Abstract
Circadian rhythms ensure that physiological processes occur at the most biologically meaningful time. The circadian timing in the gastrointestinal tract involves interlocking transcriptional and translational feedback loops that culminate in the rhythmic expression and activity of a set of clock genes and related hormones. The suprachiasmatic nucleus and peripheral core molecular clocks oscillate every 24 hours and are responsible for the periodic activity of various segments and transit along the gastrointestinal tract. Environmental cues may alter or reset these rhythms to align them with new circumstances. Colonic motility also follows a circadian rhythm with reduced nocturnal activity. Healthy humans have normal bowel motility during the day, frequently following awakening or following a meal, with minimal activity during the night. Maladjusted circadian rhythms in the bowel have been linked to digestive pathologies, including constipation and irritable bowel syndrome. Our advanced knowledge of the link between the circadian clock and gastrointestinal physiology provides potential therapeutic approaches for the treatment of gastrointestinal diseases. This review seeks to establish evidence for the correlation between circadian rhythm, bowel movements and digestive health, and examine the implications of disrupted circadian rhythms on gut physiology.
Key Words: circadian rhythm, bowel motility, clock genes, gastrointestinal tract
Many organisms have circadian clocks that anticipate daytime and establish endogenous 24-hour rhythms, which organize their physiology and behavior.1,2 The circadian cycle is orchestrated by a central pacemaker in the brain that resets the clocks in peripheral organs to control expression of key genes throughout the day.3 Circadian rhythms, driven by cell-autonomous biological clocks, take cues from cycles of light and dark, hormone levels or from the metabolic status of the individual.3,4 The first recorded reports of circadian control and the gastrointestinal system date back to the second or third century BC, where hunger was used as a time signal.5 Circadian rhythms control several gastrointestinal functions ranging from gastric enzyme and fluid production to small intestine nutrient absorption, and gastric/gut motility. Environmental cues alter or reset these rhythms to align with new circumstances.5,6 The most documented external cue, exposure to light, does not drive the circadian rhythm of the gut but merely resets the 24-hour cycle.5,6
NORMAL GUT PHYSIOLOGY
The gastrointestinal system is governed by a circadian rhythm characterized by quiescence during the night, rapidly elevated activity at the time of awakening and increased activity throughout the day.7 This rhythm, and associated endogenous clocks, prepares the body for anticipated stimuli such as feeding.8,9
DISRUPTED GUT PHYSIOLOGY
Disrupted circadian rhythms in the bowel have been linked to gastrointestinal conditions including constipation and irritable bowel syndrome (IBS).10,11 For example, differences in colonic pressure activity12,13 and time of bowel movements13,14 are observed in constipated patients versus healthy persons depending on the time of day. Similarly, differences in the frequency of bowel contractions15 and the sensitivity of rectal mechanoreceptors14 have been reported in irritable bowel syndrome-constipation (IBS-C) patients relative to healthy controls with respect to morning versus evening hours. These observations and their implications for gastrointestinal health are discussed in more detail later. Figure 1 depicts normal and disrupted circadian control of the gastrointestinal tract.
FIGURE 1.
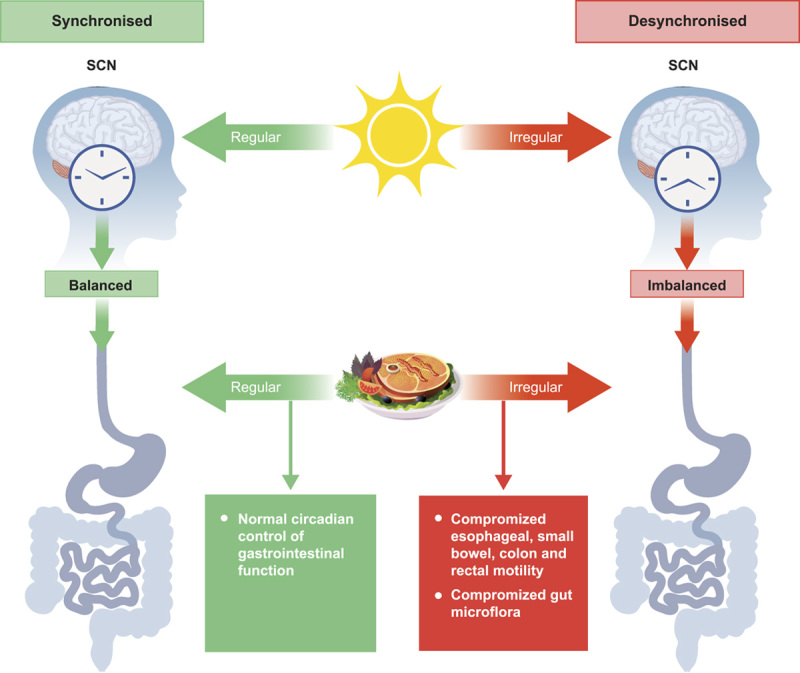
Normal and disrupted circadian control of the gastrointestinal tract. SCN indicates suprachiasmatic nucleus.
The objectives of this review are to establish evidence for the correlation between circadian rhythm, bowel movements, and digestive health, and implications of disrupted circadian rhythms on gut physiology.
METHODOLOGY
A systematic literature review was conducted by searching the Medline database (PubMed; November 1, 2017) to collate relevant published data on circadian rhythms and the gastrointestinal system. Studies included trials (randomized controlled trials, quasi-experimental and preexperimental), observational studies or reviews. Only articles written in English and published in peer-reviewed international journals were selected.
In phase 1, a search string was constructed to identify relevant articles:
(“gastrointestinal [All Fields] OR “gut” [All Fields] OR (“intestines” [MeSH Terms] OR “intestines” [All Fields] OR “bowel” [All Fields]) OR (“abdomen” [MeSH Terms] OR “abdomen” [All Fields] OR “abdominal” [All Fields]) AND (“feces” [MeSH Terms] OR “feces” [All Fields] OR “stool” [All Fields]) OR “faeces” [All Fields] OR (“faecal” [All fields] OR “faecal” [All Fields]) OR (“movement” [MeSH Terms] OR “movement” [All Fields]) OR (“defaecation” [All Fields] OR “defecation” [MeSH Terms] OR “defecation” [All Fields]) OR “motility” [All Fields] OR excretion [All Fields]) AND (“circadian rhythm” [MeSH Terms] OR (“circadian” [All Fields] AND “rhythm” [All Fields]) OR “circadian rhythm” [All Fields]) OR (“chronobiology disorders” [MeSH Terms] OR (“chronobiology” [All Fields] AND “disorders” [All Fields]) OR “chronobiology disorders” [All Fields]) OR (“circadian clocks” [MeSH Terms] OR (“circadian” [All Fields] AND “clocks” [All Fields]) OR “circadian clocks” [All Fields]) OR (circadian [All Fields] AND regulation [All Fields]) OR (chronobiology [All Fields] AND disruption [All Fields]) OR (clock [All Fields] AND (“genes” [MeSH Terms] OR “genes” [All Fields] AND “humans”[MeSH Terms]).
In phase 2, search results were manually filtered for relevance based on their title and the inclusion/exclusion criteria included in the abstract. This was carried out by 2 independent reviewers and discrepancies were resolved by a third reviewer. Relevance was defined as coverage of the circadian rhythms or underlying mechanisms influencing the digestive system and its health.
In phase 3, data extracted from the long list were reviewed by 2 independent reviewers and discrepancies were resolved by a third reviewer. The results are shown in Figure 2.
FIGURE 2.

