Abstract
Background
Studies suggest that a diet rich in omega‐3 essential fatty acids may have beneficial anti‐inflammatory effects for chronic conditions such as cystic fibrosis. This is an updated version of a previously published review.
Objectives
To determine whether there is evidence that omega‐3 polyunsaturated fatty acid supplementation reduces morbidity and mortality and to identify any adverse events associated with supplementation.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group's Trials Register comprising references identified from comprehensive electronic database searches and handsearches of relevant journals and abstract books of conference proceedings. Date of last search: 01 April 2020.
We also searched online study registries and contacted authors. Date of last search: 12 February 2020.
Selection criteria
Randomised controlled trials in people with cystic fibrosis comparing omega‐3 fatty acid supplements with placebo.
Data collection and analysis
Two authors independently selected studies for inclusion, extracted data and assessed the risk of bias of the studies. The quality of the evidence was assessed using GRADE.
Main results
The searches identified 23 studies; five studies with 106 participants (children and adults) were included; duration of studies and interventions differed. Two studies compared omega‐3 fatty acids to olive oil for six weeks; one study compared omega‐3 fatty acids and omega‐6 fatty acids to control capsules (customised fatty acid blends) for three months; one study compared a liquid dietary supplement containing omega‐3 fatty acids to one without for six months; and one study compared omega‐3 fatty acids to a placebo for 12 months. Three studies had a low risk of bias for randomisation, but the risk was unclear in the remaining two studies; all studies had an unclear risk of bias for allocation concealment. Three of the studies adequately blinded participants; the risk of bias for selective reporting was high in one study and unclear for four studies.
Two studies reported the number of respiratory exacerbations. At three months, one study (43 participants) reported no change in antibiotic usage. At 12 months the second study (15 participants) reported a reduction in the number of pulmonary exacerbations and cumulative antibiotic days in the supplement group compared to the previous year (no data for the control group); very low‐quality evidence means we are unsure whether supplementation has any effect on this outcome.
With regards to adverse events, one six‐week study (12 participants) reported no difference in diarrhoea between omega‐3 or placebo capsules; the very low‐quality evidence means we are unsure if supplementation has any effect on this outcome. Additionally, one study reported an increase in steatorrhoea requiring participants to increase their daily dose of pancreatic enzymes, but three studies had already increased pancreatic enzyme dose at study begin so as to reduce the incidence of steatorrhoea. One study (43 participants) reported stomach pains at three months (treatment or control group not specified). One six‐week study (19 participants) reported three asthma exacerbations leading to exclusion of participants since corticosteroid treatment could affect affect essential fatty acid metabolism.
Four studies reported lung function. One six‐week study (19 participants) reported an increase in forced expiratory volume in one second (FEV1) (L) and forced vital capacity (FVC) (L), but the very low‐quality evidence means we are unsure if supplementation has any effect on lung function. The remaining studies did not report any difference in lung function at three months (unit of measurement not specified) or at six months and one year (FEV1 % predicted and FVC % predicted).
No deaths were reported in any of the five studies. Four studies reported clinical variables. One study reported an increase in Schwachman score and weight alongside a reduction in sputum volume with supplementation compared to placebo at three months (data not analysable). However, three studies reported no differences in either weight at six weeks, in body mass index (BMI) standard deviation (SD) score at six months (very low‐quality evidence) or BMI Z score at 12 months.
Three studies reported biochemical markers of fatty acid status. One study showed an increase from baseline in both EPA and DHA content of serum phospholipids in the omega‐3 group compared to placebo at three months and also a significant decrease in n‐6/n‐3 ratio in the supplement group compared to placebo; since the quality of the evidence is very low we are not certain that these changes are due to supplementation. One six‐month cross‐over study showed a higher EPA content of the neutrophil membrane in the supplement group compared to the placebo group, but, no difference in DHA membrane concentration. Furthermore, the leukotriene B4 to leukotriene B5 ratio was lower at six months in the omega‐3 group compared to placebo. A one‐year study reported a greater increase in the essential fatty acid profile and a decrease in AA levels in the treatment arm compared to placebo.
Authors' conclusions
This review found that regular omega‐3 supplements may provide some limited benefits for people with cystic fibrosis with relatively few adverse effects: however, the quality of the evidence across all outcomes was very low. The current evidence is insufficient to draw firm conclusions or recommend routine use of these supplements in people with cystic fibrosis. A large, long‐term, multicentre, randomised controlled study is needed to determine any significant therapeutic effect and to assess the influence of disease severity, dosage and duration of treatment. Future researchers should note the need for additional pancreatic enzymes when providing omega‐3 supplementation or olive oil placebo capsules. More research is required to determine the exact dose of pancreatic enzyme required.
Plain language summary
The use of omega‐3 supplements in people with cystic fibrosis
Review question
We reviewed the evidence about the effect of giving omega‐3 supplements to people with cystic fibrosis.
Background
In people with cystic fibrosis recurring cycles of infection and inflammation are thought to worsen lung function. Studies suggest that omega‐3 fatty acids, such as those derived from fish oils, may work to counter the inflammation and may be of benefit in chronic inflammatory diseases including cystic fibrosis. This is an updated version of the review.
Search date
The evidence is current to: 01 April 2020.
Study characteristics
This review includes five small studies which compare omega‐3 supplements to a different supplement without omega‐3. In total there were 106 participants, including both children and adults. The studies lasted between six weeks and 12 months.
Key results
Due to the very low‐quality evidence, we are uncertain whether the following effects are due to supplementation or not. One 12‐month study reported a reduction in pulmonary exacerbations and antibiotic use when taking omega‐3 supplementation compared to placebo and one three‐month study reported no change in antibiotic use during the study period. Few side effects were reported in any of the studies. One six‐week study reported that lung function and clinical status improved when taking omega‐3 supplements. Sputum levels were also noted to be reduced in this six‐week study. Three studies reported no difference in lung function when taking omega‐3 supplementation compared to placebo. Two longer studies found that people taking omega‐3 supplements showed definite increases in levels of essential fatty acids in their white blood cell membranes and also in levels of phospholipids (molecules that provide structure and protection to cells) measured in blood samples.
We are uncertain whether regular omega‐3 supplements benefit people with cystic fibrosis and whether they cause side effects, or not. We are not able to draw firm conclusions or recommend the routine use of these supplements in people with cystic fibrosis. Larger and longer studies are needed to assess the clinical benefit of omega‐3 supplementation and to determine the appropriate dosage.
Quality of the evidence
We judged the quality of the evidence to be very low due to very low participant numbers, low event rates, limited reporting and poor study design. There was no consistency with regards to the time points when results were reported or the measurements used for the same outcome in different studies.
Summary of findings
Summary of findings for the main comparison. Summary of findings: omega‐3 fatty acid supplementation compared with placebo for cystic fibrosis.
| Omega‐3 fatty acid supplementation compared with placebo for cystic fibrosis | ||||||
|
Patient or population: children and adults with cystic fibrosis Settings: outpatients Intervention: oral omega‐3 supplementation (EPA or DHA, or both) Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Omega‐3 supplementation | |||||
|
Number of pulmonary exacerbations (median number of exacerbations during the study) Follow‐up: 12 months |
The number of exacerbations in the placebo group was greater than in the omega‐3 group (3.5 versus 1.7 (range 1 ‐ 3)). | N/A | 13 (1) | ⊕⊝⊝⊝ very lowa,b | The authors only report the number of exacerbations in the supplemented group compared to the 12 months prior to the trial. Extra data was provided by the study authors to allow a between group comparison. This outcome was not included in the study protocol. |
|
|
Adverse events: diarrhoea Follow‐up: 6 weeks |
1 study reported drop out due to diarrhoea. 2 out of 7 participants in the fish oil group dropped out and 2 out of 5 participants in the placebo group, OR 0.6 (0.05 to 6.79). | OR 0.6 (0.05 to 6.79) | 12 (1) | ⊕⊝⊝⊝ very lowc,d | Other adverse events included stomach pains (5/35 participants) but the intervention arm wasn't specified (Keen 2010). | |
|
FEV1 % predicted Follow‐up: 6 months |
The mean FEV1 % predicted was 64% in the control group. | The mean FEV1 % predicted in the intervention group was 2% higher (19.1% lower to 23.11% higher) | MD 2.00 (19.11 to 23.11) | 17 (1) | ⊕⊝⊝⊝ very lowb,e | A further study (n = 16) reported a significant increase from baseline in the EPA group compared to the control group measured in L compared to the placebo group (P = 0.06) (Lawrence 1993). Two studies reported no difference in FEV1 % predicted or lung function (measurement not stated) between groups (Hanssens 2016; Keen 2010) |
|
FVC % predicted Follow‐up: 6 months |
The mean FVC % predicted in the control group was 81%. | The mean FVC % predicted in the intervention group was 1% lower (16.65 % lower to 14.65 % higher). | MD ‐1.00 (‐16.65 to 14.65) | 17 (1) | ⊕⊝⊝⊝ very lowb,e | 1 study reported a significant rise in FVC (L) in the EPA group (P = 0.01) (Lawrence 1993). 2 studies reported no difference in FVC between groups, but no data were available for analysis (Hanssens 2016; Keen 2010). |
|
Growth and nutrition: BMI SD score Follow‐up: 6 months |
No significant difference was seen between the PUFA group and the placebo group after 6 months. | MD 0.00 (95 % CI ‐0.64 to 0.64) | 29 (1) | ⊕⊝⊝⊝ very lowb,e | A further study reported on BMI but reported only that BMI z scores remained stable throughout the study (Hanssens 2016). | |
|
Biochemical markers of essential fatty acid status: EPA and DHA % content of neutrophil membrane Follow‐up: 6 months |
1 study reported a higher EPA content of the neutrophil membrane in the omega‐3 PUFA‐supplemented group compared to the placebo group, MD 0.90 (95% CI 0.46 to 1.34). In the same study, no difference was observed in DHA membrane concentration between groups, MD 0.10 (95% CI ‐0.45 to 0.65) |
29 (1) | ⊕⊝⊝⊝ very lowb,e | At 6 months, Keen reported a significant increase from baseline in both EPA and DHA content of serum phospholipids in the omega‐3 supplemented group compared to placebo, MD 0.70 (95% CI 0.42 to 0.98) and MD 1.10 (95% CI 0.39 to 1.81), respectively (Keen 2010). | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: body mass index; CI: confidence interval; DHA: docosahexaenoic acid; EPA: eicosapentaenoic acid; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; MD: mean difference; OR: odds ratio; PUFA: polyunsaturated fatty acid; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded twice due to risk of bias within the included study for this outcome. There was uncertainty around allocation concealment and blinding and a high risk of bias due to selective reporting.
bDowngraded once due to imprecision from very low participant numbers and low event rates.
cDowngraded once due to risk of bias within the included study. It was unclear whether allocation was concealed and whether the outcomes were predefined as there was no protocol available.
dDowngraded twice due to very low participant numbers (n = 12) and low event rates.
eDowngraded twice due to risk of bias within the trial. The risk of bias was unclear across several domains including; randomisation, allocation concealment, incomplete outcome assessment and selective reporting.
Background
Description of the condition
Cystic fibrosis (CF) is the most common life‐threatening genetically inherited disease in populations of Northern European descent, affecting approximately one in 2500 births (CF Trust 2006; UK CF Registry 2018). Pulmonary inflammation is believed responsible for the progressive loss of lung function that is the major cause of morbidity and mortality in CF (Konstan 1996). In response to lung infections, with organisms such as Pseudomonas aeruginosa, neutrophils (white blood cells) accumulate within the airways, producing proteolytic enzymes and oxidants which mediate the inflammatory response (Wilmott 2000). These neutrophils contribute to the thick and viscous secretions characteristic of CF, leading to mucus plugging of the smaller airways and further cycles of infection and inflammation. Treatment with anti‐inflammatory drugs, including corticosteroids (Cheng 1999; CF Trust 2015) and non‐steroidal anti‐inflammatory agents (Lands 2007), have been shown to have some benefit.
