Abstract
Neurological soft signs (NSS) are often found in patients with schizophrenia. A wealth of neuroimaging studies have reported that NSS are related to disturbed cortical-subcortical-cerebellar circuitry in schizophrenia. However, the association between NSS and brain network abnormalities in patients with schizophrenia remains unclear. In this study, the graph theoretical approach was used to analyze brain network characteristics based on structural magnetic resonance imaging (MRI) data. NSS were assessed using the Heidelberg scale. We found that there was no significant difference in global network properties between individuals with high and low levels of NSS. Regional network analysis showed that NSS were associated with betweenness centrality involving the inferior orbital frontal cortex, the middle temporal cortex, the hippocampus, the supramarginal cortex, the amygdala, and the cerebellum. Global network analysis also demonstrated that NSS were associated with the distribution of network hubs involving the superior medial frontal cortex, the superior and middle temporal cortices, the postcentral cortex, the amygdala, and the cerebellum. Our findings suggest that NSS are associated with alterations in topological attributes of brain networks corresponding to the cortical-subcortical-cerebellum circuit in patients with schizophrenia, which may provide a new perspective for elucidating the neural basis of NSS in schizophrenia.
Keywords: neurological soft signs, gray matter, structural brain network, schizophrenia, graph theoretical approach, network characteristics
Introduction
Neurological soft signs (NSS), subtle neurological abnormalities of motor and sensory function, are frequently found in patients with schizophrenia.1–5 As one potential target feature of neurological abnormalities for psychosis,6 the neural basis of NSS has long been of interest to neuroscientists.2 Traditionally, NSS are defined as minor and non-localizable neurological abnormalities.2,7,8 With neuroimaging technologies, accumulating evidence suggests that NSS may be associated with morphometric or functional deficits in specific brain regions. Previous structural magnetic resonance imaging (sMRI) studies have shown that NSS in patients with schizophrenia are correlated with altered gray matter (GM) morphometric characteristics mainly involving the prefrontal cortex, the superior temporal cortex, the precentral area, the postcentral cortex, the thalamus, the basal ganglia and the cerebellum.9–12 Results from functional magnetic resonance imaging (fMRI) studies have also shown that NSS are correlated with abnormal activation at the prefrontal, the precentral, the postcentral and the insula cortices in schizophrenia.13–15
There is increasing evidence suggesting the presence of abnormal functional and structural connectivity in patients with schizophrenia. Findings from resting-state functional connectivity (rsFC) studies demonstrated that there were significant hyper- and hypo-connectivities in multiple regions.16–19 Studies using diffusion tensor imaging (DTI) and structural MRI also reported altered white matter (WM) tracts or abnormalities in diffusion tensor properties,20–22 as well as altered anatomical configuration of brain networks23–25 in patients with schizophrenia, which may provide anatomical evidence for functional dysconnectivity in schizophrenia.
However, although NSS and disorganized brain structural/functional connectivity in schizophrenia have frequently been reported, few studies have examined how brain network disconnectivities are linked to NSS in schizophrenia. Limited empirical findings based on DTI reveal that the structural organization of WM at the network level contributes to NSS in healthy controls.26 Functional MRI studies have found that NSS are correlated with altered functional connectivity in multiple brain networks in healthy controls27 and in patients with schizophrenia.28,29 However, to date, the network-level neural basis of NSS have been investigated primarily based on DTI or functional MRI.26–29 Due to the clear associations between NSS and GM morphometric abnormalities in patients with schizophrenia,9–12 brain connectivity based on GM volume will also be critical to explain the occurrence of NSS. Several previous studies have also stressed the importance of studying brain network characteristics based on structural MRI.30,31 Moreover, network attributes from structural MRI data are broadly consistent with functional networks, suggesting a degree of topological isomorphism between whole-brain structural and functional networks.32,33 Therefore, the examination of networks from structural MRI could provide an anatomical basis of brain functional connectivity. In addition, in the present study, we included the cerebellum in constructing our networks. The cerebellum is critical for coordinating complex motor functions4,26,34 and is often found to be associated with NSS in patients with schizophrenia.35,36 The inclusion of the cerebellum in our analysis enabled us to comprehensively examine the neural basis of NSS at the network level.
In the present study, graph theory-based approaches were used to examine structural network characteristics based on GM morphometry and their associations with NSS. Graph theory is a powerful method for quantifying the brain as a complex network.37 It provides unique insight into the structural architecture of the brain by testing topological parameters to assess the integration, segregation, and centrality of the brain network. We thus extended our previous work by exploring whether and how NSS were associated with brain structural morphometry at the network level. Based on previous findings, we hypothesized that NSS would be correlated with abnormal GM structural networks responsible for motor and sensory function involving the frontal, the temporal, the pre- and postcentral areas and the cerebellum. We expected that a thorough characterization of the relationship between NSS and GM structural networks may help to enhance our understanding of the neural basis of NSS at the network level.
Materials and Methods
Participants
In the present study, we made use of data from a previous study examining the association between NSS and GM morphometry in patients with schizophrenia.38 One hundred one (63 males) patients with schizophrenia were recruited from the Department of Psychiatry, the University of Heidelberg. The diagnoses of the schizophrenia patients were established using the German version of the Structured Clinical Interview for DSM-IV.39
The severity of schizophrenia symptoms was rated using the Brief Psychiatric Rating Scale (BPRS).40 All of the patients were in-patients and right-handed. Their mean age was 27.36 ± 7.73 years and the mean education level was 11.41 ± 1.61 years. All patients were treated with second-generation antipsychotics (mean daily dose in chlorpromazine equivalence = 678.38 mg, SD = 375.23 mg). None of the patients had a history of neurological disorder. The study was approved by the Ethics Committee of the Medical Faculty, the University of Heidelberg. All participants gave written informed consent.