Overview of the search process and resulting publications.
PHYSIOLOGY OF CIRCADIAN RHYTHMS IN THE GASTROINTESTINAL TRACT
The molecular machinery synchronizing circadian rhythms in the gut is believed to be controlled by 2 complementary systems: a central mechanism in the brain and organ-specific peripheral mechanisms.5
Central and Peripheral Mechanisms
In humans, the suprachiasmatic nucleus (SCN) in the ventral hypothalamus acts as the central circadian pacemaker which resets itself using light signals transmitted via the retinohypothalamic tract.5 The SCN communicates with peripheral tissues via neural and humoral pathways, most prominently via corticosteroids and melatonin.6 The SCN also controls the circadian release of digestive peptides, including vasoactive intestinal polypeptide and gastrin-releasing peptide.16
Peripheral circadian clocks generally operate to a phase that is 4 hours behind the central clock. Local cues, such as food intake, are used to reset or entrain the circadian rhythm of the gastrointestinal system, independently of signaling from the SCN.5,6,13 Even when the SCN is rendered inactive due to a lesion, circadian rhythms remain in the gastrointestinal system, suggesting the system is primed to anticipate alternating levels of demand between day and night.5,6
Clock Genes
Clock genes are a group of genes that are controlled by molecular feedback mechanisms.13 They are expressed rhythmically in epithelial cells of the colon and in neurons in the myenteric plexus which have a significant role in coordinating colonic motility.5,13,17 Clock genes are self-regulating, facilitating their 24-hour rhythm, and modulate the activity of other genes at specific times during each day.13 Hoogerwerf and colleagues investigated measures of colonic motility in wild-type mice, per1per2 double-knockout mice, and neuronal nitric oxide synthase knockout mice. Assessments consisted of a combination of in vivo and ex vivo methods.6 Persistence of rhythmicity in stool output, intracolonic pressure changes, and tissue contractility in wild-type mice under constant darkness and the absence of this rhythmicity in per1per2 double-knockout and neuronal knockout mice confirmed that these measures are circadian and controlled by endogenous clock-driven processes.6 Examples of clock genes include the helix-loop-helix transcription factors clock and bmal1, per1, per2, and per3 genes, and the cryptochromes cry1 and cry2.13 Approximately 8% to 10% of genes in peripheral organs are ultimately controlled by clock genes.13 In humans, single nucleotide polymorphisms in clock genes have been associated with changes in gastrointestinal motility.18 The CLOCK3111T/C single nucleotide polymorphism is associated with significantly lower dominant frequency on electrogastrography (EGG) recordings compared with subjects with wild-type (TT) clock genes, indicating slower gastric motility.18 The PER3 variable-number tandem-repeat polymorphism (either 4 or 5 repeats, 54 nucleotides in length), which is also associated with circadian rhythm, has been shown to exacerbate CLOCK3111T/C-related slowing of gastric motility.19
Entrainment
Light is an essential external cue for entrainment of the central circadian clock located in the SCN.20 In addition, there are peripheral clocks in nearly all tissues and cells of the body that are directly entrained by food, independent of the SCN.20,21 Although the mechanisms involved in entrainment have not been definitively identified, potential candidates include hormonal signaling (eg, melatonin), neural signaling, food intake, and body temperature regulation.20,21
Influence of Light on Gastrointestinal Circadian Rhythms
When subjects are exposed to dim-light versus bright light conditions during the day, less carbohydrate from the evening meal is absorbed in the cecum, although the circadian phases in each group appear to be consistent.22 Furthermore, baseline running spectral total power (ie, total spectral power of each frequency region on an EGG for 30 min) is comparable between subjects exposed to dim or bright light conditions. Taken together, evidence suggests that exposure to dim-light conditions during the daytime suppresses the digestion of an evening meal, resulting in malabsorption of dietary components.22
Influence of Food on Gastrointestinal Circadian Rhythms
Peripheral clocks are influenced by timing cues related to food intake. Restricting feeding synchronizes clock gene expression in the gastrointestinal system, independently of central control via the SCN.6 Furthermore, feeding may send metabolic cues to the SCN, entraining the central circadian rhythm.6 Studies in rodents show that rhythmic presentation of food to rats with SCN lesions can entrain anticipatory wheel running suggesting the existence of a food-mediated entrainment oscillator, semi-independent of the SCN.23 Food in contact with the gastrointestinal epithelium has been ruled out as an entrainment mechanism, although humoral mechanisms or food-related entrainment mediated by a corticotrophin-releasing hormone is a possibility.5,6
Influence of Exercise on Gastrointestinal Circadian Rhythms
Graded aerobic exercise has been shown to decrease phasic colonic motility in healthy, untrained subjects24 but after exercise there was a resurgence of predominantly propagating pressure waves, suggesting that exercise may enhance stool transit.24 Similarly, acute physical exercise increases both high amplitude propagated contractions (HAPCs) and low amplitude propagated contractions (LAPCs) in healthy individuals.25 It has been demonstrated that voluntary exercise can shift the SCN clock or modulate the photic synchronization of circadian rhythms.26 A simulated night work-study indicated that moderate-intensity exercise during the night shift produced larger circadian phase shifts compared with a sedentary control condition.27 Other groups indicated that low-intensity and high-intensity exercise during the night influenced circadian rhythms by the following day.28,29 As discussed earlier, these changes in circadian parameters can have profound effects on gastrointestinal motility.