Description of the intervention
Mucus may also prevent pancreatic enzymes reaching the intestine and lead to malabsorption (especially fat malabsorption), diarrhoea and failure to thrive (Hunt 1985; Imrie 1975). The importance of growth and nutrition on survival in CF is well established (Corey 1998; Dodge 1988; Gaskin 1982). Dietary strategies concentrate upon providing a high energy and high protein diet, together with pancreatic enzyme replacement therapy (CF Trust 2016). Despite this, there are many people with CF with sub‐optimal nutritional absorption who continue to require fat soluble vitamins on a daily basis (Benabdeslam 1998; CF Trust 2016).
How the intervention might work
It has been hypothesised that essential fatty acid deficiency may contribute to the development of respiratory disease in infants, even before clinical signs become apparent (Lloyd‐Still 1996). Furthermore, animal models suggest that phenotypic changes in the CF‐affected organs of lung, pancreas and intestine may be due to a defect in essential polyunsaturated fatty acid metabolism (Freedman 1999).
In humans, the polyunsaturated fatty acids (PUFA) linoleic acid (18:2 omega‐6, or n‐6) and alpha‐linolenic (18:3 omega‐3, or n‐3) are 'essential' for normal growth and function; the only source is dietary. The nomenclature refers to their chemical structure.
Research into the omega‐3 series of essential polyunsaturated fatty acids stems from the observation that the native Inuit (Eskimo) of Greenland (who consume a traditional diet rich in fish oils) have a very low incidence of some of the chronic inflammatory immune‐based disorders commonly found in Europe and North America (Corcoran 1937; Osterud 1995). Fish oils are the richest dietary source of the metabolically active omega‐3 fatty acid derivatives eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA); however alternative and novel sources are currently being researched. Omega‐3 fatty acids have been shown to play an important role in the integrity of cellular membranes, where they exert a profoundly anti‐inflammatory response. Some of the beneficial effects of omega‐3 fatty acids on inflammatory disease can be explained by a decrease in the production of pro‐inflammatory metabolites from the omega‐6 fatty acid family and an increase in the biologically less‐active omega‐3 end products (Gaszo 1989). Studies suggest that these fatty acids can exert anti‐inflammatory effects which may benefit a range of chronic inflammatory diseases, including CF.
Why it is important to do this review
As has been discussed above, the absorption of fatty acids may be impaired in people with CF for a number of reasons and it is therefore possible that supplementation with omega‐3 fatty acids may prove to be an effective treatment although details of dosage and administration remain to be elucidated. This is an update of previous versions of the review (Beckles‐Willson 2002; Oliver 2010; Oliver 2011; Oliver 2013; Oliver 2016).
Objectives
To determine whether there is evidence that omega‐3 polyunsaturated fatty acid supplementation reduces morbidity and mortality. To identify any adverse events associated with omega‐3 polyunsaturated fatty acid supplementation in CF.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), quasi‐randomised trials, and cross‐over trials.
Types of participants
People with CF, of any age and severity, diagnosed clinically and by sweat or genetic testing.
Types of interventions
Dietary supplementation of omega‐3 essential fatty acids of any dosage, frequency and duration compared with placebo in people with CF. The supplements contain omega‐3 fatty acids in the form of eicosapentaenoic acid (EPA) or docosahexaenoic acid (DHA), or both. Studies were included if they compared the effect of this intervention with a placebo with low omega‐3 or omega‐6 fatty acid content, such as olive oil.
Types of outcome measures
Primary outcomes
-
Number of respiratory exacerbations including:
hospitalisations
number of courses of antibiotics given (oral and intravenous) (moved from secondary outcomes in a post hoc change)
Adverse events and dropouts
-
Lung function including
per cent predicted forced expiratory volume in one second (FEV1)
forced vital capacity (FVC)
Secondary outcomes
Quality of life
Number of deaths
Clinical variables including indices of growth or nutrition
Bronchial responsiveness as measured by any provocation testing
Biochemical markers of essential fatty acid status including plasma, platelet and erythrocyte (red blood cell) levels of EPA or DHA or both, plus omega‐3 to omega‐6 fatty acid ratio
Search methods for identification of studies
We did not restrict the searches by language, year or publication status.
Electronic searches
Relevant studies were identified from the Group's cystic fibrosis trials register using the terms: omega‐3 fatty acids.
The Group's Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching through the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the website.
In addition, the original review team performed electronic searches of CINAHL and EMBASE (from 1995 to April 2007) (Appendix 1). When the current review team took on this review, these searches were no longer run. The current review team have searched the online registries clinicaltrials.gov and the WHO ICTRP (Appendix 2).
Date of the most recent search of the Group's Cystic Fibrosis Trials Register: 01 April 2020.
Searching other resources
The reference lists of all studies identified have also been checked. The first author of each paper was contacted and invited to identify any other published or unpublished studies that might be relevant.
Data collection and analysis
Selection of studies
For the current version of the review, two authors (HW, CS) independently selected studies to be included in the review. If there had been any disagreement, they would have resolved this by discussion.
Data extraction and management
The two authors (HW, CS for 2019 update onwards) independently extracted data onto data acquisition forms. Authors discussed all stages of data extraction and interpretation and there were no disagreements to resolve.
They grouped outcome data into those measured at six and 12 weeks and at six and 12 months from baseline. For future updates of this review, if data are reported at any other time periods, they will consider reporting these as well.
Since hospitalisations are often used as a marker for respiratory exacerbations, if the authors include a study which reports hospitalisations in addition to or instead of exacerbations, they will include this information in the review under the first primary outcome ‘Number of respiratory exacerbations’.
Assessment of risk of bias in included studies
Two authors (HW, CS) assessed each trial using the domain‐based evaluation as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The authors assessed the following domains as low risk of bias, unclear risk of bias or high risk of bias.
Randomisation (low risk ‐ random number table, computer‐generated lists or similar methods; unclear risk ‐ described as randomised, but no details given; high risk ‐ e.g. alternation, the use of case record numbers, and dates of birth or day of the week).
Concealment of allocation (low risk ‐ e.g. list from a central independent unit, on‐site locked computer, identically appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed opaque envelopes; unclear risk ‐ not described; high risk ‐ if allocation sequence was known to, or could be deciphered by the investigators who assigned participants or if the trial was quasi‐randomised).
Blinding (of participants, personnel and outcome assessors) (low risk ‐ e.g. there was no blinding, but we judge that the outcome and the outcome measurement are not likely to be influenced by lack of blinding, or at least outcome assessors were blinded; unclear risk ‐ not described; high risk ‐ e.g. no or incomplete blinding, and the outcome or outcome measurement is likely to be influenced by lack of blinding, or blinding was attempted, but likely to have been broken).
Incomplete outcome data (Whether investigators used an intention‐to‐treat analysis) (low risk ‐ e.g. no missing data, or missing data have been imputed using appropriate methods; unclear risk ‐ e.g. insufficient reporting of attrition/exclusions; high risk ‐ e.g. reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups).
Selective outcome reporting (low risk ‐ e.g. the study protocol is available and all of the studies pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; unclear risk ‐ e.g. insufficient information to permit judgement; high risk ‐ e.g. not all of the study's pre‐specified primary outcomes have been reported).
Other potential sources of bias (low risk ‐ the study appears to be free of other sources of bias; unclear risk ‐ e.g. insufficient information to assess whether an important risk of bias exists; high risk ‐ e.g. had a potential source of bias related to the specific study design used, or had extreme baseline imbalance).
The authors compared assessments and resolved any inconsistencies by discussion.
Measures of treatment effect
For binary outcomes, the authors have calculated a pooled estimate of the treatment effect for each outcome across studies using the odds ratio (OR) (the odds of an outcome among treatment allocated participants to the corresponding odds among controls) and 95% confidence intervals (CIs). For continuous outcomes, they recorded either mean change from baseline for each group or mean post‐treatment or intervention values and standard deviations for each group. Then, where appropriate, they have calculated a pooled estimate of treatment effect by calculating the mean difference (MD) and 95% CIs.
Unit of analysis issues
When conducting a meta‐analysis combining results from cross‐over studies the authors planned to use the methods recommended by Elbourne (Elbourne 2002). Limited availability of data would mean, we would only able to either use only the first‐arm data or to treat the cross‐over studies as if they are parallel studies. Elbourne states that this approach will produce conservative results as it does not take into account within‐patient correlation (Elbourne 2002). Also each participant will appear in both the treatment and control group, so the two groups will not be independent. For one cross‐over study included in the review, the authors were not able to access the first‐arm data, and so they have treated the study as if it were parallel (Panchaud 2006). If they are able to obtain a correlation co‐efficient for future updates of this review, they will analyse the data more appropriately.
Dealing with missing data
For future updates of the review, in order to allow an intention‐to‐treat analysis, the authors will seek data on the number of participants by allocated treatment group, irrespective of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded from treatment or follow up.
The review authors have requested missing data from the primary investigators of two studies on several occasions (Koletzko 2000; Romano 1997); however, up until 2007 there was no response. They have therefore excluded these studies and do not plan to contact the authors of them again in the future.
We required and requested additional data from the authors of one study regarding the detail of the protocol and the results table (Hanssens 2016). We also required additional data to analyse comparisons between the two CF groups in the included Henderson study; however, a reply from the author has not been received to date (Henderson 1994).
Assessment of heterogeneity
For future updates of the review, if the authors are able to present combined data from a sufficient number of studies (at least four), they will test for heterogeneity between study results using the I² statistic (Higgins 2003). For this measure of consistency of results across studies values range from 0% to 100%. They plan to categorise heterogeneity in a simple way such that if the I² value is around 25% or below, they consider heterogeneity to be low; if the value is around 50%, they will consider it moderate; if the value is around 75% or above, they will consider it high.
Assessment of reporting biases
The review authors checked that the study investigators reported on all the outcomes they stated they planned to measure in the full publications of their studies. When the authors include a sufficient number of studies, they will attempt to assess whether this review is subject to publication bias by using a funnel plot. If they detect asymmetry, they will explore causes other than publication bias.
The review authors also checked for selective outcome reporting by comparing the protocols of the included studies (where available) to the final paper to ensure that the investigators reported all outcomes measured. If the study protocols were not available, the review authors compared the 'Methods' section to the 'Results' section in the final published paper.
Data synthesis
The review authors have analysed their data using a fixed‐effect model. However, if for future updates they identify moderate or high degrees of heterogeneity, they will analyse the data using a random‐effects model.
Subgroup analysis and investigation of heterogeneity
If the authors identify moderate or high degrees of heterogeneity and they are able to included sufficient studies in the review (at least four), they plan to investigate this by performing subgroup analyses (e.g. children versus adults and severity of existing lung disease).
Sensitivity analysis
If the authors identify moderate or high degrees of heterogeneity and they are able to include sufficient studies in the review, they also plan a sensitivity analysis comparing trials with or without cross‐over design.
Summary of findings tables
In line with current Cochrane guidance, at the 2020 update the authors have presented the outcomes listed below in a summary of findings table (Summary of findings table 1). The authors selected these outcomes based on relevance to clinicians and consumers.
Number of respiratory exacerbations
Adverse events
FEV1 % predicted
FVC % predicted
Clinical variables of growth and nutrition
Biochemical markers of essential fatty acid status
For each outcome we report the illustrative risk with and without the intervention, magnitude of effect (RR or MD), numbers of trials and participants addressing each outcome and a grade of the overall quality of the body of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) with comments (Schünemann 2011a).
Results
Description of studies
Results of the search
The literature searches identified 23 studies, including five clinical trials identified from online trials registries. Five studies involving 106 participants with CF met the inclusion criteria (Hanssens 2016; Henderson 1994; Keen 2010; Lawrence 1993; Panchaud 2006). For the 2020 update, one abstract previously listed as awaiting classification was a related reference to a full published study which met the inclusion criteria (Hanssens 2016). One further abstract previously listed under awaiting classification did not have sufficient information to include it in the review; we contacted the author for further information but no response was received, therefore it was excluded (O'Sullivan 2011). A total of 13 studies published as abstracts or full papers were excluded with reasons; (Alicandro 2013; Christophe 1992; Katz 1996; Koletzko 2000; Kurlandsky 1994; Lloyd‐Still 2006; O'Connor 2016; O'Sullivan 2011; Pastor 2019; Romano 1997; Starling 1988; van Biervliet 2008; Vericel 2019). The five clinical trials identified from online trials registries were also excluded; (EUCTR2006‐004155038‐BE, NCT02518672; NCT02646995; NCT02690857; NCT03045198). Please also see the PRISMA diagram generated for this process (Figure 1).
