Abstract
Background:
Evidence-based, procedure-specific guidelines for prescribing opioids are urgently needed to optimize pain relief while minimizing excessive opioid prescribing and potential opioid diversion in our communities. A multidisciplinary panel at our institution recently developed procedure-specific guidelines for discharge opioid prescriptions for common orthopaedic surgical procedures. The purpose of this study was to evaluate postoperative opioid prescription quantities, variability, and 30-day refill rates before and after implementation of the guidelines.
Methods:
This retrospective cohort study was conducted at a single academic institution from December 2016 to March 2018. Guidelines were implemented on August 1, 2017, with a recommended maximum opioid prescription quantity for 14 common orthopaedic procedures. Patients who underwent these 14 procedures during the period of December 2016 to May 2017 made up the pre-guideline cohort (n = 2,223), and patients who underwent these procedures from October 2017 to March 2018 made up the post-guideline cohort (n = 2,300). Opioid prescription quantities were reported as oral morphine equivalents (OME), with medians and interquartile ranges (IQRs). Four levels were established for recommended prescription maximums, ranging from 100 to 400 OME.
Results:
In the pre-guideline cohort, the median amount of prescribed opioids across all procedures was 600 OME (IQR, 390 to 863 OME), which decreased by 38% in the post-guideline period, to a median of 375 OME (IQR, 239 to 400 OME) in the post-guideline cohort (p < 0.001). The 30-day refill rate did not change significantly, from a rate of 24% in the pre-guideline cohort to 25% in the post-guideline cohort (p = 0.43). Multivariable analysis demonstrated that guideline implementation was the factor most strongly associated with prescriptions exceeding guideline maximums (odds ratio [OR] = 9.9; p < 0.001). Age groups of <80 years (OR = 2.0 to 2.4; p < 0.001) and males (OR = 1.2; p = 0.025) were also shown to have higher odds of exceeding guideline maximums.
Conclusions:
Procedure-specific guidelines are capable of substantially decreasing opioid prescription amounts and variability. Furthermore, the absence of change in refill rates suggests that pain control remains similar to pre-guideline prescribing practices. Evidence-based guidelines are a readily employable solution that can drive rapid change in practice and enhance the ability of orthopaedic surgeons to provide responsible pain management.
Orthopaedic surgeons represent the third-highest opioid-prescribing specialty in all of medicine1. In response, the American Academy of Orthopaedic Surgeons (AAOS) has focused on curbing opioid prescriptions through targeted efforts to educate patients and surgeons alike in responsible opioid use, with resources such as the online “Pain Relief Toolkit.”1
Evidence-based, procedure-specific guidelines for postoperative opioid prescriptions are critically needed to optimize pain relief and minimize excessive opioid prescribing and potential opioid diversion in our communities. Our institution recently evaluated opioid prescribing practices for pain management following common procedures across multiple subspecialties, with total knee arthroplasty (TKA) and total hip arthroplasty (THA) ranking as number 1 and number 2, respectively, in the amount of opioids prescribed, variability in the amount prescribed, and refill rate as measured in oral morphine equivalents (OME)2.
Current prescribing practices for post-discharge pain are often based on an arbitrary number of tablets chosen at the discretion of the treating surgeon3. Regulatory bodies have taken steps to place limits on this approach to pain management. The U.S. Centers for Disease Control and Prevention (CDC) have proposed that opioid prescriptions for acute pain should not exceed 7 days4, and >20 states have passed laws limiting initial prescribing, either by duration or OME. Massachusetts has similarly restricted acute-pain opioid prescriptions to 7 days5. Minnesota recently passed legislation limiting initial acute-pain opioid prescriptions to 7 days (with a provision for provider discretion for a longer prescription duration), although at the time of this writing, it had not yet been signed into law6. New guidelines such as these are driven in large part by CDC data demonstrating markedly elevated risk of opioid dependence when prescription duration exceeded 5 days7. Despite the new legislation, safe and appropriate parameters for pain management after musculoskeletal surgery remain unclear.
