Abstract
Securing economically and ecologically significant molluscs, as our oceans warm due to climate change, is a global priority. South eastern Australia receives warm water in a strengthening East Australia Current and so resident species are vulnerable to elevated temperature and marine heat waves. This study tested whether prior exposure to elevated temperature can enhance resilience of oysters to ocean warming. Two Australian species, the flat oyster, Ostrea angasi, and the Sydney rock oyster, Saccostrea glomerata, were obtained as adults and “heat shocked” by exposure to a dose of warm water in the laboratory. Oysters were then transferred to elevated seawater temperature conditions where the thermal outfall from power generation was used as a proxy to investigate the impacts of ocean warming. Shell growth, condition index, lipid content and survival of flat oysters and condition of Sydney rock oysters were all significantly reduced by elevated seawater temperature in the field. Flat oysters grew faster than Sydney rock oysters at ambient temperature, but their growth and survival was more sensitive to elevated temperature. “Stress inoculation” by heat shock did little to ameliorate the negative effects of increased temperature, although the survival of heat-shocked flat oysters was greater than non-heat shocked oysters. Further investigations are required to determine if early exposure to heat stress can enhance resilience of oysters to ocean warming.
Introduction
Climate change, the result of anthropogenic activities such as the burning of fossil fuels and deforestation, has exponentially increased the concentration of carbon dioxide (CO2) and other greenhouse gasses in the atmosphere [1]. Since the onset of the industrial revolution, atmospheric partial pressure of CO2 (pCO2) has increased from 280 ppm to 410 ppm causing global warming with direct impacts on the oceans [1,2]. As a result, the world’s oceans have warmed by 0.68°C and for the East Australian coast are predicted to increase by up to 4°C by 2050 and 6°C before 2100 [3,4]. Ocean warming and the increased incidence of heatwaves (abnormal high temperatures over multiple days [5]) negatively impacts diverse species [6]. Between 1925 and 2016 there has been a 54% annual increase in the duration of marine heatwaves worldwide [7]. Climate change is also impacting ocean stratification, currents, salinity, pH, sea level and increasing the frequency of extreme events [1,7,8].
Increasing frequency of thermal stress events will have consequences for fitness and survival of marine species and there is particular concern for habitat engineers such as oysters [6,9]. If molluscs are to persist during this century along the southeast coast of Australia and in similar “hot spots” around the globe, they will need to be resilient to marine heat waves and habitat warming. Previous work has found that organisms can build resilience to environmental stress, through prior exposure to stress. [10,11]. Rather like a vaccination, exposure to mild stress in early life has been observed to increase resilience to stress in a diverse array of organisms such as bacteria, plants, insects, mammals and fish [10, 11, 12, 13, 14].
There were greater survival rates in the tidepool fish Oligocottus maculosus after exposure to a +12°C heat stress when exposed to subsequent stressful levels of high salinity and low oxygen concentration [10]. The magnitude of the shock and recovery time played an important role in the stress response later in life [10]. Baltic Sea mussels Mytilus edulis, exposed to heat shock (+16°C) and then exposed to cadmium (20 μg L-1) produced heat shock proteins faster than mussels not exposed to heat stress [15]. Stress resistance may be enabled by production of protective heat shock proteins (e.g. HSP 70), although this is energetically costly. The mechanisms behind stress inoculation, are complex and likely not limited to production of heat shock proteins. Other processes such as alterations in metabolism and epigenetics are also thought to be involved [16, 17].
While mobile species can migrate changing their distribution as the ocean warms, sessile species are vulnerable because they are unable to move and the dispersive larval stages are often short-lived [18,19]. Studies have suggested that sessile organisms such as oysters, which form the basis of aquaculture across the globe, will be impacted by elevated temperature, because of the energetic cost to physiological performance from climate change stress [20, 21]. Significant mortality has been reported for the north American oyster Crassostrea virginica exposed to elevated temperature, due to impacts on energetic reserves [22]. Reduced gametogenesis in M. galloprovincialis has also been directly connected to warming [23]. In oysters, parental exposure to stress (in this case ocean acidification) increased resilience of their larva, and this trait carried over to adulthood [24,25].
The flat oyster, Ostrea angasi and the Sydney rock oyster Saccostrea glomerata are native to south eastern Australia [26, 27], where they historically formed extensive reefs and are the basis of a USD $30 million aquaculture industry [28,29]. Saccostrea glomerata is an intertidal species that occurs along the east and west coast of Australia with a current upper sea surface temperature (SST) range of 24–26°C [28]. Ostrea angasi is distributed in shallow subtidal sheltered waterways along a similar range with a current upper SST temperature range of 22–24°C [27], however, this northern (warm) range is likely curtailed by historic overharvesting and introduced parasites in New South Wales (Polydora spp.) [27]. Ostrea angasi are mostly found sub-tidally in comparatively stable thermal conditions [30]. The distributions of these two species overlap for >1000 kms of eastern Australian coastline and are both currently the focus of reef restoration efforts in the region [31,32]. Both species are known to be vulnerable to acidification [33,34,35] and warming [33,36]. South-eastern Australia receives warm water from the Coral Sea through the southerly flow of the East Australian Current (EAC) [37,38]. The EAC is strengthening and bringing more warm water to south-eastern Australia making the region an ocean warming “hot spot” with sea surface temperatures increasing 3–4 times the global average along with increased incidence of marine heat waves [7,38,39].
The purpose of this study was to test the hypothesis that early exposure to heat stress or heat shock can be used as a mechanism to build resilience of O. angasi and S. glomerata to subsequent long-term exposure to warmed seawater. We used the thermal outfall from a power generating station as a proxy for ocean warming conditions as in previous studies eg. [40] where seawater can be warmed 10–15°C above ambient summer temperatures. Due to their different thermal ranges, distributions and habitats we predicted that S. glomerata would be more resilient than O. angasi to elevated temperature. As momentum gains to restore oyster reefs [31], knowledge of oyster responses and how to build resilience is needed to ensure sustainability of restoration efforts and the aquaculture industry.
Methods
Adult (approximately two years old) Ostrea angasi and Saccostrea glomerata were obtained from an oyster farm at Merimbula Lake (Merimbula Gourmet Oysters; 36°89’ 85”S, 149°88’ 46”E) where the water temperature at collection was 20°C. Approximately 200 oysters per species were transported to Port Stephens Fisheries Institute (PSFI; 32°44’47”S, 152°03’30”E), in New South Wales, Australia, during the Austral autumn 2018. All oyster movements followed the NSW Fisheries Management (Aquaculture) Regulations. The initial mean shell height was 69.68 ± S.E. 0.34 mm for O. angasi and 69.86 ± S.E. 0.33 mm for S. glomerata. After arrival at PSFI the oysters were placed in 40 L tubs with seawater supplied from a 750 L tank at 20°C. Oysters were fed a mixture of microalgae cultured on-site containing 50% Chaetoceros muelleri and 50% Tisochrysis lutea at a concentration equivalent to 2 x 109 cells oyster-1 d-1 [41]. The initial mean (± S.E.) condition index for O. angasi and S. glomerata were 4.12 ± 0.42 g and 4.30 ± 0.39 g (n = 6), respectively (see below for methods).
Heat shock
To determine if exposure to heat shock would confer subsequent resilience to long term exposure to elevated temperature, the following heat shock protocol was used. The oysters were divided into two sub-groups; one “control” and a “heat shocked” groups per species into 750 L tanks. Heat shock was administered by exposure to an elevated temperature of 26°C for 18 hours and then 28°C for 6 more hours by slowly ramping up the temperature using aquarium heaters (Titan G2 1500 W). This was an initial +6°C (from 20°C to 26°C) and a further increase of +2°C (from 26°C to 28°C). The 28°C maximum represents a 3.5°C increase on mean (last 12 years) summer water temperatures in Port Stephens, and 1°C increase on the maximum summer daily water temperatures on record [42]. There was no mortality following heat shock treatment. Following the 24 hours at elevated temperature, the seawater was returned to ambient (20°C) over a 12-hour period. Oysters were submerged in ambient water overnight in the laboratory. On the following day, all oysters were placed in baskets and left submerged at ambient conditions, in the adjacent estuary of PSFI (Tilligerry creek, Port Stephens) which remained at 20°C for one week. After this period, they were removed and shell height was measured with a digital calliper.
A total of 40 oysters were randomly placed into each of six baskets (SEAPA Co. Edwardstown South Australia, 600 x 250 x100 mm) divided into four compartments with 10 “control” O.angasi and 10 “control” S. glomerata which were exposed to ~20°C at all times, 10 “heat-shocked” O. angasi and 10 “heat shocked” S. glomerata, which were exposed to elevated temperature for 24 hours. 60 heat shocked and 60 non-heat shocked oysters of each species were spread across six baskets. The baskets were transported and deployed into Lake Macquarie (33°.07’94”, 151°.54’85”, Fig 1A).
