Abstract
Aims
Left ventricular assist device (LVAD) therapy improves the haemodynamics of advanced heart failure patients. However, it is unknown whether haemodynamic optimization improves haemocompatibility-related adverse events (HRAEs). This study aimed to assess HRAEs in patients with optimized haemodynamics.
Methods and results
Eighty-three outpatients [aged 61 (53–67) years, 50 male] underwent a haemodynamic ramp test at 253 (95–652) days after LVAD implantation, and 51 (61%) had optimized haemodynamics (defined as central venous pressure < 12mmHg, pulmonary artery wedge pressure < 18mmHg, cardiac index > 2.2L/min/m2) following LVAD speed adjustment. One-year survival free of any HRAEs (non-surgical bleeding, thromboembolic event, pump thrombosis, or neurological event) was achieved in 75% of the optimized group and in 44% of the non-optimized group (hazard ratio 0.36, 95% confidence interval 0.18–0.73, P = 0.003). The net haemocompatibility score, using four escalating tiers of hierarchal severity to derive a total score for events, was significantly lower in the optimized group than the non-optimized group (1.02 vs. 2.00 points/patient; incidence rate ratio 0.51, 95% confidence interval 0.29–0.90, P = 0.021).
Conclusion
Left ventricular assist device patients in whom haemodynamics can be optimized had greater freedom from HRAEs compared to those without optimized haemodynamics.
Keywords: Ramp, Heart failure, HeartMate
Introduction
The improvement of continuous-flow left ventricular assist device (LVAD) technologies has resulted in a paradigm shift in the therapeutic strategy for patients with stage D heart failure (HF) from crisis support to long-term performance-based goals.1 However, LVAD technologies have brought us various unique haemocompatibility-related adverse events (HRAEs) such as non-surgical bleeding and thrombosis through the interaction between the artificial pump interface and blood, leading to activation or destruction of circulating blood elements.2 Consequently, HRAEs are a major cause of disability, death, and readmissions during LVAD support.3
The current management of HRAEs relies on the adjustment of anticoagulation and antiplatelet medications. Reduction of these therapies may increase the risk of thrombotic events,4,5 whereas increasing these therapies may lead to a higher risk of bleeding events.6 Given the antithetical nature of managing HRAEs in these patients, dose adjustment may not be enough to mitigate this serious issue.
Although existing guidelines have few specific recommendations regarding optimization of LVAD speed,7 we have recently demonstrated the utility of the haemodynamic ramp test to characteriize the patient’s haemodynamic profile and achieve haemodynamic optimization.8 There have been no prospective studies confirming the prognostic implication of optimized haemodynamics in LVAD patients, but it is possible that stabilization of haemodynamics may contribute to suppressing HRAEs. Accordingly, this study hypothesized that the rate of HRAEs and aggregate net burden of HRAEs are lower in patients with optimized haemodynamic profiles.
Methods
Patient selection
In 2014, the senior author of this paper (N.U.) established a prospective haemodynamic ramp test database with informed consent, prospective data collection during the ramp study by a study coordinator (D.R.) and a specified follow-up protocol. Clinically stable LVAD outpatients were enrolled in this prospective study and followed for 1 year. Written informed consent was obtained from all participants before the ramp test. The study protocol was approved by the University of Chicago Institutional Review Board.
Ramp test protocol
Patients underwent an echocardiographic and haemodynamic LVAD speed ramp study in the cardiac catheterization laboratory as detailed previously.8 Right heart catheterization was used to measure intracardiac pressures over a range of LVAD speeds. The recorded haemodynamic parameters included central venous pressure (CVP) and pulmonary artery wedge pressure (PAWP). Cardiac output and cardiac index (CI) were calculated by the indirect Fick method. Echocardiographic parameters such as left ventricular end-diastolic diameter and valvular conditions were collected as detailed previously.9
During the ramp test, device speeds were increased by 100 rpm increments for HVAD patients and 400 rpm increments for HeartMate II patients up to the maximum speed settings for each device, and haemodynamic measurements were performed at each LVAD speed. At the conclusion of each test, the attending cardiologist reviewed the data and the device was set at the speed which yielded an optimal haemodynamic profile, which required all three of the following: CVP < 12mmHg, PAWP < 18mmHg, and CI > 2.2L/min/m2.7
Follow-up protocol
Patients were followed at the set LVAD speed for 1 year following ramp study. All patients received guideline-directed medical therapy including aspirin and warfarin with an international normalized ratio (INR) of 2.0–3.0. During the 1-year follow-up period, data including deaths and hospital readmissions due to HRAEs were collected and validated by two independent researchers (T.I. and D.R.). Events were classified according to INTERMACS definitions.
Haemocompatibility-related clinical adverse events
Clinical adverse events attributable to LVAD-related bleeding or thrombosis were classified as an HRAE:
Nonsurgical bleeding: gastrointestinal or other nonsurgical bleeding episodes.
Neurological events: stroke or other neurological events such as seizures.
Thromboembolic events: pump thrombosis that was medically or surgically treated, or arterial thrombosis with or without organ involvement.
Haemocompatibility score
A tiered hierarchal score [haemocompatibility score (HCS)] was calculated for each patient by weighing each event considering its escalating clinical relevance,10 to determine the aggregate net burden of HRAEs, instead of assessing each type of event. The definitions of each tier and weighted score are shown in Appendix Table A1. For example, one or two events of non-surgical gastrointestinal bleeding episodes are considered as mild events (Tier I) and have only one point. In contrast, disabling stroke is considered as a severe event (Tier IIIB) and has four points.
