Abstract
Glutamate (Glu), gamma amino-butyric acid (GABA), and excitatory/inhibitory (E/I) imbalance have inconsistently been implicated in the etiology of schizophrenia. Elevated Glu levels in language regions have been suggested to mediate auditory verbal hallucinations (AVH), the same regions previously associated with neuronal hyperactivity during AVHs. It is, however, not known whether alterations in Glu levels are accompanied by corresponding GABA alterations, nor is it known if Glu levels are affected in brain regions with known neuronal hypo-activity. Using magnetic resonance spectroscopy (MRS), we measured Glx (Glu+glutamine) and GABA+ levels in the anterior cingulate cortex (ACC), left and right superior temporal gyrus (STG), and left inferior frontal gyrus (IFG), in a sample of 77 schizophrenia patients and 77 healthy controls. Two MRS-protocols were used. Results showed a marginally significant positive correlation in the left STG between Glx and AVHs, whereas a significant negative correlation was found in the ACC. In addition, high-hallucinating patients as a group showed decreased ACC and increased left STG Glx levels compared to low-hallucinating patients, with the healthy controls in between the 2 hallucinating groups. No significant differences were found for GABA+ levels. It is discussed that reduced ACC Glx levels reflect an inability of AVH patients to cognitively inhibit their “voices” through neuronal hypo-activity, which in turn originates from increased left STG Glu levels and neuronal hyperactivity. A revised E/I-imbalance model is proposed where Glu-Glu imbalance between brain regions is emphasized rather than Glu-GABA imbalance within regions, for the understanding of the underlying neurochemistry of AVHs.
Keywords: Magnetic resonance imaging (MRI), MR spectroscopy (MRS), schizophrenia, hallucinations, auditory verbal hallucinations, glutamate, GABA, Glx, excitatory, inhibitory (E/I) imbalance model
Introduction
Auditory verbal hallucinations (AVHs) is a key symptom in schizophrenia, and refers to auditory experiences of “hearing a voice,” in the absence of an external auditory source.1,2 Neuronal underpinnings of AVHs are to date not fully understood, although imbalance between excitatory and inhibitory influences (E/I imbalance) in the brain have been proposed as potential mechanisms (ref.3 for a review), and appear to exist on multiple levels including large-scale cognitive networks (between regions) and small-scale neuronal circuits (within regions). For large-scale cognitive networks, evidence suggests E/I imbalance between language and cognitive control regions in schizophrenia patients. fMRI meta-analyses have suggested (hyper-)activation during AVHs in a bilateral fronto-temporal network including speech perception (superior temporal gyrus [STG]4) and speech production (Broca’s area in inferior frontal gyurs [IFG]) areas.1,5 Furthermore, a consistent finding in schizophrenia patients is hypo-activity in prefrontal cortex (eg, anterior cingulate cortex, ACC).6 These findings go along with findings of impaired cognitive control/executive functions and regulation of attention in these patients.7–9 Based on findings from behavioral and neuroimaging studies, Hugdahl10 proposed a 2-fold model of the pathophysiology underlying AVHs involving neuronal hyper-excitation in temporal lobe speech perception regions leading to the perception of a “voice” in a bottom-up way, and frontal lobe top-down failure in suppressing the bottom-up input (c.f.11,12). The aim of the present study was to investigate any underlying neurotransmitter excitatory-inhibitory correspondence to the bottom-up and top-down imbalance found at the cognitive/behavioral level of explanation (cf.13).
Dysfunction of glutamatergic and/or gamma amino-butyric acid (GABA)-ergic neurotransmitter systems is implicated in the etiology of schizophrenia, and could result in regional imbalances of excitation and inhibition. Dysfunction of glutamatergic transmission has been attributed to hypo-function of the N-methyl-D_aspartate (NMDA)-type glutamate (Glu) receptor, and NMDA receptor dysfunction on GABAergic neurons could lead to disinhibition and increase of Glu release.14 Administration of the NMDA antagonist ketamine to healthy volunteers has produced symptoms comparable to that of schizophrenia patients,15–17 and exacerbated psychotic symptoms (including auditory hallucinations) in schizophrenia patients.17,18 In support of deficits in GABAergic neurotransmission is the consistent postmortem finding of lowered expression of glutamatergic acid decarboxylase 67 (GAD67) mRNA (eg,19–21), which codes for a protein involved in the conversion of Glu to GABA (for a review see ref.22). In vivo studies of altered Glu and GABA levels are further needed in confirming a Glu-GABA imbalance hypothesis of schizophrenia.
Glu and GABA concentration levels can be measured in vivo by hydrogen magnetic resonance spectroscopy (1H-MRS). MRS takes advantage of the magnetic properties of the hydrogen proton. The surroundings of the hydrogen proton(s), ie, the molecule in which the proton is bound, affect its magnetic properties.23 As a result, it is possible to identify signals from different molecules. Multiple studies have investigated Glu and GABA concentrations in schizophrenia patients applying MRS, although results are inconsistent. A recent meta-analysis of Glu24 concluded of generally elevated Glu, glutamine (Gln), or of the composite signal, Glx, in sub-cortical regions in schizophrenia patients. For the ACC, significantly elevated Glx levels were found only for a high-risk population. A recent meta-analysis on GABA25 concluded with no overall difference between patients and controls in spite of single studies confirming low GABA levels in patients in the ACC.26,27 One reason for inconsistent results in MRS studies might be different symptom profiles in patients across samples.24
Only a few studies have investigated Glu in relation to auditory hallucinations. Elevated Glu levels have been found associated with auditory hallucination severity in the left STG,28 and left IFG.28,29 Whether these hallucination-related Glu alterations are accompanied by GABAergic changes within the same regions—as predicted by the E/I imbalance model—has not yet been investigated. It should also be noted that both left STG1,4 and left IFG1 have been related to neuronal hyperactivity during hallucinations in schizophrenia patients. Whether similar relationships exist between AVH and Glu in regions related to hypo-activity is unknown. In fact, one might consider that there also exist Glu-Glu imbalances and/or GABA-GABA imbalances between regions associated with respectively hyper- and hypo-activity, but this remains to be tested.
