Abstract
BACKGROUND
Full-face aesthetic treatment involving several treatment modalities may improve facial aesthetic outcome.
OBJECTIVE
To evaluate clinical outcomes and patient perceptions of monotherapy with either abobotulinumtoxinA (ABO) or hyaluronic acid (HA) filler followed by full-face combination treatments of ABO, HA filler, and skin-boosting HA (RSB).
MATERIALS AND METHODS
Subjects aged 35 to 50 years were randomized to monotherapy with 50 s.U ABO in the glabella or ≤1 mL HA filler in the nasolabial folds (NLFs)/cheeks. At Month 6 and Month 12, all subjects received combination treatment with ≤50 s.U ABO in the glabella, ≤2 mL HA filler in the NLFs/cheeks (and other facial areas as applicable), and ≤1 mL RSB (additional RSB treatment at Month 7). Assessments included global facial aesthetic appearance and improvement, first impression, perceived age, wrinkle severity, satisfaction questionnaires, and adverse events.
RESULTS
Repeated full-face combination treatment with ABO, HA filler, and RSB was associated with considerably higher levels of aesthetic improvement and subject satisfaction than monotherapy with ABO or HA filler. Improvement rate of glabellar lines was increasing with each treatment.
CONCLUSION
Repeated combination treatment achieved greater change in global facial aesthetic appearance than monotherapy. Aesthetic improvement and subject satisfaction was high and increased with each treatment. All treatments were well tolerated.
The most common injectable aesthetic treatments today are botulinum toxin type A (BoNT-A)1–5 and hyaluronic acid (HA) fillers.6–11
In routine clinical practice, patients commonly receive monotherapy with either BoNT-A or HA filler(s), whereas some patients receive combination treatment, either at the same time or in a staged fashion depending on patient priorities, budget, and number of indications to be addressed.12–15 Data were collected on subjects receiving abobotulinumtoxinA (ABO) (Azzalure; Ipsen Biopharm Limited, Wrexham, United Kingdom), or HA products; either NASHA fillers (Restylane Lidocaine; Galderma Aesthetics, Uppsala, Sweden and/or Restylane Lyft Lidocaine), or OBT fillers (Restylane Refyne and/or Restylane Defyne), as monotherapy. This was followed by 2 full-face combination treatments (ABO, HA filler, and skin-boosting HA [RSB; Restylane Skinboosters Vital Lidocaine]) to provide guidance to practitioners regarding patient outcomes achieved with combination treatment. To the best of the authors' knowledge, this is the first study combining BoNT-A, HA fillers, and RSB.
Methods
Study Design
The authors present data from an 18-month multicenter study (Clinicaltrials.gov ID: NCT02297503; Figure 1). This study compared clinical efficacy, patient outcomes, and safety of monotherapy (ABO or HA filler; randomly assigned 1:1, stratified by center) with repeated combination treatments (ABO, HA filler, and RSB) administered in sequence after monotherapy. The study was conducted at 3 private aesthetic clinics in France and Sweden. Subjects were recruited using advertisement and among the clinics' patients. The study protocol conformed to the Declaration of Helsinki and was approved by independent ethics committees.
Figure 1.
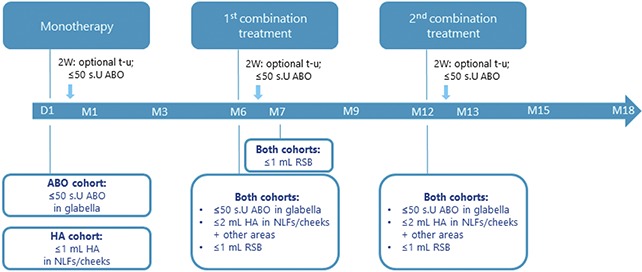
Study design and treatments. D, day; M, month; T-u, touch-up; W, weeks.
