Abstract
It shows that detrimental exposures and conditions in mothers can lead to the development of obesity and type 2 diabetes in offspring. This can lead to a vicious cycle of metabolic dysfunction, where rising rates of obesity, pre-diabetes, and diabetes in individuals of reproductive age, propagating risks to subsequent generations. It is well established that regular exercise has important health benefits for people with obesity and type 2 diabetes. Recently, increasing studies aim to examine the effects of maternal exercise on metabolic health in offspring. This review aims to demonstrate the evidence linking maternal exercise during critical periods of development and its implications for glucose metabolism in offspring, including intervention timing, sexual dimorphism, different exercise type, and intensity. Then we further examine the potential role of epigenetic modifications in this process.
Keywords: Epigenetics, Maternal exercise, Metabolic health, Offspring
Introduction
The incidence of obesity and type 2 diabetes has risen rapidly over recent years worldwide. Recently, the International Diabetes Federation declared that 9.3% of adults aged 20 to 79 years (a staggering 463 million people) are living with diabetes in 2019 and the number will rise to 700 million by 2045 worldwide. In particular, one in six live births (20 million annually) is affected by hyperglycemia in pregnancy, 84% of whom have gestational diabetes mellitus, putting these individuals at risk for a host of diabetes-associated complications.[1] The World Health Organization reported that 50% of women of childbearing ages, and 20% to 25% of pregnant women in Europe were affected by overweight or obesity.[2] It is estimated that 10% of global health expenditure is spent on diabetes (USD 760 billion).[1] As such, obesity, type 2 diabetes, and their complications pose an enormous burden on healthcare and on the individual.
In recent years, it has become increasingly clear that susceptibility to obesity and type 2 diabetes is strongly influenced by exposure to an adverse intrauterine environment during early development, which is known as “metabolic programming.” A combination of human epidemiology studies and rodent studies has clearly established that the nutritional status of the mother during pregnancy is a critical factor influencing the development of obesity and type 2 diabetes in offspring. Maternal under-nutrition, as experienced during the Dutch Famine between 1944 and 1945, resulted in the development of obesity in the offspring in adulthood.[3] Our previous studies showed that low birth weight has been associated with an increased risk of obesity, impaired glucose tolerance, and type 2 diabetes.[4–7] Interestingly, we also found maternal obesity is also a risk factor for obesity and diabetes in offspring.[8–12] Thus, gestational diabetes mellitus and maternal obesity can significantly increase the incidence of impaired glucose metabolism in offspring. However, the intervention measure is particularly restricted. Our review aims to demonstrate the evidence linking maternal exercise during critical periods of development and its implications for glucose metabolism in offspring, and then further examine the potential role of epigenetic modifications in this process.
Implications of Human Studies: Maternal Exercise
It is well established that exercise has important health benefits for people with obesity and type 2 diabetes, and regular exercise is one of the most effective strategies to treat type 2 diabetes.[13] Importantly, emerging data in humans suggest that physical exercise during pregnancy can improve the health of offspring in infancy and childhood.[14,15] Moderate exercise during pregnancy is very beneficial to the health of both the mother and the fetus. Several human studies aimed to examine the beneficial effects of maternal exercise on pregnancy outcome and metabolic health in progeny. It showed that exercise during pregnancy protected against the development of hypertension, excessive weight gain, and pre-eclampsia of the mother while also protected against macrosomia as well as low birth weight in the offspring.[15,16] It also indicated that regular exercise during pregnancy can effectively reduce the risks of gestational diabetes in overweight and obese pregnant women.[17,18] These studies indicate that maternal exercise can effectively reduce the occurrence of pregnancy outcome. Maternal exercise also has beneficial effects in progeny. Weight-bearing exercise during pregnancy results in lower body weight of children at age 5, without adverse postnatal effects.[14] Another study recruiting 5125 children found that weight gain during pregnancy significantly increased the risk of obesity in the child at 8 years of age, and proper exercise during pregnancy was effective in reducing body mass index level in child.[19] However, these studies only focused on the effects in childhood, the long-term effects of glucose metabolism is still not clear.
Implications of Rodent Experiments: Maternal Exercise and Glucose Metabolism in Offspring
While these important studies in humans strongly suggest that exercise during pregnancy is important for the health of young offspring, it has not been possible to determine if maternal exercise in humans can result in lower rates of diabetes or obesity in adulthood or middle-age, which is the time point of high-risk for development of metabolic diseases. Therefore, investigations using rodent models have been imperative in delineating the effects of maternal exercise on offspring as they age.
