Abstract
Background
Converging evidence implicates the anterior hippocampus in the proximal pathophysiology of schizophrenia. Although resting state functional connectivity (FC) holds promise for characterizing anterior hippocampal circuit abnormalities and their relationship to treatment response, this technique has not yet been used in first-episode psychosis (FEP) patients in a manner that distinguishes the anterior from posterior hippocampus.
Methods
We used masked-hippocampal-group-independent component analysis with dual regression to contrast subregional hippocampal–whole brain FC between healthy controls (HCs) and antipsychotic naïve FEP patients (N = 61, 36 female). In a subsample of FEP patients (N = 27, 15 female), we repeated this analysis following 8 weeks of second-generation antipsychotic treatment and explored whether baseline FC predicted treatment response using random forest.
Results
Relative to HC, untreated FEP subjects displayed reproducibly lower FC between the left anteromedial hippocampus and cortical regions including the anterior cingulate and insular cortex (P < .05, corrected). Anteromedial hippocampal FC increased in FEP patients following treatment (P < .005), and no longer differed from HC. Random forest analysis showed baseline anteromedial hippocampal FC with four brain regions, namely the insular–opercular cortex, superior frontal gyrus, precentral gyrus, and postcentral gyrus predicted treatment response (area under the curve = 0.95).
Conclusions
Antipsychotic naïve FEP is associated with lower FC between the anterior hippocampus and cortical regions previously implicated in schizophrenia. Preliminary analysis suggests that random forest models based on hippocampal FC may predict treatment response in FEP patients, and hence could be a useful biomarker for treatment development.
Keywords: first-episode psychosis, hippocampus, insula, cingulate, antipsychotic, resting state
Introduction
Hippocampal abnormalities are among the most consistent biological findings in schizophrenia.1–5 The hippocampal formation is a heterogeneous region, containing distinct subcomponents including the dentate gyrus, cornu ammonis subfields, and subiculum; in addition, the function, gene expression, and anatomical connectivity of these subcomponents vary along the anterior–posterior hippocampal axis.6–8 Converging evidence points toward involvement of anterior hippocampal structures in ultrahigh-risk (UHR) and first-episode psychosis (FEP) stages of the illness. Previous work in UHR and FEP subjects reported that reduced volume and higher resting blood flow are localized to the anterior hippocampus.9–14 Further, rodent ventral (homologous to human anterior) hippocampal neurons are affected by rodent models of psychosis, eg, the methylazoxymethanol acetate model, such that the interactions of these neurons with subcortical and prefrontal regions involved in salience attribution are altered in a manner that could contribute to psychotic symptoms and cognitive deficits in human.15,16 Therefore, characterizing hippocampal interactions with extrinsic brain regions may provide biomarkers useful for developing early interventions in FEP.
Resting state functional connectivity (FC), a measure of interregional coherence in low-frequency fluctuations in the BOLD (blood-oxygen-level-dependent) functional magnetic resonance imaging (fMRI) signal, has frequently been used to study brain network abnormalities in schizophrenia, including predicting response to antipsychotic medication in FEP patients.17,18 To date, few studies have characterized FC of the hippocampus in antipsychotic naive FEP patients by using techniques that differentiate FC along the anterior–posterior axis; however, several studies have assessed hippocampal–brain FC in chronic schizophrenia patients, both unmedicated and medicated.19–21
In this study, we aimed to characterize subregional hippocampal–whole brain FC in antipsychotic naïve FEP subjects using masked-hippocampal-group-independent component analysis (ICA) with dual regression.22 This data-driven technique reproducibly segments the hippocampus into independent components (ICs) that occupy distinct subregions along the anterior–posterior axis and display distinct patterns of whole brain FC.22,23 Resting state functional magnetic resonance imaging (fMRI) scans were acquired in a cohort of FEP patients at baseline, prior to commencing second-generation antipsychotic (SGA) medication, and following 8 weeks of this treatment; healthy control (HC) participants matched for age and gender were scanned at the same intervals. We hypothesized that relative to HC, unmedicated FEP patients would show altered anterior hippocampal–whole brain FC. As an exploratory analysis, we assessed the effect of SGA medication on hippocampal FC, and used a random forest (RF) model to determine whether baseline hippocampal FC could predict response to SGA treatment.
Methods and Materials
Study Design, Setting, Participants, and Antipsychotic Medication
Data were acquired as part of a larger study ascertaining FEP biomarkers, from which results were previously published.2 Between March 5, 2013, and October 8, 2014, individuals with non-affective FEP were recruited from the Shanghai Mental Health Centre early psychosis program. HCs group matched by age and sex were recruited by advertisement. Eligible FEP participants met criteria for schizophrenia or schizophreniform disorder but not for any other Axis I disorder, according to a full Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (SCID DSM-IV-TR), were psychotropic medication naive, and were experiencing a first-episode of psychosis. Baseline (“T1”) clinical and neuroimaging assessments were made prior to FEP patients commencing SGA medication according to standard clinical practice; follow-up (“T2”) assessments occurred following 8 weeks of this treatment (see table 1 and supplementary methods for medication details). Eligible HCs, also psychotropic medication naïve, were assessed with the SCID (DSM-IV-TR non-patient version) to exclude any Axis I disorder. All participants provided written informed consent, were between 16 and 40 years old, were Mandarin-speaking Han Chinese individuals living in the Shanghai metropolitan area, were right-handed, had completed at least 9 years of school, were medically stable, were free from substance abuse (according to self-report) and suicidal ideation, and had no contraindications to MRI. The study was approved by institutional review boards at Shanghai Mental Health Center and NYU School of Medicine. Symptom assessment scales included the Brief Psychiatric Rating Scale (BPRS) and the Scale for the Assessment of Negative Symptoms (SANS), see supplementary methods for details.
Table 1.
