Table of Contents

A. EXECUTIVE SUMMARY
Updated US consensus guidelines for management of cervical screening abnormalities are needed to accommodate the 3 available cervical screening strategies: primary human papillomavirus (HPV) screening, cotesting with HPV testing and cervical cytology, and cervical cytology alone. New data indicate that a patient's risk of developing cervical precancer or cancer can be estimated using current screening test results and previous screening test and biopsy results, while considering personal factors such as age and immunosuppression. Routine screening applies only to asymptomatic individuals who do not require surveillance for prior abnormal screening results.
The 2012 consensus guidelines were the first to be based on the principle of equal management for equal risk, specifically, the risk of a patient developing cervical cancer, estimated by the surrogate end point of the 5-year risk of cervical intraepithelial neoplasia (CIN) grade 3 (CIN 3) or more severe diagnoses (CIN 3+), regardless of which test combinations yielded this risk level. Introduction of risk-based guidelines in 2012 was a conceptual breakthrough, but the recommendations retained a continued reliance on complicated algorithms and insufficiently incorporated screening history. With a more nuanced understanding of how previous results affect risk, and more variables to consider, the 2019 guidelines further align management recommendations with current understanding of HPV natural history and cervical carcinogenesis. More frequent surveillance, colposcopy, and treatment are recommended for patients at progressively higher risk, whereas those at lower risk can defer colposcopy, undergo follow-up at longer surveillance intervals, and, when at sufficiently low risk, return to routine screening. Clearly defined risk thresholds to guide management are designed to continue functioning appropriately when population-level prevalence of CIN 3+ decreases because of HPV vaccination and also as new screening and triage tests are introduced. The revised guidelines provide a framework for incorporating new data and technologies as ongoing incremental recommendation revisions, minimizing the time needed to implement changes that are beneficial to patient care.
B. INTRODUCTION
This is the fourth American Society of Colposcopy and Cervical Pathology (ASCCP)-sponsored consensus guidelines for management of cervical cancer screening abnormalities, after the original consensus conferences in 20011 and subsequent updates in 20062 and 2012.3 An interim guidance publication providing management recommendations for primary HPV screening was released in 2015.4 This document updates and replaces all previous guidance. The key difference between 2019 guidelines and previous versions is the change from primarily test results–based algorithms (e.g., “Colposcopy is recommended for patients with HPV-positive atypical squamous cells of undetermined significance [ASC-US], low-grade squamous intraepithelial lesion [LSIL],” etc.) to primarily “risk-based” guidelines (e.g., “Colposcopy is recommended for any combination of history and current test results yielding a 4.0% or greater probability of finding CIN 3+,” etc.). See Box 1 for essential changes. Tables of risk estimates for possible combinations of current screening test results and screening history (including unknown history) have been generated from a prospective longitudinal cohort of more than 1.5 million patients followed for more than a decade at Kaiser Permanente Northern California (KPNC). All KPNC estimates of risk underlying guideline decisions are detailed in the accompanying article by Egemen et al.5 The applicability of these risk estimates to other United States regions and populations has been confirmed in other data sets from screening programs and clinical trials.6 Many patients, especially those with minor abnormalities, can be managed by identifying their risk level using Tables 1A to 5B in Egemen et al5 and linking it to a recommended clinical action (return to routine screening, surveillance with repeat testing at 1- or 3-year intervals, colposcopy, or treatment). To facilitate use of these tables, the same information will be accessible via smartphone app (for purchase) and web (no cost) through http://www.asccp.org. Decision aids may facilitate use of the tables.7 Common abnormalities are managed using risk estimates outlined in Section E, and rare abnormalities are managed via the result-specific consensus recommendations outlined in Sections G-K.
Box 1. Essential Changes From Prior Management Guidelines
1) Recommendations are based on risk, not results.
-
Recommendations of colposcopy, treatment, or surveillance will be based on a patient's risk of CIN 3+ determined by a combination of current results and history (including unknown history). The same current test results may yield different management recommendations depending on the history of recent past test results.
2) Colposcopy can be deferred for certain patients.
Repeat HPV testing or cotesting at 1 year is recommended for patients with minor screening abnormalities indicating HPV infection with low risk of underlying CIN 3+ (e.g., low-grade cytologic abnormalities after a documented negative screening HPV test or cotest).
-
At the 1-year follow-up test, referral to colposcopy is recommended if results remain abnormal.
3) Guidance for expedited treatment is expanded (i.e., treatment without colposcopic biopsy).
Expedited treatment was an option for patients with HSIL cytology in the 2012 guidelines; this guidance is now better defined.
For nonpregnant patients 25 years or older, expedited treatment, defined as treatment without preceding colposcopic biopsy demonstrating CIN 2+, is preferred when the immediate risk of CIN 3+ is ≥60%, and is acceptable for those with risks between 25% and 60%. Expedited treatment is preferred for nonpregnant patients 25 years or older with high-grade squamous intraepithelial lesion (HSIL) cytology and concurrent positive testing for HPV genotype 16 (HPV 16) (i.e., HPV 16–positive HSIL cytology) and never or rarely screened patients with HPV-positive HSIL regardless of HPV genotype.
-
Shared decision-making should be used when considering expedited treatment, especially for patients with concerns about the potential impact of treatment on pregnancy outcomes.
4) Excisional treatment is preferred to ablative treatment for histologic HSIL (CIN 2 or CIN 3) in the United States. Excision is recommended for adenocarcinoma in situ (AIS).
5) Observation is preferred to treatment for CIN 1.
-
Treatment remains acceptable for patients with repeat diagnoses of CIN 1 persisting 2 years or more.
6) Histopathology reports based on Lower Anogenital Squamous Terminology (LAST)/World Health Organization (WHO) recommendations for reporting histologic HSIL should include CIN 2 or CIN 3 qualifiers, i.e., HSIL(CIN 2) and HSIL (CIN 3).
7) All positive HPV tests, regardless of genotype, should have additional reflex triage testing performed from the same laboratory specimen (e.g., reflex cytology).
Additional testing from the same laboratory specimen is recommended because the findings may inform colposcopy practice. For example, those with HSIL cytology and concurrent positive testing for HPV genotype 16 qualify for expedited treatment.
HPV 16 or 18 infections have the highest risk for CIN 3 and occult cancer, so additional evaluation (e.g., colposcopy with biopsy) is necessary even when cytology results are negative.
-
If HPV 16 and 18 testing is positive, and additional laboratory testing of the same sample is not feasible, the patient should proceed directly to colposcopy.
8) Continued surveillance with HPV testing or cotesting at 3-year intervals for at least 25 years is recommended after treatment of histologic HSIL, CIN 2, CIN 3, or AIS. Continued surveillance at 3-year intervals beyond 25 years is acceptable for as long as the patient's life expectancy and ability to be screened are not significantly compromised by serious health issues.
The 2012 guidelines recommended return to 5-year screening intervals and did not specify when screening should cease. New evidence indicates that risk remains elevated for at least 25 years, with no evidence that treated patients ever return to risk levels compatible with 5-year intervals.
-
Surveillance with cytology alone is acceptable only if testing with HPV or cotesting is not feasible. Cytology is less sensitive than HPV testing for detection of precancer and is therefore recommended more often. Cytology is recommended at 6-month intervals when HPV testing or cotesting is recommended annually. Cytology is recommended annually when 3-year intervals are recommended for HPV or cotesting.
9) Human papilloma virus assays that are Food and Drug Administration (FDA)-approved for screening should be used for management according to their regulatory approval in the United States. (Note: all HPV testing in this document refers to testing for high-risk HPV types only).
For all management indications, HPV mRNA and HPV DNA tests without FDA approval for primary screening alone should only be used as a cotest with cytology, unless sufficient, exceptionally rigorous data are available to support primary HPV testing in management.
The minimum amount of data required to generate a recommendation will include the patient's age and current test results, as we recognize that previous screening history is often not known. Increased precision of management guidance will be possible if information is available on test results within the past 5 years and previous precancer treatment within the past 25 years.3 Current results and past history are designed to generate the patient's risk estimate from data tables.5 Risk estimates are available for the following clinical situations: abnormal screening test results with unknown history, abnormal screening test results with medical record documentation of a preceding negative HPV test or cotest, surveillance of previous abnormal screening test results that did not require immediate colposcopic referral (e.g., follow-up after an HPV-positive cytology negative result), colposcopy/biopsy results, and follow-up surveillance tests after colposcopy or after treatment for, or resolution of, high-grade abnormalities (e.g., CIN 2+).
The recognition that persistent HPV infection is necessary for developing precancer and cancer (defined as CIN 3+, which includes diagnoses of CIN 3, AIS, and cancer) underlies the 2019 guideline update. Prospective longitudinal data indicate that when a new abnormal screening test result follows a negative HPV test or cotest within the past 5 years, the estimated risk of CIN 3+ is reduced by approximately 50%.8 A negative cytology result within 3 years of a new abnormal screening test, however, does not confer a similar reduction in risk.9 The 2019 guidelines also recognize that a colposcopic examination performed according to accepted standards (e.g., using the KPNC colposcopy protocol or the ASCCP Colposcopy Standards10) confirming low-grade or normal histology reduces a patient's estimated risk of having precancer/cancer in the next 2 years.11 This allows patients with an HPV-positive ASC-US or LSIL result at their 1-year follow-up visit after a colposcopy confirming normal- or low-grade histology to return for repeat HPV or cotesting in 1 more year, rather than immediately return to colposcopy. Thus, incorporating a patient's history of previous HPV tests and colposcopy/biopsy results will permit detection and treatment of CIN 3+ while avoiding unnecessary interventions for patients with new HPV infections who are at lower risk.12
C. GUIDING PRINCIPLES
Guidelines are based on several guiding principles. The first 4 guiding principles are new for 2019, whereas the others are from the 2012 guidelines. As the 2012 guidelines are familiar to providers, we changed management recommendations only when new evidence favored an altered management strategy. Note that management guidelines apply only to patients with current or previous abnormal screening test results; screening guidelines for individuals in the general population, that are not being followed for a screening abnormality, are addressed elsewhere.13,14
New 2019 Principles
1. HPV–based testing is the basis for risk estimation. The term HPV-based testing is used throughout this document and refers to use of either primary HPV testing alone or HPV testing in conjunction with cervical cytology (cotesting).
Characteristics of HPV infections, including HPV type and the duration of infection, determine a patient's risk of CIN 3+.15–18 Although cytology has high specificity (apart from ASC-US) and can be helpful when estimating immediate risk, its lower sensitivity and lower negative predictive value compared with HPV testing reduces its utility for long-term risk prediction.9 The results of HPV tests alone or in conjunction with cytology are used to guide recommendations that allow lengthening of follow-up intervals and deferral of colposcopy for low-risk results. Of note, risk estimates underlying the 2019 management guidelines are based on HPV DNA testing.
2. Personalized risk-based management is possible with knowledge of current results and past history. A patient's risk of having or developing CIN 3+ is estimated based on current and previous results, as well as history of previous precancer treatment. Management recommendations use thresholds of risk.19 Recommendations of routine screening, 1-year or 3-year surveillance, colposcopy, or treatment correspond to a risk stratum, a range of risk for CIN 3+. The lower threshold of each risk stratum, called Clinical Action Threshold, defines the level at which the management recommendation changes. The Clinical Action Thresholds for each risk stratum were determined through the consensus process. Risks were estimated for all combination of current results and past history (including unknown history) for which adequate data were available at KPNC. Management can be determined via look-up tables,5 and use of the tables can be facilitated using decision aids.
3. Guidelines must allow updates to incorporate new test methods as they are validated, and to adjust for decreasing CIN3+ risks as more patients who received HPV vaccination reach screening age. The field of cervical cancer prevention is rapidly evolving, with new technologies being continually validated. Data on the validation of new technologies are being published frequently, and risk reduction from HPV vaccination is increasing as vaccine coverage increases and vaccinated individuals age into screening cohorts. Up to now, guideline revisions have required full consensus conferences, which are time-consuming, expensive, and not compatible with the rapid evolution of the field. The 2019 guidelines build a framework that allows incorporation of new technologies and modified strategies without requiring full consensus conferences, so that revisions may rapidly incorporate new findings and be quickly disseminated to optimize patient care.
Clinical Action Thresholds for management created through the 2019 consensus process will remain in place, but as new tests become available and more long-term data accrue, the test combinations used to reach these thresholds will change. For example, at the 2019 consensus conference, HPV vaccination levels in the United States population currently 25 years or older were deemed too low to warrant incorporating HPV vaccination into the 2019 management recommendations. However, this is expected to change in the near future as more vaccinated patients, who have lower CIN 3+ risk, reach the age of 25 years and additional data accrue demonstrating the impact of vaccination on the CIN 3+ risk associated with abnormal test result combinations. The framework outlined here will allow guideline modification as robust data become available and are publicized. Because Clinical Action Thresholds remain constant, new data can be added while the Clinical Action Thresholds remain unchanged. This design is intentional to reduce clinician confusion associated with frequently changing guidelines.
4. Colposcopy practice must follow guidance detailed in the ASCCP Colposcopy Standards.10 Colposcopy with targeted biopsy remains the primary method of detecting precancers requiring treatment. Because patients are managed less aggressively after a colposcopic examination where CIN grade 2 or higher (CIN 2+) is not found, maximizing detection of CIN 2+ at each colposcopy visit is paramount. Evidence-based practice recommends that biopsies be taken of all discrete acetowhite areas, usually 2 to 4 biopsies at each colposcopic examination. For those at lowest risk, defined as less than HSIL cytology, no evidence of HPV 16/18 infection, and a completely normal colposcopic impression (i.e., no acetowhitening, metaplasia, or other visible abnormality, and a fully visualized squamocolumnar junction), untargeted (random) biopsies are not recommended and patients with a completely normal colposcopic impression can be observed without biopsy. To ensure that CIN 2+ is not missed, the ASCCP Colposcopy Standards emphasize the need for biopsies even when the colposcopic impression is normal but any degree of acetowhitening, metaplasia, or other abnormality is present.
2012 Principles Carried Forward
5. The primary goal of screening and management is cancer prevention through detection and treatment of cervical precancer. Numerous population-level studies indicate that incidence and mortality from cervical cancer decrease as detection and treatment of high-grade histologic cervical abnormalities (generally defined as CIN 2+) increases.20,21 Timely detection and treatment of the highest grade of precancers (CIN 3/AIS) have been the benchmark used for previous guidelines3 and remain the primary goal of the 2019 management guideline; a secondary goal (because of the relative rarity of this finding in the United States) is early diagnosis of cervical cancer to reduce related morbidity and mortality. A patient's risk of having or developing CIN 3+ is estimated based on current and previous results, as well as history of previous precancer treatment. Management recommendations are guided by risk thresholds.19 Recommendations of routine screening, 1- or 3-year surveillance, colposcopy, or treatment each correspond to a risk stratum. These risk strata (ranges of risk for CIN 3+) are defined by Clinical Action Thresholds that were determined through the consensus process (Section E).
6. Guidelines apply to all individuals with a cervix. Guidelines apply to women and transgender men with a cervix, including individuals who have undergone supracervical hysterectomy. Risk estimates were validated in individuals of diverse racial, ethnic, and socioeconomic backgrounds and shown to be comparable.6 Though not the primary focus of the 2019 guidelines, management recommendations are also provided for patients who have undergone hysterectomy with removal of the cervix and who have a previous diagnosis of histologic HSIL, CIN 2, CIN 2/3, CIN 3, and/or AIS, irrespective of whether the hysterectomy was performed for precancer treatment or another indication.
7. Equal management for equal risk. History and current test results are used to calculate a patient's current and future risk of CIN 3+. Similar risks are managed similarly, regardless of the combination of results/history used to estimate the risk.
8. Balancing benefits and harms. Providing the best care means balancing cancer prevention with overtesting and overtreatment. Preventing all cervical cancers is unfortunately not an achievable goal. Interventions to prevent cervical cancer can cause harm. The 2019 guidelines are designed to maximize cervical cancer prevention and minimize harms from overtesting and overtreating by managing patients according to their current and future risks of CIN 3+. High-risk patients require closer follow-up to maximize detection of CIN 3+, whereas low-risk patients require fewer tests and procedures.
9. Guidelines apply to asymptomatic patients that require management of abnormal cervical screening test results. Patients with symptoms such as abnormal uterine or vaginal bleeding or a visibly abnormal-appearing cervix require appropriate diagnostic testing as this may be a sign of cancer.22 This evaluation may include cervical cytology, colposcopy, diagnostic imaging, and cervical, endocervical, or endometrial biopsy. Guidelines cannot cover all clinical situations and clinical judgment is advised, especially in those circumstances which are not covered by the 2019 guidelines.
10. Guidelines are intended for use in the United States. Appropriate management may differ in countries with limited follow-up capabilities, less availability of colposcopy, limited pathology infrastructure, or different views of the trade-offs between cancer risk, cost, and overtesting/overtreatment.
D. METHODS
D.1 Process and Timeline
The ASCCP and National Cancer Institute (NCI) established a Memorandum of Understanding in January 2017 to undertake the work of this guideline update. As with the previous 2001, 2006, and 2012 guidelines,1–3 NCI produced risk data and other scientific support for the consensus guideline process. The ASCCP sponsored the consensus effort to develop and ratify the guidelines. Stakeholder organizations representing best practice in the United States were identified and invited to participate. These included medical professional societies, patient advocacy groups, and federal agencies integral to cervical cancer screening and management of abnormal results (see Table 1). Participation of the stakeholder organizations included identifying organization representatives and, for nongovernment participants, sponsoring their travel to consensus conferences. Representatives from 19 organizations attended the initial meeting in February 2018. At that time, 7 working groups were convened. In previous consensus conferences, working groups considered specific test outcomes (e.g., ASC-US/HPV-positive) and special populations. In contrast, the 7 working groups for the 2019 guidelines were organized with the goal of establishing consensus Clinical Action Thresholds.
TABLE 1.
Participating Organizations
The treatment group evaluated which risk levels of CIN 3+ warrant expedited treatment without confirmatory biopsy, as well as addressing treatment-related issues.
The colposcopy group considered the threshold for colposcopy referral.
The surveillance group created a hierarchy of retesting at shorter intervals than currently recommended for routine screening with either HPV primary testing or cotesting (5 years) and also examined when patients could return to routine screening. Patients undergoing surveillance include those with minimally abnormal screening results not requiring colposcopy (e.g., HPV-positive Negative for Intraepithelial Lesion or Malignancy [NILM]), after colposcopy with low-grade results, or after treatment for high-grade abnormality.
The risk modification group evaluated factors that might change a patient's estimated risk or management, focusing on pregnancy and immunosuppression.
The high value care group performed decision analyses related to proposed management strategies and will continue to assess value as the 2019 guidelines are implemented.
The new technologies group evaluated laboratory terminology and emerging technologies specifically related to management.
The communications group created and reviewed relevant content for public communication to both clinicians and the lay public about the guidelines and the development process.
Working groups were composed of 2 to 8 members, including representatives of participating stakeholder organizations, content experts, and nonclinician representatives of patient advocacy organizations. Working groups met regularly from summer 2018 through fall 2019 to review data and develop guidelines for management. The consensus process was overseen by a 23-person steering committee convened by the ASCCP and was directed by a leadership team consisting of 1 NCI representative (M.S.) and 2 ASCCP representatives (R.G., R.P.). Because the guidelines represent a paradigm shift, the guidelines process included a deliberate and extensive process of stakeholder engagement. These included patient and provider surveys, a consensus meeting to review preliminary guidelines, and a 6-week open public comment period before the final consensus voting meeting in October 2019.23
D.2 Choice of CIN 3+ as Main Clinical End Point for Risk Estimates
For the management guidelines, we chose CIN 3+ as the best surrogate for cancer risk. The definition of CIN 3+ as used in these guidelines includes CIN 3, AIS, and the rare cases of invasive cervical cancer that are found in screening programs. These management guidelines consider CIN 3+ risk at the time point relevant for the clinical action being considered—Clinical Action Thresholds for colposcopy and treatment consider immediate risks of CIN 3+, whereas longer-term surveillance recommendations use 5-year risks.