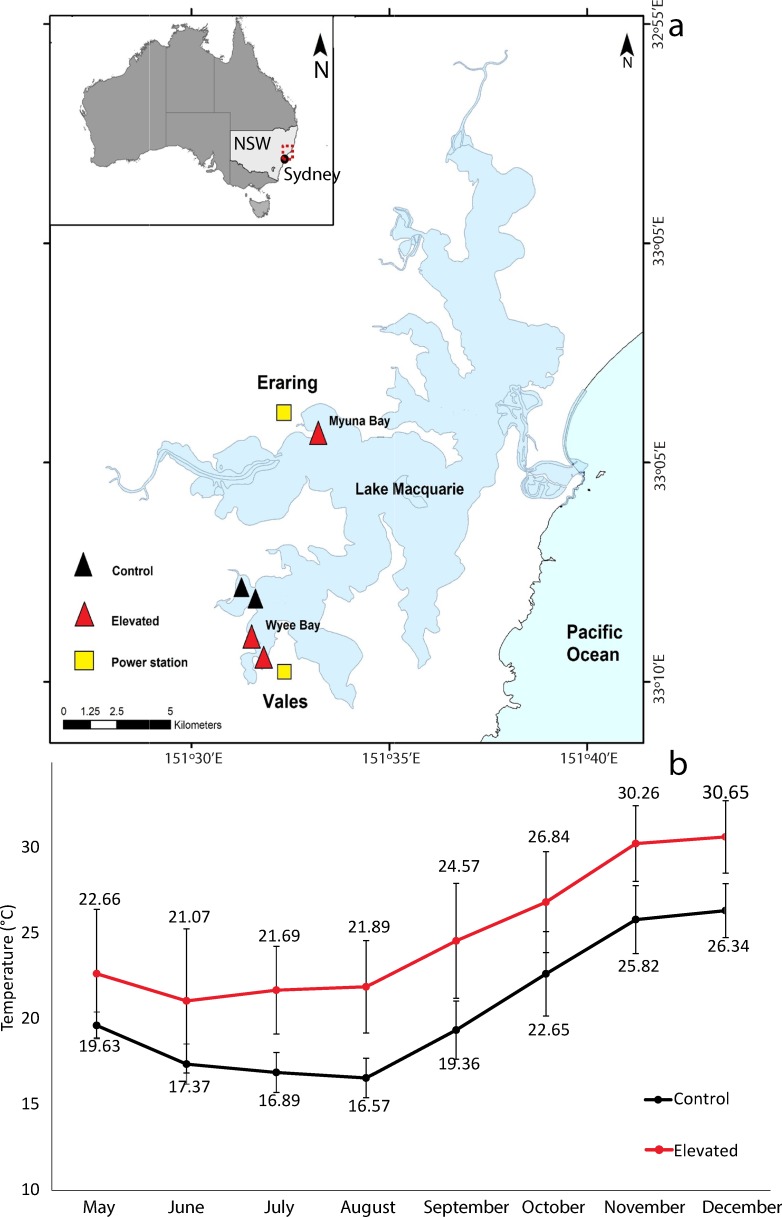
Fig 1. Map of study location and temperatures over study period.
a) Map of Lake Macquarie, New South Wales (NSW), with map a of Australia (top left) showing the field locations where the baskets were deployed and then retrieved following approximately seven months. Yellow squares represent the warm seawater outfall of two power stations (Eraring and Vales power stations). Black triangles are the ambient (control) locations and the red triangles are the elevated locations (total 5 baskets). b) Mean monthly temperatures ± S.D. at control (ambient) and elevated temperature locations in Lake Macquarie, NSW from May to December 2018 (approximately seven months). Temperature data was measured every 30 minutes at 1.10m depth.
Field location
To determine the response of oysters in the real world of elevated seawater temperature, we used warmed seawater released into a saline coastal lake by two power stations at Lake Macquarie, NSW. Lake Macquarie is a large coastal body of water in the centre of east Australian warming “hot spot”. Lake Macquarie is connected to the ocean and has daily tidal exchange. There is little freshwater input from the surrounding catchment [43]. Two coal fired power stations are located 23 kilometres apart on the shore of Lake Macquarie. Eraring power station is located in Myuna Bay (33° 4'2.92"S, 151°33'19.13"E) and Vales Point power station is located in Wyee Bay (33° 9'30.65"S, 151°31'48.37"E). Both stations use seawater from Lake Macquarie for cooling. The seawater is circulated for cooling and then released back into the estuary with no other treatment at a maximum of 37.5°C as per licence requirements (NSW Environment Protection Licences 761; 1429). One location was selected near each power station outfall of the Eraring and Vales Point power stations in Lake Macquarie during May 2018 (autumn). A control location was also selected that represented the ambient mean temperature within Lake Macquarie which was not warmed by a power station. At each location, two baskets were deployed within 20m of each other. Each individual basket was attached to a 10 Kg concrete brick and contained a total of 40 oysters from both species and treatments (control/non heat shock and heat shock) and were deployed at a depth of 1.10 m by boat. A scientific collection permit was obtained from NSW Department of Planning, Investment and Environment (Permit No: F97/109-7.1), which allowed for S. glomerata and O. angasi to be deployed in Lake Macquarie.
Temperature data were collected every 30 minutes by Hobo loggers (HOBO MX Pendant Temperature, Onset) which were within the baskets amongst the oysters. Study locations were visited five times over seven months (late autumn to early summer) to download temperature data and renew the loggers. Oysters were deployed in Lake Macquarie for approximately seven months. At the end of the deployment (7 months) five baskets were retrieved; two from the ambient (control) location and three from elevated locations; two from Wyee Bay near Vales power station and one from Rocky Point near Earing power station (120 oysters from elevated temperature and 80 oysters from ambient temperature). One basket was lost at Rocky Point. Once retrieved, oysters were transferred by boat in aerated seawater (collected from the site of retrieval for those oysters) to a temporary processing station (33° 5'8.94"S, 151°30'13.01"E) on the shore of Lake Macquarie. Here, shell growth, condition index, standard metabolic rate and survival of oysters was measured. Total lipid and profile were measured in the laboratory.
Shell growth and condition index
To determine if exposure to heat shock confers subsequent resilience to long term exposure to elevated temperature on growth and condition index measurements of oysters were done at the end of seven months of exposure in the field experiment.
There was no difference between shell height of oysters randomly allocated into heat shock and control (non- heat shock) treatments (One-way ANOVA comparing heat shock vs non-heat shock for each species, n = 60; O. angasi = p > 0.05; S. glomerata = p > 0.05) at day zero. Final shell growth was then calculated as the difference between the final size of each individual oyster at seven months from an overall initial mean size of oysters per basket (n = 10). The difference in shell growth was calculated by the formula:
Where shell growth (SG) is the difference between final individual shell height (SH1) in millimetres and the mean initial shell height (MSH0) divided by time (t) in days.
The condition index of oysters was measured at the end of the experiment. Oysters were shucked, and body tissue and shell of individuals were dried in oven at 60°C for two days, to determine the dry weight (grams). The condition index (Ci) of oysters was then calculated by the formula [44,45]:
Standard Metabolic Rate (SMR)
To determine if exposure to heat shock would confer subsequent resilience to long term exposure to elevated temperature on standard metabolic rate (SMR), the SMR of 9–11 oysters of each species, treatment and basket (total 56 oysters; heat shock and control/non-heat shock) were measured at the end of the experiment using the methods of Parker et al. [33]. Measurements were done adjacent to the locations of collection to minimise stress of transport and to use seawater from Lake Macquarie.
To calculate SMR, oxygen consumption was measured by a closed respirometry system (OXY-10 PreSens, AS1 Ltd, Regensburg, Germany). Seawater was collected from Lake Macquarie and filtered through 0.47 μm glass filter paper before being used to fill respirometry chambers. Respirometers were built to accommodate the maximum oyster size (745ml and 830 ml). Each respirometer was connected to a fibre optic probe for measurement of dissolved oxygen in seawater. The probe was previously calibrated using two O2 concentration points (0% and 100% oxygen saturation of seawater) following the methods of Parker et al. [33]. Oysters were gently cleaned of any fouling organisms before placed in filtered seawater (adjusted to the corresponding treatment levels). The time that individuals took to lower the oxygen concentration by 20% (~1.2 O2 mg L-1) was recorded. Following the procedure of Parker et al. [33], only the time that the oyster is open and actively respiring (determined by observed decreasing oxygen) is used to calculate SMR. This is done to guard against the oyster remaining closed from handling stress. After each trial, each container was rinsed clean with filtered seawater (0.47 μm) and wiped clean with paper towel. After measurement the oysters were removed from the container and shucked to separate body tissues and shell. The tissue was then dried in an oven at 60°C for three days to measure their constant dry body tissue and shell weight in grams (±0.0001g, Analytical Balance Sartorius Research). Standard metabolic rates (SMR) were calculated by the formula:
where SMR is the oxygen consumption normalized to 1 g of dry tissue mass (mg O2 g-1 dry tissue mass h-1, Vr is the volume of the respirometry chamber minus the volume of the oyster (L), ΔCwO2 is the change in water oxygen concentration measured (mg O2L-1), Δt is measuring time (h) and bw is the dry tissue mass (g) of the oyster.