The current study, therefore, aimed to explore whether Glu-GABA imbalances are associated with severity of AVHs in schizophrenia patients in language (left and right STG, and left IFG) and executive (ACC) regions. The left and right STG and ACC regions were examined with Protocol 1 (n = 39); and the left IFG and STG were examined with Protocol 2 (n = 38). The 2 MR protocols used did not differ with regard to the aims of the study. We hypothesized that relationships between AVH and Glu and GABA levels would be region-specific, such that positive Glu and negative GABA correlations with AVH would be seen in the STG and IFG regions, reflecting increased excitation (hyperactivity) in the language regions, while the reverse relationship should be found for the ACC region reflecting decreased excitation (hypo-activity) in executive/cognitive control regions.
Methods
Participants
Seventy-seven schizophrenia patients (mean age 29.83, SD 11.48) and 77 matched controls (mean age 30.23, SD 10.23) underwent MRS scanning (some tested repeatedly at 2 different time points, see table 1 for sample details). The patients were recruited via the Bergen Psychosis Study 2. Two MRS data collection protocols, including different voxel placements, were used. One protocol (hereafter referred to as Protocol 1) included MRS recordings from the left STG, right STG, and ACC and was used for 39 patients and 39 controls. The second protocol (hereafter referred to as Protocol 2) included MRS recordings from the left STG and left IFG and was used for 38 patients and 38 controls (see figure 1 for voxel placement). Data from the 2 protocols were pooled in the analyses.
Table 1.
Regional Concentration Means and SDs of Glx, Glutamate, Glutamine, GABA (Institutional Units), and Data Quality Parameters of the Patients and Controls That Were Included in Data Analysis
| Patients | Controls | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mis/ Excl. | Mean | SD | N | Mis/ Excl. | Mean | SD | |||
| LSTG | P | Glx | 92 (76) | -/1 | 15.73 | 4.10 | 77 | -/- | 15.87 | 4.19 |
| Glutamate | 81 (67) | 13.23 | 2.13 | 60 | 13.61 | 2.0 | ||||
| Glutamine | 81 (67) | 3.60 | 2.54 | 60 | 4.06 | 2.63 | ||||
| SNR | 92 (76) | 39.55 | 12.17 | 77 | 40.81 | 12.28 | ||||
| FWHM | 92 (76) | 0.07 | 0.02 | 77 | 0.07 | 0.02 | ||||
| MP | GABA | 83 (68) | 2/8 | 4.95 | 0.97 | 75 | -/2 | 4.82 | 1.08 | |
| SNR | 83 (68) | 21.51 | 5.94 | 75 | 20.53 | 6.36 | ||||
| FWHM | 83 (68) | 0.07 | 0.02 | 75 | 0.07 | 0.02 | ||||
| LIFG | P | Glx | 48 (37) | 1/- | 16.93 | 2.86 | 38 | -/- | 16.18 | 3.23 |
| Glutamate | 46 (36) | 14.28 | 1.88 | 34 | 14.35 | 1.8 | ||||
| Glutamine | 46 (36) | 3.12 | 1.64 | 34 | 2.75 | 1.5 | ||||
| SNR | 48 (37) | 42.48 | 9.02 | 38 | 47.29 | 6.79 | ||||
| FWHM | 48 (37) | 0.06 | 0.01 | 38 | 0.05 | 0.01 | ||||
| MP | GABA | 47 (37) | 1/1 | 5.47 | 0.77 | 38 | -/- | 5.4 | 0.84 | |
| SNR | 47 (37) | 27.68 | 3.93 | 38 | 28.45 | 4.16 | ||||
| FWHM | 47 (37) | 0.06 | 0.02 | 38 | 0.06 | 0.02 | ||||
| ACC | P | Glx | 43 (38) | 1/- | 20.18 | 3.33 | 37 | -/2 | 19.83 | 3.59 |
| Glutamate | 36 (33) | 17.09 | 1.80 | 33 | 16.55 | 1.69 | ||||
| Glutamine | 36 (33) | 4.22 | 2.39 | 33 | 4.31 | 2.28 | ||||
| SNR | 43 (38) | 40.51 | 7.26 | 37 | 41.46 | 6.03 | ||||
| FWHM | 43 (38) | 0.06 | 0.02 | 37 | 0.06 | 0.01 | ||||
| MP | GABA | 42 (37) | 1/1 | 4.44 | 0.83 | 38 | -/1 | 4.59 | 0.82 | |
| SNR | 42 (37) | 18.41 | 6.15 | 38 | 19.05 | 5.50 | ||||
| FWHM | 42 (37) | 0.05 | 0.01 | 38 | 0.06 | 0.01 | ||||
| RSTG | P | Glx | 42 (37) | 2/- | 22.30 | 3.25 | 38 | -/1 | 21.14 | 4.31 |
| Glutamate | 40 (35) | 16.40 | 1.96 | 35 | 15.34 | 2.51 | ||||
| Glutamine | 40 (35) | 6.95 | 3.49 | 35 | 7.08 | 3.66 | ||||
| SNR | 42 (37) | 33.71 | 9.51 | 38 | 31.76 | 8.13 | ||||
| FWHM | 42 (37) | 0.07 | 0.02 | 38 | 0.08 | 0.02 | ||||
| MP | GABA | 37 (32) | 2/5 | 4.63 | 1.13 | 37 | -/2 | 4.43 | 1.28 | |
| SNR | 37 (32) | 14.05 | 4.93 | 37 | 14.22 | 4.84 | ||||
| FWHM | 37 (32) | 0.08 | 0.03 | 37 | 0.09 | 0.03 | ||||
Note: LSTG, left superior temporal gyrus; LIFG, left inferior frontal gyrus; ACC, anterior cingulate cortex; RSTG, right superior temporal gyrus; P, PRESS sequence; MP, MEGA-PRESS sequence; Mis, missing spectra; Excl, excluded spectra; SNR, signal-to-noise ratio; FWHM, full-width at half maximum (linewidth). N refers to number of observations (as some patients were tested repeatedly at 2 sessions), and in parentheses the number of unique subjects. Number of participants differ across regions and groups (patients and controls) due to missing or excluded (low quality) spectra for single measurements. N also differ between Glx and glutamate/glutamine due to cases where the composite signal was inseparable.