Eligibility Criteria
Eligible subjects were aged between 35 and 50 years, had mild-to-moderate nasolabial folds (NLFs)16 and moderate-to-severe glabellar lines (GL) at maximum frown17 and were likely to benefit from combined injection treatments. All subjects signed an informed consent form before initiation of any study-specific activity.
Main exclusion criteria were (1) treatment of lateral canthal lines or forehead lines required; (2) obvious facial sagging; (3) facial treatment with HA, collagen, or BoNT-A during the past 12 months, or other procedures inducing an active dermal response (e.g., laser, intense pulsed light, chemical peeling, microdermabrasion, and retinoids) within the past 6 months; (4) facial surgery or treatment with non-HA or noncollagen filler; (5) neuromuscular junctional disorders, history of dysphagia, signs of eyelid ptosis or compensatory frontalis muscle activity, history of autoimmune disease or known hypersensitivity to HA, BoNT-A, lidocaine hydrochloride, or other amide-type anesthetics; and (6) damaged or scarred facial skin, active skin disease, inflammation, or related conditions.
Treatment
Monotherapy
AbobotulinumtoxinA Cohort
Subjects received ≤50 s.U ABO intramuscularly in 5 injection points (10 s.U [0.05 mL]/injection point) in the glabella: one in the procerus; 2 in each corrugator. Optional touch-up with ≤50 s.U was allowed after 2 weeks if needed.
Hyaluronic Acid Cohort
Subjects were injected in NLFs and/or cheeks with ≤1 mL HA fillers. No touch-up was allowed.
Combination Treatment
At Month 6 and Month 12, subjects in both cohorts received ≤50 s.U ABO in the glabella, ≤2 mL HA filler in NLFs and/or cheeks (and other areas as applicable if there was product left), and ≤1 mL RSB (Figure 1). Optional touch-up with ≤50 s.U ABO was allowed after 2 weeks. A second RSB treatment (≤1 mL) was given at Month 7.
Treatment Procedure
Injections were done in accordance with the Summary of Product Characteristics (ABO) and Instructions for Use (HA fillers and RSB) that were valid at the time of the study. Use of local anesthesia was by decision of the investigators.
Assessments
Blinded evaluations were performed by physicians.
(1) Blinded evaluation of global facial aesthetic appearance achieved with combination treatment compared to monotherapy; standardized photographs (frontal view at rest and at maximum frown, and half profile view [45° angle, right and left] at rest; taken using a Canon 700D camera with standardized equipment) from Month 1 (1 month after monotherapy) were compared with corresponding photographs from Month 7 (1 month after first combination treatment);
(2) Blinded evaluation of global facial aesthetic appearance by comparing photographs from Month 1, Month 7, and Month 13 (1 month after second combination treatment);
(3) Evaluation of aesthetic improvement using the 5-grade Global Aesthetic Improvement Scale (GAIS).18 Blinded evaluators used subject photographs (frontal view at rest), whereas subjects and investigators could do the assessment live with a mirror;
(4) First impression; blinded evaluation of subject photographs (frontal view at rest) regarding 8 categories (social skills, academic performance, dating success, occupational success, attractiveness, financial success, relationship success, and athletic success) on a 10-graded scale;
(5) Perceived age assessments; blinded evaluation of subject photographs (frontal view at rest);
(6) GL wrinkle severity assessments, at rest and at maximum frown using a validated 5-grade scale17 (investigators);
(7) Satisfaction questionnaires (subjects and Investigators);
(8) Adverse events (AEs).
Statistical Methods
The sample size was based on the assumption that 80% of subjects would show superior global facial aesthetic appearance after combination treatment than after monotherapy. Two analysis populations were defined for the study: the safety population (subjects who were injected in at least one injection point [ABO cohort] or one NLF/cheek [HA cohort]) and the intention-to-treat population (primary analysis population for efficacy analyses; subjects who were injected in GL [ABO cohort] or both NLFs/cheeks [HA cohort]).
