Abstract
Background:
Osteoporotic fragility fractures frequently occur at the distal part of the radius. This suggests that initial osteoporosis evaluation at this site may inform screening and treatment to prevent additional fractures. The purpose of this study was to investigate the utility of distal forearm dual x-ray absorptiometry (DXA) as a screening tool to assess the risk of fragility fractures at the distal part of the radius.
Methods:
This retrospective, case-control study included postmenopausal women who had sustained a distal radial fracture (fracture group, n = 110) and postmenopausal women with no history of fracture (control group, n = 95). DXA measurements at the spine, hip, and distal part of the forearm (ultra-distal, mid-distal, and one-third distal sections) were compared between the groups on the basis of bone mineral density (BMD), T-score, and the proportion of patients with a T-score of ≤–2.5 standard deviations (SD). We also investigated the regional differences on the basis of T-score among the skeletal sites. Furthermore, the reliability of distal forearm DXA measurements was validated by assessing the statistical correlation (r) with volumetric BMD by computed tomography (CT).
Results:
Compared with the control group, the fracture group showed significantly lower BMD and T-scores and higher proportions of patients with a T-score of ≤–2.5 SD at the ultra-distal, mid-distal, and one-third distal forearm; however, the spine and hip measurements did not differ significantly between the 2 groups. With respect to regional differences, in the fracture group, T-scores were significantly lower and the proportions of patients with a T-score of ≤–2.5 SD were significantly higher for the 3 distal forearm sites compared with the spine and hip. DXA measurements at all 3 of the distal forearm regions exhibited high correlation with volumetric BMD by CT (r = 0.83 to 0.92).
Conclusions:
Some postmenopausal women were found to exhibit bone loss preferentially at the distal part of the radius, which may render them vulnerable to fragility fractures. Forearm DXA for the assessment of local bone loss may demonstrate benefit in screening for those at risk for distal radial fractures and facilitate the early identification of patients who require intervention for osteoporosis.
Level of Evidence:
Prognostic Level III. See Instructions for Authors for a complete description of levels of evidence.
Fragility fractures in postmenopausal women are typically caused by osteoporosis and occur in areas exhibiting a greater tendency for bone loss, such as the spine, hip, or wrist1-4. Of these, distal radial fractures occur at younger ages than do other osteoporotic fractures; the incidence in the perimenopausal period is approximately 2-fold higher than that at other sites5-9. A history of wrist fracture is associated with a considerably increased risk of future fractures; several studies have emphasized the importance of screening as a preventive measure against future osteoporosis-related fractures in these patients6,8-11.
Despite this clear association, the National Osteoporosis Foundation treatment guidelines and the diagnostic classification of the World Health Organization (WHO) recommend assessment of bone mineral density (BMD) by dual x-ray absorptiometry (DXA) at either the spine or hip as the gold standard for osteoporosis screening12-14. The forearm is only targeted when the spine and hip are unmeasurable or spine and hip BMD data are unusable or uninterpretable12,13. This poses a diagnostic dilemma during assessment of patients with fragility fractures: although fractures due to osteoporosis occur frequently at the distal part of the radius, distal forearm BMD screening by DXA is not prioritized.
Low bone density is a widely accepted major risk factor for fragility fractures15-17; in addition, a decrease in local BMD tends to correlate more closely with local fracture prevalence13. However, in postmenopausal women who experience distal radial fractures, there is a paucity of evidence that supports preferential bone loss at the distal part of the radius compared with that at other skeletal sites. Therefore, the utility of distal forearm DXA for the screening of distal radial fractures is still unclear.
In the present study, we hypothesized that some postmenopausal women may exhibit low bone density at the distal part of the forearm but similar bone density at the spine and hip compared with their counterparts with no history of fracture; further, these patients may have a higher risk of distal radial fracture. If the hypotheses were to hold, then distal forearm DXA for the assessment of local bone deterioration has the potential to be used as a screening tool for patients at risk for these fractures. Such a strategy may facilitate the early identification of patients who require intervention for osteoporosis. The purpose of the study was to compare DXA measurements at the spine, hip, and distal part of the forearm between postmenopausal women with a recent distal radial fracture and control subjects without a fracture; in addition, we investigated the regional difference in measurements on the basis of T-score among the different skeletal sites (primary end points). As secondary end points, we aimed to verify whether these comparisons were relevant for subjects not meeting the osteoporosis criterion at the spine and hip. Lastly, we assessed the reliability of DXA measurements by comparison with local bone density as assessed using clinical computed tomography (CT) in patients with fractures.
Materials and Methods
Study Design and Setting
This retrospective case-control study was conducted in accordance with the tenets of the Declaration of Helsinki and received approval from the institutional review board; the requirement for written informed consent from the participants was waived by the board.