Total lipid and lipid profile
To determine if exposure to heat shock and elevated temperature influences energy allocation, total lipid and lipid profiles were analysed. Body tissues of the oysters were placed in centrifuge tubes and frozen for analysis of total lipid content and lipid classes. The tissues were kept at -22°C for transport and then stored at -80°C until analysis. The tissues were then freeze dried (Alpha 1–4 LSCbasic, Martin Christ, Germany) and weighed in a microbalance (±0.0001g; Sartorius CPA225D). Lipids were extracted overnight using a modified Bligh & Dyer [46] one-phase methanol-chloroform-water extraction (2:1:0.8 v/v/v). The phases were separated by the addition of chloroform-water (final solvent ratio, 1:1:0.9 v/v/v methanol-chloroform-water). The total solvent extract (TSE) was concentrated using rotary evaporation at 40°C.
An aliquot of the TSE was analysed using an Iatroscan MK VI TH10 thin- layer chromatography-flame ionization detector (TLC-FID) analyser (Tokyo, Japan) to quantify individual lipid classes [47,48]. Samples were applied in duplicate to silica gel SIII chromarods (5μm particle size) using 1 μl micropipettes. Chromorods were developed in a glass tank lined with pre-extracted filter paper. The primary solvent system used for the lipid separation was hexane-diethyl ether-formic acid (60:15:1.5), a mobile phase resolving non-polar compounds such as steryl ester (SE), triacylglycerol (TAG), free fatty acids (FFA), monoacylglycerol (MAG) and diacylglycerol (DAG). After development, the chromorods were oven dried and analysed immediately to minimize absorption of atmospheric contaminants. The FID was calibrated for each compound class (phosphatidylcholine (PL), cholesterol (Chol), cholesteryl palmitate (SE), palmitic acid (FFA), monopalmitin (MAG), dipalmitin (DAG), tripalmitin (TAG)). Peaks were quantified on an IBM compatible computer using DAPA Scientific software (Kalamunda, Western Australia, Australia). TLC-FID results are generally reproducible with a coefficient of variance of up to 3.46% of individual class abundances [49].
Survival
Oyster survival was determined after seven months deployment by emptying baskets one section at a time (to avoid mixing) and counting the total number of live oysters.
Data analysis
Statistical analyses were done using PRIMER v6+ software using either a three or two factor nested PERMANOVA (PRIMER v6+). This analysis was selected because it is robust to unbalanced designs [50].
For shell growth, condition index, SMR, and total lipids, data were analysed using a three factor PERMANOVA with “heat shock” as fixed factor with two levels (heat shock or control), “temperature” as fixed factor with two levels (ambient and elevated), and “basket” as random factor with two levels (basket 1 and basket 2) nested in temperature and heat shock. The analysis used 9999 permutations and alpha was set at P < 0.05 to be considered as statistically different. The percentage survival at seven months was analysed using a two factor PERMANOVA with heat shock as fixed factor with two levels (heat shock or control) and temperature as fixed factor with two levels (ambient and elevated).
The composition of lipid profiles were fourth root transformed to limit the influence of large numbers [50] and analysed using a four factor multivariate PERMANOVA using the same model as above; with heat shock as fixed factor with two levels (Heat shock or control), temperature as fixed factor with two levels (control and elevated), and basket as random factor with two levels (basket 1 and basket 2) nested in temperature and heat shock. The analysis used 9999 permutations and alpha was set at P < 0.05 to be considered as statistically different. Tabulated results for all statistical tests can be found in S1–S3 Tables.
Results
Temperature
The average temperature over seven months at the ambient location was 20.06°C ± 3.85 (mean ± S.D.) and the average temperature at the elevated locations (Wyee Bay, Rocky Point) was 24.56°C ± 4.59 (Fig 1B). The highest daily temperature experienced by oysters deployed at elevated temperature locations was 32.81 ± 0.39°C in spring (November) and lowest daily average for the ambient location during the experiment was 14.92°C ± 0.65 (mean ± S.D) in winter (August).
Shell growth and condition index
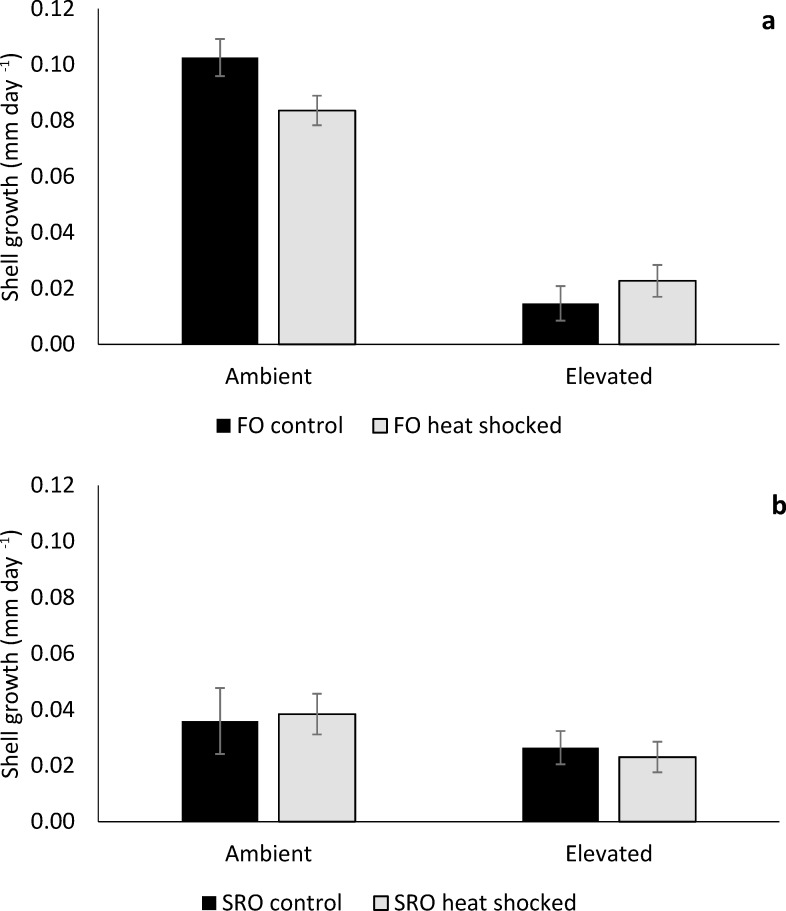
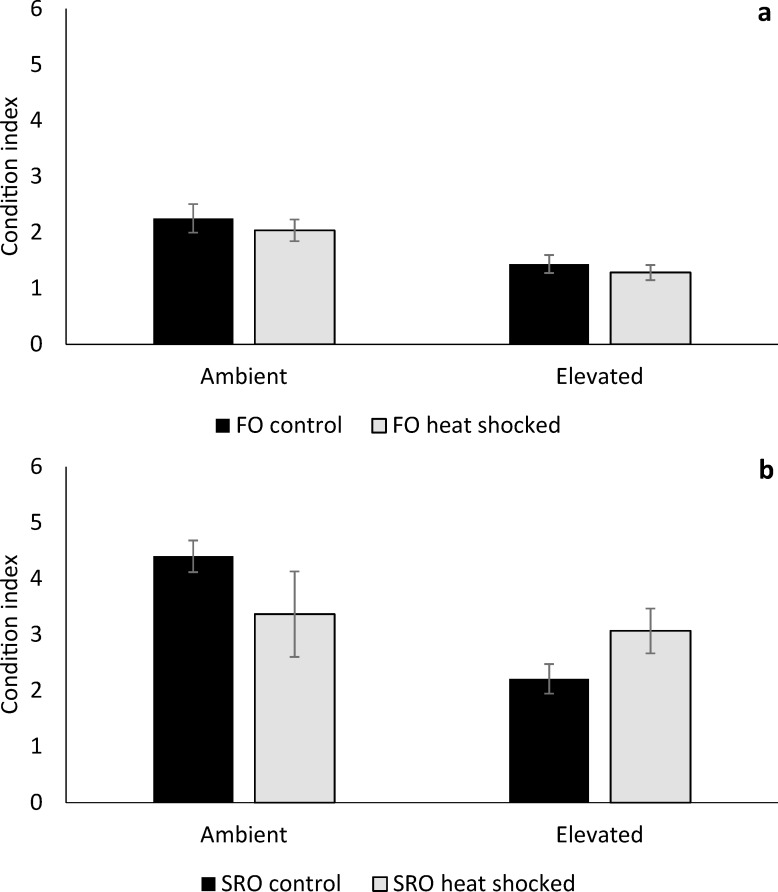
Shell growth of O. angasi was almost ten-fold greater at ambient temperature compared to elevated temperature (Fig 2A). At ambient temperatures, mean shell growth (mm day-1) of non-heat shocked flat oysters was 0.10 ± 0.01 mm day-1 (mean ± S.E), which was significantly greater than heat shocked oysters, which grew 0.08 ± 0.01 mm day-1 (mean ± S.E). (Fig 2A, PERMANOVA; Heat shock x Temperature: F4,69 = 25.84, P < 0.001). At elevated temperatures, mean shell growth (mm day-1) of non-heat shocked flat oysters was 0.01 ± 0.001 mm day-1 (mean ± S.E) compared to heat shocked flat oysters which grew 0.02 ± 0.01 mm day-1 (Fig 2A). Shell growth of S. glomerata was not affected by temperature or heat shock (Fig 2B, PERMANOVA; P > 0.05). Ostrea angasi grew an order of magnitude greater than S. glomerata under ambient conditions, however, under elevated temperature there was little growth of either species < 0.04 mm day-1 (mean ± S.E) (Fig 2A and 2B). The condition index of both O. angasi (PERMANOVA; Temperature: F1, 28 = 7.87, P = 0.04) and S. glomerata (PERMANOVA; Temperature: F1, 19 = 8.29, P = 0.02) was significantly lower at elevated temperature (Fig 3A and 3B) with no effect of heat shock treatment.
