Abstract
Purpose:
Bronchoalveolar fluid (BALF) and plasma biomarkers are often endpoints in early phase randomized trials (RCTs) in acute respiratory distress syndrome (ARDS). With ARDS mortality decreasing, we analyzed baseline biomarkers in samples from contemporary ARDS patients participating in a prior RCT and compared these to historical controls.
Materials and Methods:
Ninety ARDS adult patients enrolled in the parent trial. BALF and blood were collected at baseline, day 4±1, and day 8±1. Interleukins−8/−6/−1β/−1 receptor antagonist/−10; granulocyte colony stimulating factor; monocyte chemotactic protein-1; tumor necrosis factor-α; surfactant protein-D; vonWillebrand factor; leukotriene B4; receptor for advanced glycosylation end products; soluble Fas ligand; and neutrophil counts were measured.
Results:
Compared to historical measurements, our values were generally substantially lower, despite our participants being similar to historical controls. For example, our BALF IL-8 and plasma IL-6 were notably lower than in a 1999 RCT of low tidal volume ventilation and a 2007 biomarker study, respectively.
Conclusions:
Baseline biomarker levels in current ARDS patients are substantially lower than 6–20 years before collection of these samples. These findings, whether from ICU care changes resulting in less inflammation or from variation in assay techniques over time, have important implications for design of future RCTs with biomarkers as endpoints.
Keywords: Acute respiratory distress syndrome (ARDS), Inflammatory mediators, Respiratory failure, Critical illness, Bronchoalveolar lavage, Plasma
INTRODUCTION
The incidence of the acute respiratory distress syndrome (ARDS) in the US is approximately 200,000 cases annually, and the current case fatality is 30–40%, but has been as high as 60% historically.(Rubenfeld et al., 2005, Stapleton et al., 2005, Maca et al., 2017) Although low tidal volume ventilation and prone positioning improve survival, there are no other known treatments that reduce ARDS mortality, and therefore there is an ongoing need for trials of novel therapeutic agents.(Network, 2000, Guerin et al., 2013)
ARDS pathogenesis is thought to result from massive activation of the proinflammatory response, with release of multiple mediators of inflammation and injury into the alveolar space and the bloodstream.(Ware and Matthay, 2000, Matthay and Zemans, 2011) Increased levels of many of these biomarkers, including interleukin (IL)-6, IL-8, tumor necrosis factor (TNF)-α, are not only present in patients with ARDS but are associated with greater mortality.(Donnelly et al., 1996, Goodman et al., 1996, Ranieri et al., 1999, Ware et al., 2001, Goodman et al., 2003, Bhatia and Moochhala, 2004, Krupa et al., 2004, Parsons et al., 2005, Mukhopadhyay et al., 2006, Allen and Kurdowska, 2014, Aisiku et al., 2016) Furthermore, plasma IL-8 and IL-6 have been found to be reduced in patients receiving lung protective ventilation compared to higher tidal volumes.(Parsons et al., 2005) Therefore, several prior early phase trials of novel therapies in ARDS have utilized bronchoalveolar fluid (BALF) or plasma biomarker measurements as endpoints.(Gadek et al., 1999, Ranieri et al., 1999, Stapleton et al., 2011)
We conducted a phase II randomized controlled trial (RCT) of fish oil versus placebo in patients with ARDS that did not find evidence of efficacy.(Stapleton et al., 2011) This RCT utilized a few selected BALF and plasma biomarkers as endpoints, and power calculations for the trial were performed using historical controls (namely, two 1999 phase II RCTs comparing lung protective versus conventional ventilation and an enteral formula containing fish oil versus a control high fat enteral formula, respectively, where BALF sampling had occurred).(Gadek et al., 1999, Ranieri et al., 1999, Pacht et al., 2003) We found not only that the baseline BALF concentrations of those selected biomarkers in our recent RCT were notably lower than the historical controls receiving lung protective ventilation in the 1999 study,(Ranieri et al., 1999) but also that plasma concentrations of key inflammatory mediators Il-6 and IL-8 were lower than a study measuring plasma cytokines from samples obtained from patients participating in the NIH ARDS Network RCT of low tidal volume versus conventional ventilation.(Parsons et al., 2005) We hypothesized that these decreased levels may be explained by various processes of care leading to reduced lung inflammation in patients with ALI, especially in the era of low tidal volume ventilation.