Our department recently established, implemented, and subsequently evaluated procedure-specific recommendations for guiding surgeons on postoperative pain management. Herein, we assess postoperative opioid prescription quantity and variability as well as 30-day refill rates following common orthopaedic surgical procedures, both before and after the implementation of the prescription guidelines.
Materials and Methods
Following institutional review board approval, this retrospective cohort study was conducted at a single academic center, from December 2016 to March 2018. Our department designed guidelines that were implemented on August 1, 2017, with a recommended maximum opioid prescription quantity for the following procedures: THA, TKA, total shoulder arthroplasty (TSA), knee arthroscopy, shoulder arthroscopy, lumbar laminectomy or laminotomy with arthrodesis, lumbar laminectomy or laminotomy without arthrodesis, open reduction and internal fixation (ORIF) for femoral-neck fracture, ORIF for ankle fracture, ORIF for distal radial fracture, ankle arthrodesis, first metatarsophalangeal arthrodesis, anterior cruciate ligament (ACL) reconstruction, and thumb basal joint reconstruction (Table I) (Appendix 1)8. Patients undergoing concurrent orthopaedic or emergency procedures (n = 74), those <18 years of age (n = 145), and those declining research authorization (n = 305) were excluded; there were no significant differences between the cohorts with respect to the rate of these exclusions. The final cohort included 2,223 patients in the pre-guideline group and 2,300 patients in the post-guideline group.
TABLE I.
Summary of Departmental Guidelines for Maximum Opioid Prescription Amounts*
| Level | Procedure | Max. OME | Tramadol (50 mg)† | Hydrocodone (5 mg)† | Oxycodone (5 mg)† | Hydromorphone (2 mg)† | Oxycodone (5 mg) + Tramadol (50 mg)† |
| 1 | Acute fracture management‡ | 100 | 20 tabs | 20 tabs | 15 tabs | 15 tabs | Oxycodone, 8 tabs |
| Carpal tunnel release‡ | Tramadol, 8 tabs | ||||||
| 2 | Knee arthroscopy | 200 | 40 tabs | 40 tabs | 25 tabs | 25 tabs | Oxycodone, 15 tabs |
| ACL reconstruction | Tramadol, 20 tabs | ||||||
| Thumb basal joint reconstruction | |||||||
| 1st MTP arthrodesis | |||||||
| Femoral-neck fracture ORIF | |||||||
| 3 | Ankle fracture ORIF | 300 | 60 tabs | 60 tabs | 40 tabs | 40 tabs | Oxycodone, 20 tabs |
| Ankle arthrodesis | Tramadol, 30 tabs | ||||||
| Shoulder arthroscopy | |||||||
| Distal radial ORIF | |||||||
| Lumbar laminectomy or laminotomy without arthrodesis | |||||||
| 4 | THA | 400 | 80 tabs | 80 tabs | 50 tabs | 50 tabs | Oxycodone, 25 tabs |
| TKA | Tramadol, 40 tabs | ||||||
| TSA | |||||||
| Lumbar laminectomy or laminotomy with arthrodesis |
OME = oral morphine equivalents (mg), tabs = tablets, ACL = anterior cruciate ligament, MTP = metatarsophalangeal, ORIF = open reduction and internal fixation, THA = total hip arthroplasty, TKA = total knee arthroplasty, and TSA = total shoulder arthroplasty.
OME derived by multiplying the conversion factor (shown as follows in parentheses) by the number of milligrams of a tablet: (0.1) 50-mg tablet tramadol = 5 OME; (1.0) 5-mg tablet hydrocodone = 5 OME; (1.5) 5-mg tablets oxycodone = 7.5 OME; (4.0) 2-mg tablets hydromorphone = 8 OME.
Examples of Level-1 conditions/procedures (not assessed in the study).