Fig 2. Shell growth of flat and Sydney rock oysters.
Mean difference in shell growth (± S.E.) for a. flat oysters, Ostrea angasi (FO control and FO heat shocked) and b. Sydney rock oysters, Saccostrea glomerata (SRO control and SRO heat shocked), exposed for seven months at ambient and elevated temperature locations at Lake Macquarie.
Fig 3. Condition index of flat and Sydney rock oysters.
Mean condition index (± S.E.) of a. flat oysters, Ostrea angasi (FO control and FO heat shocked) and b. Sydney rock oysters, Saccostrea glomerata (SRO control and SRO heat shocked) exposed for seven months at ambient and elevated locations at Lake Macquarie.
Standard Metabolic Rate (SMR)
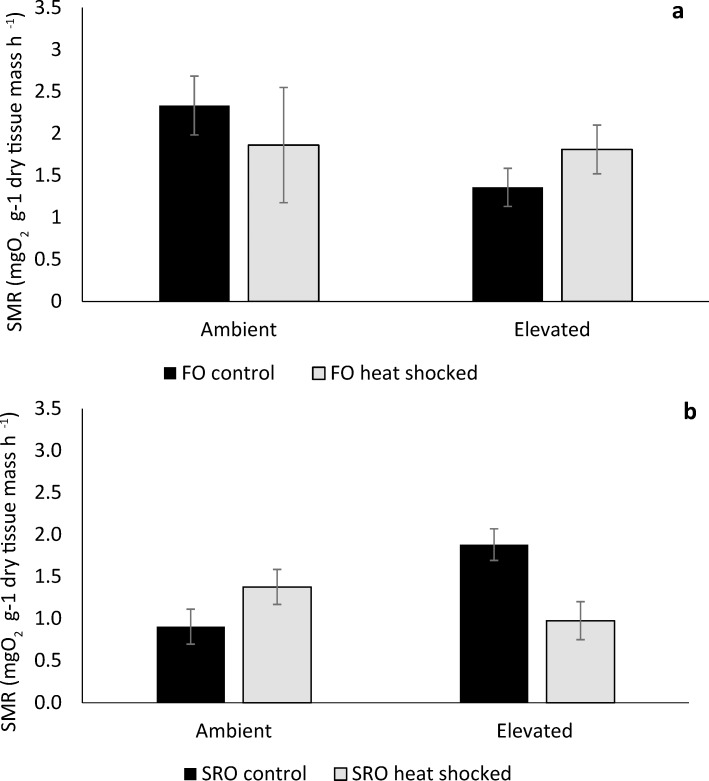
Standard Metabolic Rate of non-heat shocked O. angasi was greater at ambient temperature, compared to elevated temperature, but this was not significant (Fig 4A; PERMANOVA; P > 0.05). SMR of, non-heat shocked S. glomerata was greater at elevated, compared to ambient temperature, but this was also not significant (Fig 4B; PERMANOVA; P > 0.05).
Fig 4. Standard metabolic rate (SMR) of flat and Sydney rock oysters.
Mean standard metabolic rate (SMR) (± S.E.) of a flat oysters, Ostrea angasi (FO control and FO heat shocked) and b. Sydney rock oysters, Saccostrea glomerata (SRO control and SRO heat shocked) exposed for seven months at ambient and elevated temperature locations at Lake Macquarie.
Total lipids and lipid profiles
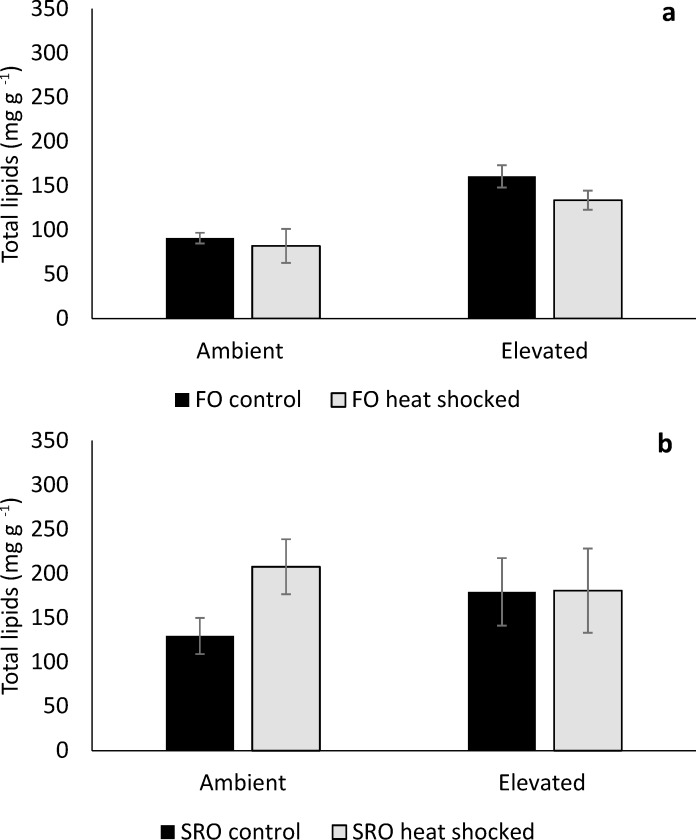
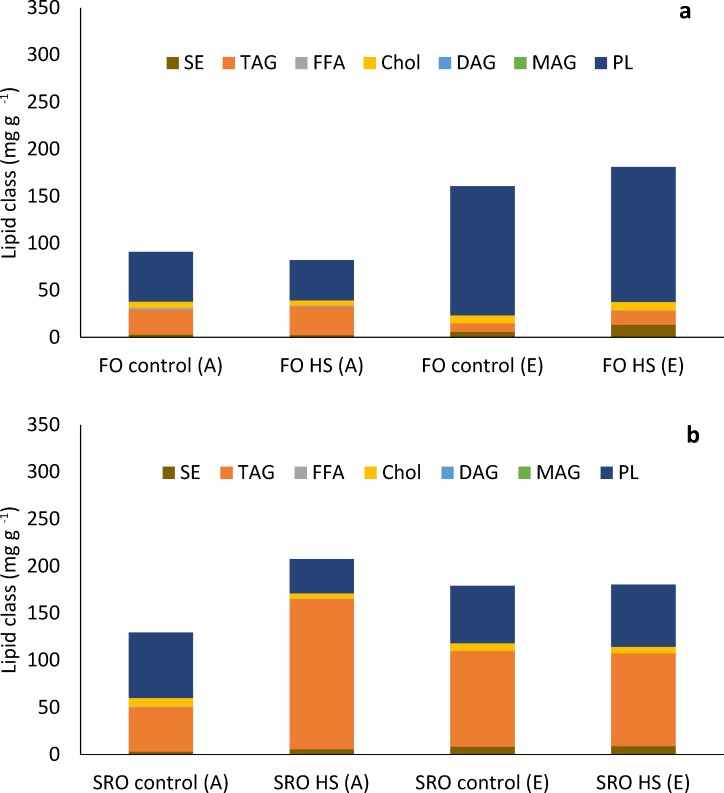
The total lipid content of O. angasi was greater at elevated compared to ambient temperature (Fig 5A, PERMANOVA; Temperature: F1, 14 = 7.55, P = 0.03). There were no effects of heat shock or temperature on total lipid content in S. glomerata (Fig 5B, P > 0.05). The lipid profile of O. angasi, was mostly driven by a significantly greater amount of phospholipids in oysters at elevated temperature (Fig 6A, PERMANOVA; Temperature: F1, 15 = 13.21, P< 0.001) and lower amounts of triacylglyceride (TAG) (Fig 6A) irrespective of heat shock. The changes in lipid profile of S. glomerata was similar across ambient and elevated temperatures, but there were significantly greater phospholipids at elevated temperature (Fig 6B, PERMANOVA; Temperature: F1, 17 = 7.31, P = 0.04).