1.
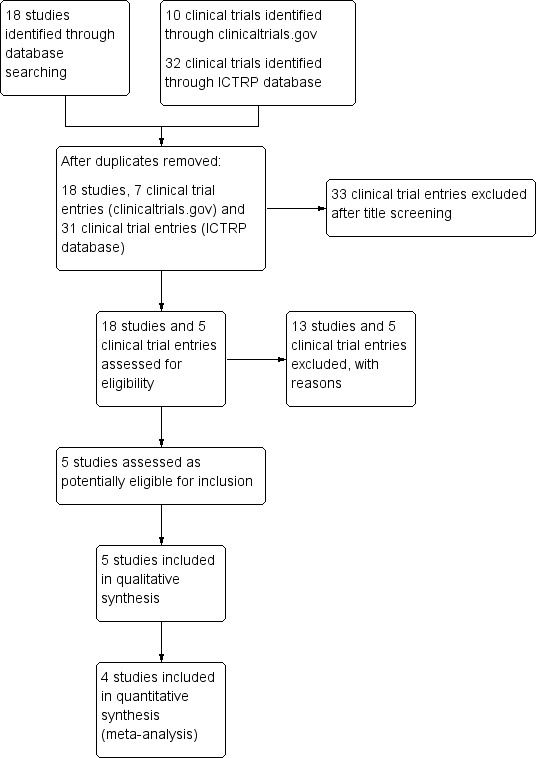
Study flow (PRISMA) diagram
Included studies
See Characteristics of included studies.
Study characteristics
All five included studies were RCTs (Hanssens 2016; Henderson 1994; Keen 2010; Lawrence 1993; Panchaud 2006). Study duration varied from six weeks (Henderson 1994) to 12 months (Hanssens 2016). The number of participants varied from 12 (Henderson 1994) to 43 (Keen 2010).
Three studies were of parallel design (Hanssens 2016; Henderson 1994; Keen 2010). One study split participants into two groups who received either an omega‐3 PUFA supplement or placebo (Hanssens 2016). Henderson split participants into four groups, two of which were in people without CF, we did not consider information from the groups without CF, as healthy participants are not eligible for inclusion in the review. The two groups of participants with CF received either active supplement or placebo (Henderson 1994). The third study randomised participants to three groups: one group received a high omega‐3 fatty acid blend (EPA and DHA); one group received a fatty acid blend containing predominantly omega‐6 fatty acids (linoleic acid (LA), arachidonic acid (AA)); the control group received a high saturated‐fatty acid (SFA) blend. We did not consider results from the group receiving the omega‐6 fatty acid intervention as this intervention was eligible for inclusion in the review (Keen 2010).
Two studies were of cross‐over design (Lawrence 1993; Panchaud 2006). Lawrence found a carry‐over effect despite a 12‐week washout period, therefore, only the results from the 16 participants who completed the first six‐week period of the study were used (Lawrence 1993). Panchaud did not include a washout period and we have treated this study as if it had a parallel design (Panchaud 2006).
Participants
All five studies included both children and adults (Hanssens 2016; Henderson 1994; Keen 2010; Lawrence 1993; Panchaud 2006), although only two studies included older adults, where the age range was stated as up to 41 years (Keen 2010), and 30.4 years (Hanssens 2016). None of the studies were very large; the number of participants in each trial ranged from 12 (Henderson 1994) to 43 (Keen 2010). There were more males in four of the studies (Hanssens 2016; Henderson 1994; Lawrence 1993; Panchaud 2006), but one more female than males in the Keen study (Keen 2010).
Four studies described participants as having pancreatic insufficiency (Hanssens 2016; Henderson 1994; Keen 2010; Panchaud 2006). Pancreatic status was not explicitly specified in one study (Lawrence 1993). Two studies stated that participants were chronically infected with Pseudomonas aeruginosa (Keen 2010; Lawrence 1993). Keen additionally described participants as having severe mutations (Keen 2010). One study described participants as homozygous for the Delta F508 mutation and already undergoing azithromycin treatment for more than three months (Hanssens 2016).
Interventions
Two studies compared omega‐3 fatty acids to olive oil control for a six‐week treatment period (Henderson 1994; Lawrence 1993). One study compared omega‐3 fatty acids to placebo control for a six‐month treatment period (Panchaud 2006). Another study compared essential fatty acid supplementation to a placebo for a three‐month treatment period (Keen 2010). One study compared omega‐3 fatty acids to placebo control for a 12‐month treatment period (Hanssens 2016).
The dose and form of omega‐3 fatty acids differed between the studies. Henderson used four 1 g capsules of fish oil, twice daily (containing a daily dose of 3.2 g EPA and 2.2 g DHA) (Henderson 1994). Lawrence used fish‐oil capsules containing a daily total of 2.7 g EPA (Lawrence 1993). Panchaud used a liquid PUFA mixture containing 0.2 g EPA and 0.1 g DHA per 200 mL (Panchaud 2006). The volume of supplementation was determined according to participant's weight; intake ranged from 200 mg to 600 mg EPA and 100 mg to 300 mg DHA per day. Keen used a customised fatty acid blend containing 21.27 % mmol EPA and 6.99 % mmol DHA and participants received 50 mg per kg body weight per day (Keen 2010). Hanssens used an omega‐3 PUFA supplementation containing 300 mg omega‐3 triglycerides from fish oil, specifically providing 100 mg DHA and 150 mg EPA. The total daily dose was calculated to correspond to 60 mg/kg body weight (Hanssens 2016).
Outcomes
All five studies reported on adverse events (Hanssens 2016; Henderson 1994; Keen 2010; Lawrence 1993; Panchaud 2006) and three on deaths (Henderson 1994; Lawrence 1993; Panchaud 2006). Two studies reported on changes in haematological indices (Henderson 1994; Lawrence 1993). Two studies presented data on serum fatty acid content (Henderson 1994; Keen 2010) and two on changes in in vitro neutrophil chemotaxis (Lawrence 1993; Panchaud 2006). Three studies reported responses to inflammatory markers and nutritional indices (Hanssens 2016; Keen 2010; Panchaud 2006). Four studies reported on lung function (Hanssens 2016; Keen 2010; Lawrence 1993; Panchaud 2006). Two studies reported on change in the number of respiratory exacerbations or antibiotic usage (Hanssens 2016; Keen 2010).
Excluded studies
See Characteristics of excluded studies.
13 studies have been excluded from the review. One study used parenteral (via blood stream), not enteral (oral) supplementation with omega‐3 fatty acids (Katz 1996). Five studies compared omega‐3 supplementation with a large omega‐6 fatty acid source, rather than a neutral placebo that contains relatively little omega‐3 or omega‐6 fatty acid such as olive oil. One study compared omega‐3 supplementation with borache oil (Christophe 1992), two studies with sunflower oil (Kurlandsky 1994; van Biervliet 2008) one study with germ oil (Alicandro 2013) and one study with corn/soy oil as placebo (Lloyd‐Still 2006). Two studies compared omega‐3 supplementation with a large omega‐6 fatty acid source (sunflower oil) rather than a neutral placebo containing relatively little omega‐3 or omega‐6 (O'Connor 2016; Vericel 2019). One study was aimed to identify a suitable approach for monitoring the incorporation of omega‐3 fatty acids in nutritional studies and was not a comparison of omega‐3 fatty acids with control (Pastor 2019).
Four studies were excluded on the basis of insufficient information and a lack of response from the studies' authors (Koletzko 2000; O'Sullivan 2011; Romano 1997; Starling 1988).
Risk of bias in included studies
Please see the risk of bias summary presented in the figures (Figure 2).
2.
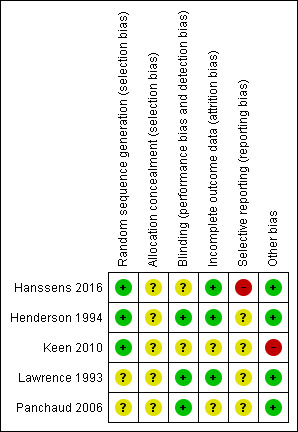
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Generation of randomisation sequence
All five studies were described as randomised, but only three studies gave details on the randomisation process and we graded these studies as having a low risk of bias (Hanssens 2016; Henderson 1994; Keen 2010). The Henderson study was randomised using a stratified randomised block design, whilst the Keen study was randomised using a random number generator (Henderson 1994; Keen 2010). The Hanssens study was randomised using a randomised block design stratified according to participant weight (Hanssens 2016). The remaining two studies did not state the randomisation technique, so were graded as having an unclear risk of bias (Lawrence 1993; Panchaud 2006).
Concealment of allocation
Allocation concealment was graded as having an unclear risk of bias for all five studies as no details were provided in the primary papers (Hanssens 2016; Henderson 1994; Keen 2010; Lawrence 1993; Panchaud 2006).
Blinding
All five studies were described as double blind, details were provided as follows. While the capsules in the Henderson study were also not described as identical, it was stated that the placebo olive oil capsules were flavoured to obtain a slight fish taste which the review authors agreed would be sufficient to blind participants (Henderson 1994). In the Lawrence study, the treatment was administered as "identical olive oil capsules" (Lawrence 1993). In the third study, the placebo treatment was not stated to be identical but it was described as the same liquid dietary supplement as the intervention but without the PUFA mixture (Panchaud 2006). We therefore attributed a low risk of bias to each of these three studies.
In two studies, the appearance of the capsules was not described and two of 12 participants (Keen 2010) complained of a fish smell in the omega‐3 treatment group. The appearance of the capsules is not described in Hanssens study but one participant withdrew from the study due to the fishy taste of the capsules (Hanssens 2016). Therefore, the risk of bias in these studies is unclear (Hanssens 2016; Keen 2010).
Incomplete outcome data
In all five studies, withdrawals from the study were discussed with explanations (Hanssens 2016; Henderson 1994; Keen 2010; Lawrence 1993; Panchaud 2006). Further details of these withdrawals are given in the tables (Characteristics of included studies). Only one study included all participants in the data analysis, which was performed according to the intention‐to‐treat principle and this study was judged to have a low risk of bias (Henderson 1994).
The remaining four studies did not employ this approach, but did describe withdrawals from the study (Hanssens 2016; Keen 2010; Lawrence 1993; Panchaud 2006). In two studies there was a low dropout rate; in one study two out of 15 participants dropped out (Hanssens 2016) and in the second three out of 19 dropped out (Lawrence 1993). We therefore assessed these two studies as having a low risk of bias. The remaining two studies were judged to have an unclear risk of bias (Keen 2010; Panchaud 2006). More than 15% of participants entering one study were excluded from data analysis and only results of 35 participants who completed the study were used in the review (Keen 2010). In the second study, some of the data from baseline and end of treatment in the placebo and treatment groups were excluded from analysis due to "technical reasons" which were not defined (Panchaud 2006).
Selective reporting
We have not been able to determine any selective reporting from the final publication of four of the included studies (unclear risk of bias); however, we have not been able to compare the full study reports to the original study protocols (Henderson 1994; Keen 2010; Lawrence 1993; Panchaud 2006).
The remaining study has a high risk of selective reporting bias (Hanssens 2016). The original study protocol refers to clinical status as an outcome, however, it does not explicitly detail which clinical parameters are to be measured as specific primary and secondary outcomes. The frequency and duration of treatment with IV antibiotics is not specifically defined as a primary outcome, but in the published paper the primary finding is of a decrease in cumulative IV antibiotic duration and in number of pulmonary exacerbations at 12 months. Furthermore, these data are presented as medians for treatment and placebo groups, but without full ranges for the placebo group (Hanssens 2016).
Other potential sources of bias
There is a potential source of bias in one of the studies that did not describe the actual dose of EPA and DHA given (Keen 2010). We have not been able to determine any other potential sources of bias in four of the included studies and judge there to be a low risk of bias (Hanssens 2016; Henderson 1994; Lawrence 1993; Panchaud 2006).