Following a review of evidence regarding historical prescribing patterns at our institution and in the existing literature, a multidisciplinary panel consisting of orthopaedic surgeons, pain-management anesthesiologists, pharmacists, and health-care policy and delivery experts developed procedure-specific guidelines for the prescribing of opioids on patient discharge. Four tiers were created within the guidelines for the categorization of specific surgical procedures (Table I). The goal of the guidelines was to create new prescription maximums that represented an approximate 30% to 50% reduction relative to historical prescription medians for each included procedure. The resultant levels and guideline implementation were previously described by our group8. Although the guidelines provided recommended discharge prescription amounts, clinicians retained the authority to prescribe higher amounts as clinically indicated, and there was no restriction on prescribing patient-requested refills.
The 2 months preceding guideline implementation and the 2 months immediately following guideline implementation were excluded from analysis as the “washout” period. The 6-month period before and the 6-month period after the washout period were compared; the pre-guideline cohort (n = 2,223) consisted of all included patients from December 2016 to May 2017, and the post-guideline cohort (n = 2,300) consisted of all included patients from October 2017 to March 2018. Patients were considered opioid-naïve if they had not used or been prescribed opioids within 90 days prior to their encounter; 72% of the entire cohort (n = 3,277) was opioid-naïve, with a similar rate of opioid naivety between the pre- and post-guideline groups (p = 0.97). Following discharge, 30-day opioid prescription refills were captured from any provider within our health system, including surgeons, emergency departments, and primary care. Opioid prescription quantities within 7 days preoperatively through the day of discharge were reported as OME, with medians and interquartile ranges (IQRs).
Demographics of the pre-guideline and post-guideline cohorts are given in Table II. No significant differences were noted between the cohorts, with the exception of the mean length of stay, which was shorter for the post-guideline group (1.9 days) compared with the pre-guideline group (2.2 days) (p < 0.001), reflective of a concurrent effort in the post-guideline period to enhance postoperative care-pathway efficiency. Overall, the mean patient age was 63 years, 51% of the patients were female, the mean body mass index (BMI) was 31 kg/m2, 95% of the patients were white, the mean final pain score at discharge was 3 of 10, 72% of the patients were opioid-naïve, 17% had a diagnosis of cancer, 14% had a diagnosis of diabetes mellitus, 10% had a diagnosis of anxiety, and 16% had a diagnosis of depression (Table II).
TABLE II.
Demographics of the Pre-Guideline and Post-Guideline Cohorts*
| Patient Factor | All (N = 4,523) | Pre-Guideline (N = 2,223) | Post-Guideline (N = 2,300) | P Value† |
| Age (yr) | 63 (18-103) | 63 (18-98) | 63 (18-103) | 0.52 |
| Female sex | 2,323 (51%) | 1,140 (51%) | 1,183 (51%) | 0.92 |
| BMI (kg/m2) | 31 (14-69) | 31 (14-69) | 31 (15-67) | 0.59 |
| Race | 0.28 | |||
| White | 4,282 (95%) | 2,114 (95%) | 2,168 (94%) | |
| Black | 59 (1%) | 30 (1%) | 29 (1%) | |
| Other | 182 (4%) | 79 (4%) | 103 (5%) | |
| Length of stay (days) | 2.0 (0-26) | 2.2 (0-26) | 1.9 (0-22) | <0.001 |
| Final pain score at discharge | 3 (0-10) | 3 (0-10) | 3 (0-10) | 0.23 |
| Preop. opioid use | 0.97 | |||
| Naïve | 3,277 (72%) | 1,610 (72%) | 1,667 (72%) | |
| Tolerant | 1,246 (28%) | 613 (28%) | 633 (28%) | |
| Cancer diagnosis | 0.18 | |||
| No | 3,773 (83%) | 1,871 (84%) | 1,902 (83%) | |
| Yes | 750 (17%) | 352 (16%) | 398 (17%) | |
| Diabetes diagnosis | 0.44 | |||
| No | 3,868 (86%) | 1,892 (85%) | 1,976 (86%) | |
| Yes | 655 (14%) | 331 (15%) | 324 (14%) | |
| Anxiety diagnosis | 0.77 | |||
| No | 4,055 (90%) | 1,996 (90%) | 2,059 (90%) | |
| Yes | 468 (10%) | 227 (10%) | 241 (10%) | |
| Depression diagnosis | 0.25 | |||
| No | 3,817 (84%) | 1,890 (85%) | 1,927 (84%) | |
| Yes | 706 (16%) | 333 (15%) | 373 (16%) |
Continuous variables are given as the mean, with the range in parentheses. Categorical variables are given as the number of patients, with the percentage of the group in parentheses.