CIRCADIAN RHYTHMS AND CONTROL IN THE GASTROINTESTINAL SYSTEM
Migrating motor complex (MMC) are waves of electrical activity that start in the stomach and move along the gut triggering peristaltic contractions.7 Hormones such as motilin and ghrelin are involved in the generation of MMCs, while others (eg, gastrin, cholecystokinin, serotonin) are involved in the generation of propagation sequence spikes.7 These processes result in peristaltic or segmental contractions in the small (duodenum, jejunum, ileum) and large intestines (colon).7 In the small bowel, the length of the interdigestive cycle is divided into 4 phases. The first phase of the MMC involves a prolonged period of quiescence (40% to 60% of total time); phase II is characterized by increased frequency of action potentials and smooth muscle contractility (20% to 30% of total time); phase III consists of a few minutes of peak electrical and mechanical activity (5 to 10 min); phase IV includes declining activity which merges with the next phase.30 The cycle is consistent throughout a 24-hour period, but the relative proportion of each cycle attributed to each phase differs between day and night. A significantly higher number of cycles at night have phases of motor quiescence compared with daytime, while the duration of the phase II period of increased frequency of action potentials and smooth muscle contractility is longer during the day versus night ensuring bowel movements occur during waking hours.30 In the colon, there is no MMC but sleep is known to strongly inhibit both propagating and nonpropagating colonic motor activity.10,30
Gastric Activity
Normal gastrointestinal motility occurs through coordinated contractions of smooth muscle which derives from 2 patterns of electrical activity across membranes of smooth muscle cells: slow waves and spike potentials. The gastric activity can be recorded by EEG, a noninvasive technique.31 The dominant EGG frequency reflects the frequency of stomach contractions.32 The dominant frequency observed in EGG recordings is believed to reflect gastric slow waves; rhythmic smooth muscle cell depolarisations that act as the gastric pacemaker to coordinate contraction. Mean dominant frequency is increased during daytime hours and decreases during sleep.33
Esophageal Activity
Esophageal function and circadian rhythms are important due to their association with gastroesophageal reflux disease (GERD).7,34 Typically, GERD patients complain about frequent night reflux symptoms. Nighttime GERD can profoundly impair the quality of life by causing pain and disturbance of sleep, that interferes with mental and physical functioning the next day.7 The upper esophageal sphincter tone changes little during sleep compared with waking hours.35 However, the motility of the esophagus is reduced during sleep, with the frequency of both primary and secondary contractions progressively diminishing from stages N1 to N3.35 N1 stage sleep refers to the transition stage between wakefulness and the deeper stages of sleep, while the N3 stage refers to deep or slow-wave sleep.35 In contrast, secondary contractions increase during rapid eye movement (REM) sleep.35 The lower esophageal sphincter has transient reductions in tone during sleep, which are conducive to reflux episodes.35
Small Intestine Activity
Motility follows a circadian rhythm with reduced nocturnal activity. Using twin intraluminal pressure-sensitive radiotelemetric capsules, Kumar et al36 demonstrated significant variations between daytime and nocturnal propagation velocities of the MMC in the small intestine. Nocturnal MMCs are less frequent and have slower velocities; reports on differences in their duration at night are conflicting, with some authors reporting shorter durations and others reporting no difference.37,38 Studies of regional velocity suggest that nocturnal differences only occur in the jejunum, and not in the ileum.37 The number of phase III periods in the jejunum is greater at night, while the overall proportion of MMCs passing through the upper gastrointestinal tract is significantly lower.37
Colonic Activity
Normal colonic motility follows a rhythm, with minimal activity during the night and increased activity during the day.6 Typical activity during the day consists of increased colonic propagation sequences compared to those occurring at night in healthy persons.10,38,39 For example, Narducci et al38 showed that HAPCs were more prevalent in the morning in a study that measured 24-hour manometric recording of colonic motor activity in healthy subjects. Furthermore, Bassotti et al10 showed that LAPC waves were constantly present with an average of about 61 events/subject/day and mean amplitude of about 20 mm Hg. More than 80% of LAPCs waves occurred during the day and were increased after meals and morning awakening (P<0.05). At night, Bampton et al39 demonstrated that there was nocturnal suppression of colonic motor activity. Comparing area under the curve data in a 2-hour period after awakening in the morning to a 2-hour period before waking showed almost a 2-fold increase in colonic activity (4874 to 8335 mm Hg; P<0.0001). The increase in colonic activity remained elevated throughout the waking hours.39
Retrograde pressure waves are less frequent than anterograde propagating pressure waves and demonstrate a lower frequency at night followed by a slightly increased frequency after awakening.39–41 Under normal physiological conditions, propagating sequences were seen to migrate in both antegrade and retrograde directions in healthy persons.39 Antegrade propagating sequences were more frequent and of greater amplitude, as measured by a prolonged multipoint recording of colonic manometry in the unprepared human colon in subjects with normal bowel habits in daytime hours. Likewise, Furukawa et al42 found retrograde propagation sequences were of significantly lower amplitude. Dinning et al43 showed that postprandial distal colonic activity consists primarily of a retrograde propagating sequences following a meal in healthy persons. These conditions may prevent premature rectal filling, while also allowing stools to remain for the appropriate amount of time for absorption of water and electrolytes. In patients with constipation, a meal failed to induce the normal increase in the distal colonic propagating sequences seen in healthy persons.44
Rectal Activity
The rectum exhibits intermittent cyclical motor activity specifically at night which has been termed the rectal motor complex or periodic rectal motor activity (PRMA). In healthy individuals, PRMA is confined largely, but not exclusively, to the rectosigmoid region and follows a distinct circadian rhythm.40,41 Rao et al40,41 demonstrated that PRMA often follows a motor event in the more proximal colon and that there is a distinct circadian rhythm suggesting that PRMA may serve as an intrinsic braking mechanism that prevents untimely flow of colonic contents. The number, duration and peak amplitude of rectal motor complexes (regular pressure fluctuations with a frequency of either 3 or 6 cycles/min) are significantly reduced at night.6,14
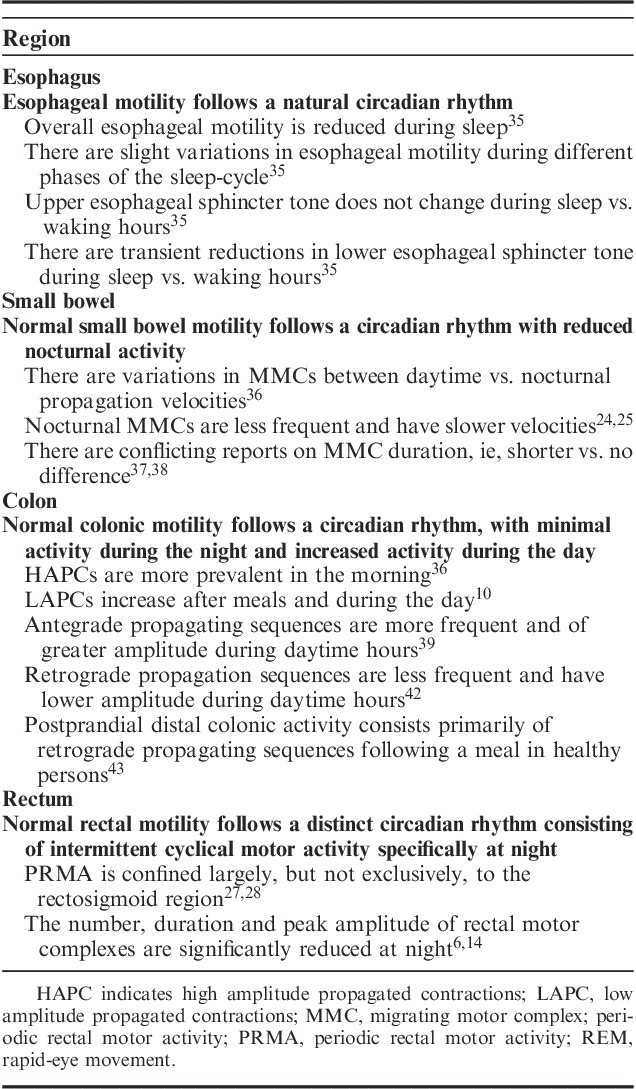
A summary of the associations between circadian rhythms and gut motility is shown in Table 1.
TABLE 1.
