Abstract
Background:
Almost one-quarter of all new HIV diagnoses in the US occur among persons ages 13-24 years. These youths have the poorest HIV Care Continuum (HCC) outcomes, yet few empirical youth-specific data are available.
Methods:
The Strategic Multisite Initiative for the Identification, Linkage and Engagement in Care of HIV-infected youth (SMILE) helped HIV-infected (mostly newly-diagnosed) youth, ages 12-24 years, link to youth-friendly care, and evaluated each milestone of the HCC (10/2012-09/2014). Numbers of HIV-infected youth referred, linked, engaged and retained in care were recorded, along with socio-demographics. Viral suppression (VS) was defined as ≥ 1 HIV viral load (VL) below the level of detection (BLD) on study. Correlates of VS were examined using Cox Proportional Hazards models.
Results:
Among 1411 HIV-infected youth, 1053 (75%) were linked, 839 (59%) engaged and 473 (34%) retained in care at adolescent healthcare sites. Antiretroviral therapy (ART) was initiated among 474 (34%) and 166 (12%) achieved VS. Predictors of VS included lower VL at baseline [aHR 1.56 (95%CI:1.32-1.89), p<0.0001], recent ART receipt [aHR 3.10 (95%CI:1.86-5.18), p<0.0001], and shorter time from HIV testing until referral to linkage coordinator [aHR 2.52 (95%CI:1.50-4.23), p=0.0005 for 7 days to 6 weeks and aHR 2.08 (95%CI:1.08-4.04), p=0.0294 for 6 weeks to 3 months compared to >3 months].
Conclusions:
Whereas this large national sample of predominately newly-diagnosed youths linked to care at similar rates as adults, they achieved disproportionately lower rates of VS. Prompt referral to youth-friendly linkage services was an independent predictor of VS. Youth-focused interventions are urgently needed to improve their HCC outcomes.
Keywords: Adolescents, HIV infection, HIV Care Continuum
INTRODUCTION
Adolescents and young adults from racial, ethnic, sexual and gender minority communities bear a disproportionate burden of HIV among youth in the United States. Recent estimates show that youth make up 21% of all new HIV diagnoses; however, nearly half of all youth living with HIV are not aware they are infected.1,2 Moreover, over the past decade or so, there have been disproportionate rates of new HIV diagnoses among racial and ethnic minority young men who have sex with men (YMSM).3 Providers for HIV-infected adolescents and young adults face substantial challenges in identifying those who are undiagnosed, linking and engaging them in care, and in helping HIV-infected youth achieve adequate adherence to their antiretroviral medications to realize health benefits.4 Given recent studies suggesting that prompt HIV treatment is important for health, survival,5,6 and the prevention of HIV transmission to others,7 tracking viral suppression has become increasingly important.
The HIV care continuum (HCC) captures important information about how well HIV-infected individuals accessed and adhered to healthcare, including antiretroviral treatment and critical support services to manage their disease.8,9 It allows HIV providers, researchers and policy makers to better understand the challenges to and facilitators of successful care outcomes. This can provide insights into what interventions might be necessary and where they should be targeted to improve health outcomes and reduce onward transmission of HIV. Whereas the HCC has been extensively studied in adults with HIV infection, little research has addressed the continuum among vulnerable populations of HIV-infected US youth who face enormous psychosocial challenges, disparities and health inequities.10 Such realities are addressed in the national HIV prevention goals.11
The Strategic Multisite Initiative for the Identification, Linkage and Engagement in Care of Youth with Undiagnosed HIV Infection (SMILE) was a collaboration between the National Institute of Child Health and Human Development (NICHD), the Centers for Disease Control and Prevention (CDC), the Health Services and Resources Administration (HRSA) and the NICHD-funded Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN) which aimed to comprehensively address multiple objectives of the national strategy for our nation’s youth. These included the reduction of new HIV infections, improvement in access to care, reduction of disparities and health inequities, and establishment of collaborative efforts across the Federal government agencies to coordinate the response to the domestic HIV epidemic.11
The HCC has been previously described for US youth using national surveillance data, yet the estimates have changed, in part due to evolving methodologies and data sources,12,13 relying on extrapolations to identify the proportions of youth to successfully achieve each milestone across the continuum.10 We present the empiric results from the first large scale systematic evaluation of the US HCC for adolescents and young adults ages 12-24 years, most of whom were newly diagnosed and new to care, using primary data from 13 major metropolitan areas.
METHODS
The SMILE Collaborative
The SMILE collaborative, previously described,14–16 supported at each ATN site a linkage to care coordinator experienced in working with adolescents. Their job was to facilitate a smooth transition from HIV testing centers where youth were diagnosed to clinical care services, focusing on linkage to and retention in the local ATN clinical sites.
Study Population
ATN 116 was the research study that evaluated the SMILE collaborative and was implemented between October 2012 and February 2015. The numbers of HIV-infected youth between ages 12 and 24 years, inclusive, who were referred for linkage services, linked to care (LTC), engaged in care (EIC), and retained in care (RIC) during defined intervals§ (Figure 1), were abstracted from clinical records, along with socio-demographics. Biomedical data obtained at the LTC visit were defined as the baseline evaluations for the purposes of this analysis.
Figure 1.