Bold indicates a significant value.
Statistical Methods
Demographics, patient characteristics, and diagnosis codes were obtained. International Classification of Diseases, Tenth Revision (ICD-10) codes were utilized, and comorbidities were grouped as cancer, anxiety, depression, and diabetes (Appendix 1). Prolonged postoperative length of stay was defined as > the 75th percentile for each procedure.
Univariate analyses were conducted to compare the pre- and post-guideline groups with respect to patient characteristics, demographics, discharge pain scores, opioid prescription quantities, and opioid refills. Multivariable logistic regression analysis was performed to observe the effect of clinical and patient factors on receiving a discharge opioid prescription that was greater than the guideline maximum. Multivariable analysis adjusted for guideline period, preoperative opioid use, age, BMI, sex, race, prolonged length of stay, anxiety, depression, diabetes, and cancer.
Univariate comparisons included Wilcoxon rank-sum and Kruskal-Wallis tests for continuous variables and chi-square and Fisher exact tests for categorical variables. Statistical analysis was performed using SAS software (version 9.4; SAS Institute). All p values were considered significant at the level of p < 0.05.
Results
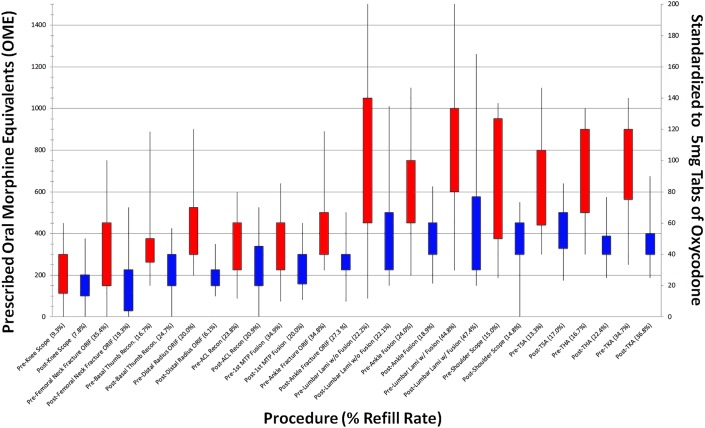
For the pre-guideline cohort, the median opioid prescription across all procedures was 600 OME (IQR, 390 to 863 OME), which decreased by 38% in the post-guideline period, to a median of 375 OME (IQR, 239 to 400 OME) for the post-guideline cohort (p < 0.001) (Table III). Although the magnitude of change varied by procedure, both the median prescribed amount and IQR decreased significantly for all 14 procedures in the post-guideline period (all p < 0.05) (Fig. 1). The largest decreases by percentage in median prescribed opioid amounts were observed for lumbar laminectomy or laminotomy without arthrodesis (57%), ORIF for distal radial fracture (52%), lumbar laminectomy or laminotomy with arthrodesis (51%), THA and TKA (48% each), and ACL reconstruction (47%) (Fig. 1). The smallest decreases by percentage in median prescribed opioid amounts were observed in knee arthroscopy (14%) and shoulder arthroscopy (19%) (Fig. 1). The 30-day refill rate did not change significantly, from a rate of 24% for the pre-guideline cohort to 25% for the post-guideline cohort (p = 0.43) (Table III). The 30-day refill rate did not increase significantly for any individual procedure (Table IV). In the pre-guideline period, the rate of cases with prescriptions below the subsequent guideline-recommended maximums was 19%; in the post-guideline period, the rate increased to 69% (p < 0.001) (Table III). In the pre-guideline period, the rate of cases with prescriptions of >200 OME (>25 tablets 5-mg oxycodone) was 92%; the rate decreased to 79% in the post-guideline period (p < 0.001) (Table III).