The randomization list and statistical analyses were performed using SAS version 9.4. Analyses of global facial aesthetic appearance, GAIS, and wrinkle severity were performed using 95% CI. First impression was presented descriptively and using the Wilcoxon signed-rank test, perceived age assessments were presented descriptively and with the paired t-test. Satisfaction questionnaires were analyzed descriptively. For the analysis of global facial aesthetic appearance, the aim was to achieve a 95% CI above 50%. Missing values were not imputed.
Results
Subject Disposition, Demographic, Baseline, and Injection Data
Figure 2 shows subject disposition. The first subject was enrolled on November 3, 2014; the last subject completed the Month 18 visit on December 17, 2016.
Figure 2.
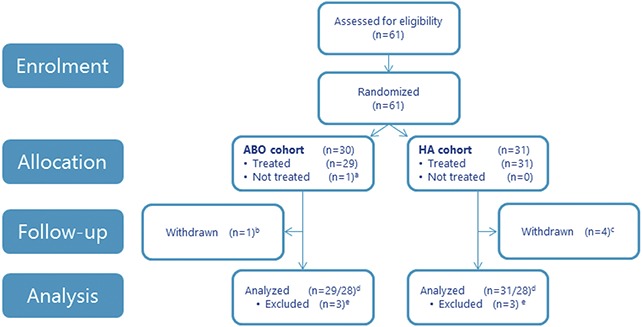
Subject disposition. aWithdrawn consent. bProhibited procedure during the study. cLost to follow-up (n = 1), AE (angina pectoris; n = 1), pregnancy (n = 1), withdrawn consent (n = 1). dSafety analyses after monotherapy, and after first and second combination treatment only included subjects who received respective treatment. ePhotographs not assessable in the blinded evaluation due to technical circumstances. ABO, abobotulinumtoxinA; HA, hyaluronic acid.
No protocol deviations were considered to significantly influence efficacy or safety assessments.
Supplemental Digital Content 1, Table S1, http://links.lww.com/DSS/A327 shows demographic and baseline data.
Supplemental Digital Content 1, Table S2, http://links.lww.com/DSS/A327 presents injection details.
Efficacy
Global Facial Aesthetic Appearance
The blinded evaluators assessed that most subjects (ABO cohort: 74%; HA filler cohort: 93%) had superior facial aesthetic appearance 1 month after first combination treatment (Month 7) compared with 1 month after monotherapy (Month 1) (Figure 3). When the evaluators compared photographs from Month 1, Month 7, and Month 13 (1 month after second combination treatment), the best result was obtained after the second combination treatment (56% of subjects), followed by first combination treatment (33%) and monotherapy (10%) (both cohorts combined, Figure 4).
Figure 3.
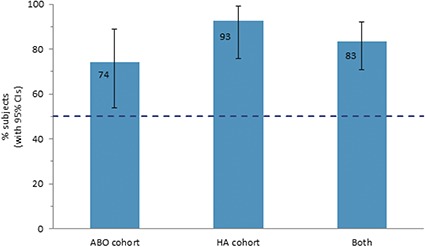
Subjects with superior global facial aesthetic appearance after first combination treatment than after monotherapy. Because the confidence interval (CI) was above the predetermined limit (50%; dashed line), it was shown that with 95% confidence, the majority of subjects in the underlying population had a superior facial aesthetic appearance after first combination treatment than after monotherapy. This conclusion was valid also when evaluating subjects in each cohort separately. ABO, abobotulinumtoxinA; HA, hyaluronic acid.
Figure 4.
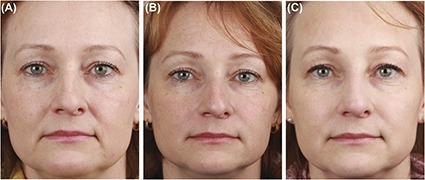
Subject photographs. Female subject aged 49 years. (A) Baseline. (B) Month 7; 1 month after first combination treatment: (1) 50 s.U ABO (glabella) (2) 2-mL HA filler (NLFs, cheeks, and marionette lines) (3) 1-mL RSB (midface); (C): Month 13; 1 month after second combination treatment: (1) 50 + 10 s.U touch-up ABO (glabella) (2) 2-mL HA filler (NLFs, cheeks and nose) (3) 1-mL RSB (lower face). ABO, abobotulinumtoxinA; HA, hyaluronic acid; NLF, nasolabial fold.