Statistical analyses
Statistical analyses were performed with SPSS Statistics 22 (SPSS Inc., Chicago, IL, USA). Two-sided P-values < 0.05 were considered statistically significant. Continuous variables were expressed as mean and standard deviation when normally distributed and compared between groups using unpaired t-test. When non-normally distributed, continuous variables were expressed as median (25%−75% quartile) and compared between groups using Mann-Whitney U test. Some variables were expressed as mean for better understanding, despite their non-normal distribution. Categorical variables were compared between groups using Fisher’s exact test.
HRAE-free survival rates were assessed using Kaplan–Meier analysis and Cox proportional hazard ratio analysis and compared between groups using log-rank test. Distributions of HRAE numbers and HCS between the two groups were compared using negative binomial regression analysis. Multivariate logistic regression analyses with a forward selection (Wald) method were performed on the variables significant in the univariate comparison analyses for the endpoint of any HRAEs.
Results
Baseline characteristics
As of April 2014, 112 patients were on active LVAD support. Of them, 33 (29%) were enrolled and underwent haemodynamic ramp testing. Among the 115 consecutive patients who received LVAD implantation between April 2014 and February 2017, 50 (43%) were enrolled (Table 1). Most of the patients were implanted as destination therapy (77%), and 47 (56%) had a non-ischaemic aetiology for HF. Ramp tests were performed at 253 (92–653) days following LVAD implantation. At baseline LVAD speed, 41 patients (49%) already had optimized haemodynamics.
Table 1.
Comparison of clinical variables between the optimized group and the non-optimized group
| Total (n = 83) | Optimized (n = 51) | Non-optimized (n = 32) | P-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 61 (53–67) | 61 (54–67) | 61 (51–72) | 0.90 |
| Male sex | 50 (60%) | 32 (63%) | 18 (56%) | 0.36 |
| Caucasian | 45 (55%) | 29 (57%) | 16 (50%) | 0.33 |
| Body mass index, kg/m2 | 29.8 ± 7.4 | 30.1 ± 7.9 | 29.3 ± 6.6 | 0.63 |
| Days before ramp test | 253 (95–652) | 210 (83–463) | 414 (146–871) | 0.30 |
| Non-ischaemic aetiology | 47 (56%) | 24 (47%) | 23 (72%) | 0.029* |
| Destination therapy | 63 (77%) | 36 (71 %) | 27 (84%) | 0.071 |
| HeartMate II LVAD | 50 (60%) | 30 (59%) | 20 (63%) | 0.17 |
| Hypertension | 44 (54%) | 29 (57%) | 15 (47%) | 0.30 |
| Diabetes mellitus | 30 (37%) | 18 (36%) | 12 (38%) | 0.47 |
| History of stroke | 11 (13%) | 6 (12%) | 5 (16%) | 0.57 |
| Atrial fibrillation | 31 (38%) | 15 (29%) | 16 (50%) | 0.038* |
| Chronic kidney disease | 17 (21%) | 11 (22%) | 6 (19%) | 0.52 |
| Haemodynamics at set LVAD speed | ||||
| LVDd, cm | 5.89 ± 1.18 | 5.97 ± 1.18 | 5.77 ± 1.19 | 0.46 |
| CVP, mmHg | 8 (5–12) | 6 (4–9) | 12 (10–16) | < 0.001* |
| PAWP, mmHg | 13.1 ± 5.2 | 11.4 ± 3.7 | 15.8 ± 6.2 | 0.001* |
| CI, L/min/m2 | 2.66 (2.31–3.00) | 2.74 (2.45–3.01) | 2.37 (2.03–2.82) | 0.041* |
| MAP, mmHg | 87.1 ± 12.5 | 88.3 ± 13.0 | 85.1 ± 11.6 | 0.28 |
| CVP < 12 mmHg | 62 (75%) | 51 (100%) | 11 (34%) | < 0.001* |
| PAWP < 18 mmHg | 71 (86%) | 51 (100%) | 20 (63%) | < 0.001* |
| CI > 2.2 L/min/m2 | 70 (84%) | 51 (100%) | 19 (59%) | < 0.001* |
| LVAD speed and medication at time of ramp test | ||||
| LVAD speed, r.p.m. | ||||
| HeartMate II LVAD (n = 53) | 9368.9 ± 437.8 | 9278.3 ± 412.4 | 9487.0 ± 450.6 | 0.086 |
| HVAD LVAD (n = 30) | 2704.7 ± 157.9 | 2699.1 ± 148.7 | 2717.8 ± 186.7 | 0.77 |
| Aspirin, mg daily | 81 (81–162) | 81 (81–325) | 81 (81–142) | 0.84 |
| INR | 2.11 ± 0.49 | 2.10 ± 0.49 | 2.14 ± 0.49 | 0.72 |
CI, cardiac index; CVP central venous pressure; INR, international normalized ratio; LVAD, left ventricular assist device; LVDd, left ventricular diastolic diameter; MAP, mean arterial pressure; PAWP, pulmonary artery wedge pressure.
P < 0.05. Variables were compared using unpaired t-test, Mann–Whitney U test, or Fisher’s exact test as appropriate.
Following LVAD speed adjustment, 51 (61%) patients achieved haemodynamic optimization. The non-optimized group had a higher frequency of non-ischaemic HF aetiology (72% vs. 47%) and atrial fibrillation (50% vs. 29%) compared with the optimized group (P < 0.05).
All patients in the optimized group satisfied three haemodynamic goals: CVP < 12 mmHg, PAWP < 18 mmHg, and CI > 2.2 L/min/m2. In the non-optimized group, 34% satisfied CVP < 12 mmHg, 63% satisfied PCWP < 18 mmHg, and 59% satisfied CI > 2.2 L/min/m2.
