05; †P ≤ 0.01; ‡P < 0.001 versus placebo. Spotting was defined as not requiring sanitary protection, whereas bleeding required sanitary protection. Cycles are 28 days in length. E2, 17β-estradiol; P4, progesterone.
FIG. 3.
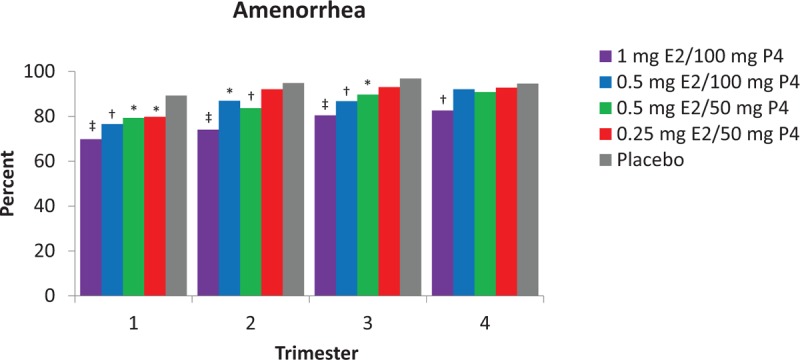
Proportion of women with amenorrhea (no bleeding or spotting) by trimester in safety population. ∗P < 0.05; †P < 0.01; ‡P < 0.001 versus placebo. E2, 17β-estradiol; P4, progesterone.
Treatment emergent bleeding-related AEs were 4.8% (n = 20) for 1 mg E2/100 mg P4, 3.3% (n = 14) for 0.5 mg/100 mg P4, 1.0% (n = 4) for 0.5 mg/50 mg P4, and 2.1% (n = 9) for 0.25 mg E2/50 mg P4. One (0.7%) bleeding AE was reported in the placebo group. Study discontinuations due to bleeding-related AEs ranged from 0.5% (0.5 mg E2/50 mg P4 dose) to 1.4% (1 mg E2/100 mg P4 dose). No women in the placebo group discontinued the study due to bleeding AEs.
Predictors of amenorrhea
Significant associations of cumulative amenorrhea (more than three cycles) were observed with age, time since LMP, and baseline E2 levels (Table 3). Older women were more likely to have amenorrhea than younger women, with the likelihood of amenorrhea increased by 27% for every 5-year increase in age (P = 0.0039). A longer time since LMP was associated with amenorrhea, with a 26% increase in amenorrhea for every 5-year increase in time since LMP (P = 0.0039). Lower baseline E2 levels were associated with increased cumulative amenorrhea; odds of amenorrhea were 171% higher with baseline E2 levels less than 5 pg/mL (P < 0.0001); and 70% higher for women with E2 levels 5 to less than 10 pg/mL (P = 0.008) than women with E2 levels of 10 pg/mL or higher. No statistically significant associations of cumulative amenorrhea were observed with race, BMI, smoking status, age at LMP, tubal ligation, parity, or baseline frequency or severity of moderate to severe VMS.
TABLE 3.
Predictors of cumulative amenorrhea (greater than three cycles) with baseline characteristics in participants of the safety population with bleeding/spotting diary data for greater than three cycles
| Parameters | OR (95% CI) | |
| Age, y | 5-y increase | 1.27 (1.08-1.50) |
| Race | White vs black | 1.21 (0.91-1.61) |
| BMI, kg/m2 | 25-<30 vs <25 | 0.99 (0.72-1.37) |
| 30+ vs <25 | 0.78 (0.55-1.11) | |
| Smoking | Current vs never | 0.91 (0.65-1.29) |
| Former vs never | 0.93 (0.67-1.27) | |
| Time since LMP | 5-y increase | 1.26 (1.08-1.47) |
| Age at LMP | 5-y increase | 0.98 (0.84-1.14) |
| Tubal ligation | Yes vs no | 0.98 (0.74-1.30) |
| Parity | Yes vs no | 1.09 (0.75-1.60) |
| Baseline E2 levels, pg/mL | <10 vs ≥10 | 2.19 (1.53-3.14) |
| 5-<10 vs ≥10 | 1.70 (1.15-2.52) | |
| <5 vs ≥10 | 2.71 (1.84-4.00) | |
| Frequency of moderate to severe VMS | ≥50/wk vs <50/wk | 0.76 (0.57-1.00) |
| ≥70/wk vs <70/wk | 1.09 (0.75-1.57) | |
| Severity of VMS | 1-Point increase | 1.17 (0.86-1.59) |
| <2.5-Point vs ≥2.5-point | 1.13 (0.81-1.59) |
OR estimates were calculated using logistic regressions. Bolded values indicate statistical significance.