Effects of interventions
See: Table 1
The effects of interventions are summarised in the summary of findings table, the quality of the evidence has been graded for pre‐defined outcomes (see above) and definitions of these gradings provided (Table 1).
Primary outcomes
1. Number of respiratory exacerbations
Two studies reported data related to this outcome (Hanssens 2016; Keen 2010); three of the studies did not measure respiratory exacerbations (Henderson 1994; Lawrence 1993; Panchaud 2006).
One study (n = 13) reported the median (range) number of pulmonary exacerbations, comparing the number of exacerbations in the study to the number in the previous 12 months (Hanssens 2016). Investigators only reported the significant change for the within‐group data for the supplement group in the main publication, but further limited data have been obtained from the author to allow a comparison between intervention and placebo (Hanssens 2016) (very low‐quality evidence from this study at this time‐point).
|
12 months prior to study begin (median (range)) |
During study (median (range)) |
|
| Omega‐3 supplement | 3.0 (1 ‐ 6) | 1.7 (1 ‐ 3) |
| Placebo | 2.0 | 3.5 |
It is important to note that the number of pulmonary exacerbations did not appear in the original study protocol, and was measured at baseline and 12 months unlike other clinical parameters in the study. Furthermore, the small number of participants in the study increases the possibility that the reduction in pulmonary exacerbations may not have been caused by omega‐3 supplementation. Also, at baseline the median (range) FEV1 % predicted of participants in the placebo group (n = 7) was lower than in the treatment group (n = 6), 65% (47 to 104) versus 83% (64 to 121), respectively (Hanssens 2016).
Hanssens also reported on the cumulative number of antibiotic therapy days (IV and oral) for participants compared to the previous 12 months and between groups (Hanssens 2016).
|
12 months up to start of study median (range) |
Antibiotic days at 9 months median (range) |
Antibiotic days at 12 months median (range) |
|
| Omega‐3 supplement | 60 days (0 ‐ 122) | 24.5 days (0 ‐ 43) | 26.5 days (0 ‐ 56) |
| Placebo | 60 days (0 ‐ 90) | 46 days (15 ‐ 92) | 65 days (15 ‐ 113) |
One further study (n = 45) reported there to be no difference in antibiotic use during the study, compared with a similar time period in the previous year; however, no data on the differences in antibiotic days were reported (Keen 2010).
2. Adverse events and dropouts
All five studies reported on adverse events (Hanssens 2016; Henderson 1994; Keen 2010; Lawrence 1993; Panchaud 2006); however, one study simply stated that overall there were no adverse effects of treatment (Panchaud 2006).
a. Steatorrhoea
One study (n = 12) reported that by six weeks, participants in both treatment and control groups needed to increase their daily dose of pancreatic enzyme capsules due to an increase in steatorrhoea and stool frequency (Henderson 1994).
In three studies prior to study start the daily pancreatic enzyme dose was increased for participants in both the intervention and placebo groups to prevent steatorrhoea; there were no reported events of stearrhoea in these three studies (Hanssens 2016; Keen 2010; Lawrence 1993).
One study did not specifically report this adverse event (Panchaud 2006).
b. Diarrhoea
In the Henderson study, two out of seven participants in the treatment group stopped fish oil supplements because of diarrhoea; the same symptoms caused two out of five participants in the placebo group to withdraw (Henderson 1994). There was no significant difference between the groups, odds ratio (OR) 0.60 (95% confidence interval (CI) 0.05 to 6.80) (Analysis 1.1) (very low‐quality evidence from this study at this time‐point).
1.1. Analysis.
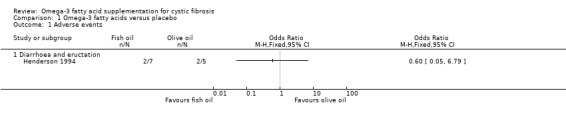
Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 1 Adverse events.
Diarrhoea was not reported in the remaining studies (Hanssens 2016; Keen 2010; Lawrence 1993; Panchaud 2006).
c. Asthma
Only one cross‐over study (n = 19) reported this event (Lawrence 1993). Three participants (one in the supplement group and two in the placebo group) had an asthma exacerbation requiring corticosteroid therapy and were excluded from analysis. The authors argued that corticosteroids affect essential fatty acid metabolism (Lawrence 1993).
d. Stomach pains
Only one study (n = 35) reported that five participants (treatment group not specified) complained of stomach pains; two participants withdrew from the study due to stomach pains (Keen 2010).
3. Lung function
Lung function was not measured in one study (Henderson 1994).
a. FEV1
There were no differences in FEV1 % predicted reported in the cross‐over Panchaud study (n = 16) at six months (Panchaud 2006), MD 2.00 (95% CI ‐19.11 to 23.11) (Analysis 1.2) (very low‐quality evidence from this study at this time‐point).
1.2. Analysis.
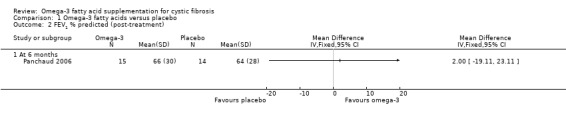
Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 2 FEV1 % predicted (post‐treatment).
One short‐term study (n = 16) reported on FEV1 (L), but investigators reported median (range) values and we are unable to analyse data in this review (Lawrence 1993). Investigators reported a significant increase from baseline in FEV1 in the EPA group, median 0.25 L (0.1 L to 0.85 L) compared with the placebo group, median ‐0.1 L (‐1.15 L to 0.24 L) (P = 0.006). Hanssens reported no difference in lung function (FEV1% predicted) between groups (Hanssens 2016). Keen also reported no difference in lung function (measure not specified) (Keen 2010).
b. FVC
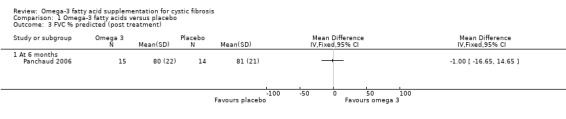
There were no differences in FVC % predicted reported in the cross‐over Panchaud study (n = 16) at six months (Panchaud 2006), MD ‐1.00 (95% CI ‐16.65 to 14.65) (Analysis 1.3) (very low‐quality evidence from this study at this time‐point).
1.3. Analysis.
Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 3 FVC % predicted (post treatment).
Lawrence (n = 16) also reported a significant rise in FVC (L) in the EPA group, median 0.6 L (‐0.1 L to 0.75 L) compared with placebo, median 0.0 L (‐0.15 L to 0.35 L) (P = 0.011) (Lawrence 1993). Hanssens reported no difference in lung function (FVC % predicted) between groups (Hanssens 2016). Keen reported no difference in lung function (measure not specified) (Keen 2010).
Secondary outcomes
1. Quality of life
This outcome was not measured in any of the studies.
2. Number of deaths
No deaths occurred in any of the studies (Hanssens 2016; Henderson 1994; Keen 2010; Lawrence 1993; Panchaud 2006).
3. Clinical variables
The Shwachman score is an overall clinical scoring system in CF, when an increase in the score indicates improvement in clinical conditions (Shwachman 1958). Lawrence reported a significant increase in Shwachman score in the EPA group, median (range) increase 5% (‐10% to 20%) compared with the placebo group, median (range) increase 0% (‐10% to 0%) (P = 0.034) (Lawrence 1993).
Nutritional parameters were recorded in four studies as exploratory outcomes (Hanssens 2016; Keen 2010; Lawrence 1993; Panchaud 2006).
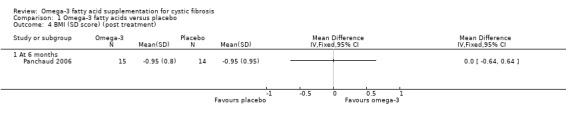
The cross‐over Panchaud study (n = 15) reported a body mass index (BMI) SD score using the nine centiles for BMI in British girls and boys as normal values and their associated co‐efficient of variation (Panchaud 2006). There was no significant difference between the PUFA group and the placebo group after six months, MD 0.00 (95% CI ‐0.64 to 0.64) (Analysis 1.4) (very low‐quality evidence from this study at this time‐point).
1.4. Analysis.
Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 4 BMI (SD score) (post treatment).
Two studies reported the median (range) change in weight (kg) which did not allow us to include the data in a meta‐analysis (Keen 2010; Lawrence 1993). Keen (n = 45) reported that at three months the median (range) change in weight in the supplement group was 1.75 kg (0.0 to 3.5; P = 0.001) and in the placebo group 1.0 kg (‐2.0 to 5.5; P = 0.004) (Keen 2010). Lawrence (n = 19) reported the median (range) change in weight at six months of 1.0 kg (‐1.0 to 3.0) in the supplement group and 0.0 kg (‐1.0 to 3.0) in the placebo group (P = 0.290) (Lawrence 1993). A further study (n = 15) reported that all participants exhibited low BMI z scores at baseline and these scores remained stable throughout the study (Hanssens 2016).
One study reported a median (range) reduction in sputum volumes in the treatment group ‐10 mL (‐50 mL to ‐5 mL) compared with placebo 0 mL (0 mL to ‐10 mL) (Lawrence 1993).
4. Bronchial responsiveness
This outcome was not measured in any of the studies.
5. Biochemical markers of essential fatty acid status
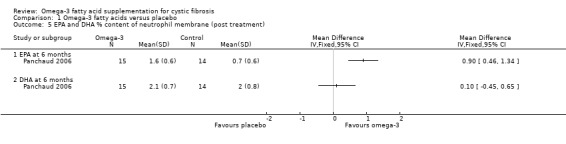
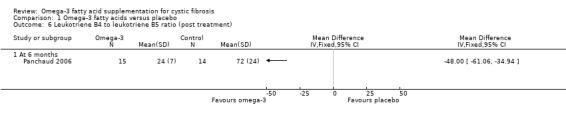
Panchaud (n = 15) reported a higher EPA content of the neutrophil membrane in the omega‐3 PUFA‐supplemented group compared to the placebo group, MD 0.90 (95% CI 0.46 to 1.34) (Analysis 1.5). However, no difference was observed in DHA membrane concentration between the study groups, MD 0.10 (95% CI ‐0.45 to 0.65) (Panchaud 2006) (Analysis 1.5) (very low‐quality evidence from this study at this time‐point). The leukotriene B4 to leukotriene B5 ratio was lower at six months in the omega‐3 PUFA‐supplemented group compared to placebo, MD ‐48.00 (95% CI ‐61.06 to ‐34.94) (Panchaud 2006) (Analysis 1.6).
1.5. Analysis.
Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 5 EPA and DHA % content of neutrophil membrane (post treatment).
1.6. Analysis.
Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 6 Leukotriene B4 to leukotriene B5 ratio (post treatment).
Keen (n = 35) reported means and standard errors (which we converted to SDs to allow analysis in RevMan) on the change from baseline in EPA and DHA content of serum phospholipids and the n6 to n3 ratio (Keen 2010). There was a significant increase from baseline in both EPA and DHA content of serum phospholipids in the omega‐3 supplemented group compared to placebo, MD 0.70 (95% CI 0.42 to 0.98) and MD 1.10 (95% CI 0.39 to 1.81) respectively (Analysis 1.7). There was also a significant decrease in n‐6/n‐3 ratio in the omega‐3 group compared to placebo, MD ‐1.42 (95% CI ‐2.30 to ‐0.54) (Analysis 1.8). Further biochemical marker data were reported by Keen, but these were reported as medians and ranges which we were not able to analyse in RevMan. Investigators reported the change from baseline in the inflammatory markers, ESR and IL‐8. At three months, ESR decreased significantly in the supplement group from a median (range) of 7 mm/h (3 to 26) at baseline to 6 mm/h (3 to 25) (P = 0.05). Similarly, at the same time‐point, the paper reports a significant decrease of IL‐8 in the supplemented group, but a lack of data for the placebo group allowed no comparison to be made (Keen 2010).
1.7. Analysis.
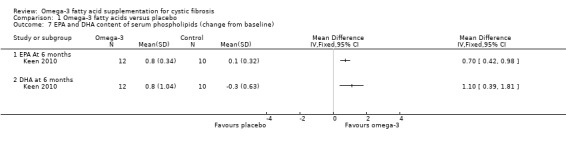
Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 7 EPA and DHA content of serum phospholipids (change from baseline).
