Abstract
Many psychiatric drugs are weak bases that accumulate in and are released from synaptic vesicles, but the functional impact of vesicular drug release is largely unknown. Here, we examine the effect of vesicular release of the anxiolytic antipsychotic drug cyamemazine on electrically evoked striatal dopamine responses with fast scan cyclic voltammetry. Remarkably, in the presence of nanomolar extracellular cyamemazine, vesicular cyamemazine release in the brain slice can increase dopamine responses 30-fold. Kinetic analysis and multiple stimulation experiments show that this occurs by inducing delayed emptying of the releasable dopamine pool. Also consistent with increased dopamine release, an antagonist (dihydro-β-erythroidine) implicates nicotinic acetylcholine receptors, which can directly cause dopamine release, in the vesicular cyamemazine effect. Therefore, vesicular release of cyamemazine can dramatically enhance dopaminergic synaptic transmission, possibly by recruiting an excitatory cholinergic input to induce an extra phase of release. More generally, this study suggests that synaptic drug release following vesicular accumulation by acidic trapping can expand psychiatric drug pharmacodynamics.
Keywords: acidic trapping, striatum, FSCV, carbon fiber electrode, neurotransmission, phenothiazine
Introduction
Many psychiatric drugs are weak amine bases, which exist in equilibrium between protonated hydrophilic and uncharged hydrophobic forms. This property facilitates their solubility throughout different tissue microenvironments, allowing them to diffuse across the blood–brain barrier to neurons to engage their receptor targets. Weak base drugs also diffuse across cell surface plasma and synaptic vesicle membranes to undergo acidic trapping.1–4 The concentration gradient predicted for antipsychotic drugs is ~2 orders of magnitude enrichment of the drug inside the synaptic vesicle relative to the extracellular concentration,3 which reflects the luminal acidic environment (pH~5.5) maintained by a proton pump and attainment of an equilibrium described by the Henderson–Hasselbalch equation. In comparison to the rapid vesicular accumulation of transmitters driven by specific active transporters, vesicular drug accumulation by acidic trapping is slow; eg, trapping of the anxiolytic antipsychotic drug cyamemazine (Cyam) in dopamine (DA) vesicles occurs over hours with nanomolar drug treatment of the striatal brain slice, where the tissue is directly accessible and baseline activity is very low due to DA neuron axons being cut, and days with in vivo treatment by injection.4 A prior study of hippocampal cultures treated with ≥5 µM haloperidol concluded that vesicular drug release contributed to inhibition of sodium channels and action potential activity.3 Although this experiment established that it is possible to see drug effects from their vesicular release, this concentration is orders of magnitude above the concentration used in patients and there is no evidence that hippocampal sodium channels are a therapeutic target for antipsychotic drugs. Thus, the medical relevance of vesicular accumulation and release of antipsychotic drugs remains in question.
Our follow-up on this issue was stimulated by the demonstration of accumulation and subsequent vesicular release of Cyam at striatal DA synapses in brain slices treated with nanomolar drug and from animals injected with the drug.4 Because Cyam, like many antipsychotic drugs inhibits D2 dopamine receptors and inhibiting D2 autoreceptors can affect DA responses in the striatum,5–7 we hypothesized that the co-release of Cyam with native transmitter could alter dopaminergic transmission; eg, vesicular antipsychotic drug release might contribute to scaling up the transient increase in DA measured electrochemically with carbon fiber electrodes. The predicted impact of vesicular drug release would be evident as a response that requires the prolonged drug exposure necessary for acidic trapping and then disappears after an intense stimulus that empties the vesicular drug pool (figure 1A). The expected reversibility of drug effects following depletion of vesicular drug pool in the continued presence of extracellular drug distinguishes vesicular drug release from delayed drug effects induced by conventional mechanisms such as gene expression, synaptic remodeling, and cell damage.