Different intervention timing of maternal exercise
Determining the optimal maternal intervention timing is an important question, which can confer maximal benefits of maternal exercise on metabolic health in offspring. Stanford et al[20] found that maternal high-fat diet during pre-conception and gestation period induce obesity, impaired glucose tolerance, and insulin resistance in offspring mice at 52 weeks of age, while the maternal voluntary wheel running during pre-conception and gestation can significantly reverse these adverse effects. They further explored the effects of maternal exercise at specific time points (pre-conception OR gestation OR pre-conception and gestation) on glucose metabolism in offspring mice. Female mice were randomly divided into four groups: exercise only during pre-conception, exercise only during gestation, exercise during both pre-conception and gestation, and sedentary. Maternal exercise only during pre-conception showed no difference in glucose tolerance of offspring at any age. Maternal exercise only during gestation improved glucose tolerance at a young age. Maternal exercise during both pre-conception and gestation improved glucose tolerance, lowered fasting insulin, and decreased body fat percentage in male offspring compared to all other three groups.[20] Sheldon et al[21] found that maternal exercise only during gestation can reduce body fat percentage and significantly improve high fat diet induced hepatic steatosis in rat offspring at 8 months of age. Maternal exercise before and during pregnancy protects adult mouse offspring from diet-induced obesity.[22] Raipuria et al[23] showed that maternal voluntary wheel running prior to mating and until lactation period decreased the metabolic risks in offspring induced by maternal obesity. These data suggests that different intervention timing of maternal exercise is important for metabolic health in offspring, and current studies suggests that both pre-conception and gestation exercise has more benefits. However, further researches about the optimal intervention timing of maternal exercise on glucose metabolism in offspring is warranted.
Sexual dimorphism in offspring of maternal exercise
Increasing evidence shows that numerous metabolic states can differentially affect male and female.[24] It indicates that the metabolic response to maternal diet may be different in male and female offspring.[25,26] Maternal exercise before and during pregnancy negates the detrimental effects of a maternal high-fat diet on the metabolic health of the male offspring.[20–23] It is imperative to examine sex differences of maternal exercise on glucose metabolism in both male and female offspring. Stanford et al further determined if maternal exercise could attenuate the detrimental effects of maternal high-fat feeding in female offspring. It showed that maternal exercise also confers benefits to female offspring, including decreasing body weight and increasing insulin sensitivity.[27] Raipuria et al[23] found that maternal voluntary wheel running improves insulin and glucose metabolism, with greater effects in male than female offspring. Carter et al[28] found that maternal voluntary exercise before mating and during pregnancy and nursing can improve glucose tolerance (at 30 and 72 weeks of age) in both male and female offspring. However, it can only reduce body fat percentage (at 39 and 68 weeks of age) in male offspring. Carter et al[29] further found that maternal voluntary wheel running can improve insulin sensitivity (at 14 months of age) in female offspring measured by euglycemic-hyperinsulinemic clamps. Eclarinal et al[30] found that maternal exercise before and during pregnancy can increase physical activity in offspring, and this effect was observed earlier in female offspring (at sexual maturation). It also showed that greater fat loss in response to a 3-week voluntary exercise program was achieved in female offspring at 300 days of age. Therefore, it indicates that the metabolic response to maternal exercise may be different in male and female offspring, and most studies indicate that the beneficial effects of maternal exercise on glucose metabolism is greater effects in male, compare with female offspring. However, more studies mainly focused on examining the sexual dimorphism in offspring of maternal exercise are needed.
Different type and intensity of maternal exercise
In addition to voluntary wheel running, treadmill was also commonly used to observe the effects of the maternal exercise on glucose metabolism in the offspring. Quiclet et al[31] showed that maternal treadmill exercise 4 weeks before as well as during gestation at a constant submaximal intensity can reduce body weight, fat content, and increase insulin sensitivity in young adult offspring at 10 weeks of age. The intensity of maternal exercise is also an important factor, which also can influence the glucose metabolism in offspring. It also indicated that maternal treadmill exercise before and during gestation at a submaximal intensity increased insulin secretory capacity in offspring at weaning.[32] Fidalgo et al[33] showed that maternal moderate-low physical training by treadmill can reverse offspring obesity, glucose intolerance, and hypercholesterolemia, caused by maternal protein restriction during gestation and lactation. In addition to voluntary wheel running and treadmill, it showed swim training of dams before and during pregnancy protected adult male offspring from diet-induced obesity and fat gain.[21,22] However, it is difficult to conclude which exercise type or intensity is the best and further studies should be investigated.
