Abstract
Cadmium (Cd) is a non-essential trace element that is widely distributed in the environment. Both geogenic and anthropogenic sources can elevate Cd concentrations in soils and groundwater, which are important for maintaining healthy supplies of food and safe drinking water. Elevated Cd doses are carcinogenic to humans. The WHO Guidelines for Drinking-Water Quality recommend a guideline value for Cd of 3 μg/L. Important anthropogenic Cd sources include mining, atmospheric deposition of combustion emissions, and the use of Cd-containing fertilizers. We document several cases of Cd pollution in soil and groundwater based on worldwide accounts. Besides anthropogenic Cd sources, Cd is also incorporated into sulfides, carbonates, and phosphorites resulting in elevated Cd concentrations in associated rock types. The crustal median Cd content is 0.2 mg/kg. In soils, Cd occurs at concentrations of 0.01 to 1 mg/kg with a worldwide mean of 0.36 mg/kg. Weathering can lead to Cd concentrations up to 5 μg/L in soil water and up to 1 μg/L in groundwater. In aqueous solutions, Cd generally occurs as the divalent Cd2+ and it is mobilized mainly in oxic, acidic conditions. Cadmium sorption is enhanced by the presence of high amounts of hydrous oxides, clay minerals, and organic matter, and its mobility is further influenced by pH, the redox state, and ionic strength of the solution. However, Cd can remain in solution as water-soluble complexes with anions, such as CdCl+ and Cd(SO4)22−, and dissolved organic matter while sorption and precipitation decrease the aqueous concentration of most other heavy metals. As a consequence, Cd is one of the most mobile heavy metals in the environment. The elevated mobilization potential, e.g., through competition and ligand induced desorption, is the reason for faster Cd release from soil into groundwater than other heavy metals. The goal of this study was to present a broad overview of the origin and concentration of Cd in groundwater, and its reaction pathways in aquatic environments. To gain an overview of the hydrochemical behavior of Cd, cases of Cd pollution in soil and groundwater, studies investigating Cd release, and information about the legal framework were compiled.
Keywords: Cadmium, Groundwater, Soil, Natural, Geogenic, Heavy metals, Toxic, Contaminant
1. Introduction
Cadmium (Cd) is one of the most toxic and mobile elements in the environment (e.g., Alloway and Jackson, 1991; Nies, 1999, 2003). It can replace calcium in minerals due to its similar ionic radius, identical charge and similar chemical behavior (e.g., Thornton, 1986). Therefore, Cd can enter the human body and accumulate to a high level in several organs (Hajeb et al., 2014; Pan et al., 2010). In contrast to other toxic elements such as mercury (Hg) and arsenic (As), Cd enters the human diet mainly through terrestrial pathways, e.g., through vegetables. In areas with both anthropogenic and geogenic elevated Cd concentrations in soil and groundwater, rice bioaccumulated Cd, leading to an elevated daily Cd uptake in China, Korea, and Jamaica (Liu et al., 2017; Sebastian and Prasad, 2014). However, Cd bioavailability is complex, for example, rice from the southern part of China contains more Cd than rice from the northern part of China. Possible reasons are the more acidic soils, an overuse of nitrogen fertilizers, pollution through irrigation and atmospheric deposition, and the cultivation of rice with a higher affinity for Cd accumulation in southern China (Chen et al., 2018; Yang et al., 2016).
Chronic Cd poisoning, termed itai-itai disease first discovered in Japan in the early 20th century, causes renal tubular dysfunction, osteomalacia, and osteoporosis due to competition with Ca and other nutrients (Aoshima, 2016; Arain et al., 2015; Khan et al., 2017). Cadmium exposure is also associated to glucose metabolism disorders, breast and lung cancer, cerebral infarction and cardiac failure (Khan et al., 2017). According to WHO (2011), the tolerable monthly Cd intake is 25 μg/kg body weight due to its long biological half-life in humans of 10 to 35 years. Cadmium uptake occurs through ingestion and inhalation and prolonged exposure may lead to various types of cancer (Pan et al., 2010). Cadmium is therefore listed as a priority hazardous substance in the European Water Framework Directive, which required management plans to cease Cd releases to the environment (EC, 2000). In addition to the European Water Framework Directive, the European Groundwater Directive required the EU member states to set a threshold value for Cd in groundwater (EC, 2006). Each member state developed their own procedures to determine a threshold value and values ranged from 0.08 to 27 μg/L; eight EU member states do not have a threshold value for Cd due to missing risk assessments (EC, 2010). In drinking water, the guideline value for Cd is set to 3 μg/L (WHO, 2011). The United States Environmental Protection Agency set the maximum contaminant level for Cd to 5 μg/L, which is the same in the European Union (UNEP, 2010) and China (Ministry of Health of China, 2006). The environmental quality standard for Cd in groundwater is 0.5 μg/L in Denmark, and 10 μg/L in Japan (UNEP, 2010) and China, respectively (Li et al., 2017).
Previous studies investigated the occurrence and behavior of Cd in soils and groundwater with respect to agricultural aspects (e.g., Bigalke et al., 2017; Grant, 2011; Holmgren et al., 1993), bioavailability (e.g., Carrillo-Gonzalez et al., 2006; Wang et al., 2010) and environmental remediation (e.g., Khan et al., 2017; Zwonitzer et al., 2003). However, most of these studies focused on specific subjects, such as local problems (e.g., Karak et al., 2015; Kozyatnyk et al., 2016), contamination issues (e.g., Akbar et al., 2006; Christensen et al., 1996; Kjeldsen et al., 2002) or interaction with a specific mineral, such as goethite (e.g., Buerge-Weirich et al., 2002; Chen et al., 2019; Wang and Xing, 2002). Most studies used modeling approaches and/or experimental settings with uncommonly high Cd concentrations for statements of hydrochemical behavior, particularly during sorption experiments (e.g., Ahmed et al., 2008; Krishnamurti and Naidu, 2003). Tabelin et al. (2018) summarized the competitive release of Cd and other trace elements from naturally contaminated rocks during construction projects.
The focus of most previous studies was the examination of chemical reactions and transport behavior of Cd stemming from its complex interactions in aquatic systems, with only a few studies (e.g., Hem, 1972; Loganathan et al., 2012; Smolders and Mertens, 2013) on the general hydrochemical behavior of Cd. Thus, there exists a lack of general knowledge about Cd sources, its geochemical behavior, its role as a competitor with other heavy metals and the resulting Cd concentrations in groundwater. This is particularly evident compared to other wellinvestigated toxic elements such as As (e.g., Smedley and Kinniburgh, 2002), Hg (Barringer et al., 2013) or Mo (Smedley and Kinniburgh, 2017).
An overview of Cd sources, abundance and distribution in the environment is presented in the following paragraphs. Furthermore, the hydrogeochemical behavior of Cd is addressed with focus on complexation, sorption, and competitive behavior in the presence of other heavy metals.
2. Cadmium content in soil water and groundwater
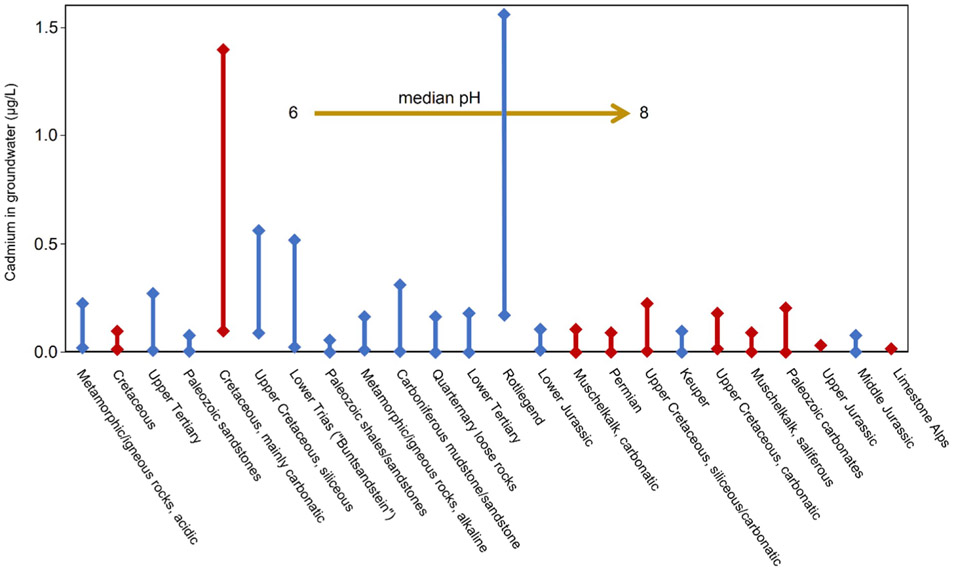
Cadmium occurs usually up to 5 μg/L in soil water (Smolders and Mertens, 2013) and up to 1 μg/L in groundwater (Naseem et al., 2014). In groundwater in Pakistan, mean Cd concentrations of 10 μg/L originated from Jurassic sulfide-bearing sedimentary rocks (Naseem et al., 2014). In Germany, background Cd concentrations in groundwater range from 0.11 μg/L in loess aquifers below arable land to 2.7 μg/L in sandy aquifers below forested lands (Duijnisveld et al., 2008). Aquifers in Germany were analyzed with respect to stratigraphy and petrography. A selection of relevant aquifer systems indicate a relation between rock type, groundwater milieu and Cd concentrations (Fig. 1). Cadmium 90th percentile as background levels ranged from less than 0.1 μg/L in groundwater, e.g., in Paleozoic, Triassic and Jurassic aquifers, to above 1 μg/L in Rotliegend, Cretaceous, and Cenozoic aquifers (BGR, 2014). Apart from calcium carbonate Cretaceous aquifers, limestone dominated aquifer systems had low Cd concentrations in groundwater. Most of them belong to aquifer systems with alkaline conditions (Fig. 1).
Fig. 1.
Cadmium concentrations in groundwater related to stratigraphic and petrographic aquifer features (BGR, 2014). Bars represent 50th percentile (lower edge) and 90th percentile (upper edge) of Cd in groundwater. Red bars show limestone dominated aquifers, blue bars show miscellaneous aquifer material. Aquifer's order indicate median pH in groundwater from acidic (left) to alkaline (right). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
A mean Cd background level of 0.2 μg/L was calculated for Irish groundwater, which increased up to 0.5 μg/L in groundwater originated from non-calcareous sediments (Tedd et al., 2017). Occasional Cd concentrations above 1 μg/L were detected in groundwater in sandstone aquifers and in unconsolidated sand and gravel aquifer systems in the western part of the United States, while Cd concentrations were below 1 μg/L in most samples, which were taken from 3124 wells in the United States (Ayotte et al., 2011). Groundwater from the glacial aquifer system in the United States shows Cd concentrations between 0.018 μg/L and 1.0 μg/L; however, 84 % of groundwater samples (N = 847) were below the detection limit (Groschen et al., 2008). Neumayer and Matthess (1977) reported for groundwater in Northern Germany a natural variation of Cd concentrations from 2 μg/L in Pleistocene glaciofluvial sediments to 20 μg/L in Holocene sands covered by marsh sediments. Wessolek and Kocher (2002) reported for road traffic influenced soil water and groundwater in Northern Germany Cd concentrations of up to 27.8 μg/L and 2.34 μg/1, respectively. In a survey of groundwater surrounding waste sites in the United States, Cd concentrations up to 6000 μg/L were found (ATSDR, 2012). Leachates from municipal solid waste landfills in the European Union can reach Cd concentrations up to 2700 μg/L (EU, 2007). Therefore, Cd concentrations above what would be considered natural background can be the result of both natural and anthropogenic processes.
3. Natural cadmium sources
3.1. Atmosphere
Significant sources of natural Cd emissions are weathering of rocks, airborne soil particles, e.g., from deserts, sea spray, forest fires, biogenic material, volcanoes, and hydrothermal vents (ATSDR, 2012; Richardson et al., 2001; UNEP, 2010). Soil particles are the predominant source of natural emissions to the atmosphere, followed by forest and bush fires, sea salt, volcanic emissions and meteoric dust. According to Pacyna and Pacyna (2001), the worldwide mean annual emission of natural Cd is about 1300 t. In contrast, the estimates of Richardson et al. (2001) are almost 41,000 t/a and thus, higher by a factor of 15. Contrary to previous studies, their calculations were based on improved data collection. Sources for anthropogenic Cd emissions are non-ferrous metal production, fossil fuel combustion, phosphate fertilizer manufacturing, iron, steel, and cement production, road dust, and municipal and sewage sludge incineration (ATSDR, 2012; Merkel and Sperling, 1998; Pacyna and Pacyna, 2001; UNEP, 2010). Anthropogenic Cd emissions have decreased by over 90 % in the last 50 years (ATSDR, 2012; Smolders and Mertens, 2013). The estimations of the natural and anthropogenic mean Cd emissions worldwide are given in Table 1.
Table 1.
Worldwide mean Cd emissions to the atmosphere in the mid-90s.
| Source | Cadmium (t/a) | Reference |
|---|---|---|
| Natural sources | a | |
| Soil particles | 24,000 | |
| Forest and bush fires | 13,000 | |
| Sea salt | 2000 | |
| Volcanoes | 1600 | |
| Meteoric dust | 1,4 × 10−4 | |
| Anthropogenic sources | b | |
| Non-ferrous metal production | 2171 | |
| Iron and steel production | 64 | |
| Stationary fossil fuel combustion | 691 | |
| Cement production | 17 | |
| Waste disposal (incineration) | 40 | |
| Sum | 43,600 |
Cadmium concentrations in the environment can be caused by wildfires and wildfire induced increases in Cd concentrations in soils and ashes have been reported (Campos et al., 2016; Demeyer et al., 2001). The examination of the long-term behavior showed decreasing Cd concentrations in the solid phase, because rainfall and a pH decrease with time-since-fire instigate desorption and mobility of Cd and other heavy metals (Bladon et al., 2014; Campos et al., 2016). Wildfires in California, for example, increased the mean Cd concentration in storm water by over two orders of magnitude (Burke et al., 2013). Cadmium concentrations in biomass ash can be up to 30 mg/kg, which provides an additional process to increase Cd concentrations in soil, because such ash is often used as a fertilizer. In the short term the bioavailable pool of Cd remains low due to an ash-induced pH increase and therefore stronger adsorption (Kepanen et al., 2005; Li et al., 2016; Perkiomaki and Fritze, 2005). With time, however, rainfall and a pH decrease will increase its bioavailability.
The total Cd deposition in Germany in 2014, for example, was 12.8 t, of which 61 % originated from transboundary and natural emissions (Ilyin et al., 2016). In the southern agricultural part of Germany, deposition of Cd in 2014 were less than 0.25 g/(ha*a). In contrast, there were maximum concentrations of 1.4 g/(ha*a) in deposition in the atmosphere in the industrial western part of Germany (Ilyin et al., 2016).
3.2. Cadmium in rocks, sediments and soils
In general, Cd concentrations in sedimentary rocks (0.01 to 2.6 mg/ kg) are higher than those in igneous rocks (0.07 to 0.25 mg/kg) or metamorphic rocks (0.11 to 1.0 mg/kg) (Hammons et al., 1978; Mar and Okazaki, 2012; Page et al., 1987; Smolders and Mertens, 2013). Average geogenic Cd concentrations in a variety of rock types are shown in Table 2.
Table 2.