Fracture Group Enrollment
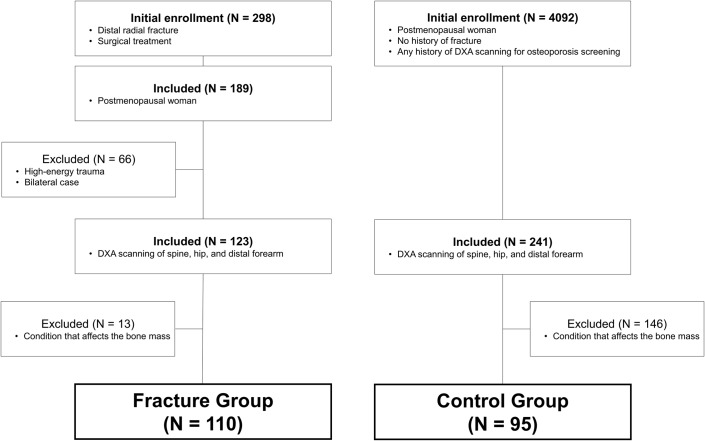
We reviewed the records of 298 consecutive patients who had sustained distal radial fractures that required surgery during the period of December 2013 to June 2017. All postmenopausal women who experienced low-energy trauma (defined as the equivalent to falling from a standing height or less) were considered for inclusion (n = 189). Patients with fractures caused by high-energy trauma (defined as the equivalent to falling from greater than a standing height, a motor vehicle accident, or a sporting injury) and patients with bilateral fractures were excluded (n = 66). Of the remaining patients (n = 123), a further inclusion criterion was that the patient had undergone DXA scanning of the spine, hip, and distal part of the forearm. Excluded were patients who had the following conditions that affect bone mass: a history of other fragility fracture(s), prior treatment for osteoporosis, exposure to glucocorticoids, severe renal disorder (i.e., estimated glomerular filtration rate [eGFR] of <15 mL/min/1.73 m2), hepatic disorder (at least double the reference value for aspartate aminotransferase [AST] or alanine aminotransferase [ALT]), diabetes, and autoimmune diseases (e.g., rheumatoid arthritis). The remaining 110 patients comprised the fracture group for this analysis (Fig. 1).
Fig. 1.
Schematic illustration of the study design and subject selection criteria. The study population consisted of 110 patients in the fracture group and 95 patients in the control group.
Control Group Enrollment
On the basis of a review of patient charts, postmenopausal women who had no history of fracture and had any history of DXA scanning for osteoporosis screening were identified for initial enrollment (n = 4,092). Of these, postmenopausal women who had undergone DXA scanning at the spine, hip, and distal part of the forearm were eligible for inclusion (n = 241). Patients with conditions that affect bone mass, as described above for the fracture group, were excluded. A total of 95 women were ultimately enrolled in the control group (Fig. 1).
Patient Characteristics
Demographic information including age, height, weight, body mass index (BMI), smoking status, and alcohol use were recorded. Data pertaining to serum levels of AST, ALT, albumin-adjusted calcium, phosphate, and eGFR, and markers of bone turnover (procollagen type-1 N-terminal propeptide [P1NP], tartrate-resistant acid phosphatase 5b [TRACP-5b], and undercarboxylated osteocalcin [ucOC]) were obtained. These data were routinely collected for fracture cases (within 48 hours of injury) and for osteoporosis screening. In the fracture group, the fracture type was classified, using the OTA/AO classification system, as intra-articular (fracture type A) or extra-articular (fracture type B or C)18,19, and hand dominance was recorded.
DXA Measurement (Areal BMD)
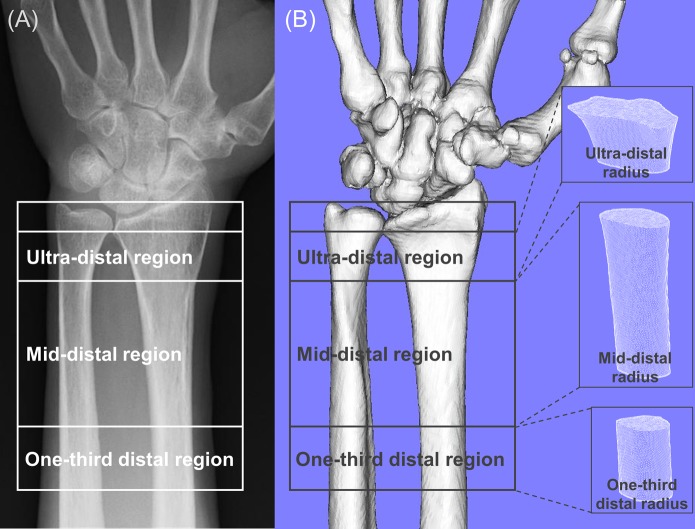
Areal BMD, measured 2-dimensionally, was assessed at the spine, hip, and distal part of the forearm using the Discovery DXA scanner (Hologic). Spine BMD was evaluated by averaging the lumbar spine values for L2-L4, after exclusion of any degenerative lesions, severe scoliosis, prevalent vertebral fractures, or postoperative sites. Hip BMD was assessed on the basis of data from the femoral neck. The distal part of the forearm was divided into 3 regions of interest (ROIs): the distalmost region (termed the “ultra-distal” radius), consisting of a 15-mm section adjacent to the end plate of the radius; the proximal region (termed the “one-third distal” radius), consisting of a 20-mm section one-third of the distance between the ulnar styloid and the olecranon; and the intermediate region (termed the “mid-distal” radius), consisting of the remaining section between the 2 aforementioned sites (Fig. 2-A)20,21. The scans were made just after injury on the unfractured side for the fracture group, and made for screening purposes before any intervention for the control group.
