Abstract
Autism spectrum disorders (ASDs) are neurodevelopmental disorders associated with atypical brain connectivity. Although language abilities vary widely, they are impaired or atypical in most children with ASDs. Underlying brain mechanisms, however, are not fully understood. The present study examined intrinsic functional connectivity (iFC) of the extended language network in a cohort of 52 children and adolescents with ASDs (ages 8–18 years), using resting-state functional magnetic resonance imaging. We found that, in comparison to typically developing peers (n = 50), children with ASDs showed increased connectivity between some language regions. In addition, seed-to-whole brain analyses revealed increased connectivity of language regions with posterior cingulate cortex (PCC) and visual regions in the ASD group. Post hoc effective connectivity analyses revealed a mediation effect of PCC on the iFC between bilateral inferior frontal and visual regions in an ASD subgroup. This finding qualifies and expands on previous reports of recruitment of visual areas in language processing in ASDs. In addition, increased iFC between PCC and visual regions was linked to lower language scores in this ASD subgroup, suggesting that increased connectivity with visual cortices, mediated by default mode regions, may be detrimental to language abilities.
Keywords: autism spectrum disorders, default mode, language, resting-state functional magnetic resonance imaging, visual cortex
Lay Summary
We examined the functional connectivity between regions of the language network in children with autism spectrum disorders (ASDs) compared to typically developing peers. We found connectivity to be intact between core language in the ASD group, but also showed abnormally increased connectivity between regions of an extended language network. Additionally, connectivity was increased with regions associated with brain networks responsible for self-reflection and visual processing.
Introduction
Autism spectrum disorders (ASDs) are neurodevelopmental disorders with high and increasing prevalence, recently estimated at 1 out of 45 children in the United States [Zablotsky, Black, Maenner, Schieve, & Blumberg, 2015]. Closely related to core symptoms in the sociocommunicative domain is language impairment. Linguistic ability serves as one of the best predictors for ASD diagnoses and functional outcome [Lombardo et al., 2015; Szatmari et al., 2015]. This highlights the importance of understanding the neurological bases of language processing in ASDs and their relation to behavioral symptomatology.
Up to 25% of children who receive ASD diagnoses never develop functional verbal language skills [Luyster, Kadlec, Carter, & Tager-Flusberg, 2008; Tager-Flusberg, Paul, & Lord, 2013]. Among those who acquire functional language, the age at which first words are spoken is on average delayed by 12–18 months compared to typically developing (TD) children [Howlin, 2003], and a wide range of verbal abilities can be observed later in life. Some individuals exhibit severe problems, such as repetitive neologisms and echolalia [speech parroting; Eigsti, de Marchena, Schuh, & Kelley, 2011]. However, even highly verbal individuals with ASDs may find some aspects of communication challenging, such as pragmatic language skills (e.g., turn taking), nonverbal communication (e.g., gesturing, facial expression), and prosody [Eigsti et al., 2011]. In a review of structural language characteristics of ASDs, Boucher [2012] suggested that the ASD language profile is highly heterogeneous and that verbal individuals display more impairment in receptive than expressive language.
Early evidence on the neural basis of language came from the study of brain lesions [Broca, 1861]. This led to the identification of two language areas with gross functional characterization: Broca’s area (left inferior frontal gyrus) for speech production, and Wernicke’s area (left posterior superior temporal cortex) for comprehension [Price, 2000]. Connected by the arcuate fasciculus [Catani, Jones, & ffytche, 2005], these regions constitute the language network in the traditional neurological model [Geschwind, 1970], although the precise anatomical location of these regions has been questioned [Mesulam, Thompson, Weintraub, & Rogalski, 2015]. Recent neuroimaging studies have shed light on a more extensive language system that includes the dorsal striatum, insula, precuneus, inferior parietal lobule (IPL), and cerebellum in addition to the classic language regions [Berl et al., 2014; Price, 2010; Rodd, Vitello, Woollams, & Adank, 2015]. Concordant with lesion findings, imaging studies have provided further support that language function is mostly lateralized to the left hemisphere in right-handed TD individuals [McAvoy et al., 2016; Nielsen, Zielinski, Ferguson, Lainhart, & Anderson, 2013].
Neuroimaging has also contributed to the understanding of biological bases of language impairments in ASDs over the past decades. Among anatomical studies, Herbert et al. [2002] found atypical asymmetries in frontal and temporal language regions in boys with ASDs. Others have reported white matter anomalies including decreased volume of the arcuate fasciculus [Moseley et al., 2016] in adults with ASDs. One study reported evidence of potential white matter compromise (increased mean diffusion) in the left superior longitudinal fasciculus, detected only in ASD individuals with language impairments [Nagae et al., 2012]. Another study [Peeva et al., 2013] found weaker structural connectivity (number of streamlines) between areas involved in speech production (left ventral premotor cortex and left supplementary motor area) in ASD adults with average language abilities.
Functional imaging studies have revealed further anomalies of language processing in ASDs, including recruitment of visual regions during language tasks [Gaffrey et al., 2007; Kana, Keller, Cherkassky, Minshew, & Just, 2006; Knaus, Silver, Lindgren, Hadjikhani, & Tager-Flusberg, 2008; Pang et al., 2016], increased activation in homologous language regions of the right hemisphere [Anderson et al., 2010; Eyler, Pierce,&Courchesne,2012;Groenetal.,2010;Herringshaw, Ammons, DeRamus, & Kana, 2016; Kleinhans, Müller, Cohen, & Courchesne, 2008; Knaus et al., 2010; Müller et al., 1999; Nielsen et al., 2014; Williams, Goldstein, & Minshew, 2006], and reduced connectivity of left inferior frontal cortex and right cerebellum with other language regions [Verly etal.,2014].
In the past decade, there has been increasing awareness that symptomatology and cognitive-behavioral impairments in ASDs require explanation at the level of distributed neural networks [Geschwind & Levitt, 2007; Müller, 2007; Rippon, Brock, Brown, & Boucher, 2007]. A method of choice in the study of functional network organization is functional connectivity (FC) MRI. FC is inferred from synchronized low frequency (<0.1 Hz) blood-oxygen level dependent (BOLD) signal fluctuations and can be measured during rest, referred to as intrinsic functional connectivity (iFC). iFC has been used to examine the language network in healthy adults, using traditional perisylvian regions (Broca’s and Wernicke’s area) as seeds [Tomasi & Volkow, 2012; Zhu et al., 2014]. Findings from these studies have shown extensive short-range left-lateralized FC for both seeds with more long-range bilateral connectivity for posterior Wernicke’s area.
The few available FC MRI studies of the language network in ASDs have generated conflicting findings. Taskbased studies reported reduced FC of the language network in ASDs [Just, Cherkassky, Keller, & Minshew, 2004; Kana et al., 2006; Knaus et al., 2008]. One small-sample iFC study found decreased language network iFC in ASD children with language impairment [Verly et al., 2014], whereas others (using iFC methods) observed mixed effects [Lee, Park, James, Kim, & Park, 2017] or even extensive overconnectivity with regions outside canonical language networks, including visual cortices [Shen et al., 2012] in individuals with ASD with and without comorbid language impairment. While methodological differences between co-activation FC and iFC may account for some inconsistencies [Müller et al., 2011; Nair et al., 2014], the evidence from previous, mostly small-sample studies (including ≤20 participants per group) remains overall inconclusive.
The present study investigated iFC of a comprehensive language network in children and adolescents with ASDs and their TD peers. We hypothesized that in ASD participants (a) iFC of the language network would be partially increased (compared to TD peers), within and outside the network, including visual cortex; (b) iFC of language regions would be less left lateralized; and (c) altered connectivity would be related to language abilities and symptom severity.
