Abstract
Background
We examined patient and study characteristics of pharmacotherapy and psychotherapy trials to establish whether the effects of these 2 treatment strategies can be compared meaningfully.
Methods
We inspected all randomized controlled trials included in 2 recent meta-analyses on antipsychotics and psychotherapy in patients with positive symptoms of schizophrenia, searching EMBASE, MEDLINE, PsycINFO, Cochrane Library, and ClinicalTrials.gov. Differences between psychotherapy and pharmacotherapy trials were analyzed with Wilcoxon–Mann–Whitney and chi-square tests.
Results
Eighty studies with 18 271 participants on antipsychotic drugs and 53 studies with 4068 participants on psychotherapy were included. Psychotherapy studies included less severely ill patients (P < .0001), with a shorter duration of illness (P = .021), lasted for a longer period (P < .0001), administered the intervention as add-on to antipsychotics (P < .0001), had higher risk of bias in some domains including blinding of outcome assessment (P < .0001), and were funded publicly more frequently (P < .0001). Antipsychotic trials had larger sample sizes (P < .0001) and more study centers (P < .0001), included more males (P = .0001), inpatients (P < .0001), and slightly older patients (P = .031), more often used diagnostic operationalized criteria (P = .006), and were sponsored by pharmaceutical companies. They did not differ in conflict of interest (P = .24).
Conclusions
We found key differences between the 2 groups of studies that encompass higher risk of bias in psychotherapy studies and the inclusion of more severe patients in drug trials. These differences imply that study and patient characteristics should be carefully taken into account before considering a network meta-analysis. In the interest of patients, psychopharmacologists and psychotherapists should optimize their treatments rather than seeing them in competition.
Keywords: schizophrenia, antipsychotics, psychotherapy, systematic review, trial-methodology
Introduction
There is controversy and ongoing debate about the appropriateness and efficacy of different treatment options for schizophrenia. The use of pharmacotherapy or psychotherapy has been supported as well as discouraged, based on contradictory evidence, with conflicting arguments brought by advocates of each treatment modality.
Pharmacological therapy with antipsychotics, considered the first-line treatment for schizophrenia, has been criticized for burdensome side-effects, high nonresponse and noncompliance rates.1,2 Furthermore, meta-analyses have suggested the efficacy of antipsychotics in terms of “clinically meaningful benefits” may have been overestimated, and adverse effects underestimated.3 Psychological treatments for schizophrenia are also being investigated by an increasing number of randomized controlled trials (RCTs), providing a more solid evidence base on the use of these interventions. They are addressing, among others, negative symptoms4, cognition,5 and social outcomes6, as well as positive symptoms, which are at the core of the disorder. Above all, cognitive behavioral therapy (CBT) has been found to be an effective intervention for positive symptoms when used in addition to pharmacological treatment with antipsychotics.7–9
Some authors went further, claiming that the use of CBT for patients with schizophrenia even without a parallel pharmacotherapy would be safe and acceptable, and found promising results when offering CBT alone.1,10 In a study by Morrison,10 CBT was compared with antipsychotics and with a combination of both; however, the trial was criticized for lacking an appropriate comparator, like a psychological placebo arm, to measure nonspecific effects of therapy.11 The administration of CBT without concomitant antipsychotic medication has been criticized and deemed to be unethical, suggesting that convincing evidence about its efficacy is lacking.12
Other authors claim that CBT is associated only with small effects that are not maintained in masked studies and argue on this basis that the efficacy of this treatment on positive symptoms of schizophrenia is not tenable.13 To the extreme, a recent editorial14 has stated that, due to poor trial methodology, the benefits of CBT might be inflated 5 or 6 times and argue that it should be offered no longer to people with schizophrenia. These arguments suffer from a major constraint: they are founded on indirect comparisons, via effect sizes compared mainly with placebo in studies on antipsychotics or with “treatment as usual” in studies on psychological interventions. The only attempt to compare a psychological intervention as monotherapy with antipsychotics is represented by a small pilot trial on CBT in first-episode patients.10 As a result, the debate on the use of pharmacological and psychotherapeutic treatments for schizophrenia thus far has been based on the assumption that findings of studies in these 2 domains are somehow comparable. However, a systematic comparison of study characteristics has never been conducted in the field of schizophrenia. In particular, quality of evidence can have an impact on funding allocation.
We aimed to fill this gap by (1) investigating whether and how patients enrolled in antipsychotic trials are different from those in psychotherapy trials; (2) investigating to what extent and how trials examining antipsychotics and trials examining psychotherapies differ from methodological, study quality, and conflict of interest points of view; (3) establishing whether the effects of these 2 treatment strategies could be compared meaningfully by means of these trials.
Methods
Study Design and Participants
We included randomized controlled trials from 2 recent systematic reviews on pharmacological15 and psychological16 treatments for schizophrenia. The systematic reviews were chosen because both were the most up-to-date and comprehensive in their respective fields, focused on acute treatment of positive symptoms and were conducted by the same team, assuring consistent methodology and the application of the same rules in data extraction and critical study appraisal.