Subject Demographics and Symptoms
| FEP Group A Baseline (n = 27) |
FEP Group A Week 8 (n = 27) |
P Value (Baseline vs Week 8) | FEP Group B Baseline (n = 34) |
Healthy Controls (n = 27) |
P Value (FEP A vs B or also vs HC) | |
|---|---|---|---|---|---|---|
| Mean (SD) or No. | ||||||
| Age, years | 24.11 (7.19) | 25.85 (8.53) | 24.46 (7.18) | > .05 | ||
| Sex | > .05 | |||||
| Female | 15 | 21 | 14 | |||
| Male | 12 | 13 | 12 | |||
| Education level, years | 11.81 (3.58) | 12.12 (2.51) | 12.69 (2.49) | > .05 | ||
| Handedness | > .05 | |||||
| Right | 27 | 34 | 25 | |||
| Left | 0 | 0 | 1 | |||
| DUP, weeks | 24.44 (16.52) | 27.16 (27.28) | > .05 | |||
| Daily CPZ average dose, mg | N/A | 488 (275.88) | N/A | N/A | N/A | |
| Treatment duration, days | 1.2 (1.94)¤ | 1.8 (2.17) | N/A | > .05 | ||
| BPRS total score | 52.56 (13.2) | 33.15 (7.23) | < .001 | 44.44 (9.26) | N/A | < .01 |
| BPRS positive score | 20.74 (5.07) | 10 (3.54) | < .001 | 18.74 (12.33) | N/A | > .05 |
| SANS composite score | 21.4 (20.51) | 15.48 (12.53) | < .05 | 18.24 (14.32) | N/A | > .05 |
Note: All but 2 patients were antipsychotic naïve. FEP, first-episode psychosis; HCs, healthy controls; DUP, duration of untreated psychosis; CPZ, chlorpromazine; BPRS, Brief Psychiatric Rating Scale; SANS, Scale for the Assessment of Negative Symptoms; N/A, not applicable.
Image Preprocessing, Masked Hippocampal Group ICA, and Dual Regression
See supplementary methods for details on all methods including acquisition parameters, preprocessing methods, as for following sections. Potential motion artifacts were controlled for by strict exclusion criteria and artifact removal according to Power et al.24 Masked hippocampal group ICA (GICA) and dual regression analyses were used to identify hippocampal ICs and map their whole brain FC, respectively. GICA is a commonly used data-driven technique in which group fMRI BOLD data concatenated across subjects is decomposed into a set of independent (uncorrelated and non-Gaussian) components, each characterized by a group-level spatial map and time course.25Masked GICA identifies ICs within a masked brain region; dual regression then measures their extrinsic FC in a multivariate manner.22,26 Masked hippocampal GICA and whole brain dual regression were performed using the mICA Toolbox (v.1.14) as in previous studies.22,23 This freely available software (www.nitrc.org/projects/mica/) streamlines implementation of FSL Melodic and Dual Regression on select brain regions. An additional Toolbox function was used to calculate split-half reproducibility (Pearson spatial correlation coefficient) of hippocampal ICs.
To establish internal reproducibility of FEP vs HC differences in hippocampal–whole brain FC, the FEP sample was split into 2 groups: 1) FEP A subjects with both T1 and T2 data available, and 2) FEP B, subjects with T1 data only. Masked hippocampal GICAs were created with data merged from all subject groups and time points relevant to each contrast, so that common ICs were used in subsequent dual regression analyses to calculate hippocampal–whole brain FC (see supplementary figure S1 GICAs 5, 7, and 8). To ensure this approach was justified, ie, that distinct group and time data yielded hippocampal ICs with a similar spatial configuration and reproducibility, GICAs were first performed within each separate group (supplementary figure S1, GICAs 1–4 and 6).
Group and Time Contrasts
Group and time contrasts in hippocampal–whole brain FC were calculated with unpaired t tests (paired for time contrasts), using FSL Randomise with 1000 permutations. Contrasts included the main effects of group (FEP A vs HC across time) and time (T1 vs T2 across HC and FEP A subjects), followed by the simple effects of group (FEP A or FEP B vs HC) at each time, and time within each group (T1 vs T2 within FEP A or HC subjects), see supplementary figure S1. Within the resulting difference maps, areas of significance were identified by threshold-free cluster enhancement,27 thresholded at P < .05 and corrected for family-wise error (FWE) rate. Clusters greater than 10 voxels were reported. The Dice similarity coefficient (DSC) was used to calculate the FEP A vs FEP B reproducibility of FEP vs HC differences in hippocampal–whole brain FC. To determine the directional basis of group and time effects, mean z scores for all significant voxels were analyzed by 2-way ANOVA with follow-up t tests. Within-group whole brain FC of hippocampal ICs that were significantly different between groups was calculated for these, and neighboring ICs, and one sample t tests were used to compare this FC between ICs. To assess baseline FC relationships to symptoms, appropriate hippocampal–whole brain voxelwise regressions were calculated using methods as earlier.
Prediction of Treatment Response
To predict treatment response in FEP A subjects, a RF model using baseline FC features was used to classify patients into “responders” vs “nonresponders”. Responders were subjects with a 35% or greater reduction in BPRS total score at T2 relative to T1 (ΔBPRS total >35%); nonresponders had ΔBPRS total <35%. This threshold was based on a median split in baseline BPRS total score. The RF model used 40 baseline FC features—ie, 40 brain regions of interest (ROIs)—to classify patients into these groups. Resulting predictive FC features were those associated with the largest area under the curve (AUC) of the receiver operating characteristic curve (ROC). The following analyses were performed to examine relationships to treatment response. First, the Kolmogorov test, which differentiates samples based on empirical distributions rather than means, was used to compare baseline FC between responders and nonresponders; second, multiple regressions including left anteromedial (LAM) FC scores from all predictive areas were calculated to determine whether baseline FC and T2-T1 change in FC predicted treatment response (ΔBPRS total or positive, as SANS did not change). To compare RF results with those from univariate analyses, voxelwise regressions were performed to identify brain regions in which baseline FC or T1-T2 change in FC correlated with ΔBPRS total or positive, and successive thresholds (P < .05, FWE corrected; P < .05 uncorrected, or P < .10, uncorrected) were used to identify the highest threshold at which at least 10 significant voxels overlapped with the relevant ROI.