Fig. 2.
Representative images of the distal part of the forearm by DXA (Fig. 2-A) and CT (Fig. 2-B). The distal part of the forearm was divided into 3 regions of interest: ultra-distal, mid-distal, and one-third distal radius.
Areal BMD was expressed in absolute terms of grams of mineral per square centimeter (g/cm2), and the T-score was calculated. The T-score is defined as the number of standard deviations (SD) of a measured BMD from the average BMD of the reference population (young-adult females)22. For analyses, osteoporosis was defined as a T-score of ≤−2.5 SD using the WHO criteria13,22; in addition, data pertaining to the proportions of patients with a T-score of ≤−2.5 SD were collected.
CT Measurement (Volumetric BMD)
We investigated the reliability of the assessment of local bone density of the distal part of the forearm by DXA measurements by comparing with volumetric BMD based on clinical CT performed routinely for fracture surgeries. Of the 110 participants in the fracture cohort, we analyzed accessible data for 63 patients who underwent preoperative CT of both forearms for evaluation of fracture type and preoperative planning. A helical-type Aquilion 64 CT scanner (Toshiba) with a low-radiation-dose technique (slice thickness, 0.65 mm; pixel size, 0.75 to 0.85 mm; scan time, 0.5 sec; scan pitch, 0.562:1; tube current, 30 mA, and tube voltage, 120 kV)23 was used with a uniform protocol, including a reconstruction algorithm. Next, image-processing MvIndex/Bone Simulator software (Orthree) was used to create 3-dimensional models of the radius, ulna, and carpal bones on the unfractured side. The radius models were divided into the 3 ROIs based on the DXA ROIs prior to the analysis of bone density at each site20,21, and bone density was computed as the pixel intensity in Hounsfield units (HUs)24. In each ROI, the HU values of all included voxels were averaged to give a result equivalent to the bone density (Fig. 2-B)25-29.
Statistical Analysis
All statistical analyses were performed using SPSS (version 24.0; IBM), with the significance level set at p < 0.05. Unless specified otherwise, continuous variables are presented as the mean and SD, while categorical variables are presented as the percentage.
A Mann-Whitney U test and Pearson chi-square test were used to compare the DXA measurements and other patient characteristics between the fracture and control groups. To detect the regional differences among the skeletal sites, T-scores and the proportions of patients with a T-score of ≤−2.5 SD were compared using a Wilcoxon signed-rank test with Bonferroni adjustments for multiple comparisons. For patients who did not meet the osteoporosis criterion at the lumbar spine and femoral neck (had a T-score of >−2.5 SD), data pertaining to the distal part of the forearm were compared between the fracture and control groups using the same statistical methods.
The reliability of forearm DXA measurements for patients with a distal radial fracture was investigated by determining the Spearman rank correlation coefficients (r) between CT measurements (volumetric BMD) and DXA measurements (both areal BMD and T-score measurements). The strength of the association was classified as slight (r < 0.2), low (0.2 ≤ r < 0.4), moderate (0.4 ≤ r < 0.7), or high (r ≥ 0.7)30.
We conducted power analyses10,31 (α = 0.05, β = 0.1, 2-tailed) to ascertain the sample size necessary to detect a BMD difference of 0.025 g/cm2 given an SD of 0.050 g/cm2. The minimum sample size was 90 patients in each of the 2 groups. Hence, our sample size was adequate to assess the primary end points.
Results
The demographic and baseline characteristics of the fracture group (n = 110; mean age, 70 ± 10 years) and the control group (n = 95; mean, 67 ± 12 years) are presented in Table I. All participants were Japanese women, and both groups were similar in their characteristics, including age and BMI; subjects differed only with respect to eGFR. Mean DXA measurements for each region are shown in the Appendix.
TABLE I.
