Abstract
Context
Alterations in gut microbiota relate to the metabolic syndrome, but have not been examined in at-risk obese youth with polycystic ovary syndrome (PCOS).
Objective
Compare the composition and diversity of the gut microbiota and associations with metabolic and hormonal measures between 2 groups of female adolescents with equal obesity with or without PCOS.
Design
Prospective, case-control cross-sectional study.
Setting
Tertiary-care center.
Participants
A total of 58 obese female adolescents (n = 37 with PCOS; 16.1 ± 0.3 years of age; body mass index [BMI] 98.5th percentile) and (n = 21 without PCOS; 14.5 ± 0.4 years of age; BMI 98.7th percentile).
Outcomes
Bacterial diversity, percent relative abundance (%RA), and correlations with hormonal and metabolic measures.
Results
Participants with PCOS had decreased α-diversity compared with the non-PCOS group (Shannon diversity P = 0.045 and evenness P = 0.0052). β-diversity, reflecting overall microbial composition, differed between groups (P < 0.001). PCOS had higher %RA of phyla Actinobacteria (P = 0.027), lower Bacteroidetes (P = 0.004), and similar Firmicutes and Proteobacteria. PCOS had lower %RA of families Bacteroidaceae (P < 0.001) and Porphyromonadaceae (P = 0.024) and higher Streptococcaceae (P = 0.047). Lower bacterial α-diversity was strongly associated with higher testosterone concentrations. Several individual taxa correlated with testosterone and metabolic measures within PCOS and across the entire cohort. Receiver operative curve analysis showed 6 taxa for which the %RA related to PCOS status and lower Bacteroidaceae conferred a 4.4-fold likelihood ratio for PCOS.
Conclusion
Alterations in the gut microbiota exist in obese adolescents with PCOS versus obese adolescents without PCOS and these changes relate to markers of metabolic disease and testosterone. Further work is needed to determine if microbiota changes are reflective of, or influencing, hormonal metabolism.
Keywords: PCOS, adolescence, obesity, metabolic syndrome
Polycystic ovary syndrome (PCOS) is a common endocrine disorder among women of reproductive age, characterized by hyperandrogenism with clinical presentations including acne, hirsutism, and irregular menses (1–5). The prevalence of PCOS is estimated to be 6% to 18% of women worldwide (2–4). PCOS manifests in adolescence, is commonly accompanied by obesity (6) and is associated with higher risk for type 2 diabetes (T2D), cardiovascular disease, nonalcoholic fatty liver disease (NAFLD), infertility, pregnancy complications, and depression (2, 4, 7–9). The etiology of PCOS is unknown but is thought to be multifactorial, comprising genetics, intrauterine environment, lifestyle factors, and more recently, potential alteration in the gut microbiota (10).
The gut microbiota has been linked to health and it plays a key role in immune modulation, pathogen prevention, host metabolism, energy uptake, fat distribution, and gut barrier protection (2–5, 8, 10, 11). The gut microbiota comprises a complex community of microorganisms, the majority of which belong to 4 bacterial phyla (Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria) (8, 12). Alterations in these 4 phyla have been associated with high fat/low fiber diet, obesity, insulin resistance, T2D and NAFLD (2–5, 7–17). A leaky gut, in which the intestinal barrier becomes compromised and permeable to toxins, antigens, and bacteria leading to passage into the blood stream, has been hypothesized to mediate the pathogenesis of the metabolic syndrome via upregulation of systemic inflammatory response (10). The systemic inflammatory response has been shown to disrupt various organ functions and could contribute to insulin resistance (2, 3, 9, 11, 18). Insulin resistance, as relates to the leaky gut hypothesis, is thought to relate in part to increased serum cytokines that can activate NF-kB, resulting in alteration of the insulin receptor subunit and impedance of insulin signaling pathway (10). Lower %RA of the phylum Bacteroidetes, in particular, is associated with metabolic disease, perhaps via this mechanism (10).
Despite the increased risk of obesity and metabolic syndrome in women with PCOS, there are limited data linking altered gut microbiota to PCOS (2–5, 19–23). Initial work demonstrated that women with PCOS, as defined by the Rotterdam criteria, have dysbiosis relative to women without PCOS, regardless of BMI (4, 5). Furthermore, rodent models of PCOS demonstrate that these alterations are independent of the effect of diet (3) and that a fecal transplant from non-PCOS donors can reestablish the menstrual cycle in PCOS rat models (2). Metabolic reproductive phenotypes were seen in female mice pretreated with antibiotics after fecal microbiome transplantation from PCOS (23). However, it is unclear if these alterations in gut microbiota are present early in the disease process or are a result of PCOS-related hyperandrogenism and insulin resistance.
Our goal, therefore, was to determine if female adolescents with PCOS and obesity have altered composition of gut microbiota compared with equally-obese female adolescents who have regular menstrual cycles. This information could provide additional directions to improve treatment and prevention of PCOS.
Materials and Methods
Participants
A total of 58 participants were included from 3 separate cross-sectional studies (APPLE [NCT02157974], n = 26; PLUM [NCT03041129], n = 16; and MISS [NCT03120871], n = 16). Thirty-seven patients were diagnosed with PCOS, and 21 did not have PCOS and had regular menses. Participants were recruited from the pediatric endocrinology and lifestyle medicine outpatient clinics at the Children’s Hospital Colorado. Inclusion criteria were female sex, age 12 to 20 years, overweight/obesity (BMI > 90th percentile), completed puberty with menses and Tanner 5 pubertal staging, and sedentary status (< 3 hours of regular exercise/week; validated with a 3-day activity recall). Exclusion criteria were blood pressure > 140/90 mmHg, hemoglobin < 9 mg/dL, serum creatinine >1.5 mg/dL, smoking, medication affecting insulin resistance (oral steroids, metformin, thiazolidinedione, atypical antipsychotics, hormonal contraceptives), antihypertensive medications, statins, pregnancy, breast feeding, and any antibiotic use in the previous 2 months. The most stringent National Institutes of Health (NIH) criteria with adolescent adaptation were used to diagnose PCOS: (1) oligomenorrhea defined as < 8 menses per year at least 2 years postmenarche and (2) clinical or biochemical signs of hyperandrogenism (24).