Results
Patient Characteristics
From 66 FEP subjects imaged at baseline, 61 met preprocessing criteria (5 were excluded due to excessive motion). Of these, 27 subjects had both T1 and T2 data (FEP A subjects), 34 subjects had T1 data only (FEP B). Twenty-seven HC subjects were selected to be age and gender matched to FEP A subjects. As shown in table 1, FEP A, FEP B, and HC subjects did not differ in age or gender composition. FEP A subjects had significantly higher symptom severity (BPRS total and BPRS positive) compared to FEP B subjects, but did not differ in other characteristics. In FEP A subjects, there was a significant reduction in BRPS total and positive scores following treatment; however, negative symptoms (SANS) did not significantly change.
Masked Hippocampal GICA
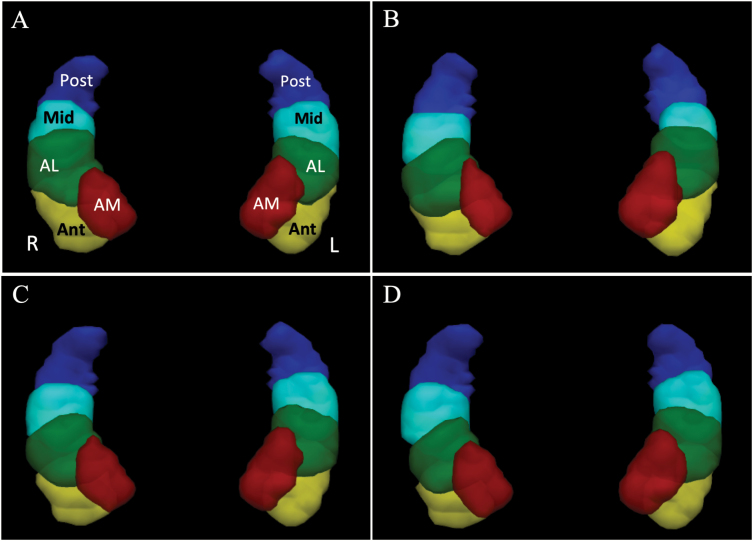
Results from masked hippocampal GICA analyses in all subjects agreed with previous studies in healthy subjects,22,28 i.e., each hippocampus contained five ICs with a similar organization in each hemisphere: one in the posterior (Post), one in the mid (Mid), and three in the anterior hippocampus (anterior, Ant; anteromedial, AM; and anterolateral, AL), (see figure 1). The zmax for each IC was both consistent with previous studies, and similar between groups and times (supplementary table S1). Likewise, split-half reproducibility was similar at baseline between HC subjects (Pearson spatial correlation coefficient, r = .77), FEP A subjects (r = .78), and FEP B subjects (r = .74).
Fig. 1.
Independent components (ICs) from masked hippocampal group ICA. Isosurfaces of z scores (thresholded 0.5–30) representing the probability of belonging to one of the 10 ICs are shown in a superior view of the masked bilateral hippocampi in MNI space. (A) Healthy controls (HCs). Each IC in the left (L) and right (R) hippocampus is labeled according to its relative position in the hippocampus. AM, anteromedial; Ant, anterior; AL, anterolateral; Mid, middle; and Post, posterior. (B) FEP group A, (C) HC + FEP group A, (D) HC + FEP group B. ICA, independent component analysis; FEP, first-episode psychosis.
Hippocampal–Whole Brain FC
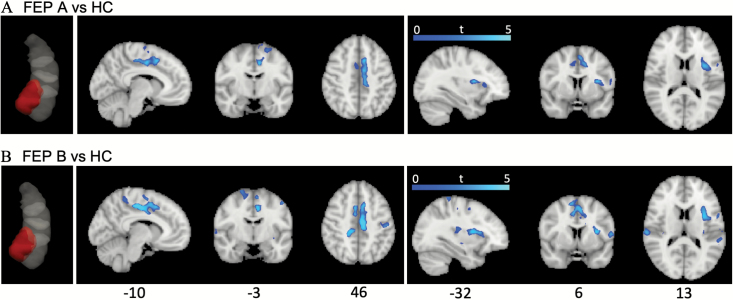
There were no significant effects of group or time at the whole brain level for subjects with follow-up (FEP A and HC), P > .05, corrected. There was, however, a significant effect of group at baseline: antipsychotic naïve FEP patients had lower FC between the LAM hippocampal IC and cortical areas in the default mode29 and salience networks,30 including the left anterior and mid-posterior insular and opercular cortices, the posterior, mid and anterior cingulate cortices, and the precentral and postcentral gyri (figure 2 and supplementary tables S2A and B). This group difference was highly reproducible between FEP A and B subjects in that the LAM IC was independently identified in both contrasts, and for areas that differed to HC in LAM–brain FC, the DSC was 0.71, indicating good reproducibility. Additional brain areas that were significant in the FEP B vs HC contrast included the right anterior and posterior insular and opercular cortices, the bilateral superior temporal gyrus and superior frontal gyrus (SFG). Baseline FC in FEP subjects was not significantly correlated with baseline BPRS total, positive, or SANS composite scores, P < .05, corrected.
Fig. 2.
Differences in hippocampal–whole brain connectivity between antipsychotic naïve FEP subjects and healthy controls (HCs). (A) FEP group A subjects compared to HCs. The left anteromedial (LAM) hippocampal IC from the group FEP A + HC sample is shown in red in leftmost figure inset. Areas of reduced functional connectivity (FC) in FEP A vs HC subjects are shown to the right. (B) FEP Group B subjects compared to HC. The LAM hippocampal IC from the group FEP B + HC sample is shown in red in leftmost figure inset. Areas of reduced FC in FEP B vs HC subjects are shown to the right. IC, independent component; FEP, first-episode psychosis. For color, please see the figure online.