NSS Assessments
NSS were assessed using the Heidelberg Scale3 after remission of acute psychotic symptoms. This scale included 5 subscales comprising 16 items with 5 items for motor coordination, 3 items for integrative functions, 2 items for complex motor tasks, 4 items for right/left and spatial orientation, and 2 items for hard signs. Ratings are given on a 0 (no prevalence) to 3 (marked prevalence) point scale (no/slight/moderate/marked abnormality). Interrater reliability (r = .88, P < .005) and internal reliability (Cronbach’s alpha = .85 for schizophrenia) were established.
To further investigate the relationship between NSS and GM structural brain networks, the whole group was divided into 2 subgroups based on their mean NSS scores, similar to the method of previous work.9,41 This method yielded 46 patients with “high” NSS (patients with more marked NSS) and 55 patients with “low” NSS (patients with no or few NSS).
Image Acquisition
Structural MRI data were obtained at the German Cancer Research Centre with a 1.5-T Magnetom Vision MR scanner (Siemens Medical Solutions, Erlangen, Germany) using a T1-weighted 3D magnetization prepared rapid gradient echo sequence (MP-RAGE, 126 coronar slices, image matrix = 256 × 256, voxel size = 0.98 mm × 0.98 mm × 1.8 mm, TR = 10 ms, TE = 4 ms, and flip angle = 12°).
Image Preprocessing
VBM8 (http://dbm.neuro.uni-jena.de/vbm) was used for imaging pre-processing and analysis in SPM8 (Wellcome Department of Imaging Neuroscience; http://www.fil.ion.ucl.ac.uk/spm) on the Matlab 2013b platform http://www.mathworks.com/products/matlab). Detailed steps of VBM analysis have been reported in previous studies.42,43 In brief, it included (1) normalization of all the brain images to a customized template; (2) segmentation of normalized brain images; and (3) modulation of the normalized GM images.
One hundred sixteen ROIs (including the cerebellum) were generated with the WFU PickAtlas Toolbox using the Automated Anatomical Labeling (AAL) atlas.44 These cerebral ROIs were re-sliced based on the normalized GM images. The REX toolbox (https://web.mit.edu/swg/software.htm) was then used to extract ROI volumes by masking ROIs to the individual modulated and normalized GM images.
Network Analysis
Network Construction
In this study, the nodes represented the extracted regional GM morphometry of the 116 ROIs by the REX toolbox, and edges were the Pearson correlation coefficients between ROIs. Structural correlation matrices (116 × 116) were then constructed by calculating the Pearson correlation coefficients between the ROIs for each group. Age, gender, length of education, and brain volume were entered as covariates to control for potential confounding effects. Each binary adjacency matrix was then derived based on the association matrix by thresholding a range of densities (Dmin: 0.02:0.5; Dmin = 0.21). The minimum density was determined in which all nodes were fully connected (Dmin = 0.21). The upper limit of the range was 0.5 as GM structural networks were less biological above this threshold.45
Global Network Measures
Clustering coefficient (C) and characteristics path length (L) are 2 key metrics for investigating the global topological properties of a network.46–48 The cluster coefficient of a node represents the number of edges between its nearest neighbors. The characteristic path length is the average shortest path passing the node and is a widely used metric for network integration. The small-world index was defined as (C/Crand)/ (L/Lrand), where Crand was the mean clustering coefficient and Lrand was the characteristic path length of the random network.49 A network with small-world index has significantly higher clustering coefficient than in random networks (C/Crand ratio > 1), and comparable characteristic path length to random networks (L/Lrand ratio ≈ 1).48,50
Regional Network Measures
Regional network characteristics were assessed based on nodal betweenness centrality for all ROIs. The betweenness of a node represents the fraction of all the shortest paths that pass through it. For each ROI, it was calculated when the network was not fragmented or fully connected at minimum density.47
Network Hubs and Modularity
Network hubs describe the importance of the nodes in a network and are crucial regulators for efficient information communication in brain networks.48 A hub also plays a key role in network resilience.47 A node was considered a hub if its betweenness was more than 2 SDs above the mean betweenness.48,51 Modularity is a metric reflecting network segregation by subdividing the network into groups of regions with minimal between-group connections and maximal within-group links.48
Statistical Analysis for Group Comparison
To investigate the differences between the 2 subgroups in global and regional network measures, parametric t-test, and nonparametric permutation test with 1000 repetitions were performed.51 For permutation testing, in each repetition, we reassigned the measures of each participant randomly to one of the 2 groups. In this case, both the original group and the randomized group had the same number of participants. Then, the difference in network measures between randomized groups was calculated across a wide range of densities. Two-tailed P values were obtained based on its percentile position.48,52 In addition, for regional measures, false discovery rate (FDR) corrected P values were also reported to correct for multiple comparisons (number of densities×number of ROIs) with P < .05 considered significant. To test the resilience of the brain network against random failure and targeted attacks, the same permutation analyses were performed. Then the group difference in network behavior was measured at each attack.
In addition to performing group difference analysis at every density, areas under a curve (AUC) and functional data analysis (FDA) were also performed for each network measure so that the between-group differences were less sensitive to the thresholding values. Finally, to investigate the potential effects of antipsychotic medications and duration of untreated psychosis on network characteristics, we further re-analyzed the data by adding them as covariates. All the networks analyses were performed using the Graph Analysis Toolbox (GAT)48 (http://brainlens.org/tools.html).
Results
Demographic Data
The clinical and demographic characteristics of the whole sample and the 2 subgroups are summarized in table 1. The NSS-high subgroup was significantly older and had lower educational attainment. To control for the potential confounding effects of these differences on brain networks, age, and educational level were included as covariates in the subsequent analysis.