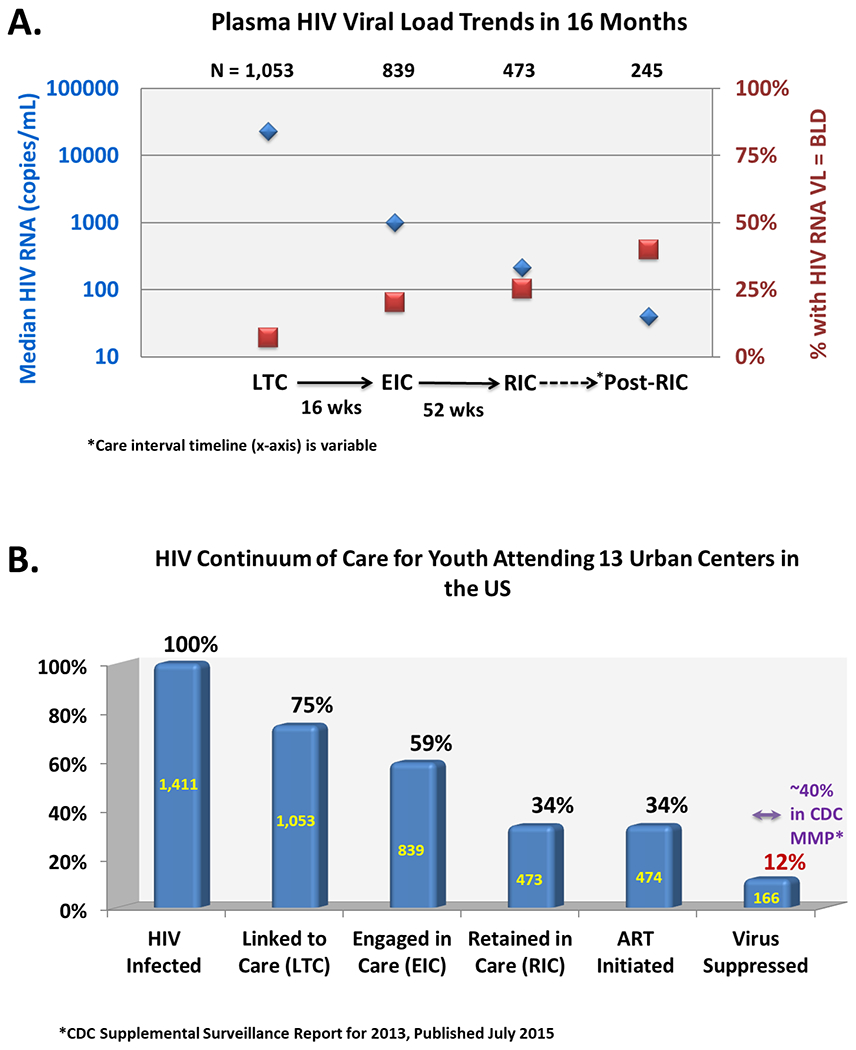
A) Proportions of youth who achieved HIV viral load suppression below the assay limit of detection at each succesive care interval after linkage and through retention, and B) the youth-specific HIV care continuum among adolescent and young adult participants attending 13 urban US centers of the NICHD-ATN-CDC-HRSA SMILE Collaborative. Care intervals along the HIV Care Continuum were defined for LTC: a clinical visit attended no later than 42 days after referral for linkage; EIC: at least one additional clinical visit attended no later than 16 weeks after LTC; RIC: at least one additional clinical visit attended no later than 52 weeks after EIC; and, *Post-RIC: a second clinical visit attended within 52 weeks after EIC, if the RIC visit occured early in the interval.
Statistical Analysis
Bivariate tests were performed using the Chi-square (or Fisher’s exact) test for categorical indicators and t-test (or nonparametric test) for continuous indicators. Viral suppression was defined as a participant having achieved a VL below the level of detection (BLD), after baseline, from at least one study visit during the SMILE participation period. Time to viral suppression was defined as the number of days from baseline to the first visit when the VL was BLD. Viral suppression was also separately calculated as the proportion of youth who achieved a VL BLD for two or more visits after baseline and those achieving a VL BLD for the last available measurement on study; however, these approaches were not used in multivariable analysis.
Study participants who never achieved BLD on any VL during the study were censored at their last available study visit. Univariate and multivariable Cox Proportional Hazards models were used to examine the covariates potentially predicting VL suppression. These included socio-demographic characteristics, risk behavior, antiretroviral therapy (ART), healthcare utilization, and ATN site data. ATN site data included whether or not there was a formal agreement between the health department and the SMILE program, specifically to provide patient identifying information to the SMILE coordinator who would then assist with linking youth to care. These agreements were categorized as formal (complete data sharing), informal or limited (to include de-identified data on a monthly basis), or no/other data sharing agreement, and they varied by jurisdiction depending on local regulation. Graphical approaches and time-dependent covariates were used to check the proportional hazard assumptions. All covariates with an unadjusted p-value of <0.15 were tested in the full multivariable model and those with an overall Type III p-value <0.05 were retained in the final model. Youth with HIV identified by SMILE but who confirmed receiving care elsewhere were excluded from this analysis.
RESULTS
1548 HIV-infected youth, ages 12 to 24 years, were identified at 13 ATN sites through SMILE between October 2012 and September 2014. Among those referred, 137 (9%) were already in care elsewhere and were excluded from further analysis. Among the remaining 1411 youth, 358 (25%) failed to link to care. Major reasons for LTC failure were insufficient contact information sharing from the testing center or other inability to locate youth (32%), youth’s refusal of LTC (11%), repeated failure to attend appointments (34%) and residing outside of jurisdiction (11%).
Among 733 participants with biomedical data at baseline, the mean age was 20.6 ± 2.3 years, most were males (80%) and non-Hispanic black (72%) and 173 (24%) had previously been in care but had been lost to follow-up and were re-linked (Table 1). Sixty four percent identified as gay, bisexual or questioning. The median HIV VL at first study visit was 23,234 copies/ml (range: BLD-107) and mean CD4 T cell count was 463 cells/µl (σ: 252), with almost 2/3 demonstrating advanced HIV with viremia > 10,000 copies/ml and CD4 T cell counts < 350 cells/µl. Male participants had a significantly higher median HIV VL compared to female and transgender youth at 30,860, 6,845 and 9,625 copies/ml, respectively (p<0.0001).