Maternal Exercise and Epigenetic Modifications
How a transient stimulus during in utero development, or pre-conception, can have lasting effects manifesting in adult life is a critical question. However, it is likely epigenetic mechanisms of inheritance play a major role [Figure 1]. Epigenetic modifications are labile alterations to DNA (eg, methylation) or DNA structure (eg, histone modifications) or non-coding RNA (eg, microRNA) that influence patterns of gene expression and are inherited from parent cells.[34,35] Interestingly, both exercise and nutrition can influence epigenetic modifications, including those in offspring. Methyl-supplementation of the maternal diet influenced DNA methylation patterns of offspring.[36,37] High fat diet feeding of pregnant mice resulted in hypermethylation of the peroxisome proliferator-activated receptorγcoactivator-1 promoter in skeletal muscle of offspring, an effect that was attenuated by maternal exercise.[38] Other studies have shown that maternal exercise alters gene expression patterns in metabolically important tissues such as liver and skeletal muscle of offspring.[21,22,39] A recent study showed that each additional hour of pre-pregnancy leisure time physical activity duration was associated with hypermethylation in C1orf212 and higher circulating miR-146b-5p. Each additional metabolic equivalent hour of early-pregnancy leisure time physical activity energy expenditure was associated with higher circulating miR-21-3p in women carrying female offspring, and lower circulating miR-146b-5p and miR-517-5pin women carrying male offspring.[40] Thus, it suggests the potential role of epigenetic inheritance in transmission of maternal exercise benefits in offspring, and it can facilitate a virtuous cycle of metabolic health, propagating benefits to subsequent generations [Figure 1].
Figure 1.
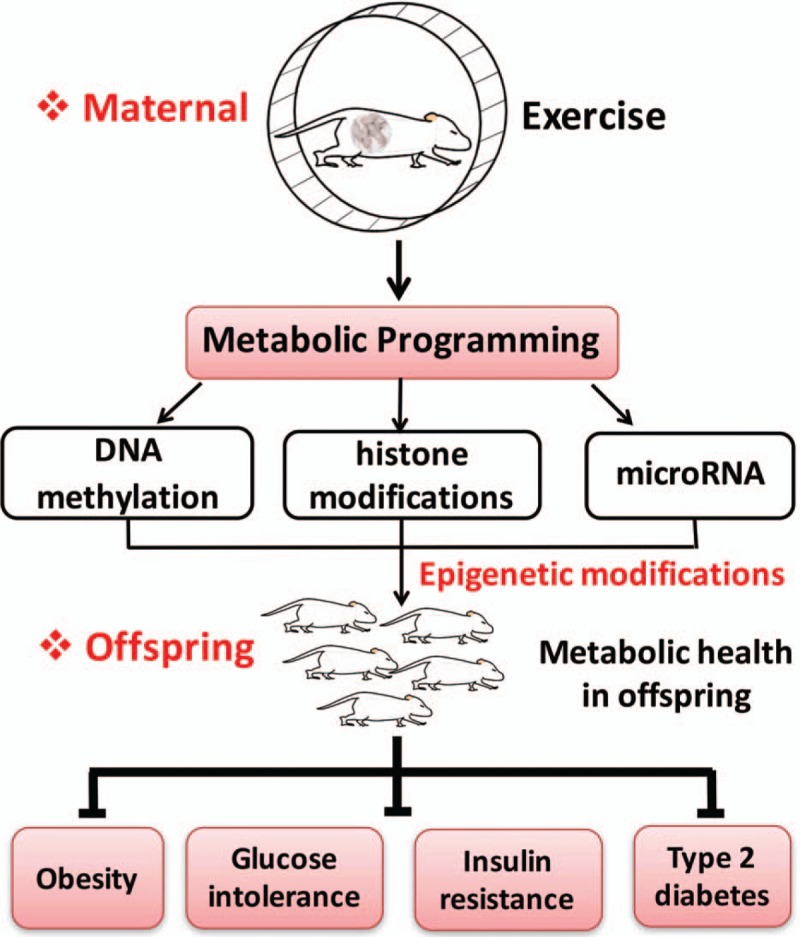
Maternal exercise and its beneficial effects on metabolic health in offspring. “Metabolic programming” indicates that maternal exercise can significantly decrease the susceptibility of obesity, glucose intolerance, insulin resistance, and type 2 diabetes in offspring. It is likely epigenetic mechanisms of inheritance play a major role, including DNA methylation, histone modifications, and non-coding RNA (eg, microRNA). This can facilitate a virtuous cycle of metabolic health, propagating benefits to subsequent generations.