Patient Data
| Characteristic | Fracture Group (N = 110) | Control Group (N = 95) | P Value |
| Age* (yr) | 70 ± 10 | 67 ± 12 | 0.206 |
| Height* (m) | 1.53 ± 0.07 | 1.53 ± 0.06 | 0.597 |
| Weight* (kg) | 53.2 ± 8.6 | 52.0 ± 9.4 | 0.378 |
| Body mass index* (kg/m2) | 22.7 ± 3.4 | 22.2 ± 3.8 | 0.354 |
| Smoker (no. [%]) | 9 (8.2%) | 6 (6.3%) | 0.609 |
| Alcohol use (no. [%]) | 14 (12.7%) | 8 (8.4%) | 0.321 |
| Serum data* | |||
| AST (IU/L) | 23.1 ± 9.7 | 22.8 ± 9.3 | 0.896 |
| ALT (IU/L) | 16.9 ± 7.3 | 18.5 ± 11.5 | 0.682 |
| Ca (mg/dL) | 9.3 ± 0.4 | 9.3 ± 0.8 | 0.088 |
| P (mg/dL) | 3.4 ± 0.5 | 3.5 ± 0.8 | 0.757 |
| eGFR (mL/min/1.73 m2) | 67.8 ± 16.9 | 60.6 ± 27.3 | 0.028† |
| Bone turnover marker* | |||
| P1NP (μg/L) | 49.8 ± 19.9 | 55.4 ± 40.2 | 0.892 |
| TRACP-5b (mU/dL) | 317.8 ± 106.7 | 359.6 ± 221.6 | 0.962 |
| ucOC (ng/mL) | 4.7 ± 3.5 | 3.9 ± 3.5 | 0.111 |
| Fracture side (no. [%]) | |||
| Dominant side | 53 (48%) | — | — |
| Nondominant side | 57 (52%) | — | — |
| Fracture type (no. [%]) | |||
| Intra-articular | 82 (75%) | — | — |
| Extra-articular | 28 (25%) | — | — |
The values are given as the mean and standard deviation. AST = aspartate aminotransferase, ALT = alanine aminotransferase, Ca = calcium (albumin-adjusted), P = phosphate, eGFR = estimated glomerular filtration rate, P1NP = procollagen type-1 N-terminal propeptide, TRACP-5b = tartrate-resistant acid phosphatase 5b, and ucOC = undercarboxylated osteocalcin.
P < 0.05; significant difference by Mann-Whitney U test.
Primary Outcome
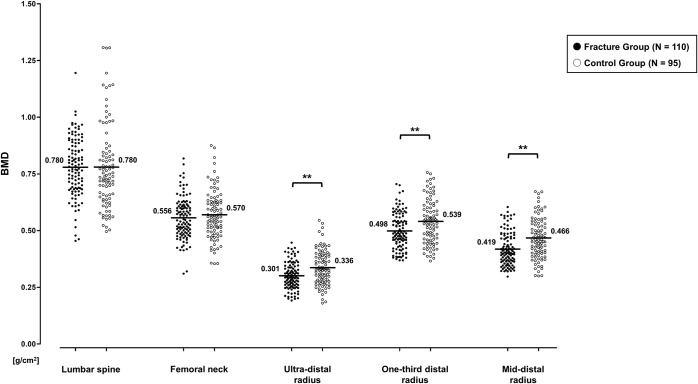
Comparisons between the fracture and control groups are shown in Figures 3 and 4. There were significant differences with respect to all distal forearm DXA measurements: measurements at the ultra-distal, one-third distal, and mid-distal radius were significantly lower with respect to BMD (p = 0.001, p = 0.002, and p < 0.001, respectively) and T-score (p < 0.001, p = 0.002, and p < 0.001, respectively) and higher with respect to the proportions of patients with a T-score of ≤−2.5 SD (p < 0.001, p = 0.002, and p = 0.002, respectively) for the fracture group than for the control group. In contrast, we did not find any significant difference in these measurements between the groups at the lumbar spine and femoral neck.
Fig. 3.
Comparison of bone mineral density (BMD) measurements between the fracture and control groups. Bars indicate mean values. **P < 0.01; significant difference by the Mann-Whitney U test, with lower BMD for the fracture group than for the control group.
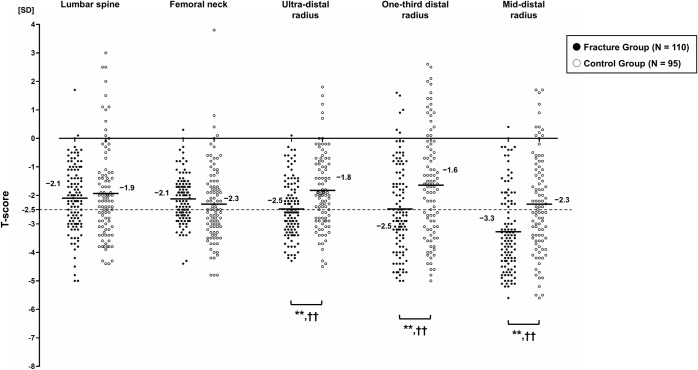
Fig. 4.
Comparison of T-score measurements between the fracture and control groups. Bars indicate mean values. The cutoff is set to −2.5 SD (dashed line). **P < 0.01; significant difference by the Mann-Whitney U test, with lower T-score for the fracture group than for the control group. ††P < 0.01; significant difference by the Pearson chi-square test, with higher proportions of patients with a T-score of ≤−2.5 SD for the fracture group than for the control group.
The regional differences among the skeletal sites are shown in Table II. Compared with the spine or hip, T-scores at the ultra-distal radius (versus spine: p = 0.012; versus hip: p < 0.001), one-third distal radius (versus spine: p = 0.036; versus hip: p = 0.024), and mid-distal radius (versus spine: p < 0.001; versus hip: p < 0.001) were significantly lower and the proportions of patients with a T-score of ≤−2.5 SD at the ultra-distal radius (versus spine: p = 0.007; versus hip: p = 0.010), one-third distal radius (versus spine: p = 0.005; versus hip: p = 0.007), and mid-distal radius (versus spine: p < 0.001; versus hip: p < 0.001) were significantly higher in the fracture group. Conversely, in the control group, T-scores of the 3 distal forearm ROIs were similar (or even higher) and proportions of those with a T-score of ≤−2.5 SD were also similar (or even lower) compared with the spine and hip.