Both reviews followed PRISMA guidelines and were preceded by protocols registered on PROSPERO (registration numbers CRD42013003342 and CRD42017067795) and published.17
Both reviews included published and unpublished RCTs, but in the review on antipsychotics studies also had to be double blind. This was not an inclusion criterion in the review on psychotherapy studies since in this case only the outcome assessors but not the therapists can be blind. In both reviews, studies with a high risk of bias for randomization and allocation concealment were excluded.
The 2 reviews had a similar focus: the one on antipsychotics included patients with acute exacerbations of schizophrenia, and the other on psychotherapy included studies recruiting participants who presented positive symptoms. Studies in first-episode patients were excluded in the psychotherapy review and were not excluded in the antipsychotic one, but no one was found. Trials conducted in patients with predominantly negative symptoms and with concomitant physical or psychiatric illnesses were excluded in both reviews. Risk of bias was independently assessed by 2 reviewers with the Cochrane Risk of Bias tool.18
Interventions and Comparators
All antipsychotics licensed in at least one country, with the exception of clozapine and of intramuscular formulations, and psychological treatments aimed at treating positive symptoms were included as interventions.
The comparators were placebo for antipsychotic studies and treatment as usual, psychological placebo, or other psychological interventions for psychotherapy studies, although the great majority compared the psychological intervention with treatment as usual (Supplementary File 7).
Search Strategy and Selection Criteria
The reviews searched multiple databases for relevant RCTs up to January 201816 and October 2016,15 without language restrictions (Supplementary File 1). Reference lists of previous reviews were also searched.
As trial methodology has changed over the years,15 we only included pharmacotherapy studies published from the publication year of the first psychotherapy trial onward (1996) to make the datasets even more comparable. The process of study selection is presented in Supplementary File 2.
Data Extracted
At least 2 reviewers among I.B., C.R., F.S., S.W., M.H., and C.L. extracted the data independently.
As patient characteristics, we extracted mean age, duration of illness in years, baseline severity measured in Positive and Negative Syndrome Scale (PANSS) equivalents, education, and marital status. As methodological characteristics, we extracted total sample, number of study centers involved, duration in weeks, administration of the intervention in monotherapy or in combination, ratio of male participants included, use of diagnostic operationalized criteria (such as Diagnostic and Statistical Manual of Mental Disorders [DSM] or International Classification of Diseases [ICD]), patients status at baseline by inclusion criteria (inpatients/outpatients), country, and comparator used. Study quality was assessed according to Cochrane Risk of Bias tool, in the following domains: randomization, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting.
We extracted data about funding (public, pharmaceutical company, other) and conflict of interest, judging a potential conflict of interest for studies funded by a pharmaceutical company in the case of antipsychotic trials and for studies conducted by the same authors who developed the treatment in psychotherapy trials (researchers’ allegiance).19
In addition, we evaluated the 2 meta-analyses with AMSTAR 2, a tool for critical assessment of systematic reviews.20
Data Analysis
Analyses were conducted in R (version 3.4.3).21 We compared the 2 samples with Wilcoxon–Mann–Whitney test for continuous variables and with chi-square test for dichotomous variables.
Because all analyses were considered exploratory rather than confirmatory, adjustments for multiple testing were not made.
Results
The process of study selection (Supplementary File 2) brought to the inclusion of 80 studies involving 18 271 participants on antipsychotics and 53 studies involving 4068 participants on psychological treatments (cognitive behavioral therapy, metacognitive training, mindfulness, acceptance and commitment therapy, experience-focused counseling, hallucination-focused integrative treatment, AVATAR therapy) (Supplementary File 8). Patient and study characteristics of drug studies and psychotherapy studies are presented in Supplementary File 7. The complete references of included studies are presented in Supplementary File 3. The reviews had a positive score on 15 and 14 out of 16 items of the AMSTAR 2 tool, respectively (Supplementary File 4).