CIN3+ was chosen as an endpoint instead of cancer because cancer is uncommon in the United States, and risk is profoundly decreased by precursor treatment. Cancers that are found in robust screening programs may represent cancers already prevalent at first screening, rare instances of aggressive or HPV-negative tumors not detectable by screening, or false negative results.24 CIN 3+ was chosen instead of CIN 2+ because it is a more pathologically reproducible diagnosis,25 the HPV type distribution in CIN 3+ lesions more closely approximates that of invasive cervical cancers than the larger range of types found in CIN 2,15–18,26 and CIN 2 has appreciable regression rates in the absence of treatment.27–29 The choice of CIN 3+ does have some limitations, as even among CIN 3/AIS lesions, risks of progression to cancer differ. Glandular lesions including AIS, lesions with HPV 16 and 18 infections, and those occurring in older patients have higher cancer risks than HPV-negative lesions and those occurring in younger patients.30
Different nomenclatures for cervical histopathology are in use in the United States. The LAST Project and the WHO recommend a 2-tiered terminology (histologic LSIL/HSIL) for reporting histopathology of HPV-associated squamous lesions, similar to the Bethesda system used for reporting cervical cytology.31,32 However, the CIN nomenclature is still commonly used, and data used to generate this set of guidelines relied on CIN nomenclature. Although no direct correlation is possible without use of the p16 biomarker, histologic HSIL is similar but not identical to CIN 2/3.33
D.3 Multiple Data Sets Used to Validate Risks
Prior guidelines relied heavily on a large prospective data set including results of cytology, HPV testing, colposcopy, histology, and follow-up outcomes from KPNC, which adopted triennial cotesting as standard practice in 2003. The KPNC data continue to be the largest, most comprehensive data source in the United States for risk estimation of combinations of HPV DNA testing and cytology. For the 2019 guidelines, several additional databases were analyzed to ensure that results are applicable to patients of diverse racial, ethnic, and socioeconomic strata. Risk estimates were compared using screening and follow-up data from clinical trials (BD Onclarity registrational trials),34,35 a state registry (New Mexico HPV Pap Registry36,37), and the Centers for Disease Control and Prevention's (CDC's) National Breast and Cervical Cancer Early Detection Program, a national program that includes many low-income and minority patients.38 The populations vary in rates of abnormal screening results and the prevalence of CIN 3+. Nonetheless, the comparison showed that the risks of CIN 3+ for the specific combination of current results and screening history were similar in that they fell within the same risk bands for management. Cheung et al6 demonstrates the similarity of CIN 3+ risks associated with screening test result combinations among the different populations of screened patients from these data sets. In summary, different populations within the United States have higher or lower rates of CIN 3+ due to factors including access to screening and HPV infection prevalence. Nonetheless, patients with similar test results and screening history combinations have largely similar CIN 3+ risk, regardless of their geographic location, race, ethnicity, or socioeconomic status.
D.4 Estimation of Risks
Details of how risks of CIN 3+ were calculated for the many combinations of test results, including longitudinal series of tests over time, are described in the accompanying Methods article.6 In brief, for each combination of past and current test results, the risk of CIN 3+ was estimated using prevalence-incidence mixture models,39 which consist of joint estimation of prevalent CIN 3+ at the time of the current testing using a logistic regression model, and incident CIN 3+ at subsequent testing using a proportional hazards model. These joint models are designed to handle verification bias and interval censoring. Verification bias in this context means that histopathologic outcomes are only available for patients referred to colposcopy; thus, CIN3+ cases that occur in the setting of false negative screening or abnormal screening tests that were not referred for colposcopy will not be detected. Interval censoring in this context means that the CIN 3+ is diagnosed at colposcopy visits, but the actual time of onset of incident CIN 3+ cannot be determined as it is typically asymptomatic and occurs between testing visits. These flexible models are designed to provide risk estimates without forcing the data into a rigid distribution assumption (e.g., Weibull).
D.5 Assigning Combinations of Test Results to Clinical Actions
For each combination of current test results and screening history (including unknown history), recommended management was determined by first estimating immediate and 5-year risk of CIN 3+. The estimated risk was compared with the proposed Clinical Action Thresholds to determine management recommendation, under the principle of “equal management for equal risk.” For example, HPV-positive ASC-US and LSIL cytology have very similar risks of CIN 3+ and are therefore managed similarly. For some rare combinations of test results, too few patients developed CIN 3+ to estimate risk with statistical certainty. In these situations, a combination of published literature, previous guidelines, and expert consensus opinion were used to develop recommendations.
D.6 Rating the Recommendations
Recommendation strength (A–E) and quality of evidence (I–III) were graded using the system that has been used for previous consensus guidelines (Table 2). Two types of evidence were considered to be strong enough to permit a level A recommendation: (a) systematic literature reviews of trials and observational studies, evaluated by the new technologies group using the QUADAS-2 adapted criteria to inform risk estimates for the guidelines40 and (b) reliable risk estimates from the KPNC prospective longitudinal cohort study. Reliable point estimates are defined as having an 80% certainty of falling within the risk bounds for the recommended management (based on the standard errors of the immediate and 5-year risk estimates) (e.g., colposcopy and surveillance respectively)6 High-quality evidence from systematic reviews and reliable risk estimates from KPNC are considered level 2 evidence. Strong recommendations against a management option (level E) rarely had substantial evidence because the obvious risk of harm precluded a clinical trial (e.g., endometrial biopsy in pregnancy). When neither primary data nor literature provided high-level evidence, previous guidelines or newly developed expert consensus opinions were used (level 3 evidence), usually leading to a C recommendation. Some recommendations are endorsements of guidelines from other organizations, which were not rated. When considering specific guideline recommendations, each group reviewed evidence derived from systematic reviews of published evidence and primary data from the KPNC cohort, assessed the strength and consistency of this evidence, and made recommendations based on quality of data and a balance of benefits and harms.
TABLE 2.
Rating the Recommendations

E. PARADIGM SHIFT: CLINICAL ACTION THRESHOLDS
This section explains the paradigm shift from results-based to risk-based guidelines. We describe the primary Clinical Action Thresholds on which management recommendations are based and the clinical situations in which these Clinical Action Thresholds are applied. For most abnormal screening results and subsequent management visits, the recommendations are based on risks estimated and validated by prospective data from large cohorts. Clinicians can use the 2019 guidelines to manage their patients via the tables in Egemen et al5 or by using an app or website designed to facilitate navigation of the tables available at http://www.asccp.org, including a no cost version. Sections G to K describe recommendations for rare clinical situations where management is based on factors other than risk estimates.
Management recommendations are based on Clinical Action Thresholds and correspond to risk strata (see Figure 1):
FIGUR'E 1.
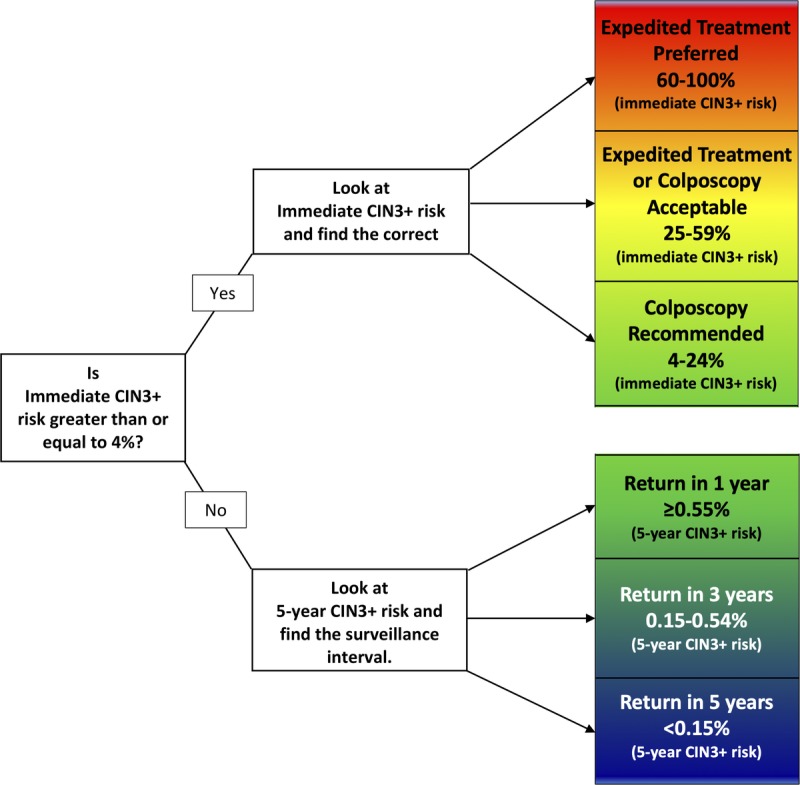
This figure demonstrates how patient risk is evaluated. For a given current results and history combination, the immediate CIN 3+ risk is examined. If this risk is 4% or greater, immediate management via colposcopy or treatment is indicated. If the immediate risk is less than 4%, the 5-year CIN 3+ risk is examined to determine whether patients should return in 1, 3, or 5 years.
The 5-year return Clinical Action Threshold approximates the risk for a patient after a negative screening test using HPV testing or cotesting in the general population, for whom retesting in 5 years is recommended by national screening guidelines.13,14 Patients with risks at or below this threshold are recommended to receive routine screening at 5-year intervals with HPV-based testing (Section E.1).
The 3-year return Clinical Action Threshold approximates the risk for a patient after a negative cervical cytology screen in the general population, for whom retesting in 3 years is recommended by national screening guidelines.13,14 Patients with risks at or below this threshold but above the 5-year threshold are recommended to receive HPV-based testing in 3 years (Section E.1).
One-year return is recommended for patients with risks above the 3-year threshold but below the Clinical Action Threshold for colposcopy (Section E.1).
The colposcopy Clinical Action Threshold approximates the risk for a patient after an HPV-positive ASC-US or LSIL screening result in the general population, for whom colposcopy is recommended in the 2012 guidelines.3 Patients with risks at or above this threshold but below the expedited treatment threshold are recommended to receive colposcopy (Section E.2).
The expedited treatment or colposcopy acceptable Clinical Action Threshold approximates the risk for a patient after an HPV-positive atypical squamous cells cannot exclude HSIL (ASC-H) cytology screening result in the general population. Patients with risks at or above this threshold but below the expedited treatment preferred threshold are recommended to receive counseling from their providers to choose between evaluation with colposcopy and biopsy or expedited treatment (Section E.3). Expedited treatment is defined as treatment without confirmatory colposcopic biopsy.
The expedited treatment preferred Clinical Action Threshold approximates the risk for a patient after an HPV 16–positive HSIL cytology screening result in the general population. It is preferred that patients with risks at or above this threshold receive expedited treatment unless they are pregnant, younger than 25 years, or have concerns about the potential effects of treatment on future pregnancy outcomes that outweigh concerns about cancer (Section E.3).
Of note, patients with histologic HSIL (CIN 2) who have chosen observation are recommended to receive colposcopy and HPV-based testing at 6-month intervals (Section I.3).
E.1 Clinical Action Thresholds Leading to Recommendation of Surveillance
Introduction
Surveillance is defined as follow-up testing at a shorter interval than that currently recommended for routine screening with either HPV primary testing or cotesting (5 years). Surveillance is recommended for patients whose risk of CIN 3+ based on current test results and screening history is higher than the risk for the general screening population, but lower than the risk at which colposcopy is recommended. Unlike colposcopy and treatment, which are performed as soon as possible after a qualifying abnormal result, surveillance entails retesting at intervals of 1 to less than 5 years. Therefore, we used the 5-year risk of CIN 3+ as the estimated risk level when assigning surveillance Clinical Action Thresholds. Surveillance intervals are defined in Figure 1 and explained in detail hereinafter. Surveillance thresholds are based on the principle of equal management for equal risks and were designed to support current screening and surveillance recommendations, which are generally accepted as a reasonable balance of benefits and harms.3 In the 2012 guidelines, intervals of 1 and 3 years were used for surveillance, with return to routine HPV-based screening at 5 years.3 Because clinicians and patients are familiar with these intervals, and review of evidence did not reveal a compelling reason to change these intervals, these intervals are retained. Note that for observation in very high-risk patients (e.g., untreated CIN2, AIS treated with conization) colposcopy and repeat testing at 6-month intervals is recommended.
Guideline: When patients have an estimated 5-year CIN 3+ risk of less than 0.15% based on past history and current test results, return to routine screening at 5-year intervals using HPV-based testing is recommended (AII).
Rationale: Using the principle of equal management for equal risk, this Clinical Action Threshold corresponds to the 5-year CIN 3+ risk after negative HPV-based screening (HPV testing or cotesting) in the general population (see Table 1A in Egemen et al5) for whom national guidelines recommend a 5-year return.13,14 Estimated 5-year CIN 3+ risks in the KPNC database after a negative HPV test and cotest are 0.14% (95% CI = 0.13%–0.15%) and 0.12% (95% CI = 0.12%–0.13%), respectively. Note that cytology alone is never recommended at 5-year intervals.
Guideline: When patients have an estimated 5-year CIN 3+ risk of 0.15% or greater but less than 0.55% based on history and current test results, repeat testing in 3 years with HPV-based testing is recommended (AII).
Rationale: Using the principle of equal management for equal risk, the 3-year return Clinical Action Threshold corresponds to the 5-year CIN 3+ risk after negative cervical cytology in the general population, for whom national guidelines recommend a 3-year return.13,14 Estimated 5-year CIN 3+ risks after a negative cytology result without HPV testing ranged from 0.33% in the KPNC population to 0.52% in the New Mexico HPV Pap Registry, to an estimated 0.45% in the screened population of the CDC's National Breast and Cervical Cancer Early Detection Program. Thus, 0.55% was considered an appropriate value for the Clinical Action Threshold. Three-year surveillance is recommended for patients whose risk falls between the 3- and 5-year follow-up thresholds. Consistent with the 2012 guidelines, patients with a low-grade cotest result (e.g., HPV-positive ASC-US or LSIL) followed by a colposcopy with results of less than CIN 2, followed in turn by a negative follow-up HPV test or cotest reach the 3-year return threshold (see Figure 2). Also consistent with previous guidelines, patients with an HPV-negative ASC-US screening result in the setting of an unknown history can return at 3 years (estimated 5 year CIN 3+ risk 0.40%).5
FIGURE 2.
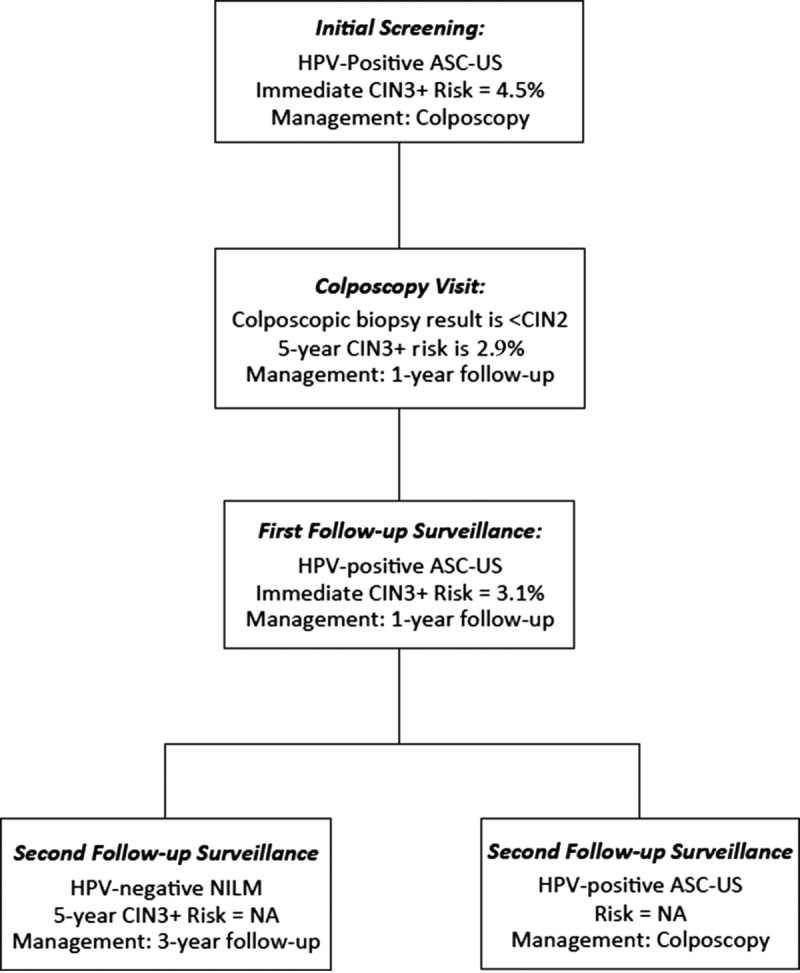
This figure demonstrates how a patient with a common low-grade screening abnormality (HPV-positive ASC-US) would be managed based on risk estimates. The initial screening result would lead to colposcopy (immediate risk 4.2%). Colposcopy of less than CIN 2 has a 5-year risk of 3.2% (1-year return). At the 1-year return visit, a second HPV-positive ASC-US result has an immediate risk of 3.1% (1-year return). If the patient has a repeat abnormal screen at the next follow-up, colposcopy is recommended. If the HPV-based test is negative, return in 3 years is recommended. NA, not applicable because stable risk estimates are not available.
Guideline: When patients have an estimated risk of CIN 3+ based on history and current results that is below the threshold for immediate colposcopy (4.0% immediate risk) and above the 3-year follow-up threshold (≥0.55% at 5 years), repeat testing in 1 year with HPV-based testing is recommended (AII).
Rationale: One-year surveillance implies close follow-up for those whose risks fall between the Clinical Action Thresholds for colposcopy and 3-year follow-up. Consistent with the 2012 consensus recommendations,3 follow-up at 1 year is recommended after screening tests showing minimal abnormalities: HPV-positive/NILM or HPV-negative/LSIL with unknown previous screening history (immediate risks 2.1% and 1.1% respectively5); 1-year surveillance is also recommended after colposcopy with biopsies of histologic LSIL (CIN 1) or less preceded by a low-grade cotest result (defined as HPV-positive LSIL, HPV-positive ASC-US, or repeated HPV-positive NILM). New data for these guidelines find that the risk of CIN 3+ is substantially reduced after a documented negative HPV primary screening test or cotest or normal colposcopic examination with biopsy confirmation of less than CIN 2.5 Based on lower CIN 3+ risks, 1-year surveillance, not colposcopy, is recommended for most patients with new HPV-positive ASC-US or LSIL results after a documented negative HPV test or cotest within an appropriate screening interval (approximately 5 years) or colposcopic examination less than CIN 2 within the past year (see Figure 2). Of note, a previous negative cytology result alone does not reduce subsequent risk like a negative HPV-based screen; therefore, cytology alone is not used to modify subsequent management recommendations.
E.2 Clinical Action Threshold Leading to Recommendation of Colposcopy
Guideline: When patients have an estimated immediate risk of diagnosis of CIN 3+ of 4.0% or greater based on history and current results, referral to colposcopy is recommended (AII).
Rationale: The following principles were used to develop the Clinical Action Threshold for referral to colposcopy: (a) colposcopy visits recommended by the threshold should yield information useful for clinical decision-making. Thus, the threshold was based on the risk of diagnosing CIN 3+ upon immediate referral to colposcopy. (b) In the absence of a compelling rationale, the colposcopy threshold should be similar to 2012 referral recommendations that are generally accepted as an appropriate balance of benefits and harms.
The 2001 consensus guidelines1 were the first to standardize the colposcopy referral threshold, referring patients with LSIL and HPV-positive ASC-US to colposcopy. This recommendation has been carried forward through revisions in 2006 and 2012.2,3 The workgroup reviewed frequently cited studies and noted that immediate risk (CIN 3+ found among patients referred directly to colposcopy) ranged from 3% to 7%.41–44 Current KPNC data were reviewed,5 and it was noted that immediate CIN 3+ risk clustered in 3 groups: (a) high-grade test results (defined as cytology ASC-H, atypical glandular cell [AGC], HSIL, or higher) having high (>25%) risk; (b) low-grade results (HPV-positive ASC-US or HPV-positive LSIL cytology with unknown previous screening history and HPV-positive NILM cytology occurring at 2 consecutive annual visits) having just over 4.0% risk; and (c) result combinations for which colposcopy has historically not been performed having risks below 4% (HPV-positive NILM cytology, HPV-negative LSIL cytology, and HPV-negative ASC-US cytology with unknown previous screening histories). The Clinical Action Threshold of a 4% immediate CIN 3+ risk was considered a reasonable balance of benefits and harms as, in a population with unknown screening history, it led to referral of HPV-positive patients with ASC-US or LSIL cytology, but not the large group of patients with HPV-positive NILM cytology.