Conclusions
Pre-conception and pregnancy are the critical periods of growth and development of humans, which are susceptible to unhealthy environment. Our study indicates that maternal exercise significantly improves the metabolic health of offspring, and raising the possibility that these effects are regulated by epigenetic mechanisms. The increasing rates of obesity and diabetes in individuals of reproductive age can initiate a vicious cycle, propagating risks to subsequent generations. The cycle of metabolic risk transmission could be greatly reduced if mothers knew they could improve their children's health by exercising before and during pregnancy. Further researches about the role and mechanism of maternal exercise on metabolic health in offspring is warranted, which can provide critical implications for the early prevention of obesity and diabetes, and ensure a healthier future for subsequent generations. It may also promote the translation to humans about the potential clinical implications of maternal exercise.
Funding
This study was supported by grants from the National Key R&D Program of China (No. 2017YFC1309603), National Key Research and Development Program of China (Nos. 2016YFA0101002 and 2018YFC2001100), National Natural Science Foundation of China (Nos. 81170736, 81570715, 81870579, and 81800703), Beijing Natural Science Foundation (Nos. 7202163 and 7184252), Medical Epigenetics Research Center, Chinese Academy of Medical Sciences (Nos. 2017PT31036, 2018PT31021), Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (No. CIFMS2017-I2M-1-008), the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (Nos. 2017PT32020 and 2018PT32001), China Diabetes Young Scientific Talent Research Project and Bethune-Merck Diabetes Research Fund of Bethune Charitable Foundation.
Conflicts of interest
None.
Footnotes
How to cite this article: Zheng J, Zhou LY, Xiao XH. Maternal exercise and its beneficial effects on glucose metabolism in offspring. Chin Med J 2020;133:863–867. doi: 10.1097/CM9.0000000000000731
References
- 1.International Diabetes Federation. IDF Diabetes Atlas, 9th edn. Brussels, Belgium: 2019. Available from: http://www.diabetesatlas.org/ [Accessed December 3, 2019] [Google Scholar]
- 2.World Health Organization. Obesity and Overweight. 2016. Available from: https://www.who.int/gho/ncd/risk_factors/overweight/en/ [Accessed December 3, 2019] [Google Scholar]
- 3.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 1976; 295:349–353. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 4.Xiao X, Zhang ZX, Cohen HJ, Wang H, Li W, Wang T, et al. Evidence of a relationship between infant birth weight and later diabetes and impaired glucose regulation in a Chinese population. Diabetes Care 2008; 31:483–487. doi: 10.2337/dc07-1130. [DOI] [PubMed] [Google Scholar]
- 5.Xiao X, Zhang ZX, Li WH, Feng K, Sun Q, Cohen HJ, et al. Low birth weight is associated with components of the metabolic syndrome. Metabolism 2010; 59:1282–1286. doi: 10.1016/j.metabol.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng J, Xiao X, Zhang Q, Wang T, Yu M, Xu J. Maternal low-protein diet modulates glucose metabolism and hepatic microRNAs expression in the early life of offspring dagger. Nutrients 2017; 9:E205.doi: 10.3390/nu9030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng J, Xiao X, Zhang Q, Yu M, Xu J, Qi C, et al. The programming effects of nutrition-induced catch-up growth on gut microbiota and metabolic diseases in adult mice. Microbiologyopen 2016; 5:296–306. doi: 10.1002/mbo3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr 2005; 146:693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 9.Rando OJ, Simmons RA. I’m eating for two: parental dietary effects on offspring metabolism. Cell 2015; 161:93–105. doi: 10.1016/j.cell.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel N, Pasupathy D, Poston L. Determining the consequences of maternal obesity for offspring health. Exp Physiol 2015; 100:1421–1428. doi: 10.1113/EP085132. [DOI] [PubMed] [Google Scholar]