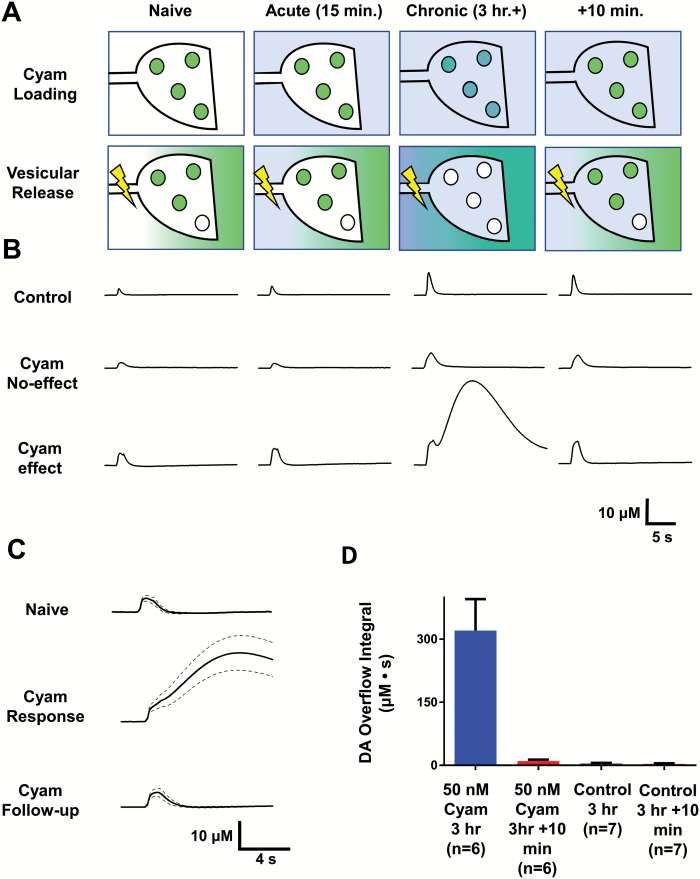
Fig. 1.
Vesicular Cyam release alters synaptic dopamine responses. (A) Model of cyamemazine (Cyam, shown in blue) distribution into cellular and extracellular compartments of dopaminergic boutons. The Naive label represents a drug-free time point; under these conditions no Cyam is loaded into vesicles, and action potentials release only DA (shown in green) from the vesicles. Under Acute conditions of drug application (15 min of superfusion), cyamemazine is present in the extracellular space. Under Chronic conditions of drug application (3+ h of superfusion), Cyam has accumulated inside the cell and been loaded into the synaptic vesicles, and action potentials release Cyam from the vesicles into the extracellular space. At +10 min after the Chronic stimulus, not enough time has passed for Cyam to be trapped in synaptic vesicles, but the ability of the boutons to release dopamine has fully returned. (B) Representative electrically evoked (10 Hz for 1 s) DA response traces under the conditions of the model shown in (A). A large and long lasting biphasic DA overflow can be observed upon vesicular release of Cyam, which then always reverses upon a follow-up stimulus at +10 min. (C) Averaged DA responses from drug naive experiments (top, n = 6), biphasic responses (middle, n = 6), and follow-up (+10 min) stimuli 10 min after biphasic responses (n = 6) with standard error of the mean (SEM) shown by dashed lines. (D) Comparison of the integrals of Cyam responses and follow-up stimulations, and for the controls. Error bars show SEM.
Here we report an effect of Cyam treatment in the nanomolar concentration range that fulfills the aforementioned criteria. However, instead of producing the expected subtle quantitative effect on phasic DA release in the brain slice,8 vesicular Cyam release induces a new delayed and prolonged phase in the DA response. Kinetic analysis and experiments show that the extra phase reflects emptying of the releasable pool, requiring excitatory cholinergic transmission to activate nicotinic acetylcholine receptors (nAChRs). Together, these results demonstrate that vesicular drug release can induce novel and dramatic drug effects.