At time 2, following 8 weeks of SGA treatment in FEP A subjects, hippocampal–whole brain FC did not differ from HC, even when the threshold was relaxed to P < .05, uncorrected. Longitudinal analysis with a 2-way ANOVA of mean z scores within the group difference map (from T1) showed a significant group × time interaction, F(1,104) = 16.36, P < .00l (see supplementary figure S2). Follow-up t tests showed that at T1, FC in FEP A subjects was lower than that in HC. Within FEP A subjects, FC increased from T1 to T2 (P < 0.005). By contrast, in HC subjects FC decreased from T1 to T2.
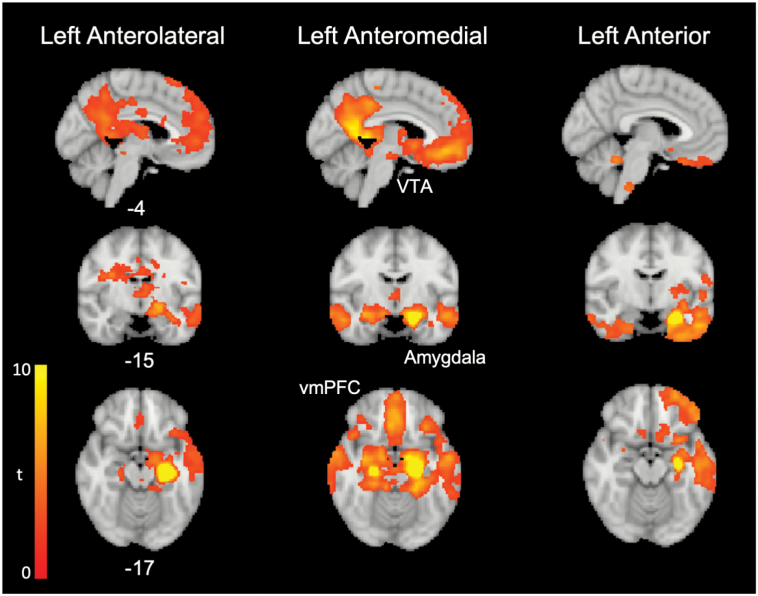
Baseline within-group whole brain FC was examined for the LAM IC, and adjacent (left anterolateral and left anterior) ICs in all FEP subjects (figure 3 and supplementary table S3A), and in HC, supplementary table S3B. For both groups, LAM–brain FC included the dorsal anterior cingulate cortex (dACC) and posterior cingulate cortex (PCC), medial prefrontal cortex, and subcortical areas including the amygdala, midline thalamus, ventral and dorsal striatum, and ventral tegmental area (VTA) (identified using masks from31), among other areas. In FEP subjects, comparison of whole brain FC between ICs in FEP subjects showed the anteromedial IC had relatively greater FC with the VTA, bilateral amygdala, medial prefrontal cortex, and subcallosal ACC, compared with anterolateral and anterior ICs (supplementary table S5).
Fig. 3.
Whole brain functional connectivity of anterior hippocampal independent components (ICs). Mean whole brain functional connectivity for the left anterolateral, left anteromedial, and left anterior hippocampal ICs is shown for FEP group A patients at baseline. FEP, first-episode psychosis; VTA, ventral tegmental area; vmPFC, ventromedial prefrontal cortex.
Prediction of Treatment Response
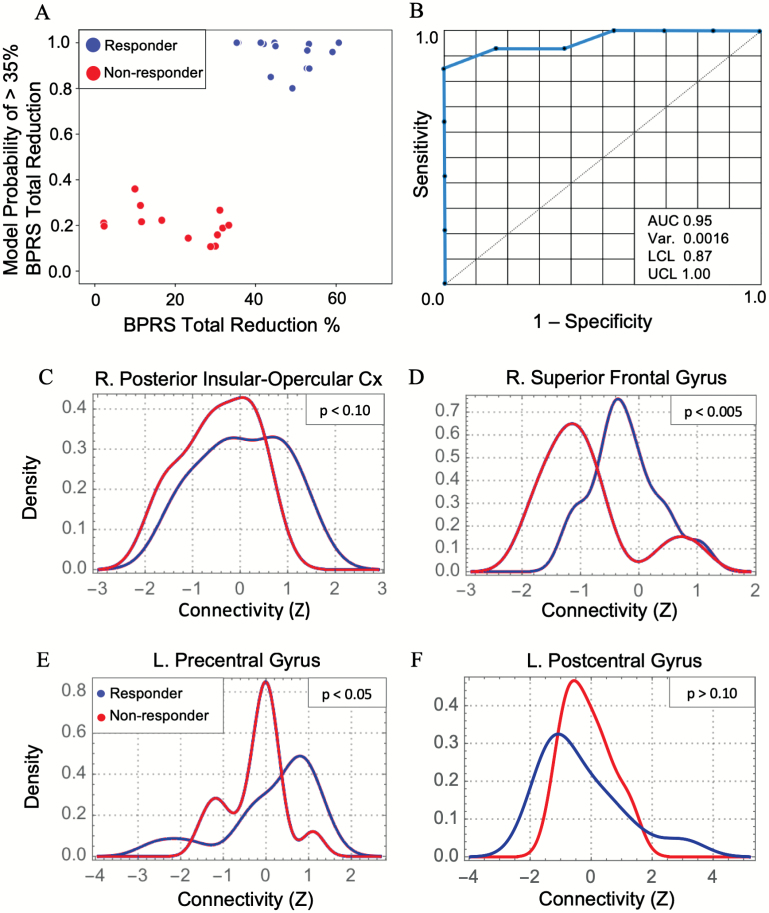
Random forest prediction of membership to responder vs nonresponder categories resulted in an ROC AUC of 0.95 and classification accuracy of 0.89 (see figure 4). Of the 40 RF features, 4 were predicted correct classification, namely the right SFG (MNI x, y, z: 16, −6, 66), left precentral gyrus (PreCG) (−42, −12, 64), right posterior insular–opercular cortex (InsOperc) (36, −14, 20), and left postcentral gyrus (PCG) (−62, −4, 30), ranked in decreasing variable importance score according to the Gini index. The Kolmogorov test of equality for the distributions of responders vs nonresponders showed that responders had significantly higher LAM FC with the InsOperc and SFG, with a trend toward higher FC with the PreCG, whereas PCG FC did not differ between groups (figure 4). Simultaneous multiple linear regression including baseline LAM FC scores from all 4 predictive brain regions significantly predicted ΔBPRS total score (P < .001), where InsOperc had a significant positive β coefficient (supplementary table S5, supplementary figure S3). In addition, ΔBPRS positive score was also predicted by the model (P < .001), where both InsOperc and SFG had significant positive β coefficients. For equivalent T2-T1 change in FC scores, the overall model was significant for both ΔBPRS total and positive scores (P < .01), with a significant negative β coefficient for InsOperc (supplementary table S5). These results indicate that greater treatment responses in both BPRS total and positive scores were correlated with a greater T1 to T2 decrease in LAM-right posterior insular-opercular cortex FC, and a greater baseline potential for this decrease to occur.