Fig. 1.
Placement of voxels in the left STG, ACC (across midline), right STG, and left IFG shown in horizontal views. Center-of-mass for the voxel localization, given in MNI space x, y, z coordinates for the left IFG: −36.8, 18.9, 11.9, left STG: −48.3, −35.9, 6.02, right STG: 50.2, −34.2, 6.11, and for the ACC: 0.389, 25.4, 33.9. The orange box illustrates the placement for a single representative subject. The red contours indicate 95%, and the green contours indicate 65% confidence regions for placement across the entire group (when mapped to a standard template). LSTG, left superior temporal gyrus; LIFG, left inferior frontal gyrus; ACC, anterior cingulate cortex; RSTG, right superior temporal gyrus.
The controls were individually matched to the patients with regard to sex and age (within ±3 y from their respective controls), except for 9 patients who were outside of the 3 years range (4–7 y). Patients and controls were in addition matched in terms of scanning protocol, and handedness, with the exception of ambidextrous patients who were matched with right-handed controls. All patients that were on medication used second-generation antipsychotic medication while some, in addition, used first-generation antipsychotics (mean defined daily dose (DDD):1.03, SD 0.62). A few patients also used anti-depressants (n = 10), mood stabilizers (n = 2), opioids (n = 1), benzodiazepines (n = 18), anticholinergic (n = 5), or ADHD medication (n = 1). All patients were diagnosed with schizophrenia spectrum disorder according to the ICD-10 diagnostic manual (World Health Organization, 1992; Norwegian translation; https://ehelse.no/standarder-kodeverk-og-referansekatalog/helsefaglige-kodeverk/kodeverket-icd-10-og-icd-11). Mean illness duration for the patient group was 4.34 years, SD 7.68. Global severity of symptoms in the patient group as assessed by the Positive and Negative Syndrome Scale (PANSS30) total score were 62.48, SD 17.63 (Positive-total 15.53, SD 5.51; Negative-total 15.03, SD 5.02; General-total 31.91, SD 9.70). The study was approved by the Regional Committee for Medical Research Ethics at the University of Bergen (REK no 2010–3387), and conducted according to the Declaration of Helsinki. All participants received oral and written information about the study before signing a written consent form.
Hallucinations were assessed with the PANSS30 interview. All PANSS-raters were trained and certified by the PANSS Institute, and satisfactory inter-rater reliability was documented. Severity of auditory hallucinations were scored from the P3 item on the PANSS positive sub-scale, ranging from 1 to 7 (1 = absent, 7 = extreme). Although the PANSS questionnaire does not explicitly distinguish between modalities, hallucinations in the auditory domain (as “voice hearing”) is most common,31,32 and the P3 item is hereafter referred to as AVH. The PANSS scores were obtained on the same day as the MRS measurements.
MR Image Acquisitions
Imaging data were acquired with a 3T GE-SignaHDx MRI scanner. See table 2 for MR acquisition parameters. Unsuppressed water reference spectra (8 repetitions) were acquired automatically after the acquisition of water-suppressed metabolite spectra in both protocols. A scanner up-grade was conducted between data collection of Protocol 1 and 2, including a change of head-coil from 8 to 32 channels.
Table 2.
MR Acquisition Parameters for Protocol 1 and 2
| N | Regions | Sequence | TR | TE | TI | FOV | Rep | Voxel size (m3) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Protocol 1 | 39 | T1 | Whole brain | 3DSPGR | 7.74 | 2.9 | 500 | 260 | 1 × 1 × 1 | |
| MRS | LSTG,RSTG, | PRESS | 1500 | 35 | 128 | STG: 24 × 40 × 30 | ||||
| MR | ACC | ACC: 40 × 40 × 25 | ||||||||
| MEGA-PRESS | 1500 | 68 | 128 | |||||||
| Protocol 2 | 38 | T1 | Whole brain | 3DSPGR | 6.8 | 2.95 | 450 | 256 | 1 × 1 × 1 | |
| MRS | LSTG, LIFG | PRESS | 1500 | 35 | 192 | LSTG: 24 × 30 × 31 | ||||
| MEGA-PRESS | 1500 | 35 | 192 | LIFG:24 × 38 × 28 |
Note: LSTG, left superior temporal gyrus; LIFG, left inferior frontal gyrus; ACC, anterior cingulate cortex; RSTG, right superior temporal gyrus; PRESS, point-resolved spectroscopy sequence; MEGA-PRESS, Mescher-Garwood PRESS; TR, repetition time; TE, echo time; TI, inversion time; FOV, field of view; Rep, repetitions. The repetitions for the MEGA-PRESS acquisition refer to the number of edit ON/OFF pairs acquired.
