Abstract
Objective:
Anorexia nervosa (AN) commonly develops during adolescence. Existing literature offers some treatment guidelines, but clear clinical criteria for initial recommendations and steps of care are needed. The aim of the present study was to develop expert consensus for a stepped-care algorithm for treatment of adolescents with AN.
Method:
The Delphi approach was used to identify clinical parameters that guide initial treatment recommendations and recommendations for transitions between levels of care. The Delphi approach provides a useful expert consensus when empirical data are limited. Individuals with at least 10 years of experience in the field of adolescent AN and membership in one of three professional organizations were recruited. Twenty-five panelists participated in three rounds of iterative online questionnaires.
Results:
Consensus was achieved on several features of a treatment algorithm. Hospitalization is recommended when medical instability, suicidality, or acute food refusal are present at any point in treatment. Family-based treatment (FBT) is recommended as the first-line treatment, with a few exceptions. Consensus was not reached on when to transition from a higher level of care to a lower level of care.
Discussion:
Expert opinion was used to develop a consensus-based algorithm for care of adolescents with AN. Future research is needed to test whether these recommendations can be used to optimize outcomes for adolescents with AN.
Keywords: adolescent, algorithm, anorexia nervosa, Delphi, treatment
1. INTRODUCTION
Anorexia nervosa (AN) commonly begins in mid- to late-adolescence (Keel & McCormick, 2010). Some studies suggest that for those adolescents who receive treatment, the remission rate is as high as 50–70% (Jagielska & Kacperska, 2017; Keel & McCormick, 2010). Identifying appropriate treatment for adolescents is an opportunity to improve overall illness outcomes, as AN in adulthood has a lower remission rate (Steinhausen, 2002), and the mortality rate increases with duration of illness (Sullivan, 1995). Furthermore, as treatment is not effective for a significant minority of young people, knowing more about effective treatment options and trajectories is essential in order to support this group of young people and their families.
Treatment recommendations for AN universally indicate that weight restoration is an essential first step (Association AP, 2006). Several professional bodies have issued advisory reports specific to the treatment of adolescents with eating disorders (National Institute for Health and Care Excellence (Excellence NIfHaC, 2017); Management of Really Sick Patients under 18 with Anorexia Nervosa [Junior MARSIPAN](Ayton, Barnnett, Beattie, et al., 2015); Society of Adolescent Health and Medicine [SAHM](Golden, Katzman, Sawyer, et al., 2015); and American Academy of Pediatrics (Rosen & American Academy of Pediatrics Committee on Adolescence, 2010)). These reports all emphasize the importance of renourishment as a treatment priority. They describe the type of expertise required for the management of adolescents with AN and offer guidance for clinicians in different treatment settings. The SAHM (Golden, Katzman, Sawyer, et al., 2015) and MARSIPAN (Ayton et al., 2015) reports provide some guidelines about factors to consider in selecting a treatment setting (e.g., “consider findings from physical examination, including degree of underweight”), and SAHM specifies medical findings that might justify hospitalization. The SAHM guidelines also recommend outpatient treatment as the first-line treatment whenever possible. Yet, these reports do not offer guidelines for treatment setting recommendations based on an individual's clinical presentation. Clinical decisionmaking is largely deferred to the expert team.
Outlining symptoms that necessitate hospitalization is a helpful step for determining level of care. The SAHM and Junior MARSIPAN guidelines begin this task by detailing specific medical conditions that may warrant hospitalization (e.g., bradycardia and hypokalemia). These recommendations need to be integrated with the SAHM suggestion that family-based treatment (FBT) is an empirically supported treatment for adolescents with AN (Golden, Katzman, Sawyer, et al., 2015). The evaluating clinician is left to determine what clinical features support treatment with FBT, and when other options, or higher levels of care, might be more useful. These guidelines do not address recommendations for second and third steps of treatment. As these reports indicate, more data are needed to be able to determine the optimal treatment course for an individual adolescent. Stepped-care guidelines are needed to determine at what point a patient should be referred to a higher level of care, such as a structured day treatment program, versus a lower level of care, such as traditional outpatient treatment. Currently, different treatment centers and clinicians use their own parameters, which may vary (Maguire et al., 2008).
Ideally, treatment recommendations are based on empirical evidence for best practices. Although there is little controversy about the need for timely treatment for adolescents, and there is agreement about the importance of renourishment, empirical support directing level-of-care decisions and treatment sequencing is limited. As such, clinical consensus can be valuable. We used the Delphi approach (Linstone & Turoff, 1975) to identify clinical parameters that determine initial treatment recommendations, as well as transitions between levels of care. This method is an established technique for developing consensus by experts in the field. The Delphi method originated as a military forecasting tool in the 1960s to pool opinions among a group of experts (Fish & Busby, 1996). It has since been applied in the medical context to address clinical questions when empirical guidelines are lacking (Bader, McDonald, & Selby, 2009; Cabral et al., 2005; Eubank et al., 2016; Fish & Busby, 1996; Hasson, Keeney, & McKenna, 2000; Jones & Hunter, 1995).
The Delphi method is a multistage process, in which panelists respond over several iterative rounds with the goal of reaching consensus. Each round builds off the last: panelists are asked to clarify and reassess their responses as they learn the views of the group. The putative strengths of this method are that opinions come from individuals who have a relevant expertise (Dalkey, Rourke, Lewis, & Snyder, 1972), that the responses are anonymous and therefore cannot be unduly influenced by interpersonal factors (Fish & Busby, 1996), and that the forces of the group can be mobilized to move the experts toward consensus. The method is particularly valuable for gaining clarity and direction around a topic for which an accepted set of standards does not yet exist (Sumison, 1998). The aim of the present study was to develop consensus for an algorithm for steps of care for adolescents with AN.