Table 1.
Clinical and Demographic Variables
| Whole Sample (n = 101) | NSS-Low (n = 55) | NSS-High (n = 46) | P-value (NSS-Low vs NSS-High) | |
|---|---|---|---|---|
| Age (y) | 27.36 (7.73) | 25.55 (6.49) | 29.52 (8.58) | .01 |
| Length of education (y) | 11.41 (1.61) | 11.82 (1.55) | 10.91 (1.55) | .004 |
| Sex (M/F) | 63/38 | 34/21 | 29/17 | .89 |
| BPRS score | 37.31 (15.69) | 35.36 (13.05) | 39.60 (18.21) | .18 |
| Duration of untreated psychosis of illness (mo) | 20.36 (39.97) | 16.22 (23.63) | 25.30 (53.24) | .29 |
| Chlorpromazine equivalents (mg/d) | 678.38 (375.23) | 639.39 (376.31) | 725.00 (372.66) | .26 |
| Heidelberg Scale total score | 15.18 (6.88) | 10.02 (3.57) | 21. 35 (4.30) | <.001 |
| Motor Coordination | 6.02 (3.85) | 3.69 (2.77) | 8.80 (3.02) | <.001 |
| Integrative function | 1.72 (1.37) | 1.15 (1.13) | 2.41 (1.31) | <.001 |
| Complex motor tasks | 2.96 (1.72) | 2.15 (1.33) | 3.93 (1.64) | <.001 |
| Spatial orientation | 2.03 (2.12) | 1.20 (1.45) | 3.02 (2.38) | <.001 |
| Hard signs | 2.45 (1.82) | 1.84 (1.51) | 3.17 (1.90) | <.001 |
Note: BPRS, Brief Psychiatric Rating Scale; SD in parenthesis.
Global Topology of Brain Structural Networks
The inter-regional association matrices and binary adjacency matrices at minimum density (Dmin = 0.11) of the NSS-High and NSS-Low groups are shown in supplementary figure S1. Changes in global network measures across a range of densities are shown in supplementary figure S2. For both groups, the normalized clustering coefficients (C/Crand ratio) were greater than 1 in a range of densities (0.11–0.5). In addition, the normalized path lengths of both groups were close to 1. Thus, the GM structural networks of both NSS-High and NSS-Low groups followed small-world properties in a range of densities.
Group Analysis on Global Network Measures
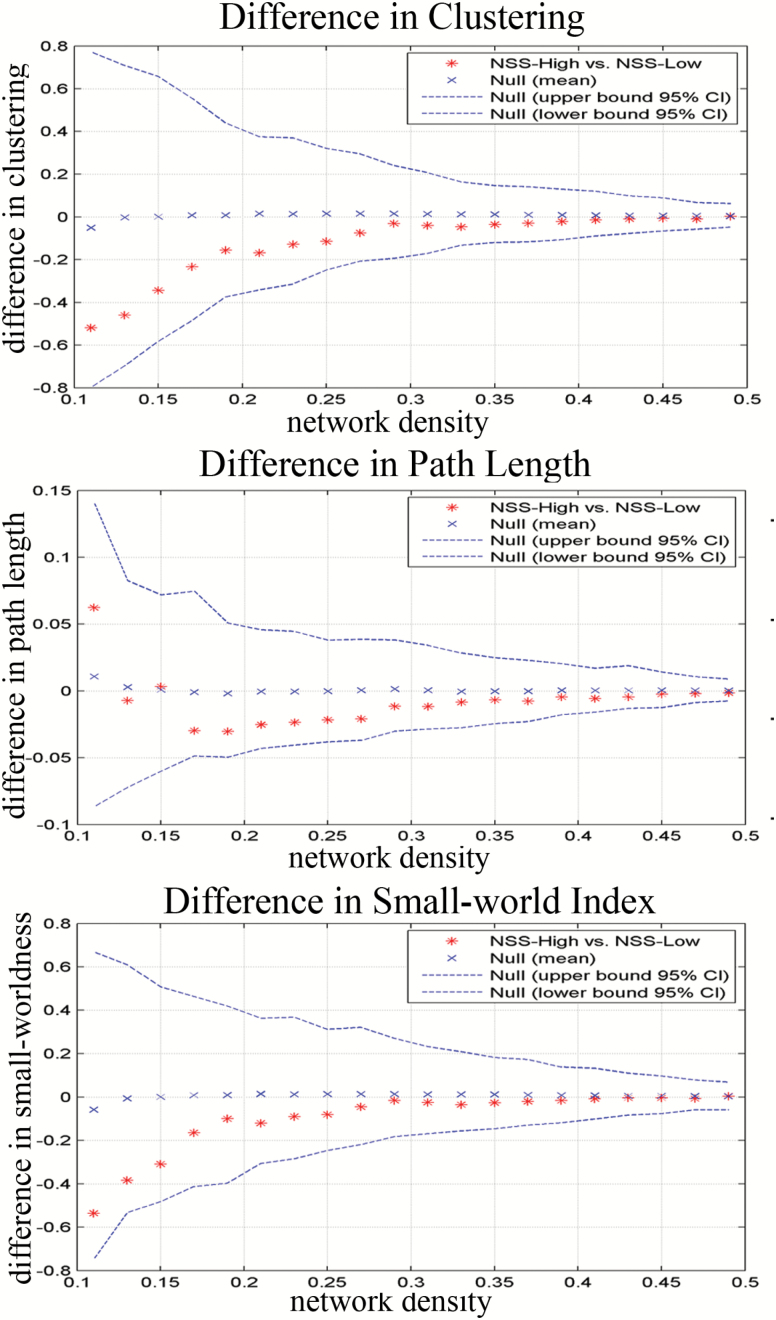
The differences in global network measures between the NSS-Low and NSS-High groups were investigated across a density range of 0.11:0.02:0.5 (figure 1). The NSS-High group network showed smaller normalized clustering across a range of densities compared with the NSS-Low group (P = .21 at Dmin). Both the NSS-Low and NSS-High groups exhibited comparable normalized path lengths across a range of densities (P = .4 at Dmin). The NSS-High group had smaller small-world indices in the network compared with the NSS-Low group, but the difference did not reach statistical significance (P = .16 at Dmin).