1.8. Analysis.
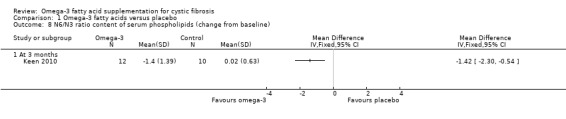
Comparison 1 Omega‐3 fatty acids versus placebo, Outcome 8 N6/N3 ratio content of serum phospholipids (change from baseline).
Hanssens reported a greater increase in the essential fatty acid profile in the treatment arm compared to placebo, but no data were available for analysis (Hanssens 2016). In the placebo group values remained stable, but after three months supplementation with omega‐3 increased erythrocyte levels of EPA (4.2 fold, P < 0.025) and DHA (1.6 fold, P < 0.05). Further increases of EPA (4.2 fold, P < 0.02) and DHA (1.9 fold, P < 0.05) were seen at six months, but no further increases were seen at 12 months (Hanssens 2016). Levels of AA decreased at three months (1.2 fold, P < 0.05) and six months (1.3 fold, P < 0.01) with a further decrease (3.5 fold, P < 0.01) seen at 12 months (Hanssens 2016).
Discussion
The most notable feature highlighted by this review was the lack of data for many of the outcomes likely to be meaningful to people with or making treatment decisions about CF. Information was limited on a number of the primary outcomes that we would have expected to find in a RCT. Across all outcomes the quality of the evidence was judged to be very low, meaning we are very uncertain whether the effects seen were due to supplementation or not.
Summary of main results
Five studies with a total of 106 participants were included in the review, but these were heterogeneous in design and duration. Three had a parallel design (Hanssens 2016; Henderson 1994; Keen 2010) and two were of cross‐over design (Lawrence 1993; Panchaud 2006). They all administered a form of omega‐3 supplementation; two studies compared this to olive oil and three to placebo. The duration of the studies lasted from six weeks to 12 months. Data were not consistently reported in a form we were able to analyse and the results we were able to present did not show consistent effects across studies.
Two studies reported on the number of pulmonary exacerbations, the review's first primary outcome, but we could not analyse the results in this review (Hanssens 2016; Keen 2010). At three months one study reported no difference in antibiotic use during the study period compared with a similar time period in the previous year (Keen 2010). The second study reported a reduction in the median (range) number of respiratory exacerbations in the treatment group compared to the 12 months prior to study begin, but did not report data for the control group (Hanssens 2016). The same study reported a greater decrease in the mean (range) cumulative number of days of antibiotic therapy (IV and oral) in the treatment group compared to the control group at both nine and 12 months (Hanssens 2016).
Few adverse events were seen with omega‐3 supplementation (Hanssens 2016; Henderson 1994; Keen 2010; Lawrence 1993; Panchaud 2006). Increased steatorrhoea was reported by one study which led to participants needing to increase their daily dose of pancreatic enzymes (Henderson 1994); but it should be noted that three further studies increased pancreatic enzyme dose prior to study begin in order to avoid steatorrhoea (Hanssens 2016; Keen 2010; Lawrence 1993). One study reported no difference between groups in incidences of diarrhoea (Henderson 1994). Three asthma exacerbations were reported by one study (one in the supplement group and two from the control group) (Lawrence 1993). Stomach pains in five participants were reported in one thee‐month study, but it was not specified in which group these occurred (Keen 2010).
Four studies reported on lung function (Hanssens 2016; Keen 2010; Lawrence 1993; Panchaud 2006), but only one cross‐over study reported data we were able to analyse (Panchaud 2006). One study reported a significant increase in FEV1 (L) in the supplement group compared to placebo at six weeks (Lawrence 1993), but two studies showed no difference between treatment and control groups in FEV1 % predicted at six months (Analysis 1.2; Panchaud 2006) or at one year (narrative only, no data presented) (Hanssens 2016). The six‐week study also reported an increase in FVC (L) in the supplement group compared with placebo (Lawrence 1993), but again this was not reflected in results for FVC % predicted at six months (Analysis 1.3; Panchaud 2006) or at one year (no data presented) (Hanssens 2016). A fourth study also reported no change in lung function (at three months), but the unit of measurement was not specified (Keen 2010).
With regards to the review's secondary outcomes, no deaths were reported in any of the five studies and quality of life and bronchial responsiveness were not reported by any study.
Each study reported different clinical variables. One study reported improved median Schwachman scores and reduced sputum volumes with supplementation compared to placebo at three months (Lawrence 1993). This study also reported no difference between groups in the median change in weight (kg) at six weeks (Lawrence 1993); however, a further study reported a greater median increase in weight (kg) in the supplement group at three months (Keen 2010). At six months, the cross‐over study showed no difference between groups in BMI SD score (Analysis 1.4; Panchaud 2006); this was also true for BMI z score as reported narratively at 12 months (Hanssens 2016).
Three studies reported on biochemical markers of essential fatty acid status (Hanssens 2016; Keen 2010; Panchaud 2006). At three months in the omega‐3 group compared to placebo, Keen showed an increase from baseline in both EPA and DHA content of serum phospholipids (Analysis 1.7) and also a significant decrease in n‐6/n‐3 ratio (Analysis 1.8). Data from this study also showed significant median decreases in ESR and IL‐8 in the supplement group at three months, but there were no data for the placebo group (Keen 2010). At six months the cross‐over Panchaud study showed a higher EPA content of the neutrophil membrane in the supplement group compared to the placebo group, but no difference in DHA membrane concentration (Analysis 1.5); furthermore, the leukotriene B4 to leukotriene B5 ratio was lower at six months in the omega‐3 group compared to placebo (Analysis 1.6). The one‐year study reported a greater increase in the essential fatty acid profile and a decrease in AA levels in the treatment arm compared to placebo; however, P values were only presented for within‐group data for the supplement group and no data for the placebo group (Hanssens 2016).
Overall completeness and applicability of evidence
The included trials recruited children and adults with CF, although sample sizes were small. The participants were mixed in terms of both gender and age. It is important to note that the effect of omega‐3 supplementation on children under five years of age was not studied in these studies.
The dose of both EPA and DHA varied considerably across the studies with DHA doses ranging from 100 mg to 2.21 g and EPA doses ranging from 150 mg to 2.7 g. One study calculated the dose of omega‐3 supplementation based on the participant's weight. The differences in dose of omega‐3 and differences in the placebo used, mean that we are unable to use these RCTs to make a generalisable finding for people with CF.
Quality of the evidence
The evidence from the studies included in this review was difficult to appraise due to very low participant numbers, low event rates and limited reporting. We judged the quality of the evidence from the studies for all five outcomes to be very low (Table 1). There was inconsistency in the weight indices measured and time‐points across the studies. Meaurements of EPA and DHA varied varied considerably across studies. Findings for pulmonary exacerbations were presented as median data without full ranges compared to placebo. Other references to pulmonary exacerbation rates were noted, but failed to provide data on the changes. Analysis of data related to FEV1 % predicted and FVC % predicted was limited with small sample sizes and limited reporting of data. The risk of bias was unclear across several domains including randomisation, allocation concealment, incomplete outcome assessment and selective reporting
Potential biases in the review process
We conducted a comprehensive literature search of online journal databases using the Cystic Fibrosis and Genetic Disorders Review Group’s Cystic Fibrosis Trials Register and also of journal conference abstracts with no restriction on publication status or language of potentially eligible studies. Although an exhaustive and comprehensive search was conducted, there is an inherent risk that studies may have been missed. Two authors independently applied the inclusion and exclusion criteria to the identified studies and excluded studies that were not relevant. Included studies were appraised for bias and data was extracted using a pre‐determined form according to the previously published review. Where disagreements existed, these were solved through mutual agreement from the authors after discussion Neither of the authors have received direct or indirect payments from the companies responsible for the development of any agents included in this review. This review has assessed all available published study data. Study authors were contacted for relevant unpublished information and individual participant data. Where unpublished data have been used, this has been noted in the review.
Agreements and disagreements with other studies or reviews
At present, we are unaware of any recently published data available about the effects of long‐term supplementation or appropriate dosage of omega‐3 fatty acids. Current nutritional management guidelines for CF do not recommend routine supplementation with omega‐3 fatty acids (CF Trust 2016).
Authors' conclusions
Implications for practice.
We conclude that the limited evidence from these five small studies is not adequate to support any change in clinical practice. The reported benefits, from the use of omega‐3 fatty acid supplements, are from small studies in which the quality of the evidence is very low and hence cannot be used to make recommendations for practice (Hanssens 2016, Henderson 1994; Keen 2010; Lawrence 1993; Panchaud 2006).
There is very little evidence to recommend that people with cystic fibrosis (CF) supplement or modify their intake of omega‐3 fatty acid supplements in order to improve control of their condition. Equally, there is an absence of evidence that they are at risk if they do so. Although the data are sparse, it would seem prudent for people with CF taking omega‐3 fatty acid supplements to take no more than the recommended dose for the general non‐CF population. Although the appropriate dose increase has not been determined, an increase in pancreatic enzymes should be considered when taking these supplements.
Implications for research.
Further large, long‐term, multicentre, randomised, controlled studies are needed in order to determine if there is a significant therapeutic effect and to assess the influence of disease severity, dosage and duration of treatment. Future researchers should note the prevalence for an increased dose of pancreatic enzymes when administering omega‐3 supplementation or oil based placebo to people with CF. The exact dose of pancreatic enzyme required is yet to be determined.
What's new
| Date | Event | Description |
|---|---|---|
| 1 April 2020 | New citation required but conclusions have not changed | A new co‐author (CS) has joined the review and the previous co‐author (CO) has stepped down. Despite the addition of one new study to this review, our conclusions remain the same. |
| 1 April 2020 | New search has been performed | A search of the Cochrane Cystic Fibrosis and Genetic Disorders Cystic Fibrosis Trials Register identified five new references which were potentially eligible for inclusion in the review. One reference was the full paper to an abstract previously listed awaiting classification and included at this update (Hanssens 2016). Two were references to a single new study that was excluded (O'Connor 2016). Two were single references to two further new studies that were also excluded (Pastor 2019; Vericel 2019). A study previously listed as awaiting assessment has been excluded (O'Sullivan 2011). A summary of findings table has been added to the review. |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 3, 2002
| Date | Event | Description |
|---|---|---|
| 5 January 2016 | New search has been performed | A search of the Cochrane Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register identified a single new reference which has been listed as 'Awaiting assessment' until further information is available (Hanssens 2016a). One study previously listed as ongoing has been completed and published; however, on closer consideration this study was not eligible for inclusion in the review (Alicandro 2013). A further study previously listed as 'Awaiting assessment' was excluded due to insufficient information and a lack of response from the author (Starling 1988). |
| 5 January 2016 | New citation required but conclusions have not changed | The previous lead author (CO) has stepped down and is now the co‐author; the former co‐author (HW) is now the lead author. No new information has been included in this updated review, hence our conclusions remain the same. |
| 26 November 2013 | New citation required but conclusions have not changed | Nikki Jahnke has stepped down as co‐author and Helen Watson is the new co‐author on this review. No new studies were included in this update of the review, therefore our conclusions remain the same. |
| 26 November 2013 | New search has been performed | A search of the Cystic Fibrosis & Genetic Disorders Group's Cystic Fibrosis Trials Register identified four new references. Three of these related to a study previously listed as ongoing, including the full paper (Alicandro 2013); and the fourth was a reference to an abstract of a new study. We have excluded the Alicandro study and the new abstract by O'Sullivan is listed as awaiting classification until we can obtain further information to allow us to include or exclude the study (O'Sullivan 2011). |
| 10 March 2011 | New citation required but conclusions have not changed | The title of the review has been amended removing 'from fish oils' and hence expanding the different potential sources of omega‐3 fatty acids to be considered for this review. One of the authors, Dr Mark Everard, has stepped down from the review team. A new author, Nikki Jahnke, joined the team for this update. Another author, Tracy N'Diaye, has not been actively involved in this update of the review and is currently not listed on the citation. |
| 10 March 2011 | New search has been performed | A search of the Cystic Fibrosis Trials Register identified eleven new references to six trials which were potentially eligible for inclusion in the review. Three of these studies were excluded (Christophe 1992; Lloyd‐Still 2006; van Biervliet 2008). One study was listed as awaiting assessment (Starling 1988). One study was listed as ongoing (Alicandro 2013). One study was included in the review (Keen 2010). |
| 11 November 2009 | Amended | Contact details updated. |
| 12 August 2009 | Amended | Contact details updated. |
| 13 June 2008 | New search has been performed | A search of the Group's Cystic Fibrosis Trials Register did not identify any studies potentially eligible for inclusion in the review. |
| 2 June 2008 | Amended | Converted to new review format. |
| 22 August 2007 | Amended | The methodological quality of the two previously included studies were also re‐assessed using the criteria described by Jüni (Jüni 2001). Two studies previously listed as 'Awaiting assessment' have been excluded from the review on the basis of insufficient information and lack of response from the primary investigators. The previous 'Synopsis' has been replaced by a 'Plain Language Summary'. The outcome measures have been re‐ordered, a number of these have been moved from primary outcomes to secondary outcomes. Given that the review has been substantively updated and following the death of the previous lead author, Naomi Beckles‐Wilson, her name has been removed from the byline of this review and her contribution recognised in the 'Acknowledgements' section. |
| 22 August 2007 | New citation required and conclusions have changed | Substantive amendment |
| 22 August 2007 | New search has been performed | One new study has been included in the review (Panchaud 2006) and the methodological quality of the study was assessed using the criteria suggested by Jüni (Jüni 2001). |
| 10 November 2004 | New search has been performed | No new references were found in the latest search of the Group's trials register. |
| 26 November 2003 | New search has been performed | No new references were found in the latest search of the Group's trials register. |
| 1 March 2002 | New search has been performed | Handsearching of several ASPEN/ESPEN and BAPEN conference proceedings were carried out by a contributor to the Group for another review. This search was not thorough. However, some RCTs were identified, including one of the references included in this review (Manner 1993), identified from the ASPEN proceedings. |
Acknowledgements
We gratefully acknowledge the assistance of Sheffield Children's Hospital Appeal funding, which supported the undertaking of the initial version of this review.