Study approval
The study protocols were approved by the University of Colorado Anschutz Institutional Review Board and the Children’s Hospital of Colorado Scientific Advisory Review Committee. Informed consent was obtained from all participants and parental consent and participant assent from all participants < 18 years old.
Physical activity
A 3-day pediatric activity recall (3DPAR) questionnaire was completed with staff assistance from all participants.
Dietary intake
A diet interview was completed using a SEARCH food frequency questionnaire (FFQ) to assess macronutrient pattern. The FFQ was modified to incorporate and represent common food choices among ethnically and regionally diverse youth from 10 to 19 years of age (25).
Laboratory measurements
Fasting glucose, sex hormone concentrations, inflammatory markers and lipid profiles were measured, but not all labs were performed in all participants due to differences in study design between the protocols. All participants had measures of total testosterone, alanine aminotransferase (ALT), and lipids. Sixteen control and 2 PCOS participants from MISS did not have measurements of HbA1c, C-peptide, complete blood count, high sensitivity-C reactive protein (hs-CRP), adiponectin, free fatty acids, or free testosterone. Glucose, insulin, and c-peptide were not included from 10 PCOS APPLE participants who had received a dose of exenatide prior to blood collection. Analyses were performed by standard methods at the University of Colorado Anschutz research core laboratory or the Children’s Hospital Colorado clinical laboratory, except where noted. Glucose was measured by a Statstrip hospital grade glucometer (Nova Biomedical, Waltham, MA). Serum insulin and adiponectin were determined by radioimmunoassay (Millipore, Billerica, MA); free fatty acids enzymatically (Wako Chemicals, Inc., Richmond, VA); HbA1c with ion-exchange high-pressure liquid chromatography (HPLC) (Bio-Rad Laboratories, Hercules, CA); ALT by P-5-P method (Vitros® 5600, Ortho Clinical Diagnostics, Raritan, NJ); total cholesterol, high density lipoprotein (HDL)-cholesterol, and triglyceride (TG) enzymatically on a Hitachi 917 autoanalyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN). Low density lipoprotein (LDL) cholesterol was calculated by the Friedewald equation; hs-CRP was measured via immunoturbidimetric assay (Beckman Coulter, Brea, CA), C-peptide via chemiluminescent immunoassay (DiaSorin, Stillwater, MN), and estradiol and progesterone via chemiluminescent immunoassay (Beckman Coulter, Brea, CA). Total testosterone was measured by HPLC/tandem mass spectrometry (Esoterix laboratories, Calbassas Hills, CA, and BRAC laboratory, Boston, MA) and free testosterone via equilibrium dialysis and sex hormone–binding globulin (SHBG) via chemiluminescent immunoassay by Esoterix laboratories (Calbassas Hills, CA).
Fecal collection and microbiome analysis
Stool samples were collected at home the day prior to blood sampling using stool collection tubes, and they were then frozen in the participant freezer. Upon return to study staff, samples were stored at −80°C until further processing. Samples were sequenced in 3 batches, with cases and controls included in each batch (Batch A, 16 cases/5 controls; Batch B, 8 cases/2 controls; Batch C, 13 cases/14 controls). Bacterial profiles were determined by broad-range analysis of 16S rRNA genes following our previously described methods (26–28). In brief, DNA was extracted from 50 to 100 mg of stool using the PowerFecal DNA isolation kit (QIAamp Powerfecal DNA kit; Qiagen INC, Hilden, Germany). Broad-range PCR amplicons were generated using barcoded primers that target the V3V4 variable region of the 16S rRNA gene: primers 338F (5′ ACTCCTACGGGAGGCAGCAG) and 806R (5′ GGACTACHVGGGTWTCTAAT). PCR products were normalized using a SequalPrepTM kit (Invitrogen, Carlsbad, CA), pooled, and quantified by Qubit Fluorometer 2.0 (Invitrogen, Carlsbad, CA). Illumina paired-end sequencing was performed on the Ilumina MiSeq platform with version v2.3.0.8 of the MiSeq Control Software and version v2.3.32 of MiSeq Reporter, using a 600-cycle version 3 reagent kit. Illumina MiSeq paired-end reads were aligned to a Homo sapiens reference genome (UCSC Hg19) with bowtie2 and matching sequences discarded (29). Remaining paired-end sequences were sorted by sample via barcodes in the paired reads with a python script. The sorted paired reads were assembled using Phrap and pairs that did not assemble were discarded. Assembled sequence ends were trimmed over a moving window of 5 nucleotides until average quality met or exceeded 20. Trimmed sequences with more than 1 ambiguity or shorter than 150 nucleotides were discarded. Potential chimeras identified with Uchime (usearch6.0.203_i86linux32) using the Schloss Silva reference sequences were removed from subsequent analyses. Assembled sequences were aligned and classified with SINA (1.3.0-r23838) using the bacterial sequences in Silva 115NR99 as reference configured to yield the Silva taxonomy. Operational taxonomic units were produced by clustering sequences with identical taxonomic assignments. From 37, 917 to 195, 588 sequence reads were generated per sample and Good’s coverage was > 99.0% for all samples.
Calculations
Insulin sensitivity was estimated using the homeostasis model assessment (HOMA) model: (fasting glucose in mg/dL fasting insulin in µIU/mL) / (405).