Patient Characteristics
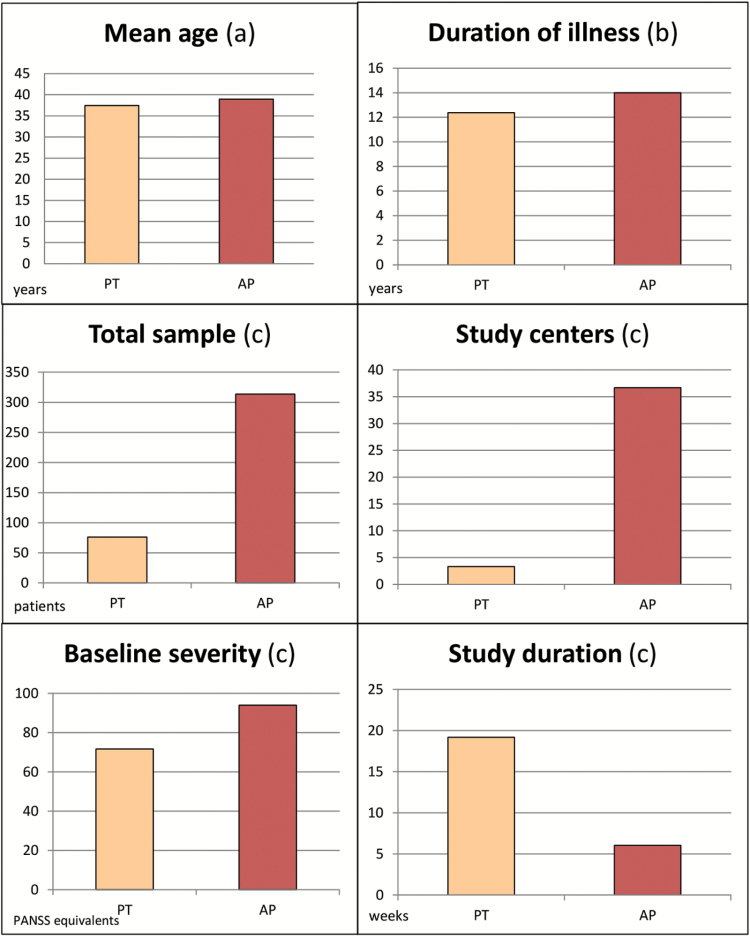
Patients enrolled in drug studies were significantly more severely ill at baseline (93.97 vs 71.72 PANSS equivalents, P < .0001), had a longer history of illness (14 vs 12.37 years, P = .021), and were older than in psychotherapy studies (38.95 vs 37.42 years, P = .031) (table 1; figure 1). Data on marital status, living conditions, and education were collected only in the studies investigating psychotherapies; this information was not reported usually in drug trials and, therefore, could not be compared among the 2 groups of studies.
Table 1.
Results of Comparison of Continuous Variables (Mann–Whitney Wilcoxon Test)
| Results of Mann–Whitney Wilcoxon Test | ||||
|---|---|---|---|---|
| Variable | Drug Studies, Mean (SD) | Psychotherapy Studies, Mean (SD) | W | P |
| Agea | 38.95 (3.78) | 37.42 (4.79) | 13 316 | .031 |
| Duration of illnessa | 14.01 (3.82) | 12.37 (4.79) | 2989.5 | .021 |
| Baseline severityb | 93.97 (7.56) | 71.72 (12.6) | 1222.5 | <.0001 |
| Total sample | 313.53 (185.07) | 76.35 (53.42) | 400.5 | <.0001 |
| Number of study centers | 36.69 (21.65) | 3.33 (2.03) | 155 | <.0001 |
| Study durationc | 6.05 (2.61) | 19.17 (12.35) | 3861.5 | <.0001 |
| Ratio of male participants | 69.96 (11.4) | 61.21 (16.5) | 1046 | .0001 |
Note: aYears.
bPANSS equivalents.
cWeeks.
Fig. 1.
Patients’ and studies’ characteristics. Wilcoxon rank sum test and chi-square test. (a) P = .031; (b) P = .021; (c) P < .0001. PT, psychotherapy; AP, antipsychotics.
Study Characteristics
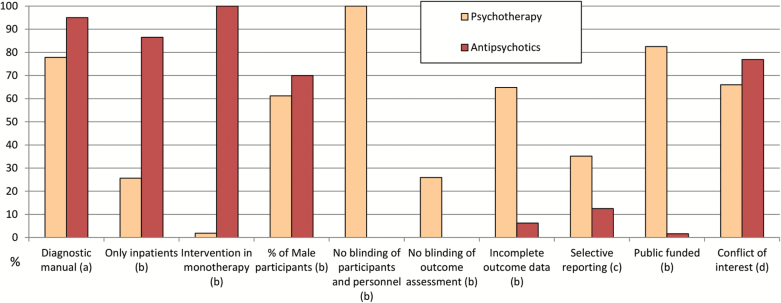
Eighty-six percent of drug studies included only inpatients at study start, whereas this was the case only for 25.64% of psychotherapy studies (P < .0001) (table 2). Studies on antipsychotics included, on average, larger samples than psychotherapy studies (313.53 vs 76.35 patients, P < .0001), involved more study centers (36.69 vs 3.33, P < .0001), and often located in different countries. Psychotherapy studies, on the contrary, were conducted mainly in only one country, and about half were conducted in the UK.
Table 2.
Results of Comparison of Dichotomous Variables (Chi-Square Test)
| Variable | Drug Studies, n/Na (%) | Psychotherapy Studies, n/Na (%) | χ 2 | P |
|---|---|---|---|---|
| Use of operationalized criteria for diagnosis | 76/80 (95) | 42/54 (77.8) | 7.53 | .006 |
| Only inpatients included | 64/74 (86.49) | 10/39 (25.64) | 39.19 | <.0001 |
| Intervention administered in monotherapy | 80/80 (100) | 1/52 (1.89) | 124.79 | <.0001 |
| High risk of bias for blinding of participants and personnel | 0/80 (0)b | 54/54 (100) | 129.88 | <.0001 |
| High risk of bias for blinding of outcome assessment | 0/80 (0)b | 14/54 (25.92) | 20.47 | <.0001 |
| High risk of bias for incomplete outcome data | 5/80 (6.25) | 35/54 (64.81) | 50.05 | <.0001 |
| High risk of bias for selective reporting | 10/80 (12.5) | 19/54 (35.19) | 8.49 | .0035 |
| Public funded | 1/61 (1.6) | 33/40 (82.5) | 67.16 | <.0001 |
| Conflict of interest | 60/78 (76.92) | 35/53 (66.04) | 1.36 | .2418 |
Note: aDenominator is not always equal to the total number of studies because only studies reporting the corresponding information were used for each analysis.
bThe studies in this review were double blinded by inclusion criteria.