Comparison in haemocompatibility-related adverse events between the optimized group and the non-optimized group
Pre-ramp rates of HRAEs were comparable between the optimized and non-optimized groups (0.89 event/year vs. 1.06 event/year, P = 0.86). There were no statistical differences in the degree of antiplatelet and anticoagulation therapy at 1,3, and 6 months, and 1 year following ramp test (Appendix Table A2).
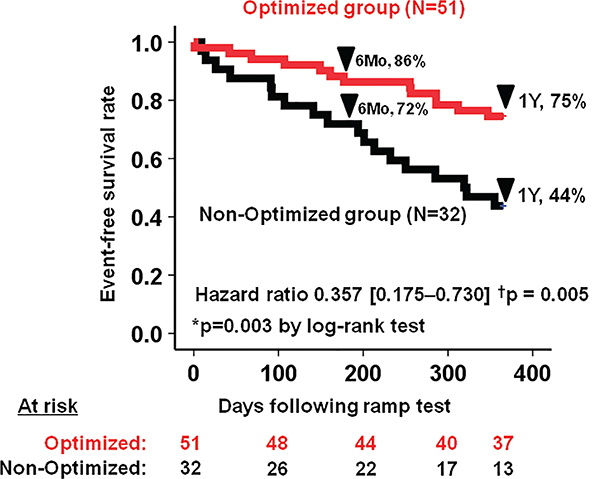
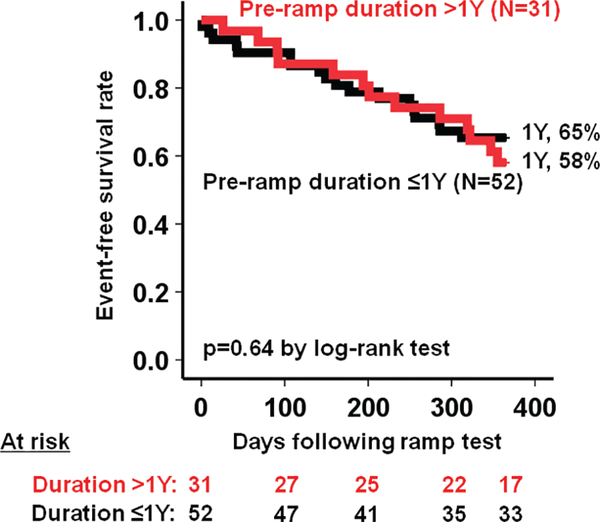
The survival free of HRAEs in the overall cohort was 81% at 6 months and 63% at 1 year. Event-free survival rate was significantly higher in the optimized group compared to the non-optimized group (75% vs. 44%, P = 0.003) (Figure 1) with a hazard ratio of 0.357 (95% confidence interval 0.175–0.730, P = 0.005). Event-free survival rates were comparable when stratified by the pre-ramp duration (> 1 year, 58% vs. ≤ 1 year, 65%, P = 0.64) (Appendix Figure A1).
Figure 1.
Haemocompatibility-related adverse events-free survival rate stratified by optimization of haemodynamics. The optimized group had 64% risk reduction considering the hazard ratio. *P < 0.05 by log-rank test; †P < 0.005 by Cox proportional hazard ratio analyses.
Among HeartMate II patients (n = 53), the optimized group still had higher 1-year event-free survival rate (70% vs. 35%, P = 0.005), whereas it did not reach statistical significance among HVAD patients at 1 year (n = 30) (81% vs. 67%, P = 0.13).
The HRAE rate was significantly lower in the optimized group than the non-optimized group (0.35 vs. 1.03 events/year, incidence rate ratio 0.34, 95% confidence interval 0.17–0.71, P = 0.004) (Table 2). Numerical percentages of both haemorrhagic and thrombotic events were lower in the optimized group compared with the non-optimized group, although the differences did not reach statistical significance. The length of stay due to HRAEs was shorter in the optimized group than the non-optimized group (P = 0.011).
Table 2.
The proportion of patients who experienced either haemorrhagic, thrombotic, or both events, and days spent in the hospital due to haemocompatibility-related adverse events
| Optimized (n = 51) | Non-optimized (n = 32) | P-value | |
|---|---|---|---|
| HRAE rate, events/year | 0.35 | 1.03 | 0.004* |
| Haemorrhagic event only | 6 (12%) | 7 (23%) | 0.18 |
| Thrombotic event only | 3 (6%) | 6 (19%) | 0.066 |
| Haemorrhagic or thrombotic event | 11 (22%) | 17 (55%) | 0.003* |
| Haemorrhagic and thrombotic event | 2 (4%) | 4 (13%) | 0.14 |
| Length of hospital stay, days | 0 (0–0) | 1 (0–18) | 0.011* |
HRAE, haemocompatibility-related adverse event.
P < 0.05. HRAE rates were compared using negative binomial regression analysis; proportions of patients who experienced each HRAE were compared using Fisher’s exact test; lengths of hospital stay were compared using Mann–Whitney U test.
Comparison in haemocompatibility score between the optimized group and the non-optimized group
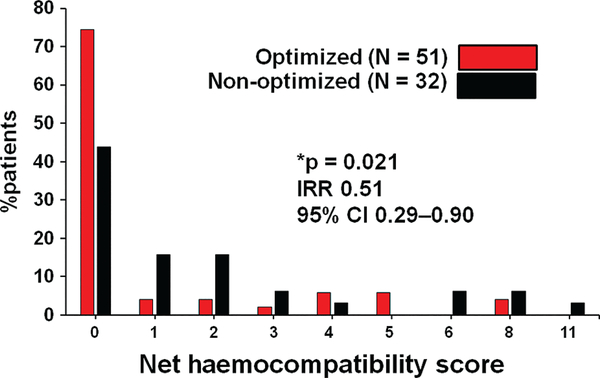
The optimized group patients had significantly lower HCS compared with the non-optimized group (1.02 vs. 2.00 points/patient, incidence rate ratio 0.51, 95% confidence interval 0.29–0.90, P = 0.021) (Figure 2).