Fig. 1.
Group differences of NSS-Low and NSS-High groups in global network characteristics threshold at a range of densities (0.11:0.02:0.5).
Similar to the observed differences across different densities, AUC analysis showed that the NSS-High group had smaller normalized clustering (P = .29), smaller small-world indices (P = .40) and comparable normalized path lengths (P = .49), but these results were not significant. The FDA analysis showed similar results as the AUC analysis.
Group Analysis on Regional Network Measures
The differences in regional network measures between the 2 groups were investigated at minimum density (Dmin = 0.11). The NSS-High group demonstrated smaller betweenness in networks involving the right superior temporal cortex and the right amygdala. However, these differences did not survive multiple comparison correction (P < .05, FDR corrected). In contrast, the NSS-High group demonstrated larger betweenness in regions including the cerebellar vermis, the right olfactory, the right inferior orbital frontal cortex, the left fusiform gyrus, and the left hippocampus. Results at the cerebellar vermis, the right olfactory and the right inferior orbital frontal cortex remained significant after multiple comparison correction (P < .05, FDR corrected).
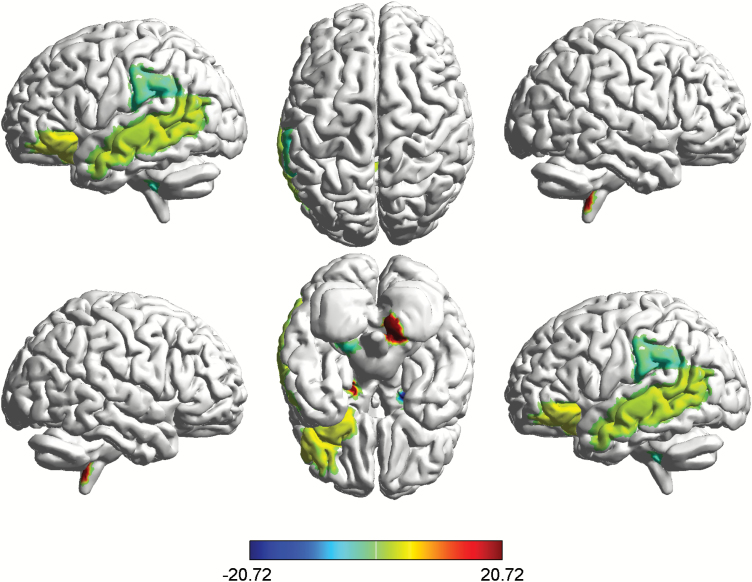
AUC analysis showed that, compared with the NSS-Low group, the NSS-High group showed smaller betweenness mainly involving the right amygdala, the left cerebellum, the left supramarginal, and the cerebellar vermis (figure 2, table 2), while betweenness was larger at the right cerebellum, the right orbital inferior frontal cortex, the left hippocampus, the left middle temporal cortex, and the cerebellar vermis (figure 2, table 2). Results found at the right amygdala, the right cerebellum, and the left hippocampus remained significant after correction for multiple comparisons (P < .05, FDR corrected). The FDA results were similar to the AUC results.
Fig. 2.
Group differences of NSS-Low and NSS-High groups in regional network characteristics. Regions with significant differences in nodal betweenness between NSS-Low and NSS-High groups. Significantly increased (hot color) and reduced (cold color) nodal betweenness were observed in NSS-High group. The results were obtained from AUC analysis across the density range of 0.11:0.02:0.5.
Table 2.
Regions With Altered Regional Betweenness Networks Based on AUC Analysis in the Density Range of 0.11:0.02:0.5
| NSS-High < NSS-Low | Right Amygdalaa | P < .001 |
|---|---|---|
| Left cerebellum | .03 | |
| Left supramarginal | .03 | |
| Cerebellar vermis | .02 | |
| NSS-High > NSS-Low | Right cerebelluma | P < .001 |
| Left hippocampusa | P < .001 | |
| Right inferior orbital frontal cortex | .01 | |
| Left middle temporal cortex | .04 | |
| Cerebellar vermis | .03 |
Note: aSurvived FDR adjustment.
Network Hubs and Modularity Analysis
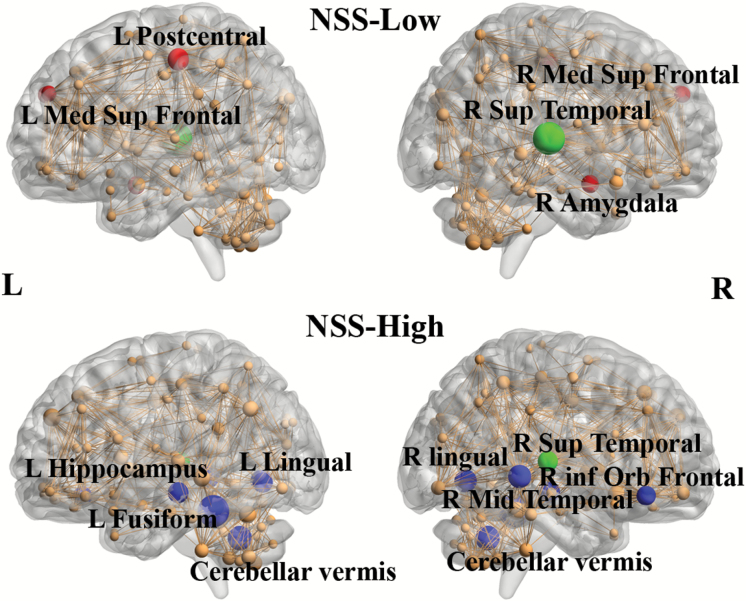
Network hubs of the NSS-Low group based on nodal betweenness at Dmin were identified at the right amygdala, the left postcentral, and the right superior temporal cortices. In the NSS-High group, network hubs were observed in the left fusiform gyrus, the left hippocampus, the bilateral lingual cortices, the right superior and middle temporal cortices and the cerebellar vermis.