BMI, body mass index; CI, confidence interval; E2, 17β-estradiol; LMP, last menstrual period; OR, odds ratio; VMS, vasomotor symptom.
Risks of bleeding with hypertensive medications
Similar risks of bleeding at any time during the study were observed between women who took hypertensives at baseline (OR 0.95, 95% CI 0.65-1.39) or during the study (OR 1.00, 95% CI 0.70-1.44) compared to those who did not take any. While the number of women who took angiotensin II receptor antagonists was very small at baseline (n = 26) and during the study (n = 37), although not significant, lower risks of bleeding were observed at baseline (OR 0.10, 95% CI 0.01-1.60) or during the study (OR 0.46, 95% CI 0.14-1.50) in women using angiotensin II receptor antagonists.
DISCUSSION
The REPLENISH trial demonstrated endometrial protection and favorable amenorrhea rates for all four doses of TX-001HR, an E2/P4 single-dose, softgel capsule, when used to treat moderate to severe VMS in menopausal women with a uterus.3 This is the first time that E2 and P4 have been combined in a single capsule for continuous use. After 1 year of TX-001HR use, the incidence of endometrial hyperplasia was less than 1%, demonstrating that endometrial protection, as defined by the FDA, was accomplished with the P4 component of TX-001HR. The high rates of cumulative amenorrhea with 1 mg E2/100 mg P4 make it a good therapeutic option for postmenopausal women seeking treatment for moderate to severe VMS and those who may be concerned about bleeding and bleeding-associated malignancy risks.
The data showing that oral TX-001HR did not cause endometrial stimulation are noteworthy because this is the first time dosages of 50 and 100 mg of P4 were shown to inhibit the stimulatory effects of E2 when taken continuously in a properly powered and randomized controlled trial. In the Postmenopausal Estrogen/Progestin Interventions trial, women taking individual capsules of 200 mg of micronized P4 cyclically 12 days out of a 28-day cycle with 0.625 mg of continuous conjugated equine estrogens had a risk of hyperplasia not different than the placebo group.1 Although the Postmenopausal Estrogen/Progestin Interventions trial was longer than the REPLENISH trial, it was not powered to show an increase in endometrial hyperplasia risk.
Prior studies evaluating HT using bioidentical E2 and P4 have required users to take two products, such as a combination of oral, transdermal, or vaginal estrogen with an oral or vaginal P4. The use of individual products, such as an estrogen patch and oral progesterone, have, however, never been systematically evaluated and may lead to an increase in the risk of endometrial cancer.5
TX-001HR is the first, single-capsule combination of E2/P4 with an FDA approval of the 1 mg E2/100 mg P4 dose for treating moderate to severe VMS in postmenopausal women with a uterus that includes P4 for endometrial protection. Previously, such a combination with good bioavailability of both E2 and P4 had been challenging to create biochemically.6 Pharmacokinetic data for TX-001HR showed that availability of the E2 and P4 was not compromised by combining them.6 Accordingly, the ES data of this single combination of E2/P4 are specific to TX-001HR and are probably not transferrable to other combinations of E2 and P4 at similar doses used together.7,8
The combination of bioidentical E2/P4 in one single capsule, as with TX-001HR, could be more convenient for women to take than separate capsules, thus, potentially increase adherence. Adherence to prescribed treatment regimens is important to ensure patient safety and treatment efficacy. It has been suggested that the failure of oral micronized P4 used as a separated agent to provide endometrial protection may be due to low compliance with or inadequate exposure to the oral P4 component,9 which should inherently improve with the use of a combined therapy. Single-dose regimens have been shown to improve patient adherence for drugs in other therapeutic areas.10-12 Thus, TX-001HR may be a more convenient HT option, as a continuous regimen of E2/P4 confirmed to inhibit the stimulatory effects of E2. Women may also prefer using a product that contains a native hormone like progesterone as opposed to using a synthetic progestogen, such as medroxyprogesterone acetate, norethisterone acetate, or drospirenone, because it has been postulated that native hormones (ie, progesterone, estradiol) have fewer risks and side effects and are equally effective for managing menopausal symptoms.13 Synthetic progestogens have been associated with a wide range of side effects,14 possibly caused by the progestins’ differential effects on target tissues.15 Furthermore, the progesterone component of TX-001HR at the doses evaluated has not been associated with any common side effects of oral progesterone (ie, somnolence, bloating).3,16
Prescribing information for five FDA-approved HT products available in the United States report 13-cycle cumulative rates of amenorrhea ranging from 23% to 49%17-21 as found in separate studies for each product; amenorrhea rates with TX-001HR ranged from 56% to 73% with E2/P4 (Table 4). In this study, the E2/P4 capsule at all doses resulted in cumulative rates of amenorrhea at or above those found with other progestogen-containing HT as reported from their respective studies. In addition, use of the E2/P4 capsule for 12 months provided endometrial protection as defined by the FDA.