Methods
Animal protocols and care procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Whole coronal striatal slices (300 μm thick, taken 0.3–1.2 mm caudal of the anterior most portion of the striatum. were prepared in icy aCSF9 from male Sprague-Dawley rats (14–40 d postnatal). This age range maintained consistency with a past optical study.4 Slices were superfused with 29–31oC aCSF (95% O2/5% CO2) containing 0.0005% vol/vol dimethylsulfoxide or 0.0005% vol/vol dimethylsulfoxide and 2 μM DHβE and/or 50 nM cyamemazine (Sigma-Aldrich, Tocris). Fast scan cyclic voltammetry (FSCV) was performed at carbon fiber microelectrodes10–12 (7-μm diameter, 200-μm length) with HDCV software10 on a WaveNeuro potentiostat (Pine Instruments). Waveforms with potential limits are 1.3 V and −0.4 or −0.5 V and a scan rate is 400 V/s were applied at 10 Hz. Carbon fibers were fully inserted into the slice at a 20° angle with the tip positioned ∼100 μm below the slice surface. DA release was evoked through optically isolated electrical stimulation (800 μA, biphasic, 2 ms per phase) performed with a stimulus isolator (A365, WPI) under the control of the potentiostat, and pulse generators (A310, WPI; AM-2100, AM Systems). Current was passed in 1 second, 10 Hz trains ~2 mm between electrode poles (MS303-1-A-SPLELECTSS, Plastics One)13 in contact with the brain surface. When multiple sites were measured, the simulating electrode was moved so that the area bracketed by the stim electrode poles did not overlap and the carbon fiber electrode was implanted equidistant between the stimulating electrode poles. DA responses were observed throughout the striatum, but the nucleus accumbens was not studied. Background subtracted DA peak current time series were converted to concentrations.14 Statistical analysis was performed with the GraphPad Prism program. The commonly observed primary DA phase was fit with a previously developed mathematical model.9,11,12,15,16
The RPOOL Model
The model includes the following parameters: RPOOL = some finite pool of dopamine vesicles that can be released in the context of evoked release upon the vesicular release of Cyam; m = a slope or ramp parameter that defines the rate of increase of release of the RPOOL; t = time; and kU = a first-order rate parameter to represent uptake of dopamine subsequent to release. The newly described DA release phase was fit with a new model based on drug-release induced depletion of the releasable DA pool (RPOOL). The RPOOL model is defined by a system of coupled differential equations:
We used the same type of direct search algorithm (Excel VBA) developed for the primary phase DA kinetic model15 to fit the data. Formulation of the RPOOL model is described in the supplementary material.
Results
As the kinetics of vesicular loading and release in striatal brain slices are established for Cyam,4 these data were used to design further brain slice experiments to investigate the impact of vesicular Cyam release on striatal dopaminergic transmission. Experiments began with superfusion with vehicle or 50 nM Cyam, a concentration below that obtained in human patient plasma, which positron emission tomography indicates produces ~75% occupancy of striatal D2 receptors.17 Then DA responses evoked by tetanic stimulation (10 Hz for 1 s) were measured with FSCV. Because we found that Cyam itself is not electroactive (eg, undetectable by the DA detection waveform at 50 μM), the presence of the drug did not interfere with DA measurements. Acute (ie, 15 min) applications of 50 nM Cyam had little impact on the monophasic DA response (figure 1B), showing that extracellular Cyam does not have a major acute effect on DA release or reuptake by the DA transporter (DAT), as is expected in the brain slice.8
After 3 hours under control conditions, DA responses in brain slices remained monophasic (figure 1B, Control). However, after 3 hours of Cyam exposure to induce vesicular trapping comparable to that seen with in vivo dosing,4 DA responses either remained monophasic or became biphasic with a dramatically delayed and prolonged extra phase (figure 1B and 1C), with cyclic voltammograms showing that the extra phase reflected increased DA (supplementary figure S1). Response variability could not be attributed to slice health as presence or absence of the delayed phase could differ between two sequentially measured, non-overlapping sites within the same slice and the monophasic DA response to a tetanus was invariably present 10 minutes after the stimulus that caused Cyam response. In initial experiments, the extra phase was detected at dorsal and ventral striatal recording sites indicating that this response was not exclusive to any one region.
To assay the effect of treatment for ≥3 hours, we assessed whether 2 non-overlapping sites (with at least 2-mm separation) could be tested sequentially per slice without encountering a biphasic response. In 7 vehicle-treated slices, all 14 sites gave monophasic responses (ie, no biphasic responses were seen). However, only 2 of 8 slices with Cyam vesicular loading had 2 test sites display only the brief monophasic responses (ie, a biphasic response was encountered in 6 of 8 slices). Therefore, the prolonged biphasic response was associated with vesicular loading with Cyam (P = .0007, chi-squared test). Consistent with an effect mediated by vesicular drug release, the extra phase induced by prolonged Cyam always reversed in follow-up tetanic stimulations delivered 10 minutes later despite the continued presence of extracellular drug (n = 6) (figure 1B and 1C). Integration of the biphasic responses and their reversals demonstrate a ~30-fold increase in the DA response is evoked immediately after vesicular Cyam release (figure 1D, 321 ± 75 vs 10.3 ± 3.2), which is in contrast to controls that displayed only a 20% change (4.5 ± 1.3 vs 3.6 ± 1.1).
As described in figure 1A, the reversal of the Cyam effect is indicative of emptying of the vesicular Cyam pool. Given the co-release of Cyam and DA,4 this suggests that the extra phase in the DA response reflects greater transmitter release rather than an inhibition of DAT, which can also prolong DA responses (figure 2A, blue dots showing the effect of the DAT inhibitor GBR12909 on evoked release). This conclusion is supported by 3 additional lines of evidence.