Cadmium contents in rocks.
| Rock type | Average Cd content (mg/kg) | Reference |
|---|---|---|
| Igneous | ||
| Granitic rocks | 0.12 | a |
| Mafic rocks | 0.11 | b |
| Ultramafic rocks | 0.02 | b |
| Obsidian | 0.25 | a |
| Basalt | 0.22 | a |
| Metamorphic | ||
| Gneisses | 0.04 | c |
| Schists | 0.02 | c |
| Sedimentary | ||
| Bituminous shale (black shales) | 0.8 | a |
| Red shales | 0.03 | b |
| Bentonite | 1.4 | a |
| Marlstone | 2.6 | a |
| Red clay | 0.56 | c |
| Shale and claystone | 1 | a |
| Limestone | 0.1 | d |
| Sandstone | 0.028 | b |
| Carbonate stone | 0.012 | b |
| Organic sediment | 0.5 | d |
| Oceanic manganese oxides | 8 | d |
| Phosphorites | 25 | d |
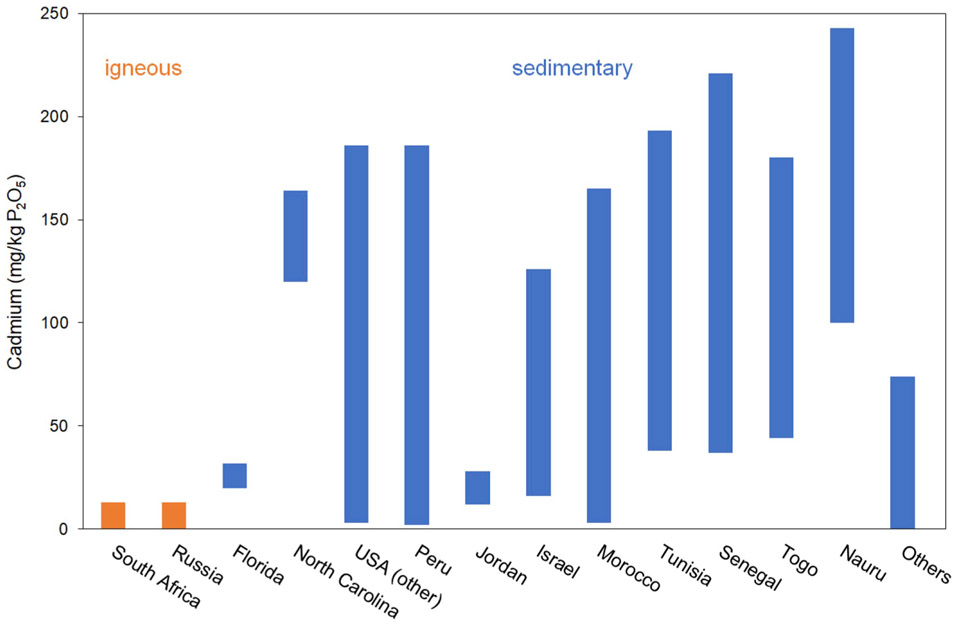
The crustal median Cd content is 0.2 mg/kg (Gong et al., 1977). Cadmium can substitute for divalent cations, such as Ca, Fe, Zn, Pb, and Co in several minerals due to its similar ionic radius, e.g., in carbonate and phosphate rocks (e.g., Merkel and Sperling, 1998; Smolders and Mertens, 2013; Thornton, 1986; Wilkin, 2007). Pyrite, for example, can contain up to 52 mg/kg Cd (Abraitis et al., 2004). Table 3 lists the Cd content in several minerals, which could be considered important for the occurrence of Cd in the environment. Cadmium can substitute for Zn in sphalerite (ZnS) or smithsonite (ZnCO3) (Merkel and Sperling, 1998; Tabelin et al., 2018; Wen et al., 2015; Zhu et al., 2013). Sulfide minerals like pyrite (FeS2) are essential constituents of reduced systems and thus, important sources and sinks for Cd (Bostick et al., 2000; Tabelin et al., 2018). Furthermore, Cd is known to be adsorbed by hydrous oxides such as Fe(III) hydrous oxide, which can be an important supply of Cd to the aqueous phase when redox conditions change from oxygenated to reducing (e.g., Descourvieres et al., 2010; Hindersmann and Mansfeldt, 2014; Li et al., 2010). Cadmium can also replace Ca in apatite, which is the main constituent of phosphorites (Gnandi and Tobschall, 2002). Consequently, Cd can be a common impurity in phosphate minerals and phosphoritic rocks, which are important for fertilizer production. The Cd content, however, varies significantly between geologic occurrences. Currently, there is no commercial means to entirely remove Cd during the production of phosphate fertilizers (Mar and Okazaki, 2012). Ranges of geogenic Cd contents in phosphates of the most important exporting countries are shown in Fig. 2. In Nauru, a Pacific island, the highest Cd contents were detected, reaching 240 mg/kg P2O5 (Mar and Okazaki, 2012). Raw phosphates from the USA and African countries also have wide ranges of Cd contents. Other exporting countries for raw phosphates are South Africa, Russia, Israel, Syria, Pakistan, Peru, and Brazil (Mar and Okazaki, 2012; Oosterhuis et al., 2000).
Table 3.
Cadmium content of minerals (in mg/kg, except where noted; modified after Thornton, 1986).
| Mineral | Composition | Range |
|---|---|---|
| Sphalerite | (Zn,Cd)S | < 2 % |
| Greenockite | CdS | 77.8 % |
| Chalcopyrite | CuFeS2 | < 110 |
| Marcasite | FeS2 | < 50 |
| Arsenopyrite | FeAsS | < 5 |
| Galena | PbS | < 3000 |
| Tetrahedrite | (Cu,Fe,Zn,Ag)12SbAs4S13 | 80 to 2000 |
| Magnetite | Fe3O4 | < 0.31 |
| Limonite | Hydrous iron oxides | < 1000 |
| Mn-Oxides | Hydrous manganese oxides | < 1000 |
| Anglesite | PbSO4 | 120 to > 1000 |
| Calcite | CaCO3 | < 1 to 23 |
| Smithonite | ZnCO3 | < 2.35 % |
| Otavite | CdCO3 | 65.18 % |
| Pyromorphite | Pb5Cl(PO4)3 | < 1 to 8 |
| Scorodite | FeAsO4 2H2O | < 1 to 5.8 |
| Apatite | Ca5(F,Cl)(PO4)3 | 0.14 to 0.15 |
| Bindheimite | Pb2Sb2O6(O,OH) | 100 to 1000 |
| Silicates | 0.03 to 5.8 |
Fig. 2.
Cadmium contents in raw phosphates by origin (Dittrich and Klose, 2008; Kharikov and Smetana, 2000; Mar and Okazaki, 2012; Oosterhuis et al., 2000; Roberts, 2014).
Like phosphorites, black shales can have an elevated Cd content due to high marine primary production and biogenic enrichment (Liu et al., 2017). Thus, weathering of black shales can be an essential geogenic source of Cd in the environment. Sulfide minerals in black shales are the primary source of Cd (Liu et al., 2017).
The concentration of Cd in a given soil is generally closely related to its abundance in the parent material, as well as input through atmospheric deposition, industrial or agricultural activities, minus output in the form of leaching, erosion, and harvested crops (Six and Smolders, 2014).
In uncontaminated soils worldwide, the average abundance of Cd is 0.36 mg/kg, although values can vary between continents, countries and soil types. For example, average concentrations are: 0.27 mg/kg in the USA (N = 3045), 0.01 mg/kg in Australia, 0.18 mg/kg in Brazil, 0.3 mg/kg in Japan, 0.2 mg/kg in Europe. In Europe, Cd concentrations are 0.3 to −1 mg/kg in Germany (N = 2947), 0.6 to 0.7 mg/kg in the UK (N = 5692), and 0.5 mg/kg in the Netherlands (N = 708) (Holmgren et al., 1993; Roberts, 2014; Smith et al., 2014; Taylor et al., 2016). Within the United States, Cd contents in uncontaminated soils exceeding 0.5 mg/kg are found in the Rocky Mountains, Great Plains and Mississippi delta, which are areas that also have elevated clay contents in the corresponding aquifer matrices (Holmgren et al., 1993; Smith et al., 2014). Within soil taxonomy, soils with organic matter (Histosols) and arid soils (Aridisols) have the highest median Cd content with 0.62 mg/kg and 0.3 mg/kg, respectively, while the strongly weathered Spodosols (0.2 mg/kg), Alfisols (0.11 mg/kg), and Ultisols (0.05 mg/kg) have lower Cd contents (Holmgren et al., 1993). Cadmium contents in soils, however, generally decrease with depth (Hiller et al., 2001; Page et al., 1987). Background levels of geogenic Cd contents in German soils were calculated as the 90th percentile depending on land use and rock genesis. They confirm the observation of decreasing Cd contents with depth independent of rock genesis. The Cd levels range from 0.06 mg/kg in sandy subsoils to 1.8 mg/kg in soils at swamp locations, 1.88 mg/kg in soils above fluviatile deposits at forest locations, and 2.0 mg/kg in sediments in the intertidal zone (LABO, 2017). Cadmium contents in soils in general depend on soil texture; elevated mean Cd contents can be found in soils with increasing contents of clay and peat (Holmgren et al., 1993; LABO, 2017).
Cadmium contents above 3 mg/kg are generally thought to indicate contaminated soil (Akbar et al., 2006). Concentration gradients in soils are common, where Cd increases with decreasing distance to industrial installations, roads and urban areas (Akbar et al., 2006; Page et al., 1987). This is comparable to Joimel et al. (2016), who reported that mean Cd contents in French soils showed an anthropization gradient of Cd concentrations with respect to land use, i.e., forest (0.13 mg/kg) < orchard and vineyard (0.18 mg/kg) < grassland (0.19 mg/kg) < farming (0.24 mg/kg) < garden (0.34 mg/kg) < urban, industrial, traffic, mining and military areas (1.30 mg/kg).
Locally Cd concentrations in soil above 3 mg/kg can be found without anthropogenic contamination. In forested areas, for example, the Cd content can reach up to 10 mg/kg due to the pedo-geochemical background (Baize and Sterckeman, 2001), while weathering of phosphorites from guano deposits produced Cd contents above 770 mg/kg in Jamaican soils (Garrett et al., 2008). In China, Korea, Finland, Sweden, and the United States, for example, black shales and associated soils show elevated Cd contents up to 42 mg/kg (Liu et al., 2017). Furthermore, oxidization of organic matter and sulfides in black shales causes acid rock drainage and thus, enhances Cd mobility (Liu et al., 2017).
In contrast to Eastern Europe, there is significantly more Cd in agricultural soils of Western Europe, which is caused by the different origin of P for fertilizers used in agriculture (Toth et al., 2016). As shown in Fig. 2, Russian magmatic Kola phosphate rock, which is the primary source of P fertilizers in Eastern Europe, has low Cd contents, while phosphate rock from Morocco, the main source of P for fertilizers in Western Europe, has elevated Cd contents (Toth et al., 2016). Birke et al. (2017) revealed another distribution pattern for Cd in agricultural soils in Europe. Besides a median Cd content of 0.18 mg/kg, Cd contents in agricultural soils differ between Northern Europe and Southern Europe, whose boundary coincides with the extension of the Quaternary glaciation. Soils in Northern Europe have median Cd contents of 0.13 mg/kg and thus, lower Cd contents compared to Southern Europe (median 0.22 mg/kg). Reasons for such a distribution pattern are differences in the amount of precipitation and drainage, lithology, weathering, grain size, and pH. However, anomalies can occur in Northern Europe due to soil mineralization, as well as in loess and clay dominated sediments and as anthropogenic overlaps (Birke et al., 2017).
Elevated Cd contents in soils and sediments are generally linked to the abundance of clay minerals, carbonates, organic matter, and hydrous oxides, as well as certain physicochemical conditions, such as elevated pH, and/or anoxic conditions (e.g., Appel and Ma, 2002; Buerge-Weirich et al., 2002; He et al., 2005). While Cd is often bound to the less stable exchangeable, carbonate and hydrous oxide fraction other heavy metals, such as Pb and Cu, are stronger bound to the organic and sulfidic fraction (Eggleton and Thomas, 2004; Zwonitzer et al., 2003). This is a likely explanation for the peculiar hydrochemical behavior and easy mobilization of Cd, when compared to other heavy metals.
4. Anthropogenic cadmium sources
Anthropogenic Cd inputs into soil and groundwater are combustion emissions, sewage sludge, landfills, traffic, metal industry, mining and incidents (Bigalke et al., 2017; Merkel and Sperling, 1998; Mirlean and Roisenberg, 2006; Sprynskyy et al., 2011). Similar to uranium (U), a common reason for elevated Cd concentrations in soil and groundwater is the use of phosphate fertilizers, which contain Cd as an impurity. This pathway of Cd addition to groundwater was investigated in the United States, Canada, Britain, Norway, Sweden, Finland, Denmark, Germany, Australia, and New Zealand (Bigalke et al., 2017; Grant, 2011; Taylor et al., 2016). The studies suggest that P fertilizer application changes soil chemistry. Additionally, Cd can potentially get transferred to the food chain and act toxic to biota. Sources for Cd can be of local or diffuse character. Local sources such as mines (e.g., Merkel and Sperling, 1998), industrials sites (e.g., Cloquet et al., 2006) or abandoned mine deposits (e.g., Monna et al., 2000) lead to elevated concentrations, however, mostly on a small spatial scale. Atmospheric emission, wastewater reuse or agricultural activities can serve as diffuse sources causing a widespread distribution of Cd in the environment (ATSDR, 2012; Knappe et al., 2008; Schuetze et al., 2003; Sprynskyy et al., 2011; UNEP, 2010).
The worldwide main Cd use, and thus primary source of Cd directed to landfills with municipal solid waste, are nickel-cadmium batteries (Khan et al., 2017; UNEP, 2010). Municipal solid wastes in Europe have Cd contents of 0.3 to 12 mg/kg, mean Cd concentrations in the leachates were estimated as 0.5 to 3.4 μg/L (EU, 2007). Additional Cd containing products are pigments, coatings and platings, stabilizers for polyvinyl chloride (PVC), and alloys (ATSDR, 2012). Table 4 lists the major anthropogenic (industrial) sources for elevated Cd in soils.
Table 4.
Cadmium contents in soils affected by industrial activities (Kabir et al., 2012).
| Source | Cadmium content | |
|---|---|---|
| Mean (mg/kg) | Max (mg/kg) | |
| Mining and metal industry | 37.6 | 289 |
| Fertilizers, chemicals, petroleum production | 0.51 | 2.13 |
| Textiles | 42.0 | 83.6 |
| Leathers | 0.63 | 1.26 |
| Nonmetallic mineral products | 25.8 | 72.0 |
The contribution range of contamination sources to the amount of Cd present in soils that is available for leachate into groundwater, is 10 to 25 % from livestock manure, 15 to 50 % from atmospheric deposition, 30 to 55 % from mineral fertilizers and 2 to 5 % from sludges and composts (Belon et al., 2012; Nicholson et al., 2003). In addition to anthropogenic activities, the natural variability in rocks and minerals can be a reason for elevated Cd in associated soils (Baize and Sterckeman, 2001; Birke et al., 2017). Since Cd is easily mobilized, soil should not necessarily be considered a permanent sink, but rather a significant temporary storage for Cd (Christensen, 1984b), which can easily affect groundwater concentrations on different timescales, e.g., annual (wet vs. dry season) or decadal (dry vs. wet years).
Besides U, Cd has the highest phosphate fertilizer to background soil ratio and thus, a high potential risk of accumulation in soils, uptake by plants or increased loss in terms of leaching (Taylor et al., 2016). However, a Swiss survey did not confirm the enrichment of Cd on arable land due to P fertilizers, as it was observed for U originated from P fertilizers. There are possible reasons for missing Cd enrichment in soil, namely the removal of Cd via harvest, interferences like Cd input as atmospheric deposition, and application of manure and sewage sludge to grassland as reference areas, which were contaminated with Cd. However, there is a significant enrichment of both Cd and U in topsoil (Bigalke et al., 2017).