Data Analysis
MRS data from the PRESS sequence were analyzed using the LCModel version 6.3-1J,33 with the standard basis-set incorporating components from 15 metabolites (Alanine, Aspartate, Creatine, γ-amino-butyric acid, Glucose, Gln, Glu, Glycerophosphorylcholine, Phosphorylcholine, Lactate, myo-inositol, N-acetylaspartate, N-acetylaspartylglutamate, scyllo-inositol, and Taurine). Basic processing of MEGA-PRESS data was achieved using in-house scripts to perform coil combination, phase correction, alignment, and residual water subtraction before subtracting edit-OFF from edit-ON parts to yield the final difference spectrum for each acquisition. This spectrum was then quantified in LCModel with the mega-press-3 spectra type, using a simulated basis set34 with Kaiser coupling constants.35 Metabolite estimates were scaled to an internal water reference, accounting for differing water concentration in the different tissue classes (GM, WM, CSF), partial volume effects, and metabolite relaxation times and differing water relaxation times between the tissue classes.36 Tissue content within the MRS voxel was estimated from the T1-image using the segmentation tool of the Statistical Parametric Mapping (SPM8) software (www.fil.ion.ucl.ac.uk/spm). Individual spectra were subject to quality control, which led to the rejection of inadequate spectra (see table 1 for excluded spectra, and spectral quality parameters, and supplementary figure S1 for spectrum examples and information on quality control procedure). For further details on analysis procedure, see ref.37 Glx and GABA+ (GABA including macromolecules) levels were used in statistical analyses.
Statistical Analysis
Glx and GABA+ values were subjected to statistical analysis using Linear mixed models available in the SPSS software package (https://www.ibm.com/analytics/spss-statistics-software). All multivariate analyses for Glx included a sample size of 77 patients and 77 controls, while GABA analyses included 76 patients and 77 controls. First, 2 multivariate models were applied to the data, with Glx and GABA+ as dependent variables, respectively, to test for overall and regional differences between patients and controls. Participants were modeled as a random factor, while group (patients vs controls) and region (left and right STG, ACC, and IFG) were entered as fixed factors, and region as a repeated measure. Similar models were estimated for subgroups of patients using a median-split into high (AVH+, P3 score > 2, n = 38) and low hallucinators (AVH-, P3 score < 2, n = 39) which resulted in 3 levels for the Group factor (AVH+, AVH-, and controls). Fisher’s LSD post hoc test was used to follow-up significant interactions. Subsequently, 2 multivariate models with Glx and GABA+ as dependent variables were run for patients only in order to test the linear relationship between regional metabolites and severity of AVHs. In these models, Region was entered as a repeated fixed factor, and P3 AVH score was entered as a regressor variable, while participants were modeled as a random factor. Post hoc testing in order to explore Region × AVHs interaction was done by splitting the multivariate regression models into lower-level models (one per region, with sample sizes: LSTG Glx = 76, GABA = 68; LIFG Glx = 37, GABA = 37; ACC Glx = 38, GABA = 37; RSTG Glx = 37, GABA = 32) while keeping the AVH variable as a regressor variable. The last analysis was thereafter repeated with the total negative symptom score, replacing the P3, as a regressor. In general, a simple model structure (scaled identity option) was chosen due to the relatively small sample size. Restricted log-likelihood test and Swartz’s Bayesian criterion showed that little was to gain in terms of model fit by choosing a more complex model structure.
Analyses were conducted to rule out the change of coil as a factor that could have biased the investigated relationship between AVH and Glx and GABA for the left STG. First, a chi-square test showed no difference between the AVH+ and AVH- groups in terms of how many patients in each group were tested with the 2 respective coils (P = .34). Second, there was no significant difference between the correlation coefficients of AVH against metabolites (Glx [P = .74], GABA [P = .16]) for data collected with the 2 respective coils.
Results
Mean and SD for Glu, Gln, Glx, and GABA+ in patients and controls are seen in table 1.
Glx
A first analysis comparing patients and controls was nonsignificant for main-effect of Glx (F(1,142.58) = 1.3, P = .26), and interaction-effect of Group × Region source (F(3,305.5) = 0.88, P = .45). The main-effect of Region was, however, significant (F(3,305.5) = 49.3, P < .001), and pairwise post hoc comparisons showed significant differences between all 4 regions (all P < .001), with regional Glx levels ranging from highest to lowest in following order: right STG, ACC, left IFG, left STG. When dividing the patients into AVH+ vs AVH- sub-groups, the main-effect of Region (F(3,298.49) = 51.27, P < .001) remained significant, and main-effect of Group (F(2, 177.87) = 0.79, P = .46) nonsignificant, but now a significant interaction-effect of Group × Region was observed (F(6, 295.24) = 2.22, P = .04; see figure 2).
Fig. 2.
Glutamate and GABA concentration levels are shown on the y-axes for healthy controls, low-hallucinating patients (AVH-), and high-hallucinating patients (AVH+) in the 4 brain regions. Error bars indicate standard error. * indicates significant post hoc comparisons at P < .05, conducted to explore the significant interaction of Group and Region. STG, superior temporal gyrus; IFG, inferior frontal gyrus; ACC, anterior cingulate cortex; IU, institutional units.
Post hoc comparisons showed that the interaction was driven by higher Glx levels in the AVH+ vs AVH- sub-group in the left STG (P = .04), while the reverse was true for the ACC, with lower Glx levels in the AVH+ sub-group compared to the AVH- sub-group (P = .03). There was also a nonsignificant trend for the AVH- sub-group to show higher Glx levels compared to the controls (P = .07).