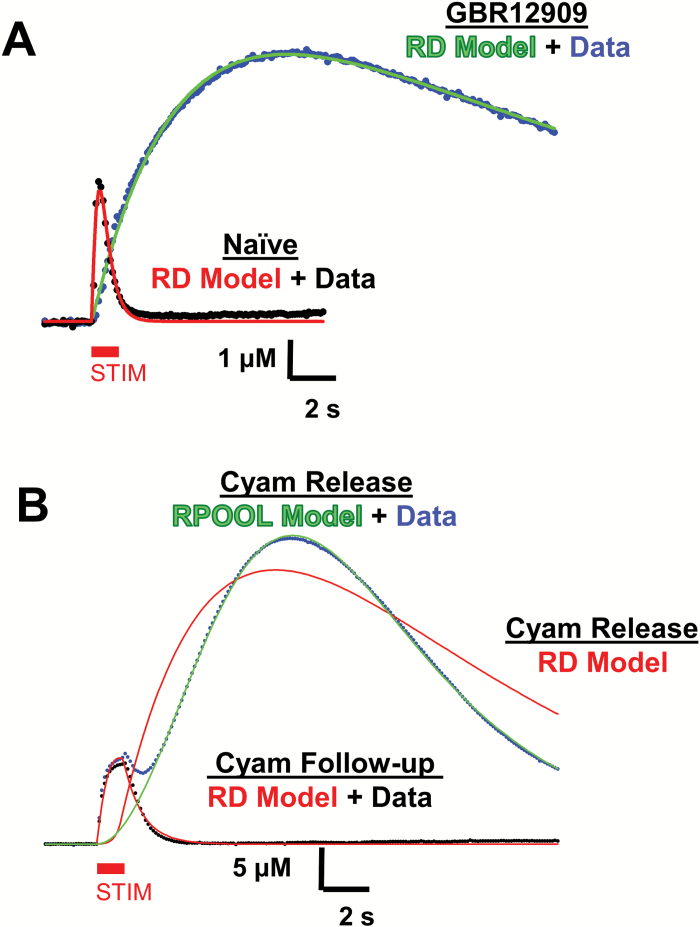
Fig. 2.
Kinetic analysis of biphasic Cyam responses. (A) Representative examples of RD model fits (naïve = red, GBR12909 = green) to an electrically evoked drug naive DA response (black dots) and a response with the DAT inhibited by 10 μM GBR12909 (blue dots). The electrical stimulus, denoted by the red bar marked STIM, is 10 pulses at 10 Hz. (B) Failure of the RD model (red line), and success of the RPOOL model (green line), to fit the second phase of a representative Cyam-induced DA response (blue dots). Success of the RD model (red line) to fit the representative follow-up DA response 10 min later (black dots) is also shown. The electrical stimulus, denoted by the red bar marked STIM, is 10 pulses at 10 Hz.
First, DA response kinetics are not compatible with a DAT effect, but can be explained by the induction of delayed release. Previously, DA responses have been described by a 2 compartment “RD” model in which DA released into an inner compartment, perhaps corresponding to the synaptic cleft, slowly escapes to either be taken up by DAT or to diffuse in the outer compartment where it can mediate volume transmission and encounter the carbon fiber electrode.15 This model fits many electrically evoked DA responses under the influence of many drugs both in brain slices and in vivo.9–11,15,18 Therefore, it is not surprising that the RD model fits DA responses under control conditions, in the presence of a DAT inhibitor and even following reversal of the Cyam effect (figure 2A and 2B, red lines). However, the vesicular Cyam-induced extra phase cannot be fit by this model (figure 2B, blue data and red line) suggesting that neither an increase in stimulus time-locked release nor a decrease in DAT activity is sufficient to explain the data. However, the extra phase time course is fit by the RPOOL model (see “Methods” section), which posits that DA release increases following cessation of stimulation until there is exhaustion of the releasable pool (figure 2B, blue data and green line). Thus, the DA response time course suggests that enhanced DA release mediates the extra phase.