Phosphate fertilizers contain an average of 77 mg Cd per kg P2O5 in the Eastern Mediterranean countries (Azzi et al., 2017), 36 mg Cd per kg P2O5 in Europe (Six and Smolders, 2014) and 60 mg Cd per kg P2O5 in Germany (Schuetze et al., 2003). In Europe, the average usage of phosphate is 43 kg/(ha*a) (Grant, 2011). In addition, the Cd input via deposition was calculated over different time scales and regions. Table 5 gives an overview of several studies about Cd inputs and outputs in soil. Depending on land use and distance to urban areas, either atmospheric deposition or application of P fertilizers are the main input of Cd.
Table 5.
Mean annual inputs and outputs of soil Cd in g/ha.
| Inputs | Outputs | Remarks (Location, period) | Reference | |||
|---|---|---|---|---|---|---|
| Atmospheric Deposition | Phosphate fertilizers | Others (e.g., manure) |
Leaching | Crop offtake | ||
| 1.7 | 5.6 | 0.64 | 0.68 | 0.28 | Germany, farming, the 1990s | Schuetze et al. (2003) |
| 5 | - | - | 0.68 | - | Germany, forest & urban, the 1990s | Schuetze et al. (2003) |
| 2.5 to 4.5 | - | - | - | - | German uplands, forest, the 1990s | Beisecker et al. (2012) |
| 2.06 | - | - | 3.35 | - | German uplands, forest, the 2000s | Beisecker et al. (2012) |
| 0.96 | 0.28 | 0.43 | 0.87 | 0.8 | Lower Saxony (Germany), farming, 1990s/2000s | Kamermann et al. (2015) |
| 4 | 0.9 | 6.3 | 1.6 | - | China, the 2000s | Luo et al. (2009) |
| 1.9 | 1.6 | - | - | - | European countries, 2000 | Grant (2011) |
| 9.8 | 1.0 | 1.4 | - | - | Belgium, the 1990s | UNEP (2010) |
| 0.25 | 0.98 | 0.56 | - | - | Farquhar et al., 1997 | Six and Smolders (2014) |
| 0.35 | 0.79 | 0.15 | 2.56 | 0.2 | European countries 2010 | Six and Smolders (2014) |
| > 0.5 | - | - | - | - | Western, Eastern and Southern Europe, 2003 | UNEP (2010) |
| 1.4 | - | - | - | - | West Germany, industry, 2014 | Ilyin et al. (2016) |
| 0.25 | - | - | - | - | South Germany, farming, 2014 | Ilyin et al. (2016) |
| 0.19 | < 0.071 | 0.148 | - | - | Farquhar et al., 1997 | UNEP (2010) |
| 0.3 | - | - | - | - | Northern Europe, 2003 | UNEP (2010) |
| 0.32 | - | - | - | - | Near the European coast, 2005 | OSPAR (2008) |
| > 0.32 | - | - | - | - | Greater North Sea, 2005 | OSPAR (2008) |
| < 0.1 | - | - | - | - | Wider Atlantic, 2005 | OSPAR (2008) |
| > 0.1 | - | - | - | - | Central Atlantic & Southern coast of Greenland, 2005 | OSPAR (2008) |
| < 0.05 | - | - | - | - | Arctic Ocean, 2005 | OSPAR (2008) |
According to Six and Smolders (2014) the average total Cd input to European soils decreased to 1.3 g/(ha*a) due to low-emission process technologies, such as off-gas and waste water treatment. At the same time, the average Cd leachate output into groundwater was 2.6 g/ (ha*a) causing a negative mass balance, which was caused by accumulation of previously elevated Cd input in soils, followed by its release to groundwater (Six and Smolders, 2014). In contrast to continental deposition rates of more than 0.3 g/(ha*a), Cd deposition decreases with distance from anthropogenic sources to below 0.05 g/(ha*a) in the Arctic Ocean (OSPAR, 2008) (Table 5).
Cadmium pollution in soil and groundwater is observed worldwide. Main groups are mining, industry, waste management, agriculture, and urban areas. An overview of documented cases of Cd contamination in soil and groundwater is given in Table 6. Example cases were selected as locations with maximum Cd levels depending on the type of pollution. Soil Cd pollution from Zn smelters, for example, can be caused by leaching of solid waste (Voglar and Lestan, 2010) or by atmospheric deposition (Bi et al., 2006), causing soil Cd concentrations of up to 344 mg/kg and 74 mg/kg, respectively. Similarly, groundwater contamination can also occur simultaneously from different sources and along different pathways at a single location, which can inhibit to identification of a significant Cd source, pathway or geogenic Cd anomaly. Consequently, other investigations, e.g., isotopic Cd fractionation (e.g., Cloquet et al., 2006; Zhu et al., 2013), are recommended.
Table 6.
Types of Cd pollution in soil and groundwater.
| Source | Type of pollution | Example case | Maximum Cd level | Reference |
|---|---|---|---|---|
| Mining | ||||
| Pb–Zn mining/refinery | 1. Atmospheric deposition and waste water | Jinding, China | Soil: 531 mg/kg | Wen et al. (2015) |
| 2. Waste water | Coeur d’Alene basin, Idaho, USA | Groundwater: 77 μg/L | Paulson (1997) | |
| Fe–Ni–Co mining | Waste material | Several sites in Albania | Soil: 14 mg/kg | Shallari et al. (1998) |
| Cu mining | Waste water | Canchaque, Peru | Soil: 499 mg/kg | Bech et al. (1997) |
| Au–Cu mining | Waste water | Bolnisi, Georgia | Soil: 121.5 mg/kg | Avkopashvili et al. (2017) |
| Au–Ag–Pb–Zn mining | Waste water | Chloride, Arizona USA | Groundwater: 19 μg/L | Rosner (1998) |
| Phosphorite mining | Mining waste, transport | Kpogamé, Hahotoé, Togo | Soil: 43 mg/kg | Gnandi and Tobschall (2002) |
| Pb mining and refinery | Atmospheric deposition | Příbram, Czech Republic | Soil: 48 mg/kg | Rieuwerts and Farago (1996) |
| As refinery | Waste material | Reppel, Belgium | Soil: 79 mg/kg | Cappuyns et al. (2002) |
| Zn smelter | 1. Waste material | Celje, Slovenia | Soil: 344 mg/kg | Voglar and Lestan (2010) |
| 2. Atmospheric deposition | Hezhang County, China | Soil: 74 mg/kg | Bi et al. (2006) | |
| Industry | ||||
| Metal industry | Atmospheric deposition | Unnao, India | Groundwater: 74 μg/L | Dwivedi and Vankar (2014) |
| Cement factory | Atmospheric deposition | Qadissiya, Jordan | Soil: 13 mg/kg | Al-Khashman and Shawabkeh (2006) |
| Ceramic industry | 1. Sewage sludge | Castellon, Spain | Soil: 72 mg/kg | Jordan et al. (2009) |
| 2. Atmospheric deposition | Yixing, China | Soil: 5.9 mg/kg | Lin et al. (2015) | |
| Textile industry | Waste water | Haridwar, India | Soil: 83.6 mg/kg Groundwater: 40 μg/L | Deepali and Gangwar (2010) |
| Pigment manufacture | Atmospheric deposition | Staffordshire, UK | Soil: 16 mg/kg | Vangronsveld et al. (2009) |
| Various (e.g., textile, electroplating) | Waste water | Coimbatore, India | Soil: 12.8 mg/kg | Malarkodi et al. (2007) |
| Waste management | ||||
| Landfill | Leachate | Taoyuan, Taiwan Alexandria, Egypt | Soil: 378 mg/kg Groundwater: 51 μg/L | Chen and Liu (2006) Abd El-Salam and Abu-Zuid (2015) |
| Brownfield | Waste water | Xiangjiang River, China | Groundwater: 474 μg/L | Li et al. (2017) |
| Electronical waste recycling | Waste water | Krishna Vihar, India | Soil: 47.7 mg/kg Groundwater: 280 μg/L | Panwar and Ahmed (2018) |
| Household wastes | Waste water | Ikare, Nigeria | Groundwater: 580 μg/L | Ololade et al. (2009) |
| Sewage and waste disposal | Waste water | Sekondi-Takoradi Metropolis, Ghana | Groundwater: 90 μg/L | Affum et al. (2015) |
| Disposal facilities | Leachate | Great lakes region, USA | Soil: 32 mg/kg | Beyer and Stafford (1993) |
| Agriculture | ||||
| P fertilizer production | Atmospheric deposition | Rio Grande, Brazil | Soil: 9.3 mg/kg Groundwater: 3 μg/L | Mirlean and Roisenberg (2006) |
| P fertilizer application | Infiltration | Cauvery River basin, India | Groundwater: 60 μg/L | Vetrimurugan et al. (2017) |
| Sewage sludge application | Irrigation | Several sites in Spain | Soil: 90 mg/kg | Moral et al. (2005) |
| Urban areas | ||||
| Road traffic | Infiltration | Celle, Germany | Groundwater: 2.34 μg/L | Wessolek and Kocher (2002) |
| Sewerage | Leakage | Rastatt, Germany | Groundwater: 5 μg/L | Eiswirth and Hotzl (1997) |
5. Hydrochemical behavior
5.1. Basics
In aqueous solution Cd generally occurs as the divalent Cd2+ cation (Smolders and Mertens, 2013). Solution pH influences Cd mobility due to metal hydrolysis, ion-pair formation, solubility of organic matter, surface charge of oxy-hydroxides, organic matter and clay edges. With increasing pH, metal retention to mineral surfaces increases via adsorption and precipitation (Appel and Ma, 2002; Buerge-Weirich et al., 2002; He et al., 2005).
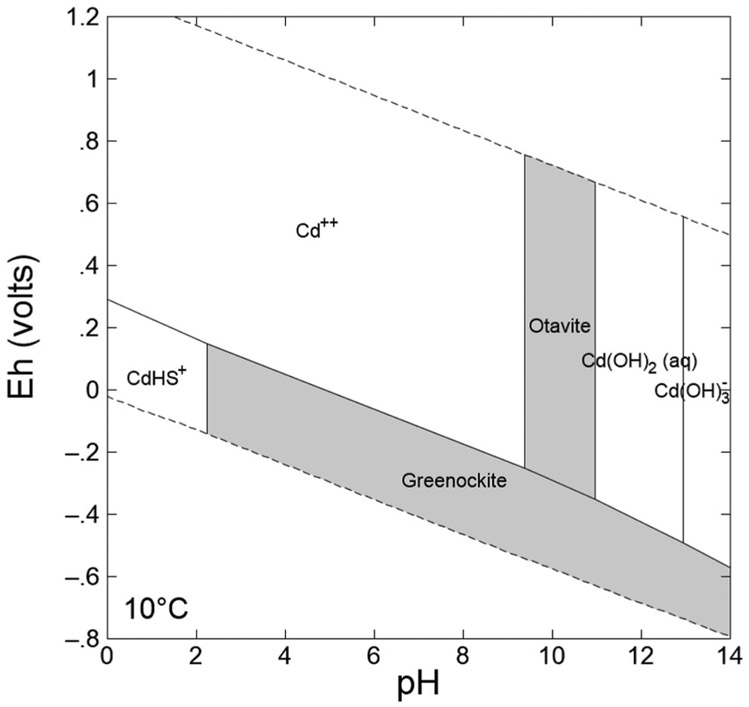
Cadmium preferentially remains in solution at a pH of less than 6.5 and under oxygenated conditions (Bruemmer et al., 1994; Merkel and Sperling, 1998). The Cd2+ ion itself is not redox-sensitive, but it is indirectly tied to redox conditions due to the formation of redox-sensitive aqueous complexes, such as CdHS+, which occurs in anoxic and sulfidic conditions, and stable precipitates, such as sphalerite (ZnS), galena (PbS), and chalcopyrite (CuFeSs), which can contain Cd as a trace element (Ditoro et al., 1990; Tabelin et al., 2018). As indicated in Fig. 3, the solubility-limiting phases of Cd are CdS, CdCO3, and Cd(OH)2. Depending on the Cd concentration, the stability fields of the Cd species can expand or contract (Ahmed et al., 2008; Brookins, 1986; Merkel and Sperling, 1998).
Fig. 3.
Eh vs. pH diagram for Cd. The concentrations of dissolved species are [Cd2+] = 10−8, [HCO3−] = 10−3, [SO42−] = 10−3.
5.2. Solubility and complexation
The environmentally mobile Cd fraction consists of water-soluble Cd, unspecific adsorbed Cd, and organo-metallic complexes (Bruemmer et al., 1994; Kabata-Pendias, 2011; Loganathan et al., 2012). The adsorbed phase consists of Cd bound at mineral surfaces or bound weakly as insoluble organo-metallic complexes. This fraction is likely responsible for transient fluctuations of Cd concentrations in natural waters. The stable Cd fraction is associated with the soil matrix or bound as surface complexes in oxy-hydroxides, organic matter, silicates, sulfides, or other stable minerals (Ahmed et al., 2008; Bruemmer et al., 1994). Furthermore, Cd is the only heavy metal with affinity for the easily solubilized fraction in typical sequential solid-phase extraction protocols (e.g., Al Husseini et al., 2013; Carlson and Morrison, 1992). This fraction includes water-soluble, exchangeable and acid-soluble components, and thus, the bioavailable Cd content. Usually, this Cd is introduced artificially via deposition; whereas, Cd originated from geogenic materials is typically present in the residual insoluble fraction (Chavez et al., 2016; Liu et al., 2017).
Cadmium forms water-soluble complexes with anions, such as CdCl+ or CdSO40, but also complexes with dissolved organic matter (DOM) (Bolan et al., 2003; Carrillo-Gonzalez et al., 2006; Gardiner, 1974; Loganathan et al., 2012). As a result, Cd can remain in solution while sorption decreases the aqueous concentration of other heavy metals. Furthermore, inorganic and organic complexation can lead to dissolution of Cd from oxy-hydroxides, phosphates, or sulfides (Beisecker et al., 2012; Carrillo-Gonzalez et al., 2006; Eggleton and Thomas, 2004; Hammons et al., 1978; Najafi and Jalali, 2015).