To validate the 4.0% Clinical Action Threshold for colposcopy, the KPNC CIN 3+ prevalent risk estimates were compared with those from other study populations with more diversity in sociodemographic characteristics including the New Mexico HPV Pap Registry,45 CDC's National Breast and Cervical Cancer Early Detection Program, and the BD Onclarity registrational trials. The 4% threshold functioned similarly.3,6
The 4.0% immediate risk Clinical Action Threshold has important implications for patients with at least 1 previous negative HPV-based test because surveillance is recommended rather than immediate colposcopy for low-grade abnormalities (HPV-positive ASC-US or LSIL) in patients whose preceding screening result was a negative HPV test or cotest within a routine screening interval (approximately 5 years).5 This additional information reduces the immediate CIN 3+ risk to approximately 2%, leading to a recommendation of 1-year surveillance instead of immediate colposcopy. Adoption of the 4.0% Clinical Action Threshold reduces the number of patients referred for colposcopy over 2 rounds of screening from an estimated 9.8%, using the 2012 ASCCP recommendations, to 8.3% using the 2019 recommendations. Exceptions to the 4.0% threshold, encompassing results with cancer risk disproportionately higher than CIN 3+ risk, are discussed in Section H.2.
E.3 Clinical Action Thresholds Leading to Recommendations of Treatment
The primary goal of treatment is cancer prevention through destruction or excision of precancerous lesions (CIN 3, AIS) to prevent the development of invasive cancer. In the only known observational study of untreated CIN 3, the long-term risk of developing invasive cancer was as high as 30% for 30 years46; progression rates could not be estimated at KPNC because of high rates of timely treatment. Because treatment is generally recommended as soon as possible after the identification of a precancerous lesion, the immediate CIN 3+ risk was used when evaluating potential thresholds. Historically, the treatment threshold has been histologic CIN 2. The LAST guidelines reports both p16-positive CIN 2 and CIN 3 as histologic HSIL. Consistent with previous guidelines, the threshold for treatment remains histologic HSIL/AIS (by LAST terminology) or CIN 2+ (by 3-tiered terminology) except in special circumstances (Sections I.3, K.1, and K.2). When considering expedited treatment versus colposcopy with biopsy, clinicians should have a thorough discussion with patients regarding the risks and benefits. Treatment without preceding histologic confirmation can be conducted in one visit among those at high immediate risk of CIN 3+. Reasons for choosing expedited treatment vary and may include personal preference, limited healthcare access, financial concerns, and cancer-related anxiety. The age cutoff of 25 years or older for recommending expedited treatment was chosen as an appropriate balance of benefits and harms due to very low cancer rates and high rates of regression of precancers among women in this age group.27,47
Guideline: For nonpregnant patients 25 years or older with an estimated immediate risk of CIN 3+ of 60% or greater based on history and current results, treatment using an excisional procedure without previous biopsy confirmation is preferred but colposcopy with biopsy is acceptable (BII).
Rationale: In the 2012 guidelines, expedited treatment (i.e., without biopsy confirmation) was an acceptable management option for HSIL cytology.3 Patients with HSIL cytology undergoing expedited treatment are diagnosed with CIN 3+ in 49% to 75% of cases.48–52 The KPNC data show similar risks: HPV-positive HSIL cytology has immediate risks of CIN 3+ and CIN 2+ of 49% and 77%, respectively.5 Two clinical situations currently exceed the 60% threshold where expedited treatment is preferred. HSIL cytology that is HPV 16–positive has an immediate CIN 3+ of 60%, CIN 2+ risks of 77%, and immediate cancer risks of 8.1%.53 In the CDC's National Breast and Cervical Cancer Early Detection Program, women with HPV-positive HSIL cytology (regardless of genotype) who were underscreened (generally defined as no screening in >5 years) had an immediate CIN 3+ risk of 64% and CIN 2+ risks of 82% (cancer risk not available). Based on the KPNC data, for clinical situations that exceed the 60% threshold, 1.7 patients will receive diagnostic excisional procedures for every CIN 3+ treated, a low rate of overtreatment.
Guideline: For nonpregnant patients 25 years or older with an estimated immediate risk of CIN 3+ 25% or greater and less than 60% based on history and current results, treatment using an excisional procedure without previous biopsy confirmation or histologic evaluation with colposcopy and biopsy are both acceptable (AII).
Rationale: The 2012 guidelines allow treatment without biopsy-proven histologic confirmation include patients who have HSIL cytology independent of HPV status. In the KPNC data set, the 25% to 59% risks strata includes patients with the following results and immediate CIN 2+/CIN 3+ risks, respectively: (a) HPV-negative HSIL cytology: 47%/25%; (b) HPV-positive ASC-H cytology: 50%/26%; (c) HPV-positive AGC (all categories): 40%/26%; and (d) HPV-positive HSIL cytology: 77%/49%. Using this threshold, 2.8 patients will undergo excisional procedures for every CIN 3+ treated.
E.4 Clinical Situations Leading to Management Recommendations
Patients with abnormal cervical cancer screening results enter management via 5 common clinical situations: (a) initial management of an abnormal screening test result (see Tables 1A, B; Egemen et al5); (b) return visit for surveillance of a previous abnormal result that did not lead to colposcopy referral (e.g., HPV-negative ASC-US), with consideration of whether to continue surveillance or refer to colposcopy (see Tables 2A–C; Egemen et al5); (c) evaluation of the colposcopic biopsy results with consideration of whether to treat or begin postcolposcopy surveillance (see Table 3; Egemen et al5); (d) managing test results at the return visit for surveillance after a colposcopic biopsy showing less than CIN 2 (Tables 4a, b; Egemen et al5); and (e) follow-up after treatment of CIN2 or CIN3 (see Tables 5a, 5b; Egemen et al5).
Recommendations are based on risks of immediate and future CIN 3+ diagnoses in light of current and past results. Regardless of the pathway by which patients enter management, equivalent risks are managed similarly. For each of the 5 clinical situations, risk tables and recommendations based on the Clinical Action Thresholds are detailed in the accompanying article by Egemen et al.5 The reader is directed to the definitive updated source of risk tables, which are freely available online (https://CervixCa.nlm.nih.gov/RiskTables). A small percentage of patients will present with a combination of results and personal characteristics requiring consideration outside of the available risk data. Management of these special situations is described in Sections G to K.
F. UPDATES RELATED TO PATHOLOGY REPORTING AND LABORATORY TESTS
Although most of the 2019 guidelines describe clinical management of patients by providers, the consensus process also addressed laboratory considerations that directly relate to results reporting and use of ancillary tests.
F.1 Statement on the Use of a 2-Tier Terminology (Histologic LSIL/HSIL) for Reporting Histopathology of Squamous Lesions of the Lower Anogenital Tract
Guideline: It is important to use p16 immunohistochemical staining according to the guidance provided by the CAP-ASCCP LAST Project.31 p16 immunohistochemistry should be used for specific indications as recommended by the LAST guidelines when interpreting the hematoxylin and eosin (H&E) slide. A positive p16 immunostain supports the diagnosis of histologic HSIL if the morphological assessment of H&E slides is consistent with CIN 2 or CIN 3. There is a risk of overcalling cervical histology results when p16 is used incorrectly. Most importantly, a morphologic CIN 1 on H&E should not be upgraded to histologic HSIL (CIN 2) even if p16 positive.
For epidemiologic and clinical management purposes, it is strongly recommended to qualify a histologic HSIL result by CIN 2 or CIN 3, according to the options given by the LAST guidelines (example histologic HSIL [CIN 2]).
Rationale: This CIN qualification can have clinical importance (e.g., to identify cases of CIN 2 in patients for whom conservative management is an acceptable option). It is also important for postvaccine surveillance studies and quality control assessments of cervical precancer that have historically relied on CIN 2 and CIN 3 end points. Furthermore, it is important for future research efforts to distinguish diagnoses of histologic HSIL (CIN 2) from HSIL (CIN 3) so that diagnostic categories are compatible with the histologic end points used for current guidelines.
In 2012, consensus recommendations were published on the use of a 2-tiered terminology for reporting histopathology of squamous lesions of the anogenital tract by the College of American Pathologists and the ASCCP.31 The central components of the LAST guidelines include a 2-tiered nomenclature that distinguishes histologic LSIL and histologic HSIL and recommendations for the use of adjunctive p16 immunohistochemistry to assist interpretation of anogenital histology. p16 is a tissue marker of HPV oncogene overexpression and transformation and can support histologic assessment.
Current guidelines are based on CIN 3 end points, the most reliable correlate of a cervical precancer. Currently, there are insufficient data to evaluate risk estimates with histologic HSIL end points. Recent studies have shown that distinguishing CIN 2 and CIN 3 within the LAST histologic HSIL group is biologically and clinically meaningful.33 Although some studies have shown that p16 immunohistochemistry improves interpretation of cervical biopsies, others have raised concerns about overuse and overdiagnosis.54–59
F.2 Updated Management of Primary HPV Screening (Replaces Interim Guidance)
Guideline: When primary HPV screening is used, performance of an additional reflex triage test (e.g., reflex cytology) for all positive HPV tests regardless of genotype is preferred (this includes tests positive for genotypes HPV 16/18) (CIII). However, if primary HPV screening test genotyping results are HPV 16 or HPV 18 positive and reflex triage testing from the same laboratory specimen is not feasible, referral for colposcopy before obtaining additional testing is acceptable (CIII). If genotyping for HPV 16 or HPV 18 is positive, and triage testing is not performed before the colposcopy, collection of an additional triage test (e.g., cytology) at the colposcopy visit is recommended (CIII).
Rationale: The US FDA approved the cobas HPV test (Roche, Indianapolis, IN), in March 2014, and the Onclarity HPV Test (Becton Dickinson, Franklin Lakes, NJ), in April 2018, for primary HPV testing for screening for patients 25 years or older.60 Both these tests offer and are approved for partial HPV genotyping. Use of primary HPV screening will likely increase in the future, as it is more effective than screening with cytology alone and performs similarly to and with lower costs than screening with cotesting.4,42 Because HPV–16 positive and HPV 18–positive test results have the highest risk of CIN 3 and occult cancers, additional diagnostic procedures are recommended for all positive test results (e.g., colposcopy with biopsy for NILM and low-grade cytology and expedited treatment for HSIL cytology that is positive for HPV type 16). This guideline replaces interim guidance (2015) for the management of a positive result for HPV primary screening, which recommended direct referral to colposcopy for HPV test results positive for HPV 16 and/or HPV 18, and performance of cytology for positive results due to other (non-16/18) high-risk HPV types.4 The immediate risk of CIN3+ in patients with HPV 16–positive and HSIL cytology exceeds the treatment threshold of 60%; therefore, these patients should be given the option for expedited treatment without preceding confirmatory biopsy (see Section E.3). Expedited treatment is only possible if cytology is performed. Therefore, reflex cytology is recommended for all HPV-positive primary screening results, regardless of HPV genotype. If reflex testing from the same laboratory specimen as the HPV test is not feasible, patients should proceed directly to colposcopy.4 In this situation, collection of an additional triage test (e.g., cytology) is recommended at the time of colposcopy to provide further information for risk-based management (e.g., if HPV 16–positive HSIL cytology is identified, treatment may be considered even if CIN 2+ is not identified on biopsy). Combining a test with high specificity (e.g., cytology when it is interpreted as HSIL) with a test with high sensitivity (i.e., HPV test) allows more precise, risk-based management of these patients.
F.3 Statement on HPV Tests Used in Management
Guideline: HPV assays should be used for management according to their regulatory approval for screening, unless there are sufficient data to support use of the assay differently (AI).
Rationale: Several HPV assays have been approved in the United States for clinical use in screening and triage.61 None of these assays have specific indications for management, but they are widely used for postcolposcopy and posttreatment surveillance. For these indications, HPV assays approved for screening should be used according to their regulatory approval. For example, when an HPV test has been approved for cotesting, it should be used in management in the context of cotesting, unless there are sufficient, exceptionally rigorous data to support use of the assay differently (e.g., as outlined in Clarke et al.40). Approved assays include target- and signal-amplification assays of HPV DNA, as well as HPV mRNA. Most FDA-approved HPV DNA assays have similar performance characteristics.62 Most assays are approved for adjunct testing with cytology (also referred to as cotesting), whereas a subset of HPV DNA assays have also been approved for primary HPV testing alone, without concomitant cytology.
G. RARE CYTOLOGY RESULTS
G.1 Evaluation of Cytology Interpreted as AGC or AIS
Guideline: For nonpregnant patients of all ages with all subcategories of AGC and AIS, except when atypical endometrial cells are specified, colposcopy is recommended regardless of HPV test result; endocervical sampling is recommended at initial colposcopy except in pregnancy (for management in pregnancy, see Section K.2) (AII). Accordingly, triage by reflex HPV testing is not recommended, and triage by repeat cytology is unacceptable (DII). Endometrial sampling is recommended in conjunction with colposcopy and endocervical sampling in nonpregnant patients 35 years or older with all categories of AGC and AIS (AII). Endometrial sampling is also recommended for nonpregnant patients younger than 35 years at increased risk of endometrial neoplasia based on clinical indications (e.g., abnormal uterine bleeding, conditions suggesting chronic anovulation, or obesity) (AII). For patients with atypical endometrial cells specified, initial evaluation limited to endometrial and endocervical sampling is preferred, with colposcopy acceptable at the time of initial evaluation. If colposcopy was deferred and no endometrial pathology is identified, additional evaluation with colposcopy is then recommended (see Figure 3).
FIGURE 3.
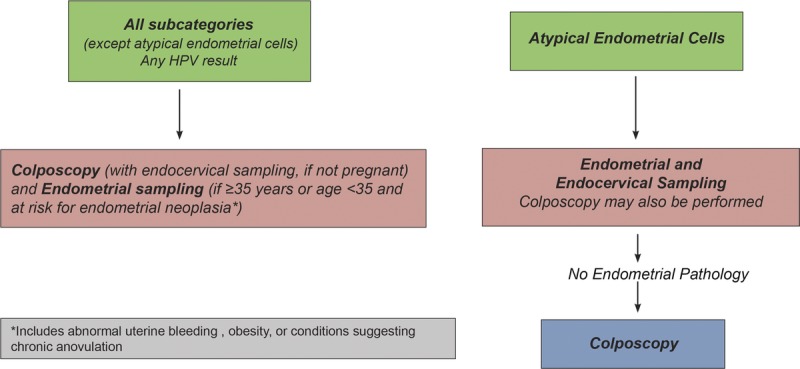
This figure describes the initial workup of AGC found on cervical cytology.
Subsequent Management
Guideline: For patients with cytology showing AGC not otherwise specified or atypical endocervical cells not otherwise specified in whom histologic HSIL (CIN 2+) or AIS/cancer is not identified, cotesting at 1 and 2 years is recommended. If both cotests are negative, repeat cotesting at 3 years is recommended. If any test is abnormal, then colposcopy is recommended (BII). If CIN 2 or CIN 3 but no glandular lesion is identified histologically for patients with cytology atypical glandular, endocervical, or endometrial cells not otherwise specified, management should be according to the 2019 guidelines for the lesion diagnosed (Section I) (CII). For patients with atypical glandular or endocervical cells “favor neoplasia” or endocervical AIS cytology, if invasive disease is not identified during initial colposcopic workup, a diagnostic excisional procedure is recommended. The diagnostic excisional procedure used in this setting should provide an intact specimen with interpretable margins (BII). Endocervical sampling above the excisional bed is preferred (BII) (see Figure 4).
FIGURE 4.
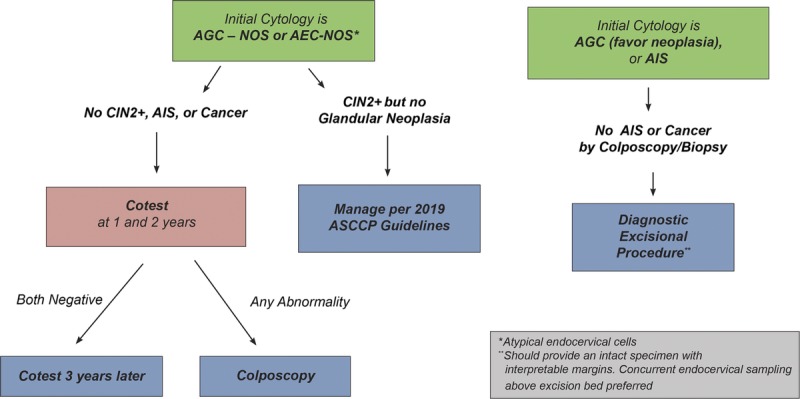
This figure describes follow-up management that should occur after the diagnostic examinations described in Figure 3.
Rationale: Atypical glandular cells on cytology is a poorly reproducible diagnostic category.63 Positive HPV test results, especially when positive for HPV type 18, can be indicative of higher risk of CIN 2+ lesions. However, colposcopy is recommended for all patients regardless of HPV result. Literature is limited, and comparisons between studies are difficult because of inconsistent use of the Bethesda system for classification of AGC.64 Atypical glandular cells can be associated with polyps and metaplasia as well as adenocarcinomas of the cervix; cancers of the endometrium, fallopian tube, ovary, and other sites are also found, especially in older women who test HPV negative.65,66 Using the Bethesda terminology, AGC, favor neoplasia, or adenocarcinoma cytology is frequently indicative of invasive or preinvasive disease.64 For this reason, diagnostic excisional procedures are recommended even when histologic HSIL or AIS has not been identified. Cytologic AGC results are associated with a histologic diagnosis of AIS in 3% to 4%, CIN 2+ in 9%, and invasive cancer in 2% to 3%.67–69 In the KPNC data, HPV-positive AGC (all categories) had an immediate CIN 3+ risk of 26% and HPV-negative AGC had an immediate CIN 3+ risk of 1.1%. Consistent with other literature, cotest results of HPV-positive AGC favor neoplasia or adenocarcinoma had an immediate CIN 3+ risk of 55%, whereas other HPV-positive AGC categories had immediate CIN 3+ risks of approximately 20%. Although endometrial cancer is rare in premenopausal patients without risk factors, the prevalence of premenopausal endometrial cancer is increasing, underscoring the importance of endometrial sampling when indicated.70,71
G.2 Unsatisfactory Cytology
Guideline: For patients with an unsatisfactory cytology result and no, unknown, or a negative HPV test result, repeat age-based screening (cytology, cotest, or primary HPV test) in 2 to 4 months is recommended (BIII). Triage using HPV testing is not recommended (DIII). Before repeat cytology, treatment to resolve atrophy or obscuring inflammation when a specific infection is present is acceptable (CIII). For patients 25 years and older who are cotested and have unsatisfactory cytology and a positive HPV test without genotyping, repeat cytology in 2 to 4 months or colposcopy is acceptable (BII). If a positive HPV test with partial genotyping is positive for HPV 16 or HPV 18, direct referral for colposcopy is recommended (BII) (see Figure 5).
FIGURE 5.
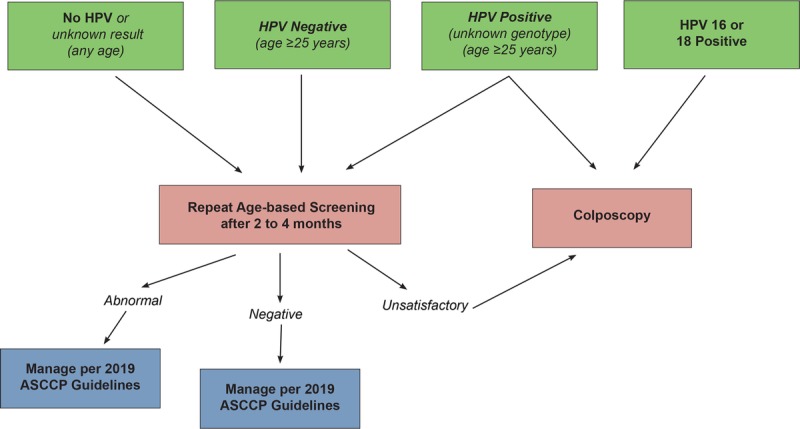
This figure describes the steps involved in clinical management of unsatisfactory cytology. Note that “unknown genotype” refers to both HPV testing without genotyping, and HPV testing where genotyping is negative for HPV 16 and 18 but positive for other high-risk HPV types.
Rationale: Literature was reviewed from 2012 to 2019, and no evidence was found to change recommendations.72–82 When cotesting is performed, a negative HPV test in the setting of an unsatisfactory cytology may reflect an inadequate sample. Although a negative HPV test (performed from the same vial as the cytology) may be adequate for testing even when the cytology cellularity is inadequate for diagnosis, interpreting the HPV result in the setting of insufficient cellularity has not been validated, which is of concern given that repeat testing is not recommended for up to 5 years after a negative HPV screen. Negative results on HPV tests that are not FDA approved for primary cervical cancer screening should not be considered valid in the absence of adequate cytology (Section F.3). In summary, a negative HPV result from a cotest with inadequate cellularity on cytology should not be interpreted as negative primary HPV test and should be repeated.