AUC analysis showed partly different results between the 2 groups. The NSS-Low group had network hubs at the right amygdala, the bilateral superior medial frontal cortices, the left postcentral cortex, and the right superior temporal cortices (figure 3). The NSS-High group had network hubs at the cerebellar vermis, the left fusiform gyrus, the left hippocampus, the bilateral lingual cortices, the right superior and middle temporal cortices and the right inferior orbital frontal cortex (figure 3). The FDA results were identical to the AUC results. Modularity analysis demonstrated that the NSS-high group had a significantly lower degree of network modularity than the NSS-Low group.
Fig. 3.
Network hubs for NSS-Low and NSS-High groups. Network hubs were labeled (2SD larger than mean betweenness). The size of the sphere means the betweenness of the corresponding region. Red color is specific to NSS-Low group. Blue color is specific to NSS-High group. Green color represents common hubs in both groups. The results were obtained from AUC analysis across the density range of 0.11:0.02:0.5.
Network Resilience
Compared with the NSS-Low group, the resilience of the network in the NSS-High group was significantly reduced due to both random failure and targeted attacks in some of the fractions of removed nodes (P < .05, see supplementary figure S3). The AUC results were smaller but not significantly so in the NSS-High group for random failure (P = .18) and targeted attacks (P = .83). FDA analysis produced similar results.
Effects of Medication and Duration of Untreated Psychosis on Networks
Medication dosage and duration of untreated psychosis were entered as additional covariates in repeated analyses to investigate their potential effects on networks. The detailed results can be found in supplementary tables S1 and S2. In brief, no significant effects on global network measures and network hubs were found and regional network measures were similar to those found in previous analyses.
Discussion
This study represents the first effort to investigate the association between NSS and GM structural networks in schizophrenia. There were 3 major findings: (1) the NSS-High and NSS-Low groups did not significantly differ in global network topologies including small-world attributes, normalized clustering, and path lengths; (2) regional network analysis demonstrated that NSS were associated with alterations in network characteristics in the frontal-temporal-cerebellum areas; and (3) different network-hub distributions were observed in the NSS-Low and NSS-High groups based on nodal betweenness.
In this study, both the NSS-High and NSS-Low groups demonstrated small-world properties across a range of densities, suggesting that the crucial characteristics of brain networks are highly preserved in both groups. Such a network, to some extent, has the ability to process functional segregation and integration of information exchange, as well as to adapt to various stimuli.53,54 Our results are in line with previous studies on small-world properties.24,51 In addition, compared with the NSS-Low group, the NSS-High group demonstrated smaller normalized clustering and comparable path lengths, resulting in a smaller small-world index across a range of densities. These differences in global network attributes mean that the NSS-High group tends to have a weaker cliquishness (local interconnectivity) and more diffused pattern of connectivity. The smaller small-world attributes in the NSS-High group suggest reduced balance between network segregation and integration, which is less optimal for information processing in the NSS-High group compared with the NSS-Low group.
Although the underlying mechanism of this NSS-related network-level abnormality is still not clear, previous findings have indicated that alterations in network characteristics in schizophrenia may be associated with WM abnormality due in part to the transection of axons.55 Combining with results from another previous DTI study showing a correlation between WM variations and NSS scores,26 it is not surprising that we observed NSS-related GM structural network alterations in patients with schizophrenia. Our results also provide additional evidence that NSS are correlated with neurological abnormalities resulting from network disorganization and impaired connections.41
Our regional network analysis showed significant differences in betweenness centrality in both groups involving the inferior orbital frontal cortex, the middle temporal cortex, the hippocampus, the supramarginal cortex, the amygdala, and the cerebellum. These results are generally consistent with previous studies investigating the morphological and neural functional correlates of NSS in patients with schizophrenia.9,10,56–58 Our findings mainly identified regions in the sensorimotor system, which are important for the control and execution of motor behavior and the integration of sensory function, and have been included as a putative domain within the National Institute of Mental Health (NIMH) Research Domain Criteria framework (RoDC).59,60 The alteration in betweenness centrality in these regions implies a change in the ability to modulate information flow and to participate in functional interactions with the corresponding regions. Therefore, NSS may represent a clinical proxy of the change in brain network architecture that putatively underlies psychotic disorders. Similarly, clinical studies found NSS to parallel the clinical course of schizophrenia and to herald chronicity, reflecting the concept of process activity in classical psychopathology.61,62
Further network analysis identified significant between-group alterations in hub distribution. The identified hub regions in the NSS-Low group involved the bilateral medial superior frontal cortex, the right superior temporal cortex, the left postcentral cortex and the right amygdala, while hub regions in the NSS-High group included the right inferior orbital frontal cortex, the right superior/middle temporal cortices, the bilateral lingual cortices, the left hippocampus, the left fusiform gyrus, and the cerebellar vermis. The 2 groups only shared one common region in the right superior temporal cortex. As previous network analysis based on structural and functional MRI data reported, altered network topological properties in patients with schizophrenia mainly involve the frontal cortex and the superior and middle temporal cortices,51,63 which is consistent with our findings. Bassett et al51 have reported that predominantly prefrontal hubs in normal brain networks are replaced by hubs at other regions, such as the temporal cortex, the insula and the cingulate in patients with schizophrenia. Hubs are key parts of efficient information communication and regulation in a network. Therefore, our results indicate a lower connectivity and a longer absolute path length between other regions and the frontal cortex in the NSS-High group, which may lead to less efficient information transmission. In addition, the alteration in hub distribution also suggests a reconfiguration of brain networks.