TABLE II.
Regional Differences Among the Skeletal Sites*
| Fracture Group (N = 110) | Control Group (N = 95) | |||||
| T-Score | P Value (Vs. Spine) | P Value (Vs. Hip) | T-Score | P Value (Vs. Spine) | P Value (Vs. Hip) | |
| Distal forearm region | ||||||
| Ultra-distal radius | −2.5 ± 1.0 | 0.012† | <0.001‡ | −1.8 ± 1.3 | >0.999 | <0.001‡ |
| 57% (63/110) | 0.007§§ | 0.010§ | 33% (31/95) | 0.229 | 0.005§§ | |
| One-third distal radius | −2.5 ± 1.6 | 0.036† | 0.024† | −1.6 ± 1.9 | 0.252 | <0.001‡ |
| 58% (64/110) | 0.005§§ | 0.007§§ | 37% (35/95) | 0.552 | 0.029§ | |
| Mid-distal radius | −3.3 ± 1.4 | <0.001‡ | <0.001‡ | −2.3 ± 1.7 | 0.066 | >0.999 |
| 71% (78/110) | <0.001§§ | <0.001§§ | 49% (47/95) | 0.224 | 0.663 | |
| Spine | ||||||
| Lumbar spine | −2.1 ± 1.2 | — | — | −1.9 ± 1.6 | — | — |
| 39% (43/110) | — | — | 41% (39/95) | — | — | |
| Hip | ||||||
| Femoral neck | −2.1 ± 0.8 | — | — | −2.3 ± 1.3 | — | — |
| 40% (44/110) | — | — | 53% (50/95) | — | — | |
T-score values are given as the mean and standard deviation and are shown with the proportion of patients in the group with a T-score of ≤−2.5 SD for the given region. Bold indicates significant p values, with lower T-scores or higher proportions of patients with a T-score of ≤−2.5 SD for the fracture group compared with the control group.
P < 0.05, and
p < 0.01; significant difference by Wilcoxon signed-rank test.
P < 0.05, and
p < 0.01; significant difference by Pearson chi-square test.
Secondary Outcome
Sixty-seven fracture and 56 control group subjects who did not meet the osteoporosis criterion (T-score of >−2.5 SD) at the spine, and 66 fracture and 45 control subjects who did not meet it at the hip, were analyzed. Results of these subgroup comparisons by distal forearm region are shown in Tables III and IV: there were significant differences with respect to all measurements except for the proportions of patients with a T-score of ≤−2.5 SD at the one-third distal radius among cases that did not meet the osteoporosis criterion for the hip (p = 0.147).
TABLE III.
Comparison of Distal Forearm DXA Measurements Among Patients Who Did Not Meet the Osteoporosis Criterion at the Spine (T-Score of >−2.5 SD)*
| BMD (g/cm2) | T-Score | |||||
| Fracture Group (N = 67) | Control Group (N = 56) | P Value | Fracture Group (N = 67) | Control Group (N = 56) | P Value | |
| Distal forearm region | ||||||
| Ultra-distal radius | 0.311 ± 0.060 | 0.369 ± 0.068 | <0.001‡ | −2.3 ± 1.1 | −1.3 ± 1.2 | <0.001‡ |
| 46% (31/67) | 13% (7/56) | <0.001§§ | ||||
| One-third distal radius | 0.518 ± 0.076 | 0.577 ± 0.094 | <0.001‡ | −2.1 ± 1.5 | −0.9 ± 1.8 | <0.001‡ |
| 48% (32/67) | 23% (13/56) | 0.005§§ | ||||
| Mid-distal radius | 0.431 ± 0.076 | 0.504 ± 0.083 | <0.001‡ | −3.0 ± 1.5 | −1.6 ± 1.6 | <0.001‡ |
| 64% (43/67) | 36% (20/56) | 0.002§§ | ||||
BMD values are given as the mean and standard deviation. T-score values are given as the mean and standard deviation and are shown with the proportion of patients in the group with a T-score of ≤−2.5 SD for the given region. Bold indicates significant p values, with lower BMD or T-scores or higher proportions of patients with a T-score of ≤−2.5 SD for the fracture group compared with the control group.
P < 0.01; significant difference by Mann-Whitney U test.
P < 0.01; significant difference by Pearson chi-square test.
TABLE IV.