TABLE 4.
Cumulative amenorrhea rates with menopausal hormone therapiesa
| Products | Doses | Cumulative amenorrhea (%) cycle 1 to cycle 13 |
| Oral | ||
| Prempro (CEE/MPA; Wyeth Pharmaceuticals Inc, Philadelphia, PA)17 | 0.625 mg/5 mg | 26 |
| 0.625 mg/2.5 mg | 23 | |
| 0.45 mg/1.5 mg | 42 | |
| 0.3 mg/1.5 mg | 45 | |
| Activella (E2/NETA; Novo Nordisk Inc, Princeton, NJ)18 | 1 mg/0.5 mg | 49 |
| Angeliq (E2/DRSP; Bayer Healthcare, Whippany, NJ)19 | 1 mg/0.5 mg | 45 |
| TX-001HR (E2/P4) | 1 mg/100 mg | 56 |
| 0.5 mg/100 mg | 68 | |
| 0.5 mg/50 mg | 69 | |
| 0.25 mg/50 mg | 73 | |
| Placebo (REPLENISH trial) | 81 | |
| Transdermal patch | ||
| CombiPatch (E2/NETA; Novartis Pharmaceuticals, East Hanover, NJ)20 | 0.05 mg/0.14 mg0.05 mg/0.25 mg | 279 |
| Climara Pro (E2/LNG; Berlex, Montville, NJ)21 | 0.045 mg/ 0.015 mg | 16 |
CEE, conjugated equine estrogens; DRSP, drospirenone; E2, 17β-estradiol; EE, ethinyl estradiol; LNG, levonorgestrel; MPA, medroxyprogesterone acetate; NETA, norethisterone acetate; P4, progesterone.
aBased on prescribing information or clinical data; not a head-to-head comparison.
Because bleeding/spotting is a major reason why women discontinue HT, both in clinical trials22 and in real-world use, knowledge of amenorrhea predictors may help clinicians counsel their patients and provide realistic expectations about potential bleeding. In the REPLENISH trial, age, time since LMP, and baseline E2 levels were predictors of cumulative amenorrhea.
Analysis of data from the Women's Health Initiative found that, among those using continuous combined 0.625 mg conjugated equine estrogen plus 2.5 mg medroxyprogesterone acetate, women with hypertension were more likely to experience endometrial spotting/bleeding than those without hypertension and women who used antihypertensive medications were more likely to experience endometrial bleeding than those not using antihypertensives.23 Women who were, however, using angiotensin II receptor antagonists or β-blockers had less endometrial spotting/bleeding than those using other antihypertensives.23 In the REPLENISH trial, the use of antihypertensive medication was not confirmed to be a risk factor for bleeding. Although not significant, a lower risk of bleeding in women using angiotensin II receptor antagonists was, however, noted and may be due to the small number of women using these antagonists. Finally, similar to the REPLENISH trial results, the Women's Health, Osteoporosis, Progestin, Estrogen study found that BMI was not significantly associated with either the mean number of bleeding days or cycles to amenorrhea.24
Study strengths of REPLENISH was that it was a double-blind, randomized, placebo-controlled, 1-year trial, designed with endometrial hyperplasia as a prospectively identified safety outcome, enrolled a large number of women, and endometrial biopsy screening excluded pre-existing endometrial pathology. Furthermore, cumulative amenorrhea and no bleeding rates were identified secondary endpoints of the trial. Our study, however, had some limitations, including the fact that all women resided in the United States, and were healthier than the general population; high-risk women were excluded from the trial based on endometrial biopsy findings at entry and results could differ from the general population. Another limitation was that bleeding risk factors were a post hoc analysis of the study. Finally, because the decision to treat was based on the presence of VMS, the influence of the VMS on vaginal bleeding risk factors cannot be excluded.