Figure 2.
Distribution of net haemocompatibility score in the optimized and non-optimized groups. The optimized group patients had lower haemocompatibility score compared with the non-optimized group patients. CI, confidence interval; IRR, incidence rate ratio. *P < 0.05 by negative binomial regression analysis.
Scores of each tier contributing to the net HCS are shown in Table 3. No patient in the optimized group experienced medically managed pump thrombosis, whereas four patients in the non-optimized group did (0 vs. 0.13 in averaged scores). Recurrent non-surgical bleeding, stroke (both non-disabling and disabling), and pump thrombosis requiring exchange tended to be rarer in the optimized group than the non-optimized group (P ~ 0.05 for all).
Table 3.
Average points per patient associated with events contributing to net haemocompatibility score
| Optimized (n = 51) | Non-optimized (n = 32) | IRR (95% CI) | P-value | |
|---|---|---|---|---|
| Tier I | ||||
| Non-surgical bleeding (≤ 2 events) | 0.10 | 0.19 | 0.52 (0.16–1.72) | 0.28 |
| Medically managed PT | 0 | 0.13 | – | – |
| Non-stroke neurological event | 0 | 0 | – | – |
| Arterial TE with no organ loss | 0 | 0 | – | – |
| Tier II | ||||
| Non-surgical bleeding (> 2 events) | 0.04 | 0.13 | 0.31 (0.09–1.04) | 0.058 |
| Non-disabling stroke | 0.08 | 0.19 | 0.42 (0.17–1.02) | 0.056 |
| Arterial TE with organ loss | 0 | 0 | – | – |
| Tier IIIA | ||||
| Surgically managed PT | 0.02 | 2.0 | 0.31 (0.08–1.25) | 0.10 |
| Tier IIIB | ||||
| Disabling stroke | 0.04 | 0.09 | 0.42 (0.17–1.02) | 0.056 |
| HC-related or inconclusive death | 0.12 | 0.13 | 0.94 (0.50–1.79) | 0.85 |
Variables are expressed as average score per patient and compared between groups using Poisson analyses.
CI, confidence interval; HC, haemocompatibility; IRR, incidence rate ratio; PT, pump thrombosis; TE, thromboembolic.
Predictors of haemocompatibility-related adverse events following ramp test
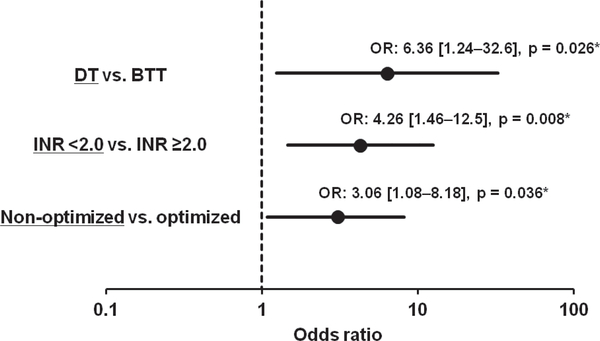
Destination therapy, HeartMate II usage, a lower dose of aspirin, and lower INR as well as non-optimized haemodynamics were associated with HRAEs in the univariate analyses (Appendix Table A3). In the multivariate analysis, destination therapy (odds ratio 6.36, 95% confidence interval 1.24–32.6), INR < 2.0 (odds ratio 4.26, 95% confidence interval 1.46–12.5), and non-optimized haemodynamics (odds ratio 3.06, 95% confidence interval 1.08–8.18) were significant predictors of HRAEs in the multivariate analysis (P < 0.05 for all) (Figure 3).
Figure 3.
Forest plot of the multivariate logistic regression analysis model for clinical variables impacting any haemocompatibility-related adverse events. BTT, bridge to transplant; DT, destination therapy; INR, international normalized ratio; OR, odds ratio. *P < 0.05 by multivariate logistic regression analysis.
Discussion
In this prospective study, we evaluated the prognostic implications of optimized haemodynamics on HRAEs in LVAD patients. Our main findings are as follows: (i) optimized haemodynamics were observed in 61 % of LVAD patients after the ramp tests; (ii) patients with optimized haemodynamics had a higher 1-year HRAE-free survival rate compared to the non-optimized group; (iii) net HCS was lower in the optimized group compared with the non-optimized group, owing to a reduction in both bleeding and thrombotic event rates; and (iv) non-optimized haemodynamics were an independent risk factor for HRAEs in the multivariate analysis along with with destination therapy and a lower INR level.
Assessment and optimization of haemodynamics by the ramp test
In total, 51% of stable ambulatory LVAD patients had abnormal haemodynamics at the baseline LVAD speed. This result challenges the ability of clinicians to rely solely on physical examination or any other standard methodologies to manage haemodynamic status, and suggests that routine haemodynamic ramp test is necessary for an accurate assessment of haemodynamics.8
Haemodynamics remained non-optimized in 39% of patients after the ramp test. The haemodynamic parameter that most commonly led to the failure of haemodynamic optimization was CVP, indicating that many of the non-optimized patients may have had a component of right ventricular dysfunction that prevented haemodynamic optimization. Whether the performance of the right ventricle was the factor that led to an increased rate of HRAEs in this group is unknown, but the identification and subsequent treatment of right ventricular dysfunction is another critical role that the haemodynamic ramp test can play in the management of these patients. Normalization of CVP levels may be an unrealistic goal with LVAD speed adjustment alone, as our team previously showed, and other management strategies such as up-titration of diuretics may be helpful.8 This study focused on optimized haemodynamics, but not the optimization of haemodynamics by the ramp test. The most effective strategy to optimize haemodynamics in LVAD patients is unknown and should be evaluated in randomized studies.