Methods
Participants
A total of 163 participants, ages 8–18 years, were recruited from the community and through ongoing collaborations with local clinicians. TD participants had no family history of ASDs or any other neurological, developmental, or psychiatric disorder. For the ASD group, only individuals with idiopathic ASDs were recruited (i.e., excluding any syndromic forms such as Fragile X or Rett syndrome). ASD diagnoses based on DSM-5 [American Psychiatric Association, 2013] criteria were confirmed using the Autism Diagnostic Interview-Revised [ADI-R; Rutter, Le Couteur, & Lord, 2003], the Autism Diagnostic Observation Schedule [ADOS or ADOS-2; Lord, Rutter, DiLavore, & Risi, 2001; Lord et al., 2012], and expert clinical judgment (coauthor IF). Participants were tested on IQ using the Wechsler Abbreviated Scale of Intelligence [Wechsler, 1999], handedness using the Edinburgh Handedness Inventory [Oldfield, 1971], and language abilities using the Clinical Evaluation of Language Fundamentals [CELF-4; Semel, Wiig, & Secord, 2004]. Groups were matched on age, nonverbal IQ, head motion, handedness, gender, and handedness by gender (Table 1). The study protocol was approved by the Institutional Review Boards of San Diego State University and University of California San Diego. Assent and informed consent were obtained from all participants and their caregivers.
Table 1.
Participant Information
| ASD (n = 52) | TD (n = 50) | ||||
|---|---|---|---|---|---|
| Gender | 8 female | 8 female | |||
| Handedness | 7 left (0 female) | 7 left (0 female) | |||
| Mean (SD) | Range | Mean (SD) | Range | T, P-value | |
| Age in years | 13.7 (2.6) | 9.2–18.0 | 13.6 (2.6) | 8.0–17.6 | 0.29, P = 0.77 |
| Head motion | |||||
| RMSD precensoring | 0.065 (0.030) | 0.019–0.148 | 0.064 (0.032) | 0.017–0.148 | −0.20, P = 0.84 |
| RMSD after denoising | 0.003 (0.002) | 0.001–0.008 | 0.003 (0.003) | 0.001–0.014 | −0.94, P = 0.34 |
| Postcensoring TP | 178 (4.2) | 158–180 | 177 (3.9) | 166–180 | 0.25, P = 0.80 |
| WASI | |||||
| Verbal IQ | 102 (17.1) | 70–147 | 108 (9.1) | 87–126 | −2.14, P = 0.04 |
| Nonverbal IQ | 106 (17.2) | 53–140 | 105 (13.3) | 62–137 | 0.34, P = 0.73 |
| Full-scale IQ | 104 (16.4) | 66–141 | 107 (11.0) | 79–130 | −1.12, P = 0.26 |
| CELF-4a | |||||
| Core language | 99 (17.5) | 56–120 | 110 (9.1) | 91–126 | −3.18, P = 0.00 |
| Receptive | 99 (16.1) | 60–131 | 104 (11.2) | 76–127 | −1.22, P = 0.23 |
| Expressive | 97 (17.1) | 55–120 | 107 (8.9) | 91–124 | −2.96, P = 0.00 |
| ADOS-2b | |||||
| Social affect | 10.2 (3.7) | 5–20 | – | – | |
| Repetitive behavior | 3.4 (1.7) | 0–8 | – | – | |
| Total | 13.5 (4.2) | 5–24 | – | – | |
| Severity | 7.5 (1.9) | 3–10 | – | – | |
| ADI-R | – | – | |||
| Social interaction | 18.4 (4.9) | 7–28 | – | – | |
| Communication | 13.4 (5.1) | 2–24 | – | – | |
| Repetitive behavior | 6.1 (2.3) | 1–12 | – | – | |
Abbreviation: TP, time-points (180 total time-points before censoring).
CELF-4 scores were not available for 11 ASD and 12 TD participants.
38 ASD participants were assessed with Module 3 and 14 participants were assessed with Module 4 of the ADOS.
After enrollment, 61 participants were excluded based on demographic or diagnostic information, or image quality. Four recruits for the ASD group did not meet full diagnostic criteria, while two TD participants were excluded for meeting diagnostic criteria for ADHD. Participants were also excluded based on unusual neuroanatomical findings (three ASD, one TD), presence of seizures or history of in utero drug exposure (two ASD), siblings with neurological conditions (two TD), excessive drowsiness during the scan (one TD), or excessive motion during MRI scanning (26 ASD, 10 TD; see below). Ten subjects (five ASD, five TD) were removed to optimally match ASD and TD groups for age, sex, handedness, nonverbal IQ, head motion, and handedness by gender (Table 1). The final sample included 52 ASD and 50 TD participants.
Data Acquisition
Imaging data were acquired on a General Electric 3T Discovery MR750 scanner with an 8-channel head coil at the University of California San Diego Center for Functional Magnetic Resonance Imaging. High-resolution structural images were collected using a standard Fast Spoiled Gradient-Echo T1-weighted sequence (172 slices; repetition time [TR] = 8.136; echo time [TE] = 3.172 msec; field of view [FOV] = 256 × 256 mm; flip angle = 8; 1 mm3 resolution). Functional T2*-weighted images were obtained using a single-shot gradient-recalled, echo-planar pulse sequence of 180 whole-brain volumes (TR = 2,000 msec; TE = 30 msec; FOV = 220 mm; flip angle = 90, 64 × 64 matrix, 3.4 mm3 resolution, 42 axial slices covering the whole brain). During the 6-min resting-state scan, participants were shown a white crosshair centered on a black background and instructed: “Keep your eyes on the cross, relax, but please stay as still as you can. Do not fall asleep.” In-bore MRI-compatible video monitoring was used to verify compliance with instructions and wakefulness.
Functional Magnetic Resonance Imaging Data Preprocessing
Functional MRI data were preprocessed and analyzed using Analysis of Functional NeuroImages software [AFNI 16.2.13; Cox, 1996]. We used a standard pipeline to unwarp, field map correct, slice timing correct, motion correct, and spatially smooth the image using a Gaussian kernel of 6 mm FWHM. FMRI software library [FSL; Smith et al. 2004] was used to normalize structural images to MNI-152 template space and segment structural images into white matter, gray matter, and cerebrospinal fluid. The segmented white matter and CSF maps were eroded by 1 voxel. Functional images were co-registered to the preprocessed structural image and transformed to 3 mm isotropic resolution. Functional time-series were bandpass filtered at 0.008–0.08 Hz using a Butterworth filter. Rootmean-squared-difference (RMSD) was calculated from six motion parameters (three translational and three rotational) to estimate in-scanner head motion. The six motion parameters and time-series from white matter and CSF, as well as their first temporal derivatives were equally bandpass filtered [Hallquist, Hwang, & Luna, 2013] and used as nuisance regressors in AFNI’s 3dDeconvolve to remove motion and noise from the functional signal.
Primary analyses were performed without global signal regression (GSR), a processing step that remains contentious. While GSR is recognized for its strengths in denoising fMRI time-series [Power, Plitt, Laumann, & Martin, 2017], it is also known to generate anticorrelations that may not be biologically meaningful [Schölvinck, Maier, Ye, Duyn, & Leopold, 2010] and has been found to distort group differences in some studies [Abbott et al., 2016; Gotts et al., 2013; Saad et al., 2012]. However, analyses including GSR are additionally presented in Supplementary Fig. 1 and Supplementary Tables 1 and 2).
Time-points with framewise displacement >0.5 mm were censored including two subsequent time-points. Blocks of time-series between censored time-points with <10 time-points remaining were also removed. All included participants retained more than 80% of their original time-points. Groups were tightly matched for head motion RMSD (Table 1).
FC Analyses
The language network was defined by regions adopted from an Activation Likelihood Estimation (ALE) analysis of 54 functional neuroimaging studies of language comprehension in both spoken and written modalities [Rodd et al., 2015]. Statistical maps from the ALE combining all 54 studies were obtained from the authors and thresholded to reduce differences in cluster volumes (to a range of 32–101 voxels). This produced a total of 14 distinct clusters used as regions of interest (ROIs) or seeds (Table 2, Supplementary Fig. 2).