Statistical analysis
The %RA of each taxon was calculated as the number of 16S rRNA sequences of a given taxon divided by the total number of 16S rRNA sequences in a patient’s sample. Differences in overall microbiome composition (β-diversity) between subsets were assessed by a nonparametric, permutation-based multivariate analysis of variance (PERMANOVA with 10,000 replicate resamplings) using Morisita-Horn dissimilarities. Three commonly used measures of α-diversity, Shannon diversity (Shannon’s entropy measure H), Shannon evenness (H/Hmax), and richness (Sobs) were calculated using rarefaction (30) and compared across groups using linear models adjusting for batch effects (31). Comparisons of %RA across groups were performed using Wilcoxon rank sum (2 groups) tests, since batch effects were not significant in any of the individual phyla, family, or genus comparisons. The P value was false discovery rate (FDR) adjusted per the number of variables for these analysis. Receiver operative curve (ROC) analyses were performed to determine if taxa could predict PCOS diagnostic status, with outputs including: ROC area under the curve (AUC), sensitivity, specificity and likelihood ratio of the bacterial taxa in predicting PCOS status. Spearman’s correlations were used to determine the relationship between %RA and metabolic and hormonal variables, with an adjusted P-value of <0.01 considered significant due to the number of tests performed. Data analyses were performed using R version 3.5.2 (32) and Sigmaplot version 13.0 (Systat Software, San Jose, CA).
Results
Clinical characteristics
Fifty-nine participants completed the baseline assessment and returned the stool sample. One PCOS subject was removed due to insufficient stool sample size. The final analyses were performed with 21 non-PCOS controls and 37 PCOS adolescents. Subject demographic, physical characteristics and laboratory measurements are summarized in Table 1. PCOS were significantly older than control (P < 0.01). The groups had similar age of menarche (P = 0.69), BMI percentile (P = 0.56), and BMI kg/m2 (P = 0.39). Race and ethnicity were similar between groups (P = 0.17). The PCOS group had a significantly more frequent family history of T2D (P < 0.05). Systolic (P = 0.11) and diastolic (P = 0.34) blood pressures were similar between groups, as were dietary fat and carbohydrate intake percentage and physical activity. Adolescents without PCOS had statistically significantly higher protein intake percentage than those with PCOS.
Table 1.
Cohort Characteristics
| Obese Control, N = 21 | Obese PCOS, N = 37 | P value | |
|---|---|---|---|
| Demographics and History | |||
| Age, years | 14.5 ± 0.4 | 16.1 ± 0.3 | <0.01 |
| Ethnicity/Race (Caucasian; Hispanic; Black; Asian), % | 14%; 71%; 14%; 0% | 32%; 43%; 19%; 5% | 0.17 |
| Menarche age, years | 11.0 (10,13) | 11.5 (11,13) | 0.69 |
| Family history of T2D, % | 38% | 73% | <0.05 |
| Physical Characteristics | |||
| BMI, kg/m2 | 35 (30.7, 39.3) | 36 (32.9, 39.7) | 0.39 |
| BMI, percentile | 98.7 (97.8, 99.3) | 98.5 (97, 99) | 0.56 |
| BMI Z-score | 2.2 ± 0.8 | 2.2 ± 0.06 | 0.53 |
| Waist:hip ratio | 0.88 ± 0.02 | 0.90 ± 0.009 | 0.28 |
| Systolic BP, mmHg | 118 (112, 132) | 124 (117, 135) | 0.11 |
| Diastolic BP, mmHg | 68 ± 1.9 | 71 ± 1.3 | 0.34 |
| 7 day Dietary Intake Recall | |||
| Fat intake, % | 34 (28, 42) | 40 (36, 43) | 0.07 |
| Protein intake, % | 18 (16, 21) | 16 (15, 18) | 0.01 |
| Carbohydrate intake, % | 46 (41, 52) | 44 (40, 50) | 0.43 |
| Physical Activity | |||
| Activity from recall survey, METS | 52.9 ± 1.6 | 54 ± 1.4 | 0.59 |
| Laboratory Measurements | |||
| Free testosterone, ng/dL | 4.0 (2.8, 4.8)§ | 8.9 (7.1, 12) | <0.001 |
| Total testosterone, ng/dL | 20 (19, 24) | 43 (33, 51) | <0.001 |
| SHBG, mmol/L | 22 (16, 39.5)§ | 19 (12, 26) | 0.36 |
| Estradiol, pg/mL | 43 (42, 68)§ | 55 (45, 84) | 0.69 |
| Progesterone, ng/dL | 0.8 (0.5, 0.9)§ | 0.6 (0.4, 0.9) | 0.27 |
| Cholesterol, mg/dL | 128 (114,148) | 140 (131, 168) | <0.01 |
| HDL, mg/dL | 35 (31, 50) | 36 (31, 44) | 0.66 |
| LDL, mg/dL | 85 (75, 108) | 108 (92, 123) | <0.01 |
| Triglycerides, mg/dL | 105 (83, 121) | 112 (96,145) | 0.12 |
| Fasting glucose, mg/dL | 90 ± 2 | 89 ± 2‡ | 0.82 |
| Fasting insulin, µU/mL | 14 (9, 21.5) | 23 (18, 39)‡ | <0.001 |
| HOMA-IR | 4.1 (2, 6) | 4.5 (3, 9)‡ | 0.15 |
| HbA1c, % | 5.2 ± 0.1§ | 5.5 ± 0.1 | <0.05 |
| C-peptide, ng/mL | 2.0 ± 0.2§ | 2.6 ± 0.2‡ | 0.14 |
| ALT, IU/mL | 25 (16, 36) | 34 (30, 44) | <0.001 |
| WBC, 109 cells/L | 7.4 ± 0.8§ | 8.0 ± 0.3 | 0.32 |
| Platelets, 108 cells/L | 328 (268, 373)§ | 317 (293, 352) | 0.83 |
| hs-CRP, mg/dL | 4.8 (0.7, 6.9)§ | 3.6 (1.2, 6.9) | 0.72 |
| Adiponectin, ng/mL | 7.3 (4.3, 10.8)§ | 6.2 (4.3, 7.5) | 0.62 |
Values are mean ± standard error of the mean, or median (25%, 75 %). Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; BP, blood pressure; HbA1c, hemoglobin A1C; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment for insulin resistance; hs-CRP, high-sensitivity C-reactive protein; LDL, low-density lipoprotein; METS, metabolic equivalents; PCOS, polycystic ovary syndrome; %RA, percent relative abundance; SHBG, sex hormone–binding globulin; T2D, type 2 diabetes; WBC, white blood cells.