Fig. 4.
Random forest (RF) prediction of treatment response. (A) RF model probability of “response” (reduction in BPRS total score >35). (B) Receiver operating curve for the RF model. (C–F) Plots show density distributions of left anteromedial hippocampal functional connectivity with the 4 brain regions that were predictive in the RF model. P values from Kolmogorov tests of distribution equality. Var, variance; LCL, lower confidence limit; UCL, upper confidence limit; R., right; L., left; Cx, cortex; AUC, area under the curve; BPRS, Brief Psychiatric Rating Scale.
Parallel univariate voxelwise analysis showed neither baseline LAM–brain FC, nor T1 to T2 change in FC were significantly correlated with symptom improvement (ΔBPRS positive or total score) at P < .05, corrected. However, at P < .05, uncorrected, baseline FC in an area of voxels overlapping the InsOperc ROI was positively correlated with both ΔBPRS total and positive scores (supplementary figure S4); in addition, a greater decrease in FC from T1 to T2 was positively correlated with both ΔBPRS total and positive scores (not shown in figure). At P < .10, these same relationships were observed for the SFG and PreCG ROIs (supplementary figure S4), but not for the PCG ROI.
Discussion
Masked Hippocampal Group ICA
In this study, we used masked hippocampal GICA with dual regression to characterize hippocampal–brain resting state FC in unmedicated FEP patients, and to examine the effects of SGA treatment. We report the first use of Masked hippocampal GICA with dual regression in a neuropsychiatric disorder. Despite the critical need to differentiate FC along the anterior–posterior hippocampal axis, there is no consensus on what configuration of subregional ROIs would best discern heterogeneity within the human hippocampus. Rodent models are unsuitable given expansion of the anterior hippocampus in primate evolution7,32,33; a binary anterior vs posterior division8 masks known differences between the anteromedial and anterolateral hippocampus,22,32–35 and multiple small ROIs are unlikely to be reproducible.36 By contrast, masked hippocampal GICA segments the hippocampus with a data-driven technique, yielding reproducible ICs consistent with rodent-human topology, ie, greater functional heterogeneity in the anterior vs posterior hippocampus.6 We used a dimensionality of 10 ICs based on previous studies22,28; also, the resulting ICs are appropriate to the current spatial resolution (80–200 voxels per IC). Hippocampal ICs in FEP subjects had a similar configuration and reproducibility to those in HCs, meaning dual regression could be performed within a group space.
FEP–Control Differences in Anterior Hippocampal–Brain FC
We report, to the best of our knowledge, the first study of FC between the hippocampus and extrinsic brain in an antipsychotic naïve FEP sample. Patients had reproducibly lower FC between an anterior hippocampal subregion and brain areas previously implicated in schizophrenia, as discussed later. That 2 independently conducted data-driven analyses converged on the LAM hippocampal IC supports the validity of this finding, and warrants discussion of this subregion’s function and anatomical connectivity. The anteromedial human hippocampus is homologous to the caudoventral tip of the rodent hippocampus (vHipp), because the anterior tip of the human hippocampus inverts medially to form the uncus in embryological development.32,33 The vHipp and functionally connected brain regions, including the VTA, midline thalamus, nucleus accumbens, amygdala, and infralimbic cortex, are implicated in rodent models of psychosis, substantiating the theory that inappropriate vHipp activation drives aberrant salience via interactions with the VTA.16,37 The anteromedial IC displayed relatively stronger FC with these brain areas, as in previous studies,22 consistent with greater poly- and monosynaptic anatomical connectivity.38–42 Although subcortical areas did not differ between FEP and controls in the resting state, aberrant anteromedial FC could nonetheless functionally affect these circuits, so these findings support the potential importance of this hippocampal subregion in human psychotic disorders.