Table 2.
Regions of Interest
| Cluster | Anatomic label | Abbreviation | Voxels | CM x | CM y | CM z |
|---|---|---|---|---|---|---|
| 1 | Left inferior frontal gyrusa | lIFG | 101 | 52 | 14 | 17 |
| 2 | Left superior temporal sulcus | lSTS | 100 | 56 | −28 | 2 |
| 3 | Bilateral dorsal precuneus | bdPrec | 99 | 0 | −67 | 44 |
| 4 | Bilateral supplementary motor area | bSMA | 91 | 2 | 16 | 52 |
| 5 | Right insula | rInsula | 82 | −37 | 23 | 6 |
| 6 | Left inferior parietal lobule | lIPL | 94 | 38 | −50 | 46 |
| 7 | Right inferior frontal sulcusa | rIFS | 91 | −43 | 17 | 30 |
| 8 | Right Heschl’s gyrus | rHG | 72 | −42 | −19 | 6 |
| 9 | Right inferior parietal lobulea | rIPL | 70 | −45 | −60 | 37 |
| 10 | Left pericentral regiona | lPC | 65 | 39 | −23 | 54 |
| 11 | Right superior temporal sulcus | rSTS | 57 | −55 | −28 | −1 |
| 12 | Left precuneus/posterior cingulate cortexa | lPrec/PCC | 44 | 5 | −64 | 24 |
| 13 | Left angular gyrus | lAG | 34 | 47 | −64 | 34 |
| 14 | Left supramarginal gyrus | lSMarG | 32 | 59 | −34 | 37 |
Regions of interests (ROI) are derived from statistical maps taken from an Activation Likelihood Estimate analysis of 54 language comprehension studies [Rodd et al., 2015]. All coordinates are listed in MNI space.
Abbreviation: CM, center of mass of each cluster.
ROIs used for whole-brain iFC analyses.
To measure within-network connectivity, average time-series data were extracted from each of the 14 ROIs in each participant and correlated with time-series from every other ROI, using Pearson’s correlation, resulting in a 14 × 14 language network connectivity matrix. The resulting coefficients were then normalized using Fisher z-transformation and entered into one-sample t-tests to examine iFC within ASD and TD groups, separately, and two-tailed t-tests to identify group differences. The results were adjusted for multiple comparisons using local false discovery rate (FDR)-correction [Efron, 2007]. Local FDR was preferred to traditional FDR due to nonuniform distribution of the obtained P-values.
Laterality of iFC in the extended language network was examined through two separate analyses. First, ipsilateral connectivity was computed by averaging each ROI–ROI pair within the same hemisphere. Next, laterality indices were calculated for each participant using the following equation: (Left – Right)/(Left + Right). Positive laterality indices signified leftward asymmetry, negative indices signified rightward asymmetry. The laterality indices were then compared between groups. In a second analysis, contralateral iFC was examined by averaging between-hemisphere ROI–ROI connections (e.g., left inferior frontal gyrus and right superior temporal sulcus) and compared between groups.
Connectivity outside the language network was examined using whole-brain iFC analyses, by correlating average time-series from each ROI with time-series from all other gray matter voxels. In an effort to reduce the number of comparisons, only ROIs that showed group differences in the within-network analyses were used for whole-brain iFC analyses (Fig. 1B,C, Table 2). The resulting whole-brain iFC maps were directly compared between groups and corrected for multiple comparisons using AFNI’s 3dttest++ -Clustsim permutation tests [Cox, Chen, Glen, Reynolds, & Taylor, 2017]. For each group, mean zscores extracted from significant clusters of between-group effects were correlated with CELF-4 Core Language, Receptive Language, and Expressive Language scores, as well as ADI-R Social and Communication scores, ADOS Communication and Social Interaction, and ADOS Total scores, while controlling for effects of age and in-scanner head motion. Results were corrected using FDR.
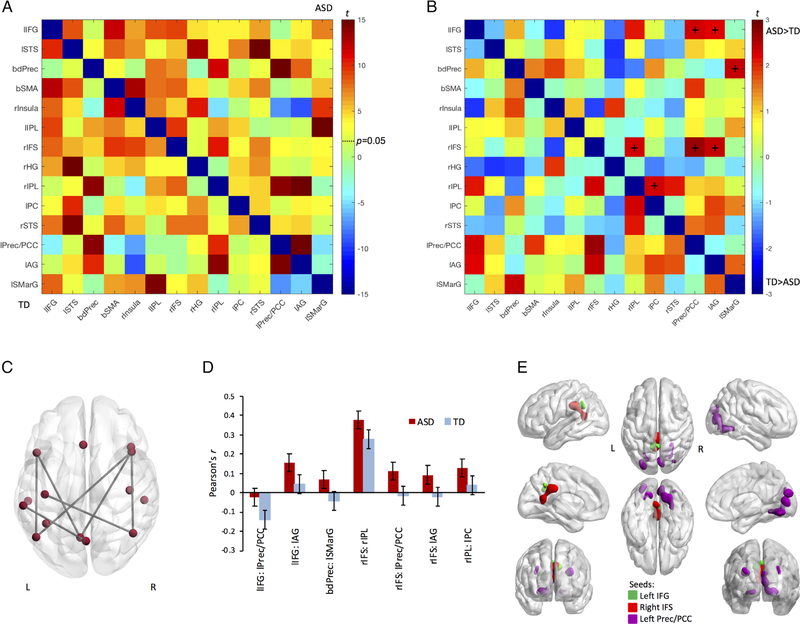
Figure 1.
Language network iFC. (A) Matrix showing results from within-group t-test of language network connectivities (warm colors represent positive t-values; cool colors represent negative t-values; critical t-value of 1.68 is marked by P = 0.05). The ASD group (top right) showed 69 connections above this threshold and the TD group showed 62 significant connections (bottom left). After multiple comparison corrections (qs < 0.05), the TD group retained 61 of the 62 significant connections, while the ASD group retained all 69 significant connections. (B) Group difference matrix for connectivities between the 14 language ROIs. + symbolizes local FDR-corrected significant difference between the groups (Cohen’s ds > 0.44, Ps < 0.03 uncorrected, qs < 0.27). (C) Glass brain rendering of significant between-group differences in language network connectivities after local FDR correction, corresponding to cells labeled + in panel B. (D) Between-group differences for ASD (red) and TD (blue) in correlations between language ROIs. Error bars signify standard error of the correlations. lIFG: left inferior frontal gyrus; lSTS: left superior temporal sulcus; lIPL: left inferior parietal lobule; lPC: left pericentral region; lPrec/PCC: left precuneus/posterior cingulate cortex; lAG: left angular gyrus; lSMarG: left supramarginal gyrus; bdPrec: bilateral dorsal precuneus; bSMA: bilateral supplementary motor area; rInsula: right insula; rIFS: right inferior frontal sulcus; rHG: right Heschl’s gyrus; rIPL: right inferior parietal lobule; rSTS: right superior temporal sulcus. (E) Clusters of between-group difference from whole-brain iFC analyses for seeds in left inferior frontal gyrus (green cluster) and right inferior frontal sulcus (red cluster). Both ROIs show higher connectivity with the posterior cingulate cortex (PCC) in the ASD group (voxel-level threshold P = 0.001, α < 0.05). For the left precuneus/PCC seed extensive overconnectivity is found in occipital cortex (purple clusters) in the ASD relative to the TD group (voxel-level P = 0.001, α < 0.01).
Results
Within-Network Analyses
One-sample t-tests showed generally high connectivity between language network ROIs in both TD and ASD groups (Fig. 1A). After correcting for multiple corrections, 69 ROI pairs in the ASD group and 61 ROI pairs in the TD group showed significant difference from zero (qs < 0.05).