§N = 7 for the control group. ‡N = 25 for the PCOS group.
Per study design, adolescents with PCOS had significantly higher free testosterone and total testosterone compared with non-PCOS controls. Participants with PCOS had significantly higher cholesterol and LDL than controls. The groups had similar SHBG, estradiol, progesterone, TG, HDL, fasting glucose and homeostasis model assessment for insulin resistance (HOMA-IR); however, the PCOS group had significantly higher fasting insulin, HbA1c, and ALT. Markers of inflammation including WBC, platelets, hs-CRP, and adiponectin were similar between groups.
PCOS had decreased biodiversity and alterations in bacterial %RA
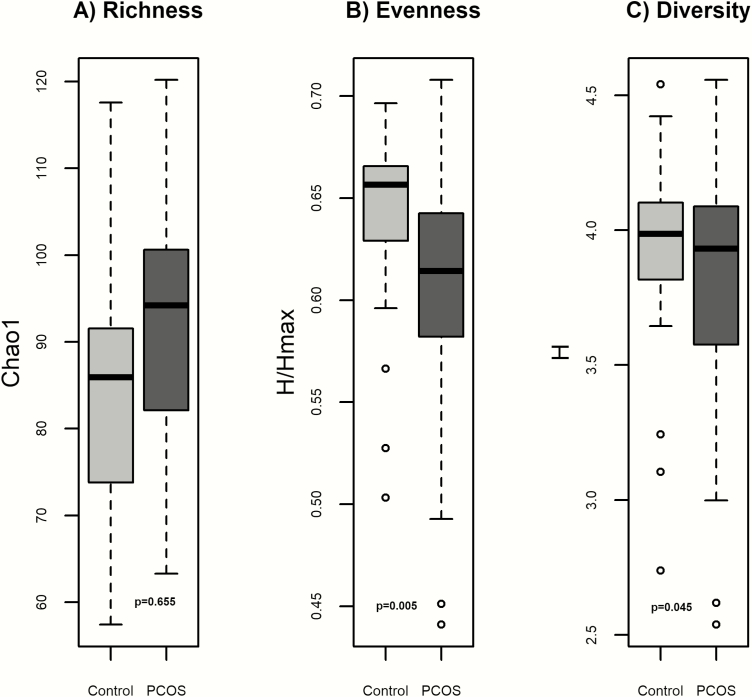
Bacterial 16S rRNA gene profiling was successful for all samples; both PCOS and OB groups had adequate depth of sequencing coverage (Good’s coverage of >99.0% for all samples). Compared with the obese participants without PCOS, those with PCOS had significantly lower α-biodiversity (Fig. 1) measured by evenness (P = 0.0052) and Shannon diversity (P = 0.045) compared with controls. The groups had similar richness (P = 0.655). β-diversity, reflecting overall gut microbial community composition, was different between groups (P < 0.001).
Figure 1.
Measures of α-biodiversity including: A) Richness (Chao1) B) Evenness (Shannon H/Hmax), C) Shannon diversity (Shannon H). Richness was similar between PCOS and obese controls. Evenness and Shannon diversity were lower in PCOS.
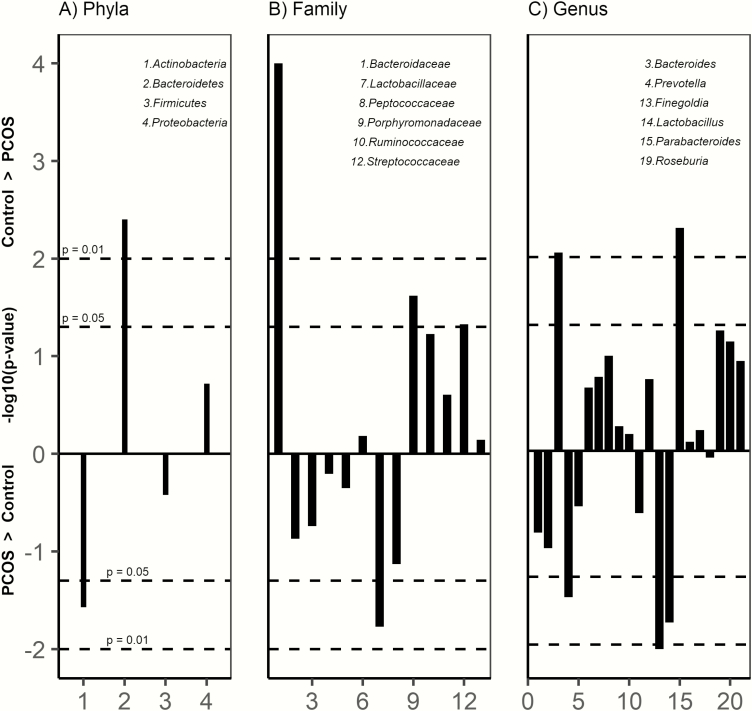
Firmicutes and Bacteroidetes were the predominant phyla in the cohort. At the phylum level (Fig. 2A), PCOS had higher %RA of Actinobacteria (P = 0.027), lower Bacteroidetes (P = 0.004), and similar Firmicutes and Proteobacteria. At the family level (Fig. 2B), adolescents with PCOS had lower %RA of Bacteroidaceae (P < 0.001) and Porphyromonadaceae (P = 0.024) and higher Streptococcaceae (P = 0.047). Six taxa at the genus level were significantly different between groups (Fig. 2C).
Figure 2.
Manhattan plot of bacterial %RA between groups at the A) Phyla B) Family and C) Genus level. Only taxa with >1% RA are included. Lines above 0 are >%RA in controls and below 0 >%RA in PCOS, with dotted horizontal lines representing P < 0.05 and P < 0.01. PCOS had significantly lower %RA of Bacteroidetes and higher Actinobacteria. At the family level, PCOS had lower %RA of Bacteroidaceae, Porphyromonadaceae, Streptococcaceae, and higher %RA of Lactobacillaceae. At the genus level, PCOS had significantly lower %RA of Bacteroides, Parabacteroides, and higher %RA of Prevotella, Finegoldia, and Lactobacillus.