Fig. 1.
Box-and-whisker plot demonstrating paired representation of postoperative opioid prescriptions for 14 procedures in the pre-guideline (red) and post-guideline (blue) periods. The boxes indicate the 25th to 75th percentile interquartile range (IQR), with the whiskers indicating the 5th to 95th percentile. Prescribed oral morphine equivalents (OME) are shown on the left y axis, and the number of 5-mg oxycodone tablets (tabs) is shown on the right y axis. The procedures (x axis) are arranged from left to right in increasing order of median post-guideline-period prescription. Scope = arthroscopy, recon = reconstruction, ORIF = open reduction and internal fixation, ACL = anterior cruciate ligament, MTP = metatarsophalangeal, lami = laminectomy or laminotomy, TSA = total shoulder arthroplasty, THA = total hip arthroplasty, and TKA = total knee arthroplasty.
TABLE III.
Prescription Quantities and Refill Status in the Pre-Guideline and Post-Guideline Cohorts*
| Patient Factor | All (N = 4,523) | Pre-Guideline (N = 2,223) | Post-Guideline (N = 2,300) | P Value† |
| OME prescribed | 388 (300-638) | 600 (390-863) | 375 (239-400) | <0.001 |
| OME prescribed > 200 | <0.001 | |||
| No | 655 (14%) | 178 (8%) | 477 (21%) | |
| Yes | 3,868 (86%) | 2,045 (92%) | 1,823 (79%) | |
| OME prescribed > procedure guideline | <0.001 | |||
| No | 1,991 (44%) | 414 (19%) | 1,577 (69%) | |
| Yes | 2,532 (56%) | 1,809 (81%) | 723 (31%) | |
| 30-day opioid refill | 0.43 | |||
| No | 3,401 (75%) | 1,683 (76%) | 1,718 (75%) | |
| Yes | 1,122 (25%) | 540 (24%) | 582 (25%) |
OME = oral morphine equivalents. Continuous variables are given as the median, with the interquartile range (IQR) in parentheses. Categorical variables are given as the number of patients, with the percentage of the group in parentheses.
Bold indicates a significant value.
TABLE IV.
Thirty-Day Opioid Prescription Refill Rate by Procedure in the Pre-Guideline and Post-Guideline Periods*
| Procedure | Pre-Guideline 30-Day Refill Rate | Post-Guideline 30-Day Refill Rate | Change in 30-Day Refill Rate | P Value |
| Knee arthroscopy | 9.3% | 7.8% | −1.5% | 0.68 |
| ORIF for femoral-neck fracture | 35.4% | 19.3% | −16.1% | 0.063 |
| Thumb basal joint reconstruction | 16.7% | 24.7% | +8.0% | 0.34 |
| ORIF for distal radial fracture | 20.0% | 6.1% | −13.9% | 0.063 |
| ACL reconstruction | 23.8% | 20.9% | −2.9% | 0.69 |
| 1st MTP arthrodesis | 34.9% | 20.0% | −14.9% | 0.084 |
| ORIF for ankle fracture | 34.8% | 27.4% | −7.4% | 0.34 |
| Lumbar lami. without arthrodesis | 22.2% | 22.1% | −0.1% | 0.98 |
| Ankle arthrodesis | 24.0% | 18.9% | −5.1% | 0.57 |
| Lumbar lami. with arthrodesis | 44.8% | 47.4% | +2.6% | 0.74 |
| Shoulder arthroscopy | 15.0% | 14.8% | −0.2% | 0.98 |
| TSA | 13.3% | 17.0% | +3.7% | 0.24 |
| THA | 16.7% | 22.4% | +5.7% | 0.15 |
| TKA | 34.7% | 36.8% | +2.1% | 0.44 |
ACL = anterior cruciate ligament, MTP = metatarsophalangeal, ORIF = open reduction and internal fixation, lami. = laminectomy or laminotomy, TSA = total shoulder arthroplasty, THA = total hip arthroplasty, and TKA = total knee arthroplasty.