We acknowledge the considerable input into the production of the protocol and initial review of the former lead author, Naomi Beckles‐Wilson, and co‐authors Dr Mark Everard and Tracy N'Diaye. In addition the contribution by the previous lead author Coleen Oliver.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategies: Embase and CINAHL (1995 to April 2007)
| Search terms |
| 1. cystic fibrosis 2. essential fatty acids 3. diet 4. nutrition 5. fish oil 6. omega‐3 fatty acid 7. n‐3 fatty acid 8. eicosapentaenoic acid 9. epa 10. docosahexaenoic acid 11. dha |
Appendix 2. Search strategies for study registries
| Database/Resource | Search terms | Date last searched |
| clinicaltrials.gov (clinicaltrials.gov) |
Cystic Fibrosis. Omega 3 Fatty Acids | 12 February 2019 |
| WHO ICTRP (apps.who.int/trialsearch/) |
Cystic Fibrosis AND Omega 3 Fatty Acids | 12 February 2019 |
Data and analyses
Comparison 1. Omega‐3 fatty acids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Diarrhoea and eructation | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FEV1 % predicted (post‐treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 FVC % predicted (post treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 BMI (SD score) (post treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 EPA and DHA % content of neutrophil membrane (post treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 EPA at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 DHA at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Leukotriene B4 to leukotriene B5 ratio (post treatment) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 EPA and DHA content of serum phospholipids (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 EPA At 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 DHA at 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 N6/N3 ratio content of serum phospholipids (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 At 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hanssens 2016.
| Methods | RCT (double‐blind placebo‐controlled). Design: parallel. Duration: 12 months. Location: multicentre (2 centres) in Belgium. |
|
| Participants | 15 people with CF, homozygous Del508, PI and over 5 years of age. Age, mean: intervention group 14 years (5.2 ‐ 26.0 years); placebo group 17.5 years (4.6 ‐ 30.4 years). Gender (M:F): intervention group (4:3); placebo group (7:1). Participants all established on azithromycin treatment for at least 3 months. |
|
| Interventions | Intervention group (n = 7): oral supplement of omega‐3, 60 mg/kg. Control group (n = 8): identical placebo. |
|
| Outcomes | Clinical and nutritional status; lung function, changes in erythrocyte levels of EPA and DHA. | |
| Notes | N = 13 completed, 6 intervention, 7 placebo. Supplement well‐tolerated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block stratification, pre‐defined block list, stratified by pharmacist. 4‐digit sequence generation to individualise participants. |
| Allocation concealment (selection bias) | Unclear risk | No mention of concealment allocation. Stratified by participant weight which could enable recruiter to identify allocation. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Identical capsules, blinded to person responsible for care, participant and outcome assessor, no description of placebo capsules and 1 dropout was due to fishy taste of capsules. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rate very simply, clearly explained and justified. 15 participants were recruited, 13 participants completed the study. Study was not analysed as ITT, authors stated they would only analyse ITT if participant attended for first 3‐month follow‐up visit. The 2 participants dropped out prior to this 3‐month follow‐up. |
| Selective reporting (reporting bias) | High risk | Outcomes both primary and secondary were modified from protocol to study. Authors acknowledged change from inflammatory biomarkers as primary outcome due to lack of facilities to test the outcome. We have concern over conclusions made regarding impact of n3 supplementation on antibiotic duration and frequency, (clinical status) as this outcome was not explicitly defined at the study protocol stage. The significant decrease in number of respiratory exacerbations is only reported in the supplement group with data not provided for the placebo group. |
| Other bias | Low risk | No additional bias identified. |
Henderson 1994.
| Methods | RCT. Design: parallel with 4 arms. Duration: 6‐weeks. Location: single centre in the USA (Children's Hospital Medical Center, Seattle). |
|
| Participants | 12 children and young adults diagnosed with CF on genotype or sweat test, pancreatic insufficiency and able to complete the spirometric tests. Age, mean (SD): age 12.2 (5.4) years. Gender: 7 males, 5 females. Plasma vitamin A and E levels within the normal range. 13 gender and age‐matched people without CF (mean (SD) age 13.4 (6.3) years), 7 males, 6 females. |
|
| Interventions | Intervention group: 8 x 1g capsules fish oil (4 capsules twice daily) containing 3.19 g EPA and 2.21 g DHA. Control: olive oil placebo capsules flavoured to obtain a slight fish taste. |
|
| Outcomes | Outcomes included in this review: number of people experiencing adverse events; number of deaths; changes in haematological indices; changes in plasma and erythrocyte levels of EPA and DHA and EPA/AA ratio. | |
| Notes | Significantly lower plasma n‐6 fatty acids (linoleic acid and AA) noted at baseline in participants with CF compared with the group who did not have CF. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a stratified randomised block design. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed in paper. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double blind ‐ while the capsules were not described as identical, it was stated that the placebo olive oil capsules were flavoured to obtain a slight fish taste which we agreed would be sufficient to blind participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals from the study were discussed with explanations (20 out of 25 randomised participants completed the study). Study included all participants in the data analysis, which was performed according to the ITT principle. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available for comparison, so unable to definitely eliminate selective reporting. |
| Other bias | Low risk | No additional bias identified. |
Keen 2010.
| Methods | RCT. Design: parallel. Duration: 3 months. Location: single centre in Sweden (west Sweden CF Centre). |
|
| Participants | 45 children and adults with "severe" CF mutations randomised; 43 commenced study and 35 participants completed the study. 8 participants discontinued the study and the inclusion parameters of these participants did not differ from those who completed the study. Age, range: 7 to 41 years. Gender: 17 males, 18 females. Disease status: 20 participants were chronically infected with Pseudomonas aeruginosa. |
|
| Interventions | Intervention group A: 50 mg/kg per day fatty acid blend capsules containing predominantly EPA and DHA. Intervention group B: placebo capsules containing predominantly saturated fatty acids. Intervention group C: 50 mg/kg per day fatty acid blend capsules containing a high proportion of linoleic acid and AA. Participants increased their pancreatic enzymes by 10% to 20% to maintain normal stools. |
|
| Outcomes | Outcomes included in this review: changes in serum phospholipid essential fatty acid content; changes in inflammatory markers; adverse effects; BMI and weight; lung function; medical treatment. | |
| Notes | Actual dose of EPA and DHA administered not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed in paper. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Described as double‐blind; treatment was assumed to be administered as identical capsules; however, 2 participants complained of fish smell in group A. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 45 participants were initially randomised, but 2 were excluded due to acute exacerbations and therefore did not enter the study. Withdrawls from study were described: 35 participants completed the study; 8 discontinued because of low compliance (n = 4), stomach pains (n = 2) and weight gain (n = 2). Study did not include all participants in the data analysis, therefore, data were not analysed as ITT. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available for comparison, so unable to definitely eliminate selective reporting. |
| Other bias | High risk | The lack of information on dosage is a potential risk of bias. |
Lawrence 1993.
| Methods | RCT. Design: cross‐over study planned. However, significant carry‐over effect of treatment noted at end of study, therefore analysis restricted to the first 6‐week treatment period. Duration: 6 weeks. Location: single centre in Australia (Sydney). |
|
| Participants | 19 adolescents and adults diagnosed with CF on genotype, sweat test, or clinically. Age, median (range): 17 years (12 years ‐ 26 years). Gender: 11 males, 5 females. 3 participants excluded due to requiring a course of corticosteroids for asthma attacks. Disease status: recruited within 4 weeks of hospitalisation for acute respiratory infection and judged to be in optimum condition for disease stage. All were Pseudomonas aeruginosa colonised and produced at least 5 mL sputum daily. |
|
| Interventions | Intervention group: capsules with 2.7 g EPA daily. Control group: identical olive oil placebo capsules. |
|
| Outcomes | Outcomes included in this review: number of people experiencing adverse events; number of deaths; changes in haematological and growth indices; changes in lung function; changes in in vitro neutrophil chemotaxis. | |
| Notes | Initial randomisation gave groups with comparable baseline characteristics except for age. The treatment group also had significantly greater weight, peripheral blood leucocyte and neutrophil counts. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Did not state the randomisation technique. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided in the primary paper. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double blind, treatment was administered as 'identical olive oil capsules'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 19 participants recruited, 3 participants were excluded from the study before analysis due to corticosteroid treatment during the first treatment period. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available for comparison, so unable to definitely eliminate selective reporting. |
| Other bias | Low risk | No additional bias identified. |
Panchaud 2006.