Optimized haemodynamics and freedom from haemocompatibility-related adverse events
The survival free of HRAEs in the overall cohort was 81% at 6 months and 63% at 1 year, which are similar to the results reported in HeartMate 3 patients (69% at 6 months) in a sub-analysis of the MOMENTUM 3 trial.11 Patients with optimal haemodynamics had an even higher HRAE-free survival rate (86% at 6 months following ramp test). It is important to note that the observational period was initiated at the time of ramp test in this study and not from device implantation as was done in the sub-analysis of MOMENTUM 3.11 Nevertheless, we can say that optimized haemodynamics have a strong positive impact on freedom from HRAEs.
Optimized haemodynamics and haemocompatibility score
When breaking down overall HRAEs, optimized haemodynamics were associated with both reduced bleeding and thrombotic events equally (Table 2). When focusing on the magnitude of HRAEs by using tiers, optimized haemodynamics reduced each tier equally except for death (Table 3). As a result of reductions in each tier, the optimized haemodynamic group had lower net HCS compared with the non-optimized group (Figure 2).
The relationship between haemodynamics and thrombotic events may be explained by the major component of failure of haemodynamic optimization in our study, i.e., elevated CVP. Right ventricular failure, which usually accompanies elevated CVP,12 results in reduced LVAD filling, lower LVAD flow, and greater degree of pump stasis. Adequate laminar flow across the blood-washed inflow bearing of the pump is critical for heat transfer, which if not maintained, can lead to bearing thrombus formation and successive systemic thrombosis.13
Bleeding events may also be explained by right ventricular failure. Elevated CVP is associated with hepatic congestion, worsened coagulopathy, and lower arterial pulsatility, leading to the formation of arteriovenous malformations and refractory bleeding.14–16 However, altered angiogenesis signal cascades may also play a significant role in the development of arteriovenous malformations.17 We should recognize that the cause of bleeding in these patients is multifactorial.
Other risk factors for haemocompatibility-related adverse events
Absence of haemodynamic optimization is not the only factor associated with HRAEs. Additionally, the ramp test is not a perfect tool to optimize haemodynamics. Similar to the sub-analysis of MOMENTUM 3,11 INR < 2.0 was another independent risk factor for HRAEs. Lower aspirin dose was identified as a risk factor in univariable analysis but did not reach statistical significance in multivariate analysis. Destination therapy, which generally indicates a sicker population, was also an independent predictor of HRAEs. It is likely that the higher frequency of destination therapy in the HeartMate II subgroup explains the overall higher rate of HRAEs in HeartMate II patients, and further accentuated the difference between optimized and non-optimized patients (driving a significant difference in event-free survival among HeartMate II patients that was not seen in HVAD patients).
Optimal management of antiplatelet and anticoagulation therapies remains controversial. Reduction in antithrombotic intensity has been implicated in the spike seen in pump thrombosis events in the HeartMate II population. However, the US-TRACE study demonstrated that in selected patients who had experienced multiple bleeding events, antiplatelet-free or anticoagulation-free therapy did not seem to increase the risk of device thrombosis but also did not significantly reduce the high bleeding event rate.4 The 2-year analysis of the European TRACE study suggested that anticoagulation therapy alone without antiplatelet therapy reduced the incidence of bleeding events without increasing the risk of thrombotic events.18
Clinical implications of the haemodynamic ramp test
Assessment of LVAD patients with haemodynamic ramp testing provides several benefits. As demonstrated in this study, haemodynamic assessment and optimization can identify a group of patients who are likely to do well with LVAD therapy and a group of patients who are more likely to experience HRAEs. This categorization can permit intensification of monitoring and medical therapy in the group that is at higher risk for complications. Furthermore, the haemodynamic ramp test also enables the optimization of therapies such as diuretics and afterload reduction in this population.
Study limitations
This study is a single-centre study with a relatively small cohort size, but includes a comprehensive haemodynamic characterization and detailed follow-up. We focused on optimal haemodynamics but not the optimization of haemodynamics by the ramp test. We cannot differentiate whether haemodynamic optimization provokes the improved outcomes or is a marker of a healthier patient population. This question can only be answered through randomization, and we eagerly anticipate the results of the ongoing RAMP-IT-UP study (NCT03021239), which is investigating the clinical outcomes associated with haemodynamic ramp testing in a randomized fashion.
We enrolled only clinically stable outpatients. Patients with early fatal or disabling adverse events did not undergo ramp testing, which may bias event rates in the studied population. However, these excluded patients are not the target of the current study, which aims to identify stable LVAD patients who are at risk of future events, and may benefit from both device and medical optimization. The implication of this study to patients with other morbidities such as decompensated HF remains uncertain. The durability of the haemodynamics that we found in this cohort is unknown. Our group has recently reported that the haemodynamic response to ramp testing remains similar on repeated tests for individual patients.19 However, whether the optimized patients in this study remained optimized, and whether the non-optimized patients remained non-optimized is unknown. We think it is important to repeat haemodynamic ramp testing at regular intervals, although the clinical benefits of this approach are unproven.