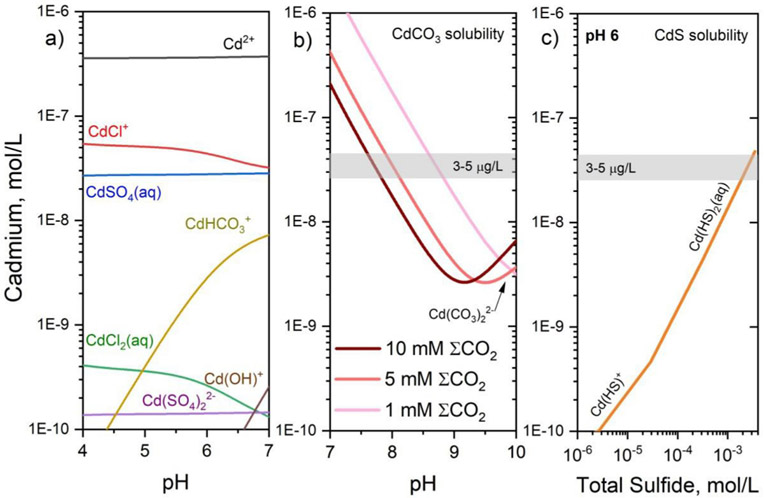
Depending on groundwater composition, 55 to 90 % of the total soluble Cd is present as divalent Cd2+ ions, while the remaining Cd is present as organic and inorganic complexes like: CdCl+ , CdCl20, CdCl3−, Cd(SO4)22−, CdSO40, CdHCO3−, CdCO30, Cd(CO3)22−, CdOH+, Cd(OH)20, Cd(OH)3−, Cd2OH3+, CdNO3+ (Baun and Christensen, 2004; Krishnamurti and Naidu, 2003; Merkel and Sperling, 1998; Sauve et al., 2000; Wilkin, 2007) (Fig. 4a). The predominance of Cd complexation with inorganic carbon increases with pH and precipitation of CdCO3 (otavite) is favorable at pH > 8 (Fig. 4b). For typical groundwater inorganic carbon concentrations, a minimum solubility for otavite occurs around pH 9 to 10; above this pH solubility increases due to the stability of the Cd(CO3)22− complex. Under anoxic and sulfidic conditions, Cd complexes occur as Cd(HS)20, Cd(HS)3−, Cd(HS)42− and CdHS+ (Astruc, 1986). The solubility of CdS (greenockite) is generally low, but equilibrium Cd concentrations increase with increasing dissolved sulfide (Fig. 4c). Complex formation is generally important in the case of low Cd concentrations and depends on ligand concentrations and the equilibrium constants for complex formation (Gardiner, 1974). The equilibrium constants for Cd, listed in Table 7, were taken from PHREEQC (Parkhurst and Appelo, 1999). The most stable Cd complexes are with chloride, carbonate, sulfate, and bisulfide anions as ligands (Fig. 4a). Previous studies dealing with Cd complex formation often used data from modeling or laboratory experiments conducted with high metal concentrations. Hence, such results may underestimate the mobility of heavy metals under natural conditions, especially with respect to the complexity of organic matter (Christensen et al., 1996).
Fig. 4.
Concentration versus pH diagrams showing a) Cd speciation from pH 4 to 7 for the composition Cd (10−6.4 M), Cl (10−3 M), ΣCO2 (10−3 M), and SO4 (10−3 M). b) Otavite solubility as a function of pH and Σ CO2 from 10−3 to 10−2 M. C) Concentration versus dissolved sulfide diagram showing the solubility of CdS at pH 6. Reference concentrations for drinking water standards are shown (3 to 5 μg/L).
Table 7.
Complex forming constants of Cd (Parkhurst and Appelo, 1999).
| Reaction | log K |
|---|---|
| Cd2+ + H2O = CdOH+ + H+ | −10.08 |
| Cd2+ + 2 H2O = Cd(OH)2 + 2 H+ | −20.35 |
| Cd2+ +3 H2O = Cd(OH)3− + 3 H+ | −33.3 |
| Cd2+ + 4 H2O = Cd(OH)42− + 4 H+ | −47.35 |
| Cd2+ + Cl− = CdCl+ | 1.98 |
| Cd2+ + 2 Cl− = CdCl20 | 2.6 |
| cd2+ + 3 cl− = CdCl3− | 2.4 |
| Cd2+ + CO32− = CdCO30 | 2.9 |
| Cd2+ + 2CO32− = Cd(CO3)22− | 6.4 |
| Cd2+ + HCO3− = CdHCO3+ | 1.5 |
| Cd2+ + SO42− = CdSO40 | 2.46 |
| Cd2+ + 2SO42− = Cd(SO4)22− | 3.5 |
| Cd2+ + F− = CdF+ | 1.1 |
| Cd2+ + 2F− = CdF20 | 1.5 |
| 2Cd2+ + H2O = Cd2OH3+ + H+ | −9.39 |
| Cd2+ + H2O + Cl− = CdOHCl0 + H+ | −7.404 |
| Cd2+ + NO3− = CdNO3+ | 0.4 |
| Cd2+ + HS− = CdHS+ | 10.17 |
| Cd2+ + 2HS− = Cd(HS)20 | 16.53 |
| Cd2+ + 3HS− = Cd(HS)3− | 18.71 |
| Cd2+ + 4HS− = Cd(HS)42− | 20.9 |
Cadmium present in sulfide minerals or bound to organic material is generally released when redox conditions change from reducing to oxidizing; this change generally causes metal sulfides to dissolve and organic matter to mineralize (Martinez et al., 2002; Simpson et al., 2000; Tabelin et al., 2018). There is also a change in the amount of Cd binding forms as groundwater conditions shift from reducing to oxidizing (Zoumis et al., 2001). During oxidation of pyrite or acid volatile sulfides (AVS), like amorphous FeS, mackinawite (FeS) or greigite (Fe3S4), Cd and other heavy metals are released. However, Cd is comparatively more mobile than, for example, Ni, Pb and Cu due to different mineral solubilities and sorption behavior, a stronger co-precipitation of Pb and Cu with iron sulfides, and the formation of stable Cd complexes (Caetano et al., 2003).
In floodplain soils, significant changes in the concentrations of Cd and other heavy metals affected by the concentrations of dissolved Fe, Mn, and S were observed. Fluctuations in the concentrations of these redox-sensitive elements can be caused by temporal dynamics of pH, redox potential, and DOC as daily cycles and longer-term seasonal influences (Husson, 2013; Shaheen et al., 2014).
Due to the influence of landfill leachate and forested land on the composition of seepage water, Cd remains in the soluble phase as labile complexes and also as organic complexes. In contrast, Cu, Cr, and Pb predominantly occur in association with colloids or as less mobile complexes. Complex formation of Cd and further heavy metals is controlled by ionic strength effects, the presence of ligands, and the presence of competing cations like Ca or other metals (Baun and Christensen, 2004; Beisecker et al., 2012; Christensen, 1985; Kjeldsen et al., 2002).
Carboxyl and phenolic functional groups are abundant in natural organic matter; however, less abundant functional groups like thiols and amines form stable complexes with Cd in DOM and soil organic matter (Karlsson et al., 2007). Furthermore, there is a component of Cd binding groups in organic matter that cause reduction of free Cd2+ (Karlsson et al., 2007). Reduced organic sulfur (DOS), which consists of thiols, is often part of the hydrophilic fraction of DOC. This fraction is more mobile than the hydrophobic fraction in the context of weak sorptive retention. In contrast to Cd, most heavy metals form complexes with the less mobile hydrophobic fraction (Kaiser and Guggenberger, 2005). Furthermore, Cd has a different selectivity than other heavy metals, because it mainly forms complexes with the neutral hydrophilic DOM fraction, while other heavy metals are bound to acidic hydrophobic and acidic hydrophilic DOM fractions (Kozyatnyk et al., 2016). At forest locations, the amount of DOS fractions and thus, of Cd in seepage water showed seasonal variations. Hydrophobic DOM is related to plant-derived material and is the most important constituent of DOS during growing seasons in summer and autumn, while the share of hydrophilic DOM related to microbial activity is more substantial in winter and spring (Kaiser and Guggenberger, 2005).
Apart from the general behavior of heavy metals that includes bonding to high molecular weight DOM, Cd belongs to a group of metals that interact more with low molecular weight DOM (Kozyatnyk et al., 2016). Furthermore, Cd tends to be replaced by other heavy metals, because its interaction with DOM is the weakest of the heavy metals and in the case of complexation with DOM, Cd bonds at weak sorption sites, which are abundant in the small DOM size fractions (Kozyatnyk et al., 2016).
5.3. Sorption
The most important parameters that control Cd solubility and mobility in aquatic environments are pH, concentration of dissolved organic and inorganic carbon (DOC, DIC), and the presence of clay and oxy-hydroxides, such as Fe, Mn, and Al (Anderson and Christensen, 1988; Appel and Ma, 2002; Gardiner, 1974; Krishnamurti and Naidu, 2003; Lin et al., 2016; Onyatta and Huang, 2006). Cadmium concentrations in groundwater are often controlled by sorption and co-precipitation rather than by chemical equilibrium (Carrillo-Gonzalez et al., 2006). As Anderson and Christensen (1988) reported, there is a correlation between the distribution coefficient Kd of Cd and the following parameters in the order: pH > cation exchange capacity > oxy-hydroxides > clay > organic matter. Furthermore, Cd sorption decreases with increasing ionic strength due to competition with other cations, decreasing activity of Cd2+, formation of ion pairs/complexes with lower sorption affinity, lower pH and changes in the electrostatic potential (Loganathan et al., 2012). Depending on temperature, Cd sorption occurs as endothermic or exothermic reactions (He et al., 2005; Karak et al., 2015).
Cadmium as a positively charged ion is adsorbed onto negatively charged mineral surfaces until the point of zero charge (PZC) is exceeded (He et al., 2005). Hence, groundwater pH is the driving factor controlling the availability of binding sites with the aquifer matrix. In Table 8, the PZC of several important minerals are listed. Sorption of Cd at pH values below the PZC is an indication of fixation via inner-sphere surface complexation or adsorption to permanent negative binding sites, e.g., at vermiculite, smectite, or organic matter surfaces (Appel and Ma, 2002). In contrast to other metals such as Pb and Cu, Cd sorption on kaolinite was inhibited at pH below 7 indicating a lower affinity for specific inner-sphere surface complexation at hydroxyl groups, ≡SOH, compared to preferred ion exchange at non-specific permanent negatively charged sites, ≡X−, resulting in higher mobility of Cd (Srivastava et al., 2005). The amount and type of surface complexation is also influenced by ionic strength. At pH 6, for example, almost 70 % of Cd is adsorbed onto kaolinite in a 0.001 M solution as ≡X2− · Cd2+ on the one hand, while Cd sorption mainly occurs as inner-sphere complex ≡SOCd+ decreased to 30 % in a 0.1 M solution on the other hand (Gu and Evans, 2008).
Table 8.
Point of zero charge of several sorbents (Langmuir, 1997; Stumm and Morgan, 1996).
| Mineral | Name | pH PZC |
|---|---|---|
| SiO2 | quartz | 2.0 |
| α-Fe2O3 | hematite | 4.2 to 6.9 |
| γ-Fe2O3 | maghemite | 6.7 |
| Fe3O4 | magnetite | 6.5 |
| α-FeOOH | goethite | 7.8 |
| 5 Fe2O3 · 9 H2O [Fe(OH)3 amorphous] | ferrihydrite | 8.5 |
| β-MnO2 | pyrolusite | 7.2 |
| δ-MnO2 | birnessite | 2.8 |
| kaolinite | kaolinite | 4.6 |
| Na feldspar | Na feldspar | 6.8 |
Usually, Cd fixation follows the two-site-sorption model. In the first step, Cd is adsorbed at highly energetic binding sites. The second step involves a slow, time dependent diffusion of Cd into the mineral matrix (Carrillo-Gonzalez et al., 2006; He et al., 2005; Loganathan et al., 2012; Strobel et al., 2005). After Smolders and Mertens (2013), a general sorption equation with surface functional groups is:
| (1) |
Co-precipitation is a possible mechanism of Cd fixation at high Cd concentrations in solution, whereas chemisorption is expected at low Cd concentrations (Ahmed et al., 2008).
Cadmium in solution can precipitate together with calcite in a solid solution:
| (2) |
In this case, Cd can substitute for Ca by forming new crystals at the mineral surface. Initially, fast adsorption of Cd occurs at the calcite surface, followed by slower diffusion of Cd into the crystal lattice. After that, formation of a calcium-cadmium carbonate solid solution occurs. These processes are controlled by pH and competition with Mg2+ within the hydrated surface layer (Davis et al., 1987). If there is bicarbonate in the liquid phase, Cd can form complexes or precipitate as otavite (Fig. 4c) and thus, decrease the pH value in poorly buffered systems (Ahmed et al., 2008):
| (3) |
At silicate surfaces, Cd sorption occurs as outer-sphere surface complexation with permanent negative loadings or as inner-sphere complexation with variable loadings. At Fe-, Mn- and Al-oxide surfaces, Cd fixation occurs by ion exchange with surface OH groups (Loganathan et al., 2012). Due to inner-sphere complexation, substantial uptake of Cd occurs at surfaces of goethite (α-FeOOH), lepidocrocite (γ-FeOOH), and pyrite (FeS2) (Parkman et al., 1999; Randall et al., 1999) as
| (4) |
or as
| (5) |
In contrast, adsorption capacity at the mackinawite (FeS) surface is lower due to surface precipitation (Parkman et al., 1999).
At phosphate mineral surfaces, Cd is bound via surface complexation or co-precipitation as a substitute for Ca2+, e.g., in hydroxy-apatite (Bolan et al., 2003):
| (6) |
Further Cd sorption or substitution occurs in P fertilizer compounds, such as fluorapatite (Ca10(PO4)6F2), tricalcium phosphate (Ca3(PO4)2), gypsum (CaSO4), and cadmium lead phosphate hydroxide ((CdPb9(PO4)6(OH)2) (Azzi et al., 2017).
However, depending on the phosphate concentration in solution, there is influence on the mobile Cd fraction. Cadmium mobility increases at low P concentrations due to pH decreases and elevated ionic strength (Grant, 2011). However, Cd can precipitate, e.g., as Cd3(PO4)2 at high P and Cd concentrations (Grant, 2011; Xiong, 1995). In the case of low Cd concentrations, phosphate induced surface complex formation can occur due to reduced PZC or co-adsorption of Cd and phosphate as an ion pair (Grant, 2011; Wang and Xing, 2002). Phosphate can block meso- and micro-pores, e.g., on the goethite surface, which inhibits Cd diffusion into the mineral structure and thus, decreases sorptive uptake (Wang and Xing, 2002). In the case of ferrihydrite, phosphate enhances Cd sorption by the formation of stable ternary complexes at the surface (Tiberg and Gustafsson, 2016):
| (7) |
Sorption onto organic matter occurs with negative carboxyl or phenol groups but also via the formation of chelate complexes (Loganathan et al., 2012). In the case of DOC, Cd sorption is pH dependent and occurs at pH > 4; whereas, sorption onto clay is pHindependent (Hammons et al., 1978). Depending on pH, type of organic matter and soil type, Cd sorption increases because of complexation with the negative binding sites of humic acids, which can sorb at positively charged soil surfaces. Cadmium sorption may decrease due to formation of soluble Cd-organic complexes (Bolan et al., 2003; Loganathan et al., 2012). Cadmium sorption onto organic matter decreases with increasing soil depth due to aging and subsequent degradation of organic matter (Mahara et al., 2007).
As Loganathan et al. (2012) reported, some experiments showed complete reversibility of Cd sorption onto soils and minerals, e.g., kaolinite and calcite, while others noted incomplete desorption due to Cd diffusion into the solid phase matrix, chemisorption on high-energy sites, and surface precipitation. Cadmium desorption increases with decreasing pH and concentration of organic ligands in solution, e.g., citrate, which enhances desorption due to complexation (Christensen, 1984a; Loganathan et al., 2012; Wasylenki et al., 2014). Cadmium desorption may also occur as pseudohysteresis, which means retarded desorption because higher activation energy is necessary for desorption than sorption. Apart from that, incomplete desorption processes to reach true equilibrium can occur due to slow reaction kinetics (Loganathan et al., 2012), because there is an initial rapid release of Cd from Al and Fe oxides that is followed by a slow release caused by strong binding of Cd, e.g., as ion exchange in the mineral structure of apatite (Strobel et al., 2005). Cadmium added by deposition, e.g., from sludge, fertilizers or lime is weakly bound, and therefore, it is mobilized during desorption. In contrast, natural Cd is dissolved slowly due to cation exchange (Strobel et al., 2005). In terms of sedimentary lithologies, Cd is mainly bound to finer-grained material such as silt and clay (Descourvieres et al., 2010).