Two-sample t-tests for group comparisons of within-network matrices revealed seven connectivity differences between language ROIs that survived local FDR adjustment (Cohen’s ds > 0.44, ps < 0.03 uncorrected, qs < 0.27; Fig. 1B,C). All seven pairs showed more positive correlations between language ROIs in the ASD group compared to the TD group. Such effects were seen for connections of both left and right inferior frontal ROIs with parietal ROIs (i.e., IPL, angular gyrus, and precuneus/posterior cingulate cortex). Higher correlations were also seen in the ASD group between the bilateral dorsal precuneus and left supramarginal gyrus, and between right IPL and the left pericentral region. Except for one connection between right inferior frontal and inferior parietal ROIs, these effects reflected correlations close to or below zero in the TD group and more positive correlations in the ASD group (Fig. 1D).
Laterality Analyses
We examined between-group differences in language network lateralization by calculating laterality indices and by examining the average connectivity of between-hemisphere language regions. We found that the laterality indices between ASD participants and TD participants did not differ significantly (t(100) = 0.69, P = 0.51). Similarly, we did not find a significant group difference in between-hemisphere language network iFC (t(100) = 0.25, P = 0.80).
Whole-Brain (Outside Network) Analyses
We selected five ROIs that were part of two or more significant connections (indicated by superscript “1” in Table 2) as seeds for further exploration in whole-brain connectivity analyses. Three of these showed significant whole-brain iFC group differences. For inferior frontal seeds bilaterally, there was increased connectivity in the ASD group with PCC (Fig. 1E). Additionally, for the left precuneus/PCC seed, four clusters of greater iFC in the ASD group were detected in occipital lobes bilaterally, including middle occipital and lingual gyri (Supplementary Table 3).
Post hoc Analysis of Effective Connectivity
Findings of overconnectivity with visual cortex from whole-brain analyses were remarkable in view of several previous ASD reports of atypical engagement of visual areas in language processing [Gaffrey et al., 2007; Kana et al., 2006; Knaus et al., 2008] and increased FC between inferior frontal gyrus (IFG) and visual cortices [Shen et al., 2012]. However, none of these explored the role of PCC. As our findings suggested that PCC might mediate connectivity between IFG and visual regions in ASDs, we performed follow-up analyses of effective connectivity, using Group Iterative Multiple Models Estimation [GIMME; Gates & Molenaar, 2012]. GIMME utilizes unified structural equation modeling in a data-driven approach. Its ability to model contemporaneous and lagged relationships between regions allows for the study of effective FC in resting-state MRI [Gates, Molenaar, Iyer, Nigg, & Fair, 2014].
We conducted four semi-confirmatory GIMME analyses that estimated prespecified directional relationships between variables and added paths as needed for each subject until the best-fit model was found. Analyses were run using the average time-series extracted from the three seeds with significant whole-brain group differences (left IFG, right IFS, and left prec/PCC ROIs), and from the corresponding significant group-effect clusters in PCC and the occipital lobes (Fig. 1E, Supplementary Table 3). The clusters in PCC (overconnectivity with inferior frontal seeds) and the left prec/PCC seed (which also showed significant overconnectivity with left IFG and right IFS in the within-network analyses) were in close proximity and were merged. The four clusters in occipital cortex (overconnectivity with left prec/PCC seed) were also merged. We tested whether connectivity between inferior frontal regions and visual cortex was mediated by PCC. In view of atypical language-related asymmetries of IFG reported in several ASD studies [Kleinhans et al., 2008; Knaus et al., 2010], right and left inferior frontal ROIs were modeled separately. We tested four mediation models that varied in directionality of connectivity, each containing four factors: left IFG, right IFS, PCC, and visual cortex (Supplementary Fig. 3). Each subject’s iterative path estimates and their fit for the prespecified model were then used to determine whether an indirect, or mediated, relationship exist for each model. Across the four models, we found that 24 out of 52 ASD participants showed significant PCC mediation of connectivity between frontal language areas and visual cortex (with 18 of the 24 showing this effect for the right inferior frontal ROI). The subgroups did not differ in age, head motion, IQ, or language abilities (ps > 0.30, uncorrected; Supplementary Table 4). A marginal difference was found in parent-reported restricted and repetitive behaviors, as measured by the ADI-R (t(43.5) = 2.16, Puncorrected = 0.04).
Relation Between Connectivity and Behavioral Measures
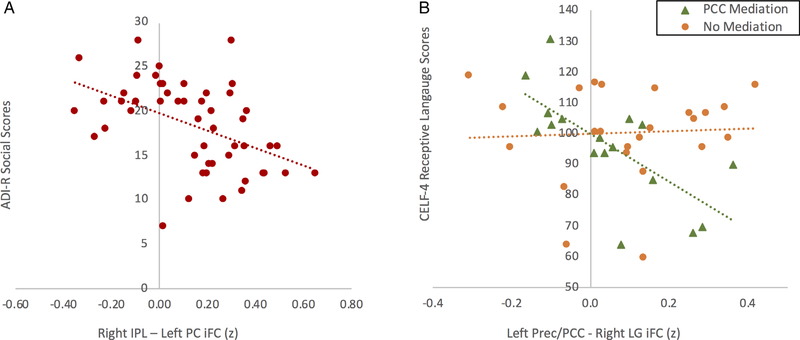
Next, we explored the relationship between connectivity (mean z) of the seven within-network connections with significant group differences (Fig. 1B,C) and measures of symptom severity and language ability. In the ASD group, iFC between right IPL and left pericentral region was negatively correlated with scores of the ADI-R Social subscale (r = −0.51, q = 0.006; Fig. 2A; Supplementary Table 5), with higher iFC linked to lower social symptom severity (controlling for age and head motion). No significant correlations between iFC pairs and language measures were found in either group (Supplementary Table 6).
Figure 2.
Correlations between brain connectivity and behavioral measures. (A) Correlation of ADI-R Social scores with iFC between right inferior parietal lobule and left pericentral region in the ASD group (r = −0.51, qFDR = 0.006), after controlling for age and in-scanner head motion. (B) Correlation of CELF-4 Receptive Language scores with iFC between the PCC/Precuneus ROI and right lingual gyrus in ASD subgroups with PCC-mediated connectivity between inferior frontal ROIs and visual cortex (shown in green [r = −0.74, qFDR = 0.022]) and without PCC mediation (shown in orange [r = 0.09, qFDR = 0.955]), after controlling for age and in-scanner head motion, and multiple comparison correction.
For whole-brain iFC results, we found no significant relationship with behavioral language measures or symptomatology in the ASD group. However, in the TD group, there was a significant negative correlation for CELF Receptive Language score and iFC between right IFS and PCC (red cluster in Fig. 1E), controlling for age and motion (r = −0.55, qFDR = 0.009; Supplementary Table 7). Similarly, higher connectivity between the PCC/Precuneus ROI and left medial occipital gyrus was linked to lower CELF Core Language scores (r = −0.56, qFDR = 0.009) and Expressive Language scores (r = −0.59, qFDR = 0.008), after correcting for multiple comparisons.
Finally, we examined the relationship between language function and whole-brain iFC in the subgroup of ASD participants who showed PCC mediation in GIMME analyses. Similar to the TD group, we found that connectivity between the PCC/precuneus ROI and visual cortex was all negatively correlated with CELF scores in this ASD subgroup. After controlling for age and head motion, and correcting for multiple comparisons, we found a significant relationship between iFC of left Precuneus/PCC with right lingual gyrus and CELF Receptive Language (r = −0.74, qFDR = 0.022; Figure 2B; Supplementary Table 7). No correlations were observed between iFC results and CELF measures in the ASD subgroup without significant PCC mediation.