Comparison of Distal Forearm DXA Measurements Among Patients Who Did Not Meet the Osteoporosis Criterion at the Hip (T-Score of >−2.5 SD)*
| BMD (g/cm2) | T-Score | |||||
| Fracture Group (N = 66) | Control Group (N = 45) | P Value | Fracture Group (N = 66) | Control Group (N = 45) | P Value | |
| Distal forearm region | ||||||
| Ultra-distal radius | 0.319 ± 0.053 | 0.374 ± 0.080 | <0.001‡ | −2.1 ± 0.9 | −1.2 ± 1.4 | <0.001‡ |
| 45% (30/66) | 18% (8/45) | 0.003§§ | ||||
| One-third distal radius | 0.525 ± 0.079 | 0.568 ± 0.104 | 0.024† | −2.0 ± 1.6 | −1.1 ± 2.0 | 0.021† |
| 42% (28/66) | 29% (13/45) | 0.147 | ||||
| Mid-distal radius | 0.445 ± 0.072 | 0.500 ± 0.093 | 0.001‡ | −2.7 ± 1.4 | −1.7 ± 1.8 | <0.001‡ |
| 56% (37/66) | 31% (14/45) | <0.001§§ | ||||
BMD values are given as the mean and standard deviation. T-score values are given as the mean and standard deviation and are shown with the proportion of patients in the group with a T-score of ≤−2.5 SD for the given region. Bold indicates significant p values, with lower BMD and T-scores and higher proportions of patients with a T-score of ≤−2.5 SD for the fracture group compared with the control group.
P < 0.05, and
p < 0.01; significant difference by Mann-Whitney U-test.
P< 0.01; significant difference by Pearson chi-square test.
Correlation analyses validated the distal forearm DXA measurements. In the ultra-distal, one-third distal, and mid-distal radius, the volumetric BMD by CT showed a high correlation with the areal BMD (r = 0.83, 0.92, and 0.83, respectively) and T-score (r = 0.83, 0.92, and 0.90, respectively) by DXA (p < 0.001 for all).
Discussion
Wrist fractures are generally believed to occur earlier than other osteoporotic fragility fractures. In addition, several studies have shown that a forearm fracture is highly associated with future risk of fracture6,8-11. Despite the clinical relevance of screening for distal radial fractures, only a few studies have sought to characterize the association between distal radial fractures and local bone loss by DXA10,31-33. Of these, some studies found global decline in BMD10, while others found regional decline in BMD at certain skeletal sites as well31-33. Hanusch et al. conducted a relevant study for men with distal forearm fractures and found that they showed not only a generalized reduction in BMD but also regional decline at the distal part of the radius33. The discrepancies with our results might be attributable to their inclusion of male participants and patients with a history of fragility fracture. To the best of our knowledge, no prior study has compared postmenopausal bone loss as assessed using DXA scanning between distal radial fracture and control cases, and among different skeletal sites.
In the present study, we retrospectively analyzed DXA measurements at different skeletal sites in postmenopausal women with fragility fractures of the distal part of the radius in comparison with women of similar age with no fracture as control subjects. Our results showed that postmenopausal bone loss in patients with fragility wrist fractures occurred preferentially at the distal part of the radius compared with that in control subjects, which supports the study hypotheses. The present findings highlight the existence of a subpopulation of postmenopausal women who exhibit preferential bone loss at the distal part of the radius, which might render them vulnerable to fracture at this site. This suggests that forearm DXA for the assessment of local bone deterioration may help screen for distal radial fractures, which is also supported by similar trends showing preferential bone loss at the distal part of the radius among subjects who did not meet the osteoporosis criterion at the spine and hip. Overall, BMD measurements at the distal part of the forearm hold promise as a screening tool for the early detection of osteoporosis in postmenopausal women; a minimally invasive, relatively low-cost screening using DXA can increase the opportunity for patients who potentially may have osteoporosis to seek intervention, thereby reducing the risk of additional fractures.
An increasing body of evidence suggests that HU measurements obtained from diagnostic CT scans can accurately characterize bone density25-29. In the present study, all distal forearm DXA findings were validated by comparison with HU values for volumetric bone-density assessment. We observed a high correlation of volumetric BMD by CT with both areal BMD and T-scores by DXA. These findings indicate the reliability of BMD measurements even if evaluated 2-dimensionally by area, which further supports the validity of the present DXA results and the study hypotheses.
This study had a number of limitations. First, the retrospective case-control study design may have introduced an element of selection bias. In particular, the study had the inherent weaknesses of the retrospective nature of chart review. The study population in the fracture group only included patients with distal radial fractures considered for surgery, rather than distal radial fractures in general, and the control subjects may not reflect the general postmenopausal population because only those who were screened for osteoporosis were recruited. This includes a sample of convenience. Second, forearm DXA might not be as accurate as DXA for spine and hip16,17,34, although use of CT data validated the reliability of DXA measurements. Furthermore, we did not take into account hand dominance during analyses; hand dominance would affect the measurements because forearm BMD is expected to differ between dominant and nondominant limbs. Third, we only analyzed data for a relatively small cohort of Japanese women. The effect of racial differences should be evaluated in a large-scale study. Fourth, we did not consider patients with low bone mass that is defined as osteopenia (−2.5 SD < T-score < −1.0 SD) for analyses13,22. The prevalence of osteopenia was relatively high in both fracture and control groups, which is different from the trend of the prevalence of osteoporosis (see Appendix). A further investigation regarding this difference is warranted.
In conclusion, some postmenopausal women were found to exhibit bone loss preferentially at the distal part of the radius, which would render them vulnerable to fragility fractures at this location. Our results highlight the existence of this subpopulation of postmenopausal women who might have a higher risk of distal radial fractures, which we believe to be a novel finding of this study. BMD measurements can be reliable, even if only evaluated 2-dimensionally by area, which further supports the validity of the present DXA results. Overall, forearm DXA for the assessment of local bone density may demonstrate benefit in screening for those at risk for distal radial fractures and facilitate the early identification of patients who require intervention for osteoporosis.