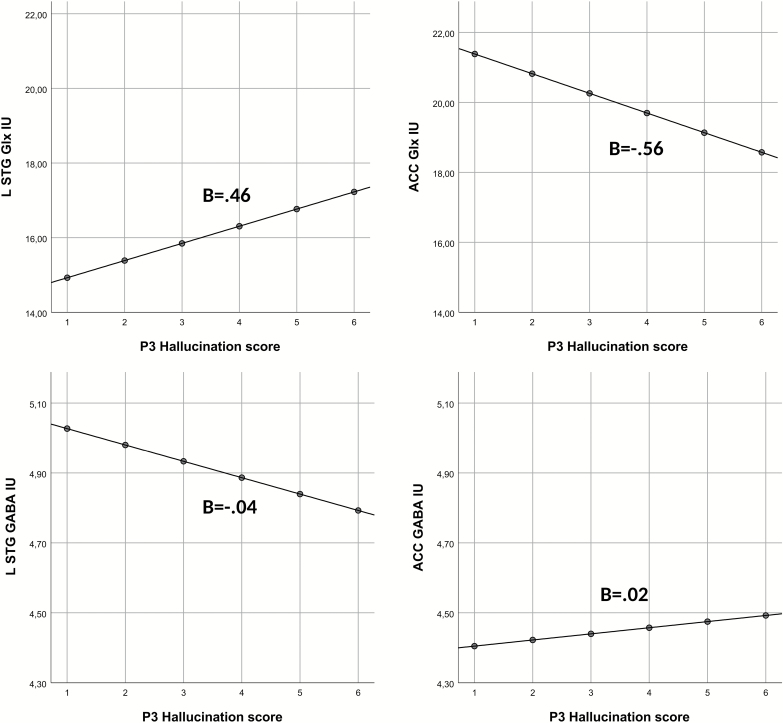
The next set of analyses involved patients only and assessed the linear relationship between AVH and Glx. Again, there was a significant main-effect of Region, (F(3,171.78) = 20.56, P < .001), while the main-effect of AVH was nonsignificant (F(1,125.4) = 0.05, P = .83, respectively). However, the interaction of Region and AVH severity was significant (F(3,170.1) = 4.38, P = .005). Post hoc analyses showed that the interaction was driven by a marginally significant positive correlation between Glx levels in the left STG and P3 score (P = .054, B = 0.46), and a significant negative correlation between Glx and the P3 score in the ACC (P = .04, B = 0.56; see figure 3). AVHs were not significantly correlated with Glx levels in the right STG (P = .93, B = −0.14) or in the left IFG (P = .79, B = −0.06).
Fig. 3.
Graphs illustrating relations between glutamate or GABA levels (y-axis) and AVHs (x-axis) in left STG and ACC. Note that fixed predicted, not raw, values are used (y-axis). LSTG, left superior temporal gyrus; ACC, anterior cingulate cortex; IU, institutional units.
Total negative symptom score was not found related to Glx.
GABA+
In the analysis comparing overall GABA+ levels for patients vs controls, only the main-effect of Region was significant (F(3,280.1) = 7.79, P < .001). Post hoc analyses showed a significant difference (P < .05) between all regions (ranged from highest to lowest levels: LIFG, LSTG, RSTG, and ACC) except for right STG compared with left STG and ACC. When splitting the patients into the AVH+ and AVH- sub-groups, there were still no significant Group or interaction-effects (figure 2). The main-effect of Region remained significant (F(3,276.31) = 7.57, P < .001). The model assessing the linear relationship between AVHs and GABA+ did not come out significant for any comparison. Lower-level models conducted for each region separately did not change these results.
A trend of a negative linear relationship between total negative symptom score and GABA+ was found (F(1, 84.99) = 3.5, P = .065) across regions.
Discussion
The current study investigated the neurochemical underpinnings of AVH by addressing Glu-GABA imbalances within selected brain regions, and Glu-Glu and GABA-GABA imbalances between regions previously associated with hyper- and hypo-activity during AVH experience. No overall differences between patients and controls were found. However, significant differences were found between patient groups, where AVH+ patients showed lower ACC and higher left STG Glx levels relative to the AVH- group (figure 2). The control group showed Glx levels which were in between those of the AVH+ and AVH- patients. These findings were confirmed by correlation analysis, where an interaction was found between brain region and AVH severity. The interaction was driven by a negative relationship between ACC Glx and the AVH score, whereas a positive relationship was shown between left STG Glx and the AVH score (figure 3).
Glu Levels: STG and IFG Voxels
As hypothesized, Glx in the left STG was positively associated with AVH severity. Although the correlation was marginally significant, previous findings by Hugdahl et al28 and Curic-Blake et al29 suggest that an a priori hypothesis could be formulated regarding the direction of the correlation. Therefore, a one-sided test could have been argued for and would, in this case, shown a significant effect.
In this sense, the present results extend the findings by Hugdahl et al28 on a larger sample. The results are also in line with previous fMRI findings showing increased activation in the left STG during AVH experiences.1,4 This is not surprising when seen in the light of functional MRS studies that show significant increase in Glx levels during stimulus processing and task execution.38,39 In this sense, left STG Glx levels might cause neuronal hyper-excitation that initiate the perceptual experience of “hearing a voice.” In spite of a positive association between left STG Glx and AVH severity, the patients did not show overall higher Glx levels as compared to controls. One could, therefore, speculate that the increased neuronal activity associated with AVHs elevates Glx to “normal levels” corresponding to that of healthy individuals. This is further supported by the finding of lowest Glx levels in AVH- group, also lower than the controls. It should, however, be noted that neuronal activity was not measured. We can therefore not rule out that Glx would rather be associated with hypo-activity due to neuronal loss as a consequence of neurotoxic effects of Glx.40 However, since AVHs have previously been associated with left STG hyperactivity, we find the results of a positive relationship between Glx and AVHs to be unlikely if Glx would cause hypo-activity.
Interestingly, the positive association between Glx and AVH experiences was restricted to the left STG, suggesting a laterality effect implicating more the left than the right hemisphere. This result is in line with several fMRI meta-analyses1,4 showing left, but not right, STG activations in patients with AVHs. Since the left hemisphere is the dominant hemisphere for language processing41–43 and since AVH are typically experienced as “voices,” 31 an increase in Glx levels in left STG only is a feasible finding.
There was a lack of significant findings for the left IFG, which contradicts 2 previous MRS studies: Hugdahl et al28 and Ćurčić-Blake et al29 both found lower Glx levels in patients relative to controls, and higher Glx levels in hallucinating relative to non-hallucinating patients. Several methodological differences between the studies could explain the discrepancies in results, such as voxel placement (more medial and anterior in former studies), or sample characteristics such as lower P3 scores in Hugdahl et al, and AVH assessed as a trait measure in Ćurčić-Blake et al. as opposed to a state measure in the current study.