Because information on BALF and plasma biomarkers may be critical for the design of phase II RCTs in ARDS patients, we therefore measured additional biomarkers of inflammation and injury in samples obtained during this trial so that these data from a more contemporary cohort of patients with ARDS could be publicly reported and made available to the scientific community for future use. Additionally, we compared our findings to historical controls.
MATERIALS AND METHODS
Participants and Study Design
This study used BALF and plasma samples obtained from 90 patients with ARDS during a phase II RCT of fish oil versus placebo (NCT00351533); a detailed description of that study has been published.(Stapleton et al., 2011) Because that RCT did not find any differences between the intervention and placebo groups, we report measurements here by combining the entire study population. Briefly, adult participants meeting acute lung injury (ALI) criteria as defined by the American-European Consensus Conference (AECC) (Bernard et al., 1994) (ratio of partial pressure of arterial oxygen to fraction of inspired oxygen [PaO2/FiO2] ≤300, chest radiograph demonstrating bilateral infiltrates consistent with pulmonary edema, and no evidence of left heart failure causing the infiltrates) were enrolled from 2006–2008 at 5 North American hospitals. Potential participants were excluded if they 1) had met ARDS criteria for ≥ 48 hrs; 2) had an expected ICU length of stay ≤48 hrs or expected survival ≤ 28 days from an underlying pre-ICU condition; 3) were unable to undergo BAL at enrollment (PaO2/FiO2 <80 on 100% FiO2, endotracheal tube too small, marked cardiovascular instability, or intracranial pressure ≥20mmHg); 4) were not able to receive enteral access; 5) were pregnant; 6) were receiving recombinant human activated protein C (rh-APC); 7) had received an enteral formula containing Ω−3 fatty acids during the ICU stay; or 8) had any of the following conditions: metastatic cancer, AIDS with CD4 < 200, post-cardiac arrest with suspected significant anoxic brain injury, past bone marrow or solid organ transplant (lung, heart, liver, pancreas, or kidney), platelets < 30,000/mm3, active bleeding, International normalized ratio (INR) > 3.0, history of ventricular tachycardia or fibrillation, or known allergy to fish. The research was approved by human subjects committees at all enrolling sites.
After informed consent and within 48 hours of ARDS onset, participants were randomized to receive enteral fish oil or 0.9% saline. Baseline demographic, laboratory, and physiologic data were recorded, and all subjects were ventilated according to local protocols mimicking the ARDS Network low tidal volume ventilation protocol.(Network, 2000)
Procedures
BALF and plasma were obtained at study entry (day 0) and on days 4±1 and 8±1. The 2nd and 3rd BALs did not occur if participants were extubated or were clinically unstable (PaO2/FiO2 <80 on 100% FiO2, hypotensive with systolic blood pressure <90mmHg, ongoing or new uncontrolled dysrhythmia, acute coronary ischemia, or intracranial pressure ≥20mmHg) for bronchoscopy. BAL was performed by passing a fiberoptic bronchoscope through the endotracheal tube. Patients were preoxygenated with 100% oxygen for 15 minutes before and during the procedure. After the bronchoscope was wedged in the right middle lobe or lingula (unless both areas were frankly purulent on inspection in which case another segment was selected), five separate 30 mL aliquots (total of 150 mL) of normal saline were instilled and recovered by hand-suction in all participants.
Measurements
The following biomarkers were measured in both BALF and plasma: IL-8, IL-6, IL-1β, IL-1 receptor antagonist (IL-1ra), IL-10, granulocyte colony stimulating factor (G-CSF), monocyte chemotactic protein-1 (MCP-1), TNF-α, surfactant protein-D (SP-D), vonWillebrand factor (vWF), and leukotriene B4 (LTB4). Neutrophil counts (PMNs) were also performed on BALF samples. BALF IL-8 was the primary endpoint for the parent RCT. Other selected biomarkers were chosen a priori as secondary endpoints for the RCT (BALF IL-6, MCP-1, LTB4, and PMNs; and plasma IL-8, IL-6, SP-D, vWF, and LTB4), and these results have been reported previously in the main RCT manuscript.(Stapleton et al., 2011) The results of other biomarkers (BALF IL-1 β, IL-1ra, IL-10, G-CSF, SP-D, vWF; and TNF-α; and plasma G-CSF, IL-1β, IL-1ra, IL-10, MCP-1, and TNF- α) have not been reported previously.