Global Aesthetic Improvement Scale
The percentage of subjects with improved GAIS score after monotherapy compared to baseline, as assessed by blinded evaluators, was 52% in the ABO cohort and 65% in the HA filler cohort. After first and second combination treatment, 76% and 90% of subjects were assessed as improved, respectively (both cohorts combined).
Global Aesthetic Improvement Scale score assessments by subjects 1 month after monotherapy resulted in improvement for all subjects (100%) in the ABO cohort and for 81% of subjects in the HA cohort. All subjects (100%) reported GAIS score improvement 1 month after both combination treatments. Global Aesthetic Improvement Scale assessment by investigators was similar to that of the subjects.
First Impression
Overall first impression (i.e., the sum of scores from all 8 categories) was similar between monotherapy and combination treatments; mean scores during the study period varied between 42.3 and 43.7, both cohorts combined.
Perceived Age
Blinded evaluators assessed that subjects looked younger after first and second combination treatment than after monotherapy (mean difference −1.8 and −2.2 years, respectively; p-value: <.001, both cohorts combined).
Subject and Investigator Satisfaction Questionnaire
Subject satisfaction was higher after combination treatments than after monotherapy. Subjects who were very or somewhat satisfied with their facial appearance increased from baseline (32%) to Month 1 (60%) and further to Months 7 and 13 (88% and 91%, respectively). Overall, satisfaction with skin quality parameters improved after the first combination treatment (when RSB was injected) compared with baseline and improved further after second combination treatment (Figure 5). One month after the first and second combination treatment, most subjects would recommend the treatment to a friend (95%–96%), and would have the treatment again (93%–98%), both cohorts combined.
Figure 5.
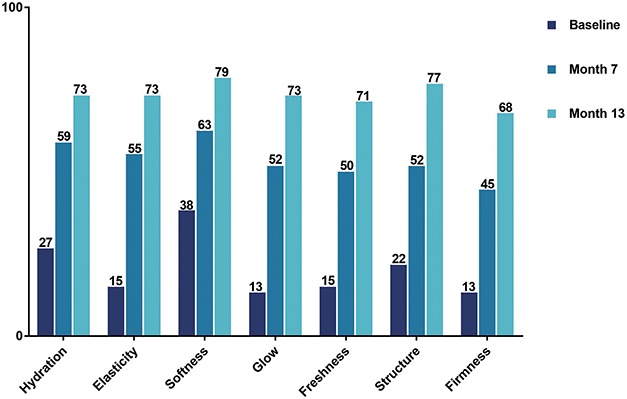
Subject satisfaction with skin quality parameters, both cohorts combined.
Investigators were very or somewhat satisfied with the overall facial aesthetic outcome for a majority of subjects 1 month after monotherapy (ABO cohort: 100%; HA cohort: 74%), and for all subjects (100%) 1 month after both combination treatments. The investigators saw a need for additional filler in most subjects (61%) 1 month after monotherapy with HA filler, whereas after the combination treatments, the need for additional filler was seen in fewer subjects (21%–25%, respectively; both cohorts combined).
Wrinkle Severity
At baseline, all subjects had mild or moderate GL at rest (except one subject who had no GL), and moderate or severe GL at maximum frown (Supplemental Digital Content 1, Table S1, http://links.lww.com/DSS/A327). Improvement in wrinkle severity of GL was higher at maximum frown (67%–100%; Months 6 and 12 excluded) than at rest (49%–77%; Months 6 and 12 excluded) (Figure 6). Six months after each treatment, improvement at rest was observed in 29% of subjects at Month 6 (ABO cohort), in 38% at Month 12, and in 49% of subjects at Month 18 (both cohorts combined). At maximum frown, improvement was observed in 25% of subjects at Month 6 (ABO cohort), in 41% at Month 12, and in 67% at Month 18 (both cohorts combined).