This is the first report of subregional hippocampal–brain FC in an antipsychotic naïve unmedicated FEP sample. Our entirely data-driven analysis showed reproducibly lower FC in FEP patients relative to controls in the PCC, dACC and insular cortex, core hubs of the default mode,29 and salience30 networks. The question of how the anterior hippocampus functionally interacts with the salience network in FEP is of interest given aberrant salience monitoring is a core feature of psychosis, and the anterior hippocampus plays a key role in salience attribution: in humans, it activates in response to mismatch or novelty,43,44 co-activates with the anterior insula during threat appraisal,45 and has strong FC with subcortical nodes involved in salience processing22,46,47; in rodents, the ventral hippocampus modulates salience according to context via interactions with the amygdala and prefrontal cortical areas.45,48,49 Although the salience network and isolated nodes (dACC and insular cortex) have been previously reported to show altered within network FC (typically hypoconnectivity) relative to controls in UHR,50,51 antipsychotic naïve FEP,52,53 and chronic medicated schizophrenia patients,54–58 few studies have assessed hippocampal FC with this network. Current findings suggest relative hypoconnectivity between the anterior hippocampus and cortical hubs in the salience network in unmedicated FEP patients. Results add to previous studies in unmedicated mixed FEP and chronic patients,21 and medicated chronic psychosis spectrum patients.19,20 These studies were not directly comparable given their use of either whole anterior and posterior ROIs,19,20 or three 6-mm diameter ROIs in the posterior, mid, and anterolateral hippocampus21; also their univariate calculation of FC is known to produce less unique patterns of brain FC compared to the current multivariate FC with dual regression59; nonetheless previous and current findings overlapped in several regards, including hypoconnectivity between the hippocampus and dACC in patients relative to controls.19–21
Time/Treatment Effects
Few studies have examined the effects of antipsychotics on hippocampal FC in schizophrenia, none in a wholly FEP sample. We did not find a main effect of treatment/time at the whole brain voxelwise level; however, within areas that differed between patients and controls, namely the cingulate and insular-opercular cortex, FEP patients displayed a significant increase in mean FC following treatment. This suggests that the remediation of patient-control differences at T2 partially resulted from increased FC in this region in patients, in addition to the T1 to T2 decrease observed in HC. This latter finding may reflect attenuation of novelty in controls at follow-up and augmentation of novelty after treatment in FEP patients, consistent with previous reports showing greater novelty-related fMRI activation in the anterior hippocampus of medicated patients compared with unmedicated patients or controls.60,61 Owing to a lack of clinical equipoise, placebo controls are not typically included in FEP treatment studies. This finding suggests that for the anterior hippocampus, control of novelty may be necessary to isolate time from medication effects. Overall, exploratory findings are broadly consistent with previous reports showing SGA treatment in FEP patients was associated with increased FC in brain areas within the salience and default mode networks,17,62 and add to regional blood flow findings63–65 in showing hippocampal function is altered by antipsychotic treatment, emphasizing the importance of isolating medication effects. Underlying mechanisms may include direct interactions with D2 dopaminergic receptors in the hippocampus,66 and improved integrity in white matter tracts arising from the hippocampus.67
Treatment Response Prediction
We report the first exploratory use of RF to predict treatment response in schizophrenia patients; a previous study used a different machine-learning method for this purpose.68 We used RF techniques previously associated with the highest accuracy in diagnostic classification,69 including an out-of-bag estimate to reduce the probability of overfitting, and recursive feature elimination (Gini index). Mechanistic analyses of predictive RF features suggest that higher baseline LAM–right posterior InsOperc cortex FC and a greater T1-T2 decrease in this FC predicted both BPRS total and positive symptom reduction, despite lower (mostly anterior) insular FC in FEP A patients relative to controls, and an increase following treatment. A previous study in unmedicated FEP patients found that higher insula-Heschl’s gyrus FC was associated with increased positive symptoms despite concurrent hypoconnectivity relative to controls, similar to the current finding (reduced FC associated with positive symptom treatment response).52 This is the first study of anterior hippocampal–insula FC in unmedicated FEP patients. Although the diverse and incompletely understood roles of both these brain areas preclude simple interpretations of FC directionality, findings merit further study. In general, univariate voxelwise analyses of baseline and T1-T2 FC change scores converged on similar areas and correlations with treatment response, but at significance levels that did not survive correction for multiple comparisons. Overall, these exploratory findings suggest that RF analysis based on hippocampal–brain FC may be useful for predicting treatment outcomes in antipsychotic naïve FEP patients.
There were several limitations to the current study. We regard the RF analysis as exploratory owing to the small sample size and lack of a replication sample. We did not explore how hippocampal FC might relate to improvement in negative symptoms, given these did not change with treatment in this study. In addition, we used a relatively stringent univariate test of group differences, which is limited in detecting nonlinear interactions. This approach allowed us to report results of masked hippocampal ICA with dual regression, (a novel technique), in a manner that could be compared with previous studies; future machine-learning based analyses may be used to classify FEP patients vs HC using this technique. Regarding posterior hippocampal FC, our results contrast with previous reports20,21 in finding no group differences. This may be because ICA assigns mutually exclusive time courses to ICs,59 and the relatively stronger FC of anterior ICs with areas such as the PCC masks posterior FC22; therefore, univariate analysis may be more appropriate for measuring posterior hippocampal FC.
In summary, we used a data-driven analysis to show that unmedicated FEP patients had reproducibly lower FC between the LAM hippocampus and cortical regions associated with the salience network, compared with controls. Future multimodal neuroimaging studies may elucidate how this hypoconnectivity relates to anterior hippocampal hyperactivity70 and reduced volume.2 Our exploratory findings suggest that altered FC may be remediated by SGA treatment, and that associated patterns of FC may predict relevant response with high accuracy. Further studies in external FEP populations may validate these findings.
Funding
This study was supported by grant R01 MH084900 from the National Institute of Mental Health (Dr Goff) and by a supplement from the United States–China Program for Biomedical Collaborative Research (Drs Goff and Wang).