Multivariable analysis demonstrated that guideline implementation was the factor most strongly associated with prescriptions exceeding guideline maximums (Table V). The odds of exceeding guideline maximums were 10 times greater in the pre-guideline period than in the post-guideline period (odds ratio [OR], 9.9 [95% confidence interval (CI), 8.6 to 11.4]; p < 0.001) (Table V). Age groups of <80 years were shown to have higher odds of exceeding guideline maximums—age of <50 years: OR, 2.3 (95% CI, 1.7 to 3.1); p < 0.001; age of 50 to 64 years: OR, 2.4 (95% CI, 1.9 to 3.1); p < 0.001; and age of 65 to 79 years: OR, 2.0 (95% CI, 1.5 to 2.5); p < 0.001 (Table V). Males had higher odds of exceeding guideline maximums than did females (OR, 1.2 [95% CI, 1.0 to 1.4]; p = 0.025) (Table V). Preoperative opioid use, BMI, race, hospital length of stay, and a diagnosis of cancer, diabetes, anxiety, or depression were not significantly associated with exceeding guideline maximums (Table V).
TABLE V.
Multivariable Adjusted Odds of Discharge Opioid Prescriptions Being Greater than Guideline Maximums
| Patient Factor | Guideline Goal Exceeded* | P Value† |
| Period | ||
| Pre-guideline | 9.9 (8.6-11.4) | <0.001 |
| Post-guideline (ref.) | ||
| Preop. opioid use | ||
| Tolerant | 0.9 (0.8-1.1) | 0.389 |
| Naïve (ref.) | ||
| Age in yr | ||
| <50 | 2.3 (1.7-3.1) | <0.001 |
| 50-64 | 2.4 (1.9-3.1) | <0.001 |
| 65-79 | 2.0 (1.5-2.5) | <0.001 |
| ≥80 (ref.) | ||
| BMI in kg/m2 | ||
| ≥30 | 1.1 (0.9-1.2) | 0.319 |
| <30 (ref.) | ||
| Sex | ||
| Male | 1.2 (1.0-1.4) | 0.025 |
| Female (ref.) | ||
| Race | ||
| Black | 1.4 (0.8-2.6) | 0.283 |
| Other | 1.0 (0.7-1.4) | 0.979 |
| White (ref.) | ||
| Length of stay | ||
| >75th percentile | 1.2 (0.9-1.5) | 0.157 |
| ≤75th percentile (ref.) | ||
| Anxiety diagnosis | ||
| Yes | 1.1 (0.8-1.4) | 0.661 |
| No (ref.) | ||
| Depression diagnosis | ||
| Yes | 1.0 (0.8-1.3) | 0.796 |
| No (ref.) | ||
| Diabetes diagnosis | ||
| Yes | 1.1 (0.9-1.3) | 0.513 |
| No (ref.) | ||
| Cancer diagnosis | ||
| Yes | 0.9 (0.8-1.1) | 0.558 |
| No (ref.) |
The values are given as the odds ratio, with the 95% confidence interval in parentheses.
Bold indicates a significant value.
Discussion
Our findings demonstrate that the formal establishment and implementation of procedure-specific guidelines for postoperative opioid prescriptions are capable of substantially decreasing prescription amount and variability. Furthermore, the observed absence of a change in refill rates suggests that stricter prescribing practices do not defer opioid volume to subsequent patient encounters and also likely allow for similar postoperative pain control as pre-guideline prescribing practices. Evidence-based, formalized guidelines are a readily employable solution that can drive rapid change in practice and enhance the ability of orthopaedic surgeons to provide responsible and uniform pain management.