G.3 Absent Transformation Zone on Screening Cytology
Guideline: For patients aged 21 to 29 years with negative screening cytology and absent endocervical cells/transformation zone component (i.e., endocervical cells or squamous metaplastic cells), routine screening is recommended (BIII). When cervical cytology alone is performed for screening, HPV testing as a triage test after negative cytology and absent endocervical cells/transformation zone component in this age group is unacceptable (DIII). For patients 30 years or older with NILM cytology and absent endocervical cells/transformation zone component and no or unknown HPV test result, HPV testing is preferred (BIII). Repeat cytology in 3 years is acceptable if HPV testing is not performed (BIII). If HPV testing is performed, manage using Clinical Action Thresholds according to 2019 consensus guidelines (see Figure 6).
FIGURE 6.
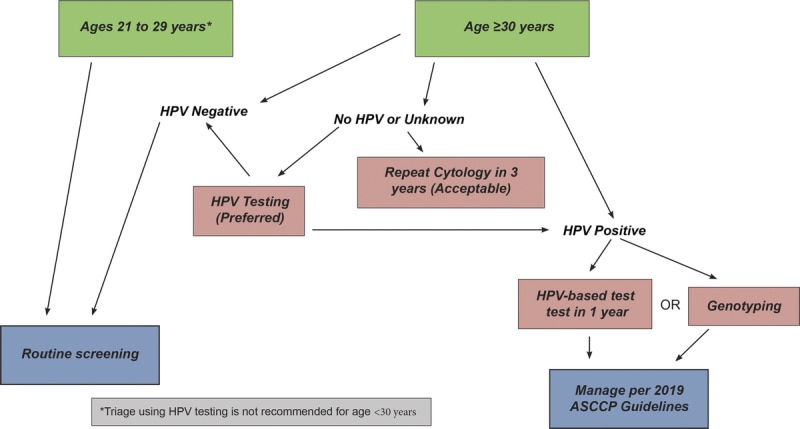
This figure describes the steps involved in clinical management of cytology that is negative for intraepithelial lesion or malignancy, but with absent transformation zone or endocervical cells.
Rationale: Literature reviewed for the 2012 guidelines indicated a lower risk of CIN 3+ for patients with absent transformation zone/endocervical cells than those with cells present, leading to a recommendation to manage these results similarly.3 The HPV testing is preferred in women 30 years or older to facilitate subsequent risk-based management. A review of the literature from 2012 to 2019 on whether the absence of a transformation zone component (TZ/EC, i.e., endocervical cells or squamous metaplastic cells) on NILM cytology slides affected patients' subsequent risks of histologic HSIL (CIN 2, CIN 3) diagnoses showed no evidence to change the 2012 recommendations.83,84
G.4 Benign Endometrial Cells in Premenopausal Patients or Benign Glandular Cells in Posthysterectomy Patients
Guideline: For asymptomatic premenopausal patients with benign endometrial cells, endometrial stromal cells, or histiocytes, no further evaluation is recommended (BII). For postmenopausal patients with benign endometrial cells, endometrial assessment is recommended (BII). For posthysterectomy patients with a cytology report of benign glandular cells, no further evaluation is recommended (BII).
Rationale: In the Bethesda system for reporting cervical cytology, cytologically benign-appearing endometrial cells are reported in women 45 years or older under the “other” general category, and follow-up left to the clinical provider. Benign glandular cells in women after hysterectomy are reported in the negative (NILM) Bethesda category. Literature review for the 2012 guidelines indicated increased risk of endometrial pathology in postmenopausal patients with endometrial cells on cytology but did not indicate increased endometrial cancer risk for premenopausal patients with benign endometrial cells in the absence abnormal uterine bleeding.3 The literature review was updated using a PubMed search for recent publications since 2012 that address benign-appearing endometrial cells in postmenopausal and glandular cells in posthysterectomy individuals. References were reviewed and no evidence was found to change the 2012 recommendations.85–93
H. COLPOSCOPY PRACTICE STANDARDS AND EXCEPTIONS TO COLPOSCOPY CLINICAL ACTION THRESHOLD
H.1 ASCCP Colposcopy Standards
The ASCCP Risk-Based Management Consensus Guidelines reaffirm that colposcopy should be practiced according to the ASCCP Colposcopy Standards.10,94 For those at lowest risk, defined as less than HSIL cytology, no evidence of HPV 16/18 infection, and a completely normal colposcopic impression (i.e., no acetowhitening, metaplasia, or other visible abnormality, and a fully visualized squamocolumnar junction), untargeted (random), biopsies are not recommended and patients with a completely normal colposcopic impression can be observed without biopsy. For those not meeting the lowest risk criteria, multiple targeted biopsies, at least 2 and up to 4, are recommended targeting all acetowhite areas to improve detection of prevalent precancers. The ASCCP Colposcopy Standards emphasize the need for biopsies even when the colposcopic impression is normal but any degree of acetowhitening, metaplasia, or other abnormality is present to ensure that CIN 2+ is not missed.94 As more patients are allowed to defer colposcopy under the ASCCP Risk-Based Management Consensus guidelines, obtaining adequate biopsies to effectively rule out CIN 2+ at each colposcopy examination is paramount.
Note that the KPNC colposcopy protocols precede the Colposcopy Standards and are based on 4-quadrant biopsies and an ECC that were widely conducted in KPNC. The recommendations against untargeted biopsies are based on the risk of occult CIN 2+ of 1% to 7% and CIN 3+ of less than 1% among patients with less than HSIL cytology, HPV 16/18 negative, and normal colposcopic impression. This indicates that management recommendations using the ASCCP Colposcopy Standards would be equivalent to those using KPNC protocols in nearly all cases. The most recent recommendations pertaining to the use of ECC are from the 2012 guidelines, restated here for clarity: ECC is preferred for non-pregnant patients when colposcopy is inadequate, in those not at lowest risk in whom no lesion is identified, and is acceptable when a lesion is seen.
H.2 Exceptions to Colposcopy Threshold
Guideline: For patients with ASC-H cytology, colposcopy is recommended regardless of HPV result (AII).
Rationale: In the KPNC data, HPV-negative ASC-H and HPV-positive ASC-H had very different CIN 3+ rates, but similar cancer rates. The HPV–positive ASC-H had an immediate CIN 3+ risk of 26% and a cancer risk of 0.92%, whereas HPV-negative ASC-H had an immediate CIN 3+ risk of 3.4%, but an immediate cancer risk of 0.69%. Because the immediate cancer risk for ASC-H is disproportionately high compared with the CIN 3+ risk, the working group carried forward the 2012 recommendations and recommended colposcopy for all patients with ASC-H, regardless of HPV test results.3
Guideline: For patients with HPV 18–positive NILM, colposcopy is recommended (AII). (Note colposcopy is also recommended for HPV 16–positive NILM, repeated here for clarity.)
Rationale: HPV 18–positive NILM had a 3.0% prevalent CIN 3+ risk, less than the Clinical Action Threshold for colposcopy. However, HPV 18–positive NILM had a disproportionately high cancer risk compared with other results: 0.2% immediately and 0.56% at 5 years. This suggests that HPV 18-related CIN 3 or AIS may be difficult to diagnose and/or more apt to rapidly progress from precancer to cancer. The elevated cancer prevalence with HPV 18 positivity has been previously noted,95 and HPV 18 is one of the most common HPV types found in invasive cervical cancers.96 Given the elevated cancer risk, referral to colposcopy is recommended.
Guideline: Colposcopy should be performed after 2 consecutive unsatisfactory screening tests (CIII).
Rationale: No new evidence was found, so the 2012 guideline was carried forward.3
I. MANAGING HISTOLOGY RESULTS
Treatment Considerations for Patients 25 Years or Older
Individuals who exceed treatment thresholds may undergo expedited treatment, defined as excisional treatment without preceding histologic confirmation. However, most patients will require both screening test and colposcopic biopsy results to determine the next step in management. The following section outlines guiding principles to consider when managing these results. Treatment guidelines are dichotomized by younger than 25 years or 25 years or older because of high spontaneous regression rates of HPV infection and CIN 2 and low incidence of cancer in those younger than 25 years. Individuals younger than 25 years are discussed under Special Populations (Section K). The term “young women” is no longer used. The consensus guidelines recognize that patients of various ages are concerned with the potential impact of treatment on future pregnancy outcomes. Shared decision-making is especially critical when individuals consider treatment of histologic HSIL (CIN 2) and abnormalities with a relatively low likelihood of underlying CIN 3+, such as histologic LSIL (CIN 1) preceded by HSIL or ASC-H cytology, or persistent histologic LSIL (CIN 1).
I.1 Management of Histologic HSIL, not Further Specified or Qualified
Histologic reporting of cervical biopsies has moved to the LAST/WHO criteria, but its uptake by pathologists has not been universal. The consensus recommendation of the LAST guidelines (Section F.1) is to qualify histologic HSIL using the CIN nomenclature (CIN 2 or CIN 3). Because of measurable regression rates for CIN 2,26 the present guidelines subdivide treatment options based on the CIN qualifiers of CIN 2 and CIN 3. However, pathology reports incorporating the LAST criteria may not specify a CIN diagnosis.
Guideline: Treatment is preferred if histologic HSIL cannot be specified (e.g., reported as histologic HSIL or histologic HSIL [CIN 2,3]) (CIII) (see Figure 7).
FIGURE 7.
This figure describes the steps involved in clinical management of histologic HSIL.
Rationale: CIN 3 is considered a direct cervical cancer precursor. If CIN 3 cannot be excluded, managing the patient as if CIN 3 is present is preferred. This conservative approach was considered safest for patients. Alternatively, the clinician could call the pathologist to further qualify the CIN equivalent and issue an additional report, then manage using the revised diagnosis.
I.2 Management of Histologic HSIL (CIN 2 or CIN 3)
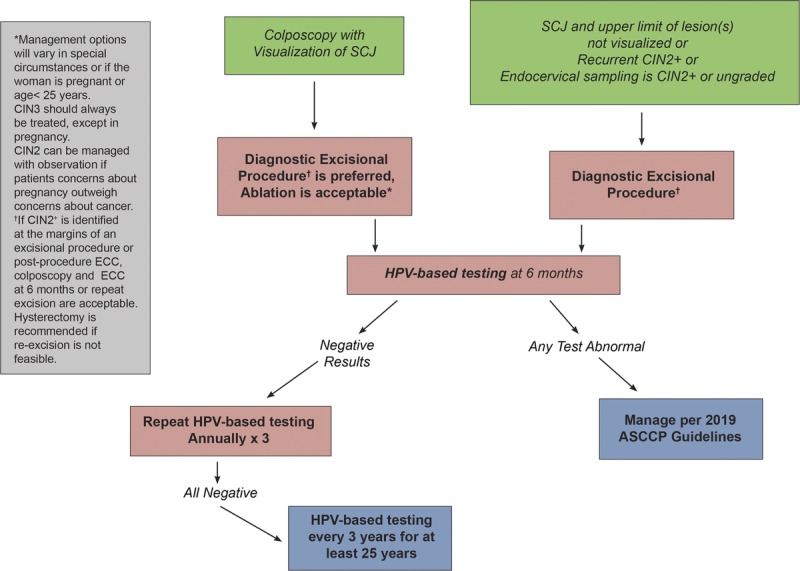
Guideline: In all nonpregnant patients with a diagnosis of histologic HSIL (CIN 3), treatment is recommended and observation is unacceptable (AII). In nonpregnant patients with histologic HSIL (CIN 2), treatment is recommended, unless the patient's concerns about the effect of treatment on future pregnancy outweigh concerns about cancer (BII). Observation is unacceptable when the squamocolumnar junction or the upper limit of the lesion is not fully visualized or when the results of an endocervical sampling, if performed, is CIN 2+ or ungraded (EIII) (see Figure 7).3
Rationale: As CIN 3 is considered an immediate cancer precursor, treatment is always recommended and observation is never acceptable, except during pregnancy (Section K.2). Observation is acceptable for CIN 2 in patients concerned about the potential effects of treatment on future pregnancy outcomes.
Guideline: When treatment of histologic HSIL is planned, excisional treatment is preferred, and treatment with ablation is acceptable (BI). Outside of the setting of a clinical research trial, nonsurgical therapies, including topical agents, therapeutic vaccines, and other biologics, are unacceptable for the treatment of histologic HSIL (CIN 2 or CIN 3) (DIII). Hysterectomy is unacceptable as primary therapy solely for the treatment of histologic HSIL (CIN 2, CIN 3, or unqualified) (EII). When considering ablative therapy, in particular cryotherapy, ablation is unacceptable in the following circumstances. as defined by the WHO: (a) the lesion extends into the canal and (b) when the lesion covers more than 75% of the surface area of the ectocervix or extends beyond the cryotip being used.97 Additional situations for which cryotherapy is not recommended include the following: (a) the squamocolumnar junction or the upper limit of any lesion is not fully visualized; (b) endocervical canal sample is diagnosed as CIN 2+ or CIN that cannot be graded; (c) after previous treatment for CIN 2+; (d) in the setting of inadequate biopsies of the cervix to confirm histologic diagnosis; and (e) if cancer is suspected (EIII).
Rationale: The WHO recommends LEEP over cryotherapy in settings where LEEP is “available and accessible.”97 In the United States, excisional treatment is used more commonly than ablation treatment for the treatment of histologic HSIL. Excisional therapy consists of loop electrosurgical excision procedure (LEEP or LLETZ), cold knife conization, and laser cone biopsy. Ablation treatment includes cryotherapy, laser ablation, and thermoablation.98 Few recent data have compared the effectiveness of excisional and ablative therapy. Most recent studies evaluating ablative therapies have been performed outside of the United States, primarily in low-resource settings. A meta-analysis of randomized trials demonstrated a CIN recurrence rate of 26.6% at 12 months after LEEP compared 31.0% for cryotherapy.99 However, another meta-analysis calculated that the recurrence rate of CIN 2–3 was 5.3% after both cryotherapy and LEEP and 1.4% after cold knife conization. More adverse events were noted with cold knife conization than with LEEP, and more with LEEP than with cryotherapy.100 A Cochrane review comparing surgical techniques for treatment of CIN concluded that no technique was clearly superior in terms of treatment failure or associated morbidity.101 However, for high-grade abnormalities, LEEP has the benefit of providing a histologic specimen, which may reveal a higher grade of squamous abnormality or a glandular abnormality, and also provides information on margin status, a predictor of CIN 2+ persistence or recurrence.102,103 Laser ablation differs from other ablative techniques and, when performed by highly experienced providers, may be appropriate in special circumstances including treatment of large cervical lesions or when lesion extends to the vagina, provided all other criteria for ablation are met.
I.3 Management of CIN 2 in Those Who Are Concerned About the Potential Effect of Treatment on Future Pregnancy Outcomes
Guideline: For patients with a diagnosis of histologic HSIL (CIN 2) whose concerns about the effects of treatment on a future pregnancy outweigh their concerns about cancer, either observation or treatment is acceptable provided the squamocolumnar junction is visible and CIN 2+ or ungraded CIN is not identified on endocervical sampling (CII) (see Figure 8). If the histologic HSIL cannot be specified as CIN 2, treatment is preferred, but observation is acceptable (CIII). For patients 25 years or older, observation includes colposcopy and HPV-based testing at 6-month intervals for up to 2 years (See Section K.1 for management age of younger than 25 years). If during surveillance, all evaluations demonstrate less than CIN 2 and less than ASC-H on 2 successive occasions, 6 months apart, subsequent surveillance should occur at 1 year after the second evaluation and use HPV-based testing. If negative on 3 consecutive annual surveillance tests, proceed to long-term surveillance (Section J.3). If CIN 2 remains present for a 2-year period, treatment is recommended (CII) (see Figure 8).
FIGURE 8.
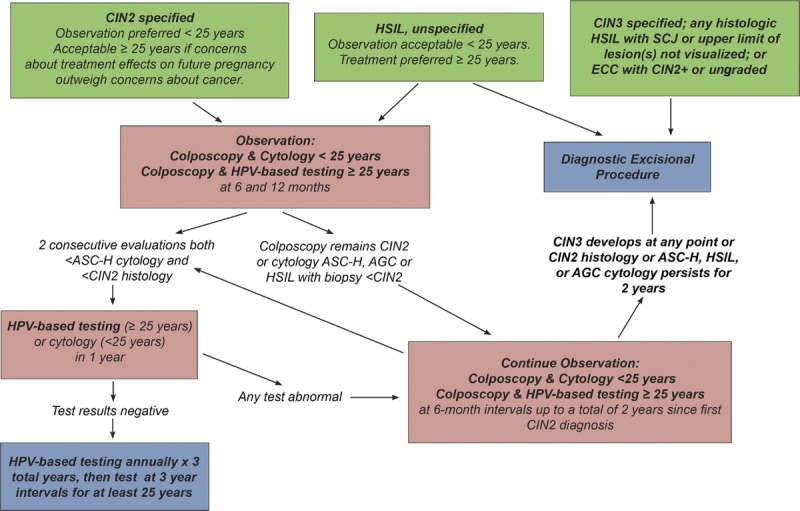
This figure describes management of CIN 2 in patients whose concerns about the effects of treatment on a future pregnancy outweigh their concerns about cancer. Also addressed is the management of histologic HSIL not further specified in women younger than 25 years, for whom observation is acceptable, and for women 25 years or older for whom treatment is preferred.
Rationale: Unlike CIN 3, which is considered a direct cancer precursor, CIN 2 has an appreciable regression rate. A systematic review and meta-analysis of studies from 1973 to 2016 indicated that among CIN 2 managed conservatively, 50% regressed, 32% persisted, and 18% progressed to CIN 3+. Notably, most regression occurred within the first 12 months, whereas rates of progression continued to increase over time. Regression rates were higher (60%) in women younger than 30 years.29 A recent study at the KPNC of 2,417 patients followed for a median of 48 months with colposcopy and cotesting at 6-month intervals found similar results: 50% regressed to CIN 1 or less, though remained in intensive surveillance for persistent HPV positivity, 30% were treated for persistence or progression, and 20% returned to routine screening. Six patients in the KPNC cohort developed cervical cancer, half of whom had significant follow-up delays.27
The primary rationale for deferring treatment of CIN 2 is the potential risk of adverse obstetric outcomes after excisional or ablative therapy; however, the magnitude of this risk is debated.104 Studies are complicated by the finding that patients with untreated CIN have a higher risk of premature delivery than the general population.105,106 Although several studies have concluded that excision is associated with increased risk of preterm birth, especially as excision depth increases,104,105,107–109 others have found no such association after adjustment for potential confounding factors.110–113 Ablation treatment seems to have little or no effect on adverse pregnancy outcomes.105,107,108,114 A Cochrane Review concluded that results should be interpreted with caution because of data being of low or very low quality.105
I.4 Management of LSIL (CIN 1) or Less Preceded by ASC-H or HSIL Cytology
Guideline: When CIN 2+ is not identified histologically after an ASC-H or HSIL cytology result, it is acceptable to review the cytologic, histologic, and colposcopic findings. If the review yields a revised interpretation, management should follow guidelines for the revised diagnosis (CIII). When CIN 2+ is not identified, HSIL cytology is managed more aggressively than ASC-H cytology. For cytology showing HSIL, but biopsy showing histologic LSIL (CIN 1) or less, either an immediate diagnostic excisional procedure or observation with HPV-based testing and colposcopy at 1 year is acceptable, provided in the latter case that the initial colposcopic examination fully visualized the squamocolumnar junction and the upper limit of any lesion, and that the endocervical sampling, if collected, was less than CIN 2 (BII). For ASC-H, if the colposcopic examination can fully visualize the squamocolumnar junction and the upper limit of any lesion and that the endocervical sampling, if collected, is negative, observation at 1 year with HPV-based testing is recommended; a diagnostic excisional procedure is not recommended (BII). For both HSIL and ASC-H cytology, if observation is elected, and all tests are negative at the 1-year visit, repeat HPV-based testing is recommended in 1 year (at 2 years from the original cytology). If all tests are negative at both the 1- and 2-year follow-up visits, return for retesting with HPV-based testing in 3 years is recommended, then proceed with long-term surveillance (Section J.3). If any test is abnormal during the observation period, repeat colposcopy is recommended, and management based on resulting biopsies is recommended. A diagnostic excisional procedure is recommended for patients with HSIL cytology results at either the 1- or 2-year visit, or ASC-H results that persist at the 2-year visit (CIII) (see Figures 9, 10).
FIGURE 9.