Discussion
Language deficits are commonly observed in ASDs, but the underlying neurobiology is not fully understood. We examined iFC of the language network in children with ASDs, using resting-state FC MRI, and found atypically increased connectivity between some language regions, in addition to increased connectivity of language ROIs with PCC and visual regions. Although several right hemisphere ROIs showed overconnectivity in the ASD group, quantitative analyses of asymmetry yielded no group differences. Effective connectivity analyses revealed a subgroup of ASD youths whose connectivity between inferior frontal language regions and visual cortex was mediated by PCC. In this subgroup, increased iFC between PCC and visual cortex was also associated with lower language abilities.
Predominant Overconnectivity of the Language Network in ASDs
FC within the language network [adopted from a large meta-analysis; Rodd et al., 2015] was predominantly greater in the ASD compared to the TD group. While seemingly at odds with some previous underconnectivity findings [Just et al., 2004; Kana et al., 2006; Knaus et al., 2008], inconsistency may be largely explained by methodological differences between co-activation FC (testing for task-driven BOLD correlations) and iFC [Müller et al., 2011; Nair et al., 2014]. One recent iFC study of language also reported underconnectivity in ASDs [Verly et al., 2014], which was, however, observed in ROIs (including right cerebellum) that largely differed from those implemented in the present study. In addition, Verly and colleagues specifically selected 19 ASD participants with a history of significant language delay and impairment, whereas the present study included 52 ASD participants with a wide range of linguistic abilities.
For all but one of the ROI–ROI pairs with increased connectivity in ASDs, group differences reflected low levels of iFC in the TD group, contrasting with more positive iFC in the ASD group (Fig. 1D). These connections may represent an extended language network of regions that do not consistently co-activate during language processing and therefore show low levels of intrinsic synchronization in the TD brain. Only one ROI pairing (right IFS and right IPL) showed positive BOLD correlations in the TD group, which were even more pronounced in the ASD group. Note, however, that overconnectivity between ROIs of the extended language network was mostly only of medium effect size, surviving only a relatively lenient local FDR correction.
For a few ROI pairings, overconnectivity in the ASD group was associated with lower sociocommunicative symptom severity (Supplementary Table 5). Specifically, atypically increased connectivity between left IFG and angular gyrus in the ASD group was associated with lower ADOS Social Communication scores. This reflects a connection between core language regions in the dominant hemisphere crucial for semantic processing, lexical selection, and other language subprocesses [Price, 2010]. However, this medium-sized effect (r = −0.32) did not survive FDR correction. More robust was an effect of increased iFC between right IPL and left pericentral cortex was associated with decreased ADI-R Social scores. Right IPL, which has traditionally been linked to spatial processing and selective attention [Husain & Nachev, 2007], has also been noted for its role in distinguishing between self and others [Ruby & Decety, 2004; Uddin, Molnar-Szakacs, Zaidel, & Iacoboni, 2006]. Left pericentral cortex is important for speech production [Price, 2010], but has also been found to activate during speech comprehension [Adank, 2012]. Findings of overconnectivity between nodes of an extended language network in ASDs may be indicative of a history of increased co-activation [Crossley et al., 2013; Lewis, Baldassarre, Committeri, Romani, & Corbetta, 2009]. More specifically, relatively mild levels of social deficits in ASD could reflect a history of high levels of effortful processing in these noncore language regions, with some compensatory effect and associated relatively mild symptomatology.
Connectivity Between Language, Visual, and Default Mode Networks
Whole-brain analyses revealed overconnectivity in the ASD group bilaterally between IFG and PCC. The PCC has been described as a “hub” with dense connectivity for information integration [Ray et al., 2014; van den Heuvel & Sporns, 2011]. Traditionally associated with the default mode network (DMN), PCC is activated during self-reflection, mentalization, and episodic memory [Hull, Jacokes, Torgerson, Irimia, & Van Horn, 2017; Washington et al., 2014]. Increased FC between DMN and language-related networks in ASDs has been previously reported in an Independent Component Analysis (ICA) study by Zhao et al. [2016], who found right hemisphere homologous language regions (e.g., IFG, AG, and supramarginal gyrus) to be overconnected with the DMN. This may suggest reduced segregation between the DMN and the language network, possibly consistent with findings of atypical crosstalk between DMN and other functional networks [Abbott et al., 2016; Fishman, Keown, Lincoln, Pineda, & Müller, 2014; Ray et al., 2014; Rudie et al., 2013; Rudie et al., 2012; Yerys et al., 2015].
The finding of increased iFC between PCC and visual cortex is consistent with previous reports of overconnectivity between the DMN and the visual network in ASDs [Washington et al., 2014; Yerys et al., 2015]. Yerys et al. [2015] found increased PCC connectivity with bilateral occipital pole, LG, and fusiform gyri. Together with the overconnectivity of the PCC and DMN discussed above, this is of special interest to language function as studies have reported increased activation of visual cortex in ASD participants during language processing [Gaffrey et al., 2007; Kana et al., 2006; Knaus et al., 2008; Pang et al., 2016].
An effective connectivity analysis of the language network in ASDs by Shen et al. [2012] detected an atypical path between left IFG and right extrastriate cortex; however, PCC was not considered in their model. In the present study, whole-brain iFC analysis revealed PCC to be overconnected with both bilateral IFG and visual cortex. Therefore, PCC was tested as a potential mediator of iFC between the frontal and visual regions—a mediation that was confirmed in almost half of the ASD participants. A negative association of robust effect size between CELF-4 scores and brain connectivity as seen in this ASD subgroup with PCC mediation suggests that connectivity between PCC and visual regions may be detrimental to language functioning. As concordant effects were seen in the TD group, this link may not be specific to ASDs. However, whereas PCC-visual connectivity was generally low in TD children, it was robust in many children with ASDs, indicating that the detrimental effect on language is common in ASDs, but uncommon in TD children. Functionally, it may indicate over-engagement of internal reflection and mental imagery in early development, contributing to increased synchronization between DMN and visual networks [Spreng, Mar, & Kim, 2009]. Accompanying overconnectivity between DMN and frontal language regions may further be associated with a history of reduced vigilance and performance efficiency during language processing [Anticevic, Repovs, Shulman, & Barch, 2010; Götting et al., 2017; Hinds et al., 2013].
Limitations
IFC findings were not robustly associated with behavioral language measures of the CELF-4. Although commonly used, CELF subtests additionally engage non-language cognitive functions such as working memory (e.g., recalling sentences), which may have limited our ability to detect links between iFC and language abilities. As with most fMRI studies of ASDs, data collection was limited to relatively high-functioning individuals who were able to lie almost motionless throughout the scan. While our aim was to include a wide range of linguistic abilities to better represent the ASD population, inclusion of children with normal-level language abilities may have weakened group-level effects and differences of brain–behavior relationships.
Conclusions
We found the intrinsic functional organization of the language network in high-functioning children and adolescents with ASDs to be characterized by partial overconnectivity, mostly involving regions of an extended network that do not show robust signal correlations in TD peers. Atypical connectivitywasdistinctfora triad ofinferiorfrontal, default mode, and visual regions. While overconnectivity with DMN (PCC) was seen for the entire ASD cohort, mediation of connectivity between inferior frontal and visual regions by PCC was seen only in an ASD subgroup, where high level of visual connectivity was associated with relatively low language abilities. Findings suggest that atypical connectivity in ASDs may predominantly affect regions of an extended network (rather than traditional regions such as Broca’s and Wernicke’s), with great heterogeneity even within the fully verbal and high-functioning segment of the spectrum.
Supplementary Material
Supplementary Figure 1. Between group differences in language network iFC across preprocessing pipelines.
Supplementary Figure 2. Regions of interest.
Supplementary Figure 3. GIMME models of PCC mediated iFC between Broca’s area and visual cortex.
Supplementary Table 1. Between group differences in language network iFC across preprocessing pipelines.
Supplementary Table 2. Significant between-group clusters from whole-brain iFC analysis across preprocessing.
Supplementary Table 3. Significant between-group clusters from whole-brain iFC analysis.