2. METHOD
Participant selection, or sampling, in the Delphi method is purposive, rather than random. This method involves recruiting individuals who are experts in the area of investigation and the participants are then considered a panel. The strength of the study depends on the strength of the panel. In order to increase the rigor of this study, inclusion criteria were used to identify and recruit participants with highly specialized knowledge of the target issue. “Expertise” was defined according to the following standards: greater than 10 years of experience treating adolescents with AN as well as membership in the Eating Disorders Research Society, the Academy for Eating Disorders, or the SAHM. To identify a range of participants meeting these criteria with geographic and disciplinary diversity, study investigators first contacted 31 eating disorders professionals in relevant disciplines in both clinical and academic settings. These individuals were asked to nominate other professionals who met the established criteria of greater than 10 years of experience and membership in one of the identified professional organizations. Panel diversity was sought in order to capture a range of treatment recommendations across geographic settings and clinical disciplines. This method identified 55 individuals across specialties, including nurses, nutritionists, social workers, psychologists, psychiatrists, and adolescent medicine physicians. All 55 were invited to participate.
In the initial contact, potential participants were informed that there would be three rounds of questions. They were also instructed that the procedure depended on their commitment to participating in all three rounds. Participants remained anonymous to one another throughout the study. At the conclusion of the study, panelists were offered the opportunity to be acknowledged in the publication.
2.1. Procedures
The Delphi method differs from traditional surveys in that panelists receive feedback as to how the panel is responding throughout the study. This encourages them to consider their own responses alongside those of the entire panel and clarify or amend their responses based on this information (Hsu & Sandford, 2007). This study involved three rounds of iterative questionnaires distributed online via Qualtrics. Round 1 consisted of open-ended questions exploring the clinical factors that form the basis of recommendations for treatment at differing levels of care.
The five Round 1 questions were:
When sitting with an adolescent with AN who is in need of weight restoration, how do you decide which level of care is most appropriate for treatment (after the diagnosis has been confirmed)?
What features and what severity levels lead you to recommend specific levels of care?
In your practice, when do you decide to switch from a lower level of care to a higher level?
In your practice, when do you decide to switch from a higher level of care to a lower level of care?
If you recommend outpatient level of care, which treatment approach or modality do you typically recommend?
In order to distinguish clinical features from systems of care or insurance-related issues, a sixth question was included to address nonclinical features that contribute to treatment recommendations:
-
6.
Are there other external factors you consider when making any of the above recommendations (such as geographic location, preference for specific clinicians, etc.)?
Responses to Round 1 were collated and systematically analyzed to identify statements for Round 2, in which participants were asked to rate levels of agreement. Whenever possible, items were taken verbatim from the respondents. Round 2 consisted of 147 statements. Panelists were asked to rate their level of agreement with each item in Round 2 on a 7-point Likert scale ranging from “Strongly Agree” to “Strongly Disagree.” Panelists were also invited to leave comments alongside their responses.
Items that did not reach consensus or reached “near consensus” (e.g., 75% agreement) in Round 2 were repeated in the next round. Round 3 consisted of 43 statements. As terminology for levels of care differs by geographic location, four levels of care were defined for use in Round 3: inpatient, structured outpatient, FBT, and other outpatient therapy. Inpatient treatment was defined as settings that provide 24 hours/7 days per week care; this definition encompasses residential treatment. Structured outpatient was defined as treatment over multiple days per week and including supervised meals but not overnight care. Based on consensus achieved in Round 2, medical instability was specifically defined in Round 3 as the presence of any of the following: bradycardia (heart rate [HR] < 40), hypotension, hyponatremia, hypokalemia, or prolonged QTc.
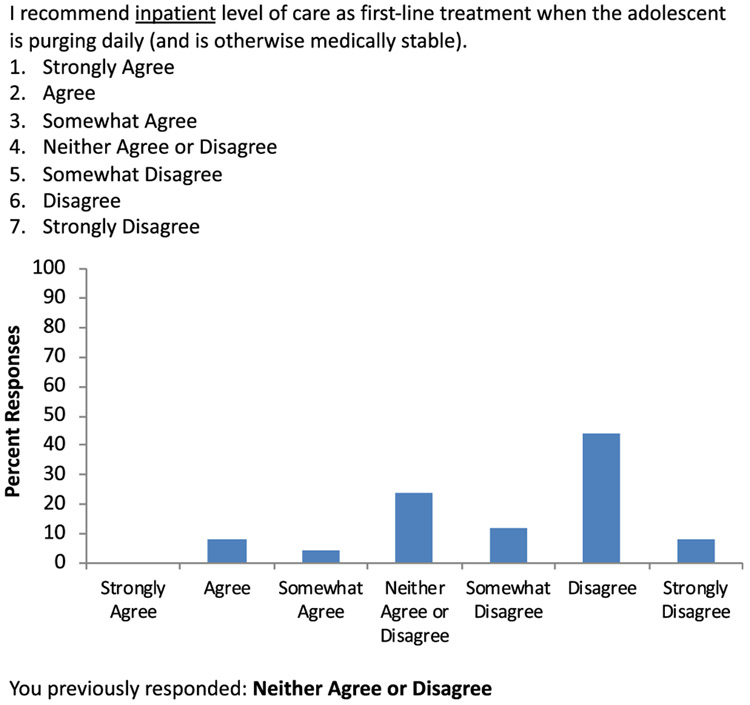
In Round 3, each statement was accompanied by an individualized display of the participant's rating from Round 2 and a histogram showing the distribution of responses for that item (Figure 1). Panelists were again provided the opportunity to leave comments.