Associations with metabolic markers within PCOS
Total testosterone and free testosterone were highly correlated with evenness and Shannon diversity measures, and free testosterone with richness (Table 2). Systolic blood pressure (SBP) was significantly associated with family Streptococcaceae. The waist-to-hip ratio was significantly associated with the phyla Firmicutes:Bacteroidetes ratio (F:B ratio) and the family Bacteroidaceae. ALT was significantly associated with the phyla F:B ratio, and the family Bacteroidaceae. TG level was significantly associated with the family Ruminococcaceae. HOMA-IR was significantly associated with family Lachnospiraceae and Veillonellaceae. Free testosterone and fasting glucose were not significantly associated with any bacterial taxa at the phylum, family, or genus levels.
Table 2.
Correlations Between Clinical Measurements and Bacterial Taxa or α-Diversity Measures
| Bacterial Measure | R | |
|---|---|---|
| PCOS Only (N = 37) | ||
| Free testosterone | Richness (Chao1) | −0.47** |
| Diversity (Shannon H) | −0.64** | |
| Evenness (Shannon H/Hmax) | −0.60** | |
| Total testosterone | Diversity (Shannon H) | −0.53** |
| Evenness (Shannon H/Hmax) | −0.52** | |
| Systolic blood pressure | (F) Streptococcaceae | 0.46 * |
| Waist:Hip ratio | (P) Firmicutes:Baceteriodetes ratio | 0.42* |
| (F) Bacteroidaceae | −0.43* | |
| ALT | (P) Firmicutes:Baceteriodetes ratio | 0.39* |
| (F) Bacteroidaceae | −0.39* | |
| Fasting triglyceride | (F) Ruminococcaceae | −0.46** |
| HOMA-IR‡ | (F) Lachnospiraceae | 0.41* |
| (F) Veillonellaceae | −0.51** | |
| All Participants (PCOS N = 37 + Control N = 21) | ||
| Free Testosterone § | Diversity (Shannon H) | −0.55** |
| Evenness (Shannon index) | −0.54** | |
| (F) Porphyromonadaceae | −0.396* | |
| Total testosterone | Diversity (Shannon H) | −0.55** |
| Evenness (Shannon H/H max) | −0.62* | |
| Systolic blood pressure | (F) Streptococcaceae | 0.38* |
| Waist:Hip ratio | (P) Bacteriodetes | −0.47* |
| (P) Firmicutes:Baceteriodetes ratio | 0.48** | |
| (F) Bacteroidaceae | −0.47* | |
| ALT | (P) Bacteriodetes | −0.43** |
| (P) Firmicutes:Baceteriodetes ratio | 0.44** | |
| (F) Bacteroidaceae | 0.47** | |
| (F) Porphyromonadaceae | −0.35* | |
| Fasting insulin‡ | (P) Bacteriodetes | −0.45** |
| (P) Firmicutes:Baceteriodetes ratio | 0.44* | |
| (F) Bacteroidaceae | −0.56** |
Correlations between clinical measures and bacterial taxa or measures of diversity with P values of <0.01 are shown. Abbreviations: ALT, alanine aminotransferase; F, family; P, phylum. *P < 0.01, **P < 0.001. §N = 7 for the control group. ‡N = 25 for the PCOS group.
Associations with metabolic markers across groups
Total testosterone was significantly associated with evenness and Shannon diversity and free testosterone was significantly correlated with evenness and Shannon diversity and family Porphyromonadaceae (Table 2). SBP was significantly associated with family Streptococcaceae. The waist-to-hip ratio is significantly associated with phyla Bacteroidetes, F:B ratio, and family Bacteroidaceae. ALT was significantly associated with phyla Bacteroidetes, F:B ratio, and families Bacteroidaceae and Porphyromonadaceae. Fasting insulin was significantly associated with phyla Bacteroidetes, and F:B ratio, and family Bacteridaceae. There were no significant associations between fasting glucose and bacterial taxa.
Taxa with significant %RA predicted PCOS status
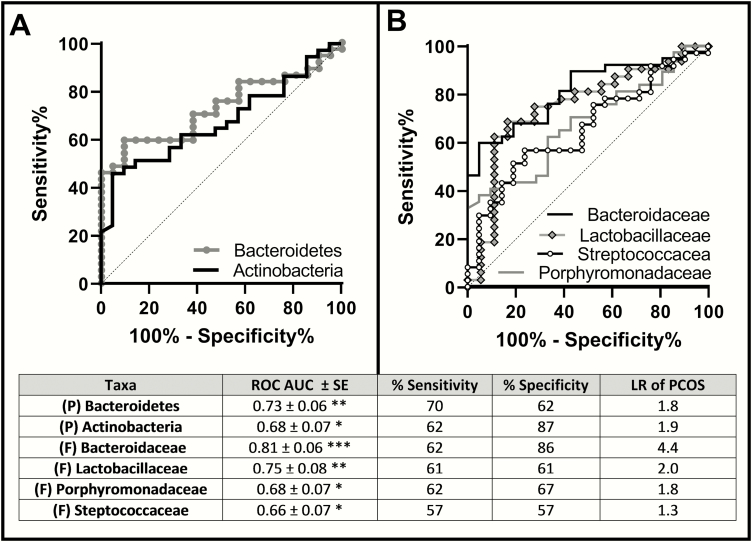
ROC analyses were performed to explore the association of significant taxa with PCOS diagnosis status at the phyla (Fig. 3A) and family (Fig. 3B) level, with ROC AUC, sensitivity and specificity, and likelihood ratios for each taxa in the included table. Genus data was not included because the %RA of bacteria at the genus level was < 1% and for some taxa, there was no classification up to the genus level. Six taxa were significantly associated with PCOS status, including the phyla Bacteroidetes (AUC 0.73 ± 0.06) and Actinobacteria (AUC 0.68 ± 0.07), and the families Lactobacillaceae (AUC 0.75 ± 0.08), Bacteroidaceae (AUC 0.81 ± 0.06), Porphyromonadaceae (AUC 0.68 ± 0.07), and Streptococcaceae (AUC 0.66 ± 0.07). Family Bacteroidaceae was most predictive, with a sensitivity of 62% and a specificity of 86%; a lower %RA of Bacteroidaceae conferred a 4.4-fold likehood ratio for PCOS diagnosis.
