Abstract
Background
Anesthesia and surgery may deteriorate liver function in patients with elevated liver enzyme levels; therefore, in these patients, choosing anesthetics with less hepatotoxicity is important.
Methods
This retrospective study investigated the effect of total intravenous anesthesia (TIVA) versus inhalation anesthesia (INHA) on the postoperative liver function in patients with preoperatively elevated liver enzyme levels (aspartate transaminase [AST] or alanine transaminase [ALT] >40 U/L) who underwent non-hepatic surgery under general anesthesia. We compared the changes in enzyme levels within 24 hrs before and after surgery.
Results
In 730 patients (TIVA: n=138; INHA: n=592), the baseline characteristics were comparable, except for higher comorbidity rates in the TIVA group. The median anesthesia and operation times were significantly longer in the TIVA group because approximately 50% of the TIVA group (vs 19.7% of the INHA group) underwent neurosurgery, which had a relatively longer operation time than other surgeries. Intraoperative hypotensive events and vasopressor use were more frequent in the TIVA group. After 1:4 propensity score matching (TIVA: n=94; INHA: n=376), the baseline characteristics and surgical variables were comparable, except for longer anesthesia time. Before matching, postoperative AST and ALT changes were significantly lower in the TIVA group than in the INHA group. After matching, only the ALT change was significantly lower after TIVA than after INHA [median (interquartile range), −16.7 (−32 to −4) % vs −12.0 (−28.6–6.5) %, P=0.025].
Conclusion
TIVA may be safer for patients with preoperatively elevated liver transaminase levels.
Keywords: alanine transaminase, aspartate transaminase, intravenous anesthetics, inhalation anesthetics, chemical and drug-induced liver injury
Introduction
Anesthesia and surgery may deteriorate liver function in patients with elevated liver enzyme levels; therefore, in these patients, choosing anesthetics with less hepatotoxicity may be important. Halothane and other halogenated inhalational anesthetics are the most commonly used anesthetic agents, and these are known to cause hepatotoxicity because of their metabolites or immunogenic factors;1–3 although the incidence of hepatotoxicity is not high.4 The latest anesthetic agents, including sevoflurane and desflurane, are associated with less hepatotoxicity, although rare cases of acute liver injury have been reported with these agents.5–7 In contrast, the possibility of liver damage from anesthetic metabolites is expected to be lower for total intravenous anesthesia (TIVA) with propofol; moreover, hemodynamic stability is noted for TIVA with propofol.8 In addition, propofol is an excellent anesthetic agent for patients with liver disease because of its short half-life even in patients with decompensated cirrhosis.9 Therefore, TIVA with propofol is likely to be safer in patients with preoperatively elevated liver transaminase levels, which might suggest liver damage. However, only a few studies have investigated the effects of TIVA compared to those of inhalation anesthesia (INHA) on the postoperative liver function in these patients.
Therefore, the present study was performed to compare the effect of TIVA and INHA on the postoperative liver function in patients with preoperatively elevated liver transaminase levels who underwent surgery under general anesthesia. We hypothesized that TIVA would induce less hepatotoxicity in terms of the liver function test (LFT) values compared to INHA after surgery under general anesthesia. In this study, we compared the changes in the aspartate transaminase (AST) or alanine transaminase (ALT) levels measured within 24 hrs before and after surgery, as well as other postoperative outcomes, such as peak AST and ALT levels within 3 days after surgery, 30-day mortality, and length of postoperative hospital stay, between the two groups.
Materials and Methods
Data Sources and Study Population
This single-center retrospective cohort study was performed at the Korea University Guro Hospital in Seoul, Republic of Korea. The study was approved by the Korea University Guro Hospital Institutional Review Board (2017GR0105) and was registered at UMIN-CTR Clinical Trial (UMIN000038949). Considering the retrospective design of this study performed by reviewing existed chart without direct patient contact and without any study-related measures that directly affected the patient, the need for obtaining informed consent was waived by the institutional review board. The patient data were anonymized and maintained with confidentiality, and this study was conducted in compliance with the Declaration of Helsinki.
We reviewed the electronic charts of all patients who underwent surgery under general anesthesia at the Korea University Guro Hospital between December 2016 and November 2017. All cases involving patients aged more than 18 years with preoperatively elevated liver enzyme levels (AST > 40 U/L or ALT > 40 U/L) within 24 hrs before non-hepatic surgeries were selected. In hepatic surgery, the surgery itself could directly affect the liver function; therefore, cases involving hepatic surgeries that offered limited scope to analyze the effects of anesthetics were excluded. Cases wherein surgeries were performed using anesthetic methods that were not clearly identified as TIVA or INHA, such as heart surgery or cesarean section, were also excluded. Cases of neuromuscular diseases were also excluded because ALT and AST could leak out of the damaged muscles and possibly confound the result. The included patients were allocated to the TIVA or INHA group according to the type of anesthetic used for the maintenance of anesthesia.
Study Outcomes
The data on the baseline characteristics were collected, including age, sex, body mass index (BMI; kg/m2), American Society of Anesthesiologists (ASA) physical status, comorbidities (hypertension, diabetes mellitus, ischemic heart disease [from stable angina to myocardial infarction], pulmonary disease, cerebrovascular disease, and cancer), type of surgery, operation time, anesthesia time, and preoperative use of any hepatoprotective agents. Fluid balance during surgery, volume of red blood cell transfusion, incidence of intraoperative hypotensive events (decrease of greater than 30% from the baseline mean arterial pressure), and use of vasopressors were assessed as intraoperative variables. Fluid balance during surgery was calculated according to the equation used in previous studies,10,11 and the period of fluid administration by the duration of anesthesia was set as follows:
Fluid balance during surgery (%) = (fluid input ‒ output in liters) × 100%/hospital admission weight (kg)/duration of anesthesia (hour).