Supplementary Material
Acknowledgments
The authors would like to acknowledge helpful discussions with Drs Eugene Laska, Babak Ardekani, and Emily Stern, and the assistance of Matthew Hollander in preparing figures. In the past 2 years, Dr Goff reported receiving research support from the National Institute of Mental Health, Stanley Medical Research Institute, and Avanir Pharmaceuticals. Dr Blessing reported receiving research support from the National Institute of Alcohol Abuse, the Department of Defense, and Tilray Pharmaceuticals. Dr Davachi and Dr Murty reported receiving research support from the National Institute of Mental Health. No other disclosures were reported. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Kraguljac NV, White DM, Reid MA, Lahti AC. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry. 2013;70(12):1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goff DC, Zeng B, Ardekani BA, et al. Association of hippocampal atrophy with duration of untreated psychosis and molecular biomarkers during initial antipsychotic treatment of first-episode psychosis. JAMA Psychiatry. 2018;75(4):370–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ho NF, Iglesias JE, Sum MY, et al. Progression from selective to general involvement of hippocampal subfields in schizophrenia. Mol Psychiatry. 2017;22(1):142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anvari AA, Friedman LA, Greenstein D, Gochman P, Gogtay N, Rapoport JL. Hippocampal volume change relates to clinical outcome in childhood-onset schizophrenia. Psychol Med. 2015;45(12):2667–2674. [DOI] [PubMed] [Google Scholar]
- 5. Heckers S, Konradi C. Hippocampal pathology in schizophrenia. Curr Top Behav Neurosci. 2010;4:529–553. [DOI] [PubMed] [Google Scholar]
- 6. Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65(1):7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15(10):655–669. [DOI] [PubMed] [Google Scholar]
- 8. Poppenk J, Evensmoen HR, Moscovitch M, Nadel L. Long-axis specialization of the human hippocampus. Trends Cogn Sci. 2013;17(5):230–240. [DOI] [PubMed] [Google Scholar]
- 9. Schobel SA, Lewandowski NM, Corcoran CM, et al. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66(9):938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schobel SA, Chaudhury NH, Khan UA, et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron. 2013;78(1):81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Allen P, Chaddock CA, Egerton A, et al. Resting hyperperfusion of the hippocampus, midbrain, and basal ganglia in people at high risk for psychosis. Am J Psychiatry. 2016;173(4):392–399. [DOI] [PubMed] [Google Scholar]
- 12. Winton-Brown T, Schmidt A, Roiser JP, et al. Altered activation and connectivity in a hippocampal-basal ganglia-midbrain circuit during salience processing in subjects at ultra high risk for psychosis. Transl Psychiatry. 2017;7(10):e1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Narr KL, Thompson PM, Szeszko P, et al. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21(4):1563–1575. [DOI] [PubMed] [Google Scholar]
- 14. McHugo M, Talati P, Woodward ND, Armstrong K, Blackford JU, Heckers S. Regionally specific volume deficits along the hippocampal long axis in early and chronic psychosis. Neuroimage Clin. 2018;20:1106–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lodge DJ, Grace AA. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J Neurosci. 2007;27(42):11424–11430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grace AA, Gomes FV. The circuitry of dopamine system regulation and its disruption in schizophrenia: insights into treatment and prevention. Schizophr Bull. 2019;45(1):148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sarpal DK, Argyelan M, Robinson DG, et al. Baseline striatal functional connectivity as a predictor of response to antipsychotic drug treatment. Am J Psychiatry. 2016;173(1):69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tarcijonas G, Foran W, Haas GL, Luna B, Sarpal DK. Intrinsic Connectivity of the Globus Pallidus: An Uncharted Marker of Functional Prognosis in People With First-Episode Schizophrenia. Schizophr Bull. 2020;46(1):184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou Y, Shu N, Liu Y, et al. Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr Res. 2008;100(1–3):120–132. [DOI] [PubMed] [Google Scholar]
- 20. Samudra N, Ivleva EI, Hubbard NA, et al. Alterations in hippocampal connectivity across the psychosis dimension. Psychiatry Res. 2015;233(2):148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kraguljac NV, White DM, Hadley N, et al. Aberrant hippocampal connectivity in unmedicated patients with schizophrenia and effects of antipsychotic medication: a longitudinal resting state functional MRI study. Schizophr Bull. 2016;42(4):1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Blessing EM, Beissner F, Schumann A, Brünner F, Bär KJ. A data-driven approach to mapping cortical and subcortical intrinsic functional connectivity along the longitudinal hippocampal axis. Hum Brain Mapp. 2016;37(2):462–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moher Alsady T, Blessing EM, Beissner F. MICA-A toolbox for masked independent component analysis of fMRI data. Hum Brain Mapp. 2016;37(10):3544–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23(2):137–152. [DOI] [PubMed] [Google Scholar]
- 26. Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31(9):3217–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. [DOI] [PubMed] [Google Scholar]
- 28. Beissner F, Preibisch C, Schweizer-Arau A, Popovici RM, Meissner K. Psychotherapy with somatosensory stimulation for endometriosis-associated pain: the role of the anterior hippocampus. Biol Psychiatry. 2018;84(10):734–742. [DOI] [PubMed] [Google Scholar]
- 29. Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–447. [DOI] [PubMed] [Google Scholar]
- 30. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Murty VP, Ballard IC, Adcock RA. Hippocampus and prefrontal cortex predict distinct timescales of activation in the human ventral tegmental area. Cereb Cortex. 2017;27(2):1660–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zeidman P, Maguire EA. Anterior hippocampus: the anatomy of perception, imagination and episodic memory. Nat Rev Neurosci. 2016;17(3):173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ding SL. Comparative anatomy of the prosubiculum, subiculum, presubiculum, postsubiculum, and parasubiculum in human, monkey, and rodent. J Comp Neurol. 2013;521(18):4145–4162. [DOI] [PubMed] [Google Scholar]
