Abstract
Collagen I interactions with integrins α1 and α2 are known to support human mesenchymal stem cell (hMSC) osteogenesis. Nonetheless, elucidating the relative impact of specific integrin interactions has proven challenging, in part due to the complexity of native collagen. In the present work, we employed two collagen-mimetic proteins – Scl2-2 and Scl2-3 – to compare the osteogenic effects of integrin α1 versus α2 signaling. Scl2-2 and Scl2-3 were both derived from Scl2-1, a triple helical protein lacking known cell adhesion, cytokine binding, and matrix metalloproteinase sites. However, Scl2-2 and Scl2-3 were each engineered to display distinct collagen-based cell adhesion motifs: GFPGER (binding integrins α1 and α2) or GFPGEN (binding only integrin α1), respectively. hMSCs were cultured within poly(ethylene glycol) (PEG) hydrogels containing either Scl2-2 or Scl2-3 for 2 weeks. PEG-Scl2-2 gels were associated with increased hMSC osterix expression, osteopontin production, and calcium deposition relative to PEG-Scl2-3 gels. These data indicate that integrin α2 signaling may have an increased osteogenic effect relative to integrin α1. Since p38 is activated by integrin α2 but not by integrin α1, hMSCs were further cultured in PEG-Scl2-2 hydrogels in the presence of a p38 inhibitor. Results suggest that p38 activity may play a key role in collagen-supported hMSC osteogenesis. This knowledge can be used toward the rational design of scaffolds which intrinsically promote hMSC osteogenesis.
Keywords: Osteogenesis, collagen-mimetic proteins, human mesenchymal stem cells, integrin α1, integrin α2
1. INTRODUCTION
Human mesenchymal stem cells (hMSCs) are considered an important cell source for bone regeneration applications due in part to their ability to be expanded in vitro and to differentiate into a number of cell lineages1. In this context, collagen I has been widely shown to support MSC osteogenic differentiation in both two-dimensional (2D) and three-dimensional (3D) environments2-8. Nonetheless, elucidating the impact of specific collagen motifs on osteogenesis toward improving bone scaffold design has proven challenging. This situation is due in large measure to the complexity of native collagen, which contains a range of signals that guide cell behavior, including cell adhesion sites, enzyme cleavage sequences, and growth factor binding regions9,10. The resulting array of biological interactions makes it difficult to deconvolute the impact of individual collagen motifs on associated cellular responses.
In an effort to overcome this obstacle, blocking peptides and blocking antibodies have been used to interrupt cell interactions with specific collagen-based sequences. These approaches have increased insight into the influence of specific motifs within the context of the remaining signals provided by the biopolymer11,12. For instance, the integrin-blocking studies conducted by Salasznyk et al. indicated that integrin α1 interactions predominantly mediated MSC matrix mineralization on collagen I-coated surfaces12. More recently, Murphy et al.13 showed that MSCs initially cultured with osteoinductive media were able to preserve their osteogenic potential following removal of osteogenic supplements if they were embedded in collagen hydrogels. However, if cell integrin α2β1 interactions with the hydrogel network were blocked, MSC calcium deposition was significantly reduced. Despite the significant advances achieved through integrin-blocking approaches, they are limited in that they do not account for the fact that the cells continue to receive remaining biopolymer signals, i.e., they do not account for the context in which the loss of signaling is occurring. This observation is significant because cells do not generally respond additively to signals they receive from their surroundings14. Thus, recognition and, if possible, control over a stimulus’ contextual presentation is critical to the development of cause-effect relationships.
To surmount the obstacles associated with biopolymer signaling context, a number of researchers have explored the use of bioactive peptides in place of full biopolymers15-17. Although the use of bioactive peptides has greatly advanced our understanding of cell responses to specific matrix motifs, this approach generally removes a critical aspect of the 3D context provided by the parent biomolecule, namely the regional protein folding18-23. For instance, GFOGER is a commonly studied collagen-derived peptide sequence that binds integrins α1β1 and α2β1. However, GFOGER peptides only exhibit native collagen-like, high-affinity binding for integrins α1β1 and α2β1 when presented within the relatively lengthy peptides (> 37 mers) capable of taking on stable triple helical structures24-28. That said, Reyes et al.26 found that triple helical GFOGER increased the expression of osteogenic markers runx2, osteocalcin, and bone sialoprotein by MC3T3-E1 osteoblastic cells to a similar extent as native collagen I. These results add to the literature base implicating integrin α1β1 and α2β1 interactions as key players in collagen I-supported MSC osteogenesis. However, lengthy bioactive peptides such as > 37 mer GFOGER are generally produced by solid-phase synthesis and provide limited capacity for the ready re-introduction of additional biopolymer signals (particularly with similar nanoscale spacing and folding)29 for studies of synergistic interactions among biopolymer signaling motifs.
In contrast, “Designer Collagens” represent a novel, recombinant protein family which can be readily modified by site-directed mutagenesis to display desired signaling motifs with precise densities, nanoscale spacing, and folding30-35. The parent protein of this collagen-mimetic family is Streptococcal collagen-like (Scl) protein 2-1 (Scl2-1), originally derived from group A Streptococcus protein Scl2.28. Scl2-1 is unique in that it contains the GXY repeats and triple helical structure of native collagen, but lacks known cell adhesion, matrix metalloproteinase, and growth factor binding sites. This feature allows the Scl2-1 protein to function as a “biological blank slate”31-34 into which desired motifs can be programmed by site directed mutagenesis35. Furthermore, Scl2-1 proteins assemble into stable triple helices in the absence of post-translational modification36,37, allowing their facile recombinant expression in bacterial culture systems30,38,39. This is in contrast to recombinantly expressed human collagens, which require expensive post-translational modification to achieve a native triple helical conformation.
Herein, we employed two “Designer Collagen” daughter proteins – namely, collagen-mimetic proteins Scl2-2 and Scl2-3 – to examine the relative impact of collagen-based integrin α1 versus integrin α2 interactions and associated downstream MAPK signaling on hMSC osteogenesis. Both Scl2-2 and Scl2-3 were recently engineered from Scl2-1 to display GFOGER-based cell adhesion motifs GFPGER or GFPGEN, respectively27,40-42. As anticipated based on the known integrin affinities of these sequences9,27,35,40,41, Scl2-2 mediates cell adhesion via binding to integrins α1 and α2, whereas Scl2-3 protein interacts only with integrin α130.
In the current studies, we first confirmed that the integrin binding associated with Scl2-2 and Scl2-3 proteins elicited expected MAPK intracellular signaling in target hMSCs. The various Scl2 proteins were then conjugated into poly(ethylene glycol) diacrylate (PEG) hydrogels, and their impact on the osteogenic progression of encapsulated hMSCs was evaluated. Since PEG hydrogels do not intrinsically promote cell adhesion43, cell interactions with PEG-Scl2 gels were initially isolated to the adhesion sites provided by the Scl2 proteins34. Following 2 weeks of culture, the levels of osteogenic transcription factors (runx2, osterix) and bone ECM components (osteopontin, calcium deposits) were analyzed in PEG-Scl2-2 and PEG-Scl2-3 constructs relative to “blank slate” PEG-Scl2-1 controls. Further studies were conducted with Scl2-2 in the presence of an inhibitor of p38 activity to elucidate the effect this MAPK shunt on integrin-mediated hMSC osteogenesis44. The understanding gained from this research will contribute to the rational development of bone scaffolds which intrinsically promote hMSC osteogenesis.
2. MATERIALS AND METHODS
2.1 Polymer and protein synthesis and characterization
2.1.1 PEG-diacrylate synthesis
PEG-diacrylate was prepared as previously described45 by combining 0.1 mmol mL−1 dry PEG (3.4 kDa, Fluka), 0.4 mmol mL−1 acryloyl chloride, and 0.2 mmol ml−1 triethylamine in anhydrous dichloromethane, followed by stirring under argon overnight. The resulting solution was washed with 2 M K2CO3 and separated into aqueous and dichloromethane phases to remove HCl. The organic phase was subsequently dried with anhydrous MgSO4, and PEG-diacrylate was precipitated in diethyl ether, filtered, and dried under vacuum. Acrylation of the PEG end hydroxyl groups was characterized by proton nuclear magnetic resonance (1H-NMR) to be ~96%.