FIGURE 1.
Sample of Round 3 question, which shows the individual's response in Round 2 as well as comparison with the group responses from Round 2
2.2. Data analysis
Responses to Round 1 were analyzed using conventional content analysis to identify discrete items (Hsieh & Shannon, 2005). Items were analyzed and grouped according to common themes. To ensure rigor, responses were coded separately by two of the study authors (S.B. and J.E.S.) to identify prevalent items or themes. There was general agreement between coders when determining themes. Items were deemed idiosyncratic if they were only attributable to a single respondent. These were not included in future rounds.
For Rounds 2 and 3, consensus was defined as 85% agreement/disagreement and “near consensus” was defined as 75% agreement/disagreement by the panel, following the methods of existing Delphi studies in eating disorders (Dawson, Rhodes, & Touyz, 2015a; Mittnacht & Bulik, 2015; Noetel, Dawson, Hay, & Touyz, 2017; Williams & Haverkamp, 2010). Agreement included participant responses of “somewhat agree,” “agree,” or “strongly agree.” Disagreement included responding “somewhat disagree,” “disagree,” or “strongly disagree.” Frequencies were calculated to determine the percentage of panelists responding in agreement or disagreement for each item.
One item in Round 3 (“I do not recommend FBT when one of the parents has an untreated substance use disorder or another psychiatric impairment”) was excluded from analyses due to a computer malfunction such that participants were not able to indicate responses.
3. RESULTS
Twenty-five individuals completed Round 1 and thereby constituted the expert panel. Study retention was 100%, with all 25 panelists completing Rounds 2 and 3. Participants included clinical psychologists (n = 11), pediatric/adolescent medicine physicians (n = 6), child/adolescent psychiatrists (n = 5), and general psychiatrists (n = 2). Despite initial invitations to eligible social workers, nurses, and dietitians, none of these professionals responded to the survey requests and therefore none were panelists. Regions represented by panelists included the Northeast United States (n = 9), the Northwest United States (n = 1), the Southwest United States (n = 5), the Southeast United States (n = 1), the United Kingdom (n = 3), the Netherlands (n = 2), Germany (n = 1), Italy (n = 1), Austria (n = 1), and Australia (n = 1). Consensus items for initial treatment recommendations and for transitions between levels of care are shown in Table 1. For each item, the table shows the mean rating (e.g., the average rating of the panel; range of 1–7) with SD, as well as the percent of agreement across the 25 panelists. Some items were worded in the affirmative and others in the negative, such that certain items achieved “disagreement consensus,” that is, consensus on what not to do. These items are presented in Table 2. Table 3 shows ratings and percent agreement for the remaining items pertaining to initial recommendations which did not achieve consensus and pertaining to transitions between levels of care. Consensus was not achieved for any items pertaining to transitioning out of a higher level of care (i.e., criteria for stepping down from inpatient care).
TABLE 1.
Consensus items
| Initial recommendations | ||||
|---|---|---|---|---|
| Item | Mean rating |
SD | %Agreement | |
| Inpatient level of care when the adolescent is at high risk for refeeding syndrome | 6.56 | 0.58 | 100 | |
| Medical instability necessitating inpatient treatment is defined by bradycardia (heart rate < 40), hyponatremia, hypokalemia, hypotension, or prolonged QTc | 6.2 | 0.71 | 100 | |
| Inpatient level of care when the adolescent is actively suicidal | 6.08 | 1.29 | 92 | |
| Family-based treatment (FBT) as a first-line treatment for adolescents | 6.08 | 1.32 | 84* | |
| Inpatient level of care when the adolescent is medically unstable | 6 | 1.47 | 88 | |
| Inpatient level of care when the parent(s) report(s) acute food refusal | 5.8 | 1.38 | 84* | |
| FBT as first-line treatment, even with co-occurring depression, OCD, or anxiety | 5.72 | 1.54 | 88 | |
| Outpatient treatment other than FBT when there is parental abuse | 5.64 | 1.22 | 80* | |
| Transitions between levels of care | ||||
| Type of transition |
Item | Mean rating |
SD | %Agreement |
| Increase | Brief medical hospitalization when an adolescent becomes medically unstable | 6.12 | 0.83 | 96 |
| Increase | Inpatient treatment when an adolescent becomes suicidal | 5.80 | 1.32 | 84* |
| No change | Additional modalities of outpatient care before recommending structured outpatient, when FBT is not succeeding (e.g., CBT, AFT) | 5.32 | 1.25 | 80* |
| Increase | Inpatient treatment when structured outpatient treatment has failed | 5.00 | 1.12 | 88 |
| Increase | Inpatient hospitalization when an adolescent is refusing to eat | 4.80 | 1.55 | 76* |
Near consensus defined as >75% agreement.
Abbreviations: CBT, cognitive behavioral therapy; AFT, adolescent-focused therapy.
TABLE 2.