5.4. Competition
Depending on the chloride concentration, formation of soluble Cd chloride complexes occurs producing species with different charges (e.g., CdCl+, CdCl20, CdCl3−, CdCl42−), which reduce Cd sorption substantially (Herms and Brummer, 1984), more so in comparison to Cu and Pb (Caetano et al., 2003). In the presence of other ligands such as sulfate and phosphate, Cd sorption increases because of the influence of cation exchange capacity and the withdrawal of competing cations such as Ca via complexation. In the presence of other heavy metals, e.g., Fe, Cd is adsorbed only to permanent negative binding sites (Loganathan et al., 2012). As opposed to Pb and Cu, Cd sorption to perthitic feldspar and muscovite is weak via outer-sphere surface complexation; whereas, Cd is bound strongly to biotite via ion exchange or inner-sphere surface complexation (Farquhar et al., 1997). In contrast to Cd, which is often bound to the easily solubilized fraction of soils and sediments, other heavy metals like Pb and Cu are often bound firmly to the organic, sulfidic, and residual fraction and thus, have a lower mobilization potential (Eggleton and Thomas, 2004; Zwonitzer et al., 2003). Some reports conclude that the fractionation depends on parent rock composition and redox potential (Liu et al., 2017; Zoumis et al., 2001).
The general order of affinity for heavy metal interaction with organic matter is as follows: Cu2+ > Cd2+ > Fe2+ > Pb2+ > Ni2+ > Co2+ > Mn2+ > Zn2+ (Bolan et al., 2003). The adsorption of Cd at pH values above eight decreases in the presence of organic ligands due to competition between the formation of organo-metallic complexes and surface adsorption, e.g., at the surface sites of goethite (Buerge-Weirich et al., 2002).
The diffusion rate of Cd into goethite is lower than that of Zn and Ni (Bruemmer et al., 1986). Cadmium sorption to Fe and Mn oxides is retarded in presence of other cations, and it decreases due to competition in the order: Ca2+ > K+ > Na+ (Christensen, 1984b; Wang et al., 2010). In the pH range 3 to 8, release of unspecific adsorbed Cd occurs, which increases with increasing salinity. An increase of ionic strength generally leads to a release of heavy metals in the order: Cd > Zn > Cu > Pb (Herms and Brummer, 1984; Kuntze et al., 1984). The presence of appropriate anions also influences sorption behavior. While Cl− and NO3− restrain Cd sorption due to the formation of soluble inorganic complexes, CO32−, H2PO4− and HSO4− enhance Cd sorption due to surface precipitation (Wang et al., 2010).
The level of mobility of heavy metals in solution in terms of varying pH and ionic strength is in the order: Cd2+ > Co2+ > Zn2+ > Ni2+ > Cu2+ > Pb2+ (Earon et al., 2012; Herms and Brummer, 1984; Hiller et al., 2001; Spark et al., 1995). Although Cd and Zn share similar geochemical behavior (Thornton, 1986), the limiting pH of Cd mobility is 6.5 and is thus higher than that of Zn (between 5.5 and 6.0) and other heavy metals (Ni 5.5; Co 5.5; Cu 4.5; Cr 4.0 to 4.5; Pb < 4) (Beisecker et al., 2012; Knappe et al., 2008).
Zinc is the most efficient competitor of Cd for sorption sites. The sorption affinity of heavy metals regarding soils and minerals such as kaolinite generally follows, with few exceptions and depending on the adsorbent and other factors, the Irving-Williams order: Hg2+ > Pb2+ > Cu2+ > Zn2+ > Ni2+ ≈ Co2+ > Cd2+ (Christensen, 1987; Gu and Evans, 2008; Loganathan et al., 2012; Ozverdi and Erdem, 2006). The enhanced sorption behavior of Pb and other competing heavy metals is caused by their smaller hydrated radius, their greater affinity for most functional groups of organic substances and their higher electronegativity (Appel and Ma, 2002). However, sorption at the calcite surface followed the selectivity sequence: Cd2+ > Zn2+ ≥ Mn2+ > Co2+ > Ni2+ ≫ Ba2+ = Sr2+ (Zachara et al., 1991).
In addition to Cd input, phosphate fertilizers application also decreases Cd sorption because of the competition of other components like NH4 or Zn, which can replace Cd from binding sites (Grant, 2011).
6. Case studies of elevated Cd concentrations in soil and groundwater
6.1. Ancient Cd contamination in Japan (itai-itai disease)
One of the most common diseases induced by Cd exposure is the itai-itai (meaning “ouch-ouch”) disease as in Toyama Prefecture, Japan, in the first half of the 20th century. The Jinzu River basin was the first known area with Cd pollution (Aoshima, 2016; ATSDR, 2012; Roberts, 2014; UNEP, 2010). The Jinzu River supplies 20 km2 of fields for paddy rice culture through several irrigation canals. Cadmium and other heavy metals originated in slag from a mine 30 km upstream. The expansion of mining activity in the Kamioka lead and zinc mine and smelting during the First and Second World War led to insufficient consideration for handling waste material and large amounts of Cd deposited for approximately 60 years (Aoshima, 2016).
Related to irrigation of the surrounding rice paddies, the soil and thus, the rice were contaminated with Cd until around 1960. The stream water was also used in households and as a drinking water supply. As a consequence, the itai-itai disease, caused by chronic Cd poisoning in combination with a low-calcium diet, became endemic among the inhabitants of the Jinzu River basin (Aoshima, 2016; ATSDR, 2012). Typical symptoms of Cd poisoning are osteoporosis and renal tubular dysfunction. In 1968, the Japanese government officially recognized the disease as being caused by Cd exposure. In an extensive survey, Cd and Zn levels in the soils were determined to be four times higher than in non-polluted samples. However, in rice samples, Cd contents were elevated 2.5 times higher than in rice from non-polluted areas. The mean Cd levels in the liver, pancreas, and thyroid of patients with the itai-itai disease were five times higher than reference cases. As a result, 15 km2 of the upper soil layer in the contaminated area had to be replaced (Aoshima, 2016).
6.2. Elevated Cd contents in ecuadorian cacao
Cacao is the most important crop in Ecuador, with a cultivated area of nearly 500,000 ha (Chavez et al., 2016). However, Cd concentrations in Ecuadorian cacao beans are the highest when compared to other cultivation areas. They exceed the critical value of 0.6 mg Cd/kg established by European Union and thus, affect worldwide chocolate production (Chavez et al., 2015). Possible reasons for Cd accumulation in soil and plants are anthropogenic influences like contamination of irrigation water by mining activities and phosphate fertilizers, but also natural sources such as volcanic ash and continental dust (Chavez et al., 2015). Nevertheless, distribution of Cd in the soil fractions affects the bioavailability of Cd, which is an indicator of the Cd source in the water and therefore in the cacao beans (Chavez et al., 2016). Apart from pH, the plant-available Cd content of the soil is the most critical parameter controlling uptake with total Cd contents up to 2.5 mg/kg (Chavez et al., 2015). As opposed to elevated Cd in soils caused primarily by weathering of parental material (e.g., Liu et al., 2017), the primary Cd pool in Ecuadorian soils are the water soluble, exchangeable, and acid-soluble fractions indicating an anthropogenic Cd source. Furthermore, acid-soluble Cd contents bound to organic matter in the soil correlates with Cd values in the cacao beans. Therefore, the dynamics of both soil pH and organic matter have to take into account for remediation of contaminated soil (Chavez et al., 2016).
6.3. Anthropogenic influences on Cd release in northwestern Germany
Groundwater in Northwestern Germany can have Cd concentrations exceeding the German threshold value of 0.5 μg/L. The assessment of a large-scale hydrogeochemical data set made it possible to investigate different aspects of Cd mobility with respect to hydrogeology, land use, and groundwater chemistry (Kubier et al., 2019; Kubier and Pichler, 2019). The main part of the area consists of sandy and gravelly Pleistocene glacial deposits and represents both, groundwater recharge areas and catchment areas for water supply. Due to the different hydrogeological settings, groundwater chemistry was heterogeneous. Reducing groundwater occurred in tidal wetlands and lowlands, while groundwater in Pleistocene glacial deposits aquifers was predominantly oxic with decreasing bicarbonate content. Most groundwater Cd concentrations above 0.5 μg/L were found in the western part of the area characteristic of intensive livestock farming and agriculture (Wriedt et al., 2019). Excessive nitrate input from fertilization can cause denitrification processes in groundwater. During autotrophic nitrate reduction, Cd, which is incorporated into the pyrite mineral structure during pyrite formation, can be released.
The study revealed that Cd concentrations in groundwater were mainly controlled by hydrogeochemical parameters. The amount of anthropogenic Cd input, in particular through the use of phosphate fertilizers, was of secondary importance. Instead, aquifer sediments can be enriched in Cd. Cadmium release was suggested to occur in terms of acidification and oxidation (Kubier and Pichler, 2019). In the Emsland region in the northwestern part of the area, pyrites in reducing aquifers were analyzed recently. Cadmium contents amounted to 300 mg/kg in median, 1600 mg/kg in maximum in the pyrites and, depending on pyrite content in the aquifer, 2.6 mg/kg in sediment (Houben et al., 2017). It is probable that these values are typical for glacial deposits, which are representative of larger parts of Northern Germany (Houben et al., 2017).
Besides influences from agriculture, elevated Cd concentrations were also related to woodland indicating Cd release from forest soils that are an effective sink for atmospherically deposited pollutants. Low pH and high concentrations of dissolved organic carbon enhance Cd transport into groundwater (Kubier et al., 2019).
7. Summary and conclusions
Cadmium is a heavy metal with specific hydrochemical characteristics causing its potential mobility in groundwater. It remains in solution at near neutral pH (< 6.5) in contrast to the typical fixation of other heavy metals. Cadmium sorption is weak in competitive situations. Cadmium tends to form stable dissolved complexes with both inorganic and organic ligands, which inhibit sorption and precipitation. In particular, its behavior with DOM can cause Cd mobility and provides a unique hydrogeochemical position for Cd relative to other heavy metals such as Zn, Ni, and Cu. Cadmium can replace Ca in the context of mineral formation and co-precipitation, and this is relevant regarding absorption into the human body.
Cadmium accumulation in soils can occur, where Cd mobility is inhibited, e.g., Cd can precipitate under anoxic conditions; Cd sorption is accompanied by enrichment of organic matter or clay. Anthropogenic sources of Cd in soils are direct input of waste material from mining and industry as well as agricultural application, e.g., as sewage sludge and phosphate fertilizers. Transport of Cd from soil into groundwater depends on hydrogeochemical factors regulating Cd mobility. Besides direct input of waste water, e.g., as runoff and leakage, or atmospheric deposition, Cd leaching from waste material, landfills, and fertilization only can happen where Cd release is promoted by replacement, formation of soluble complexes, acidification, or oxidation. Mining wastes usually go together with oxidation reactions and subsequently strongly decreased pH. Excessive N fertilization also decreases soil pH, which is associated with increased ionic strength and enhanced Cd mobility. Summarizing data on the number of soil and groundwater systems with Cd problems cannot be provided, because there exist only case studies dealing with specific contamination issues (Table 6). Further information on the amount of impacted areas and Cd fluxes from selected sites into groundwater are not available. Besides, the range and partially absence of national threshold values for Cd in soil and groundwater makes it difficult to identify impacted areas on an international scale.
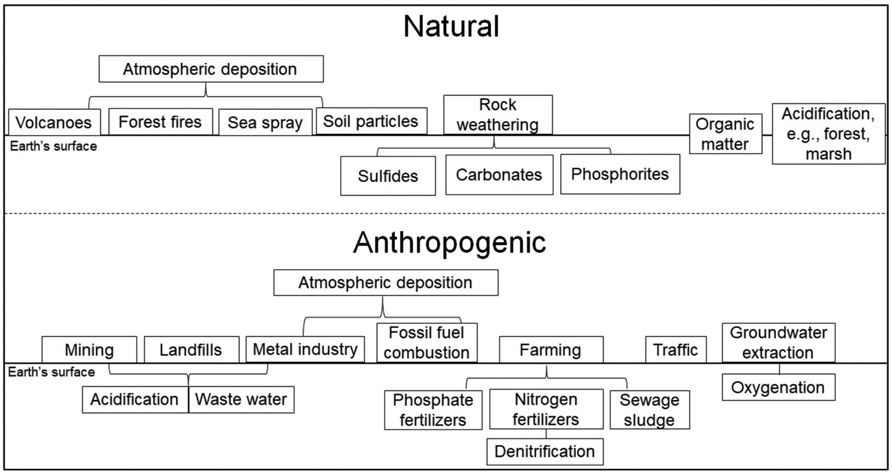
An overview of the most relevant Cd sources and influences on Cd release into soil and groundwater is given in Fig. 5. Atmospheric Cd deposition can be caused by anthropogenic and natural sources. Many reports dealing with Cd deposition consider higher estimations of Cd input from anthropogenic sources. More recent studies, however, calculated a natural amount of Cd deposition exceeding the anthropogenic amount, especially with respect to increasing off-gas cleanings. Consequently, atmospheric deposition of natural sources accounts 93 % of Cd emissions, especially from soil particles and wild fires (Table 1). Natural Cd release from weathering occurs in sulfidic and carbonatic systems due to Cd accumulation in sulfides, e.g., pyrite, and exchange of Ca in carbonates. The resulting Cd concentrations in groundwater depend on groundwater milieu. Cadmium release from calcareous systems and subsequent transport in groundwater is only possible in acidic waters (Fig. 1). Acidification can be related to natural influences, e.g., forest and marsh, but also caused by anthropogenic input, e.g., acid mine drainage. Anthropogenic Cd input from phosphate fertilizers into soil, for example, can exceed atmospheric deposition (Table 5). Drivers for Cd mobility are soil texture, content of organic matter, land use, and hydrochemical milieu. Soils with increasing amount of fine-grained material, organic matter, swamp and forest as land use as well as moderate pH and redox can contain elevated Cd contents. Cadmium leachate is controlled by quantity and quality of seepage; organic and inorganic ligands promote mobility of Cd bound in soluble complexes. In soils that are sandy and poor in organic matter feature low sorption capacity, Cd transport into groundwater, and a subsequent reduction of previously accumulated Cd can occur.
Fig. 5.
Cadmium sources and influences on Cd release into groundwater.
Cadmium pollution of soil and groundwater is a worldwide problem that affects resources for food and drinking water mainly in Asia and Africa. Further efforts to clean waste water, inhibit leachate of contaminated material, e.g., in landfills and mines, and reduce use of Cd contaminated phosphate fertilizers are necessary to decrease anthropogenic Cd output. As Fig. 2 indicates, the amount of Cd input into soil as an impurity in phosphate fertilizers depends on the origin. Thus, changes in fertilizer management do not consequently result in decreasing crop yields.
Depending on time scale, geographic position and land use, there are different scenarios for the quantity of Cd input into groundwater and the amounts of various sources, mainly deposition and P fertilizers. Apart from that, anthropogenic Cd input occurred in some areas in the past, which led to delayed Cd leaching into groundwater from soil and aquifer solids. There are other anthropogenic influences, e.g., fertilization and acidification, altering the hydrogeochemistry and thus enhancing the release of natural occurring Cd. The outline of these processes can be abstracted as the following scenarios of Cd release.
“Natural origin and release of Cd.” Elevated Cd concentrations in groundwater are linked to rock types with increased Cd contents, e.g., sulfides. Cadmium is released in the context of weathering or naturally caused acidification.
“Anthropogenically induced release of naturally occurring Cd.” In this case, Cd originates from natural sources, but its release is caused by anthropogenic influences, e.g., atmospheric deposition or acidification linked to denitrification of nitrogen fertilizers.
“Anthropogenic Cd input.” According to the most likely reason for elevated Cd in groundwater, Cd originated from P fertilizers and atmospheric deposition. Further entries are linked to industrial activities and traffic.