We compared the changes (% increase or decrease) in the liver enzyme levels (ALT and AST) measured within 24 hrs before and after surgery. The liver enzyme level measured at the time closest to the start of the surgery within 24 hrs before surgery was considered the preoperative value, and the level measured at the time closest to the end of the surgery within 24 hrs after surgery was considered the postoperative value. Moreover, the follow-up enzyme level was measured within 3 days after surgery and was used to calculate the peak AST and ALT levels within 3 days after surgery. The follow-up period was set to 3 days according to the method for causality assessment of adverse drug reactions score which values higher score for the time onset of reaction within 3 days.12 As a subset analysis, the patients with postoperative ALT level increase of more than 200 U/L being 5 times the upper limit of the normal range (40 U/L) that corresponded to the cutoff level of ALT elevation with clinically significant drug-induced liver injury were inspected.12,13 The primary outcome was the change in the ALT level within 24 hrs before and after surgery, and the secondary outcomes were the change in the AST level within 24 hrs before and after surgery, peak AST and ALT levels within 3 days after surgery. Additional postoperative outcomes, including 30-day mortality and the length of postoperative hospital stay, which was calculated from the day of the surgery to the day of discharge, were compared between the two groups as secondary outcomes.
Statistical Analysis
For comparisons, the Mann–Whitney U-test was used for continuous variables, including age, BMI, anesthesia and operation times, volume of red blood cell transfusion, estimated blood loss, fluid balance, length of postoperative hospital stay, and AST and ALT levels, whereas the chi-square test or Fisher’s exact test was used for categorical variables, including sex, ASA physical status, comorbidities, suspected cause of elevated LFT values, use of hepatoprotective agents, type of surgery, red blood cell transfusion rate, hypotensive events, use of intraoperative vasopressors, 30-day mortality. Data are presented as median with interquartile ranges for continuous variables and as numbers and percentages for categorical variables.
Propensity scores were derived using separate logistic regression models including confounding factors, such as sex, age, BMI, ASA physical status, preoperative hepatoprotective medications, and type of surgery. Propensity scores were matched to obtain matched patient pairs for reducing selection bias and the effect of confounding factors. A 1:4 matching with a 0.1 caliper by using the nearest neighbor method was applied to avoid significant data loss and to increase analytical precision because unmatched data would be discarded.
Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA), and the R program (version 3.5.2; R Development Core Team, Vienna, Austria; www.r-project.org) was used as a propensity score matching tool. P values were two-sided, and a P value of less than 0.05 was considered statistically significant.
Results
Baseline Characteristics
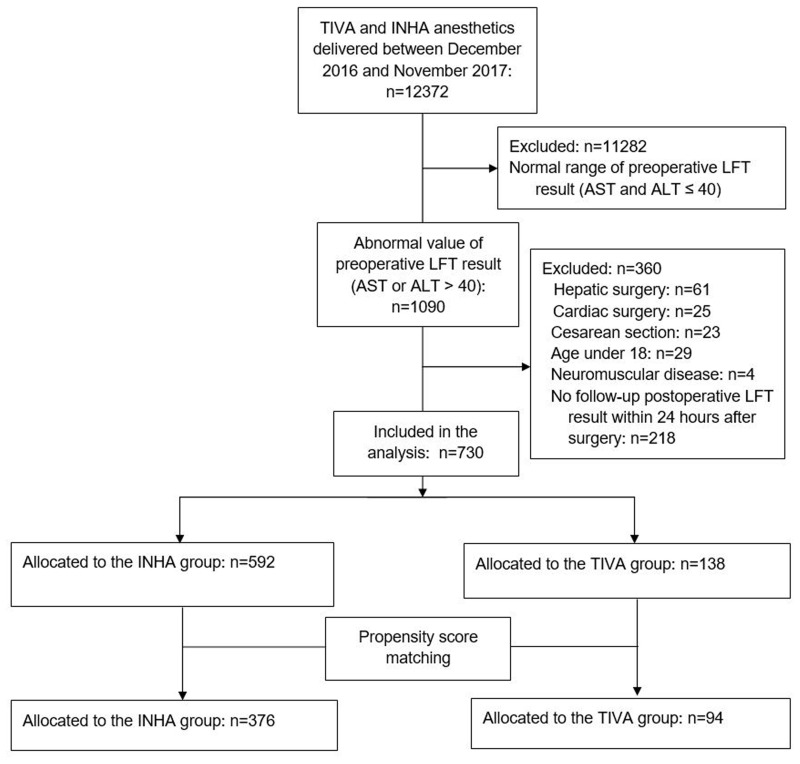
A total of 12,372 patients was operated under general anesthesia between December 2016 and November 2017. Among these, 11,282 patients with preoperative LFT values within the normal range were excluded. Thereafter, we excluded 29 patients aged <18 years, 109 patients who were undergoing hepatic surgery (n = 61), cardiac surgery (n = 25), and cesarean section (n = 23), 4 patients with neuromuscular disease, and 218 patients whose LFT values were not measured within 24 hrs after surgery. Finally, 730 patients were analyzed in this study. The flowchart illustrating the selection of the study population is shown in Figure 1.
Figure 1.
Flowchart for study population selection.
Abbreviations: TIVA, total intravenous anesthesia; INHA, inhalation anesthesia; LFT, liver function test; AST, aspartate transaminase; ALT, alanine transaminase.
Of these 730 patients, 138 (18.9%) were allocated to the TIVA group and 592 (81.1%) to the INHA group (434 in desflurane and 158 in sevoflurane). Demographic data, including age, BMI, sex, and suspected cause of elevated liver enzyme levels, were comparable between the TIVA and INHA groups; however, the proportion of patients with ASA physical status ≥III was higher in the TIVA group. Preoperative comorbidities were also comparable between the two groups, except for the greater incidence of cerebrovascular disease in the TIVA group (Table 1).
Table 1.