2.1.2 Expression and purification of Scl2 proteins
Scl2-2 and Scl2-3 were previously engineered by introducing DNA sequences encoding for GFPGER or for GFPGEN, respectively, into the plasmid encoding for Scl2-1 via site-directed mutagenesis35. The resulting Scl2-2 and Scl2-3 proteins have previously been demonstrated to maintain the capacity of Scl2-1 to spontaneously assemble into stable triple-helical structures of ∼120 kDa and to specifically engage integrins α1/α2 or integrin α1, respectively9,27,35,40,41. Scl2-1, Scl2-2, and Scl2-3 proteins were each recombinantly expressed in E. coli BL21 (Novagen) as previously described and were purified by affinity chromatography on a HisTrap HP column followed by a HiTrap Q column (GE Healthcare)35. Protein purity was confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) followed by Coomassie Blue staining. Thereafter, each expressed protein was dialyzed against double deionized water, lyophilized, and stored at −20 °C until use.
2.1.3 Synthesis of acrylate-derivatized Scl2 proteins
In order to conjugate the different Scl2 proteins within the PEG-diacrylate hydrogel networks, Scl2-1, Scl2-2 and Scl2-3 were reacted with acrylate-PEG-succinimidyl valerate (ACRL-PEG-SVA, 3.4 kDa, Laysan Bio) at a 1:2 molar ratio for 2 h in 50 mM sodium bicarbonate buffer, pH 8.545. The resulting acrylate-derivatized products were purified by dialysis against double deionized water using a 10 kDa membrane, lyophilized, and stored at −20 °C until use. Acrylation of the target proteins was confirmed by gel electrophoresis30.
2.2 Cell culture
Cryopreserved primary hMSCs (Lonza) at passage 2 were thawed and expanded in monolayer culture per manufacturer protocols. Prior to encapsulation, cells were maintained at 37 °C and 5% CO2 in MesenPRO RS medium (Gibco, Life Technologies) supplemented with 2% MesenPRO RS growth supplement (Gibco, Life Technologies). These donor cells had been confirmed by Lonza to be CD44+, CD105+, CD29+, CD166+, CD14−, CD34−, and CD45− and to undergo adipogenic, chondrogenic, or osteogenic differentiation under inductive culture conditions. Cells at passage 5–6 were harvested and allocated either for cell signaling studies or for hydrogel encapsulation.
2.3 Flow cytometry analysis of integrin subunits
Prior to their use in cell signaling or encapsulation studies, hMSC expression of the integrin subunits α1 and α2 was assessed by flow cytometry using the Agilent 2100 Bioanalyzer microfluidic system. Briefly, hMSCs were resuspended in staining buffer (1% BSA in PBS) at 200,000 cells mL−1, placed on ice, and exposed to 2 μM Calcein AM (Life Technologies) for 30 min. The cells were then exposed for 30 min to 10 μg mL−1 of appropriate primary antibody (integrin α1: clone FB12, integrin α2: clone P1E6; Millipore) or IgG control in staining buffer. Thereafter, 10 μg mL−1 secondary antibody (Alexafluor 647 conjugated donkey anti-mouse IgG; Jackson Immunoresearch) diluted in staining buffer was applied for 30 min. Finally, the cell pellet was rinsed twice with 1 mL staining buffer, resuspended at 1×106 cells mL−1 in loading buffer, and then applied to the flow cytometry microfluidic chip (Agilent 2100 Bioanalyzer). For each antibody, flow assessment was run in triplicate. Additionally, to confirm the multipotent character of the hMSCs, flow cytometry was performed for the following surface markers: CD105, CD44, CD14, and CD45 prior to encapsulation. As expected, hMSCs were positive for CD105 and CD44, but were negative for CD14 and CD45 (Supplementary Figure 1).
2.4 Effect of Scl2 proteins on hMSC MAPK signaling
To gain insight into the cell signaling induced by MSC interactions with Scl2-2 and Scl2-3 proteins, the activation of the three primary MAPK shunts in MSCs exposed to Scl2-2 or Scl2-3 was assessed relative to the “blank slate” Scl2-1 control per Humtsoe et al.39
2.4.1 Cell seeding
In brief, Scl2-1, Scl2-2, or Scl2-3 protein solutions were prepared in phosphate buffered saline (PBS, pH 7.4; Sigma) at 100 μg mL−1, followed by filter sterilization using a 0.22 μm PVDF membrane (Millipore). A 12-well plate was then coated with Scl2-1, Scl2-2, or Scl2-3 by incubating each well with 1 ml of the appropriate protein solution overnight at 4 °C. Immediately prior to cell seeding, the wells were washed with PBS to remove unadsorbed protein.
Human MSCs were adapted to serum-free Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Life Technologies) overnight, after which they were harvested by exposure to 3 mM EDTA solution in PBS (pH 7.4). Cells were pelleted, washed, and then resuspended in serum-free DMEM and maintained in suspension for 1 h at room temperature. Representative images of hMSCs interacting with Scl2-1, Scl2-2, and Scl2-3 coated tissue culture polystyrene are given in Supplementary Figure 2.
The resulting cell suspension was subsequently applied to the Scl2 protein-coated wells at 10,000 cells cm−2. Cells were then allowed to interact with the coated surfaces for 30 min at 37 °C/5% CO2, after which they were lysed with cell extraction buffer supplemented with protease and phosphatase inhibitors (1% Triton, 0.1% SDS, 10 mM sodium fluoride, 1 mM sodium pyrophosphate, 2 mM sodium orthovanadate, 1X protease cocktail (Thermo Scientific) in PBS) for 30 min at 4 °C. Protein levels in the resulting lysates were measured using the Bio-Rad DC protein assay.
2.4.2 Western blot analyses
Protein lysates were used to evaluate the activation of ERK1/2, p38, and JNK MAPK pathways via Western blots. Further details regarding the antibodies employed are given in Supplementary Table 1. For each antibody examined, equal amounts of protein (10-20 μg per lane) were loaded into each well of a 10% polyacrylamide gel, and proteins were separated by electrophoresis. Before loading the samples onto the gel, protein solutions were concentrated using 3,000 MWCO Amicon filter units (Millipore), and proteins were denatured by addition of β-mercaptoethanol and heating at 95 °C for 10 min. After electrophoresis, proteins were transferred to a nitrocellulose membrane (Thermo Scientific).
Following blocking with 5% bovine serum albumin (BSA; Fraction V, Fisher Scientific) in Tris-buffered saline containing 0.1% Tween-20 (TBST: 25 mM Tris-HCl, pH 7.5, 137 mM NaCl, 0.1% Tween 20) for 1 h at room temperature, the blot was briefly rinsed with TBST. Each blot was incubated with the appropriate primary antibody diluted in TBST containing 5% BSA overnight at 4 °C. Appropriate horse radish peroxidase- or alkaline phosphatase-conjugated secondary antibody (Jackson Immunoresearch) diluted in TBST solution containing 5% BSA was then applied for 1 h at room temperature. Bound antibody was then detected using a Luminol reagent (SCBT) or AP chemiluminescent solution (Novex). Imaging was performed using the molecular imager Chemidoc XRS system (Biorad), and band integrated optical density was quantified using Adobe Photoshop. Although evaluating the signaling induced by Scl2-2 relative to Scl2-3 was the primary focus of these studies, the ERK1/2, p38, and JNK activation induced by Scl2-2 and Scl2-3 coated surfaces is shown alongside that induced by collagen I coated surfaces in Supplementary Figure 3A for the purpose of comparison.
2.5 Influence of Scl2 proteins on osteogenic progression in 3D
2.5.1 Cell-laden hydrogel disc fabrication and culture
Hydrogels were fabricated by preparing: 1) a 20 wt% 3.4 kDa PEG-diacrylate solution in HEPES-buffered saline (HBS) and 2) individual solutions of 10 mg mL−1 acrylate-derivatized Scl2-1, Scl2-2, or Scl2-3 in double deionized water. A 300 mg mL−1 solution of UV photoinitiator 2,2-dimethoxy-2-phenyl-acetophenone in N-vinylpyrrolidone was added at 2 (v/v)% to the PEG-diacrylate solution. The PEG-diacrylate and protein solutions were sterilized separately by filtration, after which each protein solution was mixed with an equal volume of the 20 wt% 3.4kDa PEG-diacrylate solution. Harvested hMSCs were resuspended in the resulting precursor solutions at 1.5×106 cells mL−1. The cell suspensions were then pipetted into molds composed of two glass plates separated by 0.75 mm polycarbonate spacers and polymerized by 2 min exposure to longwave UV light (Spectroline, ~6 mW cm−2, 365 nm). The resulting hydrogels were removed from each mold, rinsed with PBS, immersed in DMEM supplemented with 10% MSC-qualified FBS and 1% PSA (PSA: 10,000 U mL−1 penicillin, 10,000 mg L−1 streptomycin, and 25 mg L−1 amphotericin; Gibco, Life Technologies), and placed at 37 °C and 5% CO2. After 24 h of immersion, the swollen hydrogel slabs were separated into uniform gel discs using sterile 8 mm biopsy punches (Miltex).