Consensus disagreement (items where >85% of panel disagreed with the statement)
| Initial recommendations | |||
|---|---|---|---|
| Item | Mean rating |
SD | %(dis)agreement |
| Inpatient treatment when the adolescent or family does not have insight into the illness | 2.72 | 1.10 | 92 |
| Structured outpatient when the adolescent is medically unstable | 2.08 | 0.91 | 88 |
| Inpatient level of care as first-line treatment when the adolescent has high anxiety | 2.04 | 1.10 | 96 |
| Transitions between levels of care | |||
| Item | Mean rating |
SD | %(dis)agreement |
| Transition from outpatient to inpatient treatment when an adolescent is engaging in excessive exercise (and is medically stable) | 2.52 | 1.16 | 84* |
| Residential treatment after inpatient medical stabilization | 2.2 | 1.44 | 80* |
| Inpatient treatment when an outpatient medication trial is unsuccessful | 1.72 | 0.74 | 100 |
Near consensus defined as >75% disagreement.
TABLE 3.
Items with no consensus
| Initial recommendations | ||||
|---|---|---|---|---|
| Item | Mean rating |
SD | %Agreement | |
| Including a dietitian in the outpatient treatment team | 5.68 | 1.55 | 72 | |
| Inpatient level of care when the adolescent's BMI is less than 70% of ideal body weight | 5.00 | 1.41 | 68 | |
| Duration of illness is not a deciding factor | 4.92 | 1.66 | 64 | |
| Inpatient treatment for a medically stable adolescent who has already tried multiple other treatments, including FBT, structured outpatient, and other outpatient | 4.36 | 1.60 | 52 | |
| Structured outpatient when parents refuse to participate in treatment and the patient's home or family environment are not conducive to treatment | 4.64 | 1.11 | 64 | |
| Inpatient level of care when an adolescent's BMI is less than 75% median BMI for age and sex | 4.16 | 1.37 | 28 | |
| Structured outpatient when FBT was not sufficient and the family needs additional guidance or support around meal times | 4.00 | 1.73 | 44 | |
| Other outpatient treatments for a medically stable adolescent | 4.00 | 1.61 | 52 | |
| Inpatient level of care when an adolescent has a substance use disorder in need of treatment | 3.84 | 0.94 | 36 | |
| Inpatient level of care when an adolescent has a history of multiple failed outpatient treatment attempts | 3.28 | 1.57 | 52 | |
| Inpatient level of care when an adolescent's parents request it | 2.96 | 1.17 | 60 | |
| Structured outpatient when the adolescent is below the 10th percentile in BMI | 2.84 | 1.37 | 60 | |
| Inpatient level of care when an adolescent is purging daily (and is otherwise medically stable) | 2.68 | 1.14 | 68 | |
| Inpatient level of care when there is nonsuicidal self-injury | 2.44 | 1.04 | 72 | |
| Transitions between levels of care | ||||
| Type of transition | Item | Mean rating |
SD | % agreement |
| Increase | Different outpatient treatment before structured outpatient program, when FBT is not succeeding | 4.72 | 1.40 | 68 |
| Increase | Different type of family therapy when FBT has not been successful | 4.56 | 1.36 | 52 |
| Increase | Structured outpatient when FBT has failed* | 4.52 | 1.42 | 56 |
| Decrease | Structured outpatient treatment following inpatient treatment | 4.24 | 1.88 | 48 |
| Decrease | Transition from a higher level of care to outpatient treatment when an adolescent is gaining weight consistently | 4.16 | 1.68 | 40 |
| Decrease | Discharge from the hospital as soon as adolescent is medically stable and non-suicidal, even if still substantially underweight | 4.16 | 2.06 | 48 |
| Decrease | Transition to outpatient treatment as soon as regular eating established | 4.04 | 1.67 | 40 |
| Increase | Structured outpatient level of treatment when FBT has stalled* | 4.04 | 1.34 | 44 |
| Increase | Transition from structured outpatient to inpatient when an adolescent is refusing meals in the program for several days in a row | 4.00 | 1.26 | 50 |
| Decrease | Transition to outpatient treatment when the adolescent is at full or close to full weight restoration | 3.52 | 2.12 | 52 |
| Increase | Transition from outpatient to a higher level of care when the adolescent has failed to gain weight at the rate of 0.5lbs/week over 6–8 weeks | 3.36 | 1.50 | 60 |
| Increase | Transition from FBT or other outpatient to higher level of care when adolescent has failed to gain weight at the rate of 1 lb/week over 4 weeks | 3.28 | 1.54 | 60 |
| Decrease | Transition to structured outpatient program after about 2 weeks of inpatient treatment | 3.20 | 1.32 | 44 |
| Increase | Inpatient hospitalization when FBT has failed | 2.92 | 1.35 | 64 |
| Increase | Transition adolescent from FBT to inpatient care when the family needs a break from the work of refeeding | 2.84 | 1.49 | 68 |
Note: Mean rating of less than 4 and %agreement >50 indicates that the majority of panelists disagreed with the item.
Abbreviations: BMI, body mass index; FBT, family-based treatment.
Overlapping statements with differing responses.
Consensus was reached on several nonclinical factors, as well. Initial treatment recommendation is influenced by the expertise level of specific treatment providers (96% agreement, M = 6.4; SD = 0.71). Panelists would recommend traveling further for treatment to obtain adolescent-specific treatment (96% agreement, M = 6.08; SD = 0.57). At the near-consensus level, panelists agreed that they recommend inpatient or structured outpatient programs that include some form of family-based care for their adolescents with AN (80% agreement, M = 5.48; SD = 1.42).
