SUMMARY
Nowadays, drug-induced sleep endoscopy is widely recognised as a valid tool for diagnosis and treatment planning of obstructive sleep apnoea syndrome (OSAS), as it allows a direct visualisation of sites and patterns of collapse of the upper airways (UA). Various classifications have been proposed in the literature to describe the events observed during DISE, including the NOHL (Nasopharynx cavity and walls, Oropharynx, Hypopharynx, Larynx) classification. This study was aimed at assessing which anatomical structures, according to the NOHL Classification, were most frequently involved in UA collapse in patients with moderate to severe OSAS, and evaluating treatment results with oral appliances (OA), in terms of AHI reduction. The study group consisted of 35 patients (29 M, 6 F, mean age 50.6, average BMI 26) with polysomnographic diagnosis of moderate to severe OSAS, subjected to DISE and classified according to the NOHL classification to identify the anatomical sites and patterns of UA collapse most frequently reported. Patients were subsequently addressed to mandibular advancing device (MAD) therapy and treatment results in terms of AHI reduction were evaluated. In the sample examined, the anatomical structures most frequently involved in the collapse of the UA were the nasopharynx cavity and walls and tongue base, with a correlation index of 0.35 (p < 0.04), while no significance was found for the retro-palatal area or larynx. Descriptive analysis revealed multilevel collapse in all patients, involving multiple anatomical structures in obstructive mechanics. In all patients, AHI reduction was observed after treatment with MAD (p < 0.00).
KEY WORDS: OSAS, DISE, NOHL classification, MAD therapy, AHI
RIASSUNTO
La drug-induced sleep endoscopy (DISE) è ampiamente riconosciuta come un valido strumento per la diagnosi e la pianificazione del trattamento della sindrome delle apnee ostruttive (OSAS), in quanto consente una visualizzazione diretta dei siti e pattern di collasso delle vie aeree superiori (VAS). In letteratura sono state proposte molteplici classificazioni per descrivere gli eventi osservati duranti la DISE, fra cui la NOHL (Nasopharynx cavity and walls, Oropharynx, Hypopharynx, Larynx) classification (Vicini 2012). Lo studio si prefigge gli obiettivi di identificare quali strutture anatomiche, definite secondo la classificazione NOHL, sono più rappresentate nel collasso delle VAS in pazienti con OSAS da moderato (15 ≤ AHI ≤ 30) a grave (AHI > 30), e valutare il risultato del trattamento con dispositivi orali (OA), in termini di riduzione dell’AHI. Il gruppo studio è composto da 35 pazienti (29 M, 6 F, età media 50,6, BMI medio 26) con diagnosi polisonnografica di OSAS da moderato a severo, sottoposti a DISE e classificati secondo la classificazione NOHL per individuare le sedi anatomiche e i pattern di collasso più frequentemente riportati. I pazienti sono stati successivamente indirizzati a terapia con Mandibular Advancing Devices (MAD), ed è stato valutato il risultato del trattamento in termini di riduzione dell’AHI. Nel campione esaminato, le strutture anatomiche più frequentemente coinvolte nel collasso delle VAS risultano essere il nasofaringe (N) e la base lingua (H), con indice di correlazione 0,35 (p < 0,04), mentre non è stata evidenziata significatività per l’area retropalatale (O) e la laringe (L). L’analisi descrittiva ha evidenziato inoltre un collasso multisede in tutti i pazienti, con coinvolgimento di più strutture nella meccanica ostruttiva. In tutti i pazienti è stata osservata una riduzione dell’AHI dopo terapia con MAD (p < 0,00), sebbene in alcuni casi non completamente risolutiva della patologia.
PAROLE CHIAVE: OSAS, DISE, classificazione NOHL, MAD, AHI
Introduction
Nowadays, oral appliance therapy (OA) is considered to be effective for patients with primary snoring and mild to moderate OSAS, and could be an alternative for severe OSAS patients who refuse positive air pressure (PAP) therapies 1-3. In the literature, however, there is still controversy about the selection criteria used to discriminate good responders to OA therapy from non-responders. Historically, patient selection has been based on AHI alone, although recent studies have focused on the use of drug-induced sleep endoscopy (DISE) in diagnosis 4.