A set of the resulting hydrogel discs was harvested for “day 0” physical property and cell density analyses, while remaining hydrogel discs were transferred to 12-well culture inserts fitted with porous membranes (BD Biosciences). Following transfer of the gel discs, 1.5 mL of DMEM supplemented with 10% MSC-qualified FBS, 1% PSA, and osteogenic supplements (0.1 μM dexamethasone, 50 μM ascorbate-2-phosphate, and 10 mM β-glycerophosphate46) was added to each well. After 24 h of culture at 37 °C and 5% CO2, the medium surrounding each sample was fully exchanged. Subsequent medium changes were performed every two days.
After 2 weeks of culture, samples were harvested from each hydrogel formulation for mechanical, average mesh size, cell density, calcium deposition, and Western blot analyses. Samples harvested for mechanical, average mesh size, and cell density assessments were evaluated according to the same protocols as the day 0 specimens. Samples collected for calcium deposition and protein analyses (n = 4-6 per formulation) were homogenized in lysis buffer (100 mM Tris-HCl, pH 7.5, 500 mM LiCl, 10 mM EDTA pH 8.0, 1% LiDS, 5 mM dithiothreitol (DTT)) using a microfuge tube-based mortar and pestle system (Kimble). The supernatant was isolated by centrifugation and stored at −80 °C until further analysis.
2.5.2 Hydrogel physical property characterization
Due to the high levels of similarity in the structures of Scl2-1, Scl2-2, and Scl2-3 proteins, it was anticipated that the PEG-Scl2-1, PEG-Scl2-2, and PEG-Scl2-3 hydrogels would have similar initial physical properties. However, to confirm this, the average mesh size and modulus of each hydrogel formulation was evaluated at both “day 0” and at the study endpoint. Following 24 h of immersion in medium (day 0) or 2 weeks of culture (endpoint), a set of 8 mm hydrogel discs (n = 3-4 per formulation) was collected for physical property assessment. A 6 mm biopsy punch was utilized to separate each 8 mm punch into an inner 6 mm disc and an outer hydrogel ring. Each inner 6 mm disc was used for average mesh size assessment per established dextran diffusion protocols47. The outer rings were allocated for mechanical assessment using a previously validated circumferential ring testing technique48. Samples remained immersed in PBS until immediately prior to mechanical analyses, and testing was completed rapidly to avoid sample dehydration. As shown in Table 1, the results indicated that average modulus and average mesh size were consistent across formulations.
Table 1.
Average tensile modulus, dextran diffusion (as an indirect measure of average mesh size), and cell density for each PEG–Scl2 hydrogel formulation both at day 0 and at the study endpoint.a
| Hydrogel formulation | Tensile modulus (kPa) |
Average mesh size (μg dextran/g-gel) |
Cell density (×106 cells/g-gel) |
|||
|---|---|---|---|---|---|---|
|
| ||||||
| Day 0 | Day 14 | Day 0 | Day 14 | Day 0 | Day 14 | |
| PEG-Scl2-1 | 161.7 ± 1.1 | 153.9 ± 3.7 | 21.8 ± 0.1 | 23.6 ± 0.3 | 1.43 ± 0.03 | 1.47 ± 0.12 |
| PEG-Scl2-2 | 169.8 ± 5.4 | 168.3 ± 0.8 | 22.0 ± 0.5 | 23.7 ± 0.6 | 1.33 ± 0.03 | 1.45 ± 0.08 |
| PEG-Scl2-3 | 162.8 ± 2.4 | 167.1 ± 3.3 | 21.0 ± 0.2 | 23.2 ± 0.2 | 1.34 ± 0.04 | 1.33 ± 0.16 |
Property results represent an average of n = 4 samples for each PEG-Scl2 formulation.
2.5.3 Cell density assessment
To assess cell density levels for each hydrogel formulation, 8 mm discs (n = 4 per formulation) were collected from each hydrogel formulation following 24 h (day 0) or 2 weeks of culture. After rinsing with PBS, hydrogel samples were digested for 72 h at 37 °C in 1 mL of 0.12 M NaOH per 0.2 g hydrogel wet weight49,50. Aliquots of the hydrolyzed samples were neutralized, and their DNA content was determined using the Invitrogen PicoGreen assay51. DNA measures were translated to cell numbers using a conversion factor of 6.6 pg DNA per cell52. Calf thymus DNA (Sigma) subjected to the same association with PEG-diacrylate and to the same digestion conditions as the samples served as the standard. The resulting DNA data (Table 1) confirmed that the density of encapsulated cells was consistent across hydrogel formulations for the duration of culture.
2.5.4 Total calcium deposition
To assess calcium deposition within each construct, the supernatants from the homogenized endpoint hydrogel discs (n = 4-6 per formulation) were evaluated using the Calcium Cresolphthalein Complexone (CPC) liquid color kit (Stanbio). Total calcium was quantified using 10 μl aliquots of each sample homogenate per the manufacturer’s protocol.
2.5.5 Western blot analyses
To evaluate the osteogenic lineage progression of hMSCs encapsulated in the various PEG-Scl2 hydrogels, Western blot analyses of the osteogenic markers runx2, osterix, and osteopontin (OPN) were performed. Further details regarding the antibodies employed are given in Supplementary Table 1. Protein levels in the endpoint samples were first determined using the CBQCA protein quantitation kit (Molecular Probes, Life Technologies). For each antibody examined, equal amounts of protein (10-20 μg per lane) were loaded into each well of a 10% polyacrylamide gel, and proteins were separated by electrophoresis. Western blot assessment was then performed as previously described, and each target protein was normalized to the reference protein β-actin.
2.6 Effect of p38 inhibition on hMSC osteogenesis
Concomitantly with the experiment comparing PEG-Scl2-2 to PEG-Scl2-3, an additional experiment assessing the effects of p38 activity inhibition on hMSC osteogenesis within PEG-Scl2-2 hydrogels was conducted. Towards this end, PEG-Scl2-2 gels were fabricated as previously described and immersed in DMEM supplemented with 10% MSC-qualified FBS and 1% PSA at 37 °C and 5% CO2 for 1 h. The hydrogels were then divided into two groups: 1) hydrogels exposed to 10 μM of p38 activity inhibitor SB203580 (Cell Signaling), which was first dissolved at 1 mM in dimethylsulfoxide (DMSO) prior to addition to the culture medium, and 2) control hydrogels exposed only to equivalent amounts of DMSO vehicle. The hydrogel discs were transferred to 12-well culture inserts fitted with a porous membrane (BD Biosciences), and medium changes were performed every two days. The culture medium consisted of DMEM supplemented with 10% MSC-qualified FBS, 1% PSA, and osteogenic supplements with either inhibitor supplementation or DMSO equivalent volume. After 2 weeks of culture, samples were harvested from each hydrogel group for cell density and Western blot analyses, which were conducted as previously described.
2.7 Statistical analyses
Data are shown as mean ± standard error of the mean. Comparison of sample means was performed on the raw data values using ANOVA followed by Tukey’s post hoc test (SPSS software), p ≤ 0.05.
3. RESULTS
The goal of the present work was to examine the relative impact of collagen-based integrin α1 versus integrin α2 interactions and associated downstream MAPK signaling on hMSC osteogenesis. To achieve this, we evaluated the osteogenic response of hMSCs exposed to PEG-Scl2-2 versus PEG-Scl2-3 hydrogels relative to “blank slate” PEG-Scl2-1 controls, as well as the role of p38 activity in this osteogenic process.
3.1 hMSC integrin subunit expression
As previously noted, Scl2-2 supports interactions with both integrin α1 and α2 subunits30,35, whereas Scl2-3 supports interactions only with integrin α135. Thus, in order to more fully evaluate hMSC responses to distinct Scl2 environments, an aliquot of the hMSC population to be encapsulated within the various PEG-Scl2 hydrogels was first assessed for integrin α1 and α2 subunit expression via flow cytometry. Figure 2 shows the percentage of cells with positive staining for a particular integrin subunit relative to their respective negative controls. The hMSCs displayed a ~ 57% positive fraction for the α1 integrin subunit, and a ~93% positive fraction for the α2 integrin subunit. These flow cytometry results are consistent with previous human bone marrow MSC literature85 and indicate that the hMSC population utilized herein should be capable of integrin-specific interactions with Scl2-2 and Scl2-3 proteins.
Figure 2.

Flow cytometry characterization of the expression of the integrin α1 and integrin α2 in the hMSC population used in the PEG-Scl2-2 versus PEG-Scl2-3 comparison experiments as well as in the p38 inhibition study (the comparison and inhibition studies were conducted simultaneously to avoid differences in initial hMSC population).