Supplementary Table 4. Subgroup information.
Supplementary Table 5. Correlations between language network iFC and ASD symptom severity.
Supplementary Table 6. Correlations between language network iFC and language scores.
Supplementary Table 7. Correlations between language network iFC results and language measures.
Acknowledgments
Thanks to the children and their parents who participated in this study. We also thank Dr. Patti Adank and colleagues for providing the statistical parametric maps from their Activation Likelihood Estimate study. This work was supported by the National Institutes of Health (R01 MH081023 and R01 MH101173 to RAM, K01 MH097972 to IF).
Footnotes
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
Contributor Information
Yangfeifei Gao, Brain Development Imaging Laboratories, Department of Psychology, San Diego State University, San Diego, California; San Diego State University, University of California, San Diego Joint Doctoral Program in Clinical Psychology, San Diego, California.
Annika Linke, Brain Development Imaging Laboratories, Department of Psychology, San Diego State University, San Diego, California.
Ruth Joanne Jao Keehn, Brain Development Imaging Laboratories, Department of Psychology, San Diego State University, San Diego, California.
Sanjana Punyamurthula, Brain Development Imaging Laboratories, Department of Psychology, San Diego State University, San Diego, California.
Afrooz Jahedi, Brain Development Imaging Laboratories, Department of Psychology, San Diego State University, San Diego, California; Computational Science Research Center, San Diego State University, San Diego, California.
Kathleen Gates, Department of Psychology, University of North Carolina, Chapel Hill, North Carolina.
Inna Fishman, Brain Development Imaging Laboratories, Department of Psychology, San Diego State University, San Diego, California; San Diego State University, University of California, San Diego Joint Doctoral Program in Clinical Psychology, San Diego, California.
Ralph-Axel Müller, Brain Development Imaging Laboratories, Department of Psychology, San Diego State University, San Diego, California; San Diego State University, University of California, San Diego Joint Doctoral Program in Clinical Psychology, San Diego, California.
References
- Abbott AE, Nair A, Keown CL, Datko M, Jahedi A, Fishman I, & Müller R-A (2016). Patterns of atypical functional connectivity and behavioral links in autism differ between default, salience, and executive networks. Cerebral Cortex, 26(10), 4034–4045. 10.1093/cercor/bhv191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adank P (2012). Design choices in imaging speech comprehension: An activation likelihood estimation (ALE) meta-analysis. NeuroImage, 63(3), 1601–1613. 10.1016/j.neuroimage.2012.07.027 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders: DSM-5 (5th ed.). Washington, DC: American Psychiatric Association. [Google Scholar]
- Anderson JS, Lange N, Froehlich A, DuBray MB, Druzgal TJ, Froimowitz MP, … Lainhart JE (2010). Decreased left posterior insular activity during auditory language in autism. American Journal of Neuroradiology, 31(1), 131–139. 10.3174/ajnr.A1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Shulman GL, & Barch DM (2010). When less is more: TPJ and default network deactivation during encoding predicts working memory performance. NeuroImage, 49(3), 2638–2648. 10.1016/j.neuroimage.2009.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl MM, Mayo J, Parks EN, Rosenberger LR, VanMeter J, Ratner NB, … Gaillard WD (2014). Regional differences in the developmental trajectory of lateralization of the language network: Developmental trajectories of lateralization of language. Human Brain Mapping, 35(1), 270–284. 10.1002/hbm.22179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher J (2012). Research review: Structural language in autistic spectrum disorder - characteristics and causes. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 53(3), 219–233. 10.1111/j.1469-7610.2011.02508.x [DOI] [PubMed] [Google Scholar]
- Broca P (1861). Remarques sur le siège de la faculté du langage articulé, suivies d’une observation d’aphémie (perte de la parole). Bulletins de La Société d’Anatomie, 2(6), 330–357. [Google Scholar]
- Catani M, Jones DK, & ffytche DH (2005). Perisylvian language networks of the human brain. Annals of Neurology, 57 (1), 8–16. 10.1002/ana.20319 [DOI] [PubMed] [Google Scholar]
- Cox RW (1996). AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research, 29(3), 162–173. [DOI] [PubMed] [Google Scholar]
- Cox RW, Chen G, Glen DR, Reynolds RC, & Taylor PA (2017). FMRI clustering in AFNI: False-positive rates redux. Brain Connectivity, 7(3), 152–171. 10.1089/brain.2016.0475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley NA, Mechelli A, Vértes PE, Winton-Brown TT, Patel AX, Ginestet CE, … Bullmore ET (2013). Cognitive relevance of the community structure of the human brain functional coactivation network. Proceedings of the National Academy of Sciences of the United States of America, 110(28), 11583–11588. 10.1073/pnas.1220826110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B (2007). Size, power and false discovery rates. The Annals of Statistics, 35(4), 1351–1377. 10.1214/009053606000001460 [DOI] [Google Scholar]
- Eigsti I-M, de Marchena AB, Schuh JM, & Kelley E (2011). Language acquisition in autism spectrum disorders: A developmental review. Research in Autism Spectrum Disorders, 5(2), 681–691. 10.1016/j.rasd.2010.09.001 [DOI] [Google Scholar]
- Eyler LT, Pierce K, & Courchesne E (2012). A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain, 135(Pt 3), 949–960. 10.1093/brain/awr364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman I, Keown CL, Lincoln AJ, Pineda JA, & Müller R-A (2014). Atypical cross talk between mentalizing and mirror neuron networks in autism spectrum disorder. JAMA Psychiatry, 71 (7),751–760. 10.1001/jamapsychiatry.2014.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaffrey MS, Kleinhans NM, Haist F, Akshoomoff N, Campbell A, Courchesne E, & Müller R-A (2007). Atypical [corrected] participation of visual cortex during word processing in autism: An fMRI study of semantic decision. Neuropsychologia, 45(8), 1672–1684. 10.1016/j.neuropsychologia.2007.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates KM, & Molenaar PCM (2012). Group search algorithm recovers effective connectivity maps for individuals in homogeneous and heterogeneous samples. NeuroImage, 63(1), 310–319. 10.1016/j.neuroimage.2012.06.026 [DOI] [PubMed] [Google Scholar]
- Gates KM, Molenaar PCM, Iyer SP, Nigg JT, & Fair DA (2014). Organizing heterogeneous samples using community detection of GIMME-derived resting state functional networks. PLoS One, 9(3), e91322 10.1371/journal.pone.0091322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, & Levitt P (2007). Autism spectrum disorders: Developmental disconnection syndromes. Current Opinion in Neurobiology, 17(1), 103–111. 10.1016/j.conb.2007.01.009 [DOI] [PubMed] [Google Scholar]
- Geschwind N (1970). The organization of language and the brain. Science, 170(3961), 940–944. [DOI] [PubMed] [Google Scholar]
- Götting FN, Borchardt V, Demenescu LR, Teckentrup V, Dinica K, Lord AR, … Walter M (2017). Higher interference susceptibility in reaction time task is accompanied by weakened functional dissociation between salience and default mode network. Neuroscience Letters, 649, 34–40. 10.1016/j.neulet.2017.03.035 [DOI] [PubMed] [Google Scholar]
- Gotts SJ, Saad ZS, Jo HJ, Wallace GL, Cox RW, & Martin A (2013). The perils of global signal regression for group comparisons: A case study of Autism Spectrum Disorders. Frontiers in Human Neuroscience, 7, 356 10.3389/fnhum.2013.00356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen WB, Tesink C, Petersson KM, van Berkum J, van der Gaag RJ, Hagoort P, & Buitelaar JK (2010). Semantic, factual, and social language comprehension in adolescents with autism: An FMRI study. Cerebral Cortex, 20(8), 1937–1945. 10.1093/cercor/bhp264 [DOI] [PubMed] [Google Scholar]