Recommendations have been provided by numerous parties to curb the crisis related to pain medications. The CDC and many states have either informally suggested or formally created 7-day limits for postoperative prescriptions4-6. While these efforts are intended to improve patient and community safety, they lack the procedure-specific context that is critical to balance safety and appropriate pain control, which varies greatly by surgical intervention. Furthermore, while based on expert opinion and guided by the literature, the efficacy of these guidelines has yet to be fully evaluated scientifically. Attaining multiple perspectives is critical to establishing broad recommendations such as prescription guidelines. Our multidisciplinary group enriched the final guidelines while creating an environment of shared ownership in the process, which enabled improved adherence through the involvement of all care team members.
Our institution recently evaluated opioid prescribing practices for pain following common procedures across multiple subspecialties, with TKA and THA ranking as number 1 and number 2, respectively, in prescription quantity, variability, and refill rate among all 25 procedures evaluated2. Subsequently, since the implementation of the above-described guidelines, we demonstrated significant decreases in opioid prescriptions across all 14 orthopaedic procedures studied, with a 48% decrease in median prescription amount for TKA and THA. Change of this speed and magnitude emphasizes the power of managing expectations and counseling patients to influence the complex perception of pain. Highlighting this point, in 2 previous studies, patients undergoing TKA and THA ranked the importance of multiple issues related to surgery, with the responses of patients in the U.S. varying from those of patients in countries in Central America. Among patients in the U.S., “pain immediately after surgery” constituted the greatest worry, while this was in the bottom half of concerns for patients in Guatemala and Nicaragua9,10. The campaign designating pain the “fifth vital sign” for the past several decades likely catalyzed the current problem in the U.S. by endorsing the notion that patients may expect immediate pain relief. Physicians and surgeons are now expected to record pain level at all visits. Furthermore, the management of pain represents an important criterion within value-based health care to assess care quality and ultimately determine compensation. While a facile strategy to accomplish this goal is titrating opioid prescriptions to achieve a pain goal, data show that even limited-term opioid use alters pain neurologic pathways and increases addiction risk, but does not necessarily result in superior pain control7. Opioid use also creates an environment for poor patient outcomes in future care, as demonstrated by elevated complication rates, cost, and resource utilization3. The positive trends observed following the implementation of prescription guidelines as well as multidisciplinary management of expectations suggest that culturally entrained expectations of pain can be successfully optimized through formal opioid counseling and prescribing.
One theoretical concern with prescription-guideline maximums is the potential impact on refill requests. However, we found minimal and clinically unimportant differences between refill rates before and after guideline implementation. Similar outcomes have been demonstrated in general surgery, with an absence of a change in refill rates following the establishment of guidelines11. However, a current gap in knowledge relates to how many tablets are ultimately used by patients, particularly those who do not request a refill. More granular data of this nature would be of benefit in refining future opioid prescribing strategies. Through a large institutional quality-improvement survey initiative, we have data showing that opioid use is much lower than guideline-suggested maximum prescribed quantities among most patients undergoing a subset of procedures included in the survey. Given this information, smaller and more targeted prescriptions are likely possible, yet an unintended consequence may be realized, namely, an increase in refill rates as prescriptions become progressively restrictive. Electronic prescriptions are an underutilized method for achieving medication quantities that align with ultimate patient consumption. The vast majority of pharmacies (>80%) in the U.S. accept opioid prescriptions electronically; yet, this option is used by <8% of providers12. Electronic prescribing was not used for any patients in this study, but future implementation of this process may bolster patient-specific and refined prescribing efforts.
Our findings should be considered in the context of certain limitations. First, the guidelines presented are subject to further refinement and change as more data become available. The current version of these guidelines has achieved initial efficacy; nevertheless, there is substantial room for improvement. We would encourage other centers considering this approach to maintain flexibility and pursue an iterative process toward continuous refinement that is responsive to new information.