The technique of DISE, developed by Croft and Pringle in 1991 5, is aimed at exploring the upper airways during pharmacologically-induced sleep using a flexible fibro-optic endoscope. DISE allows direct visualisation of obstruction sites and patterns of collapse in patients reporting snoring or OSAS, and can be useful in discriminating subjects who would benefit from surgical management from those who would not. During DISE it is also possible to mimic the effects of OA therapy by advancing the mandible with a gentle manoeuvre (pull up), in order to identify any improvements in UA patency or snoring. Patients showing a sufficient increase in UA dimension, as well as a reduction in snoring may be valid candidates for mandibular advancing devices (MAD) as a primary therapy or combined with surgery.
In the literature, many authors have developed different grading systems to describe the anatomical structures involved in UA collapse in OSAS patients 6,7, even though in this study we focused on the NOHL classification published by Vicini in 2012 8. According to the NOHL classification, grading and pattern of obstruction can be evaluated at four different anatomic levels: N (Nasopharynx cavity and walls), O (Oropharynx – retro-palatal space), H (Hypopharynx - tongue base) and L (Larynx - epiglottis). Grading of obstruction for nasopharynx cavities and pharyngeal walls can be classified as follows:
Grade 1: 0-25% collapse;
Grade 2: 25-50% collapse;
Grade 3: 50-75 % collapse;
Grade 4: total collapse during Muller manoeuvre (100%).
The grading system described can be applied for both awake and sleep endoscopy; furthermore, it is possible to define the pattern of pharyngeal collapse (for O and H), as transversal (t), antero-posterior (ap) or concentric (c). The presence of any laryngeal (L) obstruction could be reported as p (positive) or n (negative), and if a consistent grade of palatine tonsillar hypertrophy is observed, it can be noted in the final report as TS (grade 3 or 4), for an instance N2O4cTS3H3apLn.
The aims of the present study are to define which anatomical structures of UA, defined according to the NOHL classification in DISE, are involved in the obstruction mechanics in moderate to severe OSA patients, and to evaluate treatment outcomes with OA therapy in terms of AHI reduction.
Materials and methods
A retrospective study was conducted on 35 patients (29 males, 6 females, mean age 56.09 ± 9.43 years, mean BMI 26.00 ± 3.52) who underwent polysomnography (PSG) and DISE to assess the presence of OSAS and who were subsequently addressed to MAD therapy.
The study group consisted of patients treated at the Otorhinolaryngology Unit of the ‘G.B. Morgagni-Pierantoni Hospital’ (Forlì, Italy) from 2007 to 2017. Subjects included in the study met the following criteria:
age over 18 years;
diagnosis of moderate (15 ≤ AHI ≤ 30) or severe OSAS (AHI > 30);
poor adherence to C-PAP and eager to find a more comfortable alternative;
patient suitable health conditions for the execution of DISE in order to clarify the diagnostic question;
potentially eligible (after an accurate examination performed by a dentist experienced in Dental Sleep Medicine) to receive MAD treatment.
Patients came to the attention of the ENT specialist with a diagnosis of moderate or severe OSAS performed by standard full-night PSG (electron-encephalogram, oculography eye tracking, electromyogram, oronasal flow, pulse oximetry, respiratory effort, position, electrocardiogram, snoring).
The tracks obtained from the recordings were interpreted by a qualified sleep technician using the diagnostic criteria of the 2007 International Classification of Sleep Disorders and the definition of hypopnea was chosen according to the 2007 AASM scoring rules 9 (reduction of the flow ≥ 50% from the baseline, duration at least 10 sec, accompanied by ≥ 3% desaturation or an arousal).
The Epworth Sleepiness Scale Questionnaire (ESS, Johns 1993) was also administered to patients to investigate the degree of daytime sleepiness prior to therapy.
In order to clarify the diagnostic question and select the most suitable therapeutic option, patients underwent DISE with sedation induced by propofol and TCI technique. Heart rate, frequency and saturation were monitored for the duration of the examination. Once adequate sedation was achieved, a flexible fibro-endoscope was introduced through one nostril and it was thus possible to observe sites and patterns of airway obstruction during induced sleep, classifying them according to the NOHL classification. The NOHL classification was used to determine which UA anatomical structure was most frequently responsible for collapse in moderate or severe OSAS patients.