3.2 Effect of Scl2 proteins on hMSC MAPK signaling
Integrin α1β1 and integrin α2β1 become activated by binding to their respective ligands, initiating intracellular signaling through the p38, ERK1/2, and JNK shunts of the MAPK pathway (Figure 1)53-55. Specifically, while both integrin α1 and α2 interactions with collagen are known to activate ERK and JNK8,56,57, only integrin α2 interactions activate the p38 shunt of the MAPK cascade above basal levels44,58. To confirm that Scl2-2 and Scl2-3 triggered expected intracellular signaling, the activation of ERK1/2, JNK, and p38 following initial hMSC binding to Scl2-2 and Scl2-3 coated-surfaces was therefore evaluated relative to Scl2-1 per established methods39,59-61.
Figure 1.
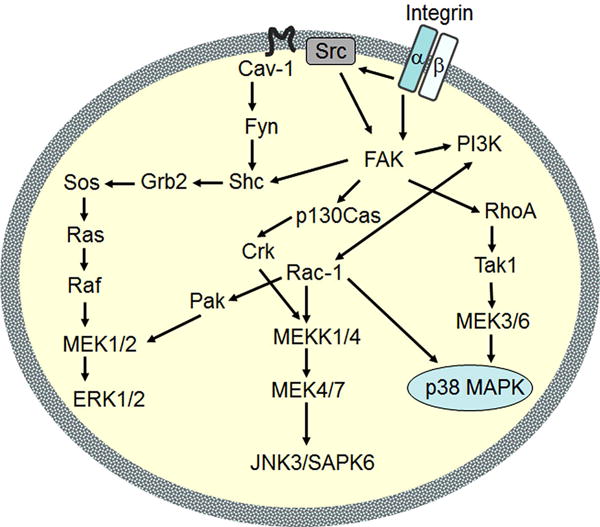
Activation of MAPK pathways through integrin interactions. ERK, JNK and p38 MAPKs are differentially activated by integrin α1β1 and α2β1 signaling. Specifically, integrin α2β1 is uniquely associated with increased p38 activation. Figure adapted from Lal et al.84
As shown in Figure 3, hMSC interactions with both Scl2-2 and Scl2-3 resulted in a ~30% increase in phosphorylated ERK1/2 (pERK1/2; p < 0.002) and a ~70-90% increase in phosphorylated JNK (pJNK; p < 0.041) relative to the basal/non-binding Scl2-1 control. In contrast, only Scl2-2 coated surfaces were associated with a ~30% increase in phosphorylated p38 (pp38) relative to the Scl2-1 controls (p = 0.017). Although the pp38 levels associated with the Scl2-3 samples appeared to be reduced relative to the Scl2-1 controls, this difference was not statistically significant (p = 0.083). Cumulatively, the above data suggest that: 1) integrin α2- and/or integrin α1-dependent adhesion to Scl2-2 and Scl2-3 led to the activation of ERK1/2 and JNK and 2) p38 activation (supported by Scl2-2 but not Scl2-3) was associated with cell adhesion through the integrin α2 subunit only, as anticipated.
Figure 3.
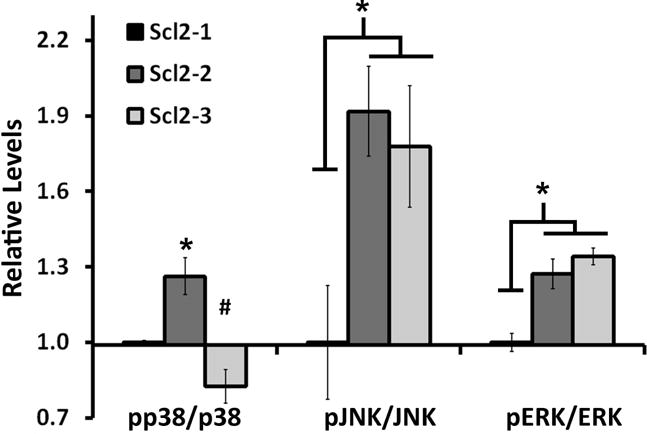
Expression of phosphorylated ERK, JNK and p38 by hMSCs seeded on Scl2-1, Scl2-2 or Scl2-3 coated surfaces. For the purpose of comparison, results for the Scl2-2 and Scl2-3 groups were normalized to the basal expression levels associated with the Scl2-1 control. Results represent an average of n = 3-4 samples per treatment group. * indicates a significant difference from Scl2-1, p < 0.05. # indicates a significant difference from Scl2-2, p < 0.05.
Given these data, the increased activation of p38 associated with Scl2-2 relative to Scl2-3 was further confirmed in 3D by evaluating hMSC signaling within PEG-Scl2-2 and PEG-Scl2-3 scaffolds 6 h following post-encapsulation (Supplementary Figure 3B). Representative images of Western blots for 2D ERK1/2, JNK, and p38 activation are given in Supplementary Figure 4.
3.3 Influence of Scl2 proteins on osteogenic progression in 3D
Along with the assessment of induced intracellular signaling, the ability of Scl2-2 and Scl2-3 to support hMSC osteogenic differentiation was evaluated. Specifically, hMSCs were encapsulated within 100 mg mL−1 (10%) PEG-diacrylate hydrogels into which either 5 mg mL−1 Scl2-2 or 5 mg mL−1 Scl2-3 had been conjugated. PEG hydrogels were utilized as the base hydrogel for the present studies due to their biological “blank slate” character43, allowing for focus on Scl2-2 or Scl2-3 as the primary source of adhesion sites initially provided by the constructs. In addition, hydrogel modulus and relative average mesh size were assessed at both the study outset and endpoint (Table 1). These measures confirmed that the physical properties of the PEG-Scl2 hydrogels were dominated by the slowly-degrading PEG component and were not a point of difference among formulations, further simplifying the attribution of differences in hMSC response to Scl2 protein identity.
After 2 weeks of culture in osteogenic medium, hMSC expression of osteogenic transcription factors runx2 and osterix as well as of the bone-associated ECM protein OPN were evaluated by Western blot for each hydrogel formulation. Representative images of the Western blots for runx2, osterix, and OPN are given in Supplementary Figure 5A. As shown in Figure 4, osterix and OPN levels were each significantly higher in the PEG-Scl2-2 formulation than in the PEG-Scl2-1 controls (p = 0.002 and p = 0.023, respectively), although no significant differences were noted with runx2 (p = 0.270). Similarly, osterix was significantly increased in the PEG-Scl2-3 formulation relative to the PEG-Scl2-1 control (p = 0.004). Although the levels of calcium deposition in the PEG-Scl2-3 constructs appeared to be lower than that in the PEG-Scl2-1 gels, this difference was not statistically significant. Overall, these data indicate that the initial integrin binding landscape provided by the scaffold critically impacts the osteogenic lineage progression of associated hMSCs.
Figure 4.
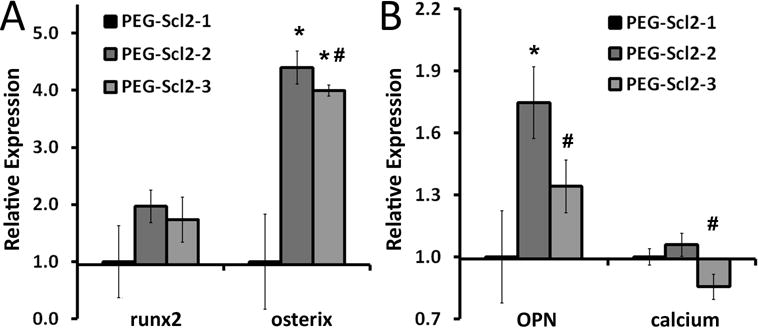
Expression of osteogenic markers (A) runx2 and osterix and (B) OPN and calcium deposition in PEG-Scl2-2 and PEG-Scl2-3 hydrogels relative to PEG-Scl2-1 controls. Results represent n = 3-6 samples per formulation. * indicates a significant difference from the PEG-Scl2-1 gel, p < 0.05; # indicates a significant difference from the PEG-Scl2-2 gel, p < 0.05.
In comparing hMSC behavior in PEG-Scl2-2 versus PEG-Scl2-3 hydrogels, no differences were noted in the levels of the early osteogenic transcription factor runx2. However, PEG-Scl2-2 hydrogels were associated with significantly higher levels of osterix (a mid-term osteogenic transcription factor, p = 0.021) as well as ~1.3-fold higher levels of OPN (p = 0.050). Assessment of construct mineralization also revealed a ~1.2-fold increase in calcium deposits in the PEG-Scl2-2 gels relative to the PEG-Scl2-3 gels (p = 0.028; Figure 4). Cumulatively, these data indicate: 1) that both PEG-Scl2-2 and PEG-Scl2-3 hydrogels supported increased osteogenesis relative to PEG-Scl2-1, perhaps due to the increased JNK and ERK1/2 signaling associated with both Scl2-2 and Scl2-3, and 2) that PEG-Scl2-2 constructs supported increased osteogenic lineage progression relative to PEG-Scl2-3 gels. Referring back to Figure 3, this latter observation may be due in part to the increased p38 signaling supported by Scl2-2 relative to Scl2-3.