Baseline Characteristics of Study Population
| Variables | Before Propensity Score Matching | After Propensity Score Matching | ||||
|---|---|---|---|---|---|---|
| INHA (n=592) | TIVA (n=138) | P value | INHA (n=376) | TIVA (n=94) | P value | |
| Age (years) | 57 (44–68) | 59.5 (47–69) | 0.342 | 57 (44–68) | 58 (43–69) | 0.930 |
| BMI (kg/m2) | 25.2 (22.5–27.7) | 24.8 (22.1–27.5) | 0.270 | 25 (23–28) | 25 (22–28) | 0.757 |
| Sex, male | 395 (66.7%) | 95 (68.8%) | 0.688 | 276 (73.4%) | 68 (72.3%) | 0.856 |
| ASA physical status | 0.044 | 0.342 | ||||
| I | 72 (12.2%) | 11 (8.0%) | 45 (12.0%) | 7 (12.0%) | ||
| II | 368 (62.2%) | 78 (56.5%) | 214 (56.9%) | 60 (63.8%) | ||
| ≥III | 152 (25.7%) | 49 (35.5%) | 117 (31.1%) | 27 (28.7%) | ||
| Comorbidities | ||||||
| Hypertension | 169 (28.5%) | 49 (35.5%) | 0.121 | 102 (27.1%) | 29 (30.9%) | 0.520 |
| Diabetes mellitus | 78 (13.2%) | 18 (13.0%) | 1.000 | 47 (12.5%) | 12 (12.8%) | 1.000 |
| Ischemic heart | 47 (7.9%) | 15 (10.9%) | 0.308 | 28 (7.4%) | 7 (7.4%) | 1.000 |
| Cerebrovascular | 42 (7.1%) | 23 (16.7%) | 0.001 | 29 (7.7%) | 10 (10.6%) | 0.402 |
| Kidney | 19 (3.2%) | 1 (0.7%) | 0.147 | 17 (4.5%) | 1 (1.1%) | 0.143 |
| Pulmonary | 30 (5.1%) | 9 (6.5%) | 0.528 | 19 (5.1%) | 7 (7.4%) | 0.447 |
| Cancer | 31 (5.2%) | 5 (3.6%) | 0.519 | 17 (4.5%) | 3 (3.2%) | 0.777 |
| Suspected cause of elevated LFT | 0.070 | 0.147 | ||||
| Viral hepatitis (B or C) | 42 (7.1%) | 4 (2.9%) | 26 (7.0%) | 3 (3.2%) | ||
| Alcoholic liver | 12 (2.0%) | 4 (2.9%) | 6 (1.6%) | 1 (1.1%) | ||
| NAFLD | 62 (10.9%) | 15 (10.9%) | 33 (8.9%) | 10 (10.6%) | ||
| Biliary disorder | 105 (17.9%) | 20 (14.5%) | 50 (13.4%) | 20 (21.3%) | ||
| Drug-induced injury | 9 (1.5%) | 6 (4.3%) | 7 (1.9%) | 34 (4.3%) | ||
| Ischemic injury | 3 (0.5%) | 0 (0%) | 2 (0.5%) | 0 (0%) | ||
| Liver trauma | 6 (1.09%) | 3 (2.2%) | 4 (1.1%) | 3 (3.2%) | ||
| Hepatic masses | 7 (1.2%) | 1 (0.7%) | 1 (0.3%) | 1 (1.1%) | ||
| Tissue injury | 24 (4.1%) | 7 (5.1%) | 19 (5.1%) | 7 (7.4%) | ||
| Liver cirrhosis | 5 (0.9%) | 5 (3.6%) | 4 (1.1%) | 2 (2.1%) | ||
| Non-specific or Un-evaluated | 313 (53.2%) | 73 (52.9%) | 220 (59.1%) | 43 (45.7%) | ||
| Hepatoprotective agent | 114 (19.5%) | 37 (27.2%) | 0.061 | 106 (28.2%) | 28 (29.8%) | 0.799 |
Notes: Values are median (interquartile range) or number of patients (%).
Abbreviations: INHA, inhalation anesthesia group; TIVA, total intravenous group; BMI, body mass index; ASA, American Society of Anesthesiologists; LFT, liver function test; NAFLD, non-alcoholic fatty liver disease.
The preoperative use of hepatoprotective agents, such as ursodeoxycholic acid, l-ornithine-l-aspartate, flavin adenine dinucleotide, and biphenyl dimethyl dicarboxylate, was not significantly different between the two groups.
After propensity score matching, 94 patients in the TIVA group and 376 in the INHA group (272 in desflurane and 104 in sevoflurane) were included for further analysis. No significant differences were observed in any of the variables associated with the baseline characteristics between the two groups (Table 1).
Analysis of Surgical Variables, LFT Changes, and Other Postoperative Outcomes Before Propensity Score Matching
The TIVA and INHA groups showed significant differences in the types of surgery. About half (52.9%) of the patients in the TIVA group underwent neurosurgeries, including brain and spine surgeries, for which the operation time was relatively longer than that for other surgeries; however, only 19.7% of the patients in the IHNA group underwent neurosurgery (Table 2). The median anesthesia and operation times were significantly longer in the TIVA group than in the INHA group (245 and 167.5 min vs 150 and 100 min, P < 0.001, respectively; Table 2).
Table 2.