Moreover, when comparing the hub distribution between the NSS-Low and NSS-High groups, there was a significant difference in that the cerebellum was a hub in the NSS-High group only. The cerebellum plays an important role in motor coordination and control.64,65 Hence, NSS have often been reported to be correlated with morphometric cerebellar abnormalities in schizophrenia.12,35,36 Our finding provides the first evidence of correlation between NSS and brain network abnormalities in the cerebellum in schizophrenia. Taken together, NSS appear to be associated with multiple cortical regions mainly involving the frontal cortex, the temporal cortex, the postcentral cortex, the amygdala and the cerebellum, which correspond to the disturbed cortico-subcortical-cerebellum circuitry that putatively underlies psychotic disorders.1
Finally, the resilience of network analysis showed that higher NSS scores were correlated with lower resilience in response to targeted attacks and random failure, suggesting a “fragile” network pattern. Modularity analysis also demonstrated that high NSS scores were associated with lower network modularity. Combining with lower clustering in the NSS-High group, our results suggest that higher levels of NSS may be associated with a more diffused network, which is less resilient to stimuli.
An important strength of this study is that our results were obtained from a large sample of first-episode patients, which increased statistical power and reliability. However, this study also has several limitations. In addition to the lack of a healthy control group, grouping NSS into high and low subgroups may obscure some nuisances in examining NSS as a continuous spectrum. Medication dosage and duration of untreated psychosis are potential confounding variables as it is difficult to assess and rule out their effects due to the collinearity between diagnosis and medication status.66 On the other hand, the first episode patients recruited had received medications only for brief periods and clinical studies have demonstrated that NSS are not the sequelae of antipsychotic treatment.67 Moreover, limited evidence has shown that network measures are independent from the effects of antipsychotic medications.66,68 An appropriately designed study (such as a randomized controlled trial) should be performed to further assess these potential effects. Finally, our participants were scanned with 1.5T MRI. As previous studies have shown that 3.0 T MRI, in general, performs as well as or better than 1.5 T MRI on clinical test parameters and imaging analysis,69,70 further studies are needed to address these issues.
In conclusion, to the best of our knowledge, this is the first study that investigates the neural basis of NSS at the network level in schizophrenia. We found that NSS are associated with alterations in brain network topological attributes corresponding to the cortical-subcortical-cerebellum circuitry in patients with schizophrenia. Our results may provide a new perspective for elucidating the neural basis of NSS in schizophrenia.
Supplementary Material
Acknowledgment
All authors report no potential conflicts of interest.
Funding
Raymond Chan was supported by a grant from the National Key Research and Development Programme (2016YFC0906402), the Beijing Training Project for the Leading Talents in Science and Technology (Z151100000315020), and the CAS Key Laboratory of Mental Health, Institute of Psychology. Li Kong was supported by the National Natural Science Foundation of China (81601170).
References
- 1. Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24(2):203–218. [DOI] [PubMed] [Google Scholar]
- 2. Heinrichs DW, Buchanan RW. Significance and meaning of neurological signs in schizophrenia. Am J Psychiatry. 1988;145(1):11–18. [DOI] [PubMed] [Google Scholar]
- 3. Schröder J, Niethammer R, Geider F-J, et al. Neurological soft signs in schizophrenia. Schizophr Res. 1992;6(1):25–30. [DOI] [PubMed] [Google Scholar]
- 4. Chan RC, Xu T, Heinrichs RW, Yu Y, Wang Y. Neurological soft signs in schizophrenia: a meta-analysis. Schizophr Bull. 2010;36(6):1089–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan RC, Xie W, Geng FL, et al. Clinical utility and lifespan profiling of neurological soft signs in schizophrenia spectrum disorders. Schizophr Bull. 2016;42(3):560–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tsuang MT, Faraone SV. The concept of target features in schizophrenia research. Acta Psychiatr Scand Suppl. 1999;395:2–11. [DOI] [PubMed] [Google Scholar]
- 7. Bombin I, Arango C, Buchanan RW. Assessment tools for soft signs. Psychiatr Ann. 2003;33(3):170–176. [Google Scholar]
- 8. Buchanan RW, Heinrichs DW. The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res. 1989;27(3):335–350. [DOI] [PubMed] [Google Scholar]
- 9. Dazzan P, Morgan KD, Orr KG, et al. The structural brain correlates of neurological soft signs in AESOP first-episode psychoses study. Brain. 2004;127(Pt 1):143–153. [DOI] [PubMed] [Google Scholar]
- 10. Hirjak D, Wolf RC, Stieltjes B, et al. Neurological soft signs and brainstem morphology in first-episode schizophrenia. Neuropsychobiology. 2013;68(2):91–99. [DOI] [PubMed] [Google Scholar]
- 11. Keshavan MS, Sanders RD, Sweeney JA, et al. Diagnostic specificity and neuroanatomical validity of neurological abnormalities in first-episode psychoses. Am J Psychiatry. 2003;160(7):1298–1304. [DOI] [PubMed] [Google Scholar]