3.4 Effect of p38 inhibition on hMSC osteogenesis in PEG-Scl2-2 hydrogels
Given the distinct osteogenic responses observed in PEG-Scl2-2 versus PEG-Scl2-3 constructs, inhibition studies were performed to gain insight into the degree to which the increased hMSC osteogenesis associated with PEG-Scl2-2 gels was due to increased p38 activation versus other potential causes. For instance, the distinct osteogenic patterns associated with the two hydrogel formulations could also be due to differences in the cell subpopulation capable of interacting with Scl2-3 – i.e., cells expressing integrin α1 (Figure 2) – versus the larger cell subpopulation capable of interacting with Scl2-2.
Toward this goal, hMSCs were encapsulated in PEG-Scl2-2 hydrogel networks, and the p38 activity was inhibited in a subset of PEG-Scl2-2 constructs using SB203580. Following 2 weeks of culture, hMSCs within the PEG-Scl2-2 hydrogels exposed to p38 activity inhibition displayed significantly lower combined levels of osterix and OPN relative to the PEG-Scl2-2 DMSO control gels (p = 0.024; Figure 5). The differences in the osterix and OPN expression patterns observed between the p38 inhibited group and the control were similar to the differences in the osterix and OPN expression patterns observed between the PEG-Scl2-2 and PEG-Scl2-3 gel groups (Figure 4 vs Figure 5). These data suggest that integrin-mediated p38 activation may play a key role in collagen-supported hMSC osteogenesis. Representative Western blot images are provided in Supplementary Figure 5B.
Figure 5.
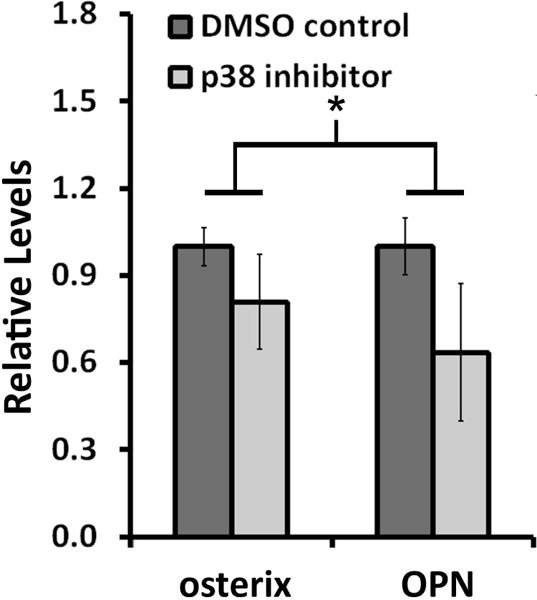
hMSC expression of osterix and OPN in PEG-Scl2-2 hydrogels exposed to p38 inhibition (SB203580 in DMSO) relative to DMSO-only controls (n=3-4 per formulation). For the purpose of comparison, measures for each marker have been normalized to the DMSO control gels. * the connected bar indicates a significant difference in overall osteogenic marker expression between treatment groups, p < 0.05.
4. DISCUSSION
Collagen I has been widely shown to support MSC osteogenic differentiation in both two and three-dimensional environments2-8. Although both α1 and α2 integrin interactions with native collagen have been associated with osteogenesis53,62-65, defined study of their respective impact has been complicated due in part to the complexity of native collagen. In the present work, we employed two “Designer Collagen” daughter proteins – namely, collagen-mimetic proteins Scl2-2 and Scl2-3 – to examine the relative impact of collagen-based integrin α1 versus integrin α2 interactions and associated downstream MAPK signaling on hMSC osteogenic lineage progression.
As previously noted, Scl2-2 proteins are known to support interactions with integrin α1 and α2 subunits30,35, whereas Scl2-3 only supports interactions with integrin α135. To confirm that expected intracellular MAPK signaling was triggered by cell interactions with these proteins, hMSCs were exposed to surfaces coated with Scl2-2 or Scl2-3 proteins and associated ERK1/2, JNK, and p38 activation was assessed. Results indicated that integrin α2- and/or integrin α1-dependent adhesion to Scl2-2 and Scl2-3 led to activation of ERK1/2 and JNK proteins (Figure 3), but that increased activation of the p38 pathway was found only in the presence of the integrin α2 interactions (i.e., Scl2-2 but not Scl2-3). These signaling results are consistent with literature in that both integrin α1 and α2 interactions with collagen are known to activate ERK and JNK8,56,57,66, but only integrin α2 interactions appear to increase p38 activation44,58,62.
Following confirmation of induced MAPK signaling, the ability of Scl2-2 versus Scl2-3 to support hMSC osteogenic differentiation in biomimetic 3D contexts was evaluated using PEG hydrogels. Both PEG-Scl2-2 and PEG-Scl2-3 hydrogels supported marked increases in the mid-term osteogenic transcription factor osterix relative to “blank slate” PEG-Scl2-1 controls (Figure 4). Runx2 and OPN also appeared to be elevated in PEG-Scl2-2 and PEG-Scl2-3 hydrogels relative to baseline PEG-Scl2-1, although these differences were only significant for PEG-Scl2-2 constructs. Given that both Scl2-2 and Scl2-3 support increased activation of ERK1/2 and JNK, the JNK and ERK1/2 MAPK shunts may play a role in the increased osterix expression associated with both PEG-Scl2-2 and PEG-Scl2-3 hydrogels relative to PEG-Scl2-1 controls. This observation would be consistent with previous studies showing that MSC osteogenesis is regulated by the activation of ERK and JNK pathways8,67-71. For instance, Jaiswal et al. demonstrated that when activation of these two pathways was inhibited, the osteogenic differentiation process was blocked68. The increased osterix expression in both PEG-Scl2-2 and PEG-Scl2-3 constructs is also in agreement with previous reports that collagen-based integrin α2β1 and integrin α1β1 interactions are osteoinductive12,26,72-74.
In comparing the osteogenesis supported by PEG-Scl2-2 versus PEG-Scl2-3 hydrogels, it was noted that PEG-Scl2-2 hydrogels stimulated hMSCs to produce significantly higher levels of osterix, OPN, and calcium deposits than PEG-Scl2-3 gels (Figure 4). Differences in the initial MAPK signaling induced by Scl2-2 and Scl2-3 may be one potential source of these distinct osteogenic trajectories. To examine the possibility that the increased p38 activation supported by Scl2-2 relative to Scl2-3 contributed to the increased osteogenesis associated with PEG-Scl2-2 hydrogels, hMSCs were encapsulated within PEG-Scl2-2 hydrogels and exposed to an inhibitor of p38 activity. Significant decreases in combined osterix and OPN levels relative to non-inhibited control cultures were noted with p38 inhibition (Figure 5). In this context, several reports have supported a critical role for the p38 MAPK cascade in regulating osteoblast differentiation75-81. Notably, Greenblatt et al.82 found that the knockout of p38 in mice resulted not only in delayed skeletal mineralization, but also in a 3-fold reduction in bone mass at 3 weeks of age, relative to normal mice. Similarly, Ortuno et al. found that osterix expression was suppressed by inhibiting the p38 pathway78.
More broadly, the present results are also consistent with a number of previous studies that have associated integrin α2 with osteogenesis26,67,73,74. For instance, Shih et al. found that integrin α2 expression favored MSC osteogenesis on collagen I-coated surfaces and that its knockdown resulted in a decreased production of the osteogenic markers osteocalcin and collagen I67. In addition, induction of osteoblastic phenotypes has been shown to be suppressed by blocking integrin α2β1 binding to collagen I74,83. Our study is complementary to these reports in that we have assessed the osteogenic effects of collagen-based integrin α2/α1 binding motifs using a recombinant Scl2 protein family that: 1) maintains the GXY repeats and triple helical structure of collagen I, and 2) allows for facile introduction of isolated as well as combinatorial signaling motifs for controlled studies of individual and synergistic interactions among signaling elements30-35.