Summary of the Associations Between Circadian Rhythm and Gut Motility
HORMONAL CONTROL OF CIRCADIAN RHYTHM IN THE GASTROINTESTINAL SYSTEM
Melatonin
Melatonin is the neuroendocrine “clock factor” generated by the pineal gland, a structure that dominates regular circadian rhythm in humans.45–47 Large amounts of melatonin are produced in the gastrointestinal system by enterochromaffin cells in the digestive mucosa, from which the highly lipophilic molecule diffuses into deeper tissue layers, including the submucosa, to act on the muscularis mucosae or the myenteric plexus.5,13,48,49 Findings that concentrations of melatonin in gastrointestinal tissue surpasses those in the blood (10- to 100-fold) and an estimated 400-fold increase in the gut compared with the pineal gland, clearly implicates an important role in digestive health.49,50 Melatonin levels increase with food intake and may be released into the systemic circulation helping to modulate other organ-specific circadian rhythms.5,48,49 Melatonin secretion in the gut is also under the direct control of clock genes in the SCN, which makes it a reliable marker of circadian rhythm phase shifts.13
GASTROINTESTINAL PATHOLOGY AND CIRCADIAN RHYTHMS
Circadian clock desynchronization often leads to functional abnormalities in the gastrointestinal system (abdominal pain, constipation, and diarrhea), and metabolic diseases (obesity, and nonalcoholic fatty liver disease), and to increased susceptibility to alcoholic liver disease due to increased intestinal membrane permeability.5,6,13,17,20,51
Circadian disruption displaces the timing of eating and normal gastrointestinal functions, such as gastric, bile, and pancreatic secretions, enzyme activity, intestinal motility, and the rate of nutrient absorption.52,53 Surveys have documented gastrointestinal problems in shift-workers in which nighttime workers complained of constipation.54,55 Altered gastrointestinal physiology and/or gut microbiota may drive gastric pathologies by increasing gastrointestinal permeability, which in turn increases the risk of injury and inflammation.20 This is supported by reports that melatonin may have a role in maintaining the integrity of the gut wall.56
Constipation
As discussed earlier, several lines of evidence suggest that altered circadian rhythm contributes to the manifestation of constipation. For example, patients with constipation demonstrate a significantly lower colonic pressure activity following waking or food consumption compared to nonconstipated subjects.5,15 This is in contrast with the normal diurnal rhythm observed in nonconstipated patients where colonic pressure is higher in the morning.12,13 Likewise, bowel movements occur most frequently in the evening rather than in the morning in patients with irregular bowel habits of just 3 to 4 times per week. Mass movements (MMs) in healthy persons have been shown to be more frequent during the day than at night, and that the percentage of subjects who expressed MM between 6 am and 2 pm was greater than those who did so between 4 pm and 4 am.57 Although the number of MM did follow a diurnal rhythm in constipated patients, it was blunted and the percentage of patients with MM was less in the morning in constipated patients compared to healthy controls.57 Prolonged manometry studies demonstrate that adults with slow transit constipation have fewer spontaneous HAPCs than healthy subjects.12,58–60 This observation will be discussed in more detail later in relation to the potential mechanism of action of the laxative medication.
There is also some evidence to suggest that the circadian clock plays a role in “traveler’s constipation.” This concept was investigated in a study that included 70 people traveling from Europe to the United States for a short stay.61 In addition to the usual questionnaires, 65 subjects kept diaries on their bowel habits, had stool samples evaluated for consistency according to a standardized methodology, and had their colonic transit time (CTT) measured after ingesting radioactive tracers.61 Nearly 40% of the subjects complained of constipation and this was most pronounced during the first days of travel. The degree of constipation correlated with the degree of jetlag.61 A potential caveat of this study could be that factors other than travel (changes in diet and physical activity) may have played a role and further studies are needed to replicate these data.
Other evidence suggests that the incidence of constipation increases during periods of fasting during the daylight. During Ramadan, many adults Muslims fast during daylight hours eliciting a change in circadian rhythm. A questionnaire measured the rate of constipation in 900 individuals and found that those who fasted for >14 days reported more “severe” or “very severe” constipation than those who fasted for <14 days.62 However, alimentation is a confounding factor, affected by the increased volume of food intake and, in some cases, the introduction of traditional dishes.
IBS
IBS is a prevalent functional gastrointestinal syndrome defined as a constellation of symptoms including abdominal pain, alternating constipation, and diarrhea.63–65 Sleep disturbances are a common comorbidity in IBS, affecting 26% to 55% of patients.48,49 Likewise, the severity of IBS may be exacerbated by poor sleep, leading to the hypothesis that IBS may be associated with a disrupted circadian rhythm.5,17,49 In a study of 399 nurses, IBS was more prevalent in rotating shift workers than in day-shift nurses. This association was found to be independent of sleep quality suggesting circadian disruption.63 The frequency of bowel contractions observed upon awakening in healthy subjects is significantly blunted in the descending colon of patients with constipation-predominant IBS (IBS-C).15 In addition, decreased sensitivity of rectal mechanoreceptors in patients with IBS-C relative to healthy controls has been reported in the morning, followed by significantly higher sensitivity during the day.14
Neurodegenerative Diseases
Circadian desynchronization (disrupted daily rhythms of physiological parameters such as sleep, activity, and hormone secretion) has been regarded as a symptom of several neurodegenerative diseases.66 Disruption in circadian rhythms has a negative impact on quality of life, cognitive performance, mental health, motor control, and metabolism. Many of these functions become impaired in neurodegenerative disorders such as Alzheimer disease, Parkinson disease, and Huntington disease, where chrono-degeneration is a common feature.67 These disorders are associated with constipation. For example, the pathophysiology of constipation in PD includes central mechanisms encompassing changes in dorsal vagal nucleus function and peripheral mechanisms with loss of dopaminergic neurons.68 It is not known whether disruptions of circadian rhythm are causal or symptomatic of neurodegenerative disorders.
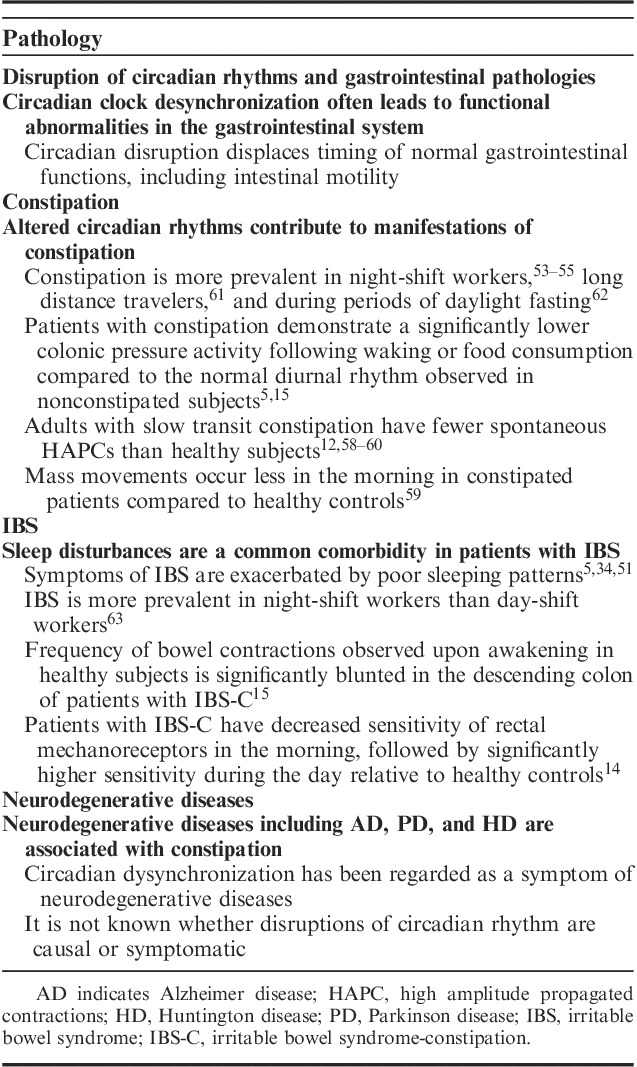
A summary of the association between compromised circadian rhythms and gastrointestinal pathologies is shown in Table 2.
TABLE 2.