In case of (1) and (2), Cd originates mainly from sorption or co-/precipitation in association with sulfide, carbonate and phosphate minerals. That is why Cd release occurs during the dissolution of these minerals according to the following three model processes (a) to (c).
-
(a)
Calcite dissolution (under acidic conditions) (equation (8)):
-
(b)
Oxidation of sulfide minerals, e.g., pyrite (under oxic conditions) (equation (9)):
Possible subsequent reaction (equation (10)):
-
(c)
Chemolithotrophic denitrification (under anoxic conditions) (equation (11)):
Possible subsequent reaction (equation (12)):
In case of (3), Cd originates from direct entry or dissolution of phosphate fertilizers. As a result, Cd is released, e.g., from fluorapatite, using soil acids, shown in reaction (d) (equation (13)):
-
(d)
Acknowledegments
This study was partly funded by Niedersächsischer Landesbetriebs für Wasserwirtschaft, Küsten- und Naturschutz (NLWKN) with funds from the State of Lower Saxony Division of Groundwater, Forschungsprojekt 62170-11-02/CD A31. We are thankful for the continued discussion of this topic with Dörte Budziak, Jörg Elbracht, Dieter de Vries and Kay Hamer. Comments by the associate editor and two anonymous reviewers helped to improve readability and focus of this review.
Footnotes
Declaration of interest
The authors certify that there is no actual or potential conflict of interest in relation to this article.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.apgeochem.2019.104388.
References
- Abd El-Salam MM, Abu-Zuid GI, 2015. Impact of landfill leachate on the groundwater quality: a case study in Egypt. J. Adv. Res 6, 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraitis PK, Pattrick RAD, Vaughan DJ, 2004. Variations in the compositional, textural and electrical properties of natural pyrite: a review. Int. J. Miner. Process 74, 41–59. [Google Scholar]
- Affum AO, Osae SD, Nyarko BJB, Afful S, Fianko JR, Akiti TT, Adomako D, Acquaah SO, Dorleku M, Antoh E, Barnes F, Affum EA, 2015. Total coliforms, arsenic and cadmium exposure through drinking water in the Western Region of Ghana: application of multivariate statistical technique to groundwater quality. Environ. Monit. Assess 187. [DOI] [PubMed] [Google Scholar]
- Ahmed IAM, Crout NMJ, Young SD, 2008. Kinetics of Cd sorption, desorption and fixation by calcite: a long-term radiotracer study. Geochem. Cosmochim. Acta 72, 1498–1512. [Google Scholar]
- Akbar KF, Hale WHG, Headley AD, Athar M, 2006. Heavy metal contamination of roadside soils of northern england. Soil Water Res. 158–163. [Google Scholar]
- Al-Khashman OA, Shawabkeh RA, 2006. Metals distribution in soils around the cement factory in southern Jordan. Environ. Pollut 140, 387–394. [DOI] [PubMed] [Google Scholar]
- Al Husseini AE, Bechet B, Gaudin A, Ruban V, 2013. Trace metal fractionation as a mean to improve on the management of contaminated sediments from runoff water in infiltration basins. Environ. Technol 34, 1255–1266. [DOI] [PubMed] [Google Scholar]
- Alloway BJ, Jackson AP, 1991. The behavior of heavy-metals in sewage sludgeamended soils. Sci. Total Environ 100, 151–176. [DOI] [PubMed] [Google Scholar]
- Anderson PR, Christensen TH, 1988. Distribution coefficients of Cd, Co, Ni, and Zn in soils. J. Soil Sci 39, 15–22. [Google Scholar]
- Aoshima K, 2016. Itai-itai disease: renal tubular osteomalacia induced by environmental exposure to cadmium - historical review and perspectives. Soil Sci. Plant Nutr 62, 319–326. [Google Scholar]
- Appel C, Ma L, 2002. Concentration, pH, and surface charge effects on cadmium and lead sorption in three tropical soils. J. Environ. Qual 31, 581–589. [PubMed] [Google Scholar]
- Arain MB, Kazi TG, Baig JA, Afridi HI, Sarajuddin, Brehman KD, Panhwar H, Arain SS, 2015. Co-exposure of arsenic and cadmium through drinking water and tobacco smoking: risk assessment on kidney dysfunction. Environ. Sci. Pollut. Res 22, 350–357. [DOI] [PubMed] [Google Scholar]
- Astruc M, 1986. Evaluation of methods for the speciation of Cadmium In: Mislin H, Ravera O (Eds.), Cadmium in the Environment. Birkhaeuser, Basel Boston Stuttgart, pp. 12–24. [Google Scholar]
- ATSDR, 2012. A Toxicological Profile for Cadmium. Agency for Toxic Substances and Disease Registry, Atlanta, pp. 430. [PubMed] [Google Scholar]
- Avkopashvili G, Avkopashvili M, Gongadze A, Gakhokidze R, 2017. Eco-monitoring of Georgia's contaminated soil and water with heavy metals. Carpath J Earth Env 12, 595–604. [Google Scholar]
- Ayotte JD, Gronberg JM, Apodaca LE, 2011. Trace Elements and Radon in Groundwater across the United States, 1992–2003, U.S. Geological Survey Scientific Investigations Report 2011–5059. U.S. Geological Survey, Reston, Virginia, pp. 115. [Google Scholar]
- Azzi V, Kazpard V, Lartiges B, Kobeissi A, Kanso A, El Samrani AG, 2017. Trace metals in phosphate fertilizers used in eastern mediterranean countries. Clean. - Soil, Air, Water 45. [Google Scholar]
- Baize D, Sterckeman T, 2001. Of the necessity of knowledge of the natural pedo-geochemical background content in the evaluation of the contamination of soils by trace elements. Sci. Total Environ 264, 127–139. [DOI] [PubMed] [Google Scholar]
- Barringer JL, Szabo Z, Reilly PA, 2013. Occurrence and mobility of mercury in groundwater In: Bradley PM (Ed.), Current Perspectives in Contaminant Hydrology and Water Resources Sustainability. InTech, Rijeka, Croatia, pp. 117–149. [Google Scholar]
- Baun DL, Christensen TH, 2004. Speciation of heavy metals in landfill leachate: a review. Waste Manag. Res 22, 3–23. [DOI] [PubMed] [Google Scholar]
- Bech J, Poschenrieder C, Llugany M, Barcelo J, Tume P, Tobias FJ, Barranzuela JL, Vasquez ER, 1997. Arsenic and heavy metal contamination of soil and vegetation around a copper mine in Northern Peru. Sci. Total Environ 203, 83–91. [Google Scholar]
- Beisecker R, Blankenburg H, Bittersohl J, Evers J, Groger J, Jakobson C, Kubal C, Meissner R, Rupp H, Schrautzer J, Seeger J, Walther W, 2012. Diffuse Stoffausträge aus Wald und naturnahen Nutzungen Working Group of the Federal States on Water Issues. Bund/Länder-Arbeitsgemeinschaft Wasser), Kassel, Gottingen, pp. 132. [Google Scholar]
- Belon E, Boisson M, Deportes IZ, Eglin TK, Feix I, Bispo AO, Galsomies L, Leblond S, Guellier CR, 2012. An inventory of trace elements inputs to French agricultural soils. Sci. Total Environ 439, 87–95. [DOI] [PubMed] [Google Scholar]
- Beyer WN, Stafford C, 1993. Survey and evaluation of contaminants in earthworms and in soils derived from dredged material at confined disposal facilities in the greatlakes region. Environ. Monit. Assess 24, 151–165. [DOI] [PubMed] [Google Scholar]
- BGR, 2014. Groundwater Background Values (HUEK200 HGW), v2.9, Hydrogeological Map of Germany 1 : 200,000 Federal Institute for Geosciences and Natural Resources, Geological Surveys of the Federal States of Germany (Bundesanstalt für Geowissenschaften und Rohstoffe, Staatliche Geologische Dienste), Hanover. [Google Scholar]
- Bi XY, Feng XB, Yang YG, Qiu GL, Lia GH, 2006. Quantitative assessment of cadmium emission from zinc smelting and its influences on the surface soils and mosses in Hezhang County, Southwestern China. Atmos. Environ 40, 4228–4233. [Google Scholar]
- Bigalke M, Ulrich A, Rehmus A, Keller A, 2017. Accumulation of cadmium and uranium in arable soils in Switzerland. Environ. Pollut 221, 85–93. [DOI] [PubMed] [Google Scholar]
- Birke M, Reimann C, Rauch U, Ladenberger A, Demetriades A, Jahne-Klingberg F, Oorts K, Gosar M, Dinelli E, Halamic J, Team GP, 2017. GEMAS: cadmium distribution and its sources in agricultural and grazing land soil of Europe - original data versus clr-transformed data. J. Geochem. Explor 173, 13–30. [Google Scholar]
- Bladon KD, Emelko MB, Silins U, Stone M, 2014. Wildfire and the future of water supply. Environ. Sci. Technol 48, 8936–8943. [DOI] [PubMed] [Google Scholar]
- Bolan NS, Adriano DC, Naidu R, 2003. Role of phosphorus in (im)mobilization and bioavailability of heavy metals in the soil-plant system. Rev. Environ. Contam. Toxicol 177 177, 1–44. [DOI] [PubMed] [Google Scholar]
- Bostick BC, Fendorf S, Fendorf M, 2000. Disulfide disproportionation and CdS formation upon cadmium sorption on FeS2. Geochem. Cosmochim. Acta 64, 247–255. [Google Scholar]
- Brookins DG, 1986. Geochemical behavior of antimony, arsenic, cadmium and thallium - eh-ph diagrams for 25-degrees-C, 1-bar pressure. Chem. Geol 54, 271–278. [Google Scholar]
- Bruemmer GW, Gerth J, Herms U, 1986. Heavy-metal species, mobility and availability in soils. Zeitschrift Fur Pflanzenernahrung Und Bodenkunde 149, 382–398. [Google Scholar]
- Bruemmer GW, Zeien H, Hiller DA, Hornburg V, 1994. Bindungsformen und Mobilität von Cadmium und Blei in Böden In: Kreysa G, Wiesner J (Eds.), Beurteilung von Schwermetallen in Boden von Ballungsgebieten: Arsen, Blei und Cadmium Internationale Expertenbeiträge und Resümee der DECHEMAArbeitsgruppe “Bewertung von Gefährdungspotenzialen im Bodenschutz”. Deutsche Gesellschaft für Chemisches Apparatewesen, Chemische Technik und Biotechnologie e, pp. 524 Frankfurt am Main. [Google Scholar]
- Buerge-Weirich D, Hari R, Xue HB, Behra P, Sigg L, 2002. Adsorption of Cu, Cd, and Ni on goethite in the presence of natural groundwater ligands. Environ. Sci. Technol 36, 328–336. [DOI] [PubMed] [Google Scholar]
- Burke MP, Hogue TS, Kinoshita AM, Barco J, Wessel C, Stein ED, 2013. Pre- and post-fire pollutant loads in an urban fringe watershed in Southern California. Environ. Monit. Assess 185, 10131–10145. [DOI] [PubMed] [Google Scholar]
- Caetano M, Madureira MJ, Vale C, 2003. Metal remobilisation during resuspension of anoxic contaminated sediment: short-term laboratory study. Water Air Soil Pollut. 143, 23–40. [Google Scholar]
- Campos I, Abrantes N, Keizer JJ, Vale C, Pereira P, 2016. Major and trace elements in soils and ashes of eucalypt and pine forest plantations in Portugal following a wildfire. Sci. Total Environ 572, 1363–1376. [DOI] [PubMed] [Google Scholar]
- Cappuyns V, Van Herreweghe S, Swennen R, Ottenburgs R, Deckers J, 2002. Arsenic pollution at the industrial site of Reppel-Bocholt (north Belgium). Sci. Total Environ 295, 217–240. [DOI] [PubMed] [Google Scholar]
- Carlson CEA, Morrison GM, 1992. Fractionation and toxicity of metals in sewagesludge. Environ. Technol 13, 751–759. [Google Scholar]
- Carrillo-Gonzalez R, Simunek J, Sauve S, Adriano D, 2006. Mechanisms and pathways of trace element mobility in soils. Adv. Agron 91, 111–178. [Google Scholar]
- Chavez E, He ZL, Stoffella PJ, Mylavarapu RS, Li YC, Baligar VC, 2016. Chemical speciation of cadmium: an approach to evaluate plant-available cadmium in Ecuadorian soils under cacao production. Chemosphere 150, 57–62. [DOI] [PubMed] [Google Scholar]
- Chavez E, He ZL, Stoffella PJ, Mylavarapu RS, Li YC, Moyano B, Baligar VC, 2015. Concentration of cadmium in cacao beans and its relationship with soil cadmium in southern Ecuador. Sci. Total Environ 533, 205–214. [DOI] [PubMed] [Google Scholar]
- Chen CM, Liu MC, 2006. Ecological risk assessment on a cadmium contaminated soil landfill - a preliminary evaluation based on toxicity tests on local species and sitespecific information. Sci. Total Environ 359, 120–129. [DOI] [PubMed] [Google Scholar]
- Chen HP, Tang Z, Wang P, Zhao FJ, 2018. Geographical variations of cadmium and arsenic concentrations and arsenic speciation in Chinese rice. Environ. Pollut 238, 482–490. [DOI] [PubMed] [Google Scholar]
- Chen YL, Ma J, Li YT, Weng LP, 2019. Enhanced cadmium immobilization in saturated media by gradual stabilization of goethite in the presence of humic acid with increasing pH. Sci. Total Environ 648, 358–366. [DOI] [PubMed] [Google Scholar]
- Christensen JB, Jensen DL, Christensen TH, 1996. Effect of dissolved organic carbon on the mobility of cadmium, nickel and zinc in leachate polluted groundwater. Water Res. 30, 3037–3049. [Google Scholar]
- Christensen TH, 1984a. Cadmium soil sorption at low concentrations .1. Effect of time, cadmium load, ph, and calcium. Water Air Soil Pollut. 21, 105–114. [Google Scholar]
- Christensen TH, 1984b. Cadmium soil sorption at low concentrations .2. Reversibility, effect of changes in solute composition, and effect of soil aging. Water Air Soil Pollut. 21, 115–125. [Google Scholar]
- Christensen TH, 1985. Cadmium soil sorption at low concentrations .4. Effect of waste leachates on distribution coefficients. Water Air Soil Pollut. 26, 265–274. [Google Scholar]
- Christensen TH, 1987. Cadmium soil sorption at low concentrations .5. Evidence of competition by other heavy-metals. Water Air Soil Pollut. 34, 293–303. [Google Scholar]
- Cloquet C, Carignan J, Libourel G, Sterckeman T, Perdrix E, 2006. Tracing source pollution in soils using cadmium and lead isotopes. Environ. Sci. Technol 40, 2525–2530. [DOI] [PubMed] [Google Scholar]
- Davis JA, Fuller CC, Cook AD, 1987. A model for trace-metal sorption processes at the calcite surface - adsorption of Cd-2+ and subsequent solid-solution formation. Geochem. Cosmochim. Acta 51, 1477–1490. [Google Scholar]
- Deepali KK, Gangwar K, 2010. Metals concentration in textile and tannery effluents, associated soils and ground water. N. Y. Sci. J 3, 82–89. [Google Scholar]
- Demeyer A, Nkana JCV, Verloo MG, 2001. Characteristics of wood ash and influence on soil properties and nutrient uptake: an overview. Bioresour. Technol 77, 287–295. [DOI] [PubMed] [Google Scholar]