Finally, for all patients a manual mandibular advancement manoeuvre (pull up) was performed, in order to mimic the effect achieved with a MAD-type device. In our sample of 35 patients, 23 showed an increase in UA volume and reduction in snoring, while the remaining 12 reported only a reduction in snoring. Since the pull up manoeuvre resulted in an improvement, in terms of increased UA patency and/or snoring in all the examined cases, all patients were considered suitable candidates for MAD treatment. The OA consists of a customisable device, removable and titled, made with precision silicone impressions (PVS) and a construction bite detected with George Gauge at 70% of the maximum protrusive. The same type of device was not used on all patients: the choice was personalised based on the patient’s characteristics.
Patients candidate for MAD therapy showed the following characteristics:
sufficient number of dental elements to support the device;
absence of high dental mobility;
good periodontal health;
absence of DTMs or functional limitations.
The devices were delivered by dentists experienced in Dental Sleep Medicine and titrated, appointment by appointment, to optimise the therapeutic result based on data from PSG or cardio-respiratory monitoring with MAD in situ during follow-up.
The final PSG was performed at the end of the titration (on average, 6 months from the start of the therapy) with MAD in situ at optimal mandibular advancement with which it was possible to obtain resolution or reduction of symptoms.
For simplification, the AHI emerged from the initial PSG (pre-treatment), was compared with the AHI resulting from the final PSG with MAD in situ, in order to observe any improvements obtained with therapy. Treatment success was expressed as a reduction in AHI below 5 (resolution of OSA) or below 10 (very mild disease), or by a percentage reduction in AHI from baseline, which is considered to be clinically significant (typically 50% AHI reduction).
Statistical analysis
Statistical analysis was conducted with the following objectives:
-
Define the association between the N, O, H, L scores and initial AHI shown by patients
In this regard, the non-parametric correlation (Spearman rank correlation) was used, with the scores (N, O, H and L) expressed as non-continuous values.
In order to define which anatomical site was potentially responsible for collapse described in the NOHL classification and which was more predictive of moderate to severe OSAS, the parameters (N, O, H and L) were analysed individually.
-
Descriptive analysis
Since the sample size may be too small to be clinically appreciable with regards to the obstruction sites detected during DISE, many tables have been provided only for descriptive illustration without any supplementary inferential analysis.
In particular, the relatively small number of the sample (35 subjects) made it necessary to neglect the role of the obstruction patterns (ap, t, c), for which only descriptive analyses were reported.
-
Difference between AHI before and after treatment
The analysis of the difference between AHI pre- and post-treatment value (used to evaluate the response to therapy) was performed with paired sample t-test.
Statistical analysis was conducted with R Statistical software (R Core Team 2018), and the significance was set with a p value < 0.05.
Results
Predictiveness of the scores N, O, H, L with respect to initial AHI
Bivariate analysis showed a Spearman correlation index between score N and the initial AHI of 0.35 (p-value 0.04). For the score O, the correlation index was instead 0.30, (p-value 0.08), and the score H exhibited a Spearman correlation index of 0.35 (p-value 0.04). The results therefore showed a statistical significance for score N and H, both with a correlation index of 0.35 (p = 0.04), while the score O was weakly significant (p = 0.08). This implies that the nasopharynx cavity and walls (N-Nose) and the tongue base (H - Hypopharynx) were the most represented anatomic areas of collapse in this sample of patients with moderate/severe OSAS.
Descriptive analysis
Tables I-III display the average AHI by score N, O and H respectively as well as the percentages of patients for each obstruction site. No analyses were performed for the score L, because no patient reported obstructions at the level of the corresponding anatomical structure (epiglottis).
Table I.
Number of patients and initial average AHI for the score N (0-4).
| Score N | |||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Mean initial AHI | 38.5 | 31.3 | 44.3 | 43.9 | 45.7 |
| Number of patients | 4 | 14 | 7 | 8 | 2 |
| % | 11.4% | 40.0% | 20.0% | 22.9% | 5.7% |
Table II.
Number of patients and initial average AHI for score O (1-3).
| Score O | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| Mean initial AHI | 30.0 | 37.7 | 46.8 | |||
| Number of patients | 5 | 23 | 7 | |||
| % | 14.3% | 65.7% | 20.0% | |||
| 1c | 2ap | 2c | 2t | 3ap | 3c | |
| Mean initial AHI | 30.0 | 52.5 | 35.2 | 29.4 | 34.7 | 48.9 |
| Number of patients | 5 | 5 | 13 | 5 | 1 | 6 |
| % | 14.3% | 14.3% | 37.1% | 14.3% | 2.9% | 17.1% |
Table III.