Surgical Variables and Other Postoperative Outcomes
| Variables | Before Propensity Score Matching | After Propensity Score Matching | ||||
|---|---|---|---|---|---|---|
| INHA (n=592) | TIVA (n=138) | P value | INHA (n=376) | TIVA (n=94) | P value | |
| Type of surgery | <0.001 | 0.065 | ||||
| Spine | 73 (12.3%) | 46 (33.3%) | 72 (19.1%) | 22 (23.4%) | ||
| Brain | 44 (7.4%) | 27 (19.6%) | 44 (11.7%) | 7 (13.7%) | ||
| Orthopedic | 224 (37.8%) | 32 (23.2%) | 153 (40.7%) | 32 (34.0%) | ||
| Abdominal | 188 (31.8%) | 23 (16.6%) | 77 (20.5%) | 23 (23.0%) | ||
| GU or OBGY | 37 (6.3%) | 2 (1.4%) | 12 (3.2%) | 2 (2.1%) | ||
| Thoracic | 13 (2.2%) | 6 (4.3%) | 8 (57.1%) | 6 (42.9%) | ||
| Others | 13 (2.2%) | 2 (1.4%) | 10 (2.7%) | 2 (2.1%) | ||
| Emergency operation | 89 (15.0%) | 26 (18.8%) | 0.299 | 59 (15.7%) | 15 (16.0%) | 1.000 |
| Anesthesia time (min) | 150 (95–235) | 245 (150–350) | <0.001 | 170 (105–262.5) | 210 (115–315) | 0.034 |
| Operation time (min) | 100 (55–180) | 167.5 (100–270) | <0.001 | 119 (63.5–204.5) | 152.5 (75–240) | 0.079 |
| RBC transfusion | 94 (15.9%) | 34 (24.6%) | 0.018 | 70 (18.6%) | 24 (25.5%) | 0.150 |
| RBC transfusion (packs) | 2.1 (1.3–3.6) | 2.0 (1.0–3.2) | 0.560 | 2.15 (1.2–3.6) | 2.0 (1.15–3.6) | 0.787 |
| Estimated blood loss (mL) | 700 (500–1000) | 800 (500–1500) | 0.222 | 800 (500–1000) | 1000 (600–1500) | 0.168 |
| Fluid balance (%) | 2.58 (1.51–4.25) | 3.22 (1.37–4.8) | 0.064 | 2.58 (1.46–4.32) | 3.22 (1.37–5.05) | 0.144 |
| Hypotensive event | 0.018 | 0.284 | ||||
| None | 438 (74.0%) | 88 (63.8%) | 268 (71.3%) | 67 (71.3%) | ||
| 1–2 | 77 (13.0%) | 29 (21.0%) | 84 (22.3%) | 17 (18.1%) | ||
| >2 | 77 (13.0%) | 21 (15.2%) | 24 (6.4%) | 10 (10.6%) | ||
| Vasopressor use | 97 (16.4%) | 35 (25.4%) | 0.019 | 65 (17.3%) | 21 (22.3%) | 0.296 |
| Post-op length of stay (days) | 7 (4–14) | 10.5 (6–23) | <0.001 | 8 (4–16) | 9 (4–20) | 0.214 |
| 30-day mortality | 16 (2.7%) | 5 (3.6%) | 0.572 | 12 (3.2%) | 3 (3.2%) | 1.000 |
Notes: Values are median (interquartile range) or number of patient. Fluid balance during surgery (%) = (cumulative fluid input ‒ output) in liter × 100/hospital admission weight (kg)/duration of anesthesia (hour) (%).
Abbreviations: INHA, inhalation anesthesia group; TIVA, total intravenous group; GU, genitourinary; OBGY, obstetrics and gynecology; RBC, red blood cell; Post-op, post-operative.
The red blood cell transfusion rate was higher in the TIVA group than in the INHA group (24.6% vs 15.9%). The volume of red blood cell transfusion, estimated blood loss, and fluid balance was not different between the groups. The incidence of hypotensive events and of the use of vasopressors during surgery was also higher in the TIVA group. The lengths of postoperative hospital stay were higher in the TIVA group, but 30-day mortality rates were comparable between the two groups (Table 2). Among the 21 patients who died, 8 patients died of bleeding; 4 patients, cancer metastasis; 4 patients, sepsis; 3 patients; respiratory failure; and 1 patient, liver cirrhosis aggravation to hepatic encephalopathy (this patient underwent burr-hole surgery under sevoflurane anesthesia).
The median values of AST and ALT changes between 24 hrs before and after surgery in the TIVA and INHA groups were negative (the postoperative values were lower than the preoperative values). Moreover, the AST and ALT level change was significantly lower in the TIVA group than in the INHA group (−8.25% vs −4.20%, P = 0.038; and −16.2% vs −10.0%, P = 0.004, respectively).
The preoperative AST and ALT levels and postoperative ALT levels were significantly higher in the TIVA group, but the postoperative AST levels were not different between the two groups. The peak AST and ALT levels within 3 days after surgery were significantly higher in the TIVA group than in the INHA group (Table 3).
Table 3.
Changes of Aspartate Transaminase and Alanine Transaminase
| Variables | Before Propensity Score Matching | After Propensity Score Matching | ||||
|---|---|---|---|---|---|---|
| INHA (n=592) | TIVA (n=138) | P value | INHA (n=376) | TIVA (n=94) | P value | |
| AST change (%) | −4.20 (−24.4–35.0) | −8.25 (−27.3–15.7) | 0.038 | −6.2 (−25–21.5) | −8.0 (−26.2–11.0) | 0.324 |
| ALT change (%) | −10.0 (−27.9–10.2) | −16.2 (−31.9– −3.8) | 0.004 | −12.0 (−28.6–6.5) | −16.7 (−32– −4) | 0.025 |
| Pre-operative | ||||||
| AST (U/L) | 44 (34–58) | 51 (38–76) | <0.001 | 45 (36–58.5) | 54 (38–82) | 0.001 |
| ALT (U/L) | 51 (41–70) | 65 (46–95) | <0.001 | 50 (39.5–69) | 75 (53–116) | <0.001 |
| Post-operative | ||||||
| AST (U/L) | 43 (31–68.5) | 51 (34–76) | 0.072 | 43 (31–66) | 52.5 (38–82) | 0.016 |
| ALT (U/L) | 46 (31–71) | 51.5 (35–88) | 0.030 | 43 (30.5–66) | 61 (39–109) | <0.001 |
| Peak value within 3 days after surgery | ||||||
| AST (U/L) | 44.5 (32–71) | 54 (38–81) | 0.008 | 44 (31–68) | 54.5 (47.5–89) | 0.013 |
| ALT (U/L) | 48 (31–73) | 57.5 (36–92) | 0.005 | 44 (31–68) | 62.5 (41–115) | <0.001 |
Notes: Values are median (interquartile range) or number of patient (%). AST change, the change in AST levels measured within 24 hrs before and after surgery; ALT change, the change in ALT levels measured within 24 hrs before and after surgery.
Abbreviations: INHA, inhalation anesthesia group; TIVA, total intravenous group; ALT, alanine transaminase; AST, aspartate transaminase; ULN, upper limit of normal.