Figure 6.
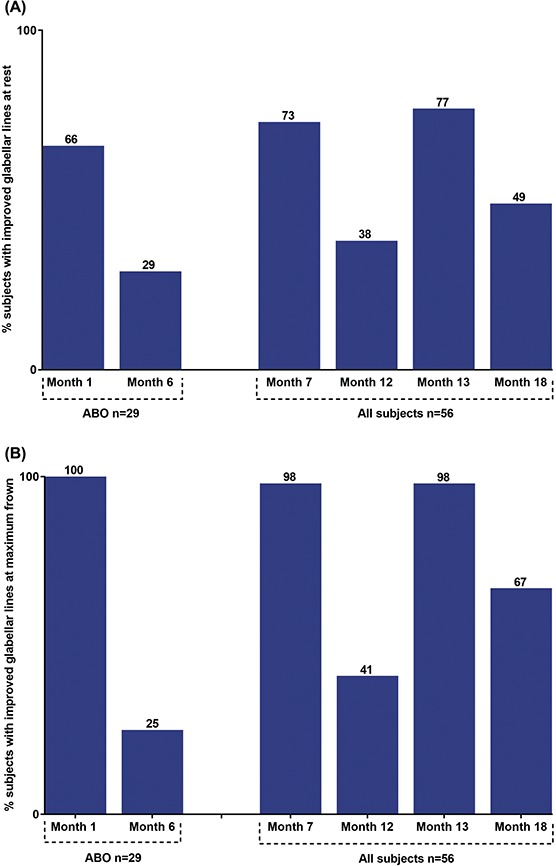
Improvement* of wrinkle severity of glabellar lines. (A) At rest. (B) At maximum frown. *At least 1 grade decrease on the scale from baseline. ABO, abobotulinumtoxinA.
Safety
Monotherapy
One subject injected with HA filler had bilateral implant-site hematomas of moderate intensity, which resolved after 15 days. No AEs were related to monotherapy with ABO. One of the AEs that were judged as unrelated to study treatment was assessed as serious (angina pectoris). One pregnancy occurred in the HA cohort. The subject was withdrawn after having completed the Month 3 assessments.
First Combination Treatment
Twenty-two subjects (39%) had 42 treatment-related AEs; implant-site bruising was most commonly reported (22 events in 11 subjects [20%]). All treatment-related AEs were mild (n = 33) or moderate (n = 9) in intensity, most resolved within 2 weeks, and none was serious. All treatment-related AEs were related to treatment with HA filler or RSB, except bilateral undesired elevation of eyebrows in one subject after ABO injection.
Second Combination Treatment
Thirteen subjects (23%) had 18 treatment-related AEs, all were related to treatment with HA filler or RSB; implant-site bruising was most commonly reported (14 events in 9 subjects [16%]). All treatment-related AEs were mild (n = 15) or moderate (n = 3) in intensity, most resolved within one week, and none was serious.
Discussion
It has become standard practice in clinical aesthetics for practitioners to complete a full facial assessment and to discuss aesthetic issues that could be addressed with skin care regime and nonsurgical intervention (including laser and injectable treatments) with their patients. The choice of treatment modality depends on patient needs, expectations, and budget; practitioner skills; and time course chosen for delivery of the treatments. Providing BoNT-A and HA filler treatment at the same time is common, but to the authors' knowledge, no study using combination treatment with BoNT-A, HA fillers, and RSB has been published. Here, the authors report data from an 18-month multicenter study comparing repeated full-face aesthetic combination treatment (ABO, HA filler, and RSB) administered in sequence after monotherapy (ABO or HA filler alone).