In all drug studies, the antipsychotic was given in monotherapy, whereas in 52 of 53 psychotherapy studies (98%), the psychological intervention was offered as add-on to the treatment as usual, which could include the administration of antipsychotics.
Studies on antipsychotics were on average also shorter (6 vs 19.17 weeks, P < .0001), included a higher ratio of male participants (69.96% vs 61.21%, P = .0001) and referred more frequently to operationalized diagnostic criteria, rather than clinical judgement alone (95% vs 77.8%, P = .06) (tables 1 and 2; figures 1 and 2). All antipsychotic trials used pill placebo as control group, whereas the comparators used in psychotherapy were multiple: other psychological interventions not focused on the treatment of positive symptoms (20.8% of the studies), inactive controls, defined as interventions intended to control for nonspecific aspects of the therapy (17%), treatment as usual (54.7%), and waiting list (13.2%).
Fig. 2.
Percentages of individual studies presenting the given characteristics. Chi-square test. (a) P = .006; (b) P < .0001; (c) P = .0035; (d) P = .24.
Study Quality
When looking at the evaluation with the Cochrane Risk of Bias tool, the first evident difference is that none of the 8 evaluated risks is greater than 25% for the drug studies, whereas 5 of the bias domains are greater for the psychotherapy studies.
In all the drug studies, patients and clinicians were blind (due to inclusion criteria of the review), where this was the case in none of the psychotherapy studies, due to the nature of the intervention. Blind assessors were employed in all drug studies, resulting in none having high risk of bias in this domain, whereas 25.92% of psychotherapy studies did (P < .0001). Sixty-four percent of psychotherapy studies were rated as having a high risk of bias concerning incomplete outcome data, not having applied intention-to-treat analysis, or other strategies to account for missing data, whereas this was the case in 6.25% of the studies on antipsychotics (P < .0001). Reporting of results also differed in the 2 groups, with psychotherapy studies being more frequently judged at high risk of bias for selective reporting (35.19% vs 12.5%, P = .0035) (table 2).
No studies were at high risk of bias for randomization and allocation concealment, by inclusion criteria of the original reviews. Detailed risk of bias judgments and criteria used are reported in Supplementary Files 5 and 6.
Conflict of Interest and Funding
Eighty-two percent of the trials on psychotherapy were funded publicly, in comparison with 1.6% of the drug studies (P < .0001).
When looking at potential conflict of interest, identified as pharmaceutical company sponsorship for drug trials and researchers’ allegiance for psychotherapy trials, we did not observe significant differences (76.92% vs 66.04%, P = .24) (table 2).
Discussion
Main Findings
We found that studies conducted to examine the efficacy of antipsychotics and psychotherapies for schizophrenia differed in many patient and study characteristics.
On average, drug studies enrolled more severely ill patients, with a longer duration of illness, and included more frequently only inpatients in comparison with psychotherapy studies. Patients were older and more likely to be males. Also, drug studies used operationalized criteria more often to make the initial diagnosis.
Psychotherapy studies had overall a higher risk of bias across all domains. They were longer, had smaller samples, involved less study centers, and they delivered the intervention primarily as add-on to pharmacological treatment.
The great majority of psychotherapy studies were funded publicly, in contrast with drug studies that were funded by the manufacturers of the drugs examined. However, the 2 groups did not differ for potential conflict of interest because many of the psychotherapy studies presented potential researchers’ allegiance.