CONCLUSIONS
In the present work, we employed two collagen-mimetic proteins – Scl2-2 and Scl2-3 – to examine the role of collagen-based integrin α1/α2 interactions on hMSC osteogenesis in a highly controlled manner. Consistent with literature, both Scl2-2 and Scl2-3 stimulated osteogenic protein expression relative to the “blank slate” parent protein Scl2-1, a collagen-mimetic protein containing no known cell adhesion, growth factor binding, or matrix metalloproteinase sites. However, PEG-Scl2-2 gels were associated with increased levels of osterix as well as increased OPN deposition and matrix mineralization relative to PEG-Scl2-3 gels. These data indicate that integrin α2 signaling may have an increased osteogenic effect relative to integrin α1. Since p38 is activated by integrin α2 but not by integrin α1, further studies were performed with Scl2-2 in the presence of an inhibitor of p38 activity. Results suggest that collagen-based integrin α2 binding plays a significant role in hMSC osteogenesis in part through its activation of the p38 MAPK pathway. The understanding gained from this research will contribute to the rational development of MSC-based bone regeneration strategies which intrinsically promote osteoblast lineage progression via modulation of specific input stimuli and/or signaling elements.
Supplementary Material
Acknowledgments
We would like to acknowledge an NSF CAREER Award and an NIH R01 for funding.
References
- 1.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science. 1999;284(5411):143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Somaiah Chinnapaka, Kumar Atul, Mawrie Darilang, Sharma Amit, Patil Suraj Dasharath, Bhattacharyya Jina, Swaminathan Rajaram, Jaganathan BG. Collagen Promotes Higher Adhesion, Survival and Proliferation of Mesenchymal Stem Cells. PLoS ONE. 2015;10(12):e0145068. doi: 10.1371/journal.pone.0145068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donzelli E, Salvadè A, Mimo P, Viganò M, Morrone M, Papagna R, Carini F, Zaopo A, Miloso M, Baldoni M, et al. Mesenchymal stem cells cultured on a collagen scaffold: In vitro osteogenic differentiation. Archives of Oral Biology. 2007;52(1):64–73. doi: 10.1016/j.archoralbio.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 4.Chamieh F, Collignon A-M, Coyac BR, Lesieur J, Ribes S, Sadoine J, Llorens A, Nicoletti A, Letourneur D, Colombier M-L, et al. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Scientific Reports. 2016;6:38814. doi: 10.1038/srep38814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan BP, Hui TY, Wong MY, Yip KHK, Chan GCF. Mesenchymal Stem Cell–Encapsulated Collagen Microspheres for Bone Tissue Engineering. Tissue Engineering Part C: Methods. 2009;16(2):225–235. doi: 10.1089/ten.tec.2008.0709. [DOI] [PubMed] [Google Scholar]
- 6.Lund AW, Bush JA, Plopper GE, Stegemann JP. Osteogenic Differentiation of Mesenchymal Stem Cells in Defined Protein Beads. Journal of biomedical materials research Part B, Applied biomaterials. 2008;87(1):213–221. doi: 10.1002/jbm.b.31098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schneider Rebekka K, Puellen Andrea, Kramann Rafael, Raupach Kerstin, Bornemann Jörg, Knuechel Ruth, Pérez-Bouza A, Neuss S. The osteogenic differentiation of adult bone marrow and perinatal umbilical mesenchymal stem cells and matrix remodelling in three-dimensional collagen scaffolds. Biomaterials. 2010;31(3):467–480. doi: 10.1016/j.biomaterials.2009.09.059. [DOI] [PubMed] [Google Scholar]
- 8.Chiu Li-Hsuan, Lai Wen-Fu T, Chang Shwu-Fen, Wong Chin-Chean, Fan Cheng-Yu, Fang Chia-Lang, Tsai Y-H. The effect of type II collagen on MSC osteogenic differentiation and bone defect repair. Biomaterials. 2014;35(9):2680–2691. doi: 10.1016/j.biomaterials.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y, Gurusiddappa S, Rich RL, Owens RT, Keene DR, Mayne R, Hook A, Hook M. Multiple binding sites in collagen type I for the integrins a1b1 and a2b1. Journal of Biological Chemistry. 2000;275(50):38981–38989. doi: 10.1074/jbc.M007668200. [DOI] [PubMed] [Google Scholar]
- 10.Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Expression and function of laminins in the embryonic and mature vasculature. Physiol Rev. 2005;85(3):979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- 11.Connelly JT, García AJ, Levenston ME. Inhibition of in vitro chondrogenesis in RGD-modified three-dimensional alginate gels. Biomaterials. 2007;28(6):1071–1083. doi: 10.1016/j.biomaterials.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Salasznyk R, Williams W, Boskey A, Batorsky A, Plopper G. Adhesion to vitronectin and collagen I promotes osteogenic differentiation of human mesenchymal stem cells. J Biomed Biotechnol. 2004;2004(1):24–34. doi: 10.1155/S1110724304306017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy KC, Hoch AI, Harvestine JN, Zhou D, Leach JK. Mesenchymal Stem Cell Spheroids Retain Osteogenic Phenotype Through α(2)β(1) Signaling. Stem Cells Translational Medicine. 2016;5(9):1229–1237. doi: 10.5966/sctm.2015-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guilak F, Cohen DM, Estes BT, Gimble JM, Liedtke W, Chen CS. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 2009;5(1):17–26. doi: 10.1016/j.stem.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mann BK, Tsai AT, Scott-Burden T, West JL. Modification of surfaces with cell adhesion peptides alters extracellular matrix deposition. Biomaterials. 1999;20(23–24):2281–2286. doi: 10.1016/s0142-9612(99)00158-1. [DOI] [PubMed] [Google Scholar]
- 16.Gobin AS, West JL. Val-ala-pro-gly, an elastin-derived non-integrin ligand: Smooth muscle cell adhesion and specificity. Journal Of Biomedical Materials Research Part A. 2003;67A(1):255–259. doi: 10.1002/jbm.a.10110. [DOI] [PubMed] [Google Scholar]
- 17.Klees RF, Salasznyk RM, Ward DF, Crone DE, Williams WA, Harris MP, Boskey A, Quaranta V, Plopper GE. Dissection of the osteogenic effects of laminin-332 utilizing specific LG domains: LG3 induces osteogenic differentiation, but not mineralization. Experimental Cell Research. 2008;314(4):763–773. doi: 10.1016/j.yexcr.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khalili-Shirazi A, Quaratino S, Londei M, Summers L, Tayebi M, Clarke AR, Hawke SH, Jackson GS, Collinge J. Protein Conformation Significantly Influences Immune Responses to Prion Protein. The Journal of Immunology. 2005;174(6):3256. doi: 10.4049/jimmunol.174.6.3256. [DOI] [PubMed] [Google Scholar]
- 19.Tran KT, Griffith L, Wells A. Extracellular matrix signaling through growth factor receptors during wound healing. Wound Repair and Regeneration. 2004;12(3):262–268. doi: 10.1111/j.1067-1927.2004.012302.x. [DOI] [PubMed] [Google Scholar]
- 20.del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Stretching Single Talin Rod Molecules Activates Vinculin Binding. Science. 2009;323(5914):638. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reilly G, Engler A. Intrinsic extracellular matrix properties regulate stem cell differentiation. J Biomech. 2010;43(1):55–62. doi: 10.1016/j.jbiomech.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8(6):457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 23.Bhattacharjee M, Schultz-Thater E, Trella E, Miot S, Das S, Loparic M, Ray A, Martin I, Spagnoli G, Ghosh S. The role of 3D structure and protein conformation on the innate and adaptive immune responses to silk-based biomaterials. Biomaterials. 2013;34(33):8161–8171. doi: 10.1016/j.biomaterials.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 24.Shekaran A, García JR, Clark AY, Kavanaugh TE, Lin AS, Guldberg RE, García AJ. Bone Regeneration using an Alpha 2 Beta 1 Integrin-Specific Hydrogel as a BMP-2 Delivery Vehicle. Biomaterials. 2014;35(21):5453–5461. doi: 10.1016/j.biomaterials.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wojtowicz AM, Shekaran A, Oest ME, Dupont KM, Templeman KL, Hutmacher DW, Guldberg RE, García AJ. Coating of Biomaterial Scaffolds with the Collagen-Mimetic Peptide GFOGER for Bone Defect Repair. Biomaterials. 2010;31(9):2574. doi: 10.1016/j.biomaterials.2009.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reyes CD, García AJ. α2β1 integrin-specific collagen-mimetic surfaces supporting osteoblastic differentiation. Journal of Biomedical Materials Research Part A. 2004;69A(4):591–600. doi: 10.1002/jbm.a.30034. [DOI] [PubMed] [Google Scholar]