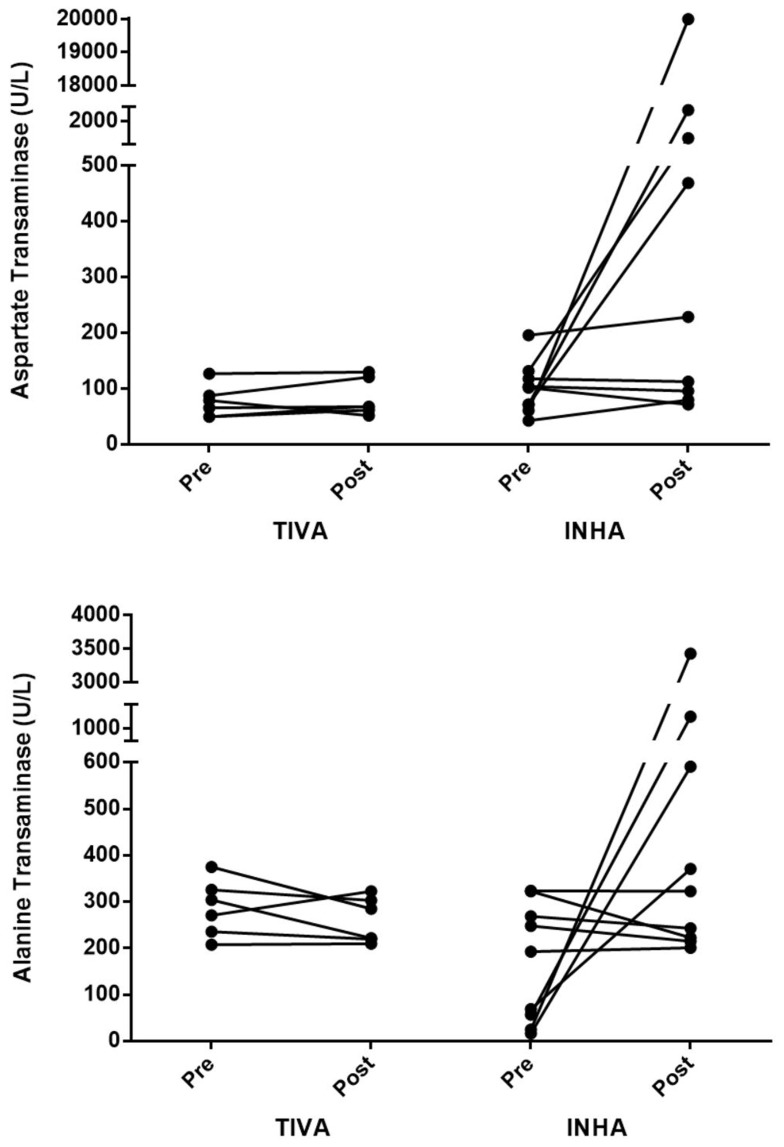
The postoperative ALT levels exceeding 200 U/L were noted in 15 patients (9 in the INHA group and 6 in the TIVA group). Two patients in the INHA group were received emergent brain surgery, and other seven patients in the INHA group and all the six patients in the TIVA group were received elective abdominal surgery. The six patients in the TIVA group had preoperative ALT levels of more than 200 U/L. In contrast, among the nine patients in the INHA group, three patients had preoperative ALT levels less than 100 U/L, but these increased to more than 200 U/L (Figure 2). The ALT levels in these 15 patients recovered to the normal range during the hospitalization period, except in one patient who underwent brain surgery under sevoflurane anesthesia and died of massive bleeding during and after the surgery.
Figure 2.
Change in aspartate transaminase and alanine transaminase levels in the 15 patients with post-operative alanine transaminase values exceeding 200 U/L.
Abbreviations: TIVA, total intravenous anesthesia; INHA, inhalation anesthesia.
Analysis After Propensity Score Matching of Surgical Variables, LFT Changes, and Other Postoperative Outcomes
After propensity score matching between the TIVA and INHA groups, no significant differences were observed in the variables that were different between the groups before matching, including the types of surgery, red blood cell transfusion rate, incidence of intraoperative hypotensive events and use of vasopressors, and length of postoperative hospital stay. The volume of red blood cell transfusion, estimated blood loss, fluid balance, and 30-day mortality rate were not different between the two groups, as observed before propensity score matching. However, the longer anesthesia time was still not compensated for despite propensity score matching (Table 2).
The median values of the changes in the AST and ALT levels within 24 hrs before and after surgery in the TIVA and INHA groups were negative (the postoperative values were lower than the preoperative values). Moreover, the ALT level changes were significantly lower in the TIVA group than in the INHA group (−16.7% vs −12.0%, P = 0.025). The AST level changes were lower in the TIVA group than in the INHA group, but the difference was not statistically significant (−8.0% vs −6.2%, P = 0.324; Table 3).
The preoperative and postoperative AST and ALT levels, and the peak AST and ALT levels within 3 days after surgery were significantly higher in the TIVA group than in the INHA group (Table 3).
Discussion
In the present study, the changes of ALT and AST level after surgery were significantly lower after TIVA than after INHA in patients with preoperatively elevated liver transaminase levels who underwent non-hepatic surgeries, despite having longer anesthesia time, higher incidence of hypotensive events, and more use of vasopressors. However, the presence of higher heterogeneity in the surgical conditions before propensity score matching should be considered. After propensity score matching, although the longer anesthesia time in the TIVA group still existed, the change of ALT level after surgery was more significantly lowered by TIVA than by INHA.
Some studies have investigated the association between the type of anesthetic agents and postoperative liver function in hepatic surgery, but their findings remain controversial.14–17 A study comparing LFT values between desflurane anesthesia and TIVA showed that liver transaminase levels were not different between the two types of anesthesia after right hepatectomy in liver donors.14 However, another study revealed isoflurane anesthesia was associated with less postoperative hepatocellular injury than was TIVA in patients with cirrhosis undergoing partial hepatectomy.15 In hepatic surgery, the reasons for postoperative liver dysfunction include surgical trauma, ischemia, hepatic mass reduction, hepatic oxygen deprivation, and stress response.18 In addition, during liver resection, hepatic inflow occlusion may be required to facilitate the procedure, and this may result in transient ischemia. This can cause liver injury and lead to postoperative liver function impairment.19 Therefore, in our study, hepatic surgery was not included in the analysis to compare the effect of the different types of anesthesia (TIVA versus INHA), in order to avoid the direct effect of hepatic surgery on liver function.