| Methods | RCT. Design: cross‐over trial with no washout period. Duration: 1 year (2 x 6‐month periods). Location: single centre in Switzerland (Lausanne CF Center). |
|
| Participants | 17 children and young adults with CF and PI; 1 participant discontinued study after 8 months for personal convenience. Age, mean (SD): 18 (9) years). Gender: 10 males, 7 females. |
|
| Interventions | Intervention Group: liquid dietary supplement containing PUFA mixture (EPA, DHA, GLA and STA). Control Group: liquid dietary supplement without PUFA mixture. Volume of supplementation was determined according to participant's weight; intake ranged from 100 mg ‐ 300 mg DHA. and 200 mg ‐ 600 mg EPA per day. |
|
| Outcomes | Outcomes included in this review: number of people experiencing adverse events; number of deaths; changes in peripheral blood neutrophil membrane composition; in vitro neutrophilic response to inflammatory stimuli; changes in in vitro neutrophil chemotaxis. | |
| Notes | Relatively low daily dose of EPA and DHA compared to previous clinical trials. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Did not state the randomisation technique. |
| Allocation concealment (selection bias) | Unclear risk | No details were provided in the primary paper. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Described as double blind, placebo treatment was not stated to be identical but it was described as the same liquid dietary supplement as the intervention but without the PUFA mixture. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 17 participants were randomised; 16 completed the study and one discontinued after 8 months for personal convenience. Due to technical reasons, data could not be analysed for several participants' samples at baseline (4 participants), after treatment with omega‐3 supplements (1) and after placebo (2). High possibility of attrition bias since the analysis of the final cohort differed from the original cohort. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available for comparison, so unable to definitely eliminate selective reporting. |
| Other bias | Low risk | No additional bias identified. |
AA: arachidonic acid BMI: body mass index CF: cystic fibrosis DHA: docosahexaenoic acid EPA: eicosapentaenoic acid GLA: gamma‐linolenic acid ITT: intention‐to‐treat PI: pancreatic insufficient PUFA: polyunsaturated fatty acids RCT: randomised controlled trial SD: standard deviation STA: stearidonic acid
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alicandro 2013 | Omega‐3 supplementation compared with large omega‐6 fatty acid source (germ oil) rather than a neutral placebo that contains relatively little omega‐3 or omega‐6 fatty acid (olive oil). |
| Christophe 1992 | Omega‐3 supplementation compared with large omega‐6 fatty acid source (borache oil) rather than a neutral placebo that contains relatively little omega‐3 or omega‐6 fatty acid (olive oil). |
| EUCTR2006‐004155038‐BE | No published outcome data available. |
| Katz 1996 | Parenteral (via blood stream), not enteral (oral) supplementation with omega‐3 fatty acids. |
| Koletzko 2000 | Eligibility unclear, attempts made to contact author for further information, but no response received. |
| Kurlandsky 1994 | Omega‐3 supplementation compared with large omega‐6 fatty acid source (sunflower oil) rather than a neutral placebo that contains relatively little omega‐3 or omega‐6 fatty acid (olive oil). |
| Lloyd‐Still 2006 | Omega‐3 supplementation compared with large omega‐6 fatty acid source (corn/soy) rather than a neutral placebo that contains relatively little omega‐3 or omega‐6 fatty acid (olive oil). |
| NCT02518672 | Omega‐3 supplementation with large omega‐6 fatty acid source (sunflower oil) rather than a neutral placebo that contains relatively little omega‐3 or omega‐6 fatty acid (olive oil). |
| NCT02646995 | Omega‐3 supplementation not compared to placebo. Fish oil compared to actively modified lipid. |
| NCT02690857 | Omega‐3 supplementation with large omega‐6 fatty acid source (sunflower oil) rather than a neutral placebo that contains relatively little omega‐3 or omega‐6 fatty acid (olive oil). |
| NCT03045198 | Clinical trial currently still in enrolment by invitation. |
| O'Connor 2016 | Omega‐3 supplementation with large omega‐6 fatty acid source (sunflower oil) rather than a neutral placebo that contains relatively little omega‐3 or omega‐6 fatty acid (olive oil). |
| O'Sullivan 2011 | Eligibility unclear. Attempts made to contact the author for further information , but no response received. |
| Pastor 2019 | Study to identify a suitable approach for monitoring the incorporation of omega‐3 fatty acids in nutritional studies, not a comparison of omega‐3 fatty acids with control. |
| Romano 1997 | Eligibility unclear, attempts made to contact author for further information, but no response received. |
| Starling 1988 | Eligibility unclear, attempts made to contact author for further information, but no response received. |
| van Biervliet 2008 | Omega‐3 supplementation compared with large omega‐6 fatty acid source (sunflower oil) rather than a neutral placebo that contains relatively little omega‐3 or omega‐6 fatty acid (olive oil). |
| Vericel 2019 | Omega‐3 supplementation with large omega‐6 fatty acid source (sunflower oil) rather than a neutral placebo that contains relatively little omega‐3 or omega‐6 fatty acid (olive oil). |
Differences between protocol and review
The former secondary outcome "number of courses of antibiotics given (oral and intravenous)" has been moved to a sub‐outcome of the primary outcome "Number of respiratory exacerbations" as often respiratory exacerbations are defined by the courses of antibiotics prescribed.
Contributions of authors
Original review NBW formulated the question, was primarily responsible for development of the protocol and writing the original review. NBW and TN'D selected the studies, graded the quality and extracted the data.
ME was consulted at all stages of the review, providing advice and support when needed.
Updates from 2007 Following the death of the lead author NBW, CO has taken on the lead and acts as guarantor of the review from 2007. The methodological quality of the included studies was re‐assessed by CO using the criteria described by Jüni (Jüni 2001) and then again to reflect the current Cochrane guidelines with regards to risk of bias.
At the update in 2011, ME stepped down from the review team and a new author, NJ joined the team. For this update also, TN'D has not been actively involved and her name does not currently appear on the citation.
At the update in 2013, NJ stepped down from the review team and a new author, HW, joined the team.
At the update in 2016, CO stepped down as lead author, but remained as a co‐author. HW became lead author.
At the update in 2020 CO stepped down as co‐author. HW remained as lead author. CS joined as co‐author.
Sources of support
Internal sources
Sheffield Children's Hospital Appeal, UK.
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Both authors: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Hanssens 2016 {published and unpublished data}
- Hanssens L, Thiebault I, Lefevre N, Malfroot A, Gaspar V, Knoop C, et al. Benefits of long‐term supplementation with omega‐3 polyunsaturated fatty acids in cystic fibrosis. Journal of Cystic Fibrosis: Official Journal of the European Cystic Fibrosis Society. 2015; Vol. 14 Suppl 1:S52. [Abstract no.: ePS05.9; CENTRAL: 1081482; CFGD Register: GN247a; CRS: 5500135000001304]
- Hanssens L, Thiebaut I, Lefevre N, Malfroot A, Knoop C, Duchateau J, et al. The clinical benefits of long‐term supplementation with omega‐3 fatty acids in cystic fibrosis patients ‐ a pilot study. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2016;108:45‐50. [CFGD Register: GN247b; DOI: 10.1016/j.plefa.2016.03.014] [DOI] [PubMed] [Google Scholar]
Henderson 1994 {published data only}
- Henderson WR. Omega‐3 supplementation in cystic fibrosis. Pediatric Pulmonology 1992;14(S8):S21.2. [CFGD Register: GN45a] [Google Scholar]
- Henderson WR Jr, Astley SJ, McCready MM, Kushmerick P, Becker JU, Ramsey B. Absorption of omega (w) ‐3 fatty acids in CF patients. Pediatric Pulmonology 1992;14(S8):311. [CFGD Register: GN45b] [DOI] [PubMed] [Google Scholar]
- Henderson WR Jr, Astley SJ, McCready MT, Kushmerick P, Casey S, Becker JW, et al. Oral absorption of omega‐3 fatty acids in patients with cystic fibrosis who have pancreatic sufficiency and in healthy control subjects. Journal of Pediatrics 1994;124(3):400‐8. [CFGD Register: GN45c] [DOI] [PubMed] [Google Scholar]
Keen 2010 {published data only}
- Keen C, Olin A, Eriksson S, Ekman A, Lindblad A, Basu S, et al. Supplementation with fatty acids influences the airway nitric oxide and inflammatory markers in patients with cystic fibrosis. Journal of Pediatric Gastroenterology and Nutrition 2010;50(5):537‐44. [CFGD Register: GN129b] [DOI] [PubMed] [Google Scholar]
- Keen C, Olin A, Eriksson S, Lindblad A, Ekman A, Basu S, et al. Supplementation with polyunsaturated fatty acids influences the inflammatory response and airway nitric oxide in patients with cystic fibrosis. European Respiratory Society Annual Congress. 2008:541s. [CFGD Register: GN129a]
Lawrence 1993 {published data only}
- Lawrence R, Sorrell T. Eicosapentaenoic acid in cystic fibrosis: evidence of a pathogenetic role for leukotriene B4. Lancet 1993;342(8869):465‐9. [CFGD Register: GN44a] [DOI] [PubMed] [Google Scholar]
- Lawrence R, Sorrell T. Modulation of abnormal neutrophil response to leukotriene B4 in the chronic pseudomonal lung infection of cystic fibrosis. Australian and New Zealand Journal of Medicine 1993;23:442. [CFGD Register: GN44b] [Google Scholar]
Panchaud 2006 {published data only}
- Panchaud A, Sauty A, Kernan Y, Decosterd LA, Buclin T, Boulat O, et al. Biological effects of a dietary omega‐3 polyunsaturated fatty acids supplementation in cystic fibrosis patients: A randomised, crossover placebo‐controlled trial. Clinical Nutrition 2006;25(3):418‐27. [CFGD Register: GN109b] [DOI] [PubMed] [Google Scholar]
- Panchaud A, Sauty A, Kernan Y, Decosterd LA, Buclin T, Roule M. Dietary Supplementation with omega 3 in cystic fibrosis (CF) patients. Journal of Cystic Fibrosis 2005;4 Supplement 1:S88. [CFGD Register: GN109a] [Google Scholar]
References to studies excluded from this review
Alicandro 2013 {published data only (unpublished sought but not used)}
- Alicandro G, Faelli N, Gagliardini R, Santini B, Magazzu G, Biffi A, et al. A randomized placebo‐controlled study on high‐dose oral algal docosahexaenoic acid supplementation in children with cystic fibrosis. Prostaglandins, Leukotrienes and Essential Fatty Acids 2013;88(2):163‐9. [CFGD Register: GN118d; ] [DOI] [PubMed] [Google Scholar]
- Alicandro G, Gagliardini R, Rise P, Santini B, Biffi A, Tirelli S, et al. Oral DHA supplementation in children with CF: a randomized placebo‐controlled study. Pediatric Pulmonology 2011;46 Suppl 34:394. [Abstract no.: 499; CFGD Register: GN118c; ] [Google Scholar]
- Alicandro G, Gagliardini R, Santini B, Rise P, Biffi A, Tirelli AS, et al. Oral DHA supplementation in children with cystic fibrosis: a randomized placebo‐controlled study. Journal of Cystic Fibrosis 2011;10 Suppl 1:S74. [Abstract no.: 290; CFGD Register: GN118b; ] [DOI] [PubMed] [Google Scholar]
- Colombo C, Dacco V, Santini B, Garliardini R, Loi S, Casartelli M, et al. DHA supplementation in children affected by cystic fibrosis: an Italian, multicentre clinical trial. Pediatric Pulmonology 2008;43 Suppl 31:427. [CFGD Register: GN118a] [Google Scholar]
Christophe 1992 {published data only}
- Christophe A, Robberecht E, Franckx H. Effects of two different dietary supplements on eicosanoid precursor fatty acids in cystic fibrosis. 11th International Cystic Fibrosis Congress, Dublin, Ireland. 1992:MP76. [CFGD Register: GN54]
EUCTR2006‐004155038‐BE {published data only}
- EUCTR2006‐004155‐38‐BE. Biochemical effects of a long‐term supplementation with omega‐3 polyunsaturated fatty acids in cystic fibrosis ‐ omega 3 study. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2006‐004155‐38‐BE (first received 23 September 2008).