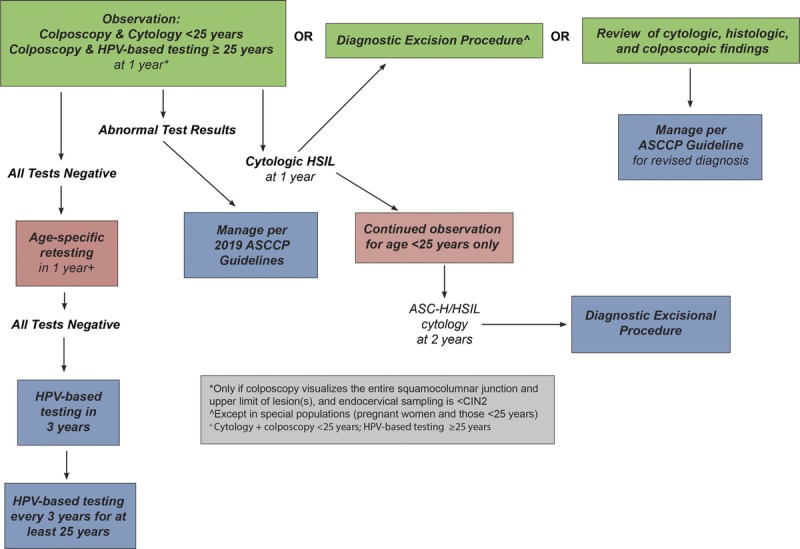
This figure describes management of histologic LSIL (CIN 1) preceded by HSIL cytology.
FIGURE 10.
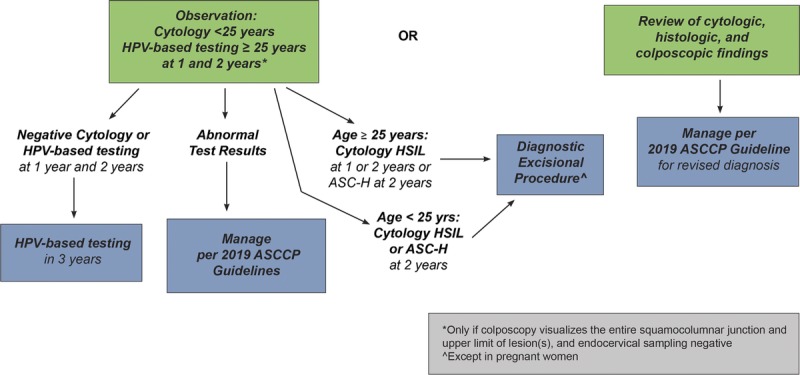
This figure describes management of histologic LSIL (CIN 1) preceded by ASC-H cytology.
Rationale: Patients with a diagnosis of histologic LSIL (CIN 1) after HSIL and ASC-H cytology have 1-year CIN 3+ risks of 3.9% and 1.4%, respectively.5 Because HSIL cytology is associated with a higher risk than ASC-H cytology, colposcopy is recommended in addition to HPV-based testing at the 1-year follow-up if excision is not elected. Failure to detect CIN 2+ at colposcopy in patients with HSIL cytology does not mean that a CIN 2+ lesion has been excluded, although occult carcinoma is unlikely. As a result, patients with HSIL cytology who do not have immediate diagnostic excision require close follow-up. Few studies of HSIL cytology managed without treatment have been reported, and follow-up in those is limited; management relies on expert opinion.3 Of note, at all colposcopic examination when no lesion is identified on the cervix, the vagina and vulva must be examined for vaginal or vulvar intraepithelial neoplasia.
I.5 Histologic LSIL (CIN 1) Diagnosed Repeatedly for at Least 2 Years
Guideline: For patients 25 years or older with histologic LSIL (CIN 1) who is diagnosed at consecutive visits for at least 2 years, observation is preferred (BII) but treatment is acceptable (CIII). If treatment is selected and the entire squamocolumnar junction and all lesions were fully visualized during colposcopic examination, either excision or ablation treatments are acceptable (CII).
Rationale: Histologic LSIL (CIN 1) is the histologic manifestation of HPV infection. CIN 1 may be associated with oncogenic (high-risk) or low-risk HPV infections and may be due to persistent infection with 1 type or sequential infections with different types. HPV 16 is less common in CIN 1 than in CIN 3.3 Histologic LSIL (CIN 1) and cytologic ASC-US/HPV+ and LSIL are the same biologically and thus should be managed similarly. Regression rates are high, especially in younger patients, and subsequent diagnosis of CIN 2+ is uncommon regardless of whether CIN 1 is found on endocervical sampling or a biopsy of the transformation zone.3,115 The KPNC data showed a similar, relatively low 5-year risk of CIN 3+ of approximately 2% when CIN 1 or no lesion was found on colposcopy/biopsy after HPV-positive cytologic ASC-US or LSIL. In the KPNC data set of individuals with CIN 1 on biopsy on 2 consecutive visits, the subsequent follow-up demonstrated that 52% were HPV negative, 48% were HPV positive, and of the HPV-positive group, 92% had NILM, ASC-US, or LSIL cytology. A study of 126 women undergoing LEEP for CIN 1 diagnosed at consecutive visits for 2 years found that 87% had CIN 1 or negative pathology, whereas 13% had histologic HSIL (CIN 2+).116 Based on these data, and considering the potential harms of treatment, the present recommendations prefer continued observation of those with histologic LSIL (CIN1) diagnosed on consecutive visits for at least 2 years. Treatment is an acceptable option based on patient preference, after shared decision-making. Because the immediate estimated CIN3+ risk is less than the 25% treatment threshold, this is considered a special situation.
I.6 Management of AIS: Adoption of Society of Gynecologic Oncology Recommendations
The Society of Gynecologic Oncology recently completed guidelines on the management of AIS; recommendations were adopted by the 2019 ASCCP Risk-Based Management Guidelines consensus committee and are summarized below. Evidence is not graded as the consensus committee did not perform primary data review.
Guideline: A diagnostic excisional procedure is recommended for all patients with a diagnosis of AIS on cervical biopsy to rule out invasive adenocarcinoma, even when definitive hysterectomy is planned. Excisional procedures should optimally remove an intact specimen to facilitate accurate interpretation of margin status. Although there is no preference for cold knife conization versus LEEP, intentional disruption of the specimen by performance of a LEEP followed by a “top hat” endocervical excision to achieve the desired specimen length is unacceptable. An excisional specimen length of at least 10 mm is preferred, and this can be increased to 18 to 20 mm for patients who are not concerned about the effect of treatment on future pregnancy. These dimensions are preferred regardless of whether hysterectomy is planned.
After the initial diagnostic procedure, hysterectomy is the preferred management for all patients who have a histologic diagnosis of AIS, although fertility-sparing management for appropriately selected patients is acceptable. For patients with confirmed AIS with negative margins on the excisional specimen, simple hysterectomy is preferred. For patients with confirmed AIS with positive margins on the excisional specimen, re-excision to achieve negative margins is preferred, even if hysterectomy is planned. For patients with AIS and persistent positive margins for whom additional excisional procedures are not feasible, either a simple or modified radical hysterectomy is acceptable. After hysterectomy, surveillance per the ASCCP surveillance guidelines for treated CIN 2+ is recommended (Section J.3).
For patients of reproductive age who desire future pregnancy, fertility-sparing management with an excisional procedure is acceptable provided that negative margins have been achieved on the excisional specimen, and the patient is willing and able to adhere to surveillance recommendations. If negative margins cannot be achieved after maximal excisional attempts, fertility-sparing management is not recommended. For patients who undergo fertility-sparing management, surveillance with cotesting and endocervical sampling is recommended every 6 months for at least 3 years, then annually for at least 2 years, or until hysterectomy is performed. For patients who have consistently negative cotesting and endocervical sampling results for 5 years, extending the surveillance interval to every 3 years starting in the sixth year of surveillance is acceptable. Small retrospective studies have shown HPV test results to be the best predictor for recurrent disease. Therefore, for patients who have consistently negative cotesting and endocervical sampling results, continued surveillance is acceptable after completion of childbearing. For patients who have had positive HPV test results or abnormal cytology/histologic results during surveillance, hysterectomy at the completion of childbearing is preferred (see Figure 11).
FIGURE 11.
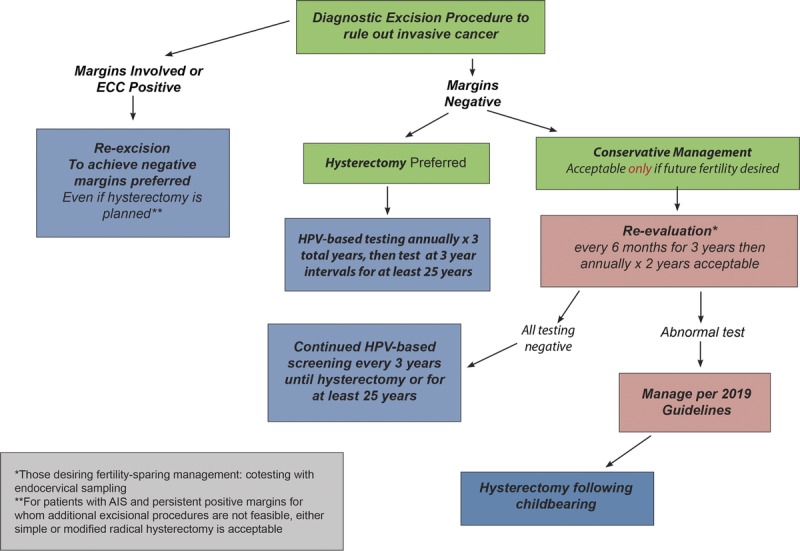
This figure describes management of AIS. This management algorithm was developed by the Society of Gynecologic Oncology and endorsed by the ASCCP Risk-Based Management Consensus process.
Rationale: The Society of Gynecologic Oncology recently conducted a literature review and is publishing recommendations for management of AIS. The ASCCP recommendations adopted the Society of Gynecologic Oncology recommendations, and additional details are provided in the Society of Gynecologic Oncology reference.117 A brief summary of the rationale is provided below. Hysterectomy is recommended for AIS for several reasons. Adenocarcinoma in situ is frequently located within the endocervical canal and colposcopic changes may be minimal; therefore, determination of the necessary length of a cervical excisional specimen may be difficult. Adenocarcinoma in situ also has a higher risk of being multifocal, so negative margins on an excisional procedure specimen do not ensure complete excision of disease. Importantly, in the setting of histologic AIS on biopsy, invasive cancer cannot be excluded without a diagnostic excisional procedure. Finally, although increased detection and treatment of squamous cell cancer precursors (e.g., CIN 3) is associated with a decrease in the incidence of invasive squamous cell carcinoma, the same has not been demonstrated for AIS.118 Because of the challenges in diagnosing and monitoring AIS, hysterectomy remains the standard treatment for AIS for patients who do not desire future pregnancy. For patients desiring future pregnancy, observation after an excisional procedure remains an option, but this carries a less than 10% risk of recurrent AIS and a small risk of invasive cancer even with negative margins. Both margin status and endocervical sampling performed at the time of excisional procedure predict residual disease and risk of invasive cancer on hysterectomy specimen. After treatment, HPV tests results are the strongest predictor for recurrent AIS.119–122
J. SURVEILLANCE AFTER ABNORMALITIES
J.1 Guidance for Specific Tests and Testing Intervals When Managing Abnormal Results
Guideline: After abnormal cervical cancer screening test results for patients 25 years or older, colposcopic biopsy results, or treatment of histologic HSIL, surveillance with either HPV testing alone or cotesting is preferred (AI). Surveillance with cervical cytology alone is acceptable only if testing with HPV or cotesting is not feasible (CIII). Cytology is recommended at 6-month intervals when 1-year intervals are recommended for HPV or cotesting, and annually when 3-year intervals are recommended for HPV or cotesting (AII). Cytology should be used for patients younger than 25 years, with transition to HPV-based testing at 25 years or older (AII).
Rationale: Individuals treated for histologic HSIL or with a recent abnormal screening test result have an elevated risk of cervical precancer warranting close follow-up.5,123 HPV testing and cotesting are more sensitive than cytology alone in detecting CIN 2+ in both the postcolposcopy and posttreatment settings.124–126 As there is marginal difference between cotesting and HPV testing alone in detection of recurrent or persistent CIN 2+, either test may be used for surveillance.126,127 Because cytology is less sensitive than HPV or cotesting, cytology must be performed more frequently to achieve similar sensitivity for the detection of CIN 3+. For example, in cases of low-grade cytology followed by colposcopy/biopsy less than CIN 2, follow-up testing at 1 year is recommended. If the follow-up test is an HPV test with negative results, the 5-year CIN 3+ risk is 0.51%, consistent with a 3-year return. However, if the follow-up test is cytology only with negative results, the 5-year CIN 3+ risk is 1.5%, consistent with a 1-year return.
J.2 Short-Term Follow-up After Treatment for Histologic HSIL
Guideline: After treatment, HPV-based testing at 6 months is preferred regardless of the margin status of the excisional specimen (BII) (see Figure 7). If HPV-based tests are positive, colposcopy and appropriate biopsies should be performed (AII). Follow-up at 6 months with colposcopy and ECC is acceptable (BIII).
When margins are positive for CIN 2+ or ECC performed at the time of the excisional procedure shows CIN 2+ in patients 25 years or older who are not concerned about the potential effect of treatment on future pregnancy outcomes, repeat excision or observation is acceptable. For observation, HPV-based testing in 6 months is preferred; it is also acceptable to perform a colposcopy and ECC at 6 months. For patients younger than 25 years or those who are concerned about the potential effect of treatment on future pregnancy outcomes, observation is recommended. (See Section J.3 for subsequent management). If recurrent histologic HSIL (CIN 2+) develops after excisional treatment, and repeat excision is not feasible or not desired, hysterectomy is recommended (see Figure 7).
Rationale: The preferential use of HPV-based testing (cotesting or HPV primary testing) is supported by evidence that posttreatment HPV testing is the most accurate predictor of treatment outcome.125 Although the relative risk of persistent or recurrent histologic HSIL (CIN 2+) is almost 5 times higher after excisional treatment with positive margins compared with negative margins (RR = 4.8; 95% CI = 3.2–7.2),103 only 56% (95% CI, 49–66%) of persistent/recurrent precancer was predicted by positive margin status. The poor ability for margin status to predict persistent/recurrent precancer argues against differentiating follow-up testing by margin status alone. In contrast, the ability of HPV-based testing to predict persistent/recurrent histologic HSIL (CIN 2+) is 91% (95% CI = 82%–96%) and does not differ significantly between patients with positive versus negative margins. The absolute risk of persistent/recurrent histologic HSIL (CIN 2+) after excision with positive margins is 17% (95% CI = 13–22%). However, repeat excisional treatment without repeat testing is considered acceptable for certain patients after appropriate counseling and consideration of age, likelihood of subsequent resolution of histologic HSIL/HPV infection, concern for the effect of treatment on future pregnancy, and ability to adhere to surveillance recommendations.
J.3 Guidance for Long-Term Follow-up After Treatment for High-Grade Histology or Cytology
Guideline: In patients treated for histologic or cytologic HSIL, after the initial HPV-based test at 6 months, annual HPV or cotesting is preferred until 3 consecutive negative tests have been obtained (AII). After the initial intensive surveillance period, continued surveillance at 3-year intervals is recommended for at least 25 years after treatment of high-grade histology (histologic HSIL, CIN 2, CIN 3, or AIS) or high-grade cytology (HSIL or persistent ASC-H) even if this is beyond the age of 65 years (BII). When patients with a history of treated high-grade histology or cytology reach the age of 65 years, if they have completed the initial 25-year surveillance period, continued surveillance at 3-year intervals is acceptable and may continue as long as the patient is in reasonably good health (BIII). Discontinuation of screening is recommended if a patient has a limited life expectancy. Management according to the highest-grade abnormality found on histology or cytology is recommended.
Rationale: According to KPNC data, the 5-year CIN 3+ risks after treatment of CIN 3 for 1, 2, and 3 negative cotests/primary HPV tests were 1.7%/2.0%, 0.68%/0.91%, and 0.35%/0.44%, respectively.5 Therefore, annual surveillance by cotesting or HPV testing is recommended until 3 negative annual HPV-based tests have been obtained. After a third negative HPV-based test, KPNC data suggest that the 5-year CIN 3+ risk remains above the 0.15% threshold for return to routine, 5-year HPV-based cervical screening. Long-term population studies support this finding, as they demonstrate a persistent twofold increase in cervical cancer risk after treatment of histologic HSIL. Risk persists for at least 25 years and seems to be increased for patients older than 50 years.123,128,129 Therefore, continued 3-year surveillance is recommended for a minimum of 25 years. As cervical cancer risk seems to remain above general population levels,123 continued screening for as long the patient remains in good health is acceptable.
J.4 Guidance for Long-Term Follow-up After Low-Grade Cytology (HPV-Positive NILM, ASC-US, or LSIL) or Histologic LSIL (CIN 1) Abnormalities Without Evidence of Histologic or Cytologic High-Grade Abnormalities
Guideline: Among patients initially diagnosed with low-grade cytology or histologic abnormalities or HPV infections, continued surveillance according to risk estimation using available data is recommended (CIII).
Rationale: The 5-year CIN 3+ risks for abnormal screening test results without evidence of cytologic or histologic HSIL followed by negative HPV-based testing were 0.51% after the first negative test and 0.23% after the second negative test. Thus, patients reach criteria for a 3-year return after the second negative HPV-based test.5 The ability to perform accurate risk estimation for 3 or more rounds of negative testing after abnormalities is limited by very small numbers of CIN 3+ diagnoses in patients with persistently negative follow-up testing after low-grade cytologic or histologic abnormalities. We estimated risk for two common scenarios related to long-term negative follow-up. The first was HPV+/NILM followed by 3 rounds of negative cotesting. At KPNC, the estimated 5-year CIN 3+ risk was 0.17% (95% CI = 0.14%–0.44%), therefore continued testing at 3-year intervals is recommended at this time. The second group included patients with low-grade abnormalities, who underwent colposcopy at which CIN2+ was not found, and then had 3 rounds of negative cotesting. This group had an estimated 5-year CIN3+ risk of 0.03% (95% CI= 0.0–0.19%), and thus does qualify for return to a 5-year interval. The 5-year CIN3+ risks for various clinical scenarios will be re-estimated as either longer-term follow-up accrue or risk modification based on genotyping are available, and publicly available tables will be modified accordingly (https://CervixCa.nlm.nih.gov/RiskTables).
K. SPECIAL POPULATIONS
Introduction: Guidelines described previously apply to the average risk individual with an intact cervix and are based primarily on screening and management data from patients aged 25 to 65 years in the KPNC population. However, several populations require special management considerations. Management of patients who are younger than 25 years, pregnant, immunosuppressed, posthysterectomy, and older than 65 years are detailed hereinafter.
K.1 Management of Patients Younger Than 25 Years
In the 2012 guidelines, patients aged 21 to 24 years were considered to be a special population. In the current guidelines, the consensus was to reference this group as “patients younger than 25 years.”
Initial Management After an Abnormal Screening Test Result
Guideline: In patients younger than 25 years with low-grade cytology screening results of LSIL, ASC-US HPV-positive, or ASC-US without HPV testing, repeat cytology alone at 1 and 2 years after the initial abnormal result is recommended (BII). Colposcopy is recommended if high-grade cytology is found at any point (HSIL, ASC-H, AGC, AIS) or if low-grade cytology persists at the 2-year follow-up visit (BII). If reflex HPV testing for ASC-US is performed and the results are negative, repeat cytology in 3 years is recommended (BII). After 2 consecutive negative cytology results, return to routine age-based screening is recommended (BII). If colposcopy is performed and the results are less than CIN 2 (i.e., histologic LSIL [CIN 1] or less), repeat cytology in 1 year (BII), and manage as above (e.g., repeat cytology for ASC-US/LSIL, colposcopy for ASC-H or higher). Clinicians should switch to using risk estimates when patients reach the age of 25 years (see Figures 12, 13).
FIGURE 12.
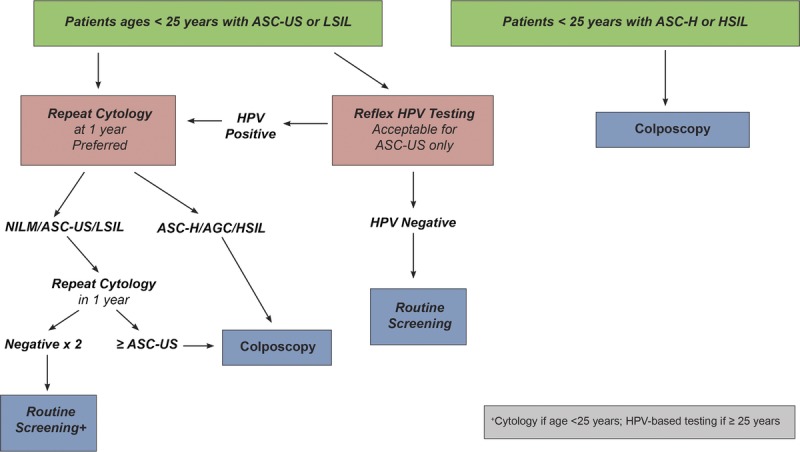
This figure describes management of cytologic abnormalities in patients younger than 25 years.