- 34. Zeidman P, Lutti A, Maguire EA. Investigating the functions of subregions within anterior hippocampus. Cortex. 2015;73:240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kahn I, Andrews-Hanna JR, Vincent JL, Snyder AZ, Buckner RL. Distinct cortical anatomy linked to subregions of the medial temporal lobe revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100(1):129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci. 2010;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lodge DJ, Grace AA. Amphetamine activation of hippocampal drive of mesolimbic dopamine neurons: a mechanism of behavioral sensitization. J Neurosci. 2008;28(31):7876–7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Legault M, Rompré PP, Wise RA. Chemical stimulation of the ventral hippocampus elevates nucleus accumbens dopamine by activating dopaminergic neurons of the ventral tegmental area. J Neurosci. 2000;20(4):1635–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Risold PY, Swanson LW. Structural evidence for functional domains in the rat hippocampus. Science. 1996;272(5267):1484–1486. [DOI] [PubMed] [Google Scholar]
- 40. Canteras NS, Swanson LW. Projections of the ventral subiculum to the amygdala, septum, and hypothalamus: a PHAL anterograde tract-tracing study in the rat. J Comp Neurol. 1992;324(2):180–194. [DOI] [PubMed] [Google Scholar]
- 41. Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Brain Res Rev. 2001;38(1–2):247–289. [DOI] [PubMed] [Google Scholar]
- 42. Vertes RP, Hoover WB. Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat. J Comp Neurol. 2008;508(2):212–237. [DOI] [PubMed] [Google Scholar]
- 43. Duncan K, Ketz N, Inati SJ, Davachi L. Evidence for area CA1 as a match/mismatch detector: a high-resolution fMRI study of the human hippocampus. Hippocampus. 2012;22(3):389–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Strange BA, Dolan RJ. Anterior medial temporal lobe in human cognition: memory for fear and the unexpected. Cogn Neuropsychiatry. 2006;11(3):198–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Suarez-Jimenez B, Bisby JA, Horner AJ, King JA, Pine DS, Burgess N. Linked networks for learning and expressing location-specific threat. Proc Natl Acad Sci USA. 2018;115(5):E1032–E1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kahn I, Shohamy D. Intrinsic connectivity between the hippocampus, nucleus accumbens, and ventral tegmental area in humans. Hippocampus. 2013;23(3):187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zheng J, Anderson KL, Leal SL, et al. Amygdala-hippocampal dynamics during salient information processing. Nat Commun. 2017;8:14413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16(2):174–182. [DOI] [PubMed] [Google Scholar]
- 49. Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. J Neurosci. 2011;31(47):17269–17277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Collin G, Seidman LJ, Keshavan MS, et al. Functional connectome organization predicts conversion to psychosis in clinical high-risk youth from the SHARP program. Mol Psychiatry. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pelletier-Baldelli A, Andrews-Hanna JR, Mittal VA. Resting state connectivity dynamics in individuals at risk for psychosis. J Abnorm Psychol. 2018;127(3):314–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pang L, Kennedy D, Wei Q, et al. Decreased functional connectivity of insular cortex in drug naïve first episode schizophrenia: in relation to symptom severity. PLoS One. 2017;12(1):e0167242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mallikarjun PK, Lalousis PA, Dunne TF, et al. Aberrant salience network functional connectivity in auditory verbal hallucinations: a first episode psychosis sample. Transl Psychiatry. 2018;8(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF. Neural primacy of the salience processing system in schizophrenia. Neuron. 2013;79(4):814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Manoliu A, Riedl V, Zherdin A, et al. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull. 2014;40(2):428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Chen X, Duan M, He H, et al. Functional abnormalities of the right posterior insula are related to the altered self-experience in schizophrenia. Psychiatry Res Neuroimaging. 2016;256:26–32. [DOI] [PubMed] [Google Scholar]
- 57. Wang D, Zhou Y, Zhuo C, et al. Altered functional connectivity of the cingulate subregions in schizophrenia. Transl Psychiatry. 2015;5:e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hare SM, Ford JM, Mathalon DH, et al. Salience-default mode functional network connectivity linked to positive and negative symptoms of schizophrenia. Schizophr Bull. 2019;45(4):892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Leech R, Braga R, Sharp DJ. Echoes of the brain within the posterior cingulate cortex. J Neurosci. 2012;32(1):215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ragland JD, Layher E, Hannula DE, et al. Impact of schizophrenia on anterior and posterior hippocampus during memory for complex scenes. Neuroimage Clin. 2017;13:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Tamminga CA, Thomas BP, Chin R, et al. Hippocampal novelty activations in schizophrenia: disease and medication effects. Schizophr Res. 2012;138(2–3):157–163. [DOI] [PubMed] [Google Scholar]
- 62. Sarpal DK, Robinson DG, Lencz T, et al. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry. 2015;72(1):5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Cropsey KL. Modulation of limbic circuitry predicts treatment response to antipsychotic medication: a functional imaging study in schizophrenia. Neuropsychopharmacology. 2009;34(13):2675–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11(5):543–550. [DOI] [PubMed] [Google Scholar]
- 65. Bolding MS, White DM, Hadley JA, Weiler M, Holcomb HH, Lahti AC. Antipsychotic drugs alter functional connectivity between the medial frontal cortex, hippocampus, and nucleus accumbens as measured by H215O PET. Front Psychiatry. 2012;3:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tomasella E, Bechelli L, Ogando MB, et al. Deletion of dopamine D2 receptors from parvalbumin interneurons in mouse causes schizophrenia-like phenotypes. Proc Natl Acad Sci USA. 2018;115(13):3476–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Reis Marques T, Taylor H, Chaddock C, et al. White matter integrity as a predictor of response to treatment in first episode psychosis. Brain. 2014;137(Pt 1):172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cao H, Dixson L, Meyer-Lindenberg A, Tost H. Functional connectivity measures as schizophrenia intermediate phenotypes: advances, limitations, and future directions. Curr Opin Neurobiol. 2016;36:7–14. [DOI] [PubMed] [Google Scholar]
- 69. Sarica A, Cerasa A, Quattrone A. Random forest algorithm for the classification of neuroimaging data in Alzheimer’s disease: a systematic review. Front Aging Neurosci. 2017;9:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lieberman JA, Girgis RR, Brucato G, et al. Hippocampal dysfunction in the pathophysiology of schizophrenia: a selective review and hypothesis for early detection and intervention. Mol Psychiatry. 2018; 23:1764–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.