As expected, overall results indicated more beneficial aesthetic outcomes of combination treatment compared with monotherapy with ABO or HA, and further improvement after second treatment compared to the first combination treatment. Thus, cumulative treatments over time resulted in better aesthetic outcomes. It is important, however, to consider the potential confounding effects from previous treatments because combination treatments were administered in sequence after monotherapy for all subjects. Including groups receiving only monotherapy versus receiving only combination treatment would have added further knowledge. However, the authors believe that the sequential treatment setup used in this study is a more accurate reflection of a real-life scenario.
All subjects improved on the GAIS after both combination treatments and after monotherapy with ABO, as assessed by subjects/investigators. Most subjects improved after monotherapy with HA filler (GAIS by investigator: 77%; GAIS by subjects: 81%).
Blinded evaluators assigned higher GAIS scores after monotherapy with HA filler than after monotherapy with ABO. This was not in line with subject/investigator assessments, where monotherapy with ABO had a higher percentage of improvement compared with HA filler, possibly due to subjects and investigators being able to do the assessment live with a mirror, whereas the blinded evaluators had 2D-photographs of subjects (frontal view, at rest) at their disposal during the assessment. The results also indicate that glabella treatment with ABO may give a more noticeable effect than HA filler in the NLFs and/or cheeks when given as monotherapy, possibly because HA treatment in these locations do not address rejuvenation or the cause of aging as effectively as ABO treatment. Global Aesthetic Improvement Scale scores in the subjects' and investigators' assessments may also reflect that the volume of filler used in the HA cohort was suboptimal.
The mean volume injected into NLFs in the monotherapy session (0.89 mL; n = 31) was similar to that injected at first combination treatment (0.84 mL; n = 53), whereas the mean volume injected at second combination treatment was lower (0.40 mL; n = 43). The mean volume injected into cheeks in the monotherapy session (0.47 mL; n = 6) was lower than that at first combination treatment (0.77 mL; n = 37) and at second combination treatment (0.99 mL; n = 40). Thus, investigators prioritized injection into NLFs in the monotherapy session, which supports the assumption that the typical 1-mL volume injected at first treatment with HA filler is suboptimal. This was also reflected in the investigator questionnaire, where investigators responded that higher HA volumes would have been preferred. These results suggest that in patients for whom it is not feasible to inject more than 1-mL HA filler, it may be beneficial to include ABO as a first step in the treatment plan.
Subjects' satisfaction with treatment and skin quality improved over time, indicating that the addition of RSB for improved skin quality was effective, although the assessment could be influenced by all products included in the combination treatments.
Improvement in wrinkle severity of GL during the study was higher at maximum frown. Higher improvement at Month 18 than at Month 12 provides further support to the benefit of repeated treatments. The percentage of subjects with at least one-grade improvement 1 month after ABO injection was higher or comparable to those in previous investigations using a 5-grade wrinkle severity assessment scale,19,20 whereas the percentage of subjects with improvement at Month 6 was lower in the authors' study (25%) than previously reported for other BoNT-A preparations (50%).20 However, 6-month data were only reported for 12 subjects in that publication. Although the percentage of subjects with at least one-grade improvement at Month 6 in a previous ABO study21 was lower (14%) than in the authors' study, these results are not directly comparable because a 4-grade scale was used for wrinkle severity assessment in the previous study.
Monotherapy and combination treatments were well tolerated and were not associated with any safety concerns. Although most treatment-related AEs were related to HA fillers and/or RSB, the combination of these 2 product categories was well tolerated. Treatment-related AEs were mild or moderate, and the majority resolved without intervention.
This study was limited by the restricted volumes of HA filler that was allowed, although these were set to reflect a real-life scenario where subjects often have limited resources. Furthermore, because combination treatments were administered in sequence after monotherapy, potential confounding effects on clinical outcome and patient perception must be considered.