Figure 3.
ROC curves of taxa associated with PCOS diagnosis status A) Phlya B) Family. Phyla Bacteroidetes and Actinobacteria, and family Bacteroidaceae, Lactobacillaceae, Prophyromonadaceae, and Streptococcaceae significantly are associated with PCOS status. *P < 0.05; **P < 0.005; ***P < 0.001. Abbreviations: AUC, area under the curve; F, family; LR, likelihood ratio of taxa predicting PCOS diagnosis; P, phyla; ROC, receiver operating curve; SE, standard error.
Discussion
Metabolic disease and PCOS has been associated with changes in the gut microbiota, but the role of dysbiosis in PCOS remains unclear. Previous studies have demonstrated that PCOS and stool microbiota changes are independently associated with increased risk for obesity and metabolic disease. We have for the first time demonstrated that obese adolescents with PCOS have an altered gut microbiome compared with adolescents without PCOS who have similar BMI, activity level, and dietary habits. We found that participants with PCOS had decreased α-diversity (evenness and diversity), which represents within-subject microbial diversity, and significantly altered microbial composition (β-diversity) compared with controls without PCOS. Differences also were noted in the %RA of several phyla, families, and genera. Furthermore, several taxa at the phylum and family levels were associated with PCOS status and were significantly correlated with metabolic biomarkers including waist-to-hip ratio, TG, ALT, fasting insulin, HOMA-IR, and SBP (Table 2).
Our overall findings are consistent with results published from studies in adult women with PCOS showing dysbiosis, although the taxa differ among the studies and none have controlled for multiple lifestyle factors. Lindheim et al reported that younger women with PCOS (median age, 22–32 years) had a significant decrease in %RA and phylogenetic diversity in the phylum Tenericutes and in an unclassified family of the phylum Bacteroidetes compared with healthy controls (4). In contrast, Liu et al reported that women with PCOS (median age, 25–33 years) had increased abundance of Bacteroides, Escherichia/Shigella, and Streptococcus, and decreased abundance of Akkermansia and Ruminococcaceae. In their study, altered bacterial composition in the gut was also associated with the clinical characteristics of PCOS (5), similar to our study. Moreover, consistent with our findings of gut microbiome correlations with metabolic disease, Jie et al found that alterations in the gut microbiome were observed in individuals with atherosclerotic cardiovascular disease (ACVD) and increased Enterobacteriaceae and Streptococcus spp. were observed in individuals with ACVD (33). Another study, conducted by Zeng et al, examined overweight women (mean age, 25 years) with insulin-resistant PCOS and with non–insulin-resistant PCOS. This study found that individuals with insulin-resistant PCOS (HOMA-IR > 2.5) had decreased α-diversity, increased pro-inflammatory Bacteroides and decreased Prevotellaceae, and association with insulin resistance and inflammation (21). In contrast, we found that participants with PCOS had decreased Bacteroides and increased Prevotella (phylum Bacteroidetes). This difference between ours and previous findings may be related to the varied BMI in previous work as compared with our cohorts, which had equal obesity; thus, in the current study, PCOS is the only variable being examined. Previous findings suggest that these particular taxa may related to obesity and metabolic syndrome (33) as well as PCOS.
Previous animal research has examined the role of gut microbiota in PCOS models. Guo et al demonstrated the interaction of sex hormones and the gut microbiome in PCOS model rats, with PCOS induced via letrozole therapy. PCOS rats had decreased %RA of several genera (Actobacillus, Ruminococcus, and Clostridium) from the phylum Firmicutes and increased abundance of Prevotella (phylum Bacteroidetes) compared with controls (2). In contrast, we found no significant difference in %RA of Firmicutes but Bacteroides were significantly less frequent in PCOS. Another study, conducted by Kelley et al, showed that nonobese letrozole-induced mice had significant weight gain and insulin resistance as well as decreased α-diversity, altered β- diversity, and changes in bacterial composition, which is consistent with our findings in humans, and that α-diversity was negatively correlated with total testosterone (3). Torres et al demonstrated that letrozole-induced mice that were co-housed with placebo mice (which can partially replicate a stool transplant) had improved PCOS phenotype compared with letrozole-induced mice co-housed with letrozole-induced mice (34), suggesting that exposure to healthy gut microbiota has a potential to improve PCOS phenotype. Similarly, Guo et al showed that treatment with Lactobacillus and fecal microbiome transplantation from healthy rats improved PCOS phenotype and normalized the gut microbiota (2).
The leaky gut hypothesis proposes that chronic inflammation via the translocation of bacteria and endotoxins leads to activation of the inflammatory cascade by activating NF-kB. This chronic state of inflammation affects multiple organs and particularly alters the insulin receptor and follicular development (10). Our results suggest a relationship between the excess testosterone in PCOS and gut microbiota. ROC analysis found several taxa that were associated with PCOS status. Furthermore, free testosterone was associated with phyla Bacteroidetes and F:B ratio, and family Porphyromonadaceae and Ruminococccaceae in the entire cohort, suggesting an interaction between serum testosterone concentrations and the gut microbiome. Insenser et al studied overweight and obese women (mean age, 23–27 years) and found that sex hormone concentration and obesity influenced the gut microbiota, α- diversity, and bacterial composition (20). Furthermore, Torres et al demonstrated that normal and overweight women (mean age, 27–29 years) with PCOS had decreased α-diversity and that α-diversity was negatively correlated with hyperandrogenism, total testosterone, and hirsutism (19).