The duration of LVAD support before the ramp test spanned a wide range. Although our protocol mandates haemodynamic ramp testing 1–3 months after LVAD implant, all patients on support underwent haemodynamic ramp testing when the protocol was initiated, resulting in the varying support durations we reported. This wide range may have set up a time bias because event rates may vary with different support durations following implantation. However, there were no statistical differences in event-free survival rates with respect to timing of the ramp test. The primary goal of the ramp test was to achieve a specific haemodynamic profile (CVP < 12mmHg, PAWP < 18mmHg, and CI > 2.2L/min/m2). However, we did not consider other factors for optimization, such as blood pressure or decoupling between diastolic pulmonary artery pressure and PAWP.20 Management protocols for each HRAE and clinical thresholds for hospital admission may vary among institutions. In our institution, patients with a suspicion of a significant HRAE are admitted and receive in-hospital treatment.
Future directions
We enrolled only HeartMate II and HVAD patients in this study. Recent results with the HeartMate 3 demonstrate an excellent survival rate and a reduction in the incidence of pump thrombosis but a persistence of many other HRAEs.20–26 There are limited data regarding haemodynamics in HeartMate 3, although one study showed that haemodynamic normalization may be achieved in the majority of patients implanted with HeartMate 3.27 It is unknown whether the results of this study can be applied to HeartMate 3 patients, and this population should be the focus of a future analysis.
Conclusion
Left ventricular assist device patients with optimized haemodynamics had a reduction in HRAEs, driven by suppression of both bleeding and thrombotic events. Haemodynamic ramp testing following LVAD implantation may help to risk stratify patients with regard to the development of HRAEs. Further study is needed to understand the full clinical impact of haemodynamic optimization during LVAD support, including impact on cognition, quality of life and health economics.
Acknowledgments
Conflict of interest: T.I. receives financial support from Postdoctoral Fellowship for Research Abroad of Japan Society for the Promotion of Science. V.J. is a consultant for Abbott. G.S. is a consultant for Medtronic. N.U. receives grant support from Abbott and Medtronic. The other authors report no conflicts of interest.
Appendix
Table A1.
Calculation of the haemocompatibility score
| Intensity | Clinical components | Score |
|---|---|---|
| Tier I: mild | ≤ 2 gastrointestinal or other non-bleeding episodes | 1 point each |
| Suspected pump thrombosis (medically treated) | ||
| Non-stroke-related neurological events | ||
| Arterial thromboembolism not resulting in organ loss | ||
| Tier II: moderate | > 2 gastrointestinal or other non-bleeding episodes | 2 points each |
| Non-disabling stroke | ||
| Arterial thromboembolism resulting in organ loss | ||
| Tier III | ||
| IIIA: moderate to severe | Pump malfunction attributable to pump thrombosis leading to reoperation for removal or replacement | 3 points each |
| IIIB: severe | Disabling stroke | 4 points each |
| Death attributable to a haemocompatibility aetiology or inconclusive |
Table A2.
Trends of antiplatelet and anticoagulation therapy
| Optimized (n = 51) | Non-optimized (n = 32) | P-value | |
|---|---|---|---|
| At 1 month | |||
| Aspirin, mg daily (n = 82) | 81 (81–325) | 81 (81–142) | 0.66 |
| INR (n = 79) | 2.27 ± 0.62 | 2.19 ± 0.75 | 0.61 |
| At 3 months | |||
| Aspirin, mg daily (n = 81) | 81 (0–162) | 81 (61–101) | 0.92 |
| INR (n = 74) | 2.31 ± 0.58 | 2.23 ± 0.63 | 0.59 |
| At 6 months | |||
| Aspirin, mg daily (n = 75) | 81 (0–162) | 81 (81–122) | 0.69 |
| INR (n = 62) | 2.13 ± 0.52 | 2.10 ± 0.42 | 0.76 |
| At 1 year | |||
| Aspirin, mg daily (n = 65) | 81 (0–162) | 81 (20–81) | 0.61 |
| INR (n = 54) | 2.11 ± 0.42 | 2.15 ± 0.33 | 0.68 |
INR, international normalized ratio.
Table A3.
Univariate analyses of factors impacting on any haemocompatibility-related adverse events
| HCS > 0 (n = 31) | HCS = 0 (n = 52) | P-value | |
|---|---|---|---|
| Demographics | |||
| Age, years | 61 (54–72) | 61 (52–67) | 0.45 |
| Age > 65 years | 9 (29%) | 20 (38%) | 0.27 |
| Male sex | 12 (39%) | 21 (40%) | 0.53 |
| Caucasian | 15 (48%) | 30 (58%) | 0.60 |
| Body mass index, kg/m2 | 28.7 ± 5.8 | 30.4 ± 8.1 | 0.31 |
| Days before ramp test | 299 (129–642) | 219 (88–665) | 0.87 |
| Ischaemic aetiology | 14 (45%) | 22 (42%) | 0.44 |
| Destination therapy | 28 (90%) | 35 (67%) | 0.006* |
| HeartMate II LVAD | 24 (77%) | 29 (56%) | 0.039* |
| Hypertension | 13 (42%) | 31 (60%) | 0.12 |
| Diabetes mellitus | 10 (32%) | 20 (38%) | 0.41 |
| History of stroke | 6 (19%) | 5 (10%) | 0.18 |
| Atrial fibrillation | 11 (35%) | 20 (38%) | 0.53 |
| Chronic kidney disease | 5 (16%) | 12 (23%) | 0.35 |
| Haemodynamics at set | |||
| LVAD speed | |||
| CVP, mmHg | 10 (4–15) | 8 (5–11) | 0.16 |
| PAWP, mmHg | 13.5 ± 5.4 | 12.9 ± 5.2 | 0.61 |
| CI, L/min/m2 | 2.49 (2.24–2.80) | 2.76 (2.41–3.16) | 0.15 |
| MAP, mmHg | 88.3 ± 13.0 | 85.0 ± 11.6 | 0.28 |
| CVP ≥ 12 mmHg | 11 (35%) | 10 (19%) | 0.084 |
| PAWP ≥ 18 mmHg | 7 (23%) | 5 (10%) | 0.10 |
| CI ≤ 2.2 L/min/m2 | 7 (23%) | 6 (12%) | 0.18 |
| Non-optimized haemodynamics | 18 (58%) | 14 (27%) | 0.005* |
| Medications at time of ramp test | |||
| Aspirin dose, mg daily | 81 (0–81) | 81 (81–325) | 0.034* |
| No aspirin administration | 7 (23%) | 8 (15%) | 0.30 |
| INR | 1.93 ± 0.49 | 2.22 ± 0.46 | 0.007* |
| INR < 2.0 | 16 (52%) | 12 (23%) | 0.008* |
CI, cardiac index; CVP, central venous pressure; HCS, haemocompatibility score; INR, international normalized ratio; LVAD, left ventricular assist device; MAP mean arterial pressure; PAWP, pulmonary artery wedge pressure.