Results from this study could potentially be used for establishing treatment plans and as support during patient consultation. To that end, a new patient assessment tool called aesthetic Global Ranking Scale22 has been developed to assist the practitioner in creating personalized treatment plans. By use of this tool in a clinical study, full-face combination treatments could be further evaluated in relation to patient needs and satisfaction.
Conclusion
Combination treatment with ABO, HA, and RSB resulted in more beneficial aesthetic outcomes compared with monotherapy with either ABO or HA in this study where repeated combination treatments were administered in sequence after monotherapy. Glabellar lines' improvement and subject satisfaction with skin quality increased over time. Monotherapy with ABO may be more beneficial to include as a first step in the treatment plan than treatment with HA filler. Both combination treatments and monotherapy were well tolerated.
Acknowledgments
Patients provided written consent for the use of their images.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.dermatologicsurgery.org).
Interim (7-month) results from this study were previously presented at the Aesthetic and Anti-aging Medicine World Congress (AMWC); April 2017; Monte Carlo, Monaco; 18-month results from this study were presented at the IMCAS Congress; February 2018; Paris, France.
Galderma funded the study and provided the study products. P. Hedén is a consultant for Allergan, Galderma, and Teoxane; C. Skoglund, C. Edwartz, and M. Norberg are employed by Galderma; P. Kestemont is a consultant for Allergan, Filorga, Galderma, Teoxane, Universkin, and Vivacy. The remaining authors have indicated no significant interest with commercial supporters.
References
- 1.Rzany B, Ascher B, Monheit G. Treatment of glabellar lines with botulinum toxin type A (Speywood Unit): a clinical overview. J Eur Acad Dermatol Venereol 2010;24(Suppl 1):1–14. [DOI] [PubMed] [Google Scholar]
- 2.Ascher B, Talarico S, Cassuto D, Escobar S, et al. International consensus recommendations on the aesthetic usage of botulinum toxin type A (Speywood Unit)—part I: upper facial wrinkles. J Eur Acad Dermatol Venereol 2010;24:1278–84. [DOI] [PubMed] [Google Scholar]
- 3.Karsai S, Adrian R, Hammes S, Thimm J, et al. A randomized double-blind study of the effect of Botox and Dysport/Reloxin on forehead wrinkles and electromyographic activity. Arch Dermatol 2007;143:1447–9. [DOI] [PubMed] [Google Scholar]
- 4.Ascher B, Rzany BJ, Grover R. Efficacy and safety of botulinum toxin type A in the treatment of lateral crow's feet: double-blind, placebo-controlled, dose-ranging study. Dermatol Surg 2009;35:1478–86. [DOI] [PubMed] [Google Scholar]
- 5.Rzany B, Dill-Müller D, Grablowitz D, Heckmann M, et al. Repeated botulinum toxin A injections for the treatment of lines in the upper face: a retrospective study of 4,103 treatments in 945 patients. Dermatol Surg 2007;33:S18–25. [DOI] [PubMed] [Google Scholar]
- 6.Rzany B, Cartier H, Kestemont P, Trevidic P, et al. Full-face rejuvenation using a range of hyaluronic acid fillers: efficacy, safety, and patient satisfaction over 6 months. Dermatol Surg 2012;38:1153–61. [DOI] [PubMed] [Google Scholar]
- 7.Weiss RA, Moradi A, Bank D, Few J, et al. Effectiveness and safety of large gel particle hyaluronic acid with lidocaine for correction of midface volume deficit or contour deficiency. Dermatol Surg 2016;42:699–709. [DOI] [PubMed] [Google Scholar]