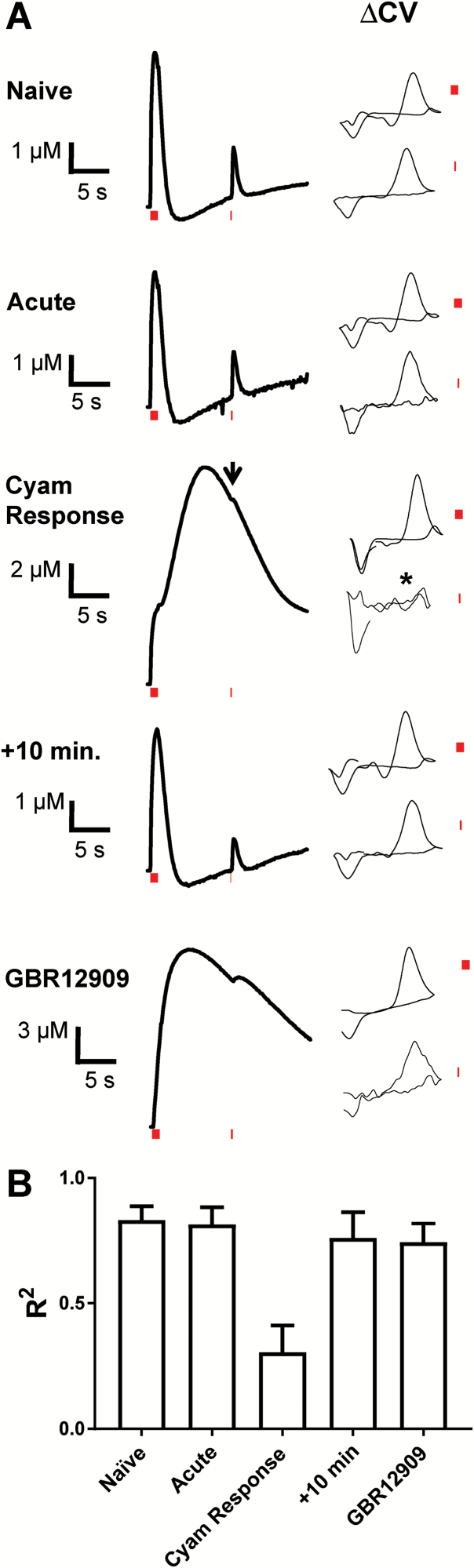
Second, the hypothesis that the Cyam-induced extra phase is caused by DA release, thereby depleting the releasable DA pool, was tested by examining responses to an added single stimulation pulse delivered 10 seconds after the 10 Hz tetanus. Responses to these test pulses were readily observed in drug naïve experiments, after acute drug application, after stimulation-induced reversal of the Cyam effect, and in the presence of the DAT inhibitor GBR12909 (figure 3A, averaged traces shown; supplementary figure S2). As expected, responses were slowed and attenuated with the DAT inhibitor reflecting reduced uptake and D2 autoreceptor activation. However, in contrast to all of the other discernible responses, there was essentially no signal evoked by the test pulse immediately following the Cyam effect (figure 3A, left; supplementary figure S2). Similarly, cyclic voltammograms in which the test pulse response was isolated by subtracting the background signal before the test pulse (figure 3A, ΔCV) showed that DA responses were evoked by the test pulses in drug naïve experiments, after acute drug application, after stimulation-induced reversal of the Cyam effect, and in the presence of the DAT inhibitor GBR12909. However, during the falling phase of the Cyam-induced prolonged response when the RPOOL model indicates that DA release is nearly complete and residual DA is clearing from the extracellular space, the test pulse did not produce a detectable DA response in the ΔCV traces (n = 3) (figure 3A, Cyam Response, arrow indicates averaged test pulse response in the extra phase and * indicates a corresponding averaged background subtracted ΔCV response) even though Cyam would antagonize D2 receptor-mediated autoinhibition. To further compare ΔCV responses, R2 correlation was calculated between the average of the 10-pulse and individual single test pulse responses for each experimental condition and compared between the data sets. This analysis showed that the single test pulse response in Cyam Response experiments was uniquely different than naïve controls (figure 3B). Therefore, the test pulse did not elicit further DA release following Cyam release. This again implies that the extra phase induced by vesicular Cyam release cannot be attributed to reduced uptake and instead is caused by enhanced release.
Fig. 3.