4. DISCUSSION
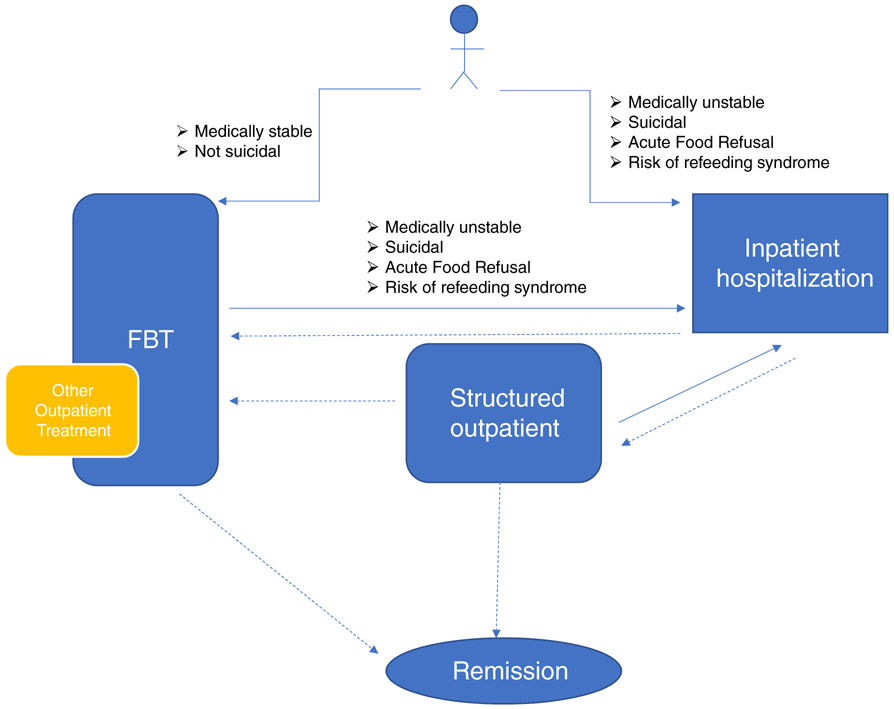
Twenty-five individuals with expertise in the treatment of adolescents with AN achieved consensus on clinical features that determine treatment recommendations using a Delphi panel method. The 100% panel retention rate provides a measure of confidence in the findings (Hasson et al., 2000). As seen in Table 1, there was consensus to a large extent for initial recommendations. The panel generally agreed that FBT should be considered first for an adolescent with AN. Inpatient treatment was recommended by the panel when the patient is medically unstable, suicidal, refusing food, or at high risk for refeeding syndrome. There was consensus about medical factors that warrant hospitalization: bradycardia (HR < 40), hypotension, hyponatremia, hypokalemia, or prolonged QTc. Consensus items can be used to begin to elaborate an algorithm for the treatment of adolescents with AN, as shown in Figure 2.
FIGURE 2.
Algorithm of care for adolescents with anorexia nervosa (AN). The Delphi panel recommends FBT as the initial treatment for an adolescent with AN unless the patient is medically unstable, suicidal, refusing food, or at high risk for refeeding syndrome (FBT is also not recommended if there is parental abuse). Adolescents should be moved to inpatient treatment if any of these clinical features emerge during treatment in either FBT or a structured outpatient setting. Panel responses also suggest that other outpatient treatments may be added to FBT or recommended instead of FBT, if response to treatment is inadequate. Decrease in level of care from inpatient to FBT is suggested when the indication for hospitalization has resolved, and eating has improved. Decrease from inpatient to structured outpatient might be recommended if food intake is inadequate, or compensatory behaviors are not controlled. Empirical research is needed to determine specific weight criteria that warrant hospitalization and to determine clinical parameters (weight gain, eating improvement, medical, and psychiatric improvement) that indicate decreases in levels of care. For this algorithm, “inpatient” was defined as settings that provide 24 hours/7 days per week care (which may include residential) and “structured outpatient” was defined as treatment over multiple days per week and including supervised meals but not overnight care
The panel consensus for treatment recommendations were largely consistent with the guidelines and goals that have been articulated in publications from professional organizations. The Junior MARSIPAN (Ayton et al., 2015) guidelines suggest considering hospitalization for medical instability and list numerous factors that might be considered. The Delphi panel consensus adds to this literature by including more specific parameters in the definition of medical instability. Furthermore, the range of expertise represented by the panel members adds to existing guidelines by confirming that there is broader expert agreement on these medical indications for hospitalization. The SAHM guidelines (Golden et al., 2015) emphasize the value of outpatient treatment for adolescents. The panel agreed at a “near-consensus” level with FBT as the first-line treatment, which is consistent with these guidelines, and with existing literature demonstrating efficacy of FBT for adolescent AN (Doyle, Le Grange, Loeb, Doyle, & Crosby, 2010; Lock, Agras, Bryson, & Kraemer, 2005; Lock et al., 2010). The panel also agreed that FBT should be recommended even when there is comorbid Obsessive-Compulsive Disorder (OCD), depression, or anxiety. The panel disagreed with the recommendation for inpatient treatment when there is “excessive exercise” in the context of medical stability. The Junior MARSIPAN guidelines refer to uncontrolled exercise as an indication for inpatient care when an adolescent is malnourished (Ayton et al., 2015), which is consistent with this panel.