Fig 5. Total lipids of flat and Sydney rock oysters.
Mean total lipids (± S.E.) for a. flat oysters, Ostrea angasi (FO control and FO heat shocked) and b. Sydney rock oysters, Saccostrea glomerata (SRO control and SRO heat shocked) exposed for seven months at ambient and elevated temperature locations (n = 5; except for HS oysters from Rocky Point–FO HS [n = 3], SRO HS [n = 2]) at Lake Macquarie.
Fig 6. Lipid profiles of flat and Sydney rock oysters.
Lipid profile of a. flat oysters, Ostrea angasi (FO control FO heat shocked) after seven months exposure at ambient and elevated temperature locations and b. Lipid profile of Sydney rock oysters Saccostrea glomerata (SRO control and SRO heat shocked) exposed for seven months at ambient and elevated temperature. Lipid classes abbreviations are: SE–steryl ester; TAG -Triacyglyceride; FFA–Free Fatty Acids; Chol–Cholesterol; DAG–Diacylglyceride; MAG–Monoglyceride and PL–Phospholipids.
Survival
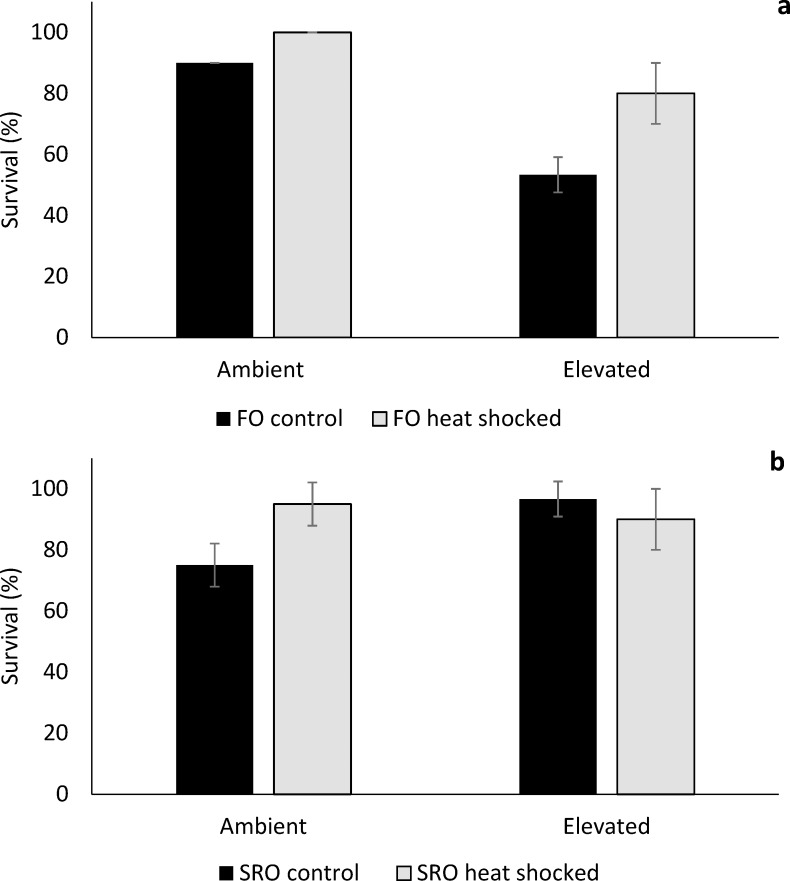
Survival of heat-shocked O. angasi was significantly greater than non-heat shocked oysters at ambient and elevated temperature (Fig 7A; PERMANOVA; Heat shock x Temperature: F1, 6 = 16.61, P = 0.04). At the ambient and elevated temperature locations, survival of O. angasi was greatest for the heat shocked oysters (Ambient, control oysters = 90% and heat shocked oysters = 100%; Elevated, control = 53% and heat shocked = 80%). Survival of S. glomerata was significantly lower for non-heat shocked oysters at ambient temperature compared to heat shocked oysters (Fig 7B, PERMANOVA; Heat shock x Temperature: F1, 6 = 16.61, P = 0.04).
Fig 7. Survival of flat and Sydney rock oysters.
Mean survival (±S.D.) of a flat oysters, Ostrea angasi (FO control and FO heat shocked) and b. Sydney rock oysters, Saccostrea glomerata, (SRO control and SRO heat shocked), exposed for seven months at ambient and elevated temperature locations at Lake Macquarie.
Discussion
Exposure to long term warming in the field had negative impacts on shell growth, condition index, and survival of O. angasi and S. glomerata. Shell growth, condition index, lipid content and profile and survival, but not SMR of O. angasi were impacted by elevated temperature. Only survival and condition index of S. glomerata was negatively impacted by elevated temperature. Flat oysters grew faster than Sydney rock oysters at ambient temperature but were more sensitive to elevated temperature. Prior exposure to heat shock did little to ameliorate the negative effects of elevated temperature. Heat shocked flat oysters, however had greater survival at elevated temperature. There was also a trend for shell growth of heat shocked flat oysters to be greater than non-heat shocked oysters at elevated temperature. SMR was not significantly impacted by elevated temperature, although once again there was a trend for SMR of flat oysters to decrease with increased temperature and for SMR of Sydney rock oysters to increase with increased temperature. The levels of TAG within the lipid profile of O. angasi was also reduced by elevated temperature, while the lipid profile for S. glomerata was not affected.
As oysters are ectothermic organisms, changes in external temperature away from their optimum causes physiological processes to become less efficient and homeostasis begins to require more energy [51]. The effects of elevated temperature on O. angasi and to some extent, S. glomerata are similar to those observed for other bivalve species. For example, Hiebenthal et al. [52] found lower growth and condition for Arctica islandica at elevated temperature (16°C) compared to the control (7.5°C) and an intermediate treatment (10°C). Condition index and survivorship of M. edulis was reduced under elevated temperature (25°C) compared with control [52]. Effects on these physiological processes, were attributed to thermal sensitivity of A. islandica to temperatures outside its distribution and to accumulation of lipofuscin, a disease related pigment [52]. Juvenile shell growth (mg d-1) of the clam Mercenaria mercenaria and Argopecten irradians, was significantly reduced by a 4°C increase in temperature (28°C) [53].
Elevations in temperature increase the SMR of marine ectotherms until a point known as the “Arrhenius Breakpoint Temperature” (ABT). When ABT is reached, SMR rapidly declines indicating that the organism can no longer meet their energetic requirements at that temperature [33]. The reduced growth of O. angasi at elevated compared to ambient temperature correlated with a trend for lower SMR, indicating that O. angasi may have experienced temperatures beyond their ABT. In this study, we found the temperatures reached at the thermal outfalls in Lake Macquarie (mean = 24.56 ±4.59, daily max = 32.81 ± 0.39°C) had no effect on the SMR of S. glomerata. Parker et al., [33] found that increased seawater temperature increased the SMR of S. glomerata. SMR of S. glomerata increased with increased temperature up to 33°C (the upper temperature treatment in that study) indicating an ABT for S. glomerata of above 33°C. Increased SMR can impact energy budget and may indicate a thermal response with extra costs needed to cover basal metabolism [33,54]. For oysters, thermal stress can also alter cardiac function, protein synthesis [54] and gametogenesis [23].