Number of patients and initial average AHI for score H (1-4).
| Score H | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||||
| Mean initial AHI | 27.5 | 33.5 | 39.5 | 59.5 | ||||||
| Number of patients | 2 | 9 | 22 | 2 | ||||||
| % | 5.7% | 25.7% | 62.9% | 5.7% | ||||||
| 1t | 2ap | 2c | 2t | 3 | 3ap | 3c | 3t | 4ap | 4t | |
| Mean initial AHI | 27.5 | 35.5 | 31.8 | 24.7 | 77.0 | 32.4 | 42.5 | 38.1 | 87.0 | 32.0 |
| Number of patients | 2 | 6 | 2 | 1 | 1 | 6 | 6 | 9 | 1 | 1 |
| % | 5.7% | 17.3% | 5.7% | 2.9% | 2.9% | 17.1% | 17.1% | 25.7% | 2.9% | 2.9% |
Difference between AHI before and after treatment with MAD
The results of the paired samples t-test showed a reduction of AHI after treatment with MAD compared to AHI pre-treatment (Fig. 1), with a significant difference (p-value = 0.00). In our sample, 40% of patients showed a final AHI < 5, while 31.4% reported a post-treatment AHI between 5 and 10. In the remaining 28.6% of patients, the AHI remained higher than 10, although there was a reduction of the initial value above 50%, indicating a good response to MAD therapy, as shown in Table IV.
Figure 1.
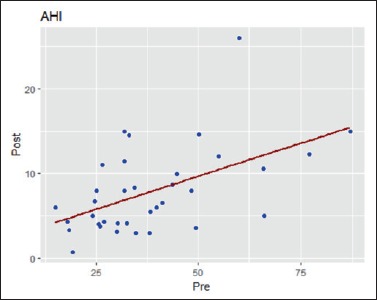
AHI values before and after MAD therapy.
Table IV.
Percentages of AHI reduction after MAD therapy compared to initial AHI.
| Initial AHI | Final AHI with MAD | AHI reduction (%) | Percentage of patients | |
|---|---|---|---|---|
| AHI post ≤ 5 | 19.2 | 0.7 | 96.4% | 40.0% |
| 38.0 | 3.0 | 92.1% | ||
| 34.7 | 3.0 | 91.4% | ||
| 30.0 | 3.1 | 89.7% | ||
| 18.3 | 3.3 | 82.0% | ||
| 49.5 | 3.6 | 92.7% | ||
| 26.0 | 3.8 | 85.4% | ||
| 25.6 | 4.0 | 84.4% | ||
| 32.4 | 4.1 | 87.3% | ||
| 30.2 | 4.1 | 86.4% | ||
| 26.9 | 4.3 | 84.0% | ||
| 18.0 | 4.3 | 76.1% | ||
| 24.2 | 5.0 | 79.3% | ||
| 66.1 | 5.0 | 92.4% | ||
| 5 < AHI post < 10 | 38.2 | 5.5 | 85.6% | 31.4% |
| 39.8 | 6.0 | 84.9% | ||
| 15.0 | 6.0 | 60.0% | ||
| 41.2 | 6.5 | 84.2% | ||
| 24.7 | 6.7 | 72.9% | ||
| 25.0 | 8.0 | 68.0% | ||
| 32.0 | 8.0 | 75.0% | ||
| 48.4 | 8.0 | 83.5% | ||
| 34.3 | 8.3 | 75.8% | ||
| 43.7 | 8.7 | 80.1% | ||
| 44.8 | 9.9 | 77.9% | ||
| AHI post ≥ 10 | 66.0 | 10.6 | 83.9% | 28.6% |
| 26.5 | 11.0 | 58.5% | ||
| 32.0 | 11.5 | 64.1% | ||
| 55.0 | 12.0 | 78.2% | ||
| 77.0 | 12.3 | 84.0% | ||
| 33.0 | 14.5 | 56.1% | ||
| 50.2 | 14.6 | 70.9% | ||
| 31.9 | 15.0 | 53.0% | ||
| 87.0 | 15.0 | 82.8% | ||
| 60.0 | 26.0 | 56.7% |
Discussion
As widely described in the literature, DISE is an excellent method to define the anatomical sites and patterns of airway obstruction in patients with OSAS, in conditions that are similar to physiological sleep 4-7,10,11.