FIGURE 13.
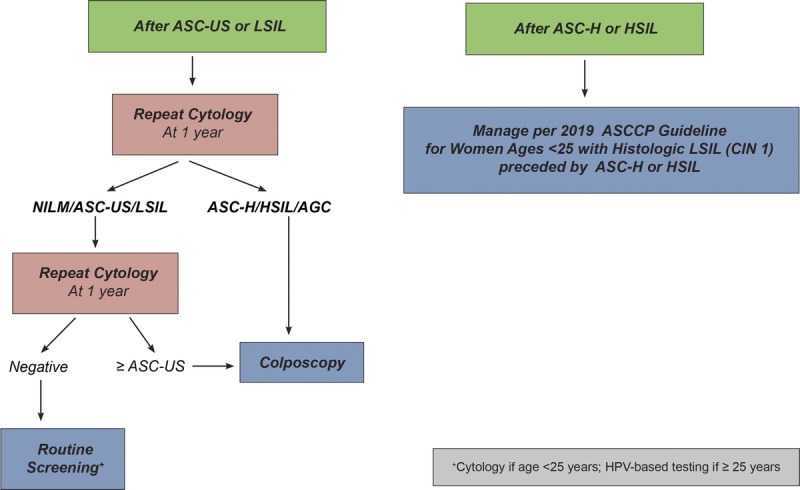
This figure describes management of histologic LSIL (CIN 1) in patients younger than 25 years.
Rationale: HPV vaccination became available in the United States in 2006, and patients at the target age for vaccination have now entered the younger than 25-year age group.130 Consequently, population-level risks of CIN 3+ for a given screening results are expected to decrease through a combination of individual and herd immunity. Observation is indicated for low-grade cytology results (ASC-US, LSIL), which are likely to represent non-16/18 HPV infections with a high probability for regression and low risk for rapid progression to cancer. Accurate risk estimation for this age group is very difficult because vaccination is rapidly changing population-level CIN 3+ risk and the conservative 2012 management guidelines recommend against colposcopy/biopsy for lesser cytology abnormalities, which limits the ability to accurately measure CIN 3+ rates in this age group. Therefore, in the absence of new compelling data to change management in this age group, the 2012 algorithms are carried forward. The guidelines outlined in this document are designed to adapt to changes in population vaccination coverage as well as new technologies, and we anticipate that incorporating HPV vaccination effects on the population-level prevalence of HPV infections will affect management recommendations in the near future.
Management of Cytology ASC-H and HSIL in Patients Younger Than 25 Years
Guideline: Colposcopy is recommended for patients younger than 25 years with ASC-H or HSIL cytology (AII). Immediate treatment without histologic confirmation is not recommended (see Figure 13).
Rationale: Although overall CIN 3+ prevalence is lower, cytology results of ASC-H are associated with higher risks of CIN 3+ than ASC-US, even in patients younger than 25.3 Therefore, colposcopy is warranted to evaluate the cervix for CIN 3+. Immediate treatment without histologic confirmation is not warranted in this population because of the high rate of resolution of CIN 2+ and the potential harms of treatment.
Management of Histology of Less Than CIN 2 Preceded by Cytology ASC-H and HSIL in Patients Younger Than 25 Years
Guideline: Observation is recommended and diagnostic excisional procedures are not recommended for patients younger than 25 years with a preceding cytology of ASC-H or HSIL and a colposcopy with biopsy of CIN 1 or less as long as the squamocolumnar junction and the upper limit of all lesions are fully visualized, the endocervical sampling is less than CIN 2, and review of histology/cytology does not change the diagnosis. Observation with colposcopy and cytology in 1 and 2 years is recommended for those with HSIL cytology. Cytology at 1 and 2 years is recommended for those with ASC-H cytology, with colposcopy recommended for ASC-US or above on repeat testing. If CIN 2+ is diagnosed, this is managed per guidelines described in the following section. If a high-grade cytologic abnormality (HSIL, ASC-H) without histologic HSIL persists for 2 years, a diagnostic excisional procedure is recommended (unless the patient is pregnant). A diagnostic excisional procedure is recommended in patients when the squamocolumnar junction or the upper limit of all lesions are not fully visualized (see Figures 9, 10).
Rationale: CIN 1 or less preceded by cytologic ASC-H or HSIL is a rare diagnosis and not well represented in the KPNC population. The rationale for conservative management of this clinical situation is discussed in Section I.4.
Management of Histologic HSIL (CIN 2 or CIN 3) for Patients Younger Than 25 Years
Guideline: In patients younger than 25 years with histologic HSIL (CIN 3), treatment is recommended, and observation is unacceptable (EII). In patients younger than 25 years with histologic HSIL (CIN 2), observation is preferred, and treatment is acceptable (BII). In patients younger than 25 years with histologic HSIL unspecified as CIN 2 or CIN 3, observation or treatment is acceptable. Observation includes colposcopy and cytology at 6-month intervals. If during surveillance of histologic HSIL, all cytology results are less than ASC-H and histology results are less than CIN 2 at 6 and 12 months, subsequent surveillance should be at 1 year after the second evaluation. If CIN 2 or unspecified histologic HSIL persists for a 2-year period, treatment is recommended. Excisional treatment is recommended when the squamocolumnar junction or the lesion(s) are not fully visualized (see Figure 8).
Rationale: Cervical cancer is uncommon in patients younger than 25 years despite the high prevalence of HPV infections and high-grade histologic lesions (especially CIN 2).16,131 Younger patients have higher rates of regression for histologic HSIL (particularly CIN 2) and lower risks of progression to invasive cancer.26,27,132,133 Therefore, less intensive management strategies that do not include HPV testing are appropriate for this population. The exception is CIN 3, which is considered a direct cervical cancer precursor and should be treated at any age.
K.2 Managing Patients During Pregnancy
Guideline: In pregnancy, management of abnormal screening results using the same Clinical Action Thresholds for surveillance and colposcopy established for nonpregnant patients is recommended (CIII). Endocervical curettage, endometrial biopsy, and treatment without biopsy are unacceptable during pregnancy (EIII). A diagnostic excisional procedure or repeat biopsy is recommended only if cancer is suspected based on cytology, colposcopy, or histology (BII). If histologic HSIL (CIN 2 or CIN 3) is diagnosed at the first colposcopy examination during pregnancy, surveillance colposcopy and testing (diagnostic cytology/HPV depending on age) is preferred every 12 to 24 weeks, but deferring colposcopy to the postpartum period is acceptable (BII). Repeat biopsy is recommended if invasion is suspected or the appearance of the lesion worsens (BII). Treatment of histologic HSIL (CIN 2 or CIN 3) during pregnancy is not recommended (DII). If AIS is diagnosed during pregnancy, referral to a gynecologic oncologist is preferred, but management by a gynecologist skilled in the colposcopic diagnosis and treatment of AIS is acceptable (CIII).
In the postpartum period, colposcopy is recommended no earlier than 4 weeks after delivery (BII). In patients diagnosed with histologic HSIL (CIN2 or CIN3) during pregnancy, if a lesion is detected at postpartum colposcopy, an excisional treatment procedure or full diagnostic evaluation (cervical cytology, HPV, and biopsy) is acceptable (BII). In the absence of a lesion on colposcopy, a full diagnostic evaluation is recommended; expedited treatment is not recommended (BII).
Rationale: Pregnancy was considered as a special population in which to consider management and treatment options that weigh the risk to fetus and mother versus the risk of missing cancer. Rate of progression to cancer is not thought to be different in pregnancy. The 2012 management guidelines for pregnant patients were considered,3 and literature published since 2012 was reviewed.134–139 The adoption of Clinical Action Thresholds in 2019 necessitated modification of the 2012 guidelines, which were based on test results. Although the risk of precancer is not known to be elevated among pregnant patients, cervical hyperemia and other physiologic changes of pregnancy may impact the likelihood of precancer and cancer detection. Colposcopist experience, specifically in the evaluation of the pregnant patient, is known to affect the ability to visually distinguish cancers from pregnancy-related changes, increasing the risk of a missed cancer diagnosis. Colposcopy by an experienced provider during pregnancy is preferred.
The intervals recommended for follow-up are relatively wide taking into consideration the experience and comfort level of the colposcopist, gestational age of the fetus, and the potential for loss to follow-up. Pregnancy does not seem to alter the risk for or rate of progression from cervical precancer to cancer, and colposcopy-directed biopsies in pregnant patients seem to be safe. The 2019 guidelines allow deferral of colposcopy for minor abnormalities in women with prior negative HPV testing or colposcopic examinations at which CIN2+ was not found. Therefore, women referred for colposcopy under the 2019 guidelines will have higher risk of prevalent CIN3+ due to either lack of prior screening or persistent HPV infections. In general, data in pregnancy are limited, however, and shared decision-making taking into account both the pregnant patient and the fetus is critical for management.
Although the risk for progression to cancer during a pregnancy is low, an estimated 11% of new mothers lose their health insurance in the postpartum period. This loss of healthcare access disproportionately affects those most at risk for cervical cancer; rates of noninsurance may be 2 to 3 times as high among low-income and minority patients, as well as those living in states that did not expand Medicaid.140 For individuals who do not qualify for health insurance before pregnancy, pregnancy care is a unique event that facilitates entry into health care coverage. However, Medicaid coverage often terminates at the end of the calendar month in which the delivery occurred or at 6 to 8 weeks postpartum. Because most deliveries in the United States are to individuals with Medicaid, pregnancy may provide an opportunity to identify cancer precursors and even cancer in this population. Healthcare access was considered when developing guidelines. Individuals who are screened infrequently or are unable to complete appropriate follow-up are at increased risk for developing cervical cancer.141
K.3 Managing Patients With Immunosuppression
Immunocompromised patients include those with HIV, solid organ transplant, or allogeneic hematopoietic stem cell transplant, as well as those with systemic lupus erythematous, and those with inflammatory bowel disease or rheumatologic disease requiring current immunosuppressive treatments. The cervical cancer screening guidelines for persons living with HIV have been supported by an increasing number of publications, including prospective studies. Although the literature for other immunosuppressed populations remains limited, these other conditions that suppress cell-mediated immunity have also been associated with virally induced cancers, including cervical cancer.142,143 Therefore, cervical cancer screening and abnormal result management recommendations for immunocompromised individuals without HIV use the guidelines developed for people living with HIV144: screening should begin within 1 year of first insertional sexual activity and continue throughout a patient's lifetime: annually for 3 years, then every 3 years (cytology only) until the age of 30 years, and then either continuing with cytology alone or cotesting every 3 years after the age of 30 years.
Guideline: In immunocompromised patients of any age, colposcopy referral is recommended for all results cytology results of HPV-positive ASC-US or higher. If HPV testing is not performed on ASC-US results, then repeat cytology in 6 to 12 months is recommended, with colposcopy referral for ASC-US or higher. For any result of ASC-US or higher on repeat cytology or if HPV positive, referral to colposcopy is recommended. For all cytology results of LSIL or worse (including ASC-H, AGC, AIS, and HSIL), referral to colposcopy is recommended regardless of HPV test result if done.
Rationale: Because of higher risk of CIN 3+ with low-grade cytologic abnormalities among HIV+ individuals, colposcopic referral is recommended for HPV-positive ASC-US.145 Lack of data at KPNC precludes risk estimation for immunosuppressed patients. Sexually active patients with HIV infection who are younger than 21 years may have a high rate of progression to precancer. No similar prospective data are available for adolescents who acquired HIV during the perinatal period, but as many as 30% of adolescents perinatally infected had ASC-US or greater on their first cervical cytology. Because of the relatively high HPV prevalence before age 30 years, HPV cotesting is not recommended for patients younger than 30 years of age with HIV.144
K.4 Managing Patients After Hysterectomy
Guideline: After a diagnosis of high-grade histology or cytology, patients may undergo hysterectomy for reasons related or unrelated to their cervical abnormalities. If hysterectomy is performed for treatment, patients should have 3 consecutive annual HPV-based tests before entering long-term surveillance. Long-term surveillance after treatment for histologic HSIL (CIN 2 or CIN 3) or AIS involves HPV-based testing at 3-year intervals for 25 years, regardless of whether the patient has had a hysterectomy either for treatment or at any point during the surveillance period (CIII). Among patients who have undergone hysterectomy but either have no previous diagnosis of CIN 2+ within the previous 25 years or have completed the 25 year surveillance period, screening is generally not recommended. However, if performed, abnormal vaginal screening test results should be managed according to published recommendations (BII).146
Rationale: The risk of high-grade vaginal intraepithelial neoplasia is elevated among patients who have had a hysterectomy for treatment of histologic HSIL.146 Although HPV testing is not FDA approved for vaginal samples, sensitivity of HPV-based testing in the setting of posthysterectomy for histologic HSIL seems superior to cytology alone.147 For patients who have undergone a hysterectomy for benign disease and are screened with cytology and/or HPV testing, ASC-US HPV-positive and LSIL cytology should be managed with follow-up in 12 months and only those with high-grade cytology (HSIL, ASC-H, AGC) should be referred immediately for vaginal colposcopy.148
K.5 Managing Patients Older Than 65 Years With a History of Prior Abnormalities
Guideline: If patients over age 65 years undergo HPV testing, cotesting, or cytology, management according to guidelines for patients aged 25 to 65 years is recommended (CII). If surveillance testing is recommended for either a history of abnormal screening results or treatment for precancer, discontinuing surveillance is unacceptable if the patient is in reasonably good health and testing is feasible (DII). Discontinuation of surveillance is recommended for patients with a limited life expectancy (EIII).
Rationale: Screening for patients older than 65 years should follow national guidelines.14,149 However, approximately 20% of cervical cancers occur in patients older than 65 years.150,151 To mitigate cancer risk in patients older than age 65 years, previous consensus management guidelines included continued testing in patients with abnormal results as well as those who do not meet exit criteria.13,14,152 Although the sensitivity of cytology, HPV testing, and colposcopy seem to be higher in premenopausal than postmenopausal patients, evidence indicates that screening in patients older than 65 years is associated with a lower risk of the subsequent development of cervical cancer.153 Because cessation of routine screening is recommended in adequately screened patients at the age of 65 years, data on the prognostic value of specific screening test results in older patients are limited. However, as cancer rates remain appreciable beyond the age of 65 years,150,151 and cancer diagnostic procedures such as mammography, breast biopsy, and colonoscopy are recommended beyond the age of 65 years,154–156 the consensus decision was to use the guidelines for patients aged 25 to 65 years in evaluating older individuals with abnormal results but without limited life expectancy. Patients with previous CIN 3+ seem to have an elevated lifetime risk of developing cervical or vaginal cancer and thus may require surveillance testing beyond the age of 65 years.123 However, patient comfort and the limitations of positioning and examining older patients should enter into the shared decision-making conversation about when to discontinue screening. Vaginal estrogen use for a limited time (3 weeks) can be considered to obtain adequate sampling.157
L. CURRENT CONSIDERATIONS AND FUTURE DIRECTIONS
L.1 Current Considerations
The 2019 guidelines are designed to take into account factors that influence Clinical Action Thresholds. Working groups considered risk factors to determine their importance for inclusion in clinical applications of the guidelines, taking into account both the magnitude of effect on the estimated risk, as well as the feasibility of collecting accurate data in clinical practice to inform management. Screening history profoundly influenced risk estimates, specifically current HPV and cytology test results, previous HPV test results, and history of histologic HSIL. Patient screening history is often not known; therefore, unknown history is considered separately as a risk factor. Additional factors were considered because of their association with cervical cancer in the literature: HPV vaccination, age, hormonal contraception use, history of sexually transmitted infection, parity, cigarette smoking, obesity, and sexual behaviors including age of first intercourse and multiple partners. HPV vaccination in adolescence (generally before the age of 18 years) does seem to reduce the risk of HPV 16/18 infections and associated histologic HSIL.158,159 However, HPV vaccination status was omitted from this revision of the guidelines because (a) management guidelines are already very conservative in the population younger than 25 years, (b) the population prevalence of on-time HPV vaccination in the 25- to 29-year-old population is currently lower than that needed for herd immunity,160 thus changing recommendations for this population as a whole is not yet warranted, and (c) making person-specific recommendations based on age at vaccine series initiation and number of doses received is impractical in the United States in the absence of linkable, comprehensive, state-based immunization registries. Overall, none of the other factors contributed clinically meaningful risk beyond that afforded by the screening factors noted previously. Therefore, additional factors were not included in risk estimates. Analyses were limited for heavy smoking history and younger than 30 years.
L.2 Future Directions
A successor to the new technologies group will be proposed to continue the consensus process, and to provide continuous future updates to guidelines as new tests become available for management. Decreases in the overall population prevalence of HPV infection, especially HPV 16/18 genotypes, are expected as individuals vaccinated as adolescents reach screening age. The guidelines outlined in this document are designed to adapt to decreases in oncogenic HPV prevalence because of HPV vaccination as well as new screening and management technologies. As data on the CIN 3+ risks associated with screening test results become available for individuals aged 25 to 29 years who received timely vaccination, we anticipate that decreases in population-level prevalence of HPV infections will affect management recommendations for this age group in the near future. In addition, new technologies that enter the market will be evaluated for their utility in improving the diagnosis and management of CIN 3+. Examples of clinically useful products would be those with increased specificity for detecting high-grade abnormalities or the ability during longitudinal follow-up to distinguish incident (new) from prevalent (persistent) HPV infections. No specific new technologies are listed as creating a comprehensive list of products in development is beyond the scope of this article.
In the near future, we will also complete analyses related to costs, benefits, and effectiveness. The high value care group laid out a future research agenda that includes simulation modeling to estimate the quality-of life and economic effects of proposed changes to managing those with abnormal cervical cancer screening test results over multiple rounds of screening.
Finally, we are tasked with disseminating these guidelines within the United States to create a new national standard of care for management of abnormal cervical cancer screening test results. Changing from recommendations that could be easily memorized by clinicians to guidelines that incorporate both current results and history is a major undertaking. However, the result of successful adoption should be reduction of unnecessary testing and invasive procedures in low-risk patients and identification of high-risk patients who will benefit from more intensive surveillance. Maximizing cancer prevention benefits while minimizing the harms of overtesting and overtreatment is a worthwhile but lofty goal, and these guidelines require more robust implementation plans than previous iterations. The process of guidelines dissemination will involve a comprehensive communications and dissemination plan using best practices for risk communication and health promotion. Components include the following: presentations at national, regional and local meetings, social media outreach to engage clinicians and medical societies, and development of promotional materials to answer frequently asked questions. Additional areas for future research include development of an evaluation and impact process for these new recommendations on clinical practices. Because low-income and minority women bear the greatest burden of cervical cancer, particular emphasis will be placed on working with these communities and the providers who serve them.
GLOSSARY
CIN 2+: this term includes CIN 2, CIN 3, AIS, and cancer
CIN 3+: this term includes CIN 3, AIS, and cancer
Clinical Action Threshold: this term refers to risk levels that prompt different clinical management strategies. For example, an immediate CIN 3+ risk of 4% is the Clinical Action Threshold for colposcopy; risks below this threshold undergo surveillance, whereas risks above this threshold, but below the expedited treatment threshold, undergo colposcopy.
Colposcopy Standards: this term refers to the ASCCP Colposcopy Standards that provide evidence-based recommendations for the practice of colposcopy
Cotesting: this term refers to screening or surveillance performed with both cytology and HPV testing.
Expedited treatment: this term means treatment without confirmatory colposcopic biopsy (e.g., see and treat).
Excisional treatment: this term includes procedures that remove the transformation zone and produce a specimen for histologic analysis, such as loop electrosurgical excision procedure (LEEP), laser cone biopsy, large loop excision of the transformation zone (LLETZ), and cold knife conization.
HPV: this term refers to human papillomavirus. Within this text, HPV refers specifically to high-risk HPV as defined by IARC, including the 12 types that are considered class 1 carcinogens, plus type 68 which is considered a class 2A carcinogen (i.e., HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68).
HPV-based testing: this term is used in this document to describe the use of either cotesting or primary HPV screening for surveillance after abnormalities. It does not apply to reflex HPV testing for triage of ASC-US cytology in this document. The HPV testing and positive HPV results discussed throughout this document refer to high-risk HPV types only.
Lower Anogenital Squamous Terminology (LAST): this term refers to 2-tiered pathology criteria for evaluating histologic specimens obtained via colposcopic biopsy
Primary HPV testing: testing with HPV testing alone as a screening or surveillance test.