- 8.Narins RS, Dayan SH, Brandt FS, Baldwin EK. Persistence and improvement of nasolabial fold correction with nonanimal-stabilized hyaluronic acid 100,000 gel particles/mL filler on two retreatment schedules: results up to 18 months on two retreatment schedules. Dermatol Surg 2008;34(Suppl 1):S2–8; discussion S8. [DOI] [PubMed] [Google Scholar]
- 9.Narins RS, Brandt FS, Dayan SH, Hornfeldt CS. Persistence of nasolabial fold correction with a hyaluronic acid dermal filler with retreatment: results of an 18-month extension study. Dermatol Surg 2011;37:644–50. [DOI] [PubMed] [Google Scholar]
- 10.Carruthers J, Klein AW, Carruthers A, Glogau RG, et al. Safety and efficacy of nonanimal stabilized hyaluronic acid for improvement of mouth corners. Dermatol Surg 2005;31:276–80. [DOI] [PubMed] [Google Scholar]
- 11.Rzany B, Bayerl C, Bodokh I, Boineau D, et al. An 18-month follow-up, randomized comparison of effectiveness and safety of two hyaluronic acid fillers for treatment of moderate nasolabial folds. Dermatol Surg 2017;43:58–65. [DOI] [PubMed] [Google Scholar]
- 12.Molina B, David M, Jain R, Amselem M, et al. Patient satisfaction and efficacy of full-facial rejuvenation using a combination of botulinum toxin type A and hyaluronic acid filler. Dermatol Surg 2015;41(Suppl 1):S325–332. [DOI] [PubMed] [Google Scholar]
- 13.Fabi SG, Goldman MP, Mills DC, Werschler WP, et al. Combining microfocused ultrasound with botulinum toxin and temporary and semi-permanent dermal fillers: safety and current use. Dermatol Surg 2016;42(Suppl 2):S168–176. [DOI] [PubMed] [Google Scholar]
- 14.Narurkar VA, Cohen JL, Dayan S, Kaminer M, et al. A comprehensive approach to multimodal facial aesthetic treatment: injection techniques and treatment characteristics from the HARMONY study. Dermatol Surg 2016;42(Suppl 2):S177–191. [DOI] [PubMed] [Google Scholar]
- 15.Carruthers J, Carruthers A. A multimodal approach to rejuvenation of the lower face. Dermatol Surg 2016;42(Suppl 2):S89–93. [DOI] [PubMed] [Google Scholar]
- 16.Narins RS, Carruthers J, Flynn TC, Geister TL, et al. Validated assessment scales for the lower face. Dermatol Surg 2012;38:333–42. [DOI] [PubMed] [Google Scholar]
- 17.Flynn TC, Carruthers A, Carruthers J, Geister TL, et al. Validated assessment scales for the upper face. Dermatol Surg 2012;38:309–19. [DOI] [PubMed] [Google Scholar]
- 18.Narins RS, Brandt F, Leyden J, Lorenc ZP, et al. A randomized, double-blind, multicenter comparison of the efficacy and tolerability of restylane versus zyplast for the correction of nasolabial folds. Dermatol Surg 2003;29:588–95. [DOI] [PubMed] [Google Scholar]
- 19.Streker M, Luebberding S, Krueger N, Harrington L, et al. Patient-reported outcomes after incobotulinumtoxinA treatment for upper facial wrinkles. Dermatol Surg 2015;41(Suppl 1):S29–38. [DOI] [PubMed] [Google Scholar]
- 20.Prager W, Rappl T. Phase IV study comparing incobotulinumtoxinA and onabotulinumtoxinA using a 1:1.5 dose-conversion ratio for the treatment of glabellar frown lines. J Cosmet Dermatol 2012;11:267–71. [DOI] [PubMed] [Google Scholar]
- 21.Ascher B, Zakine B, Kestemont P, Baspeyras M, et al. A multicenter, randomized, double-blind, placebo-controlled study of efficacy and safety of 3 doses of botulinum toxin A in the treatment of glabellar lines. J Am Acad Dermatol 2004;51:223–33. [DOI] [PubMed] [Google Scholar]
- 22.Jain R, Huang P, Ferraz RM. A new tool to improve delivery of patient-engaged care and satisfaction in facial treatments: the Aesthetic Global Ranking Scale. J Cosmet Dermatol 2017;16:132–43. [DOI] [PubMed] [Google Scholar]