Appendix
Supporting material provided by the authors is posted with the online version of this article as a data supplement at jbjs.org (http://links.lww.com/JBJSOA/A137).
Acknowledgments
Note: The authors thank Toshiyuki Kataoka and Yukihiko Yasui for their contributions in collecting the data, and Ryoji Nakao for providing technical support. The authors also acknowledge the contribution to the study conception and design of Shingo Abe, Ryoya Shiode, and Arisa Kazui.
Footnotes
Investigation performed at the Graduate School of Medicine, Osaka University, Suita, and the Japan Community Health Care Organization Hoshigaoka Medical Center, Hirakata, Japan
Disclosure: The authors indicated that no external funding was received for any aspect of this work. On the Disclosure of Potential Conflicts of Interest forms, which are provided with the online version of the article, one or more of the authors checked “yes” to indicate that the author had a relevant financial relationship in the biomedical arena outside the submitted work (http://links.lww.com/JBJSOA/A136).
References
- 1.Khosla S, Riggs BL, Atkinson EJ, Oberg AL, McDaniel LJ, Holets M, Peterson JM, Melton LJ., 3rd Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone Miner Res. 2006. January;21(1):124-31. Epub 2005 Oct 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Macdonald HM, Nishiyama KK, Kang J, Hanley DA, Boyd SK. Age-related patterns of trabecular and cortical bone loss differ between sexes and skeletal sites: a population-based HR-pQCT study. J Bone Miner Res. 2011. January;26(1):50-62. [DOI] [PubMed] [Google Scholar]
- 3.Riggs BL, Melton LJ., Iii 3rd, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004. December;19(12):1945-54. Epub 2004 Sep 20. [DOI] [PubMed] [Google Scholar]
- 4.Rozental TD, Deschamps LN, Taylor A, Earp B, Zurakowski D, Day CS, Bouxsein ML. Premenopausal women with a distal radial fracture have deteriorated trabecular bone density and morphology compared with controls without a fracture. J Bone Joint Surg Am. 2013. April 3;95(7):633-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK. Bone loss and bone size after menopause. N Engl J Med. 2003. July 24;349(4):327-34. [DOI] [PubMed] [Google Scholar]
- 6.Barrett-Connor E, Sajjan SG, Siris ES, Miller PD, Chen YT, Markson LE. Wrist fracture as a predictor of future fractures in younger versus older postmenopausal women: results from the National Osteoporosis Risk Assessment (NORA). Osteoporos Int. 2008. May;19(5):607-13. Epub 2007 Dec 6. [DOI] [PubMed] [Google Scholar]
- 7.Bengnér U, Johnell O. Increasing incidence of forearm fractures. A comparison of epidemiologic patterns 25 years apart. Acta Orthop Scand. 1985. April;56(2):158-60. [DOI] [PubMed] [Google Scholar]
- 8.Cuddihy MT, Gabriel SE, Crowson CS, O’Fallon WM, Melton LJ., 3rd Forearm fractures as predictors of subsequent osteoporotic fractures. Osteoporos Int. 1999;9(6):469-75. [DOI] [PubMed] [Google Scholar]
- 9.Eastell R. Forearm fracture. Bone. 1996. March;18(3)(Suppl):203S-7S. [DOI] [PubMed] [Google Scholar]
- 10.Kanterewicz E, Yañez A, Pérez-Pons A, Codony I, Del Rio L, Díez-Pérez A. Association between Colles’ fracture and low bone mass: age-based differences in postmenopausal women. Osteoporos Int. 2002. October;13(10):824-8. [DOI] [PubMed] [Google Scholar]
- 11.Mallmin H, Ljunghall S, Persson I, Naessén T, Krusemo UB, Bergström R. Fracture of the distal forearm as a forecaster of subsequent hip fracture: a population-based cohort study with 24 years of follow-up. Calcif Tissue Int. 1993. April;52(4):269-72. [DOI] [PubMed] [Google Scholar]
- 12.The International Society for Clinical Densitometry. 2015 ISCD official positions—adult. 2015. June Accessed 2019 Sep 27. https://www.iscd.org/official-positions/2015-iscd-official-positions-adult/ [Google Scholar]
- 13.Cosman F, de Beur SJ, LeBoff MS, Lewiecki EM, Tanner B, Randall S, Lindsay R; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014. October;25(10):2359-81. Epub 2014 Aug 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanis JA, Melton LJ, 3rd, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994. August;9(8):1137-41. [DOI] [PubMed] [Google Scholar]
- 15.Kanis JA, Johnell O, Oden A, Dawson A, De Laet C, Jonsson B. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001. December;12(12):989-95. [DOI] [PubMed] [Google Scholar]
- 16.Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR; Osteoporotic Fractures Research Group. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res. 2003. November;18(11):1947-54. [DOI] [PubMed] [Google Scholar]