- Hallquist MN, Hwang K, & Luna B (2013). The nuisance of nuisance regression: Spectral misspecification in a common approach to resting-state fMRI preprocessing reintroduces noise and obscures functional connectivity. NeuroImage, 82, 208–225. 10.1016/j.neuroimage.2013.05.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert MR, Harris GJ, Adrien KT, Ziegler DA, Makris N, Kennedy DN, … Caviness VS (2002). Abnormal asymmetry in language association cortex in autism. Annals of Neurology, 52(5), 588–596. 10.1002/ana.10349 [DOI] [PubMed] [Google Scholar]
- Herringshaw AJ, Ammons CJ, DeRamus TP, & Kana RK (2016). Hemispheric differences in language processing in autism spectrum disorders: A meta-analysis of neuroimaging studies. Autism Research, 9(10), 1046–1057. 10.1002/aur.1599 [DOI] [PubMed] [Google Scholar]
- Hinds O, Thompson TW, Ghosh S, Yoo JJ, WhitfieldGabrieli S, Triantafyllou C, & Gabrieli JDE (2013). Roles of default-mode network and supplementary motor area in human vigilance performance: Evidence from real-time fMRI. Journal of Neurophysiology, 109(5), 1250–1258. 10.1152/jn.00533.2011 [DOI] [PubMed] [Google Scholar]
- Howlin P (2003). Outcome in high-functioning adults with autism with and without early language delays: Implications for the differentiation between autism and Asperger syndrome. Journal of Autism and Developmental Disorders, 33 (1), 3–13. 10.1023/A:1022270118899 [DOI] [PubMed] [Google Scholar]
- Hull JV, Jacokes ZJ, Torgerson CM, Irimia A, & Van Horn JD (2017). Resting-state functional connectivity in autism spectrum disorders: A review. Frontiers in Psychiatry, 7, 205 10.3389/fpsyt.2016.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M, & Nachev P (2007). Space and the parietal cortex. Trends in Cognitive Sciences, 11(1), 30–36. 10.1016/j.tics.2006.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, & Minshew NJ (2004). Cortical activation and synchronization during sentence comprehension in high-functioning autism: Evidence of underconnectivity. Brain, 127(Pt 8), 1811–1821. 10.1093/brain/awh199 [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Cherkassky VL, Minshew NJ, & Just MA (2006). Sentence comprehension in autism: Thinking in pictures with decreased functional connectivity. Brain, 129(Pt 9), 2484–2493. 10.1093/brain/awl164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans NM, Müller R-A, Cohen DN, & Courchesne E (2008). Atypical functional lateralization of language in autism spectrum disorders. Brain Research, 1221, 115–125. 10.1016/j.brainres.2008.04.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus TA, Silver AM, Kennedy M, Lindgren KA, Dominick KC, Siegel J, & Tager-Flusberg H (2010). Language laterality in autism spectrum disorder and typical controls: A functional, volumetric, and diffusion tensor MRI study. Brain and Language, 112(2), 113–120. 10.1016/j.bandl.2009.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus TA, Silver AM, Lindgren KA, Hadjikhani N, & Tager-Flusberg H (2008). fMRI activation during a language task in adolescents with ASD. Journal of the International Neuropsychological Society, 14(6), 967–979. 10.1017/S1355617708081216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Park B-Y, James O, Kim S-G, & Park H (2017). Autism spectrum disorder related functional connectivity changes in the language network in children, adolescents and adults. Frontiers in Human Neuroscience, 11, 418 10.3389/fnhum.2017.00418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CM, Baldassarre A, Committeri G, Romani GL, & Corbetta M (2009). Learning sculpts the spontaneous activity of the resting human brain. Proceedings of the National Academy of Sciences, 106(41), 17558–17563. 10.1073/pnas.0902455106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Pierce K, Eyler LT, Carter Barnes C, Ahrens-Barbeau C, Solso S, … Courchesne E (2015). Different functional neural substrates for good and poor language outcome in autism. Neuron, 86(2), 567–577. 10.1016/j.neuron.2015.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore P, & Risi S (2001). Autism diagnostic observation schedule. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Lord C, Rutter M, DiLavore PS, Risi S, Gotham K, & Bishop SL (2012). Autism diagnostic observation schedule, second edition (ADOS-2) manual (Part I): modules 1–4. Torrance, CA: Western Psychological Services. [Google Scholar]
- Luyster RJ, Kadlec MB, Carter A, & Tager-Flusberg H (2008). Language assessment and development in toddlers with autism spectrum disorders. Journal of Autism and Developmental Disorders, 38(8), 1426–1438. 10.1007/s10803-007-0510-1 [DOI] [PubMed] [Google Scholar]
- McAvoy M, Mitra A, Coalson RS, d’Avossa G, Keidel JL, Petersen SE, & Raichle ME (2016). Unmasking language lateralization in human brain intrinsic activity. Cerebral Cortex, 26(4), 1733–1746. 10.1093/cercor/bhv007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam M-M, Thompson CK, Weintraub S, & Rogalski EJ (2015). The Wernicke conundrum and the anatomy of language comprehension in primary progressive aphasia. Brain, 138(8), 2423–2437. 10.1093/brain/awv154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley RL, Correia MM, Baron-Cohen S, Shtyrov Y, Pulvermüller F, & Mohr B (2016). Reduced volume of the arcuate fasciculus in adults with high-functioning autism spectrum conditions. Frontiers in Human Neuroscience, 10, 10 10.3389/fnhum.2016.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller RA, Behen ME, Rothermel RD, Chugani DC, Muzik O, Mangner TJ, & Chugani HT (1999). Brain mapping of language and auditory perception in high-functioning autistic adults: A PET study. Journal of Autism and Developmental Disorders, 29(1), 19–31. [DOI] [PubMed] [Google Scholar]
- Müller R-A (2007). The study of autism as a distributed disorder. Mental Retardation and Developmental Disabilities Research Reviews, 13(1), 85–95. 10.1002/mrdd.20141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller R-A, Shih P, Keehn B, Deyoe JR, Leyden KM, & Shukla DK (2011). Underconnected, but how? A survey of functional connectivity MRI studies in autism spectrum disorders. Cerebral Cortex, 21(10), 2233–2243. 10.1093/cercor/bhq296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagae LM, Zarnow DM, Blaskey L, Dell J, Khan SY, Qasmieh S, … Roberts TPL (2012). Elevated mean diffusivity in the left hemisphere superior longitudinal fasciculus in autism spectrum disorders increases with more profound language impairment. American Journal of Neuroradiology, 33(9), 1720–1725. 10.3174/ajnr.A3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Keown CL, Datko M, Shih P, Keehn B, & Müller R-A (2014). Impact of methodological variables on functional connectivity findings in autism spectrum disorders: FcMRI methods in autism. Human Brain Mapping, 35(8), 4035–4048. 10.1002/hbm.22456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JA, Zielinski BA, Ferguson MA, Lainhart JE, & Anderson JS (2013). An evaluation of the left-brain vs. rightbrain hypothesis with resting state functional connectivity magnetic resonance imaging. PLoS One, 8(8), e71275 10.1371/journal.pone.0071275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JA, Zielinski BA, Fletcher P, Alexander AL, Lange N, Bigler ED, … Anderson JS (2014). Abnormal lateralization of functional connectivity between language and default mode regions in autism. Molecular Autism, 5(1), 8 10.1186/2040-2392-5-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC (1971). The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia, 9(1), 97–113. [DOI] [PubMed] [Google Scholar]