- 27.Knight CG, Morton LF, Peachey AR, Tuckwell DS, Farndale RW, Barnes MJ. The collagen-binding A-domains of integrins alpha(1)beta(1) and alpha(2)beta(1) recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. J Biol Chem. 2000;275(1):35–40. doi: 10.1074/jbc.275.1.35. [DOI] [PubMed] [Google Scholar]
- 28.Reyes CD, Garcia AJ. Engineering integrin-specific surfaces with a triple-helical collagen-mimetic peptide. Journal Of Biomedical Materials Research Part A. 2003;65A(4):511–523. doi: 10.1002/jbm.a.10550. [DOI] [PubMed] [Google Scholar]
- 29.Benoit DSW, Anseth KS. The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials. 2005;26(25):5209–5220. doi: 10.1016/j.biomaterials.2005.01.045. [DOI] [PubMed] [Google Scholar]
- 30.Cosgriff-Hernandez E, Hahn MS, Russell B, Wilems T, Munoz-Pinto D, Browning MB, Rivera J, Hook M. Bioactive hydrogels based on Designer Collagens. Acta Biomater. 2010;6(10):3969–77. doi: 10.1016/j.actbio.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Caswell CC, Barczyk M, Keene DR, Lukomska E, Gullberg DE, Lukomski S. Identification of the first prokaryotic collagen sequence motif that mediates binding to human collagen receptors, integrins alpha2beta1 and alpha11beta1. J Biol Chem. 2008;283(52):36168–75. doi: 10.1074/jbc.M806865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caswell CC, Han R, Hovis KM, Ciborowski P, Keene DR, Marconi RT, Lukomski S. The Scl1 protein of M6-type group A Streptococcus binds the human complement regulatory protein, factor H, and inhibits the alternative pathway of complement. Mol Microbiol. 2008;67(3):584–96. doi: 10.1111/j.1365-2958.2007.06067.x. [DOI] [PubMed] [Google Scholar]
- 33.Caswell CC, Lukomska E, Seo NS, Hook M, Lukomski S. Scl1-dependent internalization of group A Streptococcus via direct interactions with the alpha2beta(1) integrin enhances pathogen survival and re-emergence. Mol Microbiol. 2007;64(5):1319–31. doi: 10.1111/j.1365-2958.2007.05741.x. [DOI] [PubMed] [Google Scholar]
- 34.Han R, Caswell CC, Lukomska E, Keene DR, Pawlowski M, Bujnicki JM, Kim JK, Lukomski S. Binding of the low-density lipoprotein by streptococcal collagen-like protein Scl1 of Streptococcus pyogenes. Mol Microbiol. 2006;61(2):351–67. doi: 10.1111/j.1365-2958.2006.05237.x. [DOI] [PubMed] [Google Scholar]
- 35.Seo N, Russell BH, Rivera JJ, Liang X, Xu X, Afshar-Kharghan V, Hook M. An engineered alpha1 integrin-binding collagenous sequence. J Biol Chem. 2010;285(40):31046–54. doi: 10.1074/jbc.M110.151357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Y, Keene DR, Bujnicki JM, Hook M, Lukomski S. Streptococcal Scl1 and Scl2 proteins form collagen-like triple helices. J Biol Chem. 2002;277(30):27312–8. doi: 10.1074/jbc.M201163200. [DOI] [PubMed] [Google Scholar]
- 37.Mohs A, Silva T, Yoshida T, Amin R, Lukomski S, Inouye M, Brodsky B. Mechanism of stabilization of a bacterial collagen triple helix in the absence of hydroxyproline. J Biol Chem. 2007;282(41):29757–65. doi: 10.1074/jbc.M703991200. [DOI] [PubMed] [Google Scholar]
- 38.Han R, Zwiefka A, Caswell CC, Xu Y, Keene DR, Lukomska E, Zhao Z, Hook M, Lukomski S. Assessment of prokaryotic collagen-like sequences derived from streptococcal Scl1 and Scl2 proteins as a source of recombinant GXY polymers. Appl Microbiol Biotechnol. 2006;72(1):109–15. doi: 10.1007/s00253-006-0387-5. [DOI] [PubMed] [Google Scholar]
- 39.Humtsoe JO, Kim JK, Xu Y, Keene DR, Hook M, Lukomski S, Wary KK. A streptococcal collagen-like protein interacts with the alpha2beta1 integrin and induces intracellular signaling. J Biol Chem. 2005;280(14):13848–57. doi: 10.1074/jbc.M410605200. [DOI] [PubMed] [Google Scholar]
- 40.Kim JK, Xu Y, Xu X, Keene DR, Gurusiddappa S, Liang X, Wary KK, Hook M. A novel binding site in collagen type III for integrins alpha1beta1 and alpha2beta1. J Biol Chem. 2005;280(37):32512–20. doi: 10.1074/jbc.M502431200. [DOI] [PubMed] [Google Scholar]
- 41.Knight CG, Morton LF, Onley DJ, Peachey AR, Messent AJ, Smethurst PA, Tuckwell DS, Farndale RW, Barnes MJ. Identification in collagen type I of an integrin alpha2 beta1-binding site containing an essential GER sequence. J Biol Chem. 1998;273(50):33287–94. doi: 10.1074/jbc.273.50.33287. [DOI] [PubMed] [Google Scholar]
- 42.Xu Y, Gurusiddappa S, Rich RL, Owens RT, Keene DR, Mayne R, Hook A, Hook M. Multiple binding sites in collagen type I for the integrins alpha1beta1 and alpha2beta1. J Biol Chem. 2000;275(50):38981–9. doi: 10.1074/jbc.M007668200. [DOI] [PubMed] [Google Scholar]
- 43.Gombotz WR, Wang GH, Horbett TA, Hoffman AS. Protein adsorption to poly(ethylene oxide) surfaces. Journal Of Biomedical Materials Research. 1991;25(12):1547–62. doi: 10.1002/jbm.820251211. [DOI] [PubMed] [Google Scholar]
- 44.Ivaska J, Reunanen H, Westermarck J, Koivisto L, Kahari VM, Heino J. Integrin alpha2beta1 mediates isoform-specific activation of p38 and upregulation of collagen gene transcription by a mechanism involving the alpha2 cytoplasmic tail. J Cell Biol. 1999;147(2):401–16. doi: 10.1083/jcb.147.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bulick AS, Munoz-Pinto DJ, Qu X, Mani M, Cristancho D, Urban M, Hahn MS. Impact of endothelial cells and mechanical conditioning on smooth muscle cell extracellular matrix production and differentiation. Tissue Eng Part A. 2009;15(4):815–25. doi: 10.1089/ten.tea.2008.0179. [DOI] [PubMed] [Google Scholar]
- 46.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13(12):4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Armstrong JK, Wenby RB, Meiselman HJ, Fisher TC. The hydrodynamic radii of macromolecules and their effect on red blood cell aggregation. Biophys J. 2004;87(6):4259–70. doi: 10.1529/biophysj.104.047746. Epub 2004 Sep 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hahn MS, McHale MK, Wang E, Schmedlen RH, West JL. Physiologic pulsatile flow bioreactor conditioning of poly(ethylene glycol)-based tissue engineered vascular grafts. Ann Biomed Eng. 2007;35(2):190–200. doi: 10.1007/s10439-006-9099-3. Epub 2006 Dec 16. [DOI] [PubMed] [Google Scholar]
- 49.Buxton AN, Zhu J, Marchant R, West JL, Yoo JU, Johnstone B. Design and characterization of poly(ethylene glycol) photopolymerizable semi-interpenetrating networks for chondrogenesis of human mesenchymal stem cells. Tissue Eng. 2007;13(10):2549–60. doi: 10.1089/ten.2007.0075. [DOI] [PubMed] [Google Scholar]
- 50.Munoz-Pinto DJ, Jimenez-Vergara AC, Gelves LM, McMahon RE, Guiza-Arguello V, Hahn MS. Probing vocal fold fibroblast response to hyaluronan in 3D contexts. Biotechnol Bioeng. 2009;104(4):821–31. doi: 10.1002/bit.22436. [DOI] [PubMed] [Google Scholar]
- 51.Munoz-Pinto DJ, Bulick AS, Hahn MS. Uncoupled investigation of scaffold modulus and mesh size on smooth muscle cell behavior. J Biomed Mater Res A. 2009;90(1):303–16. doi: 10.1002/jbm.a.32492. [DOI] [PubMed] [Google Scholar]
- 52.Gregory TR. Nucleotypic effects without nuclei: genome size and erythrocyte size in mammals. Genome. 2000;43(5):895–901. doi: 10.1139/g00-069. [DOI] [PubMed] [Google Scholar]
- 53.Yamada KM. Integrin signaling. Matrix Biol. 1997;16(4):137–41. doi: 10.1016/s0945-053x(97)90001-9. [DOI] [PubMed] [Google Scholar]
- 54.Assoian RK, Schwartz MA. Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr Opin Genet Dev. 2001;11(1):48–53. doi: 10.1016/s0959-437x(00)00155-6. [DOI] [PubMed] [Google Scholar]