- Descourvieres C, Hartog N, Patterson BM, Oldham C, Prommer H, 2010. Geochemical controls on sediment reactivity and buffering processes in a heterogeneous aquifer. Appl. Geochem 25, 261–275. [Google Scholar]
- Ditoro DM, Mahony JD, Hansen DJ, Scott KJ, Hicks MB, Mayr SM, Redmond MS, 1990. Toxicity of cadmium in sediments - the role of acid volatile sulfide. Environ. Toxicol. Chem 9, 1487–1502. [Google Scholar]
- Dittrich B, Klose R, 2008. Schwermetalle in Düngemitteln Sächsisch.es Landesamt für Umwelt Landwirtschaft und Geologic, Dresden, pp. 41. [Google Scholar]
- Duijnisveld WHM, Godbersen L, Dilling J, Gäbler H-E, Utermann J, Klump G, Scheeder G, 2008. Ermittlung flächenrepräsentativer Hintergrundkonzentrationen prioritärer Schadstoffe im Bodensickerwasser Federal Institute for Geosciences and Natural Resources (Bundesanstalt fur Geowissenschaften und Rohstoffe, Hanover, pp. 163. [Google Scholar]
- Dwivedi AK, Vankar PS, 2014. Source identification study of heavy metal contamination in the industrial hub of Unnao, India. Environ. Monit. Assess 186, 3531–3539. [DOI] [PubMed] [Google Scholar]
- Earon R, Olofsson B, Renman G, 2012. Initial effects of a new highway section on soil and groundwater. Water Air Soil Pollut. 223, 5413–5432. [Google Scholar]
- EC, 2000. Directive 2000/60/EC of the european parliament and of the council of 23 October 2000 establishing a framework for community action in the field of water policy, 1327/1. Off. J. Eur. Communities 72 22/December/2000. [Google Scholar]
- EC, 2006. Directive 2006/118/CE of the european parliament and of the council of 12 december 2006 on the protection of groundwater against pollution and deterioration, L 182/19. Off. J. Eur. Communities 13 27/December/2006. [Google Scholar]
- EC, 2010. Commission Staff Working Document Accompanying the Report from the Commission in Accordance with Article 3.7 of the Groundwater Directive 2006/118/EC on the Establishment of Groundwater Threshold Values. European Commission, pp. 43. [Google Scholar]
- Eggleton J, Thomas KV, 2004. A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ. Int 30, 973–980. [DOI] [PubMed] [Google Scholar]
- Eiswirth M, Hotzl H, 1997. The impact of leaking sewers on urban groundwater. Groundw. Urban Environ I, 399–404. [Google Scholar]
- EU, 2007. European Union Risk Assessment Report – Cadmium Oxide and Cadmium Metal Part I – Environment Office for Official Publications of the European Communities, Luxembourg, pp. 608. [Google Scholar]
- Farquhar ML, Vaughan DJ, Hughes CR, Charnock JM, England KER, 1997. Experimental studies of the interaction of aqueous metal cations with mineral substrates: lead, cadmium, and copper with perthitic feldspar, muscovite, and biotite. Geochem. Cosmochim. Acta 61, 3051–3064. [Google Scholar]
- Gardiner J, 1974. Chemistry of cadmium in natural-water .1. Study of cadmium complex-formation using cadmium specific-ion electrode. Water Res. 8, 23–30. [Google Scholar]
- Garrett RG, Porter ARD, Hunt PA, Lalor GC, 2008. The presence of anomalous trace element levels in present day Jamaican soils and the geochemistry of Late-Miocene or Pliocene phosphorites. Appl. Geochem 23, 822–834. [Google Scholar]
- Gnandi K, Tobschall HJ, 2002. Heavy metals distribution of soils around mining sites of cadmium-rich marine sedimentary phosphorites of Kpogame and Hahotoe (southern Togo). Environ. Geol 41, 593–600. [Google Scholar]
- Gong H, Rose AW, Suhr NH, 1977. Geochemistry of cadmium in some sedimentaryrocks. Geochem. Cosmochim. Acta 41, 1687–1692. [Google Scholar]
- Grant CA, 2011. Influence of phosphate fertilizer on cadmium in agricultural soils and crops. Pedologist 3, 143–155. [Google Scholar]
- Groschen GE, Arnold TL, Morrow WS, Warner KL, 2008. Occurrence and Distribution of Iron, Manganese, and Selected Trace Elements in Ground Water in the Glacial Aquifer System of the Northern United States, U.S. Geological Survey Scientific Investigations Report 2009–5006. U.S. Geological Survey, Reston, Virginia, pp. 89. [Google Scholar]
- Gu XY, Evans LJ, 2008. Surface complexation modelling of Cd(II), Cu(II), Ni(II), Pb(II) and Zn(II) adsorption onto kaolinite. Geochem. Cosmochim. Acta 72, 267–276. [Google Scholar]
- Hajeb P, Sloth JJ, Shakibazadeh S, Mahyudin NA, Afsah-Hejri L, 2014. Toxic elements in food: occurrence, binding, and reduction approaches. Compr. Rev. Food Sci. Food Saf 13, 457–472. [DOI] [PubMed] [Google Scholar]
- Hammons AS, Huff EJ, Braunstein HM, Drury JS, Shriner CR, Lewis EB, Whitfield BL, Towill LE, 1978. Reviews of the Environmental Effects of Pollutants: IV. Cadmium United States Environmental Protection Agency, Oak Ridge National Laboratory, Cincinnati, pp. 253. [Google Scholar]
- He ZL, Xu HP, Zhu YM, Yang XE, Chen GC, 2005. Adsorption-desorption characteristics of cadmium in variable charge soils. J Environ Sci Heal 40, 805–822. [DOI] [PubMed] [Google Scholar]
- Hem JD, 1972. Chemistry and occurrence of cadmium and zinc in surface water and groundwater. Water Resour. Res 8, 661–679. [Google Scholar]
- Herms U, Brummer G, 1984. Solubility and retention of heavy-metals in soils. Zeitschrift Fur Pflanzenernahrung Und Bodenkunde 147, 400–424. [Google Scholar]
- Hiller DA, Winzig G, Dornauf C, 2001. Bodenchemische Untersuchungen von Versickerungsanlagen als Grundlage für eine nachhaltige Niederschlagswasserbewirtschaftung im Sinne des Boden- und Grundwasserschutzes, Abschlussbericht an das Ministerium für Umwelt und Naturschutz, Landwirtschaft und Verbraucherschutz des Landes Nordrhein-Westfalen. Univ. Essen, pp. 94. [Google Scholar]
- Hindersmann I, Mansfeldt T, 2014. Trace element solubility in a multimetal-contaminated soil as affected by redox conditions. Water Air Soil Pollut. 225. [Google Scholar]
- Holmgren GGS, Meyer MW, Chaney RL, Daniels RB, 1993. Cadmium, lead, zinc, copper, and nickel in agricultural soils of the united-states-of-America. J. Environ. Qual 22, 335–348. [Google Scholar]
- Houben GJ, Sitnikova MA, Post VEA, 2017. Terrestrial sedimentary pyrites as a potential source of trace metal release to groundwater - a case study from the Emsland, Germany. Appl. Geochem 76, 99–111. [Google Scholar]
- Husson O, 2013. Redox potential (Eh) and pH as drivers of soil/plant/microorganism systems: a transdisciplinary overview pointing to integrative opportunities for agronomy. Plant Soil 362, 389–417. [Google Scholar]
- Ilyin I, Gusev A, Rozovskaya O, Strijkina I, 2016. Transboundary Pollution by Heavy Metals and Persistent Organic Pollutants in 2014 – Germany, EMEP/MSC-E Data Note 5/2016. pp. 33. [Google Scholar]
- Joimel S, Cortet J, Jolivet CC, Saby NPA, Chenot ED, Branchu P, Consales JN, Lefort C, Morel JL, Schwartz C, 2016. Physico-chemical characteristics of topsoil for contrasted forest, agricultural, urban and industrial land uses in France. Sci. Total Environ 545, 40–47. [DOI] [PubMed] [Google Scholar]
- Jordan MM, Montero MA, Pina S, Garcia-Sanchez E, 2009. Mineralogy and distribution of Cd, Ni, Cr, and Pb in biosolids-amended soils from castellon province (NE, Spain). Soil Sci. 174, 14–20. [Google Scholar]
- Kabata-Pendias A, 2011. Trace Elements in Soils and Plants, 4 ed. CRC Press, Boca raton [Google Scholar]
- Kabir E, Ray S, Kim KH, Yoon HO, Jeon EC, Kim YS, Cho YS, Yun ST, Brown RJC, 2012. Current status of trace metal pollution in soils affected by industrial activities. Sci. World J 10.1100/2012/916705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser K, Guggenberger G, 2005. Dissolved organic sulphur in soil water under Pinus sylvestris L. and Fagus sylvatica L. stands in northeastern Bavaria, Germany - variations with seasons and soil depth. Biogeochemistry 72, 337–364. [Google Scholar]
- Kamermann D, Groh H, Hoeper H, 2015. Schwermetallein- und -austräge niedersachsischer Boden-Dauerbeobachtungsflächen, GeoBerichte 30 State Authority of Mining, Energy and Geology (Landesamt für Bergbau, Energie und Geologie; ). pp. 56 Hanover. [Google Scholar]
- Karak T, Paul RK, Das S, Das DK, Dutta AK, Boruah RK, 2015. Fate of cadmium at the soil-solution interface: a thermodynamic study as influenced by varying pH at South 24 Parganas, West Bengal, India. Environ. Monit. Assess 187. [DOI] [PubMed] [Google Scholar]
- Karlsson T, Elgh-Dalgren K, Bjorn E, Skyllberg U, 2007. Complexation of cadmium to sulfur and oxygen functional groups in an organic soil. Geochem. Cosmochim. Acta 71, 604–614. [Google Scholar]
- Kepanen A, Lodenius M, Tulisao E, Hartikainen H, 2005. Effects of different wood ashes on the solubility of cadmium in two boreal forest soils. Boreal Environ. Res 10, 135–143. [Google Scholar]
- Khan MA, Khan S, Khan A, Alam M, 2017. Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ 601, 1591–1605. [DOI] [PubMed] [Google Scholar]
- Kharikov AM, Smetana VV, 2000. In: Heavy Metals and Radioactivity in Phosphate Fertilizers: Short Term Detrimental Effects, IFA Technical Conference, New Orleans, Louisiana, pp. 10. [Google Scholar]
- Kjeldsen P, Barlaz MA, Rooker AP, Baun A, Ledin A, Christensen TH, 2002. Present and long-term composition of MSW landfill leachate: a review. Crit. Rev. Environ. Sci. Technol 32, 297–336. [Google Scholar]
- Knappe F, Mohler S, Ostermayer A, Lazar S, Kaufmann C, 2008. Vergleichende Auswertung von Stoffeinträgen in Böden über verschiedene Eintragspfade Federal Environmental Agency (Umweltbundesamt; ), Dessau-Roßlau, pp. 382. [Google Scholar]
- Kozyatnyk I, Bouchet S, Bjorn E, Haglund P, 2016. Fractionation and size-distribution of metal and metalloid contaminants in a polluted groundwater rich in dissolved organic matter. J. Hazard Mater 318, 194–202. [DOI] [PubMed] [Google Scholar]
- Krishnamurti GSR, Naidu R, 2003. Solid-solution equilibria of cadmium in soils. Geoderma 113, 17–30. [Google Scholar]
- Kubier A, Hamer K, Pichler T, 2019. Cadmium background levels in groundwater in an area dominated by agriculture in Northwestern Germany. Integr. Environ. Assess. Manag (submitted for publication). [DOI] [PubMed] [Google Scholar]
- Kubier A, Pichler T, 2019. Cadmium in groundwater – A synopsis based on a large hydrogeochemical data set. Sci. Total Environ 689, 831–842. [DOI] [PubMed] [Google Scholar]
- Kuntze H, Herms U, Pluquet E, 1984. Schwermetalle in Böden. Bewertung und Gegenmaßnahmen. Geol. Jahrb 75, 715–736. [Google Scholar]
- LABO, 2017. Background values for inorganic and organic parameters in soil In: Working Group of the Federal States on Soil Protection (Bund/Länder-Arbeitsgemeinschaft Bodenschutz; ), 4 ed. pp. 41. [Google Scholar]
- Langmuir D, 1997. Aqueous Environmental Geochemistry. Prentice Hall, New Jersey. [Google Scholar]
- Li F, Qiu ZZ, Zhang JD, Liu WC, Liu CY, Zeng GM, 2017. Investigation, pollution mapping and simulative leakage health risk assessment for heavy metals and metalloids in groundwater from a typical brownfield, Middle China. Int. J. Environ. Res. Public Health 14, 768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XX, Rubaek GH, Sorensen P, 2016. High plant availability of phosphorus and low availability of cadmium in four biomass combustion ashes. Sci. Total Environ 557, 851–860. [DOI] [PubMed] [Google Scholar]
- Li YC, Ge Y, Zhang CH, Zhou QS, 2010. Mechanisms for high Cd activity in a red soil from southern China undergoing gradual reduction. Aust. J. Soil Res 48, 371–384. [Google Scholar]
- Lin LQ, Cong L, Yun WH, Yang J, Ming H, Wan ZB, Kai C, Lei H, 2015. Association of soil cadmium contamination with ceramic industry: a case study in a Chinese town. Sci. Total Environ 514, 26–32. [DOI] [PubMed] [Google Scholar]
- Lin ZB, Schneider A, Sterckeman T, Nguyen C, 2016. Ranking of mechanisms governing the phytoavailability of cadmium in agricultural soils using a mechanistic model. Plant Soil 399, 89–107. [Google Scholar]
- Liu YZ, Xiao TF, Perkins RB, Zhu JM, Zhu ZJ, Xiong Y, Ning ZP, 2017. Geogenic cadmium pollution and potential health risks, with emphasis on black shale. J. Geochem. Explor 176, 42–49. [Google Scholar]
- Loganathan P, Vigneswaran S, Kandasamy J, Naidu R, 2012. Cadmium sorption and desorption in soils: a review. Crit. Rev. Environ. Sci. Technol 42, 489–533. [Google Scholar]
- Luo L, Ma YB, Zhang SZ, Wei DP, Zhu YG, 2009. An inventory of trace element inputs to agricultural soils in China. J. Environ. Manag 90, 2524–2530. [DOI] [PubMed] [Google Scholar]
- Mahara Y, Kubota T, Wakayama R, Nakano-Ohta T, Nakamura T, 2007. Effects of molecular weight of natural organic matter on cadmium mobility in soil environments and its carbon isotope characteristics. Sci. Total Environ 387, 220–227. [DOI] [PubMed] [Google Scholar]
- Malarkodi M, Krishnasamy R, Kumaraperumal R, Chitdeshwari T, 2007. Characterization of heavy metal contaminated soils of Coimbatore district in Tamil Nadu. Agronomy 6, 147–151. [Google Scholar]
- Mar SS, Okazaki M, 2012. Investigation of Cd contents in several phosphate rocks used for the production of fertilizer. Microchem. J 104, 17–21. [Google Scholar]
- Martinez CE, McBride MB, Kandianis MT, Duxbury JM, Yoon SJ, Bleam WF, 2002. Zinc-sulfur and cadmium-sulfur association in metalliferous peats evidence from spectroscopy, distribution coefficients, and phytoavailability. Environ. Sci. Technol 36, 3683–3689. [DOI] [PubMed] [Google Scholar]
- Merkel BJ, Sperling B, 1998. Hydrogeochemische Stoffsysteme Teil II, Schriftenreihe des Deutschen Verbandes für Wasserwirtschaft und Kulturbau e. V. Wirtschafts- und Verl.-Ges. Gas und Wasser, Bonn. [Google Scholar]