Summary of the Association Between Compromised Circadian Rhythms and Gastrointestinal Pathologies
THERAPEUTIC BENEFIT OF MODULATING THE CIRCADIAN RHYTHM IN THE GASTROINTESTINAL SYSTEM
A concept has emerged that pharmacological control of the circadian clock may provide a novel therapeutic strategy for gastrointestinal disorders. Chronopharmacological techniques (chronotherapy) aim to optimize the efficiency of a drug via timed administration following a circadian cycle.69 The timing of treatment in coordination with the body-clock may increase the desired effect of drugs, lower the dose and decrease toxicity.69
Melatonin
Melatonin has been considered as a potential co-adjuvant treatment for some gastrointestinal diseases (eg, IBS-C).49 Studies have shown that melatonin has regulatory effects on gastrointestinal tract motility and sensation that may improve bowel habits and alleviate abdominal pain or distension in IBS patients.49 For example, low-dose melatonin treatment accelerates intestinal transit time whereas high doses decrease gut motility.70,71 The finding that low dose melatonin accelerates intestinal transit time suggests that it could be beneficial in the treatment of IBS-C. For example, this action could also be of benefit for the treatment of constipation alone, although studies investigating this potential have yet to be performed.
Song et al72 investigated the effects of melatonin on abdominal pain in IBS patients who had concurrent sleep disturbances. Melatonin administered at a dose of 3 mg for 2 weeks attenuated abdominal pain and reduced rectal pain sensitivity. Therapeutic efficacy was associated with heightened pressure and volume thresholds for both urgency and pain sensations in melatonin-treated patients.72 Melatonin did not influence sleep parameters, including total sleep time, sleep latency, sleep efficiency, sleep onset latency, arousals, duration of stages 1 to 4, REM sleep, and REM onset latency.72 The authors concluded that the beneficial effects of melatonin in IBS may be related to its action on gut visceral hypersensitivity and independent of its action on sleep.72
A study by Lu et al73 showed that nightly treatment with melatonin (3 mg) for 8 weeks improved mean IBS scores compared with placebo in female patients with IBS. Response rates, defined as the percentage of subjects achieving mild-to-excellent improvement in IBS symptoms, were higher in melatonin-treated patients. Symptoms of sleep disturbance and anxiety/depression scores were comparable between groups.73
Melatonin levels have also been shown to influence CTT in control subjects and patients with IBS.74 Participants were treated daily with melatonin (3 mg) or placebo for 8 weeks, followed by a 4-week washout, and then placebo or melatonin in the reverse order for a second 8-week period. Melatonin treatment induced an increase in CTT in control subjects. In contrast to baseline CTT in control subjects, CTT in constipated IBS patients was prolonged.74 The CTT did not change in IBS patients after melatonin treatment.74 This study highlights the complexity of mechanisms involved in the effects of melatonin and whether the beneficial effects are more related to rectal physiology. As discussed earlier, there is a fine balance between increasing or reducing CTT via melatonin treatment, and this is largely governed by dose and the specific methodology employed in each trial.
Even though placebo-controlled studies investigating melatonin suffer from considerable heterogeneity in methodology, an extensive literature review found that melatonin improved abdominal pain in many studies, with some studies showing improvements in quality of life.50
Probiotics
Research has demonstrated that the bacteria in the gastrointestinal tract vary over the course of a day, with the relative abundances of bacterial taxa, the proximity of bacteria to the colonic epithelium, and microbial metabolism all exhibiting diurnal rhythms.75 Furthermore, the gut microbiome appears to have a reciprocal relationship with the circadian clock and eating habits in humans.16,76
It has been proposed that disturbances in diurnal bowel function in chronic constipation may lead to changes in colonic flora.77 These alterations in the intestinal microflora could alter the metabolic homeostasis of the colon with resultant changes in the concentration of physiological substances that may influence the motor and secretory functions of the bowel, a theory that was first suggested over 30 years ago.78 This proposal has been confirmed by evidence showing the ability of probiotics to stimulate the motility of the large bowel and to normalize the intestinal microflora in patients with constipation.79–82 Probiotic supplementation was also shown to alleviate constipation-related symptoms: patients defecated with more frequency and ease, and normalized stool consistency in constipated patients.83 It is plausible that products released by the abnormal flora may contribute to the colonic motility changes that lead to constipation. The observation that the normalization of the intestinal microflora, associated with probiotic therapy, is accompanied by a stimulating effect on colonic motility further supports this concept.
Oral Laxatives
As therapeutic agents, modern laxatives are safe to use, and severe reactions are rarely reported.84 Current laxatives promote defecation by increasing bulk, decreasing stool consistency (softening) or stimulating colon motility through several mechanisms. Laxatives, in addition to interventions like dietary fiber, fluid, etc. are key treatments for constipation.
As discussed throughout this review, a potential player in the manifestation of constipation is a disruption of circadian rhythms in the gut. A strategy conducive to the reinstatement of these rhythms in constipated patients could be to encourage the natural gastrointestinal functionality that occurs in healthy subjects (eg, peristalsis, optimal osmotic pressure). Colonic HAPCs are essential for facilitating the transit of colonic contents over long distances, leading to defecation. Prolonged manometry studies demonstrate that adults with constipation have fewer spontaneous HAPCs than healthy subjects.60 Studies have shown that intraluminal infusion of bisacodyl, a stimulant laxative that acts directly on colonic musculature, can elicit HAPCs in humans.57,85,86 This effect of bisacodyl was first established over 50 years ago.87 It has been demonstrated that bisacodyl increased the number of propagated HAPCs as measured by colonic manometry in more recent studies in constipated patients.88,89 Bisacodyl-induced HAPCs are quantitatively and qualitatively similar to naturally accumulating HAPCs in healthy persons.86,89 Further evidence for a favorable action of bisacodyl linked to circadian control of gut functionality is found in a study by Kamm et al90 where the onset of action of bisacodyl was demonstrated to be 12 hours after administration. When taken at night, this property mimics the naturally occurring circadian rhythm promoting peristalsis and the secretion of fluid in the gut leading to the urge to pass stools in the morning, as is the case in healthy subjects.13,14
Collectively, these characteristics are not shared by some other commonly used laxatives include bulking agents, osmotic agents, stool softeners and prokinetics.67,91–93 Although these treatments are effective, there is little evidence to suggest that they influence the reduction of naturally occurring HAPCs seen in constipated patients, and they have variable onsets of action that do not necessarily follow the body’s natural rhythm.94–96
As pointed out earlier, probiotics have been shown to stimulate motility of the large bowel, relieve constipation-related symptoms (eg, reduced frequency of bowel evacuation and compromised stool consistency) and concurrently normalize changes in the intestinal microflora seen in patients with constipation.79–82 In a small study (n=12) by Khalif et al77 revealed significant changes in the composition of the fecal microflora among constipated patients. At baseline, a suppression of major species of the obligate microflora was paralleled by an increased pool of potentially pathogenic microorganisms: common Escherichia coli in 40.3% of the cases, atypical E. coli in 19.3%, Staphylococcus aureus in 33.3% and Enterobacteria in 21% of cases.77 These changes were most pronounced among those who were most severely constipated and demonstrated the slowest transit through the large bowel; concentrations of E. coli and Candida were increased by 10- to 100-fold in more than half of the patients in this subgroup.77 Normalization of evacuatory function by bisacodyl treatment was accompanied by a relative normalization of the microflora.77 Three months after the end of treatment, the values for most microorganisms had returned to pretreatment “diseased state” levels. Bisacodyl did not influence potentially toxic bacteria (eg, Clostridium, E. coli or Enterobacteria).77 Although this study was performed in a small number of patients, it does provide a plausible link between the gut microbiome and constipation, particularly as the microbiota returned to an imbalanced state following cessation of treatment.
A summary of the therapeutic benefit of modulating the circadian rhythm in the gastrointestinal system is shown in Table 3.
TABLE 3.