Among the numerous classifications that can be used in DISE, the NOHL classification proposed by Vicini 8 was chosen for the present study of the anatomical sites involved in the collapse of the UA. In a recent systematic review (Amos 2018) 12, it was found that there is no consensus in the literature regarding the use of a specific classification for the description of obstructive events observed in DISE, while the grading systems used most are the VOTE classification (38.6%), followed by the classifications of Pringle and Croft (15.9%), NOHL (9.1%) and Bachar (4.5%).
Identification of the anatomical sites responsible for the obstruction and their description turns out to be of fundamental importance to clarify the diagnostic question and select the most appropriate therapeutic option, especially when a surgical solution is required.
The information obtained by DISE is also valuable for selection of patients for MAD treatment 14-16: in particular, it was observed by Cistulli et al. that MADs showed greater efficacy when tongue base obstruction occurred,, while retro-palatal collapse was the main cause of lack of or incomplete response to therapy 16-18.
However, in a study by Friedman et al. it was shown that, contrary to the hypothesis that tongue base obstruction would predict a good response to MAD therapy, OA achieved reasonable response and cure rates in patients with primary retro-palatal, retro-lingual or epiglottis obstruction 19.
This study proposes assessment of sites most responsible for airway collapse in patients with moderate to severe OSAS, to define which structure (between N, O, H and L) described in the classification appears to be more represented and therefore associated with high values of AHI pre-treatment. The results of statistical analysis showed significance for scores N and H (both with correlation index 0.35 and p value 0.04), while score O exhibited a correlation index of 0.30, with p value 0.08 and therefore weakly significant. The L score was not analysed, since all the patients included in the sample scored 0.
N and H scores, emerged as significant from the analysis, refer to the anatomical regions of the nasopharynx cavity and walls and the tongue base, which, according to the results of this study, would be more represented in the dynamics of obstruction in patients with moderate/severe OSAS, while the role of site O (retro-palatal area) is weakly significant.
It has, however, been highlighted in several studies that most obstructions occur at the retro-palatal level. In particular, in the study by Vroegop in 2014 15, 81% of obstructions occurred at the retro-palatal level, followed by multilevel collapse (68.2%) with involvement of the palate and tongue base (25%). The high prevalence of retro-palatal collapse was motivated, according to the authors, by the high frequency of snoring (due to vibration of soft palate tissues), as a nocturnal symptom commonly reported by OSAS patients.
The results achieved in the present study differ from what has just been described probably due to the retrospective design and the small sample size (35 patients). Furthermore, different classification systems have been used in DISE by previous authors (VOTE classification), which can lead to a different description of the anatomical sites.
However, the descriptive analysis of the sample showed that airway collapse was always multilevel in patients with high AHI values, as observed in other studies 10,13. The involvement of several sites in the dynamics of obstruction poses the problem of the therapeutic choice, often requiring combined therapies (MAD + CPAP / MAD + surgery).
Regarding the parameter L, no patient reported a collapse at the level of the epiglottis in the sample investigated. Before the advent of DISE, it was estimated that 12% of adult patients with OSAS showed epiglottis collapse 20. However, Torre et al. asserted in a systematic review that this aspect has long been underestimated and that, thanks to DISE, it will be possible to better clarify the role of the epiglottis in obstructive events of OSAS patients 21.
Unfortunately, it was not possible to define which obstruction pattern was more frequent for O and H, because the small number of subjects included in the sample made it possible to calculate only descriptive statistics.
In the literature, it has been found that high values of AHI and BMI are more frequently associated with complete retro-palatal collapse with concentric pattern, as well as a greater probability of complete collapse at the hypopharynx (including epiglottis), with lateral pattern 13, motivated both by the accumulation of fat at the side of the pharynx.