A few studies have investigated the effects of different types of anesthetic agents, especially intravenous versus inhalational agents, on postoperative liver function after non-hepatic surgery, but the effects have remained unclear. Two studies performed on patients with preoperative LFT values in the normal range showed no difference in ALT level changes after surgery between sevoflurane anesthesia and TIVA.20,21 Unlike these previous studies, our study included patients whose LFT values were not within normal limits 24 hrs prior to non-hepatic surgery.
Liver injury, whether acute or chronic, eventually causes an increase in serum transaminase levels. Both AST and ALT are highly concentrated in the liver. AST is also diffusely present in the heart, skeletal muscles, kidneys, brain, and red blood cells, but ALT has low concentrations in the skeletal muscles and kidneys. Therefore, an increase in ALT levels is more specific to liver injury.22 This is why we considered ALT level as the primary outcome in our study.
Halogenated inhalational anesthetics can cause metabolic hepatocellular injuries in humans, ranging from simple ALT elevations to fulminant hepatitis and fatal hepatic necrosis.23 No cases of fulminant hepatitis after surgery were noted in our cohort, but 15 patients (2%) had significant liver injury, with an ALT level increase of more than 200 U/L (exceeding 5 times the upper limit of the normal range) which implies clinically significant drug-induced liver injury.12 Of these 15 patients, 9 were in the INHA group and 6 in the TIVA group. However, in the INHA group, 3 patients had preoperative ALT levels under 100 U/L, while in the TIVA group, the preoperative ALT levels of all 6 patients were already more than 200 U/L. This finding suggests that TIVA might be safer than INHA in these patients.
Postoperative LFT values tend to increase immediately after surgery under general anesthesia in patients with normal preoperative LFT values.20,21 However, in our cohort study, postoperative LFT values tended to decrease, and a similar tendency has been shown by a previous retrospective study of 91 patients with preoperatively elevated liver enzyme levels.24 We speculated one of the reasons was that in patients with elevated liver enzyme levels, the health-care providers tried to optimize the patients’ hepatic function as much as possible before surgery and sometimes delayed the surgery until the patients’ conditions recovered if the surgery was not urgent. The use of hepatoprotective agents is an option for optimizing hepatic function. More than 20% of patients in our cohort were treated using hepatoprotective agents. Their use was not significantly different in both the groups, and this seemed to similarly affect both the groups.
The greater postoperative decrease in the ALT levels in the TIVA group than in the INHA group may be associated with the effect of propofol itself. Propofol has been shown to have organ-protective effects owing to its anti-inflammatory, immune-modulatory, and antioxidant properties.25,26 Additionally, propofol increases total hepatic blood flow in both hepatic arterial and portal venous circulation,27,28 whereas volatile anesthetics decrease the mean arterial pressure and hepatic blood flow.29,30 Although the pathophysiology of liver injury following exposure to halogenated anesthetics is mainly attributed to their metabolism to hepatotoxic trifluoroacylated hepatic protein adducts by cytochrome P450 2E1 in genetically predisposed individuals, anesthetics can also affect the liver function by decreasing the cardiac output and total hepatic blood flow.31
Before propensity score matching of the cohort, the number of neurosurgeries, including brain and spine surgeries, was larger in the TIVA group. This is because intraoperative neurophysiological monitoring, such as motor-evoked potentials, was usually adopted in neurosurgery, which was performed under TIVA while avoiding inhalational anesthetics because INHA interferes with such monitoring.32
Preoperative AST and ALT levels were higher in the TIVA group than in the INHA group. This reflects the practice of health-care providers at our center, wherein they avoid INHA but prefer TIVA in patients with high LFT values owing to concerns about the risk of volatile anesthetic-induced hepatotoxicity. To compensate for the different preoperative values, we compared the outcome as the change (%) in the values before and after exposure to anesthetics rather than the actual LFT values.
Although propensity score matching was performed to compensate for the heterogeneity of preoperative conditions, the longer duration of anesthesia in the TIVA group still remained. Although the elimination kinetic profile of propofol is similar in patients with liver disease and normal patients, the mean clinical recovery time after the discontinuation of infusions may be longer in patients with liver disease.33 Considering that the longer duration of anesthesia can be disadvantageous to the clinical outcomes in patients with liver disease,34 conversely, this result may prove the superiority of TIVA over INHA from the perspective of liver function in patients with preoperatively elevated liver transaminase levels.
Ziser et al retrospectively investigated the records of 733 patients with liver cirrhosis who underwent surgery, except liver transplantation, during a 11-year period.35 The 30-day mortality rate after surgery was 11.6%. Among the patients who had died, postoperative bleeding, renal failure, and sepsis were commonly noted. In our cohort, the 30-day mortality rate was 2.9% (21/730), and postoperative bleeding, cancer metastasis, and sepsis were the main reasons for mortality. A patient with underlying liver cirrhosis who had received INHA (sevoflurane) died of hepatic encephalopathy due to underlying liver cirrhosis aggravation, even though the patient’s AST level increased only up to 100 U/L and the ALT level up to 41 U/L after surgery. Patients with chronic liver cirrhosis often have only slightly elevated serum AST and ALT levels,36 as seen in the above case. In this regard, AST and ALT lack some sensitivity in detecting chronic liver disease. To assess overall liver function, integrating information, including albumin, bilirubin, alkaline phosphatase, and gamma-glutamyl transpeptidase levels, prothrombin time, and symptoms of ascites or encephalopathy, could be helpful. However, owing to the retrospective nature of this study, integrating such information was difficult; therefore, LFT values were assessed as these are the most commonly adopted laboratory values for evaluating liver injury.