P < 0.05. Variables were compared using unpaired t-test, Mann–Whitney U test, or Fisher’s exact test as appropriate.
Figure A1.
Haemocompatibility-related adverse event-free survival rate stratified by the pre-ramp duration.
References
- 1.Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB, Naftel DC. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant 2017;36:1080–1086. [DOI] [PubMed] [Google Scholar]
- 2.Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation 2012;125:3038–3047. [DOI] [PubMed] [Google Scholar]
- 3.Forest SJ, Bello R, Friedmann P, Casazza D, Nucci C, Shin JJ, D’Alessandro D, Stevens G, Goldstein DJ. Readmissions after ventricular assist device: etiologies, patterns, and days out of hospital. Ann Thorac Surg 2013;95:1276–1281. [DOI] [PubMed] [Google Scholar]
- 4.Katz JN, Adamson RM, John R, Tatooles A, Sundareswaran K, Kallel F, Farrar DJ, Jorde UP, TRACE Study. Safety of reduced anti-thrombotic strategies in HeartMate II patients: a one-year analysis of the US-TRACE study. J Heart Lung Transplant 2015;34:1542–1548. [DOI] [PubMed] [Google Scholar]
- 5.Nassif ME, LaRue SJ, Raymer DS, Novak E, Vader JM, Ewald GA, Gage BF. Relationship between anticoagulation intensity and thrombotic or bleeding outcomes among outpatients with continuous-flow left ventricular assist devices. Circ Heart Fail 2016;9:e002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saeed O, Shah A, Kargoli F, Madan S, Levin AP, Patel SR, Jermyn R, Guerrero C, Nguyen J, Sims DB, Shin J, D’Alessandro D, Goldstein DJ, Jorde UP. Antiplatelet therapy and adverse hematologic events during Heart Mate II support. Circ Heart Fail 2016;9:e002296. [DOI] [PubMed] [Google Scholar]
- 7.Feldman D, Pamboukian SV, Teuteberg JJ, Birks E, Lietz K, Moore SA, Morgan JA, Arabia F, Bauman ME, Buchholz HW, Deng M, Dickstein ML, El-Banayosy A, Elliot T, Goldstein DJ, Grady KL, Jones K, Hryniewicz K, John R, Kaan A, Kusne S, Loebe M, Massicotte MP, Moazami N, Mohacsi P, Mooney M, Nelson T, Pagani F, Perry W, Potapov EV, Eduardo Rame J, Russell SD, Sorensen EN, Sun B, Strueber M, Mangi AA, Petty MG, Rogers J; International Society for Heart and Lung Transplantation. The 2013 International Society for Heart and Lung Transplantation guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant 2013;32:157–187. [DOI] [PubMed] [Google Scholar]
- 8.Uriel N, Sayer G, Addetia K, Fedson S, Kim GH, Rodgers D, Kruse E, Collins K, Adatya S, Sarswat N, Jorde UP, Juricek C, Ota T, Jeevanandam V, Burkhoff D, Lang RM. Hemodynamic ramp tests in patients with left ventricular assist devices. JACC Heart Fail 2016;4:208–217. [DOI] [PubMed] [Google Scholar]
- 9.Uriel N, Morrison KA, Garan AR, Kato TS, Yuzefpolskaya M, Latif F, Restaino SW, Mancini DM, Flannery M, Takayama H, John R, Colombo PC, Naka Y, Jorde UP. Development of a novel echocardiography ramp test for speed optimization and diagnosis of device thrombosis in continuous-flow left ventricular assist devices: the Columbia ramp study. J Am Coll Cardiol 2012;60:1764–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehra MR. The burden of haemocompatibility with left ventricular assist systems: a complex weave. Eur Heart J 2019;40:673–677. [DOI] [PubMed] [Google Scholar]
- 11.Uriel N, Colombo PC, Cleveland JC, Long JW, Salerno C, Goldstein DJ, Patel CB, Ewald GA, Tatooles AJ, Silvestry SC, John R, Caldeira C, Jeevanandam V, Boyle AJ, Sundareswaran KS, Sood P, Mehra MR. Hemocompatibility-related outcomes in the MOMENTUM 3 trial at 6 months: a randomized controlled study of a fully magnetically levitated pump in advanced heart failure. Circulation 2017;135:2003–2012. [DOI] [PubMed] [Google Scholar]
- 12.Lampert BC, Teuteberg JJ. Right ventricular failure after left ventricular assist devices. J Heart Lung Transplant 2015;34:1123–1130. [DOI] [PubMed] [Google Scholar]
- 13.Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Myers S, Acker MA, Rogers J, Slaughter MS, Stevenson LW. Pump thrombosis in the Thoratec HeartMate II device: an update analysis of the INTERMACS registry. J Heart Lung Transplant 2015;34:1515–1526. [DOI] [PubMed] [Google Scholar]
- 14.Sparrow CT, Nassif ME, Raymer DS, Novak E, LaRue SJ, Schilling JD. Pre-operative right ventricular dysfunction is associated with gastrointestinal bleeding in patients supported with continuous-flow left ventricular assist devices. JACC Heart Fail 2015;3:956–964. [DOI] [PubMed] [Google Scholar]