Interpretation of Findings
The severity of illness of the patients enrolled by the 2 kinds of studies is drastically different. With a baseline PANSS of 94, patients in drug studies can be considered markedly ill (score of 5) on the Clinical Global Impression Scale (CGI), whereas patients in the psychotherapy studies are on average not even moderately ill (a score of 71 on the PANSS corresponds to less than 4 on the CGI).22 More acutely and severely ill patients seemed, in general, not to have been enrolled in psychotherapy studies. This reflects real-world practice, where psychotherapies are probably not as feasible with patients in severe acute states, who might not have the minimum ability and readiness to collaborate. We also found that psychotherapy was almost always given in addition to usual care, that would typically include antipsychotics; only one psychotherapy study attempted to deliver cognitive behavioral therapy to patients who were not receiving concomitant antipsychotic medication.1 This finding is also consistent with what happens in clinical practice, where psychotherapy is offered usually as an add-on to antipsychotics. However, the possibility that CBT could be delivered without concomitant medication in people with schizophrenia is under scrutiny, and there have been attempts to provide CBT as monotherapy1 and to compare directly CBT as monotherapy with antipsychotics.10
The fact that participants enrolled in studies on antipsychotics were more often inpatients can be seen as a severity marker and, in this way, it is consistent with the higher severity of illness found in these patients. However, trials on antipsychotics might have been conducted more frequently in inpatient settings to better monitor for the onset of side effects and to control closely patients on placebo. Patients in drug studies were also older and had a longer history of illness; the difference was significant but not large, and in this case would favor psychotherapy trials because response rates are usually lower with more chronic patients.23
Drug studies usually involved many centers and were able to reach large samples, unlike psychotherapy trials, which had smaller samples; this could lead to greater effect sizes in psychotherapy trials,24 since larger trials have usually smaller effect sizes.25 However, in our reviews the effect size for overall symptoms was 0.47 for antipsychotics vs placebo (all double-blind studies)15 and 0.36 for CBT vs treatment as usual (11 of 13 studies rater-blind).16 Big samples are important also for better generalizability of results; however, to involve a large number of patients, participants in multicenter drug trials are often recruited by advertisements, attracting the so-called “professional patients,” and this might represent a problem in terms of external validity of the results.26
The higher ratio of male participants in drug studies may reflect the greater willingness of women to initiate psychotherapy27 or of men to enroll in a drug trial; it might have been easier for psychotherapy studies to enroll women instead of men. Because the prevalence of schizophrenia is approximately equal in men and women, psychotherapy studies appear to be more representative of this population from the gender point of view. The choice of an appropriate control group is of crucial importance and is complicated especially for psychotherapy studies. The most frequent situation in the included studies was that the psychological treatment was given in addition to usual care, whereas the control group just continued to be treated as usual. Because usual care can be different in different settings, ranging from a comprehensive package of interventions to almost no care at the other extreme, special attention must be paid such that this condition is comparable in the different studies. About 13% of the studies compared the experimental treatment with a waiting list; this might be problematic because this control has been associated with overestimation of the experimental condition.24,28 This has been defined “a nocebo effect” because the patients in the waiting list group receive the subtle message that only once they receive the treatment, in the future, will an improvement be expected from them. Seventeen percent of the studies adopted a psychological placebo as comparator, in which patients receive attention for the same amount of hours, and thus the change can be attributed to the specific therapeutic components of the therapy.
The use of placebo as control can be problematic also because the side effects associated with antipsychotic treatment might result in unblinding; regrettably, this is rarely tested in clinical trials.29 A possible alternative would be the use of an active placebo, which produces side effects similar to the ones of the experimental compound. An ongoing review is investigating the role of barbiturates and benzodiazepines as an active placebo in comparison with antipsychotics.30
From a methodological point of view in studies on psychological interventions, the blinding of clinicians and patients is not possible. This is a general limitation of psychotherapy studies that cannot be overcome. What would be possible is a blind outcome assessor, but still 25% of the studies did not employ one, resulting in an evaluation of high risk of bias in this domain.
Few psychotherapy studies applied an intention-to-treat analysis or other approaches to deal with missing data, which is relevant given that completer analysis can lead to overestimation of effect sizes.31 On average, psychotherapy studies showed a lower quality also in other domains. In addition, on average 2 of 10 studies on psychotherapy did not use operationalized criteria for the initial diagnosis, on which basis the patients were enrolled in the studies; this could represent a problem in the validity of results.
These results are in line with a previous overview of reviews by Huhn et al,24 that analyzed methodological characteristics of pharmacological and psychotherapy trials across different psychiatric conditions.
We argue that there is still considerable room for methodological improvement in studies about psychological treatments; specific guidelines for this kind of studies have been suggested and discussed.32 Improving the quality of psychotherapy studies is crucial to have a higher confidence in their results also because sources of bias have been found to influence dramatically the effect sizes.33,34 On the other side, drug trials suffer from many limitations, as well. In addition to those discussed above, dropout rates (37.2%)15 are higher compared with the ones found in CBT studies (around 15.4%).16
The majority of drug studies were funded by pharmaceutical companies. We have found that trials comparing 2 antipsychotics are prone to “industry bias.” 35 But, placebo-controlled trials conducted by pharmaceutical companies had, on average, smaller effect sizes.24 In contrast, the majority of psychotherapy studies were funded by public grants or institutions. However, more than the half were conducted by authors with a potential conflict of interest, which has been defined as researchers’ allegiance to the experimental intervention.19 If on one side it is reasonable that authors who develop a new psychological treatment want to test it in a trial, their potential vested interest must be taken into account when considering the results. Findings of (underpowered) sensitivity analyses controlling for this variable have conflicting results.16,36
Psychotherapy studies were longer because usually more weeks are needed for a psychological treatment to show an effect (eg, according to NICE guidelines CBT should be provided for around 16 sessions to patients with schizophrenia).37 However, the optimal duration of psychotherapy is still not clear. There are no studies that compare long and short term CBT (as assessed by a Cochrane Review,38 which found 0 studies). An ongoing meta-analysis is investigating the duration of different psychotherapies across different psychiatric conditions.39 On the contrary, it has been shown that the largest symptoms’ reduction is seen typically with antipsychotics within the first weeks of treatments.40,41 On the other side, there is the hope that psychotherapy induces lasting cognitive and behavioral changes and evaluating to which extent this happens is meaningful.33 Forty-three of 53 studies about psychotherapies (81.13%) included a follow-up after the end of the treatment (ranging from some weeks to 5 years) (Supplementary File 7). Such a follow-up would be difficult in drug studies because patients in the placebo-groups would receive medication likely in the meantime and because it is already known that many patients will relapse once antipsychotics have been stopped.42 Still, it could be interesting to observe these patients in time with a naturalistic follow-up.