CONCLUSIONS
Use of TX-001HR for 1 year in the REPLENISH trial resulted in endometrial protection, with an incidence rate of hyperplasia less than 1% with no incidence of endometrial cancer. The cumulative rates of amenorrhea increased over time with all doses of TX-001HR. Women who were older, or with a less recent LMP, and women with lower baseline E2 concentrations were less likely to experience bleeding while using E2/P4. The 1 mg E2/100 mg P4 dose of a single-capsule, continuous regimen of bioidentical E2/P4 was approved by FDA, based on its efficacy and safety data (including ES) from the REPLENISH trial, for treatment of moderate to severe VMS in postmenopausal women with a uterus.
Acknowledgments
The authors would like to acknowledge the medical writing assistance of Liz Jennison, MD and Kathleen Ohleth, PhD of Precise Publications, LLC, which was supported by TherapeuticsMD.
Footnotes
∗This paper was presented at the North American Menopause Society annual meeting, October 11-14, 2017, Philadelphia, PA and the 16th World Congress on Menopause, June 6-9, 2018, Vancouver, Canada.
Funding/support: TherapeuticsMD sponsored the study and provided support for the medical writing assistance of Liz Jennison, MD and Kathleen Ohleth, PhD of Precise Publications, LLC.
Clinical trial registration: https://clinicaltrials.gov/ct2/show/NCT01942668.
Financial disclosure/conflicts of interest: S.R.G. is on the advisory board of AbbVie, Allergan, IBSA, Pfizer, and TherapeuticsMD, and consults for Cook ObGyn and Cooper Surgical. D.F.A. (within the past 3 years) has received research support from Actavis, Bayer Healthcare, Endoceutics, Glenmark, Merck, Radius Health, Shionogi, Actavis, Glenmark, Agile, InnovaGyn, Pfizer, Sermonix, Teva Women's Healthcare, and TherapeuticsMD; has served as consultant to AbbVie, Actavis, Agile Therapeutics, Bayer Healthcare, Endoceutics, Exeltis, InnovaGyn, Merck, Pfizer, Radius Health, Sermonix, Shionogi, Teva Women's Healthcare, and TherapeuticsMD. J.H.P. consults for Pfizer, Shionogi, and TherapeuticsMD, and has stock options with TherapeuticsMD. S.M., S.G., and B.B. are employees of TherapeuticsMD with stock/stock options. B.B. is also a Board member of TherapeuticsMD.