- 12. Thomann PA, Wüstenberg T, Santos VD, Bachmann S, Essig M, Schröder J. Neurological soft signs and brain morphology in first-episode schizophrenia. Psychol Med. 2009;39(3):371–379. [DOI] [PubMed] [Google Scholar]
- 13. Rubia K, Russell T, Bullmore ET, et al. An fMRI study of reduced left prefrontal activation in schizophrenia during normal inhibitory function. Schizophr Res. 2001;52(1-2):47–55. [DOI] [PubMed] [Google Scholar]
- 14. Schröder J, Essig M, Baudendistel K, et al. Motor dysfunction and sensorimotor cortex activation changes in schizophrenia: a study with functional magnetic resonance imaging. Neuroimage. 1999;9(1):81–87. [DOI] [PubMed] [Google Scholar]
- 15. Zhao Q, Li Z, Huang J, et al. Neurological soft signs are not “soft” in brain structure and functional networks: evidence from ALE meta-analysis. Schizophr Bull. 2014;40(3):626–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liang M, Zhou Y, Jiang T, et al. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17(2):209–213. [DOI] [PubMed] [Google Scholar]
- 17. Pankow A, Deserno L, Walter M, et al. Reduced default mode network connectivity in schizophrenia patients. Schizophr Res. 2015;165(1):90–93. [DOI] [PubMed] [Google Scholar]
- 18. Martino M, Magioncalda P, Yu H, et al. Abnormal resting-state connectivity in a substantia nigra-related striato-thalamo-cortical network in a large sample of first-episode drug-naive patients with schizophrenia. Schizophr Bull. 2017;44(2):419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walther S, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Viher PV. Aberrant hyperconnectivity in the motor system at rest is linked to motor abnormalities in schizophrenia spectrum disorders. Schizophr Bull. 2017;43(5):982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu J, Wang C, Liu F, Qin W, Li J, Zhuo C. Alterations of functional and structural networks in schizophrenia patients with auditory verbal hallucinations. Front Hum Neurosci. 2016;10:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buchsbaum MS, Joseph F, Buchsbaum BR, et al. Diffusion tensor imaging in schizophrenia. Bio Psychiatry 2005;23(4):255–273. [DOI] [PubMed] [Google Scholar]
- 22. Ellison-Wright I, Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res. 2009;108(1-3):3–10. [DOI] [PubMed] [Google Scholar]
- 23. Zhang Y, Lin L, Lin CP, et al. Abnormal topological organization of structural brain networks in schizophrenia. Schizophr Res. 2012;141(2-3):109–118. [DOI] [PubMed] [Google Scholar]
- 24. Palaniyappan L, Marques TR, Taylor H, et al. Globally efficient brain organization and treatment response in psychosis: a connectomic study of gyrification. Schizophr Bull. 2016;42(6):1446–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palaniyappan L, Park B, Balain V, Dangi R, Liddle P. Abnormalities in structural covariance of cortical gyrification in schizophrenia. Brain Struct Funct. 2015;220(4):2059–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hirjak D, Thomann PA, Wolf RC, et al. White matter microstructure variations contribute to neurological soft signs in healthy adults. Hum Brain Mapp. 2017;38(7):3552–3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thomann PA, Hirjak D, Kubera KM, Stieltjes B, Wolf RC. Neural network activity and neurological soft signs in healthy adults. Behav Brain Res. 2015;278:514–519. [DOI] [PubMed] [Google Scholar]
- 28. Dong D, Wang Y, Chang X, Luo C, Yao D. Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr Bull. 2018;44(1):168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galindo L, Bergé D, Murray GK, et al. Default mode network aberrant connectivity associated with neurological soft signs in schizophrenia patients and unaffected relatives. Front Psychiatry. 2017;8:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sporns O. The human connectome: a complex network. Ann N Y Acad Sci. 2011;1224:109–125. [DOI] [PubMed] [Google Scholar]
- 31. Lerch JP, Worsley K, Shaw WP, et al. Mapping anatomical correlations across cerebral cortex (MACACC) using cortical thickness from MRI. Neuroimage. 2006;31(3):993–1003. [DOI] [PubMed] [Google Scholar]
- 32. Salvador R, Suckling J, Coleman MR, Pickard JD, Menon D, Bullmore E. Neurophysiological architecture of functional magnetic resonance images of human brain. Cereb Cortex. 2005;15(9):1332–1342. [DOI] [PubMed] [Google Scholar]
- 33. Achard S, Salvador R, Whitcher B, Suckling J, Bullmore E. A resilient, low-frequency, small-world human brain functional network with highly connected association cortical hubs. J Neurosci. 2006;26(1):63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44(2):489–501. [DOI] [PubMed] [Google Scholar]
- 35. Bottmer C, Bachmann S, Pantel J, et al. Reduced cerebellar volume and neurological soft signs in first-episode schizophrenia. Psychiatry Res. 2005;140(3):239–250. [DOI] [PubMed] [Google Scholar]
- 36. Thomann PA, Roebel M, Dos Santos V, Bachmann S, Essig M, Schröder J. Cerebellar substructures and neurological soft signs in first-episode schizophrenia. Psychiatry Res. 2009;173(2):83–87. [DOI] [PubMed] [Google Scholar]
- 37. Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009;10(3):186–198. [DOI] [PubMed] [Google Scholar]
- 38. Heuser M, Thomann PA, Essig M, Bachmann S, Schröder J. Neurological signs and morphological cerebral changes in schizophrenia: an analysis of NSS subscales in patients with first episode psychosis. Psychiatry Res. 2011;192(2):69–76. [DOI] [PubMed] [Google Scholar]
- 39. Wittchen HU, Wunderlich U, Gruschwitz S, Zaudig M.. SKID-I:Strukturiertes Klinisches Interview Für DSM-IV. Göttingen, Germany: Hogrefe; 1997. [Google Scholar]