In subjects treated with MAD, an important reduction of the AHI with respect to the initial value was observed in all patients, underlining the confirmed efficacy of MADs in OSAS treatment. In particular, in the sample of patients examined, obstruction was more frequently observed at tongue base level (H), which could motivate the success of MAD therapy. Furthermore, it is interesting to observe how, since the study group consists of moderate/severe OSAS patients, MAD therapy often fails to completely resolve the disease (AHI is often maintained > 5). Patients who did not completely respond to MAD therapy, apart from having a very high initial AHI value, reported high scores for the N and/or the O parameters, highlighting once again a multilevel obstruction dynamic that often requires a multidisciplinary approach and evaluation for possible surgery addressed to the sites involved in UA collapse (nasal cavity and walls or palate). It should also be specified that for moderate or severe OSAS patients, the gold standard therapeutic option always remains CPAP. However, the poor adherence to this solution may lead adoption of MAD therapy alone or combined with surgery. Finally, it is noted that patients with moderate or severe OSAS often show comorbidities associated with OSAS, which may affect the final result of the therapy.
The present study has numerous limitations, starting from its retrospective design and relatively small number of patients involved. It would be interesting, however, to extend the sample and repeat the DISE with MAD in situ to observe the behaviour of the airway in response to mandibular advancement, in order to provide more precise results about the predictability of treatment with OA.
Conclusions
Within the limits of this study, it is possible to draw the following conclusions:
DISE appears to be the gold standard exam for diagnosis of obstruction sites in patients with OSAS;
among the structures described in the NOHL classification, the nasopharynx cavity and walls (N) and tongue base (H) were the obstruction sites most frequently reported in this sample of patients with moderate to severe OSAS;
although MAD therapy is not the gold standard, it is effective in reducing initial AHI in patients with moderate to severe OSAS and contributes to a reduction in symptoms;
the multilevel nature of the collapse observed in patients with moderate or severe OSAS often requires combined treatments (e.g. MAD + CPAP, MAD + surgery), emphasising the need for a multidisciplinary approach.
Figures and tables
References
- 1.Tan YK, L’Estrange PR, Luo YM, et al. Mandibular advancement splints and continuous positive airway pressure in patients with obstructive sleep apnoea: a randomized cross-over trial. Europ J Orthod 2002;24:239-49. https://doi.org/10.1093/ejo/24.3.239 10.1093/ejo/24.3.239 [DOI] [PubMed] [Google Scholar]
- 2.Randerath WJ, Heise M, Hinz R, et al. An individually adjustable oral appliance vs continuous positive airway pressure in mild to moderate obstructive sleep apnea syndrome. Chest 2002;122:569-75. https://doi.org/10.1378/chest.122.2.569 10.1378/chest.122.2.569 [DOI] [PubMed] [Google Scholar]
- 3.Ferguson KA, Ono T, Lowe AA, et al. A randomized crossover study of an oral appliance vs nasal-continuous positive airway pressure in the treatment of mild-moderate obstructive sleep apnea. Chest 1996;109:1269-75. https://doi.org/10.1378/chest.109.5.1269 10.1378/chest.109.5.1269 [DOI] [PubMed] [Google Scholar]
- 4.De Corso E, Bastanza G, Della Marca G, et al. Drug-induced sleep endoscopy as a selection tool for mandibular advancement therapy by oral device in patients with mild to moderate obstructive sleep apnoea. Acta Otorhinolaryngol Ital 2015;35:426-32. https://doi.org/10.14639/0392-100X-959 10.14639/0392-100X-959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Croft CB, Pringle MB. Sleep nasendoscopy: a technique of assessment in snoring and obstructive sleep apnoea. Clin Otolaryngol Allied Sci 1991;16:504-9. https://doi.org/10.1111/j.1365-2273.1991.tb01050.x 10.1111/j.1365-2273.1991.tb01050.x [DOI] [PubMed] [Google Scholar]