Although obesity is a result of an interaction between diet, lifestyle, environment and genetic factors, recent studies have revealed the key role of the gut microbiota in the pathogenesis of metabolic disorders, including obesity (11, 14, 35). Obesity is commonly seen in PCOS, suggesting the role of obesity in increasing the severity of PCOS and other cardiometabolic diseases (6). Intestinal bacteria degrade complex glycans and provide the host with various metabolites including short chain fatty acids (SCFAs). SCFAs are associated with increased bacterial fermentation in the distal gut resulting in increased energy harvest and adiposity (11). In health, about 10% of total energy is obtained from SCFAs (36). The gut microbiota of obese individuals has increased energy extraction by SCFAs, and has reduced taxonomic diversity compared with lean individuals (11, 16). A clinical study in humans that compared 263 individuals (obese, overweight, lean, and anorexic) found Bacteroidetes and Firmicutes, which comprise a large amount of the microbiome, were associated with obesity (37). Transplantation of obese gut microbiota into germ-free mice induces obesity (3, 15, 16), suggesting the close interaction between the gut microbiota and the development of obesity. The existing studies in women with PCOS include a range of BMIs, and statistically adjust for differences in weight. By closely matching our control and PCOS groups for BMI z-score, which is a measure that is adjusted for age, as well as having a very similar absolute BMI, we were able to detect differences based on disease status without the confounder of weight.
Gut dysbiosis is also associated with the metabolic syndrome, and we found that certain genera were significantly correlated with metabolic markers within PCOS only and across the entire cohort. Dysbiosis-induced SCFA production increases inflammation and is associated with risk markers for future cardiometabolic disease, including elevated TG and LDL, decreased HDL, and elevated blood pressure, insulin resistance, serum glucose, and liver enzymes (11, 38). SCFAs have been shown to affect glucose, cholesterol, and lipid metabolism (18) and to induce adipose tissue to lipogenesis, TG storage, and energy storage (13). Diet-induced alterations of the gut microbiota result in disruption of the intestinal barrier and translocation of endotoxins, resulting in a state of metabolic endotoxemia (39). Furthermore, upregulation of inflammation also leads to increased lipid storage (4), promoting adiposity and visceral fat accumulation resulting in metabolic disorders. This suggests a relationship between inflammation and PCOS, as PCOS biochemical imbalances also insulin resistance and low-grade inflammation (3–5, 10). Conversely, fecal transplantation, as well as prebiotic and probiotic bacteria, have been shown to ameliorate metabolic effects in obesity and reestablish the gut microbiota (40). Future directions would also include analysis of SCFA content and species and their relationship to PCOS.
Several unique strengths characterize this study. Our cohorts are similar in terms of BMI, pubertal stage, and lifestyle measures. This is the first time that the gut microbiota has been described in any PCOS cohort defined by the NIH criteria, which identifies a more metabolically at-risk population, in addition to the first presentation of data in adolescents. A limitation to the study was lack of adequate stool sample to measure SCFAs, which may have provided a better understanding of functional changes in PCOS-associated microbiota. Furthermore, the correlation analysis with free testosterone is missing several samples, and perhaps further differences would be discovered with a larger sample size. Future studies could include participants of normal weight, with and without PCOS, to further delineate the effect of obesity in PCOS and the gut microbiome.
In summary, the composition of the gut microbiota is altered in obese youth with PCOS, independent of obesity, with decreased α-diversity and significant alteration in overall microbial community composition (i.e., β-diversity). PCOS status is associated with altered %RA of several families and phylum-level taxa and several taxa are correlated with testosterone and metabolic disease markers. These alterations are present in adolescents with an average age of 14 to 16 years, suggesting that the microbiota may play a key role in metabolic disease in obese female adolescents with PCOS. Further work is needed to better understand the relationship between androgens and the microbiome, especially as a potentially new avenue for therapy.
Acknowledgments
We thank the participants and their families, the Colorado Clinical Translational Research Center nurses and staff.
Financial Support: M.C.G.: National Institute of Diabetes and Digestive and Kidney Diseases K23DK107871, Doris Duke Foundation 2015212, Boettcher Webb-Waring Foundation; M.C.G., D.N.F., C.E.R., D.I.: University of Colorado GI & Liver Innate Immune Program; M.M.K.: Center for Women’s health research; this research was also supported by National Institutes of Health/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Award Grant Number UL1 TR002535.
Author Contributions: B.J. researched data, performed DNA extractions and sequencing, performed statistical analysis, analyzed the data, and wrote the manuscript. D.N.F. performed statistical analysis, edited, and revised the manuscript. L.P. and L.S. performed statistical analysis and edited the manuscript. M.M.K. and K.J.N. edited and revised the manuscript. Y.G.R. enrolled subjects, collected clinical data and fecal samples. C.E.R. performed the microbiome bioinformatics. D.I. assisted with DNA extraction, sequencing, and edited the manuscript. K.J.N and M.C.G conceived and designed the study. M.C.G designed the study, researched data, performed statistical analysis and wrote the manuscript.
Glossary
Abbreviations
- ALT
alanine aminotransferase
- AUC
area under the curve
- BMI
body mass index
- HbA1c
hemoglobin A1C
- HDL
high-density lipoprotein
- HOMA-IR
homeostasis model assessment for insulin resistance
- hs-CRP
high-sensitivity C-reactive protein
- LDL
low-density lipoprotein
- NAFLD
nonalcoholic fatty liver disease
- PCOS
polycystic ovary syndrome
- %RA
percent relative abundance
- ROC
receiver operative curve
- SBP
systolic blood pressure
- SCFA
short chain fatty acids
- SHBG
sex hormone–binding globulin
- T2D
type 2 diabetes
- TG
triglycerides
Additional Information
Disclosure Summary: The authors declare nothing to disclose
Data Availability: The datasets are available from the corresponding author upon reasonable request.