Although the recommendations from the panel consensus are useful, there are also noteworthy areas where consensus was not achieved. For example, there was no consensus around a low-weight standard that would necessitate hospitalization. Published guidelines for hospitalization have included specific weight criteria. The SAHM guidelines recommend hospitalization when an adolescent weighs less than 75% of median body mass index (BMI) for age and sex, whereas the Junior MARSIPAN guidelines recommend hospitalization when weight is less than 70% of median BMI or if recent weight loss was greater than 1 kg per week for 2 consecutive weeks. In the absence of consensus, we examined whether there was a majority opinion. The majority (68%) agreed with a statement suggesting hospitalization when weight was less than 70% of ideal body weight (Table 3), but a majority indicate “Neither agree nor disagree” to hospitalization if BMI was less than 75% median BMI. In the comments, many panelists (~50%) indicated that weight alone would not be a determinant for hospitalization. This lack of consensus around a weight criterion highlights that, despite the widespread use of BMI and weight cutoffs as the primary determinant for hospitalization, experts do not universally agree with this practice. Of note, some of the research on outpatient FBT for adolescents required hospitalization if the participant's weight fell below 75% ideal body weight (Lock et al., 2010) or enrolled participants after a medical hospitalization (Lock et al., 2005). As such, the data that support the utility of FBT may or may not apply for patients at lower weights.
Additionally, the panel did not achieve consensus regarding initial treatment recommendations specifically around purging behavior. Existing guidelines list the presence of uncontrollable purging as an indication that hospitalization may be warranted (Ayton et al., 2015; Golden, Katzman, Sawyer, et al., 2015). This panel had a majority (68%) disagreeing with recommendation of inpatient care for a medically stable adolescent who is purging daily, and there was no discussion on the panel about purging later in treatment.
A notable finding from this study was the challenge for the panel in identifying criteria for discharge from the hospital or from structured outpatient treatment. There were six separate items that included criteria for decreasing level of care. The statement “I recommend discharge from the hospital as soon as the adolescent is medically stable and non-suicidal, even if he/she is still substantially underweight” received only 48% agreement, with a fairly neutral mean rating of 4.2 (where 1 indicated strongly agree, 4 was neutral, and 7 indicated strongly disagree). Likewise, the panel did not agree that transition to outpatient is warranted when the adolescent is gaining weight consistently or discharge “as soon as regular eating is established” (40% agreed with the statement, with a fairly neutral mean rating of 4.2). The statement “transition to outpatient when the adolescents is at full or close to full weight restoration” also had split decisions, with a mean rating of 3.5, suggesting that panel leaned toward disagreement (52% concurrence). These responses potentially highlight a tension between the importance of clinical stability and the importance of minimizing hospital time for an adolescent, with individual differences in resolving this clinically. Another factor that may have contributed to a lack of consensus was differences of opinion about what type of treatment is recommended after inpatient care (i.e., structured outpatient versus other outpatient). The challenge in finding consensus for this important question underscores the need for more longitudinal outcome data. There is some evidence to suggest that transitioning adolescents to outpatient FBT as soon as possible after medical stabilization is cost effective (Madden, Hay, & Touyz, 2015) and important for long-term success (Hay et al., 2014). There are also some data that might influence treatment decisions that did not come up in this panel discussion. For example, early weight gain in outpatient treatment has been shown to have prognostic significance (Doyle et al., 2010), and there are early data indicating utility of an adaptive protocol that can intensify FBT for initial nonresponders (Lock, Le Grange, Agras, et al., 2015). Figure 2 includes proposed criteria for decreases in levels of care, based on panelists’ responses. More outcomes research is needed to determine the optimal time for transitioning an adolescent to lower levels of care and if/when to recommend discharge to structured day treatment.
As noted, panelists agreed that FBT should be recommended as the first-line treatment even in the context of comorbid OCD, depression, or anxiety. Along the same lines, the panel unanimously disagreed (96%) that inpatient treatment should be recommended as the first-line treatment when the adolescent displays high anxiety, suggesting that clinicians feel strongly that an adolescent is best treated as an outpatient in all but a few specific circumstances. The panel failed to reach consensus on whether to recommend inpatient treatment when the adolescent has comorbid substance use. Some comments indicated that programs are not always suited to dual diagnosis care, and that parents may be able to incorporate treatment of both conditions in a behavior plan. Finally, the panel did not achieve consensus on treatment recommendations in the presence of nonsuicidal self-injury, although the majority (72%) indicated agreement with recommending inpatient care. Several explained that hospitalization may not be needed as symptoms can be managed successfully by a skilled outpatient provider, and one individual raised the concern that hospitalization can be contraindicated in some cases. Overall, the panel's recommendations regarding comorbidity point to a general preference for outpatient FBT and treating co-occurring conditions in the context of the eating disorder treatment.
Panelists reported several nonclinical factors that influence treatment recommendations, including the level of expertise of providers and degree of family engagement. There was also consensus that providers will only recommend inpatient or structured outpatient facilities that include some form of family-based care. Panelists agreed that if there is a waitlist for outpatient care, they would recommend inpatient treatment, suggesting that clinicians feel strongly about the urgency of treatment for adolescents with AN. Taken together, these results suggest that current treatment recommendations can be influenced by nonclinical factors, which may confound efforts to evaluate outcomes.
The strengths of the current study come from the strengths of the panel. The panelists were selected by purposive sampling wherein experts were identified by their peers, which mitigated sampling bias. The final list of experts included individuals from a range of disciplines and both clinical and academic settings. Additionally, we achieved 100% retention for all rounds and all items from the 25 panelists.