Summary of the Therapeutic Benefit of Modulating Circadian Rhythm in the Gastrointestinal System

CONCLUSIONS
There is convincing evidence that the gastrointestinal system is governed by a circadian rhythm with both central and peripheral inputs.17,33,37,40,41,97 The molecular basis for circadian timing encompasses interlocking transcriptional/translational feedback loops that terminate in the rhythmic expression and activity of a set of clock genes and related hormones in the gastrointestinal tract. A significant increase in bowel activity occurs around the time of waking, suggesting the bowel is primed to evacuate in the morning.6 The bowel remains significantly more active during the day than the night.38–41,97–99
Studies have indicated that patients with disrupted circadian rhythms present with gastrointestinal symptoms/disorders, most notably constipation, and IBS.48–50,53,62 Some studies of circadian rhythms in the gastrointestinal system have been hampered by the inability to adequately control external cues, such as light-dark cycles or food intake in many clinical studies.77 Nevertheless, constipation has been associated with circadian rhythm-dependant differences in colonic transit following waking or food consumption, and frequency of bowel movements compared to nonconstipated patients.12,14 Likewise, IBS-C patients present with diurnal differences in the frequency of bowel contractions observed upon awakening and decreased sensitivity of rectal receptors in the morning versus the rest of the day.14,15
As our understanding of the circadian regulation of gastrointestinal health and disease increases, it is anticipated that there will be the further development of therapeutic interventions associated with chronobiology. Facilitating morning evacuation in constipated patients could further help to restore the circadian rhythm of MM. Further studies are required to demonstrate the beneficial effects that laxatives may have on circadian rhythms in healthy adults.
ACKNOWLEDGMENT
The authors acknowledge Hamell Communications Ltd, Richmond, UK for providing medical writing assistance, funded by Sanofi-Aventis Groupe.
Footnotes
H.D.: Bicodex, Ipsen: scientific advisor. B.C.: Kyowa Kirin: speaker; Biocodex: speaker; Abbott International: speaker; Ipsen: board member. L.S.: Takeda, MSD, AbbVie, Kiowa Kirin: teaching conferences; MSD, Takeda, Medtronic: research grants; Takeda, Ferring: consultant fees.
REFERENCES
- 1.Schibler U, Sassone-Corsi P. A web of circadian pacemakers. Cell. 2002;111:919–922. [DOI] [PubMed] [Google Scholar]
- 2.Stratmann M, Schibler U. Properties, entrainment, and physiological functions of mammalian peripheral oscillators. J Biol Rhythms. 2006;21:494–506. [DOI] [PubMed] [Google Scholar]
- 3.Dibner C, Schibler U. Circadian timing of metabolism in animal models and humans. J Intern Med. 2015;277:513–527. [DOI] [PubMed] [Google Scholar]
- 4.Wever RA. Characteristics of circadian rhythms in human functions. J Neural Transm Suppl. 1986;21:323–373. [PubMed] [Google Scholar]
- 5.Bron R, Furness JB. Rhythm of digestion: keeping time in the gastrointestinal tract. Clin Exp Pharmacol Physiol. 2009;36:1041–1048. [DOI] [PubMed] [Google Scholar]
- 6.Hoogerwerf WA, Shahinian VB, Cornélissen G, et al. Rhythmic changes in colonic motility are regulated by period genes. Am J Physiol Gastrointest Liver Physiol. 2010;298:G143–G150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konturek PC, Brzozowski T, Konturek SJ. Gut clock: implication of circadian rhythms in the gastrointestinal tract. J Physiol Pharmacol. 2011;62:139–150. [PubMed] [Google Scholar]
- 8.Johnston JD. Physiological responses to food intake throughout the day. Nutr Res Rev. 2014;27:107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaughn B, Rotolo S, Roth H. Circadian rhythm and sleep influences on digestive physiology and disorders. ChronoPhysiol Ther. 2014;4:67–77. [Google Scholar]
- 10.Bassotti G, Clementi M, Antonelli E, et al. Low-amplitude propagated contractile waves: a relevant propulsive mechanism of human colon. Dig Liver Dis. 2001;33:36–40. [DOI] [PubMed] [Google Scholar]
- 11.Wells M, Roth L, McWilliam M, et al. A cross-sectional study of the association between overnight call and irritable bowel syndrome in medical students. Can J Gastroenterol. 2012;26:281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao SS, Sadeghi P, Beaty J, et al. Ambulatory 24-hour colonic manometry in slow-transit constipation. Am J Gastroenterol. 2004;99:2405–2416. [DOI] [PubMed] [Google Scholar]
- 13.Hoogerwerf WA. Biologic clocks and the gut. Curr Gastroenterol Rep. 2006;8:353–359. [DOI] [PubMed] [Google Scholar]
- 14.Shemerovskii KA. Circadian rhythm of rectal reactivity in individuals with regular and irregular bowel evacuation function. Bull Exp Biol Med. 2002;134:565–567. [DOI] [PubMed] [Google Scholar]
- 15.Clemens CH, Samsom M, Van Berge Henegouwen GP, et al. Abnormalities of left colonic motility in ambulant nonconstipated patients with irritable bowel syndrome. Dig Dis Sci. 2003;48:74–82. [DOI] [PubMed] [Google Scholar]
- 16.Kaczmarek JL, Thompson SV, Holscher HD. Complex interactions of circadian rhythms, eating behaviors, and the gastrointestinal microbiota and their potential impact on health. Nutr Rev. 2017;75:673–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoogerwerf WA. Role of biological rhythms in gastrointestinal health and disease. Rev Endocr Metab Disord. 2009;10:293–300. [DOI] [PubMed] [Google Scholar]
- 18.Yamaguchi M, Kotani K, Sakane N, et al. The CLOCK 3111T/C SNP is associated with morning gastric motility in healthy young women. Physiol Behav. 2012;107:87–91. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi M, Kotani K, Tsuzaki K, et al. Circadian rhythm genes CLOCK and PER3 polymorphisms and morning gastric motility in humans. PLoS One. 2015;10:e0120009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forsyth CB, Voigt RM, Burgess HJ, et al. Circadian rhythms, alcohol and gut interactions. Alcohol. 2015;49:389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barclay JL, Husse J, Bode B, et al. Circadian desynchrony promotes metabolic disruption in a mouse model of shift work. PLoS One. 2012;7:e37150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sone Y, Hyun KJ, Nishimura S, et al. Effects of dim or bright-light exposure during the daytime on human gastrointestinal activity. Chronobiol Int. 2003;20:123–133. [DOI] [PubMed] [Google Scholar]
- 23.Comperatore CA, Stephan FK. Effects of vagotomy on entrainment of activity rhythms to food access. Physiol Behav. 1990;47:671–678. [DOI] [PubMed] [Google Scholar]
- 24.Rao SS, Beaty J, Chamberlain M, et al. Effects of acute graded exercise on human colonic motility. Am J Physiol Gastrointest Liver Physiol. 1999;276:G1221–G1226. [DOI] [PubMed] [Google Scholar]
- 25.Cheskin LJ, Crowell MD, Kamal D, et al. The effects of acute exercise on colonic motility. J Gastrointest Motil. 1992;4:173–177. [Google Scholar]