Table 1.
Clinical Characteristics of SMILE Participants with Biomedical Data
| N* | Total** | %, (SD), (Range) | |
|---|---|---|---|
| Mean age (years) (SD) | 733 | 20.6 | (2.3) |
| Birth gender | 732 | ||
| Male | 589 | 80% | |
| Female | 143 | 20% | |
| Current gender | 727 | ||
| Male | 569 | 78% | |
| Female | 148 | 20% | |
| Transgender (M to F) | 10 | 1% | |
| Sexual orientation | 729 | ||
| Heterosexual | 198 | 27% | |
| Bisexual | 69 | 9% | |
| Homosexual | 395 | 54% | |
| Questioning/Unsure | 7 | 1% | |
| Declined to answer | 60 | 8% | |
| Race/Ethnicity | 701 | ||
| Non-Hispanic white | 42 | 6% | |
| Non-Hispanic black | 507 | 72% | |
| Hispanic | 123 | 18% | |
| Other | 29 | 4% | |
| Mode of HIV acquisition | 727 | ||
| Perinatal | 47 | 6% | |
| Heterosexual Contact | 38 | 5% | |
| Injection Drug Use (IDU) | 3 | 0.4% | |
| Male to Male Sexual (MSM) Contact | 455 | 63% | |
| MSM Contact and IDU | 12 | 2% | |
| Uncertain / Unknown | 126 | 17% | |
| Declined to answer | 46 | 6% | |
| Received medical care for HIV before | 730 | ||
| Yes | 173 | 24% | |
| No | 471 | 65% | |
| Declined to answer | 86 | 12% | |
| Received ART at most recent visit | 733 | ||
| Yes | 115 | 16% | |
| No | 618 | 84% | |
| Baseline viral load (copies /mL) | 733 | ||
| Mean (SD) | 152,217 | (743,971) | |
| Median (Range) | 23,234 | (5-107) | |
| Baseline CD4 cell count (cells/mL) | 733 | ||
| Mean (SD) | 463.2 | (251.9) | |
| Median (Range) | 422 | (2–1,530) | |
| Used ≥ 1 substance in past 6 months*** | 732 | ||
| Yes | 383 | 52% | |
| No | 349 | 48% | |
| Had sex with an anonymous partner in past 6 months | 732 | ||
| Yes | 91 | 12% | |
| No | 641 | 88% | |
| Engaged in transactional sex | 732 | ||
| Yes | 27 | 4% | |
| No | 705 | 96% | |
| Had ≥ 1 STI in past 6 months | 732 | ||
| Yes | 182 | 25% | |
| No | 550 | 75% | |
N = 733 SMILE participants with available biomedical data (at least a single viral load and CD4 count available at LTC (Baseline)); may differ for some variables based on missing data.
Includes youth linked to care only, youth who linked and went on to complete the other stages of the COC, and who had at least a single viral load and CD4 count available at LTC (Baseline).
Substances assessed included alcohol, barbiturates, club drugs, hallucinogens, inhalants, sedative hypnotics, amphetamines, cannabis, cocaine, heroin, methadone, other opiates and street hormones.
The median HIV VL of the cohort decreased as individuals reached successive milestones of the HCC (p<0.0001), along with increases in the proportion of those at each milestone whose HIV VL was below the assay limit of detection (p<0.0001) (Figure 1a). Of the 1411 HIV-infected youth referred to the ATN LTC coordinator, 1053 (75%) were linked to care. Of those linked to care, 839 (80%) engaged in care and 473 (45%) were retained in care at an ATN site. ART was initiated among 474 of the 1053 adolescents who linked to care (45%); of those, 166 (35%) achieved viral suppression at least once during the study period and 152 (32%) had a suppressed VL at the last available measurement on study. Among these 166 youth, 114 (69%) achieved viral suppression during only one visit, an additional 52 (31%) achieved suppression for 2 or more visits. The HCC is shown in Figure 1b using the total sample of HIV-infected youth referred for care as the denominator. Among this group of predominantly newly diagnosed youth, 75% were linked to care, 59% were engaged in care, 34% each were retained in care and started on ART, and 12% achieved viral suppression after a median follow-up of 4.8 months.
The unadjusted relationship of socio-demographics, risk behavior, ART, healthcare utilization, and data sharing agreements (between ATN sites and local public health authorities) to viral suppression outcomes are shown in Table 2. There was a positive relationship (HR: 95% CI; p value) between viral suppression and recent receipt of ART (2.54: 1.59-4.05; <0.001), lower VL at LTC (1.33: 1.15-1.56; 0.0002), and access to case management services provided by non-ATN staff (1.67: 1.02-2.73; 0.04) and a negative relationship with recent substance use (0.68: 0.48-0.95; 0.024). In addition, relative to having a formal data sharing plan, having a limited plan with sharing of de-identified information only [3.21: 1.80-5.71; <0.001] or having informal or no data sharing plans [2.88: 1.67-4.99; 0.002]) were associated with viral suppression; as was having a longer time between the EIC visit and the RIC visit (specifically, when the RIC visit was between 6 months to 1 year from the EIC visit), compared to a shorter time period (1 to 3 months [3.37: 1.23-9.19; 0.018]).
Table 2.