The releasable DA pool is depleted by vesicular Cyam release. (A) Left, averaged dopamine response traces using a single test pulse 10 s after the initial (10 pulse, 10 Hz) tetanic stimulus to test for inhibition of release. The periods of stimulation are denoted by red bars. SEMs are omitted for clarity. Naive (n = 5, 5 slices), Acute Cyam (n = 5, 5 slices), Cyam response (n = 3, 3 slices), +10 min follow-up to Cyam-induced extra phase (n = 3, 3 slices), GBR12909 (n = 6, 3 slices). Right, averaged background subtracted cyclic voltammograms (ΔCVs) (arbitrary scale) are supplied for the DA peaks resulting from the 10 pulse and 1 pulse stimulations. The background is in each case taken from the time point immediately preceding the stimulus. In Cyam Response arrow indicates averaged test pulse response in the extra phase and * indicates a corresponding averaged background subtracted ΔCV response. (B) R2 comparisons of background-subtracted voltammogram shapes for the single pulse administered 10 s after the 10 Hz stimulus. Note that that dopamine is uniquely not detected by the single pulse after the induction of Cyam vesicular release.
Third, support for enhanced release came from implicating a known excitatory synaptic mechanism in the extra phase of DA responses. Specifically, we focused on nAChRs because their activation can enhance electrically evoked phasic release, optogenetic stimulation of the cholinergic interneurons is sufficient to evoke DA release in the striatum, and injection of nAChR agonist nicotine in vivo causes striatal DA release.19–23 Therefore, involvement of nAChRs was tested by applying the competitive nicotinic antagonist DHβE (dihydro-β-erythroidine at 2 µM) to the slice for the last 30 minutes of the Cyam loading period. Acidic trapping of DHβE (pKa 7.3) has not been detected,24 thereby excluding acidic trapping of the nicotinic antagonist as a confounding factor. Importantly, the extra DA phase was not seen in any of the 7 slices (with 2 sites per slice) treated with DHβE and Cyam (figure 4). Furthermore, the magnitude of responses in DHβE was statistically indistinguishable from naïve preparations, but different from Cyam responses (P < .0001, Tukey’s post-test following ANOVA). The simplest interpretation of these data is that the extra DA phase induced by Cyam released at synapses (potentially containing DA and/or other neurotransmitters such as ACh, GABA, and glutamate), requires nAChRs. Because nAChRs are known to enable action-potential independent DA release and are necessary for the extra phase, this result provides a fourth piece of evidence supporting the conclusion that the extra phase of the DA response is caused by enhanced DA release, not reduced uptake.
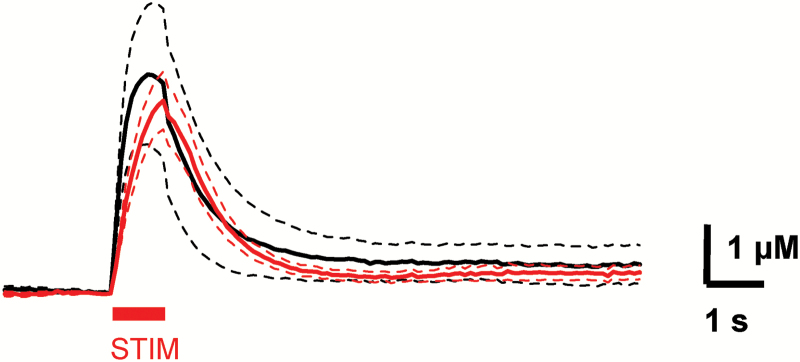
Fig. 4.
Nicotinic cholinergic receptors are needed for the vesicular Cyam effect.
Dopamine response traces for Naive (black, n = 7) and after 3+ h of Cyam with 30 min of co-incubation with 2 μM DHβE at 2 recording sites per slice (red, n = 14, 7 slices). The dashed lines are the SEM. Note the absence of the Cyam-induced extra phase in the DA response. The electrical stimulus, denoted by the red bar marked STIM, is 10 pulses at 10 Hz.
Discussion
Here vesicular Cyam release was shown to induce a dramatic increase in synaptic DA release by a mechanism that is not engaged by the steady state nanomolar extracellular drug concentration. This effect is interesting because it is large and could involve recruitment of an excitatory activation of nAChRs, which is required for the extra phase of DA release. The involvement of nicotinic excitation may also contribute to the heterogeneity in Cyam’s action. We suggest that the co-release of Cyam with DA preserves some D2 receptor inhibition, but promotes D1 receptor activation. This may contribute to therapeutic drug action or side effects. Most importantly, this study suggests that acidic trapping, which depends on drug pKa and membrane permeability,25 and effects of synaptic vesicular drug release should be considered in the development of psychiatric drugs and their administration protocols, as well as for fully understanding their mechanisms of action.