This study also had several limitations. The panel is missing the perspective and expertise of nurses, social workers, and dietitians, and is geographically limited to the United States, Western Europe, and Australia. As is inherent in the approach, the results are limited by the questions initially proposed in Round 1. This means that there may be issues related to treatment recommendations that were not captured in this study. For example, the panel did not address any treatment recommendations that may be specifically related to the presence of compensatory behaviors. Additionally, some items recommended changes in treatment when one modality was unsuccessful, but the specific clinical parameters were not defined. That is, the panel achieved consensus that inpatient treatment was recommended when structured outpatient treatment was unsuccessful, yet it is possible that panelists had different clinical criteria in mind. This is similar to challenges in the field in defining remission and recovery (Dawson, Rhodes, & Touyz, 2015a). In our algorithm in Figure 2, we have broadly defined failure to respond to treatment as referring to weight, medical, or other psychiatric symptoms. Due to a computer error, we do not have Round 3 results regarding recommendations in the setting of parental psychiatric impairment. Results from Round 2 show that the majority of the panel (19/25 respondents) agreed with this statement. This is a near-consensus level (76%) agreement. The only other item that addressed parental impairment was about parental abuse, and there was consensus that FBT was not indicated in that setting. It is reasonable to consider that the presence of psychiatric impairment in the parent might have been a consensus indication for an outpatient treatment other than FBT.
Most importantly, the Delphi method yields expert consensus but does not yield empirical evidence. More research is needed to test these recommendations and to address missing steps in the algorithm. Most glaringly, it remains unknown at what point discharge from the hospital is recommended, under what conditions structured day treatment might be beneficial, and what type of outpatient treatment is most useful when FBT is not. The lack of agreement on treatment recommendations may stem from reliance on clinical experiences, because outcome data do not yet exist. Panel responses suggest that a study that examines clinical outcomes associated with treating to various clinical targets (e.g., different BMI or BMI percentiles) would be useful.
In summary, the current study offers a consensus-based algorithm for care of adolescents with AN. There is expert consensus that treatment of adolescents with AN should begin with FBT if possible and that increased supervision in a structured setting (including inpatient) is warranted when medical severity or suicidality is present. There are majority opinions that inform suggestions for transitions to higher levels of care when renourishment goals are not met. There is, however, surprisingly little convergence around transitions from higher levels of care to lower levels of care. Empirical data are needed to test whether these recommendations can be used to optimize outcomes for adolescents with AN.
ACKNOWLEDGMENTS
This Delphi study would not have been possible without the time and expertise of the panel members who participated. The authors would like to formally acknowledge the panelists, who gave their consent to be recognized, including Douglas Bunnell, PhD, Angela Doyle, PhD, Ivan Eisler, PhD, Sara Forman, MD, Ian Frampton, PhD, Guido Frank, MD, Neville Golden, MD, Angela Guarda, MD, Beate Herpertz-Dahlmann, MD, Tom Hildebrandt, PsyD, Cynthia Kapphahn, MD, Andreas Karwautz, MD, Daniel Le Grange, PhD, Katharine Loeb, PhD, Andrea Marks, MD, Scott Moseman, MD, Lauren Muhlheim, PsyD, Dasha Nicholls, MD, Rollyn Ornstein, MD, Rebecka Peebles, MD, Jennifer Thomas, PhD, Alix Timko, PhD, Stephen Touyz, PhD, Eric van Furth, PhD, and Annemarie van Elburg, MD, PhD. Tracey Wade was the action editor for this article.
Funding information
Hilda and Preston Davis Foundation; National Institute of Mental Health, Grant/Award Number: MH110445
Footnotes
Section Editor: Tracey Wade
REFERENCES
- Association AP. (2006). Treatment of patients with eating disorders,third edition. American Journal of Psychiatry, 163(7 Suppl), 4–54. [PubMed] [Google Scholar]
- Ayton A, Barnnett R, Beattie M, Golden B, Hudson L, Le Grice S, … Wood D (2015). Summary of junior MARSIPAN: Management of really sick patients under 18 with anorexia nervosa. United Kingdom: Royal College of Psychiatrists. [Google Scholar]
- Bader P, McDonald P, & Selby P (2009). An algorithm for tailoring pharmacotherapy for smoking cessation: Results from a Delphi panel of international experts. Tobacco Control, 18(1), 34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral D, Katz JN, Weinblatt ME, Ting G, Avorn J, & Solomon DH (2005). Development and assessment of indicators of rheumatoid arthritis severity: Results of a Delphi panel. Arthritis and Rheumatism, 53(1), 61–66. [DOI] [PubMed] [Google Scholar]
- Dalkey NC, Rourke DL, Lewis R, & Snyder D (1972). Studies in the quality of life: Delphi and decision-making. Lexington, MA: Lexington Books. [Google Scholar]
- Dawson L, Rhodes P, & Touyz S (2015a). Defining recovery from anorexia nervosa: A Delphi study to determine expert practitioners' views. Advances in Eating Disorders, 3(2), 165–176. [Google Scholar]