Katz 1996 {published data only}
- Katz DP, Manner T, Furst P, Askanazi J. The use of an intravenous fish oil emulsion enriched with omega‐3 fatty acids in patients with cystic fibrosis. Nutrition 1996;12(5):334‐9. [CFGD Register: GN66a] [DOI] [PubMed] [Google Scholar]
- Manner T, Guida L, Katz DP, Askanazi J, Schlotzer E, Wiesse S. Parenteral fish oil administration in patients with cystic fibrosis. Clinical Nutrition 1992;11(11):40. [CFGD Register: GN66c] [Google Scholar]
- Manner T, Katz DP, Askanazi J, Schlotzer E, Furst P. Parenteral fish oil administration in patients with cystic fibrosis. 17th Clinical Congress of ASPEN (American Society for Parenteral and Enteral Nutrition). 1993:440. [CFGD Register: GN66b]
Koletzko 2000 {published data only}
- Koletzko B, Tuxen‐Mengedoht M, Muller I, Demmelmair H, Stern M, Steffan J. Polyunsaturated fatty acids improve outcome of cystic fibrosis patients. 13th International Cystic Fibrosis Conference; 2000 June 4‐8; Stockholm. 2000:78. [CFGD Register: GN65b]
- Tuxen‐Mengedoht M, Koletzko B, Demmelmair H, Knapp V, Stern M. Fish oil therapy in cystic fibrosis (CF): A randomised clinical trial. Clinical Nutrition 1999;18(Suppl 1):54. [CFGD Register: GN65a] [Google Scholar]
- Tuxen‐Mengedoht M, Koletzko B, Muller I, Demmelmair H, Knapp V, Stern M, et al. Fish‐oil therapy in mucoviscidosis: A randomised double‐blind study [Fischol‐Therapie bei Mukiviszidose: Eine randomisierte Doppelblindstudie]. Monatsschrift Fur Kinderheilkunde 1999;147(Suppl 2):107. [CFGD Register: GN65c] [Google Scholar]
Kurlandsky 1994 {published data only}
- Kurlandsky LE, Bennink MR, Webb PM, Ulrich PJ, Baer LJ. The absorption and effect of dietary supplementation with omega‐3 fatty acids on serum leukotriene B4 in patients with cystic fibrosis. Pediatric Pulmonology 1994;18(4):211‐7. [CFGD Register: GN46] [DOI] [PubMed] [Google Scholar]
Lloyd‐Still 2006 {published data only}
- Lloyd‐Still J, Powers C, Hoffman D, Arterburn L, Benisek D, Lester L. Bioavailability and safety of an algal DHA triglyceride in cystic fibrosis. Pediatric Research 2001;49(4 Suppl):455a. [CFGD Register: GN92d] [Google Scholar]
- Lloyd‐Still J, Powers C, Hoffman D, Boyd‐Trull K, Lester L, Benisek D, et al. A randomised controlled study examining the bioavailability and safety of an algal docosahexaenoic acid (DHA) triacylglycerol in cystic fibrosis patients. Pediatric Pulmonology 2004;38(Suppl 27):331. [CFGD Register: GN92c] [Google Scholar]
- Lloyd‐Still J, Powers C, Hoffman D, Boyd‐Trull K, Lester L, Benisek D, et al. Bioavailability and safety of a high dose of docosahexaenoic acid triacylglycerol of algal origin in cystic fibrosis patients: a randomised controlled study. Nutrition 2006;22(1):36‐46. [CFGD Register: GN92e] [DOI] [PubMed] [Google Scholar]
- Lloyd‐Still J, Powers C, Hoffman D, Boyd‐trull K, Arterburn L, Benisek D, et al. Blood and tissue essential fatty acids after docosahexaenoic acid supplementation in cystic fibrosis. Pediatric Pulmonology 2001;32(Suppl 22):263. [CFGD Register: GN92b] [Google Scholar]
- Powers CA, Lloyd‐Still J, Hoffman D, Arteburn L, Benisek D, Lester L. Lipid soluble antioxidant status during supplementation with algal docosahexaenoic acid triglyceride in CF. Abstracts of the 24th European Cystic Fibrosis Conference. 2001:P133. [CFGD Register: GN92a]
NCT02518672 {published data only}
- NCT02518672. Pro‐resolving effect of MAG‐DHA in cystic fibrosis (PREMDIC). clinicaltrials.gov/ct2/show/NCT02518672 (first received 10 August 2015).
NCT02646995 {published data only}
- NCT02646995. Lipid formulation to increase the bioavailabilty of fatty acids in cystic fibrosis (CF) patients. clinicaltrials.gov/ct2/show/NCT02646995 (first received 6 January 2016).
NCT02690857 {published data only}
- NCT02690857. Study of docosahexanoic acid in patients with cystic fibrosis (CF) (OMEGAMUCO). clinicaltrials.gov/ct2/show/NCT02690857 (first received 24 Feb 2016).
NCT03045198 {published data only}
- NCT03045198. Effect of azithromycin on fatty acids in CF. clinicaltrials.gov/ct2/show/NCT03045198 (first received 07 February 2017).
O'Connor 2016 {published data only}
- O'Connor MG, Thomsen K, Brown RF, Laposata M, Seegmiller A. Elevated prostaglandin e metabolites and abnormal plasma fatty acids at baseline in pediatric cystic fibrosis patients: a pilot study. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2016;113:46‐9. [CFGD Register: GN250b] [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'connor MG, Thomsen K, Brown RF, Laposata M, Seegmiller AC. DHA supplementation in pediatric CF patients: a randomized, double‐blind clinical trial.. Pediatric Pulmonology. 2015; Vol. Suppl 34:401. [Abstract no.: 582; CFGD Register: GN250a]
O'Sullivan 2011 {published data only}
- O'Sullivan B, Baker D, Borowitz D, Comeau A, Cleveland R, Freedman S. The effect of formula fortified with docosahexaenoic acid (DHA) on infants with CF. Pediatric Pulmonology 2011;46 Suppl 34:401. [Abstract no.: 519; CFGD Register: GN229; ] [Google Scholar]
Pastor 2019 {published data only}
- Pastor O, Guzman‐Lafuente P, Serna J, Munoz‐Hernandez M, Lopez Neyra A, Garcia‐Rozas P, et al. A comprehensive evaluation of omega‐3 fatty acid supplementation in cystic fibrosis patients using lipidomics. Journal of Nutritional Biochemistry 2019;63:197‐205. [CFGD Register: GN277; DOI: 10.1016/j.jnutbio.2018.09.026] [DOI] [PubMed] [Google Scholar]
Romano 1997 {published data only}
- Romano L, Gandino M, Fiore P, Shepherd D, Casciaro R, Coccia C, et al. Study on feasibility and results of midterm dietary supplementation in omega‐3 fatty acids. 21st European Cystic Fibrosis Conference; 1997; Davos. 1997:167. [CFGD Register: GN67]
Starling 1988 {published data only}
- Starling MB, Elliot RB. EPA and cystic fibrosis. Excerpta Medica, Asia Pacific Congress Series. 10th International Cystic Fibrosis Congress. 1988:74. [CFGD Register: GN59]
van Biervliet 2008 {published data only}
- Biervliet S, Devos M, Delhaye J, Biervliet J, Robberecht E, Christophe A. Oral DHA supplementation in F508 homozygous cystic fibrosis patients. Prostaglandins, Leukotrienes and Essential Fatty Acids 2008;78(2):109‐15. [CFGD Register: GN116] [DOI] [PubMed] [Google Scholar]
Vericel 2019 {published data only}
- Vericel E, Mazur S, Colas R, Delaup V, Calzada C, Reix P, et al. Moderate intake of docosahexaenoic acid raises plasma and platelet vitamin E. Prostaglandins, Leukotrienes, and Essential Fatty Acids 2016;115:41‐7. [CFGD Register: GN264; DOI: 10.1016/j.plefa.2016.10.008] [DOI] [PubMed] [Google Scholar]
Additional references
Benabdeslam 1998
- Benabdeslam H, Garcia I, Bellon G, Gilly R, Revol A. Biochemical assessment of the nutritional status of cystic fibrosis patients treated with pancreatic enzyme extracts. American Journal of Human Nutrition 1998;67(5):912‐8. [DOI] [PubMed] [Google Scholar]
CF Trust 2006
- Littlewood J, Green M, Stannard W. Finding Out. CF Trust Factsheet 2006.
CF Trust 2015
- UK CF Trust. CF Trust Factsheet. Steroid treatment in cystic fibrosis. www.cysticfibrosis.org.uk/˜/media/documents/life‐with‐cf/publications/factsheets/factsheet‐steroid‐treatment‐2016.ashx (accessed 10 January 2020).
CF Trust 2016
- UK CF Trust. CF Trust Consensus Documents: Nutritional Management of Cystic Fibrosis. September 2016. www.cysticfibrosis.org.uk/˜/media/documents/the‐work‐we‐do/care/consensus‐documents‐with‐old‐address/nutritional‐management‐of‐cystic‐fibrosis‐sep‐16.ashx?la=en (accessed 10 January 2020).
Cheng 1999
- Cheng K, Ashby D, Smyth R. Oral steroids for cystic fibrosis. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI: 10.1002/14651858.CD000407] [DOI] [PubMed] [Google Scholar]
Corcoran 1937
- Corcoran AC, Rabinowitch IM. A study of the blood lipids and blood proteins in Canadian Eastern Arctic Eskimos. Biochemistry Journal 1937;31:343‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Corey 1998
- Corey M, McLaughlin FJ, Williams M, Levison H. A comparison of survival, growth and pulmonary function in patients with cystic fibrosis in Boston and Toronto. Journal of Clinical Epidemiology 1998;41(6):583‐91. [DOI] [PubMed] [Google Scholar]
Dodge 1988
- Dodge JA. Nutritional requirement in cystic fibrosis: a review. Journal of Pediatric Gastroenterology Nutrition 1988;7(Suppl 1):S8‐11. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Freedman 1999
- Freedman SD, Alvarez JG. Pathogenesis of pancreatic disease in cystic fibrosis. Pediatric Pulmonology 1999;Suppl 19:129. [Google Scholar]
Gaskin 1982
- Gaskin K, Gurwitz D, Durie P, Corey M, Levison H, Forstner G. Improved respiratory prognosis in patients with normal fat absorption. Journal of Pediatrics 1982;100(6):857‐62. [DOI] [PubMed] [Google Scholar]
Gaszo 1989
- Gazso A, Kaliman J, Horrobin DF, Sinzinger H. Effects of omega‐3 fatty acids on the prostaglandin system in healthy probands. Wiener Klinische Wochenschrift 1989;101(8):283‐8. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA on behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hunt 1985
- Hunt B, Geddes DM. Newly diagnosed cystic fibrosis in middle and later life. Thorax 1985;40(1):23‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Imrie 1975
- Imrie J, Fagan D, Sturgess J. Quantitative evaluation of the development of the exocrine pancreas in cystic fibrosis and controlled subjects. American Journal Pathology 1975;95(3):697‐708. [PMC free article] [PubMed] [Google Scholar]
Jüni 2001
- Jüni P, Douglas G, Altman G, Egger M. Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Konstan 1996
- Konstan MW. Treatment of airway inflammation in cystic fibrosis. Current Opinion in Pulmonary Medicine 1996;2(6):452‐6. [PubMed] [Google Scholar]
Lands 2007
- Lands LC, Stanojevic S. Oral non‐steroidal anti‐inflammatory drug therapy for lung disease in cystic fibrosis. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD001505.pub2] [DOI] [PubMed] [Google Scholar]
Lloyd‐Still 1996
- Lloyd‐Still JD, Bibus DM, Powers CA, Johnson SB, Holman RT. Essential fatty acid deficiency and predisposition to lung disease in cystic fibrosis. Acta Paediatrica 1996;85(12):1426‐32. [DOI] [PubMed] [Google Scholar]
Osterud 1995
- Osterud B, Elvevoll E, Barstad H, Brox J, Halvorsen H, Lia K, et al. Effect of marine oils supplementation on coagulation and cellular activation in whole blood. Lipids 1995;30(12):1111‐8. [DOI] [PubMed] [Google Scholar]
Schünemann 2011a
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH on behalf of the Cochrane Applicability and Recommendations Methods Group. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shwachman 1958
- Shwachman H, Kulczycki LL. Long term study of 105 patients with cystic fibrosis. American Journal of Diseases of Children 1958;96(1):6‐10. [DOI] [PubMed] [Google Scholar]
UK CF Registry 2018
- UK CF Trust. UK Cystic Fibrosis Registry Annual Data Report 2018. www.cysticfibrosis.org.uk 2019.
Wilmott 2000
- Wilmott RW, Khurana‐Hershui G, Stark JM. Current concepts on pulmonary host defence mechanisms in children. Current Opinion in Pediatrics 2000;12(3):187‐93. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Beckles‐Willson 2002
- Beckles‐Willson NNR, Elliott T, Everard MML. Omega‐3 fatty acids (from fish oils) for cystic fibrosis. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD002201.pub2] [DOI] [PubMed] [Google Scholar]
Oliver 2010
- Oliver C, Everard M, N'Diaye T. Omega‐3 fatty acids (from fish oils) for cystic fibrosis. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD002201.pub2] [DOI] [PubMed] [Google Scholar]
Oliver 2011
- Oliver C, Jahnke N. Omega‐3 fatty acids for cystic fibrosis. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD002201.pub3] [DOI] [PubMed] [Google Scholar]
Oliver 2013
- Oliver C, Watson H. Omega‐3 fatty acids for cystic fibrosis. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD002201.pub4] [DOI] [PubMed] [Google Scholar]
Oliver 2016
- Oliver C, Watson H. Omega‐3 fatty acids for cystic fibrosis. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD002201.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]