- 11.Zheng J, Zhang Q, Mul JD, Yu M, Xu J, Qi C, et al. Maternal high-calorie diet is associated with altered hepatic microRNA expression and impaired metabolic health in offspring at weaning age. Endocrine 2016; 54:70–80. doi: 10.1007/s12020-016-0959-9. [DOI] [PubMed] [Google Scholar]
- 12.Zheng J, Xiao X, Zhang Q, Yu M, Xu J, Wang Z, et al. Maternal and post-weaning high-fat, high-sucrose diet modulates glucose homeostasis and hypothalamic POMC promoter methylation in mouse offspring. Metab Brain Dis 2015; 30:1129–1137. doi: 10.1007/s11011-015-9678-9. [DOI] [PubMed] [Google Scholar]
- 13.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clapp JF., 3rd Morphometric and neurodevelopmental outcome at age five years of the offspring of women who continued to exercise regularly throughout pregnancy. J Pediatr 1996; 129:856–863. doi: 10.1016/s0022-3476(96)70029-x. [DOI] [PubMed] [Google Scholar]
- 15.Barakat R, Pelaez M, Cordero Y, Perales M, Lopez C, Coteron J, et al. Exercise during pregnancy protects against hypertension and macrosomia. Randomized Clinical Trial. Am J Obstet Gynecol 2015; 214:649.e1–649.e8. doi: 10.1016/j.ajog.2015.11.039. [DOI] [PubMed] [Google Scholar]
- 16.Perales M, Artal R, Lucia A. Exercise during pregnancy. JAMA 2017; 317:1113–1114. doi: 10.1001/jama.2017.0593. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Wei Y, Zhang X, Zhang Y, Xu Q, Sun Y, et al. A randomized clinical trial of exercise during pregnancy to prevent gestational diabetes mellitus and improve pregnancy outcome in overweight and obese pregnant women. Am J Obstet Gynecol 2017; 216:340–351. doi: 10.1016/j.ajog.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 18.Wang C, Wei Y, Zhang X, Zhang Y, Xu Q, Su S, et al. Effect of regular exercise commenced in early pregnancy on the incidence of gestational diabetes mellitus in overweight and obese pregnant women: a randomized controlled trial. Diabetes Care 2016; 39:e163–164. doi: 10.2337/dc16-1320. [DOI] [PubMed] [Google Scholar]
- 19.Mourtakos SP, Tambalis KD, Panagiotakos DB, Antonogeorgos G, Arnaoutis G, Karteroliotis K, et al. Maternal lifestyle characteristics during pregnancy, and the risk of obesity in the offspring: a study of 5,125 children. BMC Pregnancy Childbirth 2015; 15:66.doi: 10.1186/s12884-015-0498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stanford KI, Lee MY, Getchell KM, So K, Hirshman MF, Goodyear LJ. Exercise before and during pregnancy prevents the deleterious effects of maternal high-fat feeding on metabolic health of male offspring. Diabetes 2015; 64:427–433. doi: 10.2337/db13-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheldon RD, Nicole Blaize A, Fletcher JA, Pearson KJ, Donkin SS, Newcomer SC, et al. Gestational exercise protects adult male offspring from high-fat diet-induced hepatic steatosis. J Hepatol 2016; 64:171–178. doi: 10.1016/j.jhep.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wasinski F, Bacurau RF, Estrela GR, Klempin F, Arakaki AM, Batista RO, et al. Exercise during pregnancy protects adult mouse offspring from diet-induced obesity. Nutr Metab (Lond) 2015; 12:56.doi: 10.1186/s12986-015-0052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raipuria M, Bahari H, Morris MJ. Effects of maternal diet and exercise during pregnancy on glucose metabolism in skeletal muscle and fat of weanling rats. PLoS One 2015; 10:e0120980.doi: 10.1371/journal.pone.0120980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clayton JA, Collins FS. Policy: NIH to balance sex in cell and animal studies. Nature 2014; 509:282–283. doi: 10.1038/509282a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleinert M, Clemmensen C, Hofmann SM, Moore MC, Renner S, Woods SC, et al. Animal models of obesity and diabetes mellitus. Nat Rev Endocrinol 2018; 14:140–162. doi: 10.1038/nrendo.2017.161. [DOI] [PubMed] [Google Scholar]