These findings raise the question of the target of Cyam that is responsible for the extra phase of DA release. Because the local concentration of Cyam transiently increases upon vesicular release, low affinity Cyam targets might be involved. One possibility is that in the context of ongoing high affinity inhibition of D2 and serotonin (5-HT) receptors by Cyam,7 transient Cyam antagonism of inhibitory M2 and M4 muscarinic autoreceptors (Ki values at 22oC of 42 nM and 12 nM, respectively, vs 5.8 nM for D27) might boost signaling from striatal cholinergic interneurons and thus promote nAChR-mediated DA release. Likewise, Cyam could act on unknown and uncharacterized targets (eg by blocking presynaptic K+ channels) to increase release of acetylcholine and other neurotransmitters. Indeed, at the locally high concentrations achieved by vesicular drug release due to emptying of the vesicular drug pool, relatively nonspecific binding of Cyam to multiple receptors could produce effects beyond those seen with cholinergic neuron activation alone to produce the extra phase of DA release for which nAChRs are necessary, but perhaps not sufficient. Such low affinity targets are not ordinarily considered to be important to drug action because they are outside the therapeutic window at steady-state, but it is possible to substantially occupy them on a transient basis following vesicular drug release at the synapse. Therefore, extensive follow-up research will be required to identify the potentially numerous Cyam targets engaged under conditions that evoke synaptic drug release.
The Cyam response is also intriguing because of the observed massive release of DA. Often, release is viewed as being supported by separate readily releasable and reserve pools of vesicles, which in DA neurons can be separated based on reliance on DA synthesis, synapsin, and sensitivity to drugs.26,27 The observed loss of single spike responses (figure 3) is consistent with an emptying of the readily releasable pool, but the data thus far do not provide insight into the reserve pool. Furthermore, in the striatum there is an extra level of complexity because not all DA vesicle clusters and varicosities are responsive to depolarization or electrical stimulation.28,29 The impact of these silent sites and the conventional reserve pool on nAChR-evoked DA release and the Cyam effect remains to be determined. However, these known aspects of the DA system provide reasonable context for both the new finding presented here and for our previous finding that Cyam continues to build up inside vesicles for days in vivo4; even though there is activity in vivo to empty some vesicles, conventional reserve pool vesicles and vesicles at silent sites may participate in the Cyam-induced nAChR dependent extra phase of DA release. The contents of such vesicles could be released via conventional exocytosis, which is favored by the implication of nAChRs, or an amphetamine-like effect. We anticipate that exploring this issue will provide insights into cholinergic stimulation of DA release and the remarkable extra phase of DA release induced when a psychiatric drug coopts synaptic release underlying neurotransmission.
Funding
This research was supported by NARSAD Distinguished Investigator Award 24295 and National Institutes of Health grants R21MH110153 and R21NS106823.
Supplementary Material
Acknowledgments
We thank Adrian Michael (University of Pittsburgh) for access to materials and equipment for manufacturing carbon fiber electrodes. The Authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Schmalzing G. The role of a transmembrane pH gradient in uptake and release of imipramine and haloperidol in synaptosomes. Mol Pharmacol. 1988;34(6):888–895. [PubMed] [Google Scholar]
- 2. Rayport S, Sulzer D. Visualization of antipsychotic drug binding to living mesolimbic neurons reveals D2 receptor, acidotropic, and lipophilic components. J Neurochem. 1995;65(2):691–703. [DOI] [PubMed] [Google Scholar]
- 3. Tischbirek CH, Wenzel EM, Zheng F, et al. Use-dependent inhibition of synaptic transmission by the secretion of intravesicularly accumulated antipsychotic drugs. Neuron. 2012;74(5):830–844. [DOI] [PubMed] [Google Scholar]
- 4. Tucker KR, Block ER, Levitan ES. Action potentials and amphetamine release antipsychotic drug from dopamine neuron synaptic VMAT vesicles. Proc Natl Acad Sci U S A. 2015;112(32):E4485–E4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Benoit-Marand M, Borrelli E, Gonon F. Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J Neurosci. 2001;21(23):9134–9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmitz Y, Schmauss C, Sulzer D. Altered dopamine release and uptake kinetics in mice lacking D2 receptors. J Neurosci. 2002;22(18):8002–8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hameg A, Bayle F, Nuss P, Dupuis P, Garay RP, Dib M. Affinity of cyamemazine, an anxiolytic antipsychotic drug, for human recombinant dopamine vs. serotonin receptor subtypes. Biochem Pharmacol. 2003;65(3):435–440. [DOI] [PubMed] [Google Scholar]