Glx Levels: ACC Voxel
As hypothesized, ACC Glx levels were found to be negatively correlated with AVH. This could be related to neuronal hypo-activity and reduced executive and cognitive control functions that has been observed in hallucinating schizophrenia patients.6,44–47 A possible interpretation is that patients with low Glx levels lack the cognitive resources necessary to inhibit the “voices” originating from the left STG, due to top-down hypo- and bottom-up hyper-excitation cf.10,11 Interestingly, no association was found between total negative symptom score and Glx levels in ACC, nor in any of the other regions, highlighting the specificity of AVHs symptoms in predicting Glx concentrations.
The finding of no overall ACC Glx level difference between patients and controls adds to previous inconsistent findings, with some studies showing higher Glx levels in patients relative to controls,48,49 other showing lower levels,50,51 and others again reporting no difference.52–54 However, when taking AVH into account, a significant group-effect appeared, driven by differences between the 2 patient sub-groups (AVH+ and AVH-). There was also a trend towards higher Glx levels in the AVH- group compared to the control group.
Thus, as for both the ACC and left STG regions, the healthy controls showed Glx levels in between the levels for the AVH+ and AVH- sub-groups. The ACC results, therefore, suggest that one reason for inconsistencies in previous studies when comparing schizophrenia patients and controls could, at least partly, be due to inter-individual heterogeneity in symptom severity, in particular, severity of AVHs. AVH should, therefore, be reported or controlled for in future MRS studies.
GABA+ Levels: All Voxels
GABA+ levels were not found to differ between patients and controls, nor were GABA+ levels associated with AVHs. Previous GABA MRS studies have shown inconsistent results, in spite of consistent postmortem findings of reduced GAD67 mRNA production.25 The current study showed that even when inter-individual and inter-regional differences were taken into account, the measurements failed to show GABAergic alterations in the patients. de Jonge et al22 suggested that other subclasses of GABAergic neurons might compensate for the reduced GABA production in parvalbumin-neurons, or could be stimulated by increased glutamergic activity. This would be a plausible explanation for the left STG findings where Glx was elevated in AVH+ patients.
Intra-Regional vs Inter-Regional E/I-Imbalances
The current study investigated the underlying neurochemistry in terms of the E/I- imbalance model for AVHs. While the results could not unambiguously confirm intra-regional Glu-GABA imbalances due to the lack of significant GABA findings, we found an inter-regional Glu-Glu imbalance associated with AVH severity. We thus propose a revised and extended E/I-imbalance model where Glu-Glu imbalance between brain regions may be more relevant than Glu-GABA imbalance within regions, for the understanding of the underlying neurochemistry of AVHs.
The suggested region-specificity regarding the relation between Glx and AVH give rise to questions of underlying mechanisms. It is increasingly accepted that NMDA receptor deficiency should result in elevated Glu and reduced GABA production in schizophrenia patients.24,55,56 If NMDA receptor deficiency, as eg, indicated by compensatory up-regulation of NMDA receptor subunits,57,58 underpin the results of the current study, they are likely to be region-specific, and Glx alterations seen in other regions secondary. One possibility is that dysfunction of NMDA-receptors in the left STG58,59 causes neuronal hyperactivity and elevated Glx levels, leading to the perceptual experience of “hearing a voice.” In a non-dysfunctional brain, the ACC would be similarly activated to suppress or inhibit the “voices” to enter awareness. In the hallucinating brain, this top-down regulation might be upset because of low ACC Glx levels, together with, or as a consequence of, “strong” bottom-up perceptual impulses, resulting in AVH. It should, however, be remembered that MRS do not measure NMDA- receptor function per se, and therefore further studies applying, eg, PET-MR, may be instrumental in disentangle cause and effect with regard to the underlying neurochemistry of AVH. Another limitation of the current study is that different numbers of patients were assessed in the 2 regions, and therefore, it remains undetermined whether the regional alterations co-exist in the same patients. With these limitations in mind, the findings may nevertheless point in new directions for the development of symptom-specific targets for pharmacological treatment interventions.
Supplementary Material
Supplementary material is available at Schizophrenia Bulletin online.
Figure S1: Examples of PRESS and MEGA-PRESS spectra from one patient and one control subject in each of the four regions. Abbreviations: STG=superior temporal gyrus, IFG=inferior frontal gyrus, ACC=anterior cingulate cortex. All spectra were subjected to quality control, before included in data analysis. A local algorithm assigned a quality score to each spectra, considering the following factors: spectral linewidth (FWHM), signal-to-noise ratio (SNR) and CRLB %SD of key metabolites in the spectrum, the magnitude of aberrant features in the individual metabolite spectrum relative to the group mean, and the magnitude of features in the residuals after fitting. This quality score does not impose strict rejection criteria (which in some instances could risk introducing systematic biases 60), but rather flags aspects of spectra of possible concern to guide visual inspection, after which spectra deemed to be of insufficient quality for meaningful assessment were rejected.
Acknowledgments
The authors want to thank all subjects participating in the study, and the MR technicians and research technicians who assisted in acquiring the data. The coauthors A.R.C., L.E., R.G., and K.H. own shares in the NordicNeuroLab Inc. company, which produces some of the add-on equipment used during data acquisition. All other authors declare no conflict of interest.
Funding
The present study was funded by grants from the Norwegian Research Council (RCN) grant nr. 13727 and the Western Norway Regional Health Authority (Helse-Vest Samarbeidsorganet) grants nr. 911820 and 911629 to E.J. The contribution of coauthors H.H., ARC, JJB, and K.H. was funded with a grant from ERC #249516.