Oysters have the capacity to store surplus energy ingested from food in the form of lipids which can assist in the persistence during stressful conditions. While the lipid profile of S. glomerata was not impacted by elevated temperature, there were significant impacts of elevated temperature on total lipids and lipid profile, especially triacylglycerides (TAG) of O. angasi. Lower lipid content may cause lower condition index of O. angasi at elevated temperatures. TAG are the primary source of stored lipid energy for bivalves [54, 55], indicating that O. angasi had begun to use stored lipid reserves. Under stressful conditions bivalves have lower lipid reserves. For example, exposure to elevated pCO2 decreased the lipid index of larvae of A. irradians, M. mercenaria and C. virginica which further declined when combined with warming [53, 56]. Lipid levels in the eggs of S. glomerata also decreased when exposed to the dual stress of elevated pCO2 and copper [57]. Habitat warming may decrease the lipid content, condition index and value of oysters further reducing the profitability of aquaculture [19]. Research on how lipids are used by oysters in response to stress could provide insights into the ramifications of living in warmer oceans.
Stress inoculation, and resilience
This study tested the hypothesis that prior-exposure of oysters to heat shock stress will build resilience to later exposure to elevated temperature. Stress inoculation leading to stress resilience has been observed in diverse phyla from bacteria to mammals e.g. [10,11]. Heat shock may help to build resilience, but at the same time have costs. For example, heat shocked O. angasi had significantly greater rates of survival at elevated temperatures, but heat shocked oysters had less growth at ambient temperature. Energy may have been used to produce heat shock proteins or other protective measures, thereby reducing energetic reserves for growth and other important physiological processes [58].
Heat shocked O. angasi had greater rates of survival at elevated temperature, and a similar trend was observed for S. glomerata which had greater than 90% survival at elevated temperature. When organisms experience stressful temperatures, they undergo a thermal response, which is energy dependent [58]. This thermal response includes producing chaperone proteins, such as energetically expensive HSPs [58,59]. Species with lower thermal tolerance might be induced to produce HSPs in response to elevated temperatures at lower levels of warming than more tolerant species, which can endure longer periods of heat stress (e.g. M. trossulus and M. galloprovincialis; [59]). Heat shock proteins have important functions when an organism is exposed to elevated temperature, including degradation of denatured proteins and prevention of misfolding, having a key function on cellular protection [60]. These responses (e.g. expression of heat shock proteins, antioxidants, increased respiration rates) all incur an energetic cost which can cause an imbalance in the energetic partitioning of individuals [33,58,61,62].
In this study, the heat shock was administered to adult oysters for a relatively short period of 24 hours. A longer time period may have allowed oysters to produce more heat shock proteins (HSP) or for epigenetic shifts to occur [58, 63]. Furthermore, positive cross-generation effects may have occurred if the heat shock had been administered to the parents of the oysters [64]. Previous research has shown that the resilience of S. glomerata can be built to environmental stressors such as ocean acidification with transgenerational exposure [24, 25]. This, however, can be limited. Parker et al., [65] found that adaptations were maladaptive under other stressors, including warming [65].
Overall, S. glomerata was found to be generally more tolerant of habitat warming than O. angasi. S. glomerata had no change in shell growth although they were in poorer condition at elevated temperature of 28–30°C. These findings are supported by previous work by Parker et al., [33] that showed 33°C was not beyond their ABT. As an intertidal species that experiences a highly dynamic thermal environment, S. glomerata could be expected to be more thermally tolerant as has been shown for other intertidal organisms [63,66]. In addition, S. glomerata is distributed further north along the east coast of Australia than O. angasi and is likely to have a greater temperature tolerance; S. glomerata can experience air temperatures in excess of 40°C during emersion at low tide [67]. The lack of effect of elevated temperature on S. glomerata indicates that the temperatures experienced in this study (heat shock; 28°C, max outfall daily max; 32.81 ± 0.39°C) did not place S. glomerata beyond their thermal limits in contrast to O. angasi, which did not cope as well.
O. angasi had the greatest growth rate at ambient conditions. The shell growth of O. angasi was over ten-fold greater than Sydney rock oysters after seven months, as expected from growth in aquaculture [68]. While O. angasi grew well at ambient conditions, growth and survival were impacted by warming. As this species lives in a relatively stable, sub-tidal habitat we expected this species to be more sensitive to warming compared to S. glomerata.
Globally and across Australia efforts are being made to restore oyster reefs [31,69,70]. Climate change will impact on oyster reef restoration [36]. Projected ocean warming for the region (4°C) as well as contemporary marine heat waves, as seen in the region recently [39] are an important consideration for reef restoration efforts in southeast Australia. Our study has shown that using a thermal outfall as a proxy for ocean warming can be useful in assessing organism responses to projected habitat warming. This approach is similar to natural laboratories using underwater CO2 vents which have successfully tested the responses of marine organisms to ocean acidification [71,72]. Our results indicate that habitat warming will be a greater threat to O. angasi than S. glomerata. As ocean warming will not act alone, oyster reef restoration is at risk from multiple stressors including ocean acidification, salinity, and other environmental pollutants which will act simultaneously [36]. These co-occurring stressors further threaten native species of oysters and other marine organisms and so mitigation strategies to build oyster resilience will be critical. Our results indicate that early exposure to stress inoculation does not enhance resilience and may not be useful strategy, especially for restoration ventures involving O. angasi.
Supporting information
a. Results of PERMANOVA for the shell growth, condition index and SMR of Ostrea angasi exposed for seven months in Lake Macquarie. b. Results of PERMANOVA for the shell growth, condition index and SMR of Saccostrea glomerata exposed for seven months in Lake Macquarie. P values were created using Monte Carlo tests. Significant values (P<0.05) are bold.
(DOCX)
a. Results of PERMANOVA for the total lipids, amount of total lipids (mg/g), amount of Triacylglycerides (TAGs; mg/g) and Phospholipids (PLs; mg/g) of Ostrea angasi exposed for seven months in Lake Macquarie. b. Results of PERMANOVA for the total lipids (mg/g), amount of Triacylglycerides (TAGs; mg/g) and Phospholipids (PLs; mg/g), of Saccostrea glomerata exposed for seven months in Lake Macquarie. P values were created using Monte Carlo tests. Significant values (P<0.05) are bold.
(DOCX)
a. Results of PERMANOVA for the percentage survival of Ostrea angasi exposed for seven months in Lake Macquarie. b. Results of PERMANOVA for the percentage survival of Saccostrea glomerata exposed for seven months in Lake Macquarie. P values were created using Monte Carlo tests. Significant values (P<0.05) are bold.
(DOCX)
(CSV)
Acknowledgments
We would like to thank Port Stephens Fisheries Institute, Dr Wayne O’Connor and the Office of Environment and Heritage for logistical support of this study. We thank Kyle Johnston and Richard Grainger for assistance in the field.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by a CNPq PhD scholarship CNPq – Conselho Nacional de Desenvolvimento Científico e Tecnológico – Brazil.