- 17.Wainwright SA, Marshall LM, Ensrud KE, Cauley JA, Black DM, Hillier TA, Hochberg MC, Vogt MT, Orwoll ES; Study of Osteoporotic Fractures Research Group. Hip fracture in women without osteoporosis. J Clin Endocrinol Metab. 2005. May;90(5):2787-93. Epub 2005 Feb 22. [DOI] [PubMed] [Google Scholar]
- 18.de Jong JJ, Willems PC, Arts JJ, Bours SG, Brink PR, van Geel TA, Poeze M, Geusens PP, van Rietbergen B, van den Bergh JP. Assessment of the healing process in distal radius fractures by high resolution peripheral quantitative computed tomography. Bone. 2014. July;64:65-74. Epub 2014 Apr 2. [DOI] [PubMed] [Google Scholar]
- 19.Meinberg EG, Agel J, Roberts CS, Karam MD, Kellam JF. Fracture and dislocation classification compendium-2018. J Orthop Trauma. 2018. January;32(Suppl 1):S1-170. [DOI] [PubMed] [Google Scholar]
- 20.Ducher G, Tournaire N, Meddahi-Pellé A, Benhamou CL, Courteix D. Short-term and long-term site-specific effects of tennis playing on trabecular and cortical bone at the distal radius. J Bone Miner Metab. 2006;24(6):484-90. [DOI] [PubMed] [Google Scholar]
- 21.Shepherd JA, Cheng XG, Lu Y, Njeh C, Toschke J, Engelke K, Grigorian M, Genant HK. Universal standardization of forearm bone densitometry. J Bone Miner Res. 2002 Apr;17(4):734-45. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group [meeting held in Rome from 22 to 25 June 1992]. 1994. Accessed 2019 Sep 27. https://apps.who.int/iris/handle/10665/39142 [PubMed] [Google Scholar]
- 23.Oka K, Murase T, Moritomo H, Goto A, Sugamoto K, Yoshikawa H. Accuracy analysis of three-dimensional bone surface models of the forearm constructed from multidetector computed tomography data. Int J Med Robot. 2009. December;5(4):452-7. [DOI] [PubMed] [Google Scholar]
- 24.Miyamura S, Oka K, Abe S, Shigi A, Tanaka H, Sugamoto K, Yoshikawa H, Murase T. Altered bone density and stress distribution patterns in long-standing cubitus varus deformity and their effect during early osteoarthritis of the elbow. Osteoarthritis Cartilage. 2018. January;26(1):72-83. Epub 2017 Oct 14. [DOI] [PubMed] [Google Scholar]
- 25.Johnson CC, Gausden EB, Weiland AJ, Lane JM, Schreiber JJ. Using Hounsfield units to assess osteoporotic status on wrist computed tomography scans: comparison with dual energy x-ray absorptiometry. J Hand Surg Am. 2016. July;41(7):767-74. Epub 2016 May 14. [DOI] [PubMed] [Google Scholar]
- 26.Rhee SH, Baek GH. A correlation exists between subchondral bone mineral density of the distal radius and systemic bone mineral density. Clin Orthop Relat Res. 2012. June;470(6):1682-9. Epub 2011 Dec 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG. Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am. 2011. June 1;93(11):1057-63. [DOI] [PubMed] [Google Scholar]
- 28.Schreiber JJ, Gausden EB, Anderson PA, Carlson MG, Weiland AJ. Opportunistic osteoporosis screening - gleaning additional information from diagnostic wrist CT scans. J Bone Joint Surg Am. 2015. July 1;97(13):1095-100. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber JJ, McQuillan TJ, Halilaj E, Crisco JJ, Weiss AP, Patel T, Kenney D, Ladd AL. Changes in local bone density in early thumb carpometacarpal joint osteoarthritis. J Hand Surg Am. 2018. January;43(1):33-8. Epub 2017 Oct 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guilford JP. Fundamental statistics in psychology and education. 3rd ed. McGraw-Hill; 1956. [Google Scholar]
- 31.Mallmin H, Ljunghall S. Distal radius fracture is an early sign of general osteoporosis: bone mass measurements in a population-based study. Osteoporos Int. 1994. November;4(6):357-61. [DOI] [PubMed] [Google Scholar]
- 32.Earnshaw SA, Cawte SA, Worley A, Hosking DJ. Colles’ fracture of the wrist as an indicator of underlying osteoporosis in postmenopausal women: a prospective study of bone mineral density and bone turnover rate. Osteoporos Int. 1998;8(1):53-60. [DOI] [PubMed] [Google Scholar]
- 33.Hanusch BC, Tuck SP, McNally RJQ, Wu JJ, Prediger M, Walker J, Tang J, Piec I, Fraser WD, Datta HK, Francis RM. Does regional loss of bone density explain low trauma distal forearm fractures in men (the Mr F study)? Osteoporos Int. 2017. October;28(10):2877-86. Epub 2017 Jul 6. [DOI] [PubMed] [Google Scholar]
- 34.Schuit SC, van der Klift M, Weel AE, de Laet CE, Burger H, Seeman E, Hofman A, Uitterlinden AG, van Leeuwen JP, Pols HA. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004. January;34(1):195-202. [DOI] [PubMed] [Google Scholar]