- 26.Challet E. Minireview: entrainment of the suprachiasmatic clockwork in diurnal and nocturnal mammals. Endocrinology. 2007;148:5648–5655. [DOI] [PubMed] [Google Scholar]
- 27.Eastman CI, Hoese EK, Youngstedt SD, et al. Phase-shifting human circadian rhythms with exercise during the night shift. Physiol Behav. 1995;58:1287–1291. [DOI] [PubMed] [Google Scholar]
- 28.Buxton OM, Frank SA, L’Hermite-Baleriaux M, et al. Roles of intensity and duration of nocturnal exercise in causing phase delays of human circadian rhythms. Am J Physiol. 1997;273(pt 1):E536–E542. [DOI] [PubMed] [Google Scholar]
- 29.Van Reeth O, Sturis J, Byrne MM, et al. Nocturnal exercise phase delays circadian rhythms of melatonin and thyrotropin secretion in normal men. Am J Physiol. 1994;266(pt 1):E964–E974. [DOI] [PubMed] [Google Scholar]
- 30.Keller J, Gröger G, Cherian L, et al. Circadian coupling between pancreatic secretion and intestinal motility in humans. Am J Physiol Gastrointest Liver Physiol. 2001;280:G273–G278. [DOI] [PubMed] [Google Scholar]
- 31.Yin J, Chen JD. Electrogastrography: methodology, validation and applications. J Neurogastroenterol Motil. 2013;19:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen JD, Richards RD, McCallum RW. Identification of gastric contractions from the cutaneous electrogastrogram. Am J Gastroenterol. 1994;89:79–85. [PubMed] [Google Scholar]
- 33.Suzuki A, Asahina M, Ishikawa C, et al. Impaired circadian rhythm of gastric myoelectrical activity in patients with multiple system atrophy. Clin Auton Res. 2005;15:368–372. [DOI] [PubMed] [Google Scholar]
- 34.Orr WC, Johnson LF, Robinson MG. Effect of sleep on swallowing, esophageal peristalsis, and acid clearance. Gastroenterology. 1984;85:814–819. [PubMed] [Google Scholar]
- 35.Collop NA, Salas RE, Delayo M, et al. Normal sleep and circadian processes. Crit Care Clin. 2008;24:449–460. [DOI] [PubMed] [Google Scholar]
- 36.Kumar D, Wingate D, Ruckebusch Y. Circadian variation in the propagation velocity of the migrating motor complex. Gastroenterology. 1986;91:926–930. [DOI] [PubMed] [Google Scholar]
- 37.Kellow JE, Borody TJ, Phillips SF, et al. Human interdigestive motility: variations in patterns from esophagus to colon. Gastroenterology. 1986;91:386–395. [DOI] [PubMed] [Google Scholar]
- 38.Narducci F, Bassotti G, Gaburri M, et al. Twenty four hour manometric recording of colonic motor activity in healthy man. Gut. 1987;28:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bampton PA, Dinning PG, Kennedy ML, et al. Prolonged multi-point recording of colonic manometry in the unprepared human colon: providing insight into potentially relevant pressure wave parameters. Am J Gastroenterol. 2001;96:1838–1848. [DOI] [PubMed] [Google Scholar]
- 40.Rao SS, Sadeghi P, Beaty J, et al. Ambulatory 24-h colonic manometry in healthy humans. Am J Physiol Gastrointest Liver Physiol. 2001;280:G629–G639. [DOI] [PubMed] [Google Scholar]
- 41.Rao SS, Sadeghi P, Batterson K, et al. Altered periodic rectal motor activity: mechanism for slow transit constipation. Neurogastroenterol Motil. 2001;13:591–598. [DOI] [PubMed] [Google Scholar]
- 42.Furukawa Y, Cook IJ, Panagopoulos V, et al. Relationship between sleep patterns and human colonic motor patterns. Gastroenterology. 1994;107:1372–1381. [DOI] [PubMed] [Google Scholar]
- 43.Dinning PG, Wiklendt L, Maslen L, et al. Quantification of in vivo colonic motor patterns in healthly human before and after a meal revealed by high-resolution fiber-optic manometry. Neurogastroenterol Motil. 2014;26:1445–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dinning PG, Wiklendt L, Maslen L, et al. Colonic motor abnormalities in slow transit constipation defined by high resolution, fibre-optic manometry. Neurogastroenterol Motil. 2015;27:379–388. [DOI] [PubMed] [Google Scholar]
- 45.Lerner AB, Case JD, Mori W, et al. Melatonin in peripheral nerve. Nature. 1959;183:1821. [DOI] [PubMed] [Google Scholar]
- 46.Brun J, Claustrat B, Saddier P, et al. Nocturnal melatonin excretion is decreased in patients with migraine without aura attacks associated with menses. Cephalagia. 1995;15:136–139. [DOI] [PubMed] [Google Scholar]
- 47.Moller M, Baeres FM. The anatomy and innervation of the mammalian pineal gland. Cell Tissue Res. 2002;309:139–150. [DOI] [PubMed] [Google Scholar]
- 48.Radwan P, Skrzydlo-Radomanska B, Radwan-Kwiatek K, et al. Is melatonin involved in the irritable bowel syndrome? J Physiol Pharmacol. 2009;60(suppl 3):67–70. [PubMed] [Google Scholar]
- 49.Esteban-Zubero E, López-Pingarrón L, Alatorre-Jiménez MA, et al. Melatonin’s role as a co-adjuvant treatment in colonic diseases: a review. Life Sci. 2017;170:72–81. [DOI] [PubMed] [Google Scholar]
- 50.Siah KTH, Wong RKM, Ho KY. Melatonin for the treatment of irritable bowel syndrome. World J Gastroenterol. 2014;20:2492–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hastings MH. Circadian rhythms: a gut feeling for time. Nature. 2002;417:391–392. [DOI] [PubMed] [Google Scholar]
- 52.Lennernäs M, Hambraeus L, Åkerstedt T. Nutrient intake in day and shift workers. Work Stress. 1994;8:332–342. [Google Scholar]
- 53.Costa G. Shift work and health: current problems and preventive actions. Saf Health Work. 2010;1:112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rutenfranz J. Occupational health measures for night and shift workers. J Hum Ergol (Tokyo). 1982;11:67–86. [PubMed] [Google Scholar]
- 55.Costa G. The impact of shift and night work on health. Appl Ergon. 1996;27:9–16. [DOI] [PubMed] [Google Scholar]
- 56.Motilva V, Cabeza J, Alarcón de la Lastra C. New issues about melatonin and its effects on the digestive system. Curr Pharm Des. 2001;7:909–931. [DOI] [PubMed] [Google Scholar]
- 57.Bassotti G, Gaburri M, Imbimbo BP, et al. Colonic mass movements in idiopathic chronic constipation. Gut. 1988;29:1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Schryver AM, Samsom M, Smout AI. Effects of a meal and bisacodyl on colonic motility in healthy volunteers and patients with slow-transit constipation. Dig Dis Sci. 2003;48:1206–1212. [DOI] [PubMed] [Google Scholar]
- 59.Bassotti G, Chistolini F, Marinozzi G, et al. Abnormal colonic propagated activity in patients with slow transit constipation and constipation-predominant irritable bowel syndrome. Digestion. 2003;68:178–183. [DOI] [PubMed] [Google Scholar]
- 60.Ancha HR, Fajardo NR, Bauman WA, et al. Absence of high amplitude propagating contractions in subjects with chronic spinal cord injury. World J Gastroenterol. 2010;16:5435–5439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mearin F, Zárate N, Sardi JA, et al. Traveler’s constipation. Am J Gastroenterol. 2003;98:507–509. [DOI] [PubMed] [Google Scholar]
- 62.Keshteli AH, Sadeghpour, Feizi A, et al. Evaluation of self-perceived changes in gastrointestinal symptoms during Ramadan fasting. J Relig Health. 2017;56:1620–1627. [DOI] [PubMed] [Google Scholar]