- 15.Jabbar HR, Abbas A, Ahmed M, Klodell CT Jr, Chang M, Dai Y, Draganov PV. The incidence, predictors and outcomes of gastrointestinal bleeding in patients with left ventricular assist device (LVAD). Dig Dis Sci 2015;60:3697–3706. [DOI] [PubMed] [Google Scholar]
- 16.Tomizawa Y, Tanaka A, Kitahara H, Sakuraba A, Uriel N, Jeevanandam V, Ota T. Preoperative right-sided cardiac congestion is associated with gastrointestinal bleeding in patients with continuous-flow left ventricular assist devices. Dig Dis Sci 2018;63:1518–1524. [DOI] [PubMed] [Google Scholar]
- 17.Tabit CE, Chen P, Kim GH, Fedson SE, Sayer G, Coplan MJ, Jeevanandam V, Uriel N, Liao JK. Elevated angiopoietin-2 level in patients with continuous-flow left ventricular assist devices leads to altered angiogenesis and is associated with higher nonsurgical bleeding. Circulation 2016;134:1 41–1 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Netuka I, Litzler PY, Berchtold-Herz M, Flecher E, Zimpfer D, Damme L, Sundareswaran KS, Farrar DJ, Schmitto JD, EU TRACE Investigators. Outcomes in HeartMate II patients with no antiplatelet therapy: 2-year results from the European TRACE study. Ann Thorac Surg 2017;103:1 262–1 268. [DOI] [PubMed] [Google Scholar]
- 19.Imamura T, Burkhoff D, Rodgers D, Adatya S, Sarswat N, Kim G, Raikhelkar J, Ota T, Song T, Juricek C, Jeevanandam V, Sayer G, Uriel N. Repeated ramp tests on stable LVAD patients reveal patient-specific hemodynamic fingerprint. ASAIO J 2018;64:701–707. [DOI] [PubMed] [Google Scholar]
- 20.Imamura T, Chung B, Nguyen A, Rodgers D, Sayer G, Adatya S, Sarswat N, Kim G, Raikhelkar J, Ota T, Song T, Juricek C, Kagan V, Jeevanandam V, Mehra M, Burkhoff D, Uriel N. Decoupling between diastolic pulmonary artery pressure and pulmonary capillary wedge pressure as a prognostic factor after continuous flow ventricular assist device implantation. Circ Heart Fail 2017;10:e003882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanke JS, Dogan G, Rojas SV, Zoch A, Feldmann C, Deniz E, Avsar M, Warnecke G, Haverich A, Schmitto JD. First experiences with HeartMate 3 follow-up and adverse events. J Thorac Cardiovasc Surg 2017; 154:173–178. [DOI] [PubMed] [Google Scholar]
- 22.Schmitto JD, Hanke JS, Rojas SV, Avsar M, Haverich A. First implantation in man of a new magnetically levitated left ventricular assist device (HeartMate III). J Heart Lung Transplant 2015;34:858–860. [DOI] [PubMed] [Google Scholar]
- 23.Zimpfer D, Netuka I, Schmitto JD, Pya Y, Garbade J, Morshuis M, Beyersdorf F, Marasco S, Rao V, Damme L, Sood P, Krabatsch T. Multicentre clinical trial experience with the HeartMate 3 left ventricular assist device: 30-day outcomes. Eur J Cardiothorac Surg 2016;50:548–554. [DOI] [PubMed] [Google Scholar]
- 24.Schmitto JD, Pya Y, Zimpfer D, Krabatsch T, Garbade J, Rao V, Morshuis M, Beyersdorf F, Marasco S, Sood P, Damme L, Netuka I. Long-term evaluation of a fully magnetically levitated circulatory support device for advanced heart failure-two-year results from the HeartMate 3 CE Mark Study. Eur J Heart Fail 2019;21:90–97. [DOI] [PubMed] [Google Scholar]
- 25.Netuka I, Sood P, Pya Y, Zimpfer D, Krabatsch T, Garbade J, Rao V, Morshuis M, Marasco S, Beyersdorf F, Damme L, Schmitto JD. Fully magnetically levitated left ventricular assist system for treating advanced HF: a multicenter study. J Am Coll Cardiol 2015;66:2579–2589. [DOI] [PubMed] [Google Scholar]
- 26.Krabatsch T, Netuka I, Schmitto JD, Zimpfer D, Garbade J, Rao V, Morshuis M, Beyersdorf F, Marasco S, Damme L, Pya Y. HeartMate 3 fully magnetically levitated left ventricular assist device for the treatment of advanced heart failure — 1 year results from the CE Mark trial. J Cardiothorac Surg 2017; 12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uriel N, Adatya S, Maly J, Kruse E, Rodgers D, Heatley G, Herman A, Sood P, Berliner D, Bauersachs J, Haverich A, Želízko M, Schmitto JD, Netuka I. Clinical hemodynamic evaluation of patients implanted with a fully magnetically levitated left ventricular assist device (HeartMate 3). J Heart Lung Transplant 2017;36:28–35. [DOI] [PubMed] [Google Scholar]