Limitations and Strengths
We acknowledge that this work suffers from some limitations. The last search conducted for the 2 sets of studies was not exactly the same (2016 for antipsychotics and 2018 for psychotherapies), but we argue that this is a minor issue because we are aware of only one antipsychotic study that was published afterwards.43 Moreover, the 2 reviews had very similar but not identical inclusion criteria; for example, in the review on psychotherapy studies, patients with first-episode were excluded, whereas in the review on drugs, they were not excluded by inclusion criteria. However, no such drug studies were found. From the interventions’ point of view, the drug review focused on the comparison with placebo, excluding head-to-head comparisons between active treatments, whereas these were not excluded in the psychotherapy review; however, no active comparisons between psychotherapies were found because the majority of the studies used standard treatment as control. Therefore, we conclude in the end the 2 sets of studies are comparable. This work also presents important strengths: it is based on 2 reviews that were conducted applying the highest standards in terms of systematic search, study selection, and data extraction, by the same team, and were based on a priori registered protocols. This rigorous methodology ensures the quality of the subsequent analyses and results.
Implications for Research and Practice
These findings have substantial implications for research. We argue that an eventual comparison of their results should consider carefully the significant differences identified in studies investigating pharmacotherapy and psychotherapy for schizophrenia. For example, one could be tempted to conduct a network meta-analysis that allows simultaneous comparison of multiple treatments, including both these kinds of interventions, as has been done for other conditions in psychiatry.44,45 In such case, the authors should thoroughly take into account the specific characteristics of the 2 kinds of trials; above all, because the patients enrolled in the studies are noticeably different regarding variables that would have a clear impact on the results (most importantly, severity of illness), there is the risk that the transitivity principle could be violated.46 It must be noted that the meaning of such a comparison would be debatable from a clinical point of view: pharmacotherapy and psychotherapy in schizophrenia are not intended as alternative treatments but are offered to different patients or at a different phase of the illness, as made clear by our results. A possible solution could be the conduction of studies with a 2 × 2 factorial design, in which patients are simultaneously randomized to different pharmacological and psychological interventions.47 Future studies on psychological interventions should aim for a more rigorous methodological quality. Enhancing the precision and credibility of the evidence is crucial for a meaningful allocation of resources. Also, it has been claimed that psychotherapy should focus on specific symptoms, rather than be evaluated as a substitute for antipsychotics.48,49 We go further and argue that, in the interest of patients, antipsychotic and psychological treatments should not be seen in competition, but rather, should be intended to be used jointly. Future studies should investigate the effect of combinations of antipsychotic and psychological treatments, to establish which patients can best profit from which synergistic association of these treatment options.
Supplementary Material
Acknowledgments
We thank colleagues who helped with data extraction, Patricia Kratochwill for full-text acquisition and proof-reading, and Yikang Zhu for screening and data extraction (Chinese studies). In the last 3 years, S.L. has received honoraria as a consultant/advisor and/or for lectures from LB Pharma, Otsuka, Lundbeck, Boehringer Ingelheim, LTS Lohmann, Janssen, Johnson and Johnson, TEVA, MSD, Sandoz, SanofiAventis, Angelini, Sunovion, Recordati, and Gedeon Richter. C.L. is the spouse of S.L.; therefore, his conflicts of interest are also relevant for her. M.H. has received speaker’s honoraria from Janssen and Lundbeck. All other authors declare no competing interests.
Funding
The original reviews whose data were reanalysed for this work were funded by the German Federal Ministry for Education and Research (Bundesministerium für Bildung und Forschung, BMBF), grant FKZ 01KG1115 (Leucht et al. 2017) and by European Union’s Horizon 2020 Research and Innovation Programme, Marie Skłodowska-Curie grant agreement no. 701717 (Bighelli et al. 2018). The funder had no role in study design, data collection, analysis or interpretation, writing of the report, or decision to submit the paper for publication.