- 40. Overall JE, Gorham DR. The brief psychiatric rating scale. Psychol Rep. 1962;10:799–812. [Google Scholar]
- 41. Gay O, Plaze M, Oppenheim C, et al. Cortex morphology in first-episode psychosis patients with neurological soft signs. Schizophr Bull. 2013;39(4):820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11(6 Pt 1):805–821. [DOI] [PubMed] [Google Scholar]
- 43. Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(1 Pt 1):21–36. [DOI] [PubMed] [Google Scholar]
- 44. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. [DOI] [PubMed] [Google Scholar]
- 45. Kaiser M, Hilgetag CC. Nonoptimal component placement, but short processing paths, due to long-distance projections in neural systems. PLoS Comput Biol. 2006;2(7):e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sporns O, Zwi JD. The small world of the cerebral cortex. Neuroinformatics. 2004;2(2):145–162. [DOI] [PubMed] [Google Scholar]
- 47. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. [DOI] [PubMed] [Google Scholar]
- 48. Hosseini SM, Hoeft F, Kesler SR. GAT: a graph-theoretical analysis toolbox for analyzing between-group differences in large-scale structural and functional brain networks. PLoS One. 2012;7(7):e40709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12(6):512–523. [DOI] [PubMed] [Google Scholar]
- 50. Lim HK, Jung WS, Aizenstein HJ. Aberrant topographical organization in gray matter structural network in late life depression: a graph theoretical analysis. Int Psychogeriatr. 2013;25(12):1929–1940. [DOI] [PubMed] [Google Scholar]
- 51. Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci. 2008;28(37):9239–9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bernhardt BC, Chen Z, He Y, Evans AC, Bernasconi N. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb Cortex. 2011;21(9):2147–2157. [DOI] [PubMed] [Google Scholar]
- 53. Yong H, Zhang C, Alan E. Structural insights into aberrant topological patterns of large-scale cortical networks in Alzheimer’s disease. J Neurosci. 2008;28:4756–4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jiang TZ, Liu Y, Yong-Hui LI. Brain networks: from anatomy to dynamics. Chin Bull Life Sci. 2009;21(2):0181–0188. [Google Scholar]
- 55. White T, Hilgetag CC. Gyrification and neural connectivity in schizophrenia. Dev Psychopathol. 2011;23(1):339–352. [DOI] [PubMed] [Google Scholar]
- 56. Kong L, Bachmann S, Thomann PA, Essig M, Schröder J. Neurological soft signs and gray matter changes: a longitudinal analysis in first-episode schizophrenia. Schizophr Res. 2012;134(1):27–32. [DOI] [PubMed] [Google Scholar]
- 57. Chan RC, Di X, McAlonan GM, Gong QY. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull. 2011;37(1):177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Schröder J, Wenz F, Schad LR, Baudendistel K, Knopp MV. Sensorimotor cortex and supplementary motor area changes in schizophrenia. A study with functional magnetic resonance imaging. Br J Psychiatry. 1995;167(2):197–201. [DOI] [PubMed] [Google Scholar]
- 59. Bernard JA, Mittal VA. Updating the research domain criteria: the utility of a motor dimension. Psychol Med. 2015;45(13):2685–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mittal VA, Bernard JA, Northoff G. What can different motor circuits tell us about psychosis? an RDoC perspective. Schizophr Bull. 2017;43(5):949–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bachmann S, Bottmer C, Weimer D, Schro¨Der J. First-episode schizophrenia: a follow-up study on neurological soft signs. Am J Psychiatry. 2008;162(12):2337–2343. [DOI] [PubMed] [Google Scholar]
- 62. Bachmann S, Degen C, Geider FJ, Schröder J. Neurological soft signs in the clinical course of schizophrenia: results of a meta-analysis. Front Psychiatry. 2014;5:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu Y, Liang M, Zhou Y, et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131(Pt 4):945–961. [DOI] [PubMed] [Google Scholar]
- 64. Ho BC, Mola C, Andreasen NC. Cerebellar dysfunction in neuroleptic naive schizophrenia patients: clinical, cognitive, and neuroanatomic correlates of cerebellar neurologic signs. Biol Psychiatry. 2004;55(12):1146–1153. [DOI] [PubMed] [Google Scholar]
- 65. Bersani G, Paolemili M, Quartini A, et al. Neurological soft signs and cerebral measurements investigated by means of MRI in schizophrenic patients. Neurosci Lett. 2007;413(1):82–87. [DOI] [PubMed] [Google Scholar]
- 66. Kaufmann T, Skåtun KC, Alnæs D, et al. Disintegration of sensorimotor brain networks in schizophrenia. Schizophr Bull. 2015;41(6):1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Schröder J. Soft signs, neuroleptic side effects, and schizophrenia. Psychiatric Annals. 2003;33(3):214–220. [Google Scholar]
- 68. Schürmann M, Järveläinen J, Avikainen S, Cannon TD, Lönnqvist J, Huttunen M, Riitta H. Manifest disease and motor cortex reactivity in twins discordant for schizophrenia. Br J Psychiatry. 2007;191(2):178–179. [DOI] [PubMed] [Google Scholar]
- 69. Hoenig K, Kuhl CK, Scheef L. Functional 3.0-T MR assessment of higher cognitive function: are there advantages over 1.5-T imaging? Radiology. 2005;234(3):860–868. [DOI] [PubMed] [Google Scholar]
- 70. Wood R, Bassett K Foerster 5th, Spry C, Tong L. 1.5 tesla magnetic resonance imaging scanners compared with 3.0 tesla magnetic resonance imaging scanners: systematic review of clinical effectiveness. CADTH Technol Overv. 2012;2(2):e2201. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.