Relationship of Viral Suppression with Risk Behavior, HIV, and LTC Case Management Related Characteristics, SMILE Collaborative, 2012-2015
| Total | BLD | >BLD | Unadjusted | Adjusted | |||
|---|---|---|---|---|---|---|---|
| N=453 | N=135 | N=318 | |||||
| n (col %) | n (row %) | n (row %) | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Used ≥ 1 substance in past 6 months* | |||||||
| Yes | 245 (54.1) | 67 (27.3) | 178 (72.7) | 0.68 (0.48 - 0.95) | 0.0237 | ||
| No | 208 (45.9) | 68 (32.7) | 140 (67.3) | 1 | |||
| Had sex with an anonymous partner in past 6 months | |||||||
| Yes | 65 (14.3) | 25 (38.5) | 40 (61.5) | 1.49 (0.97 - 2.31) | 0.0719 | ||
| No | 388 (85.7) | 110 (28.4) | 278 (71.6) | 1 | |||
| Engaged in transactional sex | |||||||
| Yes | 16 (3.5) | 7 (43.8) | 9 (56.3) | 2.06 (0.95 - 4.43) | 0.0657 | ||
| No | 437 (96.5) | 128 (29.3) | 309 (70.7) | 1 | |||
| Received medical care for HIV before | |||||||
| Yes | 92 (22.3) | 13 (14.1) | 79 (85.9) | 1 | |||
| No | 321 (77.7) | 109 (34.0) | 212 (66.0) | 1.56 (0.88 - 2.79) | 0.1278 | ||
| Receiving ART at most recent visit | |||||||
| Yes | 281 (62.0) | 114 (40.6) | 167 (59.4) | 2.54 (1.59 - 4.05) | <.0001 | 3.10 (1.86 - 5.18) | <.0001 |
| No | 172 (38.0) | 21 (12.2) | 151 (87.8) | 1 | 1 | ||
| Baseline viral load (Log10) | |||||||
| Continuous (decrease) | 442 (100.0) | 130 (29.4) | 312 (70.6) | 1.33 (1.15 – 1.56) | 0.0002 | 1.56 (1.32 – 1.89) | <.0001 |
| Person conducting LTC activities | |||||||
| LTC OW | 374 (82.7) | 112 (29.9) | 262 (70.1) | 1 | |||
| Other ATN staff | 16 (3.5) | 4 (25.0) | 12 (75.0) | 1.25 (0.46 - 3.39) | 0.6627 | ||
| Non-ATN staff | 62 (13.7) | 19 (30.6) | 43 (69.4) | 1.67 (1.02 - 2.73) | 0.0401 | ||
| Data sharing plan | |||||||
| Formal data sharing | 98 (21.6) | 16 (16.3) | 82 (83.7) | 1 | 1 | ||
| Limited, Sharing of de-identified information monthly | 141 (31.1) | 47 (33.3) | 94 (66.7) | 3.21 (1.80 - 5.71) | <.0001 | 2.33 (1.22 - 4.47) | 0.0106 |
| No formal, informal, no data sharing, and others | 214 (47.2) | 72 (33.6) | 142 (66.4) | 2.88 (1.67 - 4.99) | 0.0002 | 2.78 (1.51 - 5.11) | 0.0010 |
| Region | |||||||
| 1=Northeast | 34 (7.5) | 14 (41.2) | 20 (58.8) | 1 | |||
| 2=Midwest | 60 (13.2) | 15 (25.0) | 45 (75.0) | 0.51 (0.24 - 1.06) | 0.0718 | ||
| 3=South | 305 (67.3) | 91 (29.8) | 214 (70.2) | 0.79 (0.45 - 1.39) | 0.4092 | ||
| 4=West | 54 (11.9) | 15 (27.8) | 39 (72.2) | 0.51 (0.24 - 1.05) | 0.0687 | ||
| Care interval** | |||||||
| LTC | 12 (2.7) | 1 (8.3) | 11 (91.7) | 1 | 1 | ||
| EIC | 118 (26.2) | 24 (20.3) | 94 (79.7) | 2.16 (0.29 - 16.00) | 0.4521 | 2.30 (0.31 - 17.16) | 0.4175 |
| RIC | 121 (26.8) | 34 (28.1) | 87 (71.9) | 1.40 (0.19 - 10.24) | 0.7406 | 1.53 (0.21 - 11.37) | 0.6757 |
| Post-RIC | 200 (44.3) | 76 (38.0) | 124 (62.0) | 0.50 (0.07 - 3.67) | 0.4974 | 0.49 (0.07 - 3.68) | 0.4882 |
| Time from HIV testing to referral | |||||||
| 0-7 days | 111 (26.2) | 34 (30.6) | 77 (69.4) | 1.17 (0.69 - 1.99) | 0.5569 | 1.64 (0.93 - 2.91) | 0.0883 |
| >7 days to 6 weeks | 146 (34.4) | 50 (34.2) | 96 (65.8) | 1.64 (1.00 - 2.69) | 0.0495 | 2.52 (1.50 - 4.23) | 0.0005 |
| >6 weeks to 3 months | 54 (12.7) | 19 (35.2) | 35 (64.8) | 1.49 (0.81 - 2.75) | 0.1960 | 2.08 (1.08 - 4.04) | 0.0294 |
| >3 months | 113 (26.7) | 23 (20.4) | 90 (79.6) | 1 | 1 | ||
| Time from referral to LTC | |||||||
| Same day (0 day) | 66 (14.6) | 17 (25.8) | 49 (74.2) | 1 | |||
| 1-7 days | 118 (26.2) | 39 (33.1) | 79 (66.9) | 0.90 (0.51 - 1.59) | 0.7110 | ||
| >7 days to <=42 days | 260 (57.6) | 77 (29.6) | 183 (70.4) | 0.84 (0.49 - 1.42) | 0.5129 | ||
| >42 days | 7 (1.6) | 2 (28.6) | 5 (71.4) | 0.75 (0.17 - 3.28) | 0.7054 | ||
| Time from EIC to RIC | |||||||
| 30 days to 3 months | 277 (81.0) | 98 (35.4) | 179 (64.6) | 1 | |||
| >3 months to 6 months | 56 (16.4) | 14 (25.0) | 42 (75.0) | 0.87 (0.49 - 1.55) | 0.6457 | ||
| >6 months to 1 year | 9 (2.6) | 4 (44.4) | 5 (55.6) | 3.37 (1.23 - 9.19) | 0.0178 | ||
BLD: Below limit of detection for the assay being used; LTC: linked to care; EIC: engaged in care; RIC: retained in care; LTC OW: linkage to care outreach worker, also referred to as LTC or linkage coordinator; ATN: Adolescent Medicine Trials Network for HIV/AIDS Interventions; ART: antiretroviral therapy
Substances assessed included alcohol, barbiturates, club drugs, hallucinogens, inhalants, sedative hypnotics, amphetamines, cannabis, cocaine, heroin, methadone, other opiates and street hormones.