References
- 1. Morrison AP, Dunn G, Turkington D, et al. Cognitive therapy for patients with schizophrenia – authors’ reply. Lancet. 2014;384(9941):401–402. [DOI] [PubMed] [Google Scholar]
- 2. Lieberman JA, Stroup TS, McEvoy JP, et al.; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. [DOI] [PubMed] [Google Scholar]
- 3. Morrison AP, Hutton P, Shiers D, et al. Antipsychotics: is it time to introduce patient choice? Br J Psychiatry. 2012;201:83–84. [DOI] [PubMed] [Google Scholar]
- 4. Cella M, Preti A, Edwards C, et al. Cognitive remediation for negative symptoms of schizophrenia: a network meta-analysis. Clin Psychol Rev. 2017;52:43–51. [DOI] [PubMed] [Google Scholar]
- 5. van Oosterhout B, Smit F, Krabbendam L, et al. Metacognitive training for schizophrenia spectrum patients: a meta-analysis on outcome studies. Psychol Med. 2016;46(1):47–57. [DOI] [PubMed] [Google Scholar]
- 6. Almerie MQ, Okba Al Marhi M, et al. Social skills programmes for schizophrenia. Cochrane Database Syst Rev. 2015;6:CD009006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wykes T, Steel C, Everitt B, et al. Cognitive behavior therapy for schizophrenia: effect sizes, clinical models, and methodological rigor. Schizophr Bull. 2008;34(3):523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zimmermann G, Favrod J, Trieu VH, et al. The effect of cognitive behavioral treatment on the positive symptoms of schizophrenia spectrum disorders: a meta-analysis. Schizophr Res. 2005;77(1):1–9. [DOI] [PubMed] [Google Scholar]
- 9. Jauhar S, Laws KR, McKenna PJ. CBT for schizophrenia: a critical viewpoint. Psychol Med. 2019;49(8):1233–1236. [DOI] [PubMed] [Google Scholar]
- 10. Morrison AP, Law H, Carter L, et al. Antipsychotic drugs versus cognitive behavioural therapy versus a combination of both in people with psychosis: a randomised controlled pilot and feasibility study. Lancet Psychiatry. 2018;5(5):411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jauhar S. Cognitive behavioural therapy – a valid alternative to antipsychotics for psychosis? Lancet Psychiatry. 2018;5(5):381–383. [DOI] [PubMed] [Google Scholar]
- 12. Mustafa FA. Stand-alone cognitive behavioural therapy is not in clinical equipoise with antipsychotic treatment. Lancet Psychiatry. 2018;5(7):540. [DOI] [PubMed] [Google Scholar]
- 13. Jauhar S, McKenna PJ, Radua J, et al. Cognitive-behavioural therapy for the symptoms of schizophrenia: systematic review and meta-analysis with examination of potential bias. Br J Psychiatry. 2014;204(1):20–29. [DOI] [PubMed] [Google Scholar]
- 14. Laws K, Gournay K. Why cognitive behavioural therapy should stop being offered to people with schizophrenia. Br J Mental Health Nurs. 2018;7(5):200–201. [Google Scholar]
- 15. Leucht S, Leucht C, Huhn M, et al.. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, Bayesian meta-analysis, and meta-regression of efficacy predictors. Am J Psychiatry. 2017;174(10):927–942. [DOI] [PubMed] [Google Scholar]
- 16. Bighelli I, Salanti G, Huhn M, et al. Psychological interventions to reduce positive symptoms in schizophrenia: systematic review and network meta-analysis. World Psychiatry. 2018;17(3):316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bighelli I, Salanti G, Reitmeir C, et al.. Psychological interventions for positive symptoms in schizophrenia: protocol for a network meta-analysis of randomised controlled trials. BMJ Open. 2018;8(3):e019280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins JPT, ed. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Chichester, UK: John Wiley & Sons; 2011. [Google Scholar]
- 19. Munder T, Brütsch O, Leonhart R, et al. Researcher allegiance in psychotherapy outcome research: an overview of reviews. Clin Psychol Rev. 2013;33(4):501–511. [DOI] [PubMed] [Google Scholar]
- 20. Shea BJ, Reeves BC, Wells G, et al.. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. http://www.R-project.org/. [Google Scholar]
- 22. Leucht S, Kane JM, Kissling W, et al. What does the PANSS mean? Schizophr Res. 2005;79(2–3):231–238. [DOI] [PubMed] [Google Scholar]
- 23. Rabinowitz J, Werbeloff N, Caers I, et al.. Determinants of antipsychotic response in schizophrenia: implications for practice and future clinical trials. J Clin Psychiatry. 2014;75(4):e308–e316. [DOI] [PubMed] [Google Scholar]
- 24. Huhn M, Tardy M, Spineli LM, et al.. Efficacy of pharmacotherapy and psychotherapy for adult psychiatric disorders: a systematic overview of meta-analyses. JAMA Psychiatry. 2014;71(6):706–715. [DOI] [PubMed] [Google Scholar]
- 25. Dechartres A, Trinquart L, Boutron I, et al. Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ. 2013;346:f2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leucht S, Hierl S, Kissling W, et al. Putting the efficacy of psychiatric and general medicine medication into perspective: review of meta-analyses. Br J Psychiatry. 2012;200(2):97–106. [DOI] [PubMed] [Google Scholar]