REFERENCES
- 1.Writing Group for the PEPI Trial Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The postmenopausal estrogen/progestin interventions (PEPI) trial. JAMA 1996; 275:370–375. [DOI] [PubMed] [Google Scholar]
- 2.US Department of Health and Human Services (FDA). Guidance for Industry: Estrogen and estrogen/progestin drug products to treat vasomotor symptoms and vulvar and vaginal atrophy symptoms--recommendations for clinical evaluation. January 2003. Available at: http://www.fda.gov/downloads/Drugs/DrugSafety/informationbyDrugClass/UCM135338.pdf Accessed December 21, 2018. [Google Scholar]
- 3.Lobo RA, Archer DF, Kagan R, et al. A 17β-estradiol-progesterone oral capsule for vasomotor symptoms in postmenopausal women: a randomized controlled trial. Obstet Gynecol 2018; 132:161–170. [DOI] [PubMed] [Google Scholar]
- 4.Bijuva (estradiol and progesterone) capsules, for oral use Prescribing Information. Boca Raton, FL: TherapeuticsMD; 2018. [Google Scholar]
- 5.Fournier A, Dossus L, Mesrine S, et al. Risks of endometrial cancer associated with different hormone replacement therapies in the E3N cohort, 1992-2008. Am J Epidemiol 2014; 180:508–517. [DOI] [PubMed] [Google Scholar]
- 6.Pickar JH, Bon C, Amadio JM, Mirkin S, Bernick B. Pharmacokinetics of the first combination 17beta-estradiol/progesterone capsule in clinical development for menopausal hormone therapy. Menopause 2015; 22:1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prometrium (Progesterone, USP) Prescribing Information. St. Petersburg, FL: Catalent Pharma Solutions; 2011. [Google Scholar]
- 8.Estrace Tablets (estradiol tablets, USP) Prescribing Information. Princeton, NJ: Bristol-Myers Squibb; 2005. [Google Scholar]
- 9.Gompel A. Micronized progesterone and its impact on the endometrium and breast vs. progestogens. Climacteric 2012; 15: suppl 1: 18–25. [DOI] [PubMed] [Google Scholar]
- 10.Sutton SS, Hardin JW, Bramley TJ, D'Souza AO, Bennett CL. Single- versus multiple-tablet HIV regimens: adherence and hospitalization risks. Am J Manag Care 2016; 22:242–248. [PubMed] [Google Scholar]
- 11.Coca A, Agabiti-Rosei E, Cifkova R, Manolis AJ, Redon J, Mancia G. The polypill in cardiovascular prevention: evidence, limitations and perspective—position paper of the European Society of Hypertension. J Hypertens 2017; 35:1546–1553. [DOI] [PubMed] [Google Scholar]
- 12.Bailey CJ, Day C. Fixed-dose single tablet antidiabetic combinations. Diabetes Obes Metab 2009; 11:527–533. [DOI] [PubMed] [Google Scholar]
- 13.Adams C, Cannell S. Women's beliefs about “natural” hormones and natural hormone replacement therapy. Menopause 2001; 8:433–440. [DOI] [PubMed] [Google Scholar]
- 14.Pickar JH, Archer DF, Kagan R, Pinkerton JV, Taylor HS. Safety and benefit considerations for menopausal hormone therapy. Expert Opin Drug Saf 2017; 1–14. [DOI] [PubMed] [Google Scholar]
- 15.Binkowska M, Woron J. Progestogens in menopausal hormone therapy. Prz Menopauzalny 2015; 14:134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kagan R, Constantine G, Kaunitz AM, Bernick B, Mirkin S. Improvement in sleep outcomes with a 17β-estradiol-progesterone oral capsule (TX-001HR) for postmenopausal women. Menopause 2019; 26:622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prempro/Premphase (conjugated estrogens/medroxyprogesterone acetate tablets) Prescribing Information. Philadelphia, PA: Wyeth Pharmaceuticals Inc; 2008. [Google Scholar]
- 18.Activella (estradiol/norethindrone acetate) tablets. Princeton, NJ: Novo Nordisk Inc; 2006. [Google Scholar]
- 19.Angeliq (drospirenone and estradiol) tablets, for oral use Prescribing Information. Whippany, NJ: Bayer Healthcare; 2005. [Google Scholar]
- 20.CombiPatch (estradiol/norethindrone acetate transdermal system) Prescribing Information. East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2005. [Google Scholar]
- 21.Climara Pro (estradiol/Levonorgestrel transdermal system) Prescribing Information. Northridge, CA: 3 M Drug Delivery Systems; 2007. [Google Scholar]
- 22.Christodoulakos GE, Botsis DS, Lambrinoudaki IV, et al. A 5-year study on the effect of hormone therapy, tibolone and raloxifene on vaginal bleeding and endometrial thickness. Maturitas 2006; 53:413–423. [DOI] [PubMed] [Google Scholar]
- 23.Sriprasert I, Beydoun H, Barnabei V, Nassir R, LaCroix AZ, Archer DF. Incidence of endometrial spotting or bleeding during continuous-combined estrogen-progestin therapy in postmenopausal women with and without hypertension. Menopause 2015; 22:1067–1075. [DOI] [PubMed] [Google Scholar]
- 24.Utian WH, Gass ML, Pickar JH. Body mass index does not influence response to treatment, nor does body weight change with lower doses of conjugated estrogens and medroxyprogesterone acetate in early postmenopausal women. Menopause 2004; 11:306–314. [DOI] [PubMed] [Google Scholar]