References
- 1. Franks S. Polycystic ovary syndrome in adolescents. Int J Obes (Lond). 2008;32(7):1035–1041. [DOI] [PubMed] [Google Scholar]
- 2. Guo Y, Qi Y, Yang X, et al. Association between polycystic ovary syndrome and gut microbiota. Plos One. 2016;11(4):e0153196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelley ST, Skarra DV, Rivera AJ, Thackray VG. The gut microbiome is altered in a letrozole-induced mouse model of polycystic ovary syndrome. Plos One. 2016;11(1):e0146509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lindheim L, Bashir M, Münzker J, et al. Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with Polycystic Ovary Syndrome (PCOS): a pilot study. Plos One. 2017;12(1):e0168390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu R, Zhang C, Shi Y, et al. Dysbiosis of gut microbiota associated with clinical parameters in polycystic ovary syndrome. Front Microbiol. 2017;8:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anderson AD, Solorzano CM, McCartney CR. Childhood obesity and its impact on the development of adolescent PCOS. Semin Reprod Med. 2014;32(3):202–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Abdou RM, Zhu L, Baker RD, Baker SS. Gut microbiota of nonalcoholic fatty liver disease. Dig Dis Sci. 2016;61(5):1268–1281. [DOI] [PubMed] [Google Scholar]
- 8. Fukuda S, Ohno H. Gut microbiome and metabolic diseases. Semin Immunopathol. 2014;36(1):103–114. [DOI] [PubMed] [Google Scholar]
- 9. Mokhtari Z, Gibson DL, Hekmatdoost A. Nonalcoholic fatty liver disease, the gut microbiome, and diet. Adv Nutr. 2017;8(2):240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tremellen K, Pearce K. Dysbiosis of Gut Microbiota (DOGMA)–a novel theory for the development of Polycystic Ovarian Syndrome. Med Hypotheses. 2012;79(1):104–112. [DOI] [PubMed] [Google Scholar]
- 11. Duranti S, Ferrario C, van Sinderen D, Ventura M, Turroni F. Obesity and microbiota: an example of an intricate relationship. Genes Nutr. 2017;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qin J, Li R, Raes J, et al. ; MetaHIT Consortium A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG. Role of gut microbiota in the aetiology of obesity: proposed mechanisms and review of the literature. J Obes. 2016;2016:7353642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ridaura VK, Faith JJ, Rey FE, et al. Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science. 2013;341(6150):1241214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. [DOI] [PubMed] [Google Scholar]
- 17. Vrieze A, Van Nood E, Holleman F, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143(4):913–6.e7. [DOI] [PubMed] [Google Scholar]
- 18. Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489(7415): 242–249. [DOI] [PubMed] [Google Scholar]
- 19. Torres PJ, Siakowska M, Banaszewska B, et al. Gut microbial diversity in women with polycystic ovary syndrome correlates with hyperandrogenism. J Clin Endocrinol Metab. 2018;103(4):1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Insenser M, Murri M, Del Campo R, Martínez-García MÁ, Fernández-Durán E, Escobar-Morreale HF. Gut microbiota and the polycystic ovary syndrome: influence of sex, sex hormones, and obesity. J Clin Endocrinol Metab. 2018;103(7):2552–2562. [DOI] [PubMed] [Google Scholar]
- 21. Zeng B, Lai Z, Sun L, et al. Structural and functional profiles of the gut microbial community in polycystic ovary syndrome with insulin resistance (IR-PCOS): a pilot study. Res Microbiol. 2019;170(1):43–52. [DOI] [PubMed] [Google Scholar]
- 22. Zhang J, Sun Z, Jiang S, et al. Probiotic Bifidobacterium lactis V9 regulates the secretion of sex hormones in polycystic ovary syndrome patients through the gut-brain axis. mSystems. 2019;4(2)e00017-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qi X, Yun C, Sun L, et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019;25(8):1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Legro RS, Arslanian SA, Ehrmann DA, et al. ; Endocrine Society Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mayer-Davis EJ, Nichols M, Liese AD, et al. ; SEARCH for Diabetes in Youth Study Group Dietary intake among youth with diabetes: the SEARCH for diabetes in youth study. J Am Diet Assoc. 2006;106(5):689–697. [DOI] [PubMed] [Google Scholar]
- 26. Liu J, Johnson R, Dillon S, et al. Among older adults, age-related changes in the stool microbiome differ by HIV-1 serostatus. Ebiomedicine. 2019;40:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Soderborg TK, Clark SE, Mulligan CE, et al. The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat Commun. 2018;9(1):4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nycz BT, Dominguez SR, Friedman D, et al. Evaluation of bloodstream infections, Clostridium difficile infections, and gut microbiota in pediatric oncology patients. Plos One. 2018;13(1):e0191232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9(4):357–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Robertson CE, Harris JK, Wagner BD, et al. Explicet: graphical user interface software for metadata-driven management, analysis and visualization of microbiome data. Bioinformatics. 2013;29(23):3100–3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schloss PD, Handelsman J. The last word: books as a statistical metaphor for microbial communities. Annu Rev Microbiol. 2007;61:23–34. [DOI] [PubMed] [Google Scholar]
- 32. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. http://www.R-project.org/. [Google Scholar]
- 33. Jie Z, Xia H, Zhong SL, et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat Commun. 2017;8(1):845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Torres PJ, Ho BS, Arroyo P, et al. Exposure to a healthy gut microbiome protects against reproductive and metabolic dysregulation in a PCOS mouse model. Endocrinology. 2019;160(5):1193–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Egshatyan L, Kashtanova D, Popenko A, et al. Gut microbiota and diet in patients with different glucose tolerance. Endocr Connect. 2016;5(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tilg H, Adolph TE. Influence of the human intestinal microbiome on obesity and metabolic dysfunction. Curr Opin Pediatr. 2015;27(4):496–501. [DOI] [PubMed] [Google Scholar]
- 37. Million M, Angelakis E, Maraninchi M, et al. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int J Obes (Lond). 2013;37(11):1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38. Schwiertz A, Taras D, Schäfer K, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring). 2010;18(1):190–195. [DOI] [PubMed] [Google Scholar]
- 39. Kelly CJ, Colgan SP, Frank DN. Of microbes and meals: the health consequences of dietary endotoxemia. Nutr Clin Pract. 2012;27(2):215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125(6):1401–1412. [DOI] [PubMed] [Google Scholar]