References
- 1. Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011;168(1):73–81. [DOI] [PubMed] [Google Scholar]
- 2. Larøi F, Aleman A.. Hallucinations - A Guide to Treatment and Management. Oxford, UK: Oxford university press; 2010. [Google Scholar]
- 3. Jardri R, Hugdahl K, Hughes M, et al. Are hallucinations due to an imbalance between excitatory and inhibitory influences on the brain? Schizophr Bull. 2016;42(5):1124–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kompus K, Westerhausen R, Hugdahl K. The “paradoxical” engagement of the primary auditory cortex in patients with auditory verbal hallucinations: a meta-analysis of functional neuroimaging studies. Neuropsychologia. 2011;49(12):3361–3369. [DOI] [PubMed] [Google Scholar]
- 5. Kühn S, Gallinat J. Quantitative meta-analysis on state and trait aspects of auditory verbal hallucinations in schizophrenia. Schizophr Bull. 2012;38(4):779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carter CS, Robertson LC, Nordahl TE, Chaderjian M, Oshora-Celaya L. Perceptual and attentional asymmetries in schizophrenia: further evidence for a left hemisphere deficit. Psychiatry Res. 1996;62(2):111–119. [DOI] [PubMed] [Google Scholar]
- 8. Løberg EM, Hugdahl K, Green MF. Hemispheric asymmetry in schizophrenia: a “dual deficits” model. Biol Psychiatry. 1999;45(1):76–81. [DOI] [PubMed] [Google Scholar]
- 9. Hugdahl K, Nygård M, Falkenberg LE, et al. Failure of attention focus and cognitive control in schizophrenia patients with auditory verbal hallucinations: evidence from dichotic listening. Schizophr Res. 2013;147(2-3):301–309. [DOI] [PubMed] [Google Scholar]
- 10. Hugdahl K. “Hearing voices”: auditory hallucinations as failure of top-down control of bottom-up perceptual processes. Scand J Psychol. 2009;50(6):553–560. [DOI] [PubMed] [Google Scholar]
- 11. Aleman A, Böcker KB, Hijman R, de Haan EH, Kahn RS. Cognitive basis of hallucinations in schizophrenia: role of top-down information processing. Schizophr Res. 2003;64(2-3):175–185. [DOI] [PubMed] [Google Scholar]
- 12. Ćurčić-Blake B, Ford JM, Hubl D, et al. Interaction of language, auditory and memory brain networks in auditory verbal hallucinations. Prog Neurobiol. 2017;148:1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hugdahl K, Sommer IE. Auditory verbal hallucinations in schizophrenia from a levels of explanation perspective. Schizophr Bull. 2018;44(2):234–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27(43):11496–11500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adler CM, Malhotra AK, Elman I, et al. Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry. 1999;156(10):1646–1649. [DOI] [PubMed] [Google Scholar]
- 16. Krystal JH, Karper LP, Seibyl JP, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51(3):199–214. [DOI] [PubMed] [Google Scholar]
- 17. Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25(4):455–467. [DOI] [PubMed] [Google Scholar]
- 18. Malhotra AK, Pinals DA, Adler CM, et al. Ketamine-induced exacerbation of psychotic symptoms and cognitive impairment in neuroleptic-free schizophrenics. Neuropsychopharmacology. 1997;17(3):141–150. [DOI] [PubMed] [Google Scholar]
- 19. Akbarian S, Kim JJ, Potkin SG, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52(4):258–266. [DOI] [PubMed] [Google Scholar]
- 20. Guidotti A, Auta J, Davis JM, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57(11):1061–1069. [DOI] [PubMed] [Google Scholar]
- 21. Thompson M, Weickert CS, Wyatt E, Webster MJ. Decreased glutamic acid decarboxylase(67) mRNA expression in multiple brain areas of patients with schizophrenia and mood disorders. J Psychiatr Res. 2009;43(11):970–977. [DOI] [PubMed] [Google Scholar]
- 22. de Jonge JC, Vinkers CH, Hulshoff Pol HE, Marsman A. GABAergic mechanisms in schizophrenia: linking postmortem and in vivo studies. Front Psychiatry. 2017;8:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Graaf R. In vivo NMR Spectroscopy. Principles and Techniques. 2nd ed. Chichester, UK: John Wiley and Sons; 2007. [Google Scholar]
- 24. Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK. Nature of glutamate alterations in schizophrenia: a meta-analysis of proton magnetic resonance sspectroscopy studies. JAMA Psychiatry. 2016;73(7):665–674. [DOI] [PubMed] [Google Scholar]
- 25. Egerton A, Modinos G, Ferrera D, McGuire P. Neuroimaging studies of GABA in schizophrenia: a systematic review with meta-analysis. Transl Psychiatry. 2017;7(6):e1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marsman A, Mandl RC, Klomp DW, et al. GABA and glutamate in schizophrenia: a 7 T ¹H-MRS study. Neuroimage Clin. 2014;6:398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rowland LM, Krause BW, Wijtenburg SA, et al. Medial frontal GABA is lower in older schizophrenia: a MEGA-PRESS with macromolecule suppression study. Mol Psychiatry. 2016;21(2):198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hugdahl K, Craven AR, Nygård M, et al. Glutamate as a mediating transmitter for auditory hallucinations in schizophrenia: a (1)H MRS study. Schizophr Res. 2015;161(2-3):252–260. [DOI] [PubMed] [Google Scholar]
- 29. Ćurčić-Blake B, Bais L, Sibeijn-Kuiper A, et al. Glutamate in dorsolateral prefrontal cortex and auditory verbal hallucinations in patients with schizophrenia: a 1H MRS study. Prog Neuropsychopharmacol Biol Psychiatry. 2017;78:132–139. [DOI] [PubMed] [Google Scholar]
- 30. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. [DOI] [PubMed] [Google Scholar]
- 31. Nayani TH, David AS. The auditory hallucination: a phenomenological survey. Psychol Med. 1996;26(1):177–189. [DOI] [PubMed] [Google Scholar]
- 32. Waters F, Collerton D, Ffytche DH, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and eye disease. Schizophr Bull. 2014;40(Suppl 4):S233–S245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. [DOI] [PubMed] [Google Scholar]