This study has some limitations. First, data quality or accuracy may be more compromised in a retrospective study than in a prospective study. Bias of nonrandomized groups and retrospective results may have been present. Second, as a single-center study, the generalizability of the results may be limited. Third, the proportion of patients who underwent TIVA was relatively small at 14.2%, and the proportion of patients who underwent neurosurgery was markedly higher in the TIVA group. Although propensity score matching was performed to compensate for this heterogeneity in conditions, many samples had to be discarded. Fourth, LFT values were followed up within 3 days after surgery; however, the hepatotoxicity may be delayed and missed at 3 days after surgery. Last, although TIVA showed superiority over INHA from the perspective of the LFT values, the other secondary outcomes, such as 30-day mortality and length of postoperative hospital stay, were not different between the groups in the propensity score-matched analysis. This result may suggest the anesthetics do not affect the long-term clinical outcomes in patients with elevated liver enzyme levels. Therefore, further well-designed studies are required to confirm the effect of anesthetics on liver function as well as long-term clinical outcomes.
Conclusion
The analysis before and after propensity score matching revealed that the change of ALT level was significantly lower after TIVA than after INHA in patients with preoperatively elevated liver transaminase levels who underwent non-hepatic surgeries. This suggests that TIVA may be safer than INHA in these patients. Nevertheless, most patients in both groups had relatively healthy livers after surgery with lower AST and ALT values than before surgery. These findings may support the use of both types of anesthesia, TIVA and INHA, in patients who exhibit abnormal liver enzyme levels. Further well-designed prospective studies are warranted to verify this finding.
Acknowledgments
We would like to thank Editage for English language editing.
Funding Statement
This work was supported by a Korea University Guro Hospital Grant (O1903961).
Data Sharing Statement
The deidentified participant data are intended to be shared by the authors upon request, please contact Seok Kyeong Oh (email address: nanprayboy@korea.ac.kr).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kenna JG, Jones RM. The organ toxicity of inhaled anesthetics. Anesth Analg. 1995;81(6 Suppl):S51–S66. doi: 10.1097/00000539-199512001-00008 [DOI] [PubMed] [Google Scholar]
- 2.Safari S, Motavaf M, Seyed Siamdoust SA, Alavian SM. Hepatotoxicity of halogenated inhalational anesthetics. Iran Red Crescent Med J. 2014;16(9):e20153–e20153. doi: 10.5812/ircmj [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Njoku D, Laster MJ, Gong DH, Eger EI 2nd, Reed GF, Martin JL. Biotransformation of halothane, enflurane, isoflurane, and desflurane to trifluoroacetylated liver proteins: association between protein acylation and hepatic injury. Anesth Analg. 1997;84(1):173–178. doi: 10.1213/00000539-199701000-00031 [DOI] [PubMed] [Google Scholar]
- 4.Berghaus TM, Baron A, Geier A, Lamerz R, Paumgartner G, Conzen P. Hepatotoxicity following desflurane anesthesia. Hepatology. 1999;29(2):613–614. doi: 10.1002/(ISSN)1527-3350 [DOI] [PubMed] [Google Scholar]
- 5.Singhal S, Gray T, Guzman G, Verma A, Anand K. Sevoflurane hepatotoxicity: a case report of sevoflurane hepatic necrosis and review of the literature. Am J Ther. 2010;17(2):219–222. doi: 10.1097/MJT.0b013e318197eacb [DOI] [PubMed] [Google Scholar]
- 6.Turillazzi E, D’Errico S, Neri M, Riezzo I, Fineschi V. A fatal case of fulminant hepatic necrosis following sevoflurane anesthesia. Toxicol Pathol. 2007;35(6):840–845. doi: 10.1080/01926230701584148 [DOI] [PubMed] [Google Scholar]
- 7.Tung D, Yoshida EM, Wang CSK, Steinbrecher UP. Severe desflurane hepatotoxicity after colon surgery in an elderly patient. Can J Anesthesia. 2005;52(2):133–136. doi: 10.1007/BF03027717 [DOI] [PubMed] [Google Scholar]
- 8.Surbatovic M, Vesic Z, Djordjevic D, et al. [Hemodynamic stability in total intravenous propofol anesthesia with midazolam coinduction versus general balanced anaesthesia in laparoscopic cholecystectomy]. Vojnosanit Pregl. 2012;69(11):967–972. doi: 10.2298/VSP1211967S [DOI] [PubMed] [Google Scholar]
- 9.Servin F, Desmonts JM, Haberer JP, Cockshott ID, Plummer GF, Farinotti R. Pharmacokinetics and protein binding of propofol in patients with cirrhosis. Anesthesiology. 1988;69(6):887–891. doi: 10.1097/00000542-198812000-00014 [DOI] [PubMed] [Google Scholar]
- 10.Oh TK, Song IA, Do SH, Jheon S, Lim C. Association of perioperative weight-based fluid balance with 30-day mortality and acute kidney injury among patients in the surgical intensive care unit. J Anesth. 2019;33(3):354–363. doi: 10.1007/s00540-019-02630-8 [DOI] [PubMed] [Google Scholar]
- 11.Balakumar V, Murugan R, Sileanu FE, Palevsky P, Clermont G, Kellum JA. Both positive and negative fluid balance may be associated with reduced long-term survival in the critically Ill. Crit Care Med. 2017;45(8):e749–e757. doi: 10.1097/CCM.0000000000002372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aithal GP, Watkins PB, Andrade RJ, et al. Case definition and phenotype standardization in drug-induced liver injury. Clin Pharmacol Ther. 2011;89(6):806–815. doi: 10.1038/clpt.2011.58 [DOI] [PubMed] [Google Scholar]
- 13.Lin J, Moore D, Hockey B, et al. Drug-induced hepatotoxicity: incidence of abnormal liver function tests consistent with volatile anaesthetic hepatitis in trauma patients. Liver Int. 2014;34(4):576–582. doi: 10.1111/liv.2014.34.issue-4 [DOI] [PubMed] [Google Scholar]