Care intervals along the HIV Care Continuum were defined for LTC: a clinical visit attended no later than 42 days after referral for linkage; EIC: at least one additional clinical visit attended no later than 16 weeks after LTC; RIC: at least one additional clinical visit attended no later than 52 weeks after EIC; and, *Post-RIC: a second clinical visit attended within 52 weeks after EIC, if the RIC visit occured early in the interval.
LTC = Linked to Care; VL = Viral Load; STI = Sexually transmitted infection
Independent predictors (aHR: 95% CI; p value) of viral suppression from the final multivariable model are also shown in Table 2. For each log10 unit decrease in VL at LTC, there was an approximately 1.6-fold increase in the likelihood of viral suppression (1.56: 1.32-1.89; <0.0001). Additional independent predictors were recent receipt of ART (3.10: 1.86-5.18; <0.0001) and either limited data sharing plans between testing centers and care providers (2.33: 1.22-4.47; 0.0106) or no formal/other sharing (2.78: 1.51-5.11; 0.001). Finally, compared to those for whom referral to care took more than 3 months from time of testing, those referred to care in shorter periods of time had twice the odds of achieving viral suppression (2.52: 1.50-4.23; 0.0005 for 1-6 weeks; 2.08: 1.08-4.04; 0.0294 for 6 weeks to 3 months). Repeating this analysis among the subset of newly diagnosed youth yielded similar results (data not shown).
DISCUSSION
This multiagency SMILE collaborative demonstrated that a large national sample of HIV-infected youth who were not in care, most with new HIV diagnoses, had high levels of plasma viremia and advanced infection at presentation. These are consistent with earlier findings17 and highlight the urgency of addressing the high proportion of undiagnosed HIV-infected youth. While youth linked to HIV care at similar rates as adults once they were diagnosed,18,19 they achieved disproportionately lower rates of viral suppression, a major contributor to morbidity and secondary transmission events.20,21 Prompt referral to youth-friendly LTC services after HIV testing independently predicted VL suppression. These findings have implications for disease progression and transmission potential among this vulnerable group.
Youth exposed to the SMILE project appeared to achieve higher rates of LTC relative to contemporaneously measured national levels.22–24 Due to the evolution in measures and indicators during the program, exact comparisons to national continuum of care data are challenging. When SMILE began in 2010, the national indicator for LTC was 3 months from time of diagnosis.12 SMILE’s 42-day milestone reflected an ambitious target consistent with emerging findings on the benefits of rapid linkage and early treatment. The national indicator was subsequently changed to 1 month,22 now slightly shorter than the SMILE period but closer than the prior 3 month indicator. The overall 75% LTC rate in SMILE compared favorably to the 2013, 2014 and 2015 national 1-month LTC rates of 66%, 67.5%, and 70.5%, respectively.22–24
Despite the high LTC rate, similar to that among HIV diagnosed adults,18,19 SMILE youth were less likely to achieve viral suppression.** Across the HCC , the proportions of persons 18-24 years linked to care, prescribed ART and virally suppressed among those with an HIV diagnosis in 2012 were 41%, 33.5% and 26%, respectively in national data22 while in SMILE, these numbers were 75%, 34% and 12% showing a substantial improvement in LTC, similar ART rates, but lower viral suppression among these harder to engage youth, who without SMILE may not have been in care. This low rate of viral suppression across the HCC may partly reflect that only 45% of those with HIV who linked to care initiated ART, which may suggest that some youth had prescriptions written but did not initiate ART; the inability to follow youth who receive care from other treatment centers; or a less aggressive clinical approach to ART initiation at the linkage visit in order to prepare these challenging, mostly newly diagnosed youth, for ART, particularly during a time when HIV treatment guidelines were still evolving to their current recommendations of universal ART.25,26 Nonetheless, the low level of viral suppression among these recently-diagnosed youth (Figure 1b), despite the advent of simpler one pill, once daily regimens, is a source of concern. It compares unfavorably with the 41.7% overall viral suppression rate among all persons living with diagnosed HIV from contemporaneous national MMP surveillance data22, or the 59.8% rate from the most recent NHSS report,13 or the age-disaggregated rates in adults older than 24 that ranged 32.4 – 56.6% and 54.2 – 62.8%, respectively.
Low rates of ART initiation and viral suppression for this sample may also reflect a higher burden of psychosocial and socioeconomic comorbidities for HIV-positive youth in the urban centers where the SMILE clinics were located. Additionally, while youth actively linked and retained in care through SMILE were mostly (> 75%) new to care and less well adjusted to their diagnosis, the NHSS-based data reports only on a minority of such youth (<30%) (9,129 newly diagnosed in 201427 among 32,149 diagnosed by end of 2014 and alive at end of 201513) with the remaining majority representing presumably better-adjusted youth established in care for at least one year from diagnosis. These youth may not require as much help in remaining engaged and suggests that possible ascertainment biases in both cohorts may explain the disparate viral suppression rates between them.