References
- 1.IPCC. Summary for policymakers In: Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Field C.B., Barros V.R., Dokken D.J., Mach K.J., Mastrandrea M.D., Bilir T.E., Chatterjee M., Ebi K.L., Estrada Y.O., Genova R.C., Girma B., Kissel E.S., Levy A.N., MacCracken S., Mastrandrea P.R., and White L.L. (eds.)]. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA: 2014:pp. 1–32. [Google Scholar]
- 2.Cheng L, Abraham J, Hausfather Z, Trenberth KE. How fast are the oceans warming? Science. 2019;363(6423):128–9. 10.1126/science.aav7619 [DOI] [PubMed] [Google Scholar]
- 3.Hobday AJ, Okey TA, Poloczanska ES, Kunz TJ, Richardson AJ. Impacts of climate change on Australian marine life. Report to the Australian Greenhouse Office, Canberra, Australia: 2006. [Google Scholar]
- 4.Lenton A, McInnes KL, O’Grady JG. Marine projections of warming and ocean acidification in the Australasian region. Australian Meteorological and Oceanographic Journal. 2015;65(1):1–28. [Google Scholar]
- 5.Hobday AJ, Alexander LV, Perkins SE, Smale DA, Straub SC, Oliver EC, et al. A hierarchical approach to defining marine heatwaves. Progress in Oceanography. 2016;141:227–38. [Google Scholar]
- 6.Ridgway K, Hill K. The East Australian Current. A marine climate change impacts and adaptation report card for Australia. 2009;5(09). [Google Scholar]
- 7.Oliver EC, Donat MG, Burrows MT, Moore PJ, Smale DA, Alexander LV, et al. Longer and more frequent marine heatwaves over the past century. Nat Commun. 2018;9(1):1324 10.1038/s41467-018-03732-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doney SC, Ruckelshaus M, Duffy JE, Barry JP, Chan F, English CA, et al. Climate change impacts on marine ecosystems. Annu Rev Mar Sci. 2012;4:11–37. [DOI] [PubMed] [Google Scholar]
- 9.Oliver EC, Benthuysen JA, Bindoff NL, Hobday AJ, Holbrook NJ, Mundy CN, et al. The unprecedented 2015/16 Tasman Sea marine heatwave. Nat Commun. 2017;8:16101 10.1038/ncomms16101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Todgham AE, Schulte PM, Iwama GK. Cross-tolerance in the tidepool sculpin: the role of heat shock proteins. Physiological and Biochemical Zoology. 2005;78(2):133–44. 10.1086/425205 [DOI] [PubMed] [Google Scholar]
- 11.Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proceedings of the National Academy of Sciences. 2006;103(8):3000–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laplace JM, Boutibonnes P, Auffray Y. Unusual resistance and acquired tolerance to cadmium chloride in Enterococcus faecalis. J Basic Microbiol. 1996;36(5):311–7. 10.1002/jobm.3620360504 [DOI] [PubMed] [Google Scholar]
- 13.Krebs RA, Feder ME. Hsp70 and larval thermotolerance in Drosophila melanogaster: how much is enough and when is more too much? J Insect Physiol. 1998;44(11):1091–101. 10.1016/s0022-1910(98)00059-6 [DOI] [PubMed] [Google Scholar]
- 14.Munne-Bosch S, Alegre L. Cross-stress tolerance and stress"memory" in plants. Environ Exp Bot. 2013;94:1–88. [Google Scholar]
- 15.Tedengren M, Olsson B, Reimer O, Brown DC, Bradley BP. Heat pretreatment increases cadmium resistance and HSP 70 levels in Baltic Sea mussels. Aquat Toxicol. 2000;48(1):1–12. [Google Scholar]
- 16.Chapple JP, Smerdon GR, Berry R, Hawkins AJ. Seasonal changes in stress-70 protein levels reflect thermal tolerance in the marine bivalve Mytilus edulis L. J Exp Mar Biol Ecol. 1998;229(1):53–68. [Google Scholar]
- 17.Huey RB, Bennett AF. Physiological adjustments to fluctuating thermal environments: an ecological and evolutionary perspective In: Morimoto RI, Tissieres A, Georgopoulos C, editors. Stress proteins in biology and medicine. Cold Spring Harbor, NY: Cold Spring Harbor Lab. Press; 1990;19:37–59. [Google Scholar]
- 18.Pechenik JA. On the advantages and disadvantages of larval stages in benthic marine invertebrate life cycles. Mar Ecol Prog Ser. 1999;177:269–97. [Google Scholar]
- 19.Gattuso J-P, Magnan A, Billé R, Cheung WW, Howes EL, Joos F, et al. Contrasting futures for ocean and society from different anthropogenic CO2 emissions scenarios. Science. 2015;349(6243):aac4722 10.1126/science.aac4722 [DOI] [PubMed] [Google Scholar]
- 20.Pörtner H-O. Integrating climate-related stressor effects on marine organisms: unifying principles linking molecule to ecosystem-level changes. Mar Ecol Prog Ser. 2012; 470:273–90. [Google Scholar]
- 21.Sokolova IM. Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. Integ and Comp Biol. 2013;53(4):597–608. [DOI] [PubMed] [Google Scholar]
- 22.Ivanina AV, Dickinson GH, Matoo OB, Bagwe R, Dickinson A, Beniash E, et al. Interactive effects of elevated temperature and CO2 levels on energy metabolism and biomineralization of marine bivalves Crassostrea virginica and Mercenaria mercenaria. Comp Biochem Physiol A Mol Integr Physiol. 2013;166(1):101–11. 10.1016/j.cbpa.2013.05.016. 10.1016/j.cbpa.2013.05.016 [DOI] [PubMed] [Google Scholar]
- 23.Fearman J, Moltschaniwskyj N. Warmer temperatures reduce rates of gametogenesis in temperate mussels, Mytilus galloprovincialis. Aquaculture. 2010;305(1–4):20–5. [Google Scholar]
- 24.Parker LM, Ross PM, O'Connor WA, Borysko L, Raftos DA, Pörtner HO. Adult exposure influences offspring response to ocean acidification in oysters. Glob Change Biol. 2012;18(1):82–92. [Google Scholar]
- 25.Parker LM O’Connor WA, Raftos DA, Pörtner H-O, Ross PM. Persistence of positive carryover effects in the oyster, Saccostrea glomerata, following transgenerational exposure to ocean acidification. PloS one. 2015;10(7):e0132276 10.1371/journal.pone.0132276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scanes E, Johnston EL, Cole VJ, O'Connor WA, Parker LM, Ross PM. Quantifying abundance and distribution of native and invasive oysters in an urbanised estuary. Aquatic Invasions. 2016;11(4):425–36. [Google Scholar]
- 27.Ogburn DM, White I, Mcphee DP. The disappearance of oyster reefs from eastern Australian estuaries—impact of colonial settlement or mudworm invasion? Coast Manage. 2007;35(2–3):271–87. [Google Scholar]
- 28.Nell JA. The history of oyster farming in Australia. Marine Fisheries Review. 2001;63(3):14–25. [Google Scholar]
- 29.NSW Department of Primary Industries, Aquaculture Production Report 2010–2011. Port Stephens, New South Wales: Department of Primary Industries, 2011. ISSN 1444-840. [Google Scholar]
- 30.Crawford C, National review of Ostrea angasi aquaculture: historical culture, current methods and future priorities, University of Tasmania Institute for Marine and Antarctic Studies, Hobart, Tasmania: 2016. [Google Scholar]
- 31.Gillies CL, Crawford C, Hancock B. Restoring Angasi oyster reefs: What is the endpoint ecosystem we are aiming for and how do we get there? Ecol Manage Restor. 2017;18(3):214–22. [Google Scholar]
- 32.McLeod I, Boström-Einarsson L, Creighton C, D’Anastasi B, Diggles B, Dwyer P, et al. Habitat value of Sydney rock oyster (Saccostrea glomerata) reefs on soft sediments. Mar and Freshw Res.2019. 10.1071/MF18197 [DOI]
- 33.Parker LM, Scanes E, O'Connor WA, Coleman RA, Byrne M, Pörtner H-O, et al. Ocean acidification narrows the acute thermal and salinity tolerance of the Sydney rock oyster Saccostrea glomerata. Mar Pollut Bull. 2017;122(1–2):263–71. 10.1016/j.marpolbul.2017.06.052 [DOI] [PubMed] [Google Scholar]
- 34.Parker LM, Ross PM, O’Connor WA. Comparing the effect of elevated pCO2 and temperature on the fertilization and early development of two species of oysters. Mar Biol. 2010;157(11):2435–52. [Google Scholar]
- 35.Cole VJ, Parker LM, O’Connor SJ, O’Connor WA, Scanes E, Byrne M, et al. Effects of multiple climate change stressors: ocean acidification interacts with warming, hyposalinity, and low food supply on the larvae of the brooding flat oyster Ostrea angasi. Mar Biol. 2016;163(5):1–17. [Google Scholar]
- 36.Pereira RRC, Scanes E, Parker LM, Byrne M, Cole VJ, Ross PM. Restoring the flat oyster Ostrea angasi in the face of a changing climate. Mar Ecol Prog Ser. 2019;625:27–39. [Google Scholar]
- 37.Ridgway K. Long‐term trend and decadal variability of the southward penetration of the East Australian Current. Geophys Res Lett. 2007;34(13). [Google Scholar]
- 38.Hobday AJ, Pecl GT. Identification of global marine hotspots: sentinels for change and vanguards for adaptation action. Rev Fish Biol Fish. 2014;24(2):415–25. [Google Scholar]
- 39.Babcock R.C., Bustamante R.H., Fulton E.A., Fulton D.J., Haywood M.D., Hobday A.J.,et al. Severe continental-scale impacts of climate change are happening now: Extreme climate events impact marine habitat forming communities along 45% of Australia’s coast. Frontiers in Marine Science, 6, 2019;p.411. [Google Scholar]
- 40.Schiel D.R., Steinbeck J.R. and Foster M.S.,. Ten years of induced ocean warming causes comprehensive changes in marine benthic communities. Ecology, 85(7), 2004; pp.1833–1839. [Google Scholar]
- 41.Nell JA O'Connor WA. The evaluation of fresh algae and stored algal concentrates as a food source for Sydney rock oyster, Saccostrea commercialis (Iredale & Roughley), larvae. Aquaculture. 1991;99(3–4):277–84. [Google Scholar]
- 42.Scanes E, Scanes P, Ross PM. Climate change rapidly warms and acidifies Australian estuaries. Nat Comms 2020 (In Press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roy P, Williams R, Jones A, Yassini I, Gibbs P, Coates B, et al. Structure and function of south-east Australian estuaries. Estuar Coast Shelf Sci. 2001;53(3):351–84. [Google Scholar]
- 44.Lucas A, Beninger PG. The use of physiological condition indices in marine bivalve aquaculture. Aquaculture. 1985;44(3):187–200. [Google Scholar]
- 45.Mann R. A comparison of methods for calculating condition index in eastern oysters Crassostrea virginica (Gmelin, 1791). J Shellfish Res. 1992;11(1):55. [Google Scholar]
- 46.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37(8):911–7. 10.1139/o59-099 [DOI] [PubMed] [Google Scholar]
- 47.Volkman JK, Nichols PD. Applications of thin layer chromatography-flame ionization detection to the analysis of lipids and pollutants in marine and environmental samples. J Planar Chromatogr. 1991;4:19–26. [Google Scholar]
- 48.Ackman R. [11] Flame ionization detection applied to thin-layer chromatography on coated quartz rods. Methods Enzymol. 72: Elsevier; 1981. p. 205–52. 10.1016/s0076-6879(81)72013-5 [DOI] [PubMed] [Google Scholar]
- 49.Sinanoglou VJ, Strati IF, Bratakos SM, Proestos C, Zoumpoulakis P, Miniadis-Meimaroglou S. On the combined application of Iatroscan TLC-FID and GC-FID to identify total, neutral, and polar lipids and their fatty acids extracted from foods. ISRN Chromatography. 2013;2013. [Google Scholar]
- 50.Anderson M, Gorley RN, Clarke RK. Permanova+ for primer: Guide to software and statisticl methods: Primer-E Limited; 2008, Plymouth, UK.