- 14.Ko JS, Gwak MS, Choi SJ, et al. The effects of desflurane and propofol-remifentanil on postoperative hepatic and renal functions after right hepatectomy in liver donors. Liver Transpl. 2008;14(8):1150–1158. doi: 10.1002/lt.v14:8 [DOI] [PubMed] [Google Scholar]
- 15.Yang LQ, Tao KM, Cheung CW, et al. The effect of isoflurane or propofol anaesthesia on liver injury after partial hepatectomy in cirrhotic patients. Anaesthesia. 2010;65(11):1094–1100. doi: 10.1111/j.1365-2044.2010.06505.x [DOI] [Google Scholar]
- 16.Ko JS, Gwak MS, Choi SJ, et al. The effects of desflurane and sevoflurane on hepatic and renal functions after right hepatectomy in living donors*. Transpl Int. 2010;23(7):736–744. doi: 10.1111/tri.2010.23.issue-7 [DOI] [PubMed] [Google Scholar]
- 17.Ko JS, Kim G, Shin YH, et al. The effects of desflurane and isoflurane on hepatic and renal functions after right hepatectomy in living donors. Transplant Proc. 2012;44(2):442–444. doi: 10.1016/j.transproceed.2012.01.016 [DOI] [PubMed] [Google Scholar]
- 18.Panis Y, McMullan DM, Emond JC. Progressive necrosis after hepatectomy and the pathophysiology of liver failure after massive resection. Surgery. 1997;121(2):142–149. doi: 10.1016/S0039-6060(97)90283-X [DOI] [PubMed] [Google Scholar]
- 19.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356(15):1545–1559. doi: 10.1056/NEJMra065156 [DOI] [PubMed] [Google Scholar]
- 20.Kim JW, Kim JD, Yu SB, Ryu SJ. Comparison of hepatic and renal function between inhalation anesthesia with sevoflurane and remifentanil and total intravenous anesthesia with propofol and remifentanil for thyroidectomy. Korean J Anesthesiol. 2013;64(2):112–116. doi: 10.4097/kjae.2013.64.2.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sahin SH, Cinar SO, Paksoy I, Sut N, Oba S. Comparison between low flow sevoflurane anesthesia and total intravenous anesthesia during intermediate-duration surgery: effects on renal and hepatic toxicity. Hippokratia. 2011;15(1):69–74. [PMC free article] [PubMed] [Google Scholar]
- 22.Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005;172(3):367–379. doi: 10.1503/cmaj.1040752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reichle FM, Conzen PF. Halogenated inhalational anaesthetics. Best Pract Res Clin Anaesthesiol. 2003;17(1):29–46. doi: 10.1053/bean.2002.0265 [DOI] [PubMed] [Google Scholar]
- 24.Sahin H, Pirat A, Arslan G. Anaesthesia and surgery in patients with abnormal preoperative liver enzymes. Eur J Anaesthesiol. 2007;24(5):465–467. doi: 10.1017/S0265021506002079 [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Lopez JM, Sanchez-Conde P, Lozano FS, et al. Laboratory investigation: effects of propofol on the systemic inflammatory response during aortic surgery. Can J Anaesth. 2006;53(7):701–710. doi: 10.1007/BF03021629 [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Conde P, Rodriguez-Lopez JM, Nicolas JL, et al. The comparative abilities of propofol and sevoflurane to modulate inflammation and oxidative stress in the kidney after aortic cross-clamping. Anesth Analg. 2008;106(2):371–378. doi: 10.1213/ane.0b013e318160580b [DOI] [PubMed] [Google Scholar]
- 27.Wouters PF, Van de Velde MA, Marcus MA, Deruyter HA, Van Aken H. Hemodynamic changes during induction of anesthesia with eltanolone and propofol in dogs. Anesth Analg. 1995;81(1):125–131. doi: 10.1097/00000539-199507000-00025 [DOI] [PubMed] [Google Scholar]
- 28.Carmichael FJ, Crawford MW, Khayyam N, Saldivia V. Effect of propofol infusion on splanchnic hemodynamics and liver oxygen consumption in the rat. A dose-response study. Anesthesiology. 1993;79(5):1051–1060. doi: 10.1097/00000542-199311000-00024 [DOI] [PubMed] [Google Scholar]
- 29.Gatecel C, Losser M-R, Payen D. The postoperative effects of halothane versus isoflurane on hepatic artery and portal vein blood flow in humans. Anesthesia Analg. 2003;96(3):740–745. doi: 10.1213/01.ANE.0000047888.55004.4B [DOI] [PubMed] [Google Scholar]
- 30.Grundmann U, Zissis A, Bauer C, Bauer M. In vivo effects of halothane, enflurane, and isoflurane on hepatic sinusoidal microcirculation. Acta Anaesthesiol Scand. 1997;41(6):760–765. doi: 10.1111/j.1399-6576.1997.tb04780.x [DOI] [PubMed] [Google Scholar]
- 31.Gelman S. General anesthesia and hepatic circulation. Can J Physiol Pharmacol. 1987;65(8):1762–1779. doi: 10.1139/y87-276 [DOI] [PubMed] [Google Scholar]
- 32.Sloan TB, Heyer EJ. Anesthesia for intraoperative neurophysiologic monitoring of the spinal cord. J Clin Neurophysiol. 2002;19(5):430–443. doi: 10.1097/00004691-200210000-00006 [DOI] [PubMed] [Google Scholar]
- 33.Servin F, Cockshott I, Farinotti R, Haberer J, Winckler C, Desmonts J. Pharmacokinetics of propofol infusions in patients with cirrhosis. Br J Anaesth. 1990;65(2):177–183. doi: 10.1093/bja/65.2.177 [DOI] [PubMed] [Google Scholar]
- 34.Sato M, Tateishi R, Yasunaga H, et al. The ADOPT-LC score: a novel predictive index of in-hospital mortality of cirrhotic patients following surgical procedures, based on a national survey. Hepatol Res. 2017;47(3):E35–e43. doi: 10.1111/hepr.v47.3 [DOI] [PubMed] [Google Scholar]
- 35.Ziser A, Plevak DJ, Wiesner RH, Rakela J, Offord KP, Brown DL. Morbidity and mortality in cirrhotic patients undergoing anesthesia and surgery. Anesthesiology. 1999;90(1):42–53. doi: 10.1097/00000542-199901000-00008 [DOI] [PubMed] [Google Scholar]
- 36.Ahmed Z, Ahmed U, Walayat S, et al. Liver function tests in identifying patients with liver disease. Clin Exp Gastroenterol. 2018;11:301–307. doi: 10.2147/CEG [DOI] [PMC free article] [PubMed] [Google Scholar]