Although the observational design of the SMILE project did not allow us to identify which, if any, aspects of SMILE (e.g., the LTC coordinator, youth-friendly services) were associated with higher linkage rates, several factors were predictive of achieving viral suppression. Promptly referring youth for linkage to care (within the 3 month interval between HIV diagnosis and linkage services), was a significant predictor of achieving viral suppression and is consistent with findings from work with adults.19 This relationship was the strongest when linkage occurred in the shortest window (7 to 42 days) within that interval (Table 2). This finding suggests that the 1 month national target for LTC is critically important for youth and underscores the recommendations found in HIV treatment guidelines that youth may benefit from more expedient and frequent clinical interactions.26,28 Cross-sectional analyses of data previously obtained in the ATN also suggested that prompt referral to linkage services after diagnosis was associated with better and more successful engagement29 and that adolescent friendly services are important for youth to successfully link to care.15 Finally, anecdotal reports and expert opinion suggest that adolescents may engage more and have better health outcomes with more intensive case management services and more frequent contact with clinic staff.30,31 Overall, these early interactions may help youth develop strong, trusting relationships with their clinicians and facilitate engagement, retention in care, and medication adherence. Programs that provide intensive case management, whether through peer navigators or other youth-friendly support personnel, may be particularly helpful in strengthening vital early bonds, by providing newly diagnosed youth with trusted confidantes who can help them deal with often unfamiliar and cumbersome steps necessary to engage in care (e.g. filling out forms, maintaining appointment times, obtaining and maintaining insurance, and securing housing, food and transportation).30,31 While these youth-focused approaches have been shown to improve care engagement and did so in SMILE, impact on virologic suppression remains an elusive target.32
That prompt receipt of ART and lower level baseline plasma viremia independently predicted VL suppression was not surprising. Together, they speak to the need for renewed testing efforts, particularly among young gay men, to identify infection earlier, as well as support for initiating and adhering to ART early after diagnosis. Several findings were unexpected, however. First, in univariate analyses, case management by non-ATN staff was associated with better virologic outcomes. This may have been because ATN outreach staff who served as LTC coordinators were more likely to be assigned to manage the more difficult cases, that is, youth with significant linkage and engagement barriers. The disappearance of this association, and that of substance use, after adjustment for confounding psychosocial covariates, and the emergence of early referral to care, may suggest that addressing unmet behavioral health needs early in the process facilitates the ability to successfully initiate and adhere to ART. The partnership between community-based organizations and clinical care sites facilitated through the SMILE collaborative may have supported this. Second, better virologic outcomes were predicted by limited or absent formal plans for data sharing between health departments and the SMILE LTC coordinators. Decisions around having or allowing formal data sharing plans were not random; they varied with jurisdictional interpretations and guidance around shared public health authority.16 It is possible they also varied with a site’s need for additional youth linkage support. This unexpected relationship may also have been affected by unmeasured structural and other psychosocial confounders (e.g., influences of the transition between entitlement programs and those employing the Affordable Care Act) but a better understanding of it will require further exploration. Nonetheless, the high rates of linkage, similar rates of ART initiation and lower rates of viral suppression compared to the MMP cohort, suggest it may have been possible that active outreach efforts by the LTC coordinators who facilitated care linkage and retention through SMILE reached and supported a population that would otherwise not have successfully accessed or engaged in care early on. Perhaps youth in SMILE mirrored more the cascade of those with HIV, diagnosed or undiagnosed, who ultimately achieved 16.3% viral suppression at that time.22
LTC failure occurred as a result of a variety of structural barriers which have remained consistent from a prior examination in ATN.17,29,33,34 Such barriers included youth’s challenges with navigating health insurance policies and entitlement programs, and accessing transportation to appointments, lack of youth friendliness of clinic space and staff, and duplication of linkage services. The two most frequent barriers to effective linkage were the inability to locate the youth to offer them referral for linkage, stemming in large part from the lack of sufficient available contact information or poor data sharing across agencies, and repeated missed appointments. Taken together with the finding that shorter time from diagnosis to linkage referral predicts viral suppression, these findings underscore the need to engage HIV-infected youth from point of first contact, to obtain as much locator information as possible, and to maintain frequent contact from the outset through electronic media (e.g. text messages, social media) and the engagement of trained peers.
Accumulating data also demonstrate the benefits of prompt ART initiation for all persons with HIV infection regardless of CD4 count, with appropriate psychosocial considerations.28 In the current study, 38% of the youth presented with CD4 counts above 500 cells/mm3 at linkage, suggesting they were more recently infected. Current recommendations are that treatment should be started, but immediate initiation yields less time for youth to adjust to their diagnosis than when treatment guidelines recommended waiting. Whether current simpler, more potent regimens may favorably influence ART initiation, adherence and viral suppression rates in youth are a focus of ongoing ATN research.35 More research and services are needed to optimally address the critical questions of how to improve treatment readiness and medication adherence among newly diagnosed youth, particularly as additional services may put an added burden on already taxed healthcare systems. Eliminating barriers at all levels and intervening to ensure prompt, youth-friendly HIV care is initiated is now more urgent than ever.
This study is not without limitations. We were not able to follow individuals who left care from agencies within the SMILE collaborative, which may have led to biases. One the one hand, the design may underestimate the rate of viral suppression by missing individuals who may have successfully engaged and initiated care elsewhere. At the same time, individuals who were not successfully adhering to treatment may have been more likely to drop out of care, thus providing follow up findings only on those who were better able to utilize treatment and care. Finally, the design of the study did not allow for assessing the representativeness of the sample. However, participants were drawn from major epicenters of the domestic HIV epidemic.