- 27. Liddon L, Kingerlee R, Barry JA. Gender differences in preferences for psychological treatment, coping strategies, and triggers to help-seeking. Br J Clin Psychol. 2018;57(1):42–58. [DOI] [PubMed] [Google Scholar]
- 28. Furukawa TA, Noma H, Caldwell DM, et al. Waiting list may be a nocebo condition in psychotherapy trials: a contribution from network meta-analysis. Acta Psychiatr Scand. 2014;130(3):181–192. [DOI] [PubMed] [Google Scholar]
- 29. Baethge C, Assall OP, Baldessarini RJ. Systematic review of blinding assessment in randomized controlled trials in schizophrenia and affective disorders 2000–2010. Psychother Psychosom. 2013;82(3):152–160. [DOI] [PubMed] [Google Scholar]
- 30. Siafis S, Davis J, Papazisis G, Leucht S.. Antipsychotic Drugs Versus Barbiturates or Benzodiazepines as Active Placebos for Schizophrenia. PROSPERO International Prospective Register of Systematic Reviews; 2018. http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018086263. Accessed April 3, 2019. [Google Scholar]
- 31. Cuijpers P, van Straten A, Bohlmeijer E, et al. The effects of psychotherapy for adult depression are overestimated: a meta-analysis of study quality and effect size. Psychol Med. 2010;40(2):211–223. [DOI] [PubMed] [Google Scholar]
- 32. Guidi J, Brakemeier EL, Bockting CLH, et al.. Methodological recommendations for trials of psychological interventions. Psychother Psychosom. 2018;87(5):276–284. [DOI] [PubMed] [Google Scholar]
- 33. Munder T, Barth J. Cochrane’s risk of bias tool in the context of psychotherapy outcome research. Psychother Res. 2018;28(3):347–355. [DOI] [PubMed] [Google Scholar]
- 34. Cuijpers P, Karyotaki E, Reijnders M, et al. Was Eysenck right after all? A reassessment of the effects of psychotherapy for adult depression. Epidemiol Psychiatr Sci. 2019;28( 1):21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heres S, Davis J, Maino K, et al. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine: an exploratory analysis of head-to-head comparison studies of second-generation antipsychotics. Am J Psychiatry. 2006;163(2):185–194. [DOI] [PubMed] [Google Scholar]
- 36. Turner DT, van der Gaag M, Karyotaki E, et al. Psychological interventions for psychosis: a meta-analysis of comparative outcome studies. Am J Psychiatry. 2014;171(5):523–538. [DOI] [PubMed] [Google Scholar]
- 37. NICE. Schizophrenia (Update) 2009. http://www.nice.org.uk/CG82. Accessed April 3, 2019.
- 38. Naeem F, Farooq S, Kingdon D. Cognitive behavioral therapy (brief vs standard duration) for schizophrenia. Schizophr Bull. 2014;40(5):958–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Juul S, Poulsen S, Lunn S, et al. Short-term versus long-term psychotherapy for adult psychiatric disorders: a protocol for a systematic review with meta-analysis and trial sequential analysis. Syst Rev. 2019;8(1):169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McMahon RP, Kelly DL, Boggs DL, et al.. Feasibility of reducing the duration of placebo-controlled trials in schizophrenia research. Schizophr Bull. 2008;34(2):292–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Samara MT, Leucht C, Leeflang MM, et al.. Early improvement as a predictor of later response to antipsychotics in schizophrenia: a diagnostic test review. Am J Psychiatry. 2015;172(7):617–629. [DOI] [PubMed] [Google Scholar]
- 42. Leucht S, Tardy M, Komossa K, et al.. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063–2071. [DOI] [PubMed] [Google Scholar]
- 43. Ishigooka J, Iwashita S, Tadori Y. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia in Japan: a 6-week, randomized, double-blind, placebo-controlled study. Psychiatry Clin Neurosci. 2018;72(9):692–700. [DOI] [PubMed] [Google Scholar]
- 44. Zhou X, Cipriani A, Zhang Y, et al.. Comparative efficacy and acceptability of antidepressants, psychological interventions, and their combination for depressive disorder in children and adolescents: protocol for a network meta-analysis. BMJ Open. 2017;7(8):e016608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bryden PA, Caldwell DM, Welton N, et al.. Network meta-analysis of the relative efficacy of pharmacological and psychological interventions in adults with obsessive compulsive disorder. Value Health. 2014;17(7):A454–A455. [DOI] [PubMed] [Google Scholar]
- 46. Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3(2):80–97. [DOI] [PubMed] [Google Scholar]
- 47. Subotnik KL, Casaus LR, Ventura J, et al.. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia. A randomized clinical trial. JAMA Psychiatry. 2015;72(8):822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Laws KR, Darlington N, Kondel TK, et al. Cognitive behavioural therapy for schizophrenia – outcomes for functioning, distress and quality of life: a meta-analysis. BMC Psychol. 2018;6(1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Birchwood M, Trower P. The future of cognitive-behavioural therapy for psychosis: not a quasi-neuroleptic. Br J Psychiatry. 2006;188:107–108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.