- 26.Samuelsson AM, Morris A, Igosheva N, Kirk SL, Pombo JM, Coen CW, et al. Evidence for sympathetic origins of hypertension in juvenile offspring of obese rats. Hypertension 2010; 55:76–82. doi: 10.1161/hypertensionaha.109.139402. [DOI] [PubMed] [Google Scholar]
- 27.Stanford KI, Takahashi H, So K, Alves-Wagner AB, Prince NB, Lehnig AC, et al. Maternal exercise improves glucose tolerance in female offspring. Diabetes 2017; 66:2124–2136. doi: 10.2337/db17-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter LG, Lewis KN, Wilkerson DC, Tobia CM, Ngo Tenlep SY, Shridas P, et al. Perinatal exercise improves glucose homeostasis in adult offspring. Am J Physiol Endocrinol Metab 2012; 303:E1061–E1068. doi: 10.1152/ajpendo.00213.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carter LG, Qi NR, De Cabo R, Pearson KJ. Maternal exercise improves insulin sensitivity in mature rat offspring. Med Sci Sports Exerc 2013; 45:832–840. doi: 10.1249/MSS.0b013e31827de953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eclarinal JD, Zhu S, Baker MS, Piyarathna DB, Coarfa C, Fiorotto ML, et al. Maternal exercise during pregnancy promotes physical activity in adult offspring. Faseb J 2016; 30:2541–2548. doi: 10.1096/fj.201500018R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quiclet C, Dubouchaud H, Berthon P, Sanchez H, Vial G, Siti F, et al. Maternal exercise modifies body composition and energy substrates handling in male offspring fed a high-fat/high-sucrose diet. J Physiol 2017; 595:7049–7062. doi: 10.1113/jp274739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quiclet C, Siti F, Dubouchaud H, Vial G, Berthon P, Fontaine E, et al. Short-term and long-term effects of submaximal maternal exercise on offspring glucose homeostasis and pancreatic function. Am J Physiol Endocrinol Metab 2016; 311:E508–E518. doi: 10.1152/ajpendo.00126.2016. [DOI] [PubMed] [Google Scholar]
- 33.Fidalgo M, Falcao-Tebas F, Bento-Santos A, de Oliveira E, Nogueira-Neto JF, de Moura EG, et al. Programmed changes in the adult rat offspring caused by maternal protein restriction during gestation and lactation are attenuated by maternal moderate-low physical training. Br J Nutr 2013; 109:449–456. doi: 10.1017/s0007114512001316. [DOI] [PubMed] [Google Scholar]
- 34.Ling C, Ronn T. Epigenetics in human obesity and type 2 diabetes. Cell Metab 2019; 29:1028–1044. doi: 10.1016/j.cmet.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radford EJ. Exploring the extent and scope of epigenetic inheritance. Nat Rev Endocrinol 2018; 14:345–355. doi: 10.1038/s41574-018-0005-5. [DOI] [PubMed] [Google Scholar]
- 36.Jin C, Zhuo Y, Wang J, Zhao Y, Xuan Y, Mou D, et al. Methyl donors dietary supplementation to gestating sows diet improves the growth rate of offspring and is associating with changes in expression and DNA methylation of insulin-like growth factor-1 gene. J Anim Physiol Anim Nutr (Berl) 2018; 102:1340–1350. doi: 10.1111/jpn.12933. [DOI] [PubMed] [Google Scholar]
- 37.McKee SE, Zhang S, Chen L, Rabinowitz JD, Reyes TM. Perinatal high fat diet and early life methyl donor supplementation alter one carbon metabolism and DNA methylation in the brain. J Neurochem 2018; 145:362–373. doi: 10.1111/jnc.14319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laker RC, Lillard TS, Okutsu M, Zhang M, Hoehn KL, Connelly JJ, et al. Exercise prevents maternal high-fat diet-induced hypermethylation of the Pgc-1alpha gene and age-dependent metabolic dysfunction in the offspring. Diabetes 2014; 63:1605–1611. doi: 10.2337/db13-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barres R, Yan J, Egan B, Treebak JT, Rasmussen M, Fritz T, et al. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab 2012; 15:405–411. doi: 10.1016/j.cmet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Badon SE, Littman AJ, Chan KCG, Tadesse MG, Stapleton PL, Bammler TK, et al. Physical activity and epigenetic biomarkers in maternal blood during pregnancy. Epigenomics 2018; 10:1383–1395. doi: 10.2217/epi-2017-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]