- 55.Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol. 2002;4(4):E65–8. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- 56.Pozzi A, Wary KK, Giancotti FG, Gardner HA. Integrin a1b1 mediates a unique collagen-dependent proliferation pathway in vivo. Journal of Cell Biology. 1998;142(2):587–594. doi: 10.1083/jcb.142.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiu L-H, Chen S-C, Wu K-C, Yang C-B, Fang C-L, Lai W-FT, Tsai Y-H. Differential effect of ECM molecules on re-expression of cartilaginous markers in near quiescent human chondrocytes. Journal of Cellular Physiology. 2011;226(8):1981–1988. doi: 10.1002/jcp.22530. [DOI] [PubMed] [Google Scholar]
- 58.Heino J. The collagen receptor integrins have distinct ligand recognition and signaling functions. Matrix Biol. 2000;19(4):319–23. doi: 10.1016/s0945-053x(00)00076-7. [DOI] [PubMed] [Google Scholar]
- 59.Sawai H, Okada Y, Funahashi H, Matsuo Y, Takahashi H, Takeyama H, Manabe T. Activation of focal adhesion kinase enhances the adhesion and invasion of pancreatic cancer cells via extracellular signal-regulated kinase-1/2 signaling pathway activation. Mol Cancer. 2005;4:37. doi: 10.1186/1476-4598-4-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Slambrouck S, Grijelmo C, De Wever O, Bruyneel E, Emami S, Gespach C, Steelant WF. Activation of the FAK-src molecular scaffolds and p130Cas-JNK signaling cascades by alpha1-integrins during colon cancer cell invasion. Int J Oncol. 2007;31(6):1501–8. [PubMed] [Google Scholar]
- 61.Sanders MA, Basson MD. Collagen IV-dependent ERK activation in human Caco-2 intestinal epithelial cells requires focal adhesion kinase. J Biol Chem. 2000;275(48):38040–7. doi: 10.1074/jbc.M003871200. [DOI] [PubMed] [Google Scholar]
- 62.Klekotka PA, Santoro SA, Zutter MM. alpha 2 integrin subunit cytoplasmic domain-dependent cellular migration requires p38 MAPK. J Biol Chem. 2001;276(12):9503–11. doi: 10.1074/jbc.M006286200. [DOI] [PubMed] [Google Scholar]
- 63.Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268(5208):233–9. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 64.Yamada KM, Miyamoto S. Integrin transmembrane signaling and cytoskeletal control. Curr Opin Cell Biol. 1995;7(5):681–9. doi: 10.1016/0955-0674(95)80110-3. [DOI] [PubMed] [Google Scholar]
- 65.Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10(2):220–31. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- 66.Popov C, Radic T, Haasters F, Prall WC, Aszodi A, Gullberg D, Schieker M, Docheva D. Integrins alpha2beta1 and alpha11beta1 regulate the survival of mesenchymal stem cells on collagen I. Cell Death Dis. 2011;28(2):71. doi: 10.1038/cddis.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shih Y-RV, Tseng K-F, Lai H-Y, Lin C-H, Lee OK. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. Journal of Bone and Mineral Research. 2011;26(4):730–738. doi: 10.1002/jbmr.278. [DOI] [PubMed] [Google Scholar]
- 68.Jaiswal RK, Jaiswal N, Bruder SP, Mbalaviele G, Marshak DR, Pittenger MF. Adult human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage Is regulated by mitogen-activated protein kinase. J Biol Chem. 2000;275:9645–9652. doi: 10.1074/jbc.275.13.9645. [DOI] [PubMed] [Google Scholar]
- 69.Hwang J-H, Byun MR, Kim AR, Kim KM, Cho HJ, Lee YH, Kim J, Jeong MG, Hwang ES, Hong J-H. Extracellular matrix stiffness regulates osteogenic differentiation through MAPK activation. PLoS ONE. 2015;10(8):e0135519. doi: 10.1371/journal.pone.0135519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kundu AK, Putnam AJ. Vitronectin and collagen I differentially regulate osteogenesis in mesenchymal stem cells. Biochem Biophys Res Commun. 2006;347(1):347–57. doi: 10.1016/j.bbrc.2006.06.110. [DOI] [PubMed] [Google Scholar]
- 71.Li C-S, Zheng Z, Su X-X, Wang F, Ling M, Zou M, Zhou H. Activation of the extracellular signal-regulated kinase signaling is critical for human umbilical cord mesenchymal stem cell osteogenic differentiation. BioMed Research International. 2016;2016:10. doi: 10.1155/2016/3764372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gronthos S, Simmons PJ, Graves SE, Robey PG. Integrin-mediated interactions between human bone marrow stromal precursor cells and the extracellular matrix. Bone. 2001;28(2):174–81. doi: 10.1016/s8756-3282(00)00424-5. [DOI] [PubMed] [Google Scholar]
- 73.Reyes CD, Petrie TA, Burns KL, Schwartz Z, García AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007;28(21):3228–3235. doi: 10.1016/j.biomaterials.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mizuno M, Fujisawa R, Kuboki Y. Type I collagen-induced osteoblastic differentiation of bone-marrow cells mediated by collagen-alpha2beta1 integrin interaction. J Cell Physiol. 2000;184(2):207–13. doi: 10.1002/1097-4652(200008)184:2<207::AID-JCP8>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 75.Suzuki A, Guicheux J, Palmer G, Miura Y, Oiso Y, Bonjour JP, Caverzasio J. Evidence for a role of p38 MAP kinase in expression of alkaline phosphatase during osteoblastic cell differentiation. Bone. 2002;30(1):91–8. doi: 10.1016/s8756-3282(01)00660-3. [DOI] [PubMed] [Google Scholar]
- 76.Noth U, Tuli R, Seghatoleslami R, Howard M, Shah A, Hall DJ, Hickok NJ, Tuan RS. Activation of p38 and Smads mediates BMP-2 effects on human trabecular bone-derived osteoblasts. Exp Cell Res. 2003;291(1):201–11. doi: 10.1016/s0014-4827(03)00386-0. [DOI] [PubMed] [Google Scholar]
- 77.Gallea S, Lallemand F, Atfi A, Rawadi G, Ramez V, Spinella-Jaegle S, Kawai S, Faucheu C, Huet L, Baron R, Roman-Roman S. Activation of mitogen-activated protein kinase cascades is involved in regulation of bone morphogenetic protein-2-induced osteoblast differentiation in pluripotent C2C12 cells. Bone. 2001 May;28(5):491–8. doi: 10.1016/s8756-3282(01)00415-x. [DOI] [PubMed] [Google Scholar]
- 78.Ortuno MRuiz-Gaspa S, Rodriguez-Carballo E, Susperregui AR, Bartrons R, Rosa JL, Ventura F. p38 regulates expression of osteoblast-specific genes by phosphorylation of osterix. J Biol Chem. 2010;285(42):31985–94. doi: 10.1074/jbc.M110.123612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thouverey C, Caverzasio J. The p38alpha MAPK positively regulates osteoblast function and postnatal bone acquisition. Cell Mol Life Sci. 2012;69(18):3115–2. doi: 10.1007/s00018-012-0983-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodríguez-Carballo E, Gámez B, Ventura F. p38 MAPK Signaling in Osteoblast Differentiation. Frontiers in Cell and Developmental Biology. 2016;4:40. doi: 10.3389/fcell.2016.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Doan T, Park K, Kim H, Park D, Kim J, Yoon T. Inhibition of JNK and ERK pathways by SP600125- and U0126-enhanced osteogenic differentiation of bone marrow stromal cells. Tissue Engineering and Regenerative Medicine. 2012;9(6):283–294. [Google Scholar]
- 82.Greenblatt MB, Shim J-H, Zou W, Sitara D, Schweitzer M, Hu D, Lotinun S, Sano Y, Baron R, Park JM, et al. The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. The Journal of Clinical Investigation. 2010;120(7):2457–2473. doi: 10.1172/JCI42285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiao G, Wang D, Benson MD, Karsenty G, Franceschi RT. Role of the alpha2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. J Biol Chem. 1998;273(49):32988–94. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- 84.Lal H, Verma SK, Foster DM, Golden HB, Reneau JC, Watson LE, Singh H, Dostal DE. Integrins and proximal signaling mechanisms in cardiovascular disease. Front Biosci. 2009;14:2307–34. doi: 10.2741/3381. [DOI] [PubMed] [Google Scholar]
- 85.Delorme B, Ringe J, Gallay N, Le Vern Y, Kerboeuf D, Jorgensen C, Rosset P, Sensebé L, Layrolle P, Häupl T, Charbord P. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood. 2008;111:2631–2635. doi: 10.1182/blood-2007-07-099622. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