- Doyle PM, Le Grange D, Loeb K, Doyle AC, & Crosby RD (2010). Early response to family-based treatment for adolescent anorexia nervosa. The International Journal of Eating Disorders, 43(7), 659–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eubank BH, Mohtadi NG, Lafave MR, Wiley JP, Bois AJ, Boorman RS, & Sheps DM (2016). Using the modified Delphi method to establish clinical consensus for the diagnosis and treatment of patients with rotator cuff pathology. BMC Medical Research Methodology, 16, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excellence NIfHaC. Eating disorders: Recognition and treatment (NICE guideline 69). 2017. [PubMed] [Google Scholar]
- Fish LS, & Busby DM (1996). The Delphi method In Moon SM (Ed.), Research Methods in Family Therapy (pp. 469–482). New York: Guilford. [Google Scholar]
- Golden NH, Katzman DK, Sawyer SM, Ornstein RM, Rome ES, Garber AK, … Kreipe RE (2015). Update on the medical management of eating disorders in adolescents. The Journal of Adolescent Health, 56(4), 370–375. [DOI] [PubMed] [Google Scholar]
- Golden NH, Katzman DK, Sawyer SM, Ornstein RM, Rome ES, Garber AK, … Kreipe RE (2015). Position paper of the Society for Adolescent Health and Medicine: Medical management of restrictive eating disorders in adolescents and young adults. The Journal of Adolescent Health, 56(1), 121–125. [DOI] [PubMed] [Google Scholar]
- Hasson F, Keeney S, & McKenna H (2000). Research guidelines for the Delphi survey technique. Journal of Advanced Nursing, 32(4), 1008–1015. [PubMed] [Google Scholar]
- Hay P, Chinn D, Forbes D, Madden S, Newton R, Sugenor L, … Royal Australian and New Zealand College of Psychiatrists. (2014). Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of eating disorders. The Australian and New Zealand Journal of Psychiatry, 48(11), 977–1008. [DOI] [PubMed] [Google Scholar]
- Hsieh HF, & Shannon SE (2005). Three approaches to qualitative content analysis. Qualitative Health Research, 15(9), 1277–1288. [DOI] [PubMed] [Google Scholar]
- Hsu CW, & Sandford BA (2007). Minimizing non-response in the Delphi process: How to respond to non-response. Practical Assessment Research and Evaluation, 12(17), 1–6. [Google Scholar]
- Jagielska G, & Kacperska I (2017). Outcome, comorbidity and prognosis in anorexia nervosa. Psychiatria Polska, 51(2), 205–218. [DOI] [PubMed] [Google Scholar]
- Jones J, & Hunter D (1995). Consensus methods for medical and health services research. BMJ, 311(7001), 376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keel PK, & McCormick L (2010). Diagnosis, assessment, and treatment planning for anorexia nervosa In Grilo CM & Mitchel JE (Eds.), The treatment of eating disorders: A clinical handbook (pp. 3–27). New York, NY: The Guilford Press. [Google Scholar]
- Linstone HT, & Turoff M (1975). The Delphi method: Techniques and applications. Boston: Addison-Wesley Pub. Co. [Google Scholar]
- Lock J, Agras WS, Bryson S, & Kraemer HC (2005). A comparison of short- and long-term family therapy for adolescent anorexia nervosa. Journal of the American Academy of Child and Adolescent Psychiatry, 44 (7), 632–639. [DOI] [PubMed] [Google Scholar]
- Lock J, Le Grange D, Agras WS, Moye A, Bryson SW, & Jo B (2010). Randomized clinical trial comparing family-based treatment with adolescent-focused individual therapy for adolescents with anorexia nervosa. Archives of General Psychiatry, 67(10), 1025–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock J, Le Grange D, Agras WS, Fitzpatrick KK, Jo B, Accurso E, … Stainer M (2015). Can adaptive treatment improve outcomes in family-based therapy for adolescents with anorexia nervosa? Feasibility and treatment effects of a multi-site treatment study. Behaviour Research and Therapy, 73, 90–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden S, Hay P, & Touyz S (2015). Systematic review of evidence for different treatment settings in anorexia nervosa. World J Psychiatry, 5 (1), 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire S, Le Grange D, Surgenor L, Marks P, Lacey H, & Touyz S (2008). Staging anorexia nervosa: Conceptualizing illness severity. Early Intervention in Psychiatry, 2(1), 3–10. [DOI] [PubMed] [Google Scholar]
- Mittnacht AM, & Bulik CM (2015). Best nutrition counseling practices for the treatment of anorexia nervosa: A Delphi study. The International Journal of Eating Disorders, 48(1), 111–122. [DOI] [PubMed] [Google Scholar]
- Noetel M, Dawson L, Hay P, & Touyz S (2017). The assessment and treatment of unhealthy exercise in adolescents with anorexia nervosa: A Delphi study to synthesize clinical knowledge. The International Journal of Eating Disorders, 50(4), 378–388. [DOI] [PubMed] [Google Scholar]
- Rosen DS, & American Academy of Pediatrics Committee on Adolescence. (2010). Identification and management of eating disorders in children and adolescents. Pediatrics, 126(6), 1240–1253. [DOI] [PubMed] [Google Scholar]
- Steinhausen HC (2002). The outcome of anorexia nervosa in the 20th century. American Journal of Psychiatry, 159(8), 1284–1293. [DOI] [PubMed] [Google Scholar]
- Sullivan PF (1995). Mortality in anorexia nervosa. The American Journal of Psychiatry, 152(7), 1073–1074. [DOI] [PubMed] [Google Scholar]
- Sumison T (1998). The Delphi technique: An adaptive research tool. British Journal of Occupational Therapy, 61(4), 153–156. [Google Scholar]
- Williams M, & Haverkamp BE (2010). Identifying critical competencies for psychotherapeutic practice with eating disordered clients: A Delphi study. Eating Disorders, 18(2), 91–109. [DOI] [PubMed] [Google Scholar]