- 8. Kennedy RT, Jones SR, Wightman RM. Dynamic observation of dopamine autoreceptor effects in rat striatal slices. J Neurochem. 1992;59(2):449–455. [DOI] [PubMed] [Google Scholar]
- 9. Hoffman AF, Spivak CE, Lupica CR. Enhanced dopamine release by dopamine transport inhibitors described by a restricted diffusion model and fast-scan cyclic voltammetry. ACS Chem Neurosci. 2016;7(6):700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bucher ES, Brooks K, Verber MD, et al. Flexible software platform for fast-scan cyclic voltammetry data acquisition and analysis. Anal Chem. 2013;85(21):10344–10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Walters SH, Robbins EM, Michael AC. Modeling the kinetic diversity of dopamine in the dorsal striatum. ACS Chem Neurosci. 2015;6(8):1468–1475. [DOI] [PubMed] [Google Scholar]
- 12. Walters SH, Robbins EM, Michael AC. Kinetic diversity of striatal dopamine: evidence from a novel protocol for voltammetry. ACS Chem Neurosci. 2016;7(5):662–667. [DOI] [PubMed] [Google Scholar]
- 13. Wu Y, Pearl SM, Zigmond MJ, Michael AC. Inhibitory glutamatergic regulation of evoked dopamine release in striatum. Neuroscience. 2000;96(1):65–72. [DOI] [PubMed] [Google Scholar]
- 14. Roberts JG, Toups JV, Eyualem E, McCarty GS, Sombers LA. In situ electrode calibration strategy for voltammetric measurements in vivo. Anal Chem. 2013;85(23):11568–11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Walters SH, Taylor IM, Shu Z, Michael AC. A novel restricted diffusion model of evoked dopamine. ACS Chem Neurosci. 2014;5(9):776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Taylor IM, Nesbitt KM, Walters SH, et al. Kinetic diversity of dopamine transmission in the dorsal striatum. J Neurochem. 2015;133(4):522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hodé Y, Reimold M, Demazières A, et al. A positron emission tomography (PET) study of cerebral dopamine D2 and serotonine 5-HT2A receptor occupancy in patients treated with cyamemazine (Tercian). Psychopharmacology (Berl). 2005;180(2):377–384. [DOI] [PubMed] [Google Scholar]
- 18. Trevathan JK, Yousefi A, Park HO, et al. Computational modeling of neurotransmitter release evoked by electrical stimulation: nonlinear approaches to predicting stimulation-evoked dopamine release. ACS Chem Neurosci. 2017;8(2):394–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7(6):583–584. [DOI] [PubMed] [Google Scholar]
- 20. Zhang H, Sulzer D. Frequency-dependent modulation of dopamine release by nicotine. Nat Neurosci. 2004;7(6):581–582. [DOI] [PubMed] [Google Scholar]
- 21. Cheer JF, Wassum KM, Sombers LA, et al. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27(4):791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Threlfell S, Lalic T, Platt NJ, Jennings KA, Deisseroth K, Cragg SJ. Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron. 2012;75(1):58–64. [DOI] [PubMed] [Google Scholar]
- 23. Wang L, Shang S, Kang X, et al. Modulation of dopamine release in the striatum by physiologically relevant levels of nicotine. Nat Commun. 2014;5:3925. [DOI] [PubMed] [Google Scholar]
- 24. Govind AP, Vallejo YF, Stolz JR, Yan JZ, Swanson GT, Green WN. Selective and regulated trapping of nicotinic receptor weak base ligands and relevance to smoking cessation. eLife 2017;6:e25651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trapp S, Rosania GR, Horobin RW, Kornhuber J. Quantitative modeling of selective lysosomal targeting for drug design. Eur Biophys J. 2008;37(8):1317–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ewing AG, Bigelow JC, Wightman RM. Direct in vivo monitoring of dopamine released from two striatal compartments in the rat. Science. 1983;221(4606):169–171. [DOI] [PubMed] [Google Scholar]
- 27. Venton BJ, Seipel AT, Phillips PE, et al. Cocaine increases dopamine release by mobilization of a synapsin-dependent reserve pool. J Neurosci. 2006;26(12):3206–3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pereira DB, Schmitz Y, Mészáros J, et al. Fluorescent false neurotransmitter reveals functionally silent dopamine vesicle clusters in the striatum. Nat Neurosci. 2016;19(4):578–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu C, Kershberg L, Wang J, Schneeberger S, Kaeser PS. Dopamine secretion is mediated by sparse active zone-like release sites. Cell. 2018;172(4):706–718.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.