Each biomarker was measured based on specific biologic rationale. Interleukin (IL)-8 is a neutrophil chemoattractant(Oppenheim et al., 1991, Goodman et al., 2003) and is decreased in the BALF of patients receiving low tidal volume ventilation,(Ranieri et al., 1999) an intervention known to improve ARDS.(Network, 2000) IL-6 activates leukocytes, affects the acute phase response, and levels correlate with severity of ARDS.(Bhatia and Moochhala, 2004) LTB4 is also a potent neutrophil chemoattractant.(Martin et al., 1989, Peters-Golden and Henderson, 2007) MCP-1 is a monocyte chemoattractant, and increasing levels have been associated with both worse lung injury and persistent inflammation in ARDS.(Goodman et al., 1996, Bautista et al., 2013) SP-D is a biomarker of alveolar epithelial injury and vWF is a marker of endothelial activation and injury; both have been associated with increased mortality in ARDS.(Ware et al., 2001, Eisner et al., 2003, Calfee et al., 2007) G-CSF may play a role in ARDS pathogenesis and baseline levels are associated with mortality in a bimodal fashion.(Suratt et al., 2009) IL-1β stimulates production of other inflammatory cytokines and plays a prominent role in the early and fibroproliferative phases of ARDS,(Goodman et al., 2003) and increased levels of IL-1β in BALF of ARDS patients are also associated with death.(Goodman et al., 1996) Low concentrations of IL-1ra in BALF from ARDS patients are associated with increased mortality.(Donnelly et al., 1996) TNF-α plays a key role in the development and progression of inflammation in ARDS.(Goodman et al., 1996, Mukhopadhyay et al., 2006) Increased plasma levels of the anti-inflammatory cytokine IL-10 are associated with death in ARDS.(Parsons et al., 1997, Parsons et al., 2005) RAGE is a marker of alveolar type I cell injury and increasing baseline levels are strongly associated with greater mortality in patients with ARDS.(Calfee et al., 2008) Finally, sFasL is involved in the regulation of epithelial cell apoptosis, is released into the airspaces of patients with ARDS, and higher BALF concentrations are significantly associated with death.(Matute-Bello et al., 1999, Lee et al., 2008)
BAL and plasma samples were transported to the laboratory immediately for analysis. BALF was filtered through sterile 100 μM filters to remove mucus. Total cell counts were performed in a hemocytometer, and differential cell counts were performed on cytospin preparations using standard techniques.(Sittipunt et al., 2001) The remaining BAL fluid was spun at 200 × g for 30 min, and the supernatant was removed and stored at −80°C.
Inflammatory biomarker assays were performed in duplicate on stored BALF and plasma samples in a central research laboratory in Seattle, Washington. Concentrations of IL-8, IL-6, IL-1β, IL-1ra, IL-10, TNF-α, G-CSF, and MCP-1 were determined using Luminex bead-based immunoassays (R&D Systems).(Haegens et al., 2007) With these assays, small amounts of sample are added to color-coded beads that are pre-coated with antibodies against each analyte of interest. Biotinylated detection antibodies against each analyte are then added, followed by phycoerythrin-conjugated streptavidin, creating an antibody-antigen sandwich. This mixture is then passed through a laser flow-based detection instrument to detect the color-coded beads and quantify the magnitude of the signal.
BAL and plasma for LTB4 analysis were treated with 4 volumes of methanol with formic acid, and LTB4 was measured by enzyme immunoassay (Cayman Chemical) after solid phase extraction on an Oasis HLB column (Waters, Milford, Mass).(Debley et al., 2007) SP-D (BioVendor), vWF (Diagnostica Stago), and RAGE and sFasL (R&D Systems) were all measured by standard enzyme-linked immunosorbent assays.
RESULTS
Participants and Baseline Characteristics
Ninety participants were enrolled in the parent RCT.(Stapleton et al., 2011) One patient was extubated before the baseline BAL was performed, leaving 89 baseline BALF samples (Figure 1). As BALs were not performed in extubated patients, and no samples were obtained from patients who had either died or were discharged, the number of BALF and plasma samples obtained on days 4±1 (n=66) and 8±1 (n=42) appropriately decreased. Hospital mortality in the trial was 21.1%.
Figure 1.