Reflex testing: this means that laboratories should perform a specific additional triage test in the setting of a positive screening test to inform the next steps in management. For example, an ASC-US cytology should trigger a reflex HPV test. New for these guidelines, a positive a positive primary HPV screening test should trigger both a reflex genotyping test (to determine the presence/absence of HPV 16/18 if that information is not included in the initial primary test result) and also a reflex cytology test to determine whether the patient would be a candidate for expedited management.
Surveillance: this term refers to repeat testing (HPV primary screening, cotesting, or cytology alone) that occurs at shorter intervals than those recommended for routine screening. For example, HPV primary testing or cotesting at intervals of less than 5 years, or cytology alone at intervals of less than 3 years.
Additional contributing authors for the ASCCP Risk Based Management Consensus Guidelines Committee
Deborah Arrindell, Washington DC
Pelin Batur, MD, Cleveland OH
Alicia Carter, MD, Burlington NC
Patty Cason, MS, FNP, Los Angeles, CA
Xiaojian Chen MS, Bethesda, MD
Li Cheung PhD, Bethesda, MD
Kim Choma, DNP, Teaneck NJ
Megan Clarke, PhD, MHS, Rockville MD
Christine Conageski, MD, Aurora CO
Miriam Cremer, MD, MPH, Cleveland, OH
Barbara Crothers, DO, Silver Spring MD
Teresa Darragh, MD, San Francisco CA
Maria Demarco, PhD, Rockville MD
Eileen Duffey-Lind, MSN, Boston, MA
Ysabel Duron, BA, San Jose CA
Didem Egemen PhD, Bethesda, MD
Carol Eisenhut, MD, MBA, Indianapolis IN
Tamika Felder, Upper Marlboro MD
Sarah Feldman, MD, MPH, Boston MA
Michael Gold, MD, Tulsa OK
Robert Goulart, MD, Springfield MA
Paul Han, MD, Portland ME
Sally Hersh, DNP, Portland OR
Aimee Holland, DNP, Birmingham AL
Eric Huang, MD, Seattle, WA
Michelle Khan, MD, MPH, San Leandro CA
Rachel Kupets, MD, Toronto ON, Canada
Margaret Long, MD, Rochester MN
Thomas Lorey MD, Berkeley, CA
Jennifer Loukissas, MPP, Bethesda MD
Jeanne Murphy, PhD, Washington DC
Amber Naresh, MD, MPH, New Orleans LA
Erin Nelson, MD, San Antonio TX
Akiva Novetsky, MD, MS, Newark NJ
Jeffrey Quinlan, MD, Bethesda, MD
Debbie Saslow, PhD, Atlanta GA
Kathryn Sharpless, MD, PhD, Portland ME
Katie Smith, MD, MS, Oklahoma City OK
Elizabeth Stier, MD, Boston MA
Colleen Stockdale, MD, MS, Iowa City IA
Sana Tabbara, MD, Washington DC
Deanna Teoh, MD, MS, Minneapolis MN
Elizabeth Unger, PhD, MD, Atlanta GA
Alan Waxman, MD, MPH, Albuquerque NM
Kelly Welch, North Falmouth, MA
Claudia Werner, MD, Dallas TX
Amy Wiser, MD, Portland OR
Rosemary Zuna, MD, Oklahoma City OK
Footnotes
R.B.P. and R. S. G. contributed equally to the development of this manuscript and are co-first authors.
The guidelines effort received support from the National Cancer Institute and ASCCP. Participating organizations supported travel for their participating representatives. All participating consensus organizations, including the primary funders, had equal and balanced roles in the consensus process including data analysis and interpretation, writing of manuscript, and decision to submit for publication. No industry funds were used in the development of these guidelines. The corresponding authors had final responsibility for the submission decision.
The National Cancer Institute (including M.S. and N.W.) receives cervical screening results at reduced or no cost from commercial research partners (Qiagen, Roche, BD, MobileODT, Arbor Vita) for independent evaluations of screening methods and strategies. A.-B.M. is an advisory board member of Merck and GSK. R.S.G. is an ASCCP consultant of Inovio Pharmaceuticals DSMB. W.K.H. is connected with Inovio Pharmaceuticals DSMB. P.E.C. has received HPV tests and assays at a reduced or no cost from Roche, Becton Dickinson, Arbor Vita Corporation, and Cepheid for research. M.H.E. has advised companies and participated in educational activities but does not receive any honoraria or payments for these activities, In some cases, his employer, Rutgers, receives payment for his time for these activities from Papivax, Cynvec, Merck, Hologic, and PDS Biotechnologies. He has been the overall PI or local PI for clinical trials from Johnson&Johnson, Pfizer, Iovance, and Inovio. Funding for these activities is for the research related costs of the trials. The other authors have declared they have no conflicts of interest.
Disclaimer: The conclusions, findings, and opinions expressed by authors contributing to this journal do not necessarily reflect the official position of the US Department of Health and Human Services, the Public Health Service, the Centers for Disease Control and Prevention, or the National Cancer Institute.
REFERENCES
- 1.Wright TC, Cox JT, Massad LS, et al. 2001 Consensus Guidelines for the Management of Women with Cervical Cytological Abnormalities. J Low Genit Tract Dis 2002;6:127–43. [DOI] [PubMed] [Google Scholar]
- 2.Wright TC, Massad LS, Dunton CJ, et al. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. Am J Obstet Gynecol 2007;197:346–55. [DOI] [PubMed] [Google Scholar]
- 3.Massad LS, Einstein MH, Huh WK, et al. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol 2013;121:829–46. [DOI] [PubMed] [Google Scholar]
- 4.Huh WK, Ault KA, Chelmow D, et al. Use of primary high-risk human papillomavirus testing for cervical cancer screening: interim clinical guidance. Gynecol Oncol 2015;136:178–82. [DOI] [PubMed] [Google Scholar]
- 5.Egemen D, Cheung LC, Chen X, et al. Risk estimates supporting the 2019 ASCCP Risk-Based Management Consensus Guidelines. J Low Genit Tract Dis 2020;24:132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung LC, Egemen, Chen, et al. A 2019 ASCCP Risk-Based Management Consensus Guidelines: methods for risk estimation, recommended management, and validation. J Low Genit Tract Dis 2020;24:90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drug and Therapeutics Bulletin. An introduction to patient decision aids. BMJ 2013;347:f4147–7. [DOI] [PubMed] [Google Scholar]
- 8.Castle PE, Kinney WK, Xue X, et al. Effect of Several negative rounds of human papillomavirus and cytology co-testing on safety against cervical cancer: an observational cohort study. Ann Intern Med 2018;168:20–9. [DOI] [PubMed] [Google Scholar]
- 9.Schiffman M, Kinney WK, Cheung LC, et al. Relative performance of HPV and cytology components of cotesting in cervical screening. J Natl Cancer Inst 2018;110:501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wentzensen N, Massad LS, Mayeaux EJ, et al. Evidence-based consensus recommendations for colposcopy practice for cervical cancer prevention in the United States. J Low Genit Tract Dis 2017;21:216–22. [DOI] [PubMed] [Google Scholar]
- 11.Demarco M, Cheung LC, Kinney WK, et al. Low risk of cervical cancer/precancer among most women under surveillance postcolposcopy. J Low Genit Tract Dis 2018;22:97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perkins RB, Schiffman M, Guido RS. The next generation of cervical cancer screening programs: making the case for risk-based guidelines. Curr Probl Cancer 2018;42:521–6. [DOI] [PubMed] [Google Scholar]
- 13.US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, et al. Screening for cervical cancer: US preventive services task force recommendation statement. JAMA 2018;320:674–86. [DOI] [PubMed] [Google Scholar]
- 14.Saslow D, Solomon D, Lawson HW, et al. American cancer society, american society for colposcopy and cervical pathology, and american society for clinical pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012;137:516–42. [DOI] [PubMed] [Google Scholar]
- 15.Elfgren K, Elfström KM, Naucler P, et al. Management of women with human papillomavirus persistence: long-term follow-up of a randomized clinical trial. Am J Obstet Gynecol 2017;216:264.e1–7. [DOI] [PubMed] [Google Scholar]
- 16.Aro K, Nieminen P, Louvanto K, et al. Age-specific HPV type distribution in high-grade cervical disease in screened and unvaccinated women. Gynecol Oncol 2019;154:354–9. [DOI] [PubMed] [Google Scholar]
- 17.Wentzensen N, Schiffman M, Dunn T, et al. Multiple human papillomavirus genotype infections in cervical cancer progression in the study to understand cervical cancer early endpoints and determinants. Int J Cancer 2009;125:2151–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wentzensen N, Schiffman M, Dunn ST, et al. Grading the severity of cervical neoplasia based on combined histopathology, cytopathology, and HPV genotype distribution among 1,700 women referred to colposcopy in Oklahoma. Int J Cancer 2009;124:964–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pauker SG, Kassirer JP. The Threshold Approach to Clinical Decision Making. N Engl J Med 1980;302:1109–17. [DOI] [PubMed] [Google Scholar]
- 20.Aareleid T, Pukkala E, Thomson H, et al. Cervical cancer incidence and mortality trends in Finland and Estonia: a screened vs. an unscreened population. Eur J Cancer 1993;29A:745–9. [DOI] [PubMed] [Google Scholar]
- 21.Andrae B, Andersson TM, Lambert PC, et al. Screening and cervical cancer cure: population based cohort study. BMJ 2012;344:e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ASCCP. Committee opinion: evaluation of the cervix in patients with abnormal vaginal bleeding. 2017. Available at: http://www.asccp.org. Accessed November 20, 2019.
- 23.Perkins RB, Fuzzell LN, Lake P, et al. Incorporating Stakeholder Feedback in Guidelines Development for the Management of Abnormal Cervical Cancer Screening Tests. J Low Genit Tract Dis 2020;24:167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castle PE, Kinney WK, Cheung LC, et al. Why does cervical cancer occur in a state-of-the-art screening program? Gynecol Oncol 2017;146:546–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carreon JD, Sherman ME, Guillén D, et al. CIN2 is a much less reproducible and less valid diagnosis than CIN3: results from a histological review of population-based cervical samples. Int J Gynecol Pathol 2007;26:441–6. [DOI] [PubMed] [Google Scholar]
- 26.Wentzensen N, Wilson LE, Wheeler CM, et al. Hierarchical clustering of human papillomavirus genotype patterns in the ASCUS-LSIL triage study. Cancer Res 2010;70:8578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silver MI, Gage JC, Schiffman M, et al. Clinical outcomes after conservative management of cervical intraepithelial neoplasia grade 2 (CIN2) in women ages 21-39 years. Cancer Prev Res (Phila) 2018;11:165–70. [DOI] [PubMed] [Google Scholar]
- 28.Moscicki AB, Ma Y, Wibbelsman C, et al. Rate of and risks for regression of cervical intraepithelial neoplasia 2 in adolescents and young women. Obstet Gynecol 2010;116:1373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tainio K, Athanasiou A, Tikkinen KAO, et al. Clinical course of untreated cervical intraepithelial neoplasia grade 2 under active surveillance: systematic review and meta-analysis. BMJ 2018;360:k499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalla Palma P, Giorgi Rossi P, Collina G, et al. The risk of false-positive histology according to the reason for colposcopy referral in cervical cancer screening: a blind revision of all histologic lesions found in the NTCC trial. Am J Clin Pathol 2008;129:75–80. [DOI] [PubMed] [Google Scholar]
- 31.Darragh TM, Colgan TJ, Cox JT, et al. The Lower Anogenital Squamous Terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. J Low Genit Tract Dis 2012;16:205–42. [DOI] [PubMed] [Google Scholar]
- 32.WHO Classification of Tumours of Female Reproductive Organs. Fourth Edition - WHO - OMS. Available at: https://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=70&codcch=4006. Accessed January 10, 2020. [PubMed]
- 33.Castle PE, Adcock R, Cuzick J, et al. Relationships of p16 immunohistochemistry and other biomarkers with diagnoses of cervical abnormalities: implications for LAST terminology. Arch Pathol Lab Med 2019. 10.5858/arpa.2019-0241-OA [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright TC, Stoler MH, Behrens CM, et al. The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol 2012;206:46.e1–11. [DOI] [PubMed] [Google Scholar]
- 35.Stoler MH, Wright TC, Parvu V, et al. The onclarity human papillomavirus trial: design, methods, and baseline results. Gynecol Oncol 2018;149:498–505. [DOI] [PubMed] [Google Scholar]
- 36.Gage JC, Schiffman M, Hunt WC, et al. Cervical histopathology variability among laboratories: a population-based statewide investigation. Am J Clin Pathol 2013;139:330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wheeler CM, Hunt WC, Cuzick J, et al. A population-based study of human papillomavirus genotype prevalence in the United States: baseline measures prior to mass human papillomavirus vaccination. Int J Cancer 2013;132:198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ekwueme DU, Uzunangelov VJ, Hoerger TJ, et al. Impact of the National Breast and Cervical Cancer Early Detection Program on cervical cancer mortality among uninsured low-income women in the U.S., 1991-2007. Am J Prev Med 2014;47:300–8. [DOI] [PubMed] [Google Scholar]
- 39.Cheung LC, Pan Q, Hyun N, et al. Mixture models for undiagnosed prevalent disease and interval-censored incident disease: applications to a cohort assembled from electronic health records. Stat Med 2017;36:3583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clarke MA, Darragh TM, Nelson E, et al. Reporting and assessing the quality of diagnostic accuracy studies for cervical cancer screening and management. J Low Genit Tract Dis 2020;24:157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med 2003;127:946–9. [DOI] [PubMed] [Google Scholar]
- 42.Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol 2015;136:189–97. [DOI] [PubMed] [Google Scholar]
- 43.Stoler MH, Wright TC, Jr., Sharma A, et al. High-risk human papillomavirus testing in women with ASC-US cytology: results from the ATHENA HPV study. Am J Clin Pathol 2011;135:468–75. [DOI] [PubMed] [Google Scholar]
- 44.Arbyn M, Roelens J, Simoens C, et al. Human papillomavirus testing versus repeat cytology for triage of minor cytological cervical lesions. Cochrane Database Syst Rev 2013;Mar 28;(3):CD008054. 10.1002/14651858.CD008054.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gage JC, Hunt WC, Schiffman M, et al. Similar risk patterns after cervical screening in two large U.S. populations: implications for clinical guidelines. Obstet Gynecol 2016;128:1248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCredie MRE, Sharples KJ, Paul C, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol 2008;9:425–34. [DOI] [PubMed] [Google Scholar]
- 47.SEER. Available at: http://seer.cancer.gov/statfacts/html/cervix.html#incidence-mortality. Accessed October 7, 2019.
- 48.Silver MI, Andrews J, Cooper CK, et al. Risk of cervical intraepithelial neoplasia 2 or worse by cytology, human papillomavirus 16/18, and colposcopy impression: a systematic review and meta-analysis. Obstet Gynecol 2018;132:725–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith HJ, Leath CA, Huh WK, et al. See-and-treat for high-grade cytology: do young women have different rates of high-grade histology? J Low Genit Tract Dis 2016;20:243–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuroki LM, Bergeron LM, Gao F, et al. See-and-treat loop electrosurgical excision procedure for high-grade cervical cytology: are we overtreating? J Low Genit Tract Dis 2016;20:247–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Numnum TM, Kirby TO, Leath CA, et al. A prospective evaluation of ‘see and treat’ in women with HSIL Pap smear results: is this an appropriate strategy? J Low Genit Tract Dis 2005;9:2–6. [DOI] [PubMed] [Google Scholar]
- 52.Cho H, Kim JH. Treatment of the patients with abnormal cervical cytology: a “see-and-treat” versus three-step strategy. J Gynecol Oncol 2009;20:164–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Demarco M, Egemen D, Raine-Bennett TR, et al. A Study of Partial Human Papillomavirus Genotyping in Support of the 2019 ASCCP Risk-Based Management Consensus Guidelines. J Low Genit Tract Dis 2020;24:144–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bergeron C, Ordi J, Schmidt D, et al. Conjunctive p16INK4a testing significantly increases accuracy in diagnosing high-grade cervical intraepithelial neoplasia. Am J Clin Pathol 2010;133:395–406. [DOI] [PubMed] [Google Scholar]
- 55.Stoler MH, Wright TC, Ferenczy A, et al. Routine use of adjunctive p16 immunohistochemistry improves diagnostic agreement of cervical biopsy interpretation: results from the CERTAIN study. Am J Surg Pathol 2018;42:1001–9. [DOI] [PubMed] [Google Scholar]
- 56.Thrall MJ. Effect of Lower Anogenital Squamous Terminology recommendations on the use of p16 immunohistochemistry and the proportion of high-grade diagnoses in cervical biopsy specimens. Am J Clin Pathol 2016;145:524–30. [DOI] [PubMed] [Google Scholar]
- 57.Reuschenbach M, Wentzensen N, Dijkstra MG, et al. p16INK4a immunohistochemistry in cervical biopsy specimens: a systematic review and meta-analysis of the interobserver agreement. Am J Clin Pathol 2014;142:767–72. [DOI] [PubMed] [Google Scholar]
- 58.Maniar KP, Sanchez B, Paintal A, et al. Role of the biomarker p16 in downgrading -IN 2 diagnoses and predicting higher-grade lesions. Am J Surg Pathol 2015;39:1708–18. [DOI] [PubMed] [Google Scholar]
- 59.Torres S, Wentzensen N, Stoler M, et al. Estimating the benefits and harms of p16 utilization on cervical biopsy interpretation in routine clinical practice. Under Review. [Google Scholar]
- 60.FDA Executive Summary New Approaches in the Evaluation for High-Risk Human Papillomavirus Nucleic Acid Detection Devices Prepared for the March 8, 2019 meeting of the Microbiology Devices Panel of the Medical Devices Advisory Committee. Available at: https://www.fda.gov/media/122799/download. Accessed November 2, 2019.
- 61.FDA. Meeting materials of the microbiology devices panel. FDA. Bethesda, MD; 2014. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/MicrobiologyDevicesPanel/UCM388564.pdf. Accessed November 10, 2019. [Google Scholar]
- 62.Arbyn M, Snijders PJ, Meijer CJ, et al. Which high-risk HPV assays fulfil criteria for use in primary cervical cancer screening? Clin Microbiol Infect 2015;21:817–26. [DOI] [PubMed] [Google Scholar]
- 63.Lee KR, Darragh TM, Joste NE, et al. Atypical glandular cells of undetermined significance (AGUS): interobserver reproducibility in cervical smears and corresponding thin-layer preparations. Am J Clin Pathol 2002;117:96–102. [DOI] [PubMed] [Google Scholar]
- 64.Levine L, Lucci JA, Dinh TV. Atypical glandular cells: new Bethesda Terminology and Management Guidelines. Obstet Gynecol Surv 2003;58:399–406. [DOI] [PubMed] [Google Scholar]
- 65.Zhao C, Florea A, Onisko A, et al. Histologic follow-up results in 662 patients with Pap test findings of atypical glandular cells: Results from a large academic womens hospital laboratory employing sensitive screening methods. Gynecol Oncol 2009;114:383–9. [DOI] [PubMed] [Google Scholar]
- 66.Verdoodt F, Jiang X, Williams M, et al. High-risk HPV testing in the management of atypical glandular cells: a systematic review and meta-analysis. Int J Cancer 2016;138:303–10. [DOI] [PubMed] [Google Scholar]
- 67.Katki HA, Schiffman M, Castle PE, et al. Five-year risks of CIN 3+ and cervical cancer among women with HPV-positive and HPV-negative high-grade pap results. J Low Genit Tract Dis 2013;17:S50–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davey DD, Neal MH, Wilbur DC, et al. Bethesda 2001 implementation and reporting rates: 2003 practices of participants in the College of American Pathologists Interlaboratory Comparison Program in Cervicovaginal Cytology. Arch Pathol Lab Med 2004;128:1224–9. [DOI] [PubMed] [Google Scholar]
- 69.Schnatz PF, Guile M, O'Sullivan DM, et al. Clinical significance of atypical glandular cells on cervical cytology. Obstet Gynecol 2006;107:701–8. [DOI] [PubMed] [Google Scholar]
- 70.Castle PE, Fetterman B, Poitras N, et al. Relationship of atypical glandular cell cytology, age, and human papillomavirus detection to cervical and endometrial cancer risks. Obstet Gynecol 2010;115:243–8. [DOI] [PubMed] [Google Scholar]
- 71.Wise MR, Jordan V, Lagas A, et al. Obesity and endometrial hyperplasia and cancer in premenopausal women: a systematic review. Am J Obstet Gynecol 2016;214:689.e1–17. [DOI] [PubMed] [Google Scholar]
- 72.Fontaine D, Narine N, Naugler C. Unsatisfactory rates vary between cervical cytology samples prepared using ThinPrep and SurePath platforms: a review and meta-analysis. BMJ Open 2012;2:e000847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Selvaggi SM. Factors contributing to high ThinPrep® Pap test unsatisfactory rates in an academic medical center laboratory. Diagn Cytopathol 2014;42:380–3. [DOI] [PubMed] [Google Scholar]
- 74.Randolph ML, Wu HH, Crabtree WN. Reprocessing unsatisfactory ThinPrep papanicolaou tests using a modified SurePath preparation technique. Cancer Cytopathol 2014;122:343–8. [DOI] [PubMed] [Google Scholar]
- 75.Mirzamani N, Chau K, Rafael O, et al. Quality assessment and improvement of ‘Unsatisfactory’ liquid-based cervicovaginal papanicolaou smears. Diagn Cytopathol 2017;45:873–7. [DOI] [PubMed] [Google Scholar]
- 76.Zhao L, Wentzensen N, Zhang RR, et al. Factors associated with reduced accuracy in Papanicolaou tests for patients with invasive cervical cancer. Cancer Cytopathol 2014;122:694–701. [DOI] [PubMed] [Google Scholar]
- 77.Quiroga-Garza G, Satrum LS, Trujillo CJ, et al. Common causes for unsatisfactory Pap tests in a high-risk population: insights into a yet unresolved problem in gynecologic cytology. J Am Soc Cytopathol 2014;3:256–60. [DOI] [PubMed] [Google Scholar]
- 78.Nygård JF, Sauer T, Nygård M, et al. CIN 2/3 and cervical cancer in an organised screening programme after an unsatisfactory or a normal Pap smear: a seven-year prospective study of the Norwegian population-based screening programme. J Med Screen 2004;11:70–6. [DOI] [PubMed] [Google Scholar]
- 79.Carozzi FM, Del Mistro A, Cuschieri K, et al. HPV testing for primary cervical screening: laboratory issues and evolving requirements for robust quality assurance. J Clin Virol 2016;76(suppl 1):S22–8. [DOI] [PubMed] [Google Scholar]
- 80.Preisler S, Rebolj M, Ejegod DM, et al. Cross-reactivity profiles of hybrid capture II, cobas, and APTIMA human papillomavirus assays: split-sample study. BMC Cancer 2016;16:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghofrani M, Zhao C, Davey DD, et al. Update on the College of American Pathologists experience with high-risk human papillomavirus proficiency testing for cytology. Arch Pathol Lab Med 2016;140:1371–4. [DOI] [PubMed] [Google Scholar]
- 82.College of American Pathologists Commission on Laboratory Accreditation. Laboratory Accreditation Program: Cytopathology Checklist 2017. http://www.cap.org/apps/docs/laboratory_accreditation/checklists/cytopathology_08.21.2017.pdf (behind firewall).