These empiric results from the first of its kind cross-agency collaborative implementing a systematic evaluation of the HCC for adolescents and young adults, ages 12-24 years, across major US metropolitan areas suggest that youth-friendly coordinators may facilitate better linkage to care from testing sites but also highlights significant gaps in achieving durable viral suppression. Leveraging the strengths of multiple agencies such as occurred in SMILE demonstrated that these collaborations are possible and may serve to improve linkage to and retention in care the for newly HIV diagnosed youth. Innovative solutions to eliminate barriers at each of the individual, provider, clinic, community and structural (e.g., health system) levels are urgently needed to most effectively address the HIV epidemic among youth in the US and most appropriately address the youth mission of the US NHAS.11
ACKNOWLEDGEMENTS
This work was supported by grants to the Adolescent Medicine Trials Network for HIV/AIDS Interventions (ATN): 5 U01 HD 40533 and 5 U01 HD 40474 from the National Institutes of Health through the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Bill Kapogiannis, MD; Sonia Lee, PhD) with supplemental funding from the National Institutes on Drug Abuse (Katherine Davenny, PhD) and Mental Health (Susannah Allison, PhD; Pim Brouwers, PhD).
The study was scientifically reviewed by the ATN Community Prevention Leadership Group. Network, scientific, and logistical support was provided by the ATN Coordinating Center (Craig Wilson, MD; Cynthia Partlow, MEd). ATN Data and Operations Center at Westat, Inc. (Jim Korelitz, PhD; Bob Harris, PhD; Barbara Driver RN, MS) provided project support and coordination. We also acknowledge the contribution of the investigators and staff at the following sites that participated in this study: University of South Florida, Tampa (Emmanuel, Straub), Children’s Hospital of Los Angeles (Belzer), Children’s National Medical Center (D’Angelo), John H. Stroger Jr. Hospital of Cook County and the Ruth M. Rothstein CORE Center (Martinez, Henry-Reid), Montefiore Medical Center (Futterman), Tulane University Health Sciences Center (Abdalian), University of Miami School of Medicine (Friedman), St. Jude’s Children’s Research Hospital (Flynn, Gaur), Baylor College of Medicine (Paul), Wayne State University (Secord), Johns Hopkins University (Agwu), Fenway Health (Mayer), University of Colorado (Reirden).
We would also like to acknowledge Drs. Lynne Mofenson, Steve Nesheim, Tim Quinn and Bernard Branson whose formative and invaluable guidance made this Federal collaborative possible.
Supported by award numbers U01HD40533 and U01HD40474 from the National Institutes of Health through the Eunice Kennedy Shriver National Institute of Child Health and Human Development with supplemental funding from the National Institutes on Drug Abuse and Mental Health.
Footnotes
Care intervals along the HIV Care Continuum were defined for LTC: a clinical visit attended no later than 42 days after referral for linkage; EIC: at least one additional clinical visit attended no later than 16 weeks after LTC; RIC: at least one additional clinical visit attended no later than 52 weeks after EIC; and, *Post-RIC: a second clinical visit attended within 52 weeks after EIC, if the RIC visit occured early in the interval.
In 2014, the national indicators and measures further evolved to collect data from the CDC’s National HIV Surveillance System (NHSS) representing broader geography and lower age strata (13-24 years) than CDC’s Medical Monitoring Project (MMP) which is more geographically limited in scope and age group (18-24 years).12 Whereas rates of viral suppression in youth have been rising, these changes reported disparate suppression rates of 33.7% using NHSS compared to 23.1% using MMP in two similar youth groups diagnosed at end of 2010 and alive at end of 2011, within the same report (tables 5a and 10, respectively).12 Interestingly, while the NHSS is broader in geographic scope and age, the denominator contained over 14,000 fewer persons than in the approach used by the MMP while the numerator of those with VL < 200 copies/ml was about the same in either those aged 18-24 years or those aged 13-24 years (7,834 vs 6,429 persons) suggesting possible differences in ascertainment contributing to the disparity in reported viral suppression. Comparing our data on viral suppression across these changes in national data is challenging, however we focus comparisons with the 2013 CDC MMP-based data as these are more methodologically similar and contemporaneous to our study and population.
The authors have no conflicts of interest to disclose.
Presented in part at the International AIDS Society Conference; July 19-22, 2015
Comments and views of the author(s) do not necessarily represent the official positions of the NICHD, NIH, CDC, HRSA or DHHS.
REFERENCES
- 1.CDC. HIV Among Youth. 2016; http://www.cdc.gov/hiv/pdf/group/age/youth/cdc-hiv-youth.pdf. Accessed June 29, 2016.
- 2.CDC. Estimated HIV incidence and prevalence in the United States, 2010–2016. HIV Surveillance Supplemental Report 2019;24(No. 1). 2019; https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-24-1.pdf. Accessed May 1, 2019.
- 3.CDC. Fact Sheet: Trends in U.S. HIV Diagnoses, 2005-2014. 2016; https://www.cdc.gov/nchhstp/newsroom/docs/factsheets/HIV-Data-Trends-Fact-Sheet-508.pdf. Accessed March 4, 2019.
- 4.Kahana SY, Jenkins RA, Bruce D, et al. Structural Determinants of Antiretroviral Therapy Use, HIV Care Attendance, and Viral Suppression among Adolescents and Young Adults Living with HIV. PLoS One. 2016;11(4):e0151106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Group ISS, Lundgren JD, Babiker AG, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Group TAS, Danel C, Moh R, et al. A Trial of Early Antiretrovirals and Isoniazid Preventive Therapy in Africa. N Engl J Med. 2015;373(9):808–822. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheever LW. Engaging HIV-infected patients in care: their lives depend on it. Clin Infect Dis. 2007;44(11):1500–1502. [DOI] [PubMed] [Google Scholar]
- 9.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zanoni BC, Mayer KH. The adolescent and young adult HIV cascade of care in the United States: exaggerated health disparities. AIDS Patient Care STDS. 2014;28(3):128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ONAP. The National HIV/AIDS Strategy for the United States: Updated to 2020. 2019; https://www.hiv.gov/federal-response/national-hiv-aids-strategy/nhas-update. Accessed March 12, 2019.