- 6.Pringle MB, Croft CB. A grading system for patients with obstructive sleep apnea based on sleep nasendoscopy. Clin Otolaryng Allied Sci 1993;18:480-4. https://doi.org/10.1111/j.1365-2273.1993.tb00618.x 10.1111/j.1365-2273.1993.tb00618.x [DOI] [PubMed] [Google Scholar]
- 7.Kezirian EJ, Hohenhorst W, De Vries N, et al. Drug-induced sleep endoscopy: the VOTE classification. Eur Arch Otorhinolaryngol 2011;268:1233-6. https://doi.org/10.1007/s00405-011-1633-8 10.1007/s00405-011-1633-8 [DOI] [PubMed] [Google Scholar]
- 8.Vicini C, De Vito A, Benazzo M, et al. The nose oropharynx hypopharynx and larynx (NOHL) classification: a new system of diagnostic standardized examination for OSAHS patients. Eur Arch Otorhinolaryngol 2012;269:1297-300. https://doi.org/10.1007/s00405-012-1965-z 10.1007/s00405-012-1965-z [DOI] [PubMed] [Google Scholar]
- 9.Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med 2012;8:597-619. https://doi.org/10.5664/jcsm.2172 10.5664/jcsm.2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.1Viana ACJ, Thuler LC, Araújo-Melo MH, et al. Drug-induced sleep endoscopy in the identification of obstruction sites in patients with obstructive sleep apnea: a systematic review. Braz J Otorhinolaryngol 2015;81:439-46. https://doi.org/10.1016/j.bjorl.2015.01.007 10.1016/j.bjorl.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blumen MB, Latournerie V, Bequignon E, et al. Are the obstruction sites visualized on drug-induced sleep endoscopy reliable? Sleep Breath 2015;19:1021-6. https://doi.org/10.1007/s11325-014-1107-5 10.1007/s11325-014-1107-5 [DOI] [PubMed] [Google Scholar]
- 12.Amos JM, Durr ML, Nardone HC, et al. Systematic review of drug-induced sleep endoscopy scoring systems. Otolaryngol Head Neck Surg 2018;158:240-8. https://doi.org/10.1177/0194599817737966 10.1177/0194599817737966 [DOI] [PubMed] [Google Scholar]
- 13.Vroegop AV, Vanderveken OM, Boudewyns AN, et al. Drug-induced sleep endoscopy in sleep-disordered breathing: report on 1,249 cases. Laryngoscope 2014; 124:797-802. https://doi.org/10.1002/lary.24479 10.1002/lary.24479 [DOI] [PubMed] [Google Scholar]
- 14.Johal A, Battagel J M, Kotecha B T. Sleep nasendoscopy: a diagnostic tool for predicting treatment success with mandibular advancement splints in obstructive sleep apnoea. Europ J Orthod 2005;27: 607-61. https://doi.org/10.1093/ejo/cji063 10.1093/ejo/cji063 [DOI] [PubMed] [Google Scholar]
- 15.Vroegop AV, Vanderveken OM, Dieltjens M, et al. Sleep endoscopy with simulation bite for prediction of oral appliance treatment outcome. J Sleep Res 2013;22:348-55. https://doi.org/10.1111/jsr.12008 10.1111/jsr.12008 [DOI] [PubMed] [Google Scholar]
- 16.Kent DT, Rogers R, Soose RJ, et al. Drug-induced sedation endoscopy in the evaluation of osa patients with incomplete oral appliance therapy response. Otolaryngol Head Neck Surg 2015;153:302-7. https://doi.org/10.1177/0194599815586978 10.1177/0194599815586978 [DOI] [PubMed] [Google Scholar]
- 17.Marklund M, Stenlund H, Franklin KA. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest 2004;125:1270-8. https://doi.org/10.1378/chest.125.4.1270 10.1378/chest.125.4.1270 [DOI] [PubMed] [Google Scholar]
- 18.Ng AT, Qian J, Cistulli PA. Oropharyngeal collapse predicts treatment response with oral appliance therapy in obstructive sleep apnea. Sleep 2006;29:666-71 [PubMed] [Google Scholar]
- 19.Friedman M, Shnowske K, Hamilton C, et al. Mandibular advancement for obstructive sleep apnea: relating outcomes to anatomy. JAMA Otolaryngol Head Neck Surg 2014;140:46-51. https://doi.org/10.1001/jamaoto.2013.5746 10.1001/jamaoto.2013.5746 [DOI] [PubMed] [Google Scholar]
- 20.Catalfumo FJ, Golz A, Westerman ST, et al. The epiglottis and obstructive sleep apnoea syndrome. J Laryngol Otol 1998;112:940-3. https://doi.org/10.1017/s0022215100142136 10.1017/s0022215100142136 [DOI] [PubMed] [Google Scholar]
- 21.Torre C, Camacho M, Liu SY, et al. Epiglottis collapse in adult obstructive sleep apnea: a systematic review. Laryngoscope 2016;126:515-23. https://doi.org/10.1002/lary.25589 10.1002/lary.25589 [DOI] [PubMed] [Google Scholar]