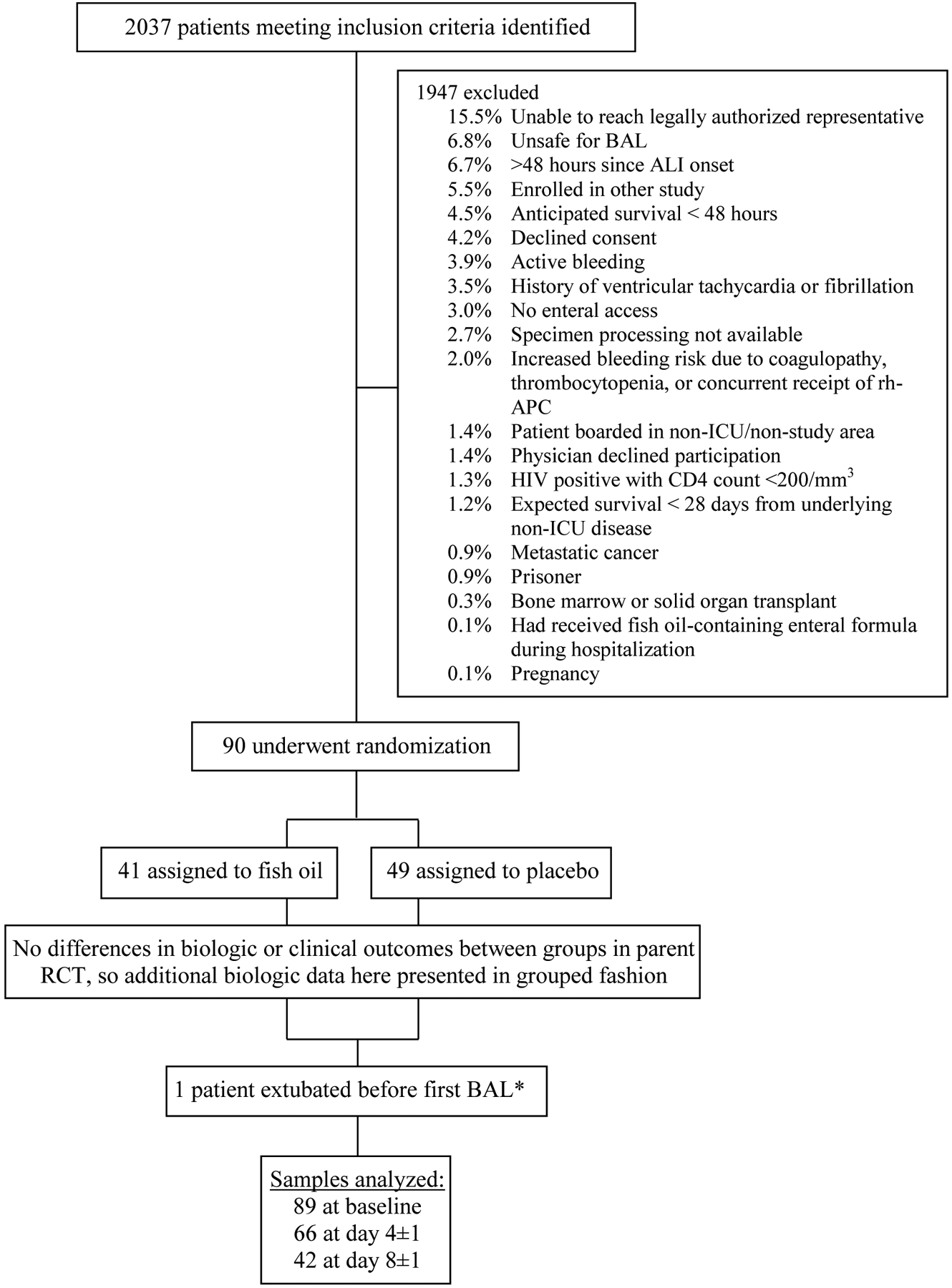
Diagram of Participant Flow and Sample Collection in Parent RCT. Patients may have had more than one reason for exclusion. *Baseline biologic available on 40 participants in the fish oil group and all 49 patients in placebo group.
Demographic and physiologic characteristics of the 90 participants are demonstrated in Table 1. Average age of participants was approximately 50 years and the majority were Caucasian men. Trauma, shock, aspiration, and sepsis were the most common ARDS risk factors. This population was quite ill with an average APACHE II score of 21.8.(Knaus et al., 1985)
Table 1.