Orthopaedic surgeons have an opportunity to take a position of leadership in addressing the national opioid crisis. Although many areas outside the realm of medicine contribute to this problem, overzealous prescribing patterns that are not guided by evidence represent a clear target for actionable change. Procedure-specific guidelines are one potential solution, and our data, along with those of other groups2,8,11, have shown initial efficacy in this regard. Early successes with the use of guidelines can inform other centers interested in adopting a similar strategy while contributing to the broader dialogue on how to adequately manage pain for our patients in a responsible manner.
Appendix
Supporting material provided by the authors is posted with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/JBJSOA/A145).
Footnotes
Investigation performed at the Mayo Clinic, Rochester, Minnesota
Disclosure: The authors indicated that no external funding was received for any aspect of this work. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work (http://links.lww.com/JBJSOA/A144).
References
- 1.Hilibrand AS, Matzkin E. American Academy of Orthopaedic Surgeons. Combatting opioid misuse. 2017. Accessed 2017 Nov 18. https://www.aaos.org/AAOSNow/2017/Jun/YourAAOS/youraaos10/?ssopc=1 [Google Scholar]
- 2.Thiels CA, Anderson SS, Ubl DS, Hanson KT, Bergquist WJ, Gray RJ, Gazelka HM, Cima RR, Habermann EB. Wide variation and overprescription of opioids after elective surgery. Ann Surg. 2017. October;266(4):564-73. [DOI] [PubMed] [Google Scholar]
- 3.Hill MV, McMahon ML, Stucke RS, Barth RJ., Jr Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017. April;265(4):709-14. [DOI] [PubMed] [Google Scholar]
- 4.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016. April 19;315(15):1624-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meier B, Tavernise S. States move to control how painkillers are prescribed. 2016. Accessed 2017 Nov 18. https://www.nytimes.com/2016/03/12/business/states-move-to-control-how-painkillers-are-prescribed.html [Google Scholar]
- 6.Opioid Prescribing Work Group, Minnesota Departments of Health, Human Services, and Labor & Industry. Minnesota Opioid Prescribing Guidelines, 1st ed 2018. Accessed 2020 Jan 22. https://mn.gov/dhs/assets/mn-opioid-prescribing-guidelines_tcm1053-337012.pdf [Google Scholar]
- 7.Shah A, Hayes CJ, Martin BC. Factors influencing long-term opioid use among opioid naive patients: an examination of initial prescription characteristics and pain etiologies. J Pain. 2017. November;18(11):1374-83. Epub 2017 Jul 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyles CCHM, Hevesi M, Trousdale ER, Ubl DS, Gazelka HM, Habermann EB, Trousdale RT, Pagnano MW, Mabry TM. The 2018 Chitranjan S. Ranawat, MD Award: developing and implementing a novel institutional guideline strategy reduced postoperative opioid prescribing after TKA and THA. Clin Orthop Relat Res. 2019. January;477(1):104-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kavolus JJ, Ritter MA, Claverie JG, Barfield WR, Lackland DT, Trousdale RT. Concerns of an itinerant surgeon: results of a Guatemalan surgical aid trip. J Arthroplasty. 2014. May;29(5):861-6. Epub 2013 Oct 16. [DOI] [PubMed] [Google Scholar]
- 10.Kavolus JJ, Ritter MA, Claverie JG, Salas MD, Kavolus CH, Trousdale RT. Cultural nuance in orthopedic foreign aid: differences in patient concerns. J Arthroplasty. 2016. January;31(1):27-30. Epub 2015 Aug 14. [DOI] [PubMed] [Google Scholar]
- 11.Hill MV, Stucke RS, McMahon ML, Beeman JL, Barth RJ., Jr An educational intervention decreases opioid prescribing after general surgical operations. Ann Surg. 2018. March;267(3):468-72. [DOI] [PubMed] [Google Scholar]
- 12.Gawande AA. It’s time to adopt electronic prescriptions for opioids. Ann Surg. 2017. April;265(4):693-4. [DOI] [PubMed] [Google Scholar]