- 12.CDC. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas—2012. HIV Surveillance Supplemental Report 2014;19(No. 3). 2014; https://www.cdc.gov/hiv/pdf/surveillance_Report_vol_19_no_3.pdf. Accessed March 12, 2019.
- 13.CDC. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2016. HIV Surveillance Supplemental Report 2018;23(No. 4). 2018; https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-23-4.pdf. Accessed March 10, 2019.
- 14.Fortenberry JD, Koenig LJ, Kapogiannis BG, Jeffries CL, Ellen JM, Wilson CM. Implementation of an Integrated Approach to the National HIV/AIDS Strategy for Improving Human Immunodeficiency Virus Care for Youths. JAMA Pediatr. 2017;171(7):687–693. [DOI] [PubMed] [Google Scholar]
- 15.Tanner AE, Philbin MM, Duval A, et al. “Youth friendly” clinics: considerations for linking and engaging HIV-infected adolescents into care. AIDS Care. 2014;26(2):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanner AE, Philbin MM, Ott MA, et al. Linking HIV+ adolescents into care: The effects of relationships between local health departments and adolescent medicine clinics. J HIV AIDS Soc Serv. 2013;12(3–4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellen JM, Kapogiannis B, Fortenberry JD, et al. HIV viral load levels and CD4+ cell counts of youth in 14 cities. AIDS. 2014;28(8):1213–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CDC. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas—2012 HIV Surveillance Supplemental Report 2014; http://www.cdc.gov/hiv/pdf/surveillance_Report_vol_19_no_3.pdf. [Google Scholar]
- 19.Hall HI, Tang T, Westfall AO, Mugavero MJ. HIV care visits and time to viral suppression, 19 U.S. jurisdictions, and implications for treatment, prevention and the national HIV/AIDS strategy. PLoS One. 2013;8(12):e84318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crepaz N, Tang T, Marks G, Mugavero MJ, Espinoza L, Hall HI. Durable Viral Suppression and Transmission Risk Potential among Persons with Diagnosed HIV Infection: United States, 2012–2013. Clin Infect Dis. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marks G, Gardner LI, Rose CE, et al. Time above 1500 copies: a viral load measure for assessing transmission risk of HIV-positive patients in care. AIDS. 2015;29(8):947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CDC. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas—2013. HIV Surveillance Supplemental Report 2015;20(No. 2). 2015; https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillancereport_vol20_no2.pdf. Accessed March 10, 2019.
- 23.CDC. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2014. HIV Surveillance Supplemental Report 2016;21(No. 4). 2016; https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-21-4.pdf. Accessed March 10, 2019.
- 24.CDC. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2015. HIV Surveillance Supplemental Report 2017;22(No. 2). 2017; https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-22-2.pdf. Accessed March 10, 2019.
- 25.DHHS. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2012; https://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL003093.pdf. Accessed March 12, 2019.
- 26.DHHS. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents. 2019; https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/21/adolescents-and-young-adults-with-hiv. Accessed March 12, 2019.
- 27.CDC. HIV Surveillance Report, 2017; vol. 29. 2018; https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2017-vol-29.pdf. Accessed March 12, 2019.
- 28.DHHS. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. 2019; https://aidsinfo.nih.gov/guidelines/html/2/pediatric-arv/78/specific-issues-in-antiretroviral-therapy-for-adolescents-living-with-hiv-infection. Accessed March 12, 2019.
- 29.Philbin MM, Tanner AE, DuVal A, et al. HIV Testing, Care Referral, and Linkage to Care Intervals Affect Time to Engagement in Care for Newly Diagnosed HIV-Infected Adolescents in 15 Adolescent Medicine Clinics in the United States. J Acquir Immune Defic Syndr. 2016;72(2):222–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hightow-Weidman LB, Smith JC, Valera E, Matthews DD, Lyons P. Keeping them in “STYLE”: finding, linking, and retaining young HIV-positive black and Latino men who have sex with men in care. AIDS Patient Care STDS. 2011;25(1):37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sitapati AM, Limneos J, Bonet-Vazquez M, Mar-Tang M, Qin H, Mathews WC. Retention: building a patient-centered medical home in HIV primary care through PUFF (Patients Unable to Follow-up Found). J Health Care Poor Underserved. 2012;23(3 Suppl):81–95. [DOI] [PubMed] [Google Scholar]
- 32.Griffith D, Snyder J, Dell S, Nolan K, Keruly J, Agwu A. Impact of a Youth-Focused Care Model on Retention and Virologic Suppression Among Young Adults With HIV Cared for in an Adult HIV Clinic. J Acquir Immune Defic Syndr. 2019;80(2):e41–e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Philbin MM, Tanner AE, Duval A, Ellen J, Kapogiannis B, Fortenberry JD. Linking HIV-positive adolescents to care in 15 different clinics across the United States: creating solutions to address structural barriers for linkage to care. AIDS Care. 2014;26(1):12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Philbin MM, Tanner AE, DuVal A, et al. Factors affecting linkage to care and engagement in care for newly diagnosed HIV-positive adolescents within fifteen adolescent medicine clinics in the United States. AIDS Behav. 2014;18(8):1501–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ATN. The Adolescent Medicine Trials Network for HIV/AIDS Interventions. 2019; https://atnweb.org/atnweb/studies. Accessed 12/15/2019.