- Ministry of Health of China, P., 2006. National Standard of the People's republic of China: Standard for Drinking Water Quality, GB 5749-2006. Standardization Administration of China, pp. 16. [Google Scholar]
- Mirlean N, Roisenberg A, 2006. The effect of emissions of fertilizer production on the environment contamination by cadmium and arsenic in southern Brazil. Environ. Pollut 143, 335–340. [DOI] [PubMed] [Google Scholar]
- Monna F, Hamer K, Leveque J, Sauer M, 2000. Pb isotopes as a reliable marker of early mining and smelting in the Northern Harz province (Lower Saxony, Germany). J. Geochem. Explor 68, 201–210. [Google Scholar]
- Moral R, Gilkes RJ, Jordan MM, 2005. Distribution of heavy metals in calcareous and non-calcareous soils in Spain. Water Air Soil Pollut. 162, 127–142. [Google Scholar]
- Najafi S, Jalali M, 2015. Effects of organic acids on cadmium and copper sorption and desorption by two calcareous soils. Environ. Monit. Assess 187. [DOI] [PubMed] [Google Scholar]
- Naseem S, Hamza S, Nawaz-ul-Huda S, Bashir E, ul-Haq Q, 2014. Geochemistry of Cd in groundwater of Winder, Balochistan and suspected health problems. Environ. Earth Sci 71, 1683–1690. [Google Scholar]
- Neumayer V, Matthess G, 1977. Schwermetalle in grundwässern der Westküste schleswig-holsteins. Vom Wasser 48, 17–39. [Google Scholar]
- Nicholson FA, Smith SR, Alloway BJ, Carlton-Smith C, Chambers BJ, 2003. An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci. Total Environ 311, 205–219. [DOI] [PubMed] [Google Scholar]
- Nies DH, 1999. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol 51, 730–750. [DOI] [PubMed] [Google Scholar]
- Nies DH, 2003. Efflux-mediated heavy metal resistance in prokaryotes. FEMS Microbiol. Rev 27, 313–339. [DOI] [PubMed] [Google Scholar]
- Ololade IA, Adewunmi A, Ologundudu A, Adeleye A., 2009. Effects of household wastes on surface and underground waters. Int. J. Phys. Sci 4, 22–29. [Google Scholar]
- Onyatta JO, Huang PM, 2006. Distribution of applied cadmium in different size fractions of soils after incubation. Biol. Fertil. Soils 42, 432–436. [Google Scholar]
- Oosterhuis FH, Brouer FM, Wijnants HJ, 2000. A Possible EU Wide Charge on Cadmium in Phosphate Fertilisers: Economic and Environmental Implications. Final report to the European Commission, Amsterdam, pp. 75. [Google Scholar]
- OSPAR, 2008. Atmospheric Deposition of Selected Heavy Metals and Persistent Organic Pollutants to the OSPAR Maritime Area (1990 - 2005). OSPAR Commission, London, pp. 99. [Google Scholar]
- Ozverdi A, Erdem M, 2006. Cu2+, Cd2+ and Pb2+ adsorption from aqueous solutions by pyrite and synthetic iron sulphide. J. Hazard Mater. 137, 626–632. [DOI] [PubMed] [Google Scholar]
- Pacyna JM, Pacyna EG, 2001. An assessment of global and regional emissions of trace metals to the atmosphere from anthropogenic sources worldwide. Environ. Rev 9, 269–298. [Google Scholar]
- Page AL, Chang AC, El-Amamy M, 1987. Cadmium levels in soils and crops in the United States In: Hutchinson TC, Meema KM (Eds.), Lead, Mercury, Cadmium and Arsenic in the Environment. Wiley, Chichester, New York, pp. 119–146. [Google Scholar]
- Pan JL, Plant JA, Voulvoulis N, Oates CJ, Ihlenfeld C, 2010. Cadmium levels in Europe: implications for human health. Environ Geochem Hlth 32, 1–12. [DOI] [PubMed] [Google Scholar]
- Panwar RM, Ahmed S, 2018. Assessment of contamination of soil and groundwater due to e-waste handling. Curr Sci India 114, 166–173. [Google Scholar]
- Parkhurst DL, Appelo CAJ, 1999. User's Guide to PHREEQC (Version 2) – A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Calculations. United States Geological Survey, Denver, pp. 312. [Google Scholar]
- Parkman RH, Charnock JM, Bryan ND, Livens FR, Vaughan DJ, 1999. Reactions of copper and cadmium ions in aqueous solution with goethite, lepidocrocite, mackinawite, and pyrite. Am. Mineral 84, 407–419. [Google Scholar]
- Paulson AJ, 1997. The transport and fate of Fe, Mn, Cu, Zn, Cd, Ph and SO4 in a groundwater plume and in downstream surface waters in the Coeur d'Alene Mining District, Idaho, USA. Appl. Geochem 12, 447–464. [Google Scholar]
- Perkiomaki J, Fritze H, 2005. Cadmium in upland forests after vitality fertilization with wood ash - a summary of soil microbiological studies into the potential risk of cadmium release. Biol. Fertil. Soils 41, 75–84. [Google Scholar]
- Randall SR, Sherman DM, Ragnarsdottir KV, Collins CR, 1999. The mechanism of cadmium surface complexation on iron oxyhydroxide minerals. Geochem. Cosmochim. Acta 63, 2971–2987. [Google Scholar]
- Richardson MG, Garrett R, Mitchell IA, Mah-Paulson M, Hackbarth T, 2001. Critical Review on Natural Global and Regional Emissions of Six Trace Metals to the atmosphere International Lead Zinc Research Organisation, the International Copper Association, and the Nickel Producers Environmental Research Association. pp. 52. [Google Scholar]
- Rieuwerts J, Farago M, 1996. Heavy metal pollution in the vicinity of a secondary lead smelter in the Czech Republic. Appl. Geochem 11, 17–23. [Google Scholar]
- Roberts TL, 2014. Cadmium and phosphorous fertilizers: the issues and the science. In: Symphos 2013 - 2nd International Symposium on Innovation and Technology in the Phosphate Industry, vol.83 pp. 52–59. [Google Scholar]
- Rosner U, 1998. Effects of historical mining activities on surface water and groundwater - an example from northwest Arizona. Environ. Geol 33, 224–230. [Google Scholar]
- Sauve S, Norvell WA, McBride M, Hendershot W, 2000. Speciation and complexation of cadmium in extracted soil solutions. Environ. Sci. Technol 34, 291–296. [Google Scholar]
- Schuetze G, Becker R, Daemmgen U, Nagel H-D, Schlutow A, Weigel H-J, 2003. Risikoabschätzung der Cadmium-Belastung für Mensch und Umwelt infolge der Anwendung von cadmiumhaltigen Düngemitteln. FAL agricultural research (Landbauforschung Voelkenrode) 2/3, 63–170. [Google Scholar]
- Sebastian A, Prasad MNV, 2014. Cadmium minimization in rice. A review. Agron. Sustain. Dev 34, 155–173. [Google Scholar]
- Shaheen SM, Rinklebe J, Rupp H, Meissner R, 2014. Temporal dynamics of pore water concentrations of Cd, Co, Cu, Ni, and Zn and their controlling factors in a contaminated floodplain soil assessed by undisturbed groundwater lysimeters. Environ. Pollut 191, 223–231. [DOI] [PubMed] [Google Scholar]
- Shallari S, Schwartz C, Hasko A, Morel JL, 1998. Heavy metals in soils and plants of serpentine and industrial sites of Albania. Sci. Total Environ 209, 133–142. [PubMed] [Google Scholar]
- Simpson SL, Apte SC, Batley GE, 2000. Effect of short-term resuspension events on the oxidation of cadmium, lead, and zinc sulfide phases in anoxic estuarine sediments. Environ. Sci. Technol 34, 4533–4537. [Google Scholar]
- Six L, Smolders E, 2014. Future trends in soil cadmium concentration under current cadmium fluxes to European agricultural soils. Sci. Total Environ 485, 319–328. [DOI] [PubMed] [Google Scholar]
- Smedley PL, Kinniburgh DG, 2002. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem 17, 517–568. [Google Scholar]
- Smedley PL, Kinniburgh DG, 2017. Molybdenum in natural waters: a review of occurrence, distributions and controls. Appl. Geochem 84, 387–432. [Google Scholar]
- Smith DB, Cannon WF, Woodruff LG, Solano F, Ellefsen KJ, 2014. Geochemical and Mineralogical Maps for Soils of the Conterminous United States, Open-File Report 2014–1082. U.S. Geological Survey, Reston, pp. 386. [Google Scholar]
- Smolders E, Mertens J, 2013. Cadmium In: Alloway JB (Ed.), Heavy Metals in Soils – Trace Metals and Metalloids in Soils and Their Bioavailability, 3 ed. Springer, Dordrecht, pp. 283–299. [Google Scholar]
- Spark KM, Johnson BB, Wells JD, 1995. Characterizing heavy-metal adsorption on oxides and oxyhydroxides. Eur. J. Soil Sci 46, 621–631. [Google Scholar]
- Sprynskyy M, Kowalkowski T, Tutu H, Cozmuta LM, Cukrowska EM, Buszewski B, 2011. The adsorption properties of agricultural and forest soils towards heavy metal ions (Ni, Cu, Zn, and Cd). Soil Sediment Contam. 20, 12–29. [Google Scholar]
- Srivastava P, Singh B, Angove M, 2005. Competitive adsorption behavior of heavy metals on kaolinite. J. Colloid Interface Sci 290, 28–38. [DOI] [PubMed] [Google Scholar]
- Strobel BW, Borggaard OK, Hansen HCB, Andersen MK, Raulund-Rasmussen K, 2005. Dissolved organic carbon and decreasing pH mobilize cadmium and copper in soil. Eur. J. Soil Sci 56, 189–196. [Google Scholar]
- Stumm W, Morgan J, 1996. Aquatic Chemistry-Chemical Equilibria and Rates in Natural Waters, 3 ed. John Wiley & Sons, Inc., New York. [Google Scholar]
- Tabelin CB, Igarashi T, Villacorte-Tabelin M, Park I, Opiso EM, Ito M, Hiroyoshi N, 2018. Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: a review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Sci. Total Environ 645, 1522–1553. [DOI] [PubMed] [Google Scholar]
- Taylor M, Kim N, Smidt G, Busby C, McNally S, Robinson B, Kratz S, Schnug E, 2016. Trace element contaminants and radioactivity from phosphate fertiliser In: Schnug E, De Kok LJ (Eds.), Phosphorus in Agriculture: 100 % Zero. Springer, Dordrecht, pp. 231–266. [Google Scholar]
- Tedd K, Coxon C, Misstear B, Daly D, Craig M, Mannix A, Williams TH, 2017. In: EPA (Ed.), Assessing and Developing Natural Background Levels for Chemical Parameters in Irish Groundwater EPA Research Programme 2014-2020 Environmental Protection Agency, Waxford, Ireland. [Google Scholar]
- Thornton I, 1986. Geochemistry of cadmium In: Mislin H, Ravera O (Eds.), Cadmium in the Environment. Birkhaeuser, Basel, Boston, Stuttgart, pp. 7–12. [Google Scholar]
- Tiberg C, Gustafsson JP, 2016. Phosphate effects on cadmium(II) sorption to ferrihydrite. J. Colloid Interface Sci 471, 103–111. [DOI] [PubMed] [Google Scholar]
- Toth G, Hermann T, Da Silva MR, Montanarella L, 2016. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int 88, 299–309. [DOI] [PubMed] [Google Scholar]
- UNEP, 2010. Final Review of Scientific Information on Cadmium. United Nations Environment Programme, pp. 201. [Google Scholar]
- Vangronsveld J, Herzig R, Weyens N, Boulet J, Adriaensen K, Ruttens A, Thewys T, Vassilev A, Meers E, Nehnevajova E, van der Lelie D, Mench M, 2009. Phytoremediation of contaminated soils and groundwater: lessons from the field. Environ. Sci. Pollut. Res 16, 765–794. [DOI] [PubMed] [Google Scholar]
- Vetrimurugan E, Brindha K, Elango L, Ndwandwe OM, 2017. Human exposure risk to heavy metals through groundwater used for drinking in an intensively irrigated river delta. Appl Water Sci 7, 3267–3280. [Google Scholar]
- Voglar GE, Lestan D, 2010. Solidification/stabilisation of metals contaminated industrial soil from former Zn smelter in Celje, Slovenia, using cement as a hydraulic binder. J. Hazard Mater 178, 926–933. [DOI] [PubMed] [Google Scholar]
- Wang KJ, Xing BS, 2002. Adsorption and desorption of cadmium by goethite pretreated with phosphate. Chemosphere 48, 665–670. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zeng X, Yu X, Zhang H, LI Z, Jin D, 2010. Adsorption behaviors of Cd2 + on Fe2O3/MnO2 and the effects of coexisting ions under alkaline conditions. Chin. J. Geochem 29, 197–203. [Google Scholar]
- Wasylenki LE, Swihart JW, Romaniello SJ, 2014. Cadmium isotope fractionation during adsorption to Mn oxyhydroxide at low and high ionic strength. Geochem. Cosmochim. Acta 140, 212–226. [Google Scholar]
- Wen HJ, Zhang YX, Cloquet C, Zhu CW, Fan HF, Luo CG, 2015. Tracing sources of pollution in soils from the Jinding Pb-Zn mining district in China using cadmium and lead isotopes. Appl. Geochem 52, 147–154. [Google Scholar]
- Wessolek G, Kocher B, 2002. Verlagerung straßenverkehrsbedingter Stoffe mit dem Sickerwasser. Techn. Univ. Berlin, Berlin, pp. 147. [Google Scholar]
- WHO, 2011. Guidelines for Drinking Water Quality, 4 ed. World Health Organization, Geneva. [Google Scholar]
- Wilkin RT, 2007. Cadmium In: Ford RG, Wilkin RT, Puls RW (Eds.), Monitored Natural Attenuation of Inorganic Contaminants in Ground Water, Volume 2, Assessment for Non-radionuclides Including Arsenic, Cadmium, Chromium, Copper, Lead, Nickel, Nitrate, Perchlorate, and Selenium. Environmental Protection Agency, Ada, Oklahoma, pp. 1–9. [Google Scholar]
- Wriedt G, de Vries D, Eden T, Federolf C, 2019. Regionalisierte Darstellung der Nitratbelastung im Grundwasser Niedersachsens. Grundwasser 24, 1–15. [Google Scholar]
- Xiong LM, 1995. Influence of phosphate on cadmium adsorption by soils. Fert. Res. 40, 31–40. [Google Scholar]
- Yang YJ, Xiong J, Chen RJ, Fu GF, Chen TT, Tao LX, 2016. Excessive nitrate enhances cadmium (Cd) uptake by up-regulating the expression of OsIRT1 in rice (Oryza sativa). Environ. Exp. Bot 122, 141–149. [Google Scholar]
- Zachara JM, Cowan CE, Resch CT, 1991. Sorption of divalent metals on calcite. Geochem. Cosmochim. Acta 55, 1549–1562. [Google Scholar]
- Zhu CW, Wen HJ, Zhang YX, Fan HF, Fu SH, Xu J, Qin TR, 2013. Characteristics of Cd isotopic compositions and their genetic significance in the leadzinc deposits of SW China. Sci. China Earth Sci 56, 2056–2065. [Google Scholar]
- Zoumis T, Schmidt A, Grigorova L, Calmano W, 2001. Contaminants in sediments: remobilisation and demobilisation. Sci. Total Environ 266, 195–202. [DOI] [PubMed] [Google Scholar]
- Zwonitzer JC, Pierzynski GM, Hettiarachchi GM, 2003. Effects of phosphorus additions on lead, cadmium, and zinc bioavailabilities in a metal-contaminated soil. Water Air Soil Pollut. 143, 193–209. [Google Scholar]