- 34. Dydak U, Jiang YM, Long LL, et al. In vivo measurement of brain GABA concentrations by magnetic resonance spectroscopy in smelters occupationally exposed to manganese. Environ Health Perspect. 2011;119(2):219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaiser LG, Young K, Meyerhoff DJ, Mueller SG, Matson GB. A detailed analysis of localized J-difference GABA editing: theoretical and experimental study at 4 T. NMR Biomed. 2008;21(1):22–32. [DOI] [PubMed] [Google Scholar]
- 36. Gasparovic C, Song T, Devier D, et al. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med. 2006;55(6):1219–1226. [DOI] [PubMed] [Google Scholar]
- 37. Hjelmervik H, Hausmann M, Craven AR, et al. Sex- and sex hormone-related variations in energy-metabolic frontal brain asymmetries: a magnetic resonance spectroscopy study. Neuroimage. 2018;172:817–825. [DOI] [PubMed] [Google Scholar]
- 38. Schaller B, Mekle R, Xin L, Kunz N, Gruetter R. Net increase of lactate and glutamate concentration in activated human visual cortex detected with magnetic resonance spectroscopy at 7 tesla. J Neurosci Res. 2013;91(8):1076–1083. [DOI] [PubMed] [Google Scholar]
- 39. Taylor R, Schaefer B, Densmore M, et al. Increased glutamate levels observed upon functional activation in the anterior cingulate cortex using the Stroop Task and functional spectroscopy. Neuroreport. 2015;26(3):107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Plitman E, Nakajima S, de la Fuente-Sandoval C, et al. Glutamate-mediated excitotoxicity in schizophrenia: a review. Eur Neuropsychopharmacol. 2014;24(10):1591–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Beaton A. Left Side, Right Side. A Review of Laterality Research. New Haven: Yale University Press; 1985. [Google Scholar]
- 42. Specht K. Neuronal basis of speech comprehension. Hear Res. 2014;307:121–135. [DOI] [PubMed] [Google Scholar]
- 43. Sperry RW. Lateral specialization in the surgically separated hemispheres. The Neurosciences: Third Study Program 1973:5–19. [Google Scholar]
- 44. Heinrichs RW, Awad AG. Neurocognitive subtypes of chronic schizophrenia. Schizophr Res. 1993;9(1):49–58. [DOI] [PubMed] [Google Scholar]
- 45. Hugdahl K, Rund BR, Lund A, et al. Brain activation measured with fMRI during a mental arithmetic task in schizophrenia and major depression. Am J Psychiatry. 2004;161(2):286–293. [DOI] [PubMed] [Google Scholar]
- 46. Paulman RG, Devous MD Sr, Gregory RR, et al. Hypofrontality and cognitive impairment in schizophrenia: dynamic single-photon tomography and neuropsychological assessment of schizophrenic brain function. Biol Psychiatry. 1990;27(4):377–399. [DOI] [PubMed] [Google Scholar]
- 47. Rund BR, Sundet K, Asbjørnsen A, et al. Neuropsychological test profiles in schizophrenia and non-psychotic depression. Acta Psychiatr Scand. 2006;113(4):350–359. [DOI] [PubMed] [Google Scholar]
- 48. Demjaha A, Egerton A, Murray RM, et al. Antipsychotic treatment resistance in schizophrenia associated with elevated glutamate levels but normal dopamine function. Biol Psychiatry. 2014;75(5):e11–e13. [DOI] [PubMed] [Google Scholar]
- 49. Kegeles LS, Mao X, Stanford AD, et al. Elevated prefrontal cortex γ-aminobutyric acid and glutamate-glutamine levels in schizophrenia measured in vivo with proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2012;69(5):449–459. [DOI] [PubMed] [Google Scholar]
- 50. Natsubori T, Inoue H, Abe O, et al. Reduced frontal glutamate + glutamine and N-acetylaspartate levels in patients with chronic schizophrenia but not in those at clinical high risk for psychosis or with first-episode schizophrenia. Schizophr Bull. 2014;40(5):1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rowland LM, Spieker EA, Francis A, et al. White matter alterations in deficit schizophrenia. Neuropsychopharmacology. 2009;34(6):1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kraguljac NV, Reid MA, White DM, den Hollander J, Lahti AC. Regional decoupling of N-acetyl-aspartate and glutamate in schizophrenia. Neuropsychopharmacology. 2012;37(12):2635–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ohrmann P, Kugel H, Bauer J, et al. Learning potential on the WCST in schizophrenia is related to the neuronal integrity of the anterior cingulate cortex as measured by proton magnetic resonance spectroscopy. Schizophr Res. 2008;106(2-3):156–163. [DOI] [PubMed] [Google Scholar]
- 54. Wood SJ, Yücel M, Wellard RM, et al. Evidence for neuronal dysfunction in the anterior cingulate of patients with schizophrenia: a proton magnetic resonance spectroscopy study at 3 T. Schizophr Res. 2007;94(1-3):328–331. [DOI] [PubMed] [Google Scholar]
- 55. Stone JM, Dietrich C, Edden R, et al. Ketamine effects on brain GABA and glutamate levels with 1H-MRS: relationship to ketamine-induced psychopathology. Mol Psychiatry. 2012;17(7):664–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Taylor SF, Tso IF. GABA abnormalities in schizophrenia: a methodological review of in vivo studies. Schizophr Res. 2015;167(1-3):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Akbarian S, Sucher NJ, Bradley D, et al. Selective alterations in gene expression for NMDA receptor subunits in prefrontal cortex of schizophrenics. J Neurosci. 1996;16(1):19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Grimwood S, Slater P, Deakin JF, Hutson PH. NR2B-containing NMDA receptors are up-regulated in temporal cortex in schizophrenia. Neuroreport. 1999;10(3):461–465. [DOI] [PubMed] [Google Scholar]
- 59. Nudmamud S, Reynolds GP. Increased density of glutamate/N-methyl-D-aspartate receptors in superior temporal cortex in schizophrenia. Neurosci Lett. 2001;304(1-2):9–12. [DOI] [PubMed] [Google Scholar]
- 60. Kreis R. The trouble with quality filtering based on relative Cramér-Rao lower bounds. Magn Reson Med. 2016;75(1):15–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.