- 63.Nojkov B, Rubenstein JH, Chey WD, et al. The impact of rotating shift work on the prevalence of irritable bowel syndrome in nurses. Am J Gastroenterol. 2010;105:842–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mearin F, Lacy BE, Chang L, et al. Bowel disorders. Gastroenterology. 2016;150:1393.e5–1407.e5. [DOI] [PubMed] [Google Scholar]
- 65.Wang B, Duan R, Duan L. Prevalence of sleep disorder in irritable bowel syndrome: a systematic review with meta-analysis. Saudi J Gastroenterol. 2018;24:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Musiek ES. Circadian clock disruption in neurodegenerative diseases: cause and effect? Front Pharmacol. 2015;6:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Videnovic A, Lazar AS, Barker RA, et al. “The clocks that time us”—circadian rhythms in neurodegenerative disorders. Nat Rev Neurol. 2014;10:683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Andrews CN, Storr M. The pathophysiology of chronic constipation. Can J Gastroenterol. 2011;25:16B–21B. [PMC free article] [PubMed] [Google Scholar]
- 69.Shetty A, Selvam TV. Review on chronotherapy: a novel drug delivery system. J Pharm Sci Bioscientific Res. 2016;6:646–653. [Google Scholar]
- 70.Bubenik GA. Gastrointestinal melatonin: localization, function, and clinical relevance. Dig Dis Sci. 2002;47:2336–2348. [DOI] [PubMed] [Google Scholar]
- 71.Drago F, Macauda S, Salehi S. Small doses of melatonin increases intestinal motility in rats. Dig Dis Sci. 2002;47:1969–1974. [DOI] [PubMed] [Google Scholar]
- 72.Song GH, Leng PH, Gwee KA, et al. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: a randomised, double blind, placebo controlled study. Gut. 2005;54:1402–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu WZ, Gwee KA, Moochhalla S, et al. Melatonin improves bowel symptoms in female patients with irritable bowel syndrome: a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2005;22:927–934. [DOI] [PubMed] [Google Scholar]
- 74.Lu WZ, Song GH, Gwee KA, et al. The effects of melatonin on colonic transit time in normal controls and IBS patients. Dig Dis Sci. 2009;54:1087–1093. [DOI] [PubMed] [Google Scholar]
- 75.Zarrinpar A, Chaix A, Yooseph S, et al. Diets and feeding pattern affect the diurnal dynamic of the gut microbiome. Cell Metab. 2014;20:1006–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liang X, FitzGerald GA. Timing of the microbes: the circadian rhythm of the gut microbiome. J Biol Rhythms. 2017;32:505–515. [DOI] [PubMed] [Google Scholar]
- 77.Khalif IL, Quigley EM, Konovitch EA, et al. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig Liver Dis. 2005;37:838–849. [DOI] [PubMed] [Google Scholar]
- 78.Borriello SP. Bacteria and gastrointestinal secretion and motility. Scand J Gastroenterol. 1984;93:115–121. [PubMed] [Google Scholar]
- 79.Ouwehand AC, Lagstrom H, Suomalainen T, et al. Effect of probiotics on constipation, fecal azoreductase activity and fecal mucin content in the elderly. Ann Nutr Metab. 2002;46:159–162. [DOI] [PubMed] [Google Scholar]
- 80.Ceresola ER, Ferrarese R, Preti A, et al. Targeting patients’ microbiota with probiotics and natural fibers in adults and children with constipation. Eur Rev Med Pharmacol Sci. 2018;22:7045–7057. [DOI] [PubMed] [Google Scholar]
- 81.Nath A, Haktanirlar G, Varga A, et al. Biological activities of lactose-derived prebiotics and symbiotic with probiotics on gastrointestinal system. Medicina (Kaunas). 2018;54:piiE18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dimidi E, Zdanaviciene A, Christodoulides S, et al. Randomised clinical trial: Bifidobacterium lactis NCC2818 probiotic vs placebo, and impact on gut transit time, symptoms, and gut microbiology in chronic constipation. Aliment Pharmacol Ther. 2019;49:251–264. [DOI] [PubMed] [Google Scholar]
- 83.Chen S, Ou Y, Zhao L, et al. Differential effects of lactobacillus casei strain Shirota on patients with constipation regarding stool consistency in China. J Neurogastroenterol Motil. 2019;25:148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McClung HJ, Potter C. Rational use of laxatives in children. Adv Pediatr. 2004;51:231–262. [PubMed] [Google Scholar]
- 85.Schang JC, Hemond M, Hebert M, et al. Changes in colonic myoelectric spiking activity during stimulation by bisacodyl. Can J Physiol Pharmacol. 1986;64:39–43. [DOI] [PubMed] [Google Scholar]
- 86.Hamid SA, Di Lorenzo C, Reddy SN, et al. Bisacodyl and high-amplitude-propagating colonic contractions in children. J Pediatr Gastroenterol Nutr. 1998;27:398–402. [DOI] [PubMed] [Google Scholar]
- 87.Hardcastle JD, Mann CV. Study of large bowel peristalsis. Gut. 1968;9:512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Borrelli O, Pescarin M, Saliakellis E, et al. Sequential incremental doses of bisacodyl increase the diagnostic accuracy of colonic manometry. J Neurogastroenterol Motil. 2016;28:1747–1755. [DOI] [PubMed] [Google Scholar]
- 89.Min YW, Ko E, Kim JH, et al. Increased tone of the human colon muscle by bisacodyl in vitro. J Neurogastroenterol Motil. 2018;24:317–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kamm MA, Mueller-Lissner S, Wald A, et al. Oral bisacodyl is effective and well-tolerated in patients with chronic constipation. Clin Gastroenterol Hepatol. 2011;9:577–583. [DOI] [PubMed] [Google Scholar]
- 91.Bouras EP, Camilleri M, Burton DD, et al. Prucalopride accelerates gastrointestinal and colonic transit in patients with constipation without a rectal evacuation disorder. Gastroenterology. 2001;120:354–360. [DOI] [PubMed] [Google Scholar]
- 92.Winge K, Rasmussen D, Werdelin L. Constipation in neurological diseases. J Neurol Neurosurg Psychiatry. 2003;74:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hsieh C. Treatment of constipation in older adults. Am Fam Physician. 2005;72:2277–2284. [PubMed] [Google Scholar]
- 94.Patel M, Schimpf MO, O’Sullivan DM, et al. The use of senna with docusate for postoperative constipation after pelvic reconstructive surgery: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol. 2010;202:479.e1–479.e5. [DOI] [PubMed] [Google Scholar]
- 95.Portalatin M, Winstead N. Medical management of constipation. Clin Colon Rectal Surg. 2012;25:12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mounsey A, Raleigh M, Wilson A. Management of constipation in older adults. Am Fam Physician. 2015;92:500–504. [PubMed] [Google Scholar]
- 97.Keller J, Layer P. Circadian pancreatic enzyme pattern and relationship between secretory and motor activity in fasting humans. J Appl Physiol. 2002;93:592–600. [DOI] [PubMed] [Google Scholar]
- 98.Hagger R, Kumar D, Benson M, et al. Colonic motor activity in slow-transit idiopathic constipation as identified by 24-h pancolonic ambulatory manometry. Neurogastroenterol Motil. 2003;15:515–522. [DOI] [PubMed] [Google Scholar]
- 99.Dinning PG, Smith TK, Scott SM. Pathophysiology of colonic causes of chronic constipation. Neurogastroenterol Motil. 2009;21:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]