- 51.Pörtner H-O, Reipschläger A, Heisler N. Acid-base regulation, metabolism and energetics in Sipunculus nudus as a function of ambient carbon dioxide level. J Exp Biol. 1998;201(1):43–55. [DOI] [PubMed] [Google Scholar]
- 52.Hiebenthal C, Philipp EE, Eisenhauer A, Wahl M. Effects of seawater pCO2 and temperature on shell growth, shell stability, condition and cellular stress of Western Baltic Sea Mytilus edulis (L.) and Arctica islandica (L.). Mar Biol. 2013;160(8):2073–87. [Google Scholar]
- 53.Talmage SC, Gobler CJ. Effects of elevated temperature and carbon dioxide on the growth and survival of larvae and juveniles of three species of northwest Atlantic bivalves. PloS one. 2011;6(10):e26941 10.1371/journal.pone.0026941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bayne B, Bayne C, Carefoot T, Thompson R. The physiological ecology of Mytilus californianus Conrad. 1. Metabolism and energy balance. Oecologia. 1976:211–28. [DOI] [PubMed] [Google Scholar]
- 55.Abad M., Ruiz C., Martinez D., Mosquera G. and Sánchez J. Seasonal variations of lipid classes and fatty acids in flat oyster, Ostrea edulis, from San Cibran (Galicia, Spain). Comparative Biochemistry and Physiology Part C: Pharmacology, Toxicology and Endocrinology, 1995;110(2). pp.109–118. [Google Scholar]
- 56.Fields PA, Zuzow MJ, Tomanek L. Proteomic responses of blue mussel (Mytilus) congeners to temperature acclimation. J Exp Biol. 2012;215(7):1106–16. [DOI] [PubMed] [Google Scholar]
- 57.Scanes E, Parker LM, O’Connor WA, Gibbs MC, Ross PM. Copper and ocean acidification interact to lower maternal investment, but have little effect on adult physiology of the Sydney rock oyster Saccostrea glomerata. Aquat Toxicol. 2018;203:51–60. 10.1016/j.aquatox.2018.07.020 [DOI] [PubMed] [Google Scholar]
- 58.Somero GN. Thermal physiology and vertical zonation of intertidal animals: optima, limits, and costs of living. Integr Comp Biol. 2002;42(4):780–9. 10.1093/icb/42.4.780 [DOI] [PubMed] [Google Scholar]
- 59.Anestis A, Lazou A, Pörtner HO, Michaelidis B. Behavioral, metabolic, and molecular stress responses of marine bivalve Mytilus galloprovincialis during long-term acclimation at increasing ambient temperature. American Journal of Physiology-Regulatory, Integr Comp Physiol. 2007;293(2):R911–R21. [DOI] [PubMed] [Google Scholar]
- 60.Sørensen JG, Kristensen TN, Loeschcke V. The evolutionary and ecological role of heat shock proteins. Ecol Lett. 2003;6(11):1025–37. [Google Scholar]
- 61.Ivanina A, Taylor C, Sokolova I. Effects of elevated temperature and cadmium exposure on stress protein response in eastern oysters Crassostrea virginica (Gmelin). Aquat Toxicol. 2009;91(3):245–54. 10.1016/j.aquatox.2008.11.016 [DOI] [PubMed] [Google Scholar]
- 62.Abele D, Heise K, Pörtner H-O, Puntarulo S. Temperature-dependence of mitochondrial function and production of reactive oxygen species in the intertidal mud clam Mya arenaria. J Exp Biol. 2002;205(13):1831–41. [DOI] [PubMed] [Google Scholar]
- 63.Somero G.N. The physiology of global change: linking patterns to mechanisms. Annual Review of Marine Science, 2012;4, pp.39–61. 10.1146/annurev-marine-120710-100935 [DOI] [PubMed] [Google Scholar]
- 64.Byrne M, Foo AS, Ross PM, Putnam HM. Limitations to cross- and multigenerational plasticity for marine invertebrates faced with global climate change. 2020 Glob. Ch. Biol 10.1111/gcb.14882 [DOI] [PubMed]
- 65.Parker L M, O'Connor W. A, Byrne M, Coleman R A, Virtue P., Dove M, et al. Adult exposure to ocean acidification is maladaptive for larvae of the Sydney rock oyster Saccostrea glomerata in the presence of multiple stressors Biol. Lett.; 13 10.1098/rsbl.2016.0798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rivest E.B., Comeau S. and Cornwall C.E. The role of natural variability in shaping the response of coral reef organisms to climate change. Current Clim Chan Reports, 2017;3(4), pp.271–281. [Google Scholar]
- 67.McAfee D., O'connor W.A. and Bishop M.J. Fast‐growing oysters show reduced capacity to provide a thermal refuge to intertidal biodiversity at high temperatures. J Animal Ecol, 2017;86(6), pp.1352–1362. [DOI] [PubMed] [Google Scholar]
- 68.Mitchell I.M., Crawford C.M. and Rushton M.J. Flat oyster (Ostrea angasi) growth and survival rates at Georges Bay, Tasmania (Australia). Aquaculture, 2000;191(4), pp.309–321. [Google Scholar]
- 69.Lipcius RN, Burke RP. Successful recruitment, survival and long-term persistence of eastern oyster and hooked mussel on a subtidal, artificial restoration reef system in Chesapeake Bay. PloS one. 2018;13(10):e0204329 10.1371/journal.pone.0204329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laing I, Walker P, Areal F. Return of the native–is European oyster (Ostrea edulis) stock restoration in the UK feasible? Aquat Liv Resour. 2006;19(3):283–7. [Google Scholar]
- 71.Rodolfo‐Metalpa R, Lombardi C, Cocito S, Hall‐Spencer JM, Gambi MC. Effects of ocean acidification and high temperatures on the bryozoan Myriapora truncata at natural CO2 vents. Mar Ecol. 2010;31(3):447–56. [Google Scholar]
- 72.Foo SA, Byrne M, Ricevuto E, Gambi MC. The carbon dioxide vents of Ischia, Italy, a natural laboratory to assess impacts of ocean acidification on marine ecosystems: an overview of research and comparisons with other vent systems. 2018. Oceanography and Marine Biology: Annual Review 56:237–310. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
a. Results of PERMANOVA for the shell growth, condition index and SMR of Ostrea angasi exposed for seven months in Lake Macquarie. b. Results of PERMANOVA for the shell growth, condition index and SMR of Saccostrea glomerata exposed for seven months in Lake Macquarie. P values were created using Monte Carlo tests. Significant values (P<0.05) are bold.
(DOCX)
a. Results of PERMANOVA for the total lipids, amount of total lipids (mg/g), amount of Triacylglycerides (TAGs; mg/g) and Phospholipids (PLs; mg/g) of Ostrea angasi exposed for seven months in Lake Macquarie. b. Results of PERMANOVA for the total lipids (mg/g), amount of Triacylglycerides (TAGs; mg/g) and Phospholipids (PLs; mg/g), of Saccostrea glomerata exposed for seven months in Lake Macquarie. P values were created using Monte Carlo tests. Significant values (P<0.05) are bold.
(DOCX)
a. Results of PERMANOVA for the percentage survival of Ostrea angasi exposed for seven months in Lake Macquarie. b. Results of PERMANOVA for the percentage survival of Saccostrea glomerata exposed for seven months in Lake Macquarie. P values were created using Monte Carlo tests. Significant values (P<0.05) are bold.
(DOCX)
(CSV)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.