- Pang EW, Valica T, MacDonald MJ, Taylor MJ, Brian J, Lerch JP, & Anagnostou E (2016). Abnormal brain dynamics underlie speech production in children with autism spectrum disorder: Abnormal speech production in ASD. Autism Research, 9(2), 249–261. 10.1002/aur.1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeva MG, Tourville JA, Agam Y, Holland B, Manoach DS, & Guenther FH (2013). White matter impairment in the speech network of individuals with autism spectrum disorder. NeuroImage, 3, 234–241. 10.1016/j.nicl.2013.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Plitt M, Laumann TO, & Martin A (2017). Sources and implications of whole-brain fMRI signals in humans. NeuroImage, 146, 609–625. 10.1016/j.neuroimage.2016.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ (2000). The anatomy of language: Contributions from functional neuroimaging. Journal of Anatomy, 197(3), 335–359. 10.1046/j.1469-7580.2000.19730335.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ (2010). The anatomy of language: A review of 100 fMRI studies published in 2009. Annals of the New York Academy of Sciences, 1191(1), 62–88. 10.1111/j.1749-6632.2010.05444.x [DOI] [PubMed] [Google Scholar]
- Ray S, Miller M, Karalunas S, Robertson C, Grayson DS, Cary RP, … Fair DA (2014). Structural and functional connectivity of the human brain in autism spectrum disorders and attention-deficit/hyperactivity disorder: A rich cluborganization study. Human Brain Mapping, 35(12), 6032–6048. 10.1002/hbm.22603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippon G, Brock J, Brown C, & Boucher J (2007). Disordered connectivity in the autistic brain: Challenges for the ‘new psychophysiology.’. International Journal of Psychophysiology, 63(2), 164–172. 10.1016/j.ijpsycho.2006.03.012 [DOI] [PubMed] [Google Scholar]
- Rodd JM, Vitello S, Woollams AM, & Adank P (2015). Localising semantic and syntactic processing in spoken and written language comprehension: An activation likelihood estimation meta-analysis. Brain and Language, 141, 89–102. 10.1016/j.bandl.2014.11.012 [DOI] [PubMed] [Google Scholar]
- Ruby P, & Decety J (2004). How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. Journal of Cognitive Neuroscience, 16(6), 988–999. 10.1162/0898929041502661 [DOI] [PubMed] [Google Scholar]
- Rudie JD, Brown JA, Beck-Pancer D, Hernandez LM, Dennis EL, Thompson PM, … Dapretto M (2013). Altered functional and structural brain network organization in autism. NeuroImage, 2, 79–94. 10.1016/j.nicl.2012.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudie JD, Shehzad Z, Hernandez LM, Colich NL, Bookheimer SY, Iacoboni M, & Dapretto M (2012). Reduced functional integration and segregation of distributed neural systems underlying social and emotional information processing in autism spectrum disorders. Cerebral Cortex, 22 (5), 1025–1037. 10.1093/cercor/bhr171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, & Lord C (2003). Autism diagnostic interview-revised (ADI-R) manual. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, & Cox RW (2012). Trouble at rest: How correlation patterns and group differences become distorted after global signal regression. Brain Connectivity, 2(1), 25–32. 10.1089/brain.2012.0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schölvinck ML, Maier A, Ye FQ, Duyn JH, & Leopold DA (2010). Neural basis of global resting-state fMRI activity. Proceedings of the National Academy of Sciences, 107, 10238–10243. 10.1073/pnas.0913110107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semel E, Wiig E, & Secord W (2004). CELF-4: Clinical evaluation of language fundamentals (4th ed.). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Shen MD, Shih P, Öttl B, Keehn B, Leyden KM, Gaffrey MS,& Müller R-A.(2012).Atypicallexicosemanticfunction of extrastriate cortex in autism spectrum disorder: Evidence from functional and effective connectivity. NeuroImage, 62(3), 1780–1791. 10.1016/j.neuroimage.2012.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, … Matthews PM (2004). Advances in functional and structural MR image analysis and implementation as FSL. 10.1016/j.neuroimage.2004.07.051.PMID:15501092. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, & Kim ASN (2009). The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. Journal of Cognitive Neuroscience, 21(3), 489–510. 10.1162/jocn.2008.21029 [DOI] [PubMed] [Google Scholar]
- Szatmari P, Georgiades S, Duku E, Bennett TA, Bryson S, & Fombonne E (2015). Developmental trajectories of symptom severity and adaptive functioning in an inception cohort of preschool children with autism spectrum disorder. JAMA Psychiatry, 72(3), 276–283. 10.1001/jamapsychiatry.2014.2463 [DOI] [PubMed] [Google Scholar]
- Tager-Flusberg H, Paul R, & Lord C (2013). Language and communication in autism In Volkmar FR, Paul R, Klin A, & Cohen D (Eds.), Handbook of autism and pervasive developmental disorders (pp. 335–364). Hoboken, NJ: John Wiley & Sons,Inc; 10.1002/9780470939345.ch12 [DOI] [Google Scholar]
- Tomasi D, & Volkow ND (2012). Resting functional connectivity of language networks: Characterization and reproducibility. Molecular Psychiatry, 17(8), 841–854. 10.1038/mp.2011.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Molnar-Szakacs I, Zaidel E, & Iacoboni M (2006). rTMS to the right inferior parietal lobule disrupts self– other discrimination. Social Cognitive and Affective Neuroscience, 1(1), 65–71. 10.1093/scan/nsl003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, & Sporns O (2011). Rich-club organization of the human connectome. The Journal of Neuroscience, 31(44), 15775–15786. 10.1523/JNEUROSCI.3539-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verly M, Verhoeven J, Zink I, Mantini D, Peeters R, Deprez S, … Sunaert S (2014). Altered functional connectivity of the language network in ASD: Role of classical language areas and cerebellum. NeuroImage, 4, 374–382. 10.1016/j.nicl.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington SD, Gordon EM, Brar J, Warburton S, Sawyer AT,Wolfe A, …VanMeter JW.(2014).Dysmaturation ofthedefaultmodenetworkinautism.HumanBrainMapping,35 (4),1284–1296. 10.1002/hbm.22252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1999). Wechsler abbreviated scale of intelligence (WASI). San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Williams DL, Goldstein G, & Minshew NJ (2006). Neuropsychologic functioning in children with autism: Further evidence for disordered complex information-processing. Child Neuropsychology, 12(4–5), 279–298. 10.1080/09297040600681190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Gordon EM, Abrams DN, Satterthwaite TD, Weinblatt R, Jankowski KF, … Vaidya CJ (2015). Default mode network segregation and social deficits in autism spectrum disorder: Evidence from non-medicated children. NeuroImage, 9, 223–232. 10.1016/j.nicl.2015.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zablotsky B, Black LI, Maenner MJ, Schieve LA, & Blumberg SJ (2015). Estimated prevalence of autism and other developmentaldisabilitiesfollowingquestionnaire changes in the 2014 National Health Interview Survey. National Health StatisticsReports,87,1–20. [PubMed] [Google Scholar]
- Zhao Y, Chen H, Li Y, Lv J, Jiang X, Ge F, … Liu T (2016). Connectome-scale group-wise consistent resting-state network analysis in autism spectrum disorder. NeuroImage, 12, 23–33. 10.1016/j.nicl.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Fan Y, Zou Q, Wang J, Gao J-H, & Niu Z (2014). Temporal reliability and lateralization of the resting-state language network. PLoS One, 9(1), e85880 10.1371/journal.pone.0085880 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Between group differences in language network iFC across preprocessing pipelines.
Supplementary Figure 2. Regions of interest.
Supplementary Figure 3. GIMME models of PCC mediated iFC between Broca’s area and visual cortex.
Supplementary Table 1. Between group differences in language network iFC across preprocessing pipelines.
Supplementary Table 2. Significant between-group clusters from whole-brain iFC analysis across preprocessing.
Supplementary Table 3. Significant between-group clusters from whole-brain iFC analysis.
Supplementary Table 4. Subgroup information.
Supplementary Table 5. Correlations between language network iFC and ASD symptom severity.
Supplementary Table 6. Correlations between language network iFC and language scores.
Supplementary Table 7. Correlations between language network iFC results and language measures.