- 83.Sultana F, English DR, Simpson JA, et al. High-grade cervical abnormalities and cervical cancer in women following a negative Pap smear with and without an endocervical component: a cohort study with 10 years of follow-up. Int J Cancer 2014;135:1213–9. [DOI] [PubMed] [Google Scholar]
- 84.Polanco Jacome EC, Maerki J, Chau K, et al. Lack of transformation zone in cervical Pap tests, should it be a concern? A quality assurance initiative. Diagn Cytopathol 2018;46:584–8. [DOI] [PubMed] [Google Scholar]
- 85.Hastings JW, Alston MJ, Mazzoni SE, et al. Frequency of adequate endometrial biopsy in evaluation of postmenopausal women with benign endometrial cells on Pap test. J Low Genit Tract Dis 2017;21:258–60. [DOI] [PubMed] [Google Scholar]
- 86.Moyer AB, El-Zaatari ZM, Thrall MJ. The effects of the Bethesda System 2014 on endometrial cell reporting and follow-up endometrial biopsies in women 45 years of age and over. J Am Soc Cytopathol 2018;7:201–4. [DOI] [PubMed] [Google Scholar]
- 87.Fischer G, Haddad M, Cormier K. Endometrial cells on Pap tests: ideal reporting is more complex than just finding the right age. Diagn Cytopathol 2017;45:587–91. [DOI] [PubMed] [Google Scholar]
- 88.Grada Z, Paquette C, Eklund CM, et al. Evaluating the age cutoff criterion for reporting benign-appearing endometrial cells in routine pap tests: an 8-year retrospective review. Acta Cytol 2017;61:194–8. [DOI] [PubMed] [Google Scholar]
- 89.Colletti SM, Tranesh GA, Nassar A. Significance of finding benign endometrial cells in women 40-45 versus 46 years or older on Papanicolaou tests and histologic follow-up. Cytojournal 2017;14:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Weiss VL, Cate F, Bloom L, et al. Age cut-off for reporting endometrial cells on a Papanicolaou test: 50 years may be more appropriate than 45 years. Cytopathology 2016;27:242–8. [DOI] [PubMed] [Google Scholar]
- 91.Izadi-Mood N, Sarmadi S, Sanii S, et al. Normal-appearing endometrial cells in Pap tests of women aged forty years or older and cytohistological correlates. Acta Cytol 2015;59:175–9. [DOI] [PubMed] [Google Scholar]
- 92.Kir G, Gocmen A, Cetiner H, et al. Clinical significance of benign endometrial cells found in papanicolaou tests of Turkish women aged 40 years and older. J Cytol 2013;30:156–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ramdall RB, Wallach RC, Cangiarella J, et al. Origin, frequency and clinical significance of glandular cells in liquid-based pap tests from patients posthysterectomy. Acta Cytol 2009;53:1–9. [DOI] [PubMed] [Google Scholar]
- 94.Wentzensen N, Schiffman M, Silver MI, et al. ASCCP Colposcopy Standards: risk-based colposcopy practice. J Low Genit Tract Dis 2017;21:230–4. [DOI] [PubMed] [Google Scholar]
- 95.Kurman RJ, Schiffman MH, Lancaster WD, et al. Analysis of individual cervical human papillomavirus types in neolasia: a possible role for type 18 in rapid progression. Am J Obstet Gynecol 1988;159:293–6. [DOI] [PubMed] [Google Scholar]
- 96.Clifford G, Franceschi S, Diaz M, et al. Chapter 3: HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine 2006;24(suppl 3):S26–34. [DOI] [PubMed] [Google Scholar]
- 97.WHO Guidelines: Use of Cryotherapy for Cervical Intraepithelial Neoplasia. Geneva: World Health Organization; 2011. Available at: http://www.ncbi.nlm.nih.gov/books/NBK138476/. Accessed January 13, 2020. [PubMed] [Google Scholar]
- 98.WHO guidelines for the use of thermal ablation for cervical pre-cancer lesions. Available at: https://apps.who.int/iris/handle/10665/329299. Accessed January 13, 2020. [PubMed]
- 99.D'Alessandro P, Arduino B, Borgo M, et al. Loop electrosurgical excision procedure versus cryotherapy in the treatment of cervical intraepithelial neoplasia: a systematic review and meta-analysis of randomized controlled trials. Gynecol Minim Invasive Ther 2018;7:145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Santesso N, Mustafa RA, Wiercioch W, et al. Systematic reviews and meta-analyses of benefits and harms of cryotherapy, LEEP, and cold knife conization to treat cervical intraepithelial neoplasia. Int J Gynecol Obstet 2016;132:266–71. [DOI] [PubMed] [Google Scholar]
- 101.Martin-Hirsch PPL, Paraskevaidis E, Bryant A, et al. Surgery for cervical intraepithelial neoplasia. Cochrane Database Syst Rev 2013;CD001318. [DOI] [PubMed] [Google Scholar]
- 102.Kalogirou D, Antoniou G, Karakitsos P, et al. Predictive factors used to justify hysterectomy after loop conization: increasing age and severity of disease. Eur J Gynaecol Oncol 1997;18:113–6. [PubMed] [Google Scholar]
- 103.Arbyn M, Redman CWE, Verdoodt F, et al. Incomplete excision of cervical precancer as a predictor of treatment failure: a systematic review and meta-analysis. Lancet Oncol 2017;18:1665–79. [DOI] [PubMed] [Google Scholar]
- 104.Kyrgiou M, Athanasiou A, Paraskevaidi M, et al. Adverse obstetric outcomes after local treatment for cervical preinvasive and early invasive disease according to cone depth: systematic review and meta-analysis. BMJ 2016;354:i3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kyrgiou M, Koliopoulos G, Martin-Hirsch P, et al. Obstetric outcomes after conservative treatment for intraepithelial or early invasive cervical lesions: systematic review and meta-analysis. Lancet 2006;367:489–98. [DOI] [PubMed] [Google Scholar]
- 106.Reilly R, Paranjothy S, Beer H, et al. Birth outcomes following treatment for precancerous changes to the cervix: a population-based record linkage study: birth outcomes following colposcopy treatments. BJOG 2012;119:236–44. [DOI] [PubMed] [Google Scholar]
- 107.Bruinsma F, Quinn M. The risk of preterm birth following treatment for precancerous changes in the cervix: a systematic review and meta-analysis: cervical dysplasia and preterm birth: a meta-analysis. BJOG 2011;118:1031–41. [DOI] [PubMed] [Google Scholar]
- 108.Bjørge T, Skare GB, Bjørge L, et al. Adverse pregnancy outcomes after treatment for cervical intraepithelial neoplasia. Obstet Gynecol 2016;128:1265–73. [DOI] [PubMed] [Google Scholar]
- 109.Miller ES, Sakowicz A, Grobman WA. The association between cervical dysplasia, a short cervix, and preterm birth. Am J Obstet Gynecol 2015;213:543.e1–4. [DOI] [PubMed] [Google Scholar]
- 110.Bruinsma F, Lumley J, Tan J, et al. Precancerous changes in the cervix and risk of subsequent preterm birth. BJOG 2006;114:70–80. [DOI] [PubMed] [Google Scholar]
- 111.Shanbhag S, Clark H, Timmaraju V, et al. Pregnancy outcome after treatment for cervical intraepithelial neoplasia. Obstet Gynecol 2009;114:727–35. [DOI] [PubMed] [Google Scholar]
- 112.Castanon A, Brocklehurst P, Evans H, et al. Risk of preterm birth after treatment for cervical intraepithelial neoplasia among women attending colposcopy in England: retrospective-prospective cohort study. BMJ 2012;345:e5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Werner CL, Lo JY, Heffernan T, et al. Loop electrosurgical excision procedure and risk of preterm birth. Obstet Gynecol 2010;115:605–8. [DOI] [PubMed] [Google Scholar]
- 114.Weinmann S, Naleway A, Swamy G, et al. Pregnancy outcomes after treatment for cervical cancer precursor lesions: an observational study. PLoS One 2017;12:e0165276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fukuchi E, Fetterman B, Poitras N, et al. Risk of cervical precancer and cancer in women with cervical intraepithelial neoplasia grade 1 on endocervical curettage. J Low Genit Tract Dis 2013;17:255–60. [DOI] [PubMed] [Google Scholar]
- 116.Lueng SOA, Vitonis A, Feldman S. Yield of loop electrosurgical excision procedure (LEEP) among patients with and without known high-grade cervical dysplasia. Soc Gynecol Oncologists 2020. [Google Scholar]
- 117.Teoh D, Musa F, Salani R, et al. Diagnosis and management of adenocarcinoma in situ: a society of gynecologic oncology evidence-based review and recommendations. Obstet Gynecol 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang SS, Sherman ME, Hildesheim A, et al. Cervical adenocarcinoma and squamous cell carcinoma incidence trends among white women and black women in the United States for 1976-2000. Cancer 2004;100:1035–44. [DOI] [PubMed] [Google Scholar]
- 119.Salani R, Puri I, Bristow RE. Adenocarcinoma in situ of the uterine cervix: a metaanalysis of 1278 patients evaluating the predictive value of conization margin status. Am J Obstet Gynecol 2009;200:182.e1–5. [DOI] [PubMed] [Google Scholar]
- 120.Lea JS, Shin CH, Sheets EE, et al. Endocervical curettage at conization to predict residual cervical adenocarcinoma in situ. Gynecol Oncol 2002;87:129–32. [DOI] [PubMed] [Google Scholar]
- 121.Costa S, Venturoli S, Negri G, et al. Factors predicting the outcome of conservatively treated adenocarcinoma in situ of the uterine cervix: an analysis of 166 cases. Gynecol Oncol 2012;124:490–5. [DOI] [PubMed] [Google Scholar]
- 122.Costa S, Venturoli S, Origoni M, et al. Performance of HPV DNA testing in the follow-up after treatment of high-grade cervical lesions, adenocarcinoma in situ (AIS) and microinvasive carcinoma. Ecancermedicalscience 2015;9:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Strander B, Andersson-Ellström A, Milsom I, et al. Long term risk of invasive cancer after treatment for cervical intraepithelial neoplasia grade 3: population based cohort study. BMJ 2007;335:1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kocken M, Uijterwaal MH, de Vries ALM, et al. High-risk human papillomavirus testing versus cytology in predicting post-treatment disease in women treated for high-grade cervical disease: a systematic review and meta-analysis. Gynecol Oncol 2012;125:500–7. [DOI] [PubMed] [Google Scholar]
- 125.Arbyn M, Ronco G, Anttila A, et al. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 2012;30(suppl 5):F88–99. [DOI] [PubMed] [Google Scholar]
- 126.Clarke M, Unger ER, Zuna R, et al. A systematic review of tests for post-colposcopy and post-treatment surveillance. J Low Genit Tract Dis 2020;24:148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cuschieri K, Bhatia R, Cruickshank M, et al. HPV testing in the context of post-treatment follow up (test of cure). J Clin Virol 2016;76(suppl 1):S56–61. [DOI] [PubMed] [Google Scholar]
- 128.Melnikow J, McGahan C, Sawaya GF, et al. Cervical intraepithelial neoplasia outcomes after treatment: long-term follow-up from the British Columbia Cohort Study. J Natl Cancer Inst 2009;101:721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kalliala I, Anttila A, Pukkala E, et al. Risk of cervical and other cancers after treatment of cervical intraepithelial neoplasia: retrospective cohort study. BMJ 2005;331:1183–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Williams WW, Lu P-J, O'Halloran A, et al. Surveillance of vaccination coverage among adult populations - United States, 2015. MMWR Surveill Summ 2017;66:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Benard VB, Watson M, Castle PE, et al. Cervical carcinoma rates among young females in the United States. Obstet Gynecol 2012;120:1117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Loopik DL, Doucette S, Bekkers RLM, et al. Regression and progression predictors of CIN2 in women younger than 25 years. J Low Genit Tract Dis 2016;20:213–7. [DOI] [PubMed] [Google Scholar]
- 133.Lee MH, Finlayson SJ, Gukova K, et al. Outcomes of conservative management of high grade squamous intraepithelial lesions in young women. J Low Genit Tract Dis 2018;22:212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.He Y, Wu YM, Wang T, et al. Perinatal outcomes of pregnant women with cervical intraepithelial neoplasia. Arch Gynecol Obstet 2013;288:1237–42. [DOI] [PubMed] [Google Scholar]
- 135.Kärrberg C, Brännström M, Strander B, et al. Colposcopically directed cervical biopsy during pregnancy; minor surgical and obstetrical complications and high rates of persistence and regression. Acta Obstet Gynecol Scand 2013;92:692–9. [DOI] [PubMed] [Google Scholar]
- 136.Mailath-Pokorny M, Schwameis R, Grimm C, et al. Natural history of cervical intraepithelial neoplasia in pregnancy: postpartum histo-pathologic outcome and review of the literature. BMC Pregnancy Childbirth 2016;16:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schaefer K, Peters D, Aulmann S, et al. Value and feasibility of LLETZ procedures for pregnant women with suspected high-grade squamous intraepithelial lesions and microinvasive cervical cancer. Int J Gynecol Obstet 2012;118:141–4. [DOI] [PubMed] [Google Scholar]
- 138.Schuster S, Joura E, Kohlberger P. Natural history of squamous intraepithelial lesions in pregnancy and mode of delivery. Anticancer Res 2018;38:2439–42. [DOI] [PubMed] [Google Scholar]
- 139.Wu YM, Wang T, He Y, et al. Clinical management of cervical intraepithelial neoplasia in pregnant and postpartum women. Arch Gynecol Obstet 2014;289:1071–7. [DOI] [PubMed] [Google Scholar]
- 140.McMorrow, Stacey and Kenney, Genevieve. Despite progress under the ACA, many new mothers lack insurance coverage. 2018. Available at: https://www.healthaffairs.org/do/10.1377/hblog20180917.317923/full/. Accessed November 22, 2019.
- 141.Downs LS, Smith JS, Scarinci I, et al. The disparity of cervical cancer in diverse populations. Gynecol Oncol 2008;109:S22–30. [DOI] [PubMed] [Google Scholar]
- 142.Moscicki AB, Flowers L, Huchko MJ, et al. Guidelines for Cervical Cancer Screening in Immunosuppressed Women Without HIV Infection. J Low Genit Tract Dis 2019;23:87–101. [DOI] [PubMed] [Google Scholar]
- 143.Chin-Hong PV, Reid GE, AST Infectious Diseases Community of Practice. Human papillomavirus infection in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transplant 2019;33:e13590. [DOI] [PubMed] [Google Scholar]
- 144.US Department of Health and Human Services. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV. 2018. Available at: https://aidsinfo.nih.gov/guidelines/html/4/adult-and-adolescent-opportunistic-infection/343/human-papillomavirus. Accessed November 25, 2019.
- 145.Robbins HA, Strickler HD, Massad LS, et al. Cervical cancer screening intervals and management for women living with HIV: a risk benchmarking approach. AIDS 2017;31:1035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Khan MJ, Massad LS, Kinney W, et al. A common clinical dilemma: management of abnormal vaginal cytology and human papillomavirus test results. Gynecol Oncol 2016;141:364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schockaert S, Poppe W, Arbyn M, et al. Incidence of vaginal intraepithelial neoplasia after hysterectomy for cervical intraepithelial neoplasia: a retrospective study. Am J Obstet Gynecol 2008;199:113.e1–5. [DOI] [PubMed] [Google Scholar]
- 148.Cong Q, Song Y, Wang Q, et al. A retrospective study of cytology, high-risk HPV, and colposcopy results of vaginal intraepithelial neoplasia patients. Biomed Res Int 2018;2018:5894801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.USPSTF. Screening for Cervical Cancer. Available at: http://www.ahcpr.gov/clinic/uspstf/uspscerv.htm 2003. Accessed April 10, 2012.
- 150.Gravitt PE, Landy R, Schiffman M. How confident can we be in the current guidelines for exiting cervical screening? Prev Med 2018;114:188–92. [DOI] [PubMed] [Google Scholar]
- 151.Feldman S, Cook E, Davis M, et al. Cervical cancer incidence among elderly women in massachusetts compared with younger women. J Low Genit Tract Dis 2018;22:314–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gage JC, Katki HA, Schiffman M, et al. The low risk of precancer after a screening result of human papillomavirus-negative/atypical squamous cells of undetermined significance papanicolaou and implications for clinical management: low precancer risk after HPV-negative/ASC-US. Cancer Cytopathol 2014;122:842–50. [DOI] [PubMed] [Google Scholar]
- 153.Rosenblatt KA, Osterbur EF, Douglas JA. Case-control study of cervical cancer and gynecologic screening: a SEER-Medicare analysis. Gynecol Oncol 2016;142:395–400. [DOI] [PubMed] [Google Scholar]
- 154.Siu AL, U.S. Preventive Services Task Force. Screening for breast cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016;164:279–96. [DOI] [PubMed] [Google Scholar]
- 155.American Cancer Society. American Cancer Society Guideline for Colorectal Cancer Screening. 2018. Available at: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/acs-recommendations.html. Accessed November 17, 2019.
- 156.US Preventive Services Task Force, Bibbins-Domingo K, Grossman DC, et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016;315:2564–75. [DOI] [PubMed] [Google Scholar]
- 157.Mayeux EJ, Cox JT. Modern Colposcopy Textbook and Atlas - New Edition - ASCCP. 3rd ed. Rockville, MD: ASCCP; 2011. [Google Scholar]
- 158.Castle PE, Xie X, Xue X, et al. Impact of human papillomavirus vaccination on the clinical meaning of cervical screening results. Prev Med 2019;118:44–50. [DOI] [PubMed] [Google Scholar]
- 159.Gertig DM, Brotherton JM, Budd AC, et al. Impact of a population-based HPV vaccination program on cervical abnormalities: a data linkage study. BMC Med 2013;11:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Drolet M, Benard E, Boily MC, et al. Population-level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis 2015;15:565–80. [DOI] [PMC free article] [PubMed] [Google Scholar]