Baseline Participant Characteristics
| n=90 | |
| Age (years)a | 49.9±16.4 |
| Male gender, n (%) | 57 (63.3) |
| Race, n (%) | |
| African-American | 3 (3.3) |
| American Indian | 2 (2.2) |
| Asian | 2 (2.2) |
| Pacific Islander | 3 (3.3) |
| White | 80 (88.9) |
| Ethnicity, n (%) | |
| Hispanic | 3 (3.3) |
| Non-Hispanic | 84 (93.3) |
| Unknown | 3 (3.3) |
| ALI risk factor, n (%) (not mutually exclusive) | |
| Trauma | 36 (40.0) |
| Shock | 36 (40.0) |
| Aspiration | 17 (18.9) |
| Sepsis | 14 (15.6) |
| Pneumonia | 12 (13.3) |
| Massive transfusion | 2 (2.2) |
| Moderate transfusion | 8 (8.9) |
| Overdose | 3 (3.3) |
| Near drowning | 1 (1.1) |
| Inhalation injury | 1 (1.1) |
| Admission Diagnosis, n (%) | |
| Trauma | 34 (37.8) |
| Sepsis | 13 (14.4) |
| Pneumonia | 9 (10.0) |
| Respiratory failure | 9 (10.0) |
| ALI/ARDS | 5 (5.6) |
| Intracranial hemorrhage | 5 (5.6) |
| Other | 15 (16.7) |
| APACHE II Scorea | 21.8±16.4 |
| PaO2/FiO2a | 164.4±58.2 |
| Tidal volume (mL/kg predicted body weight)a | 7.2±1.7 |
| Plateau pressure (cm H20)a | 24.6±5.5 |
| PEEP (cm H20)a | 9.1±4.0 |
| Lung Injury Score(Murray et al., 1988) a | 2.6±0.6 |
Data presented as mean ± standard deviation
Abbreviations: ALI = acute lung injury; ARDS = acute respiratory distress syndrome; APACHE = Acute Physiologic and Chronic Health Evaluation; PaO2/FiO2 = ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen; PEEP = positive end expiratory pressure.
Biomarker Results
Results of BALF and plasma biomarker measurements are shown in Table 2, as mean ± standard deviation (SD). Even though most biomarker values are non-normally distributed, we chose not to depict them as median and intraquartile range because power calculations are much easier to perform with mean ± standard deviation. As indicated by the large standard deviations, variability was quite high for many of the biomarkers. In general, biomarker concentrations tended to decrease over the 3 sampling times, as would be expected.
Table 2.
Bronchoalveolar Lavage Fluid and Plasma Biomarker Measurements
| Biomarker* (mean±SD) |
Site | Study Entry (baseline) n=89 |
Day4±1 n=66 |
Day 8±1 n=42 |
|---|---|---|---|---|
| G-CSF | BALF | 611.6± 1967.3 | 173.2±349.8 | 168.2±360.1 |
| Plasma | 2213.6±14658.4 | 209.4±1472.1 | 27.8±33.9 | |
| IL-1β | BALF | 238.5±1370.4 | 193.2±838.4 | 301.8±736.2 |
| Plasma | 9.0±1.8 | 8.7±0.4 | 8.8±0.01 | |
| IL-1ra | BALF | 3905.6±7336.4 | 3682.3±7395.1 | 2909.7±5006.3 |
| Plasma | 6727.0±17427.5 | 3799.9±6193.0 | 3189.9±3077.7 | |
| IL-6 | BALF | 796.0±2320.5 | 245.6±753.8 | 151.7±353.6 |
| Plasma | 403.5±1829.8 | 103.1±407.5 | 48.9±57.4 | |
| IL-8 | BALF | 2407.6±8870.3 | 1909.9±3687.7 | 3511.9±8116.9 |
| Plasma | 86.1±419.2 | 42.0±l 12.5 | 43.7±133.9 | |
| IL-10 | BALF | 7.4±13.4 | 4.8±5.0 | 10.6±38.9 |
| Plasma | 39.6±159.2 | 16.2±13.7 | 15.0±7.5 | |
| LTB4 | BALF | 126.9±496.2 | 37.6±43.5 | 71.0±108.5 |
| Plasma | 47.6±61.2 | 41.3±25.9 | 44.5±38.7 | |
| MCP-1 | BALF | 1122.9±2094.4 | 643.2±1259.5 | 333.7±523.4 |
| Plasma | 318.8±333.7 | 205.5±180.8 | 163.7±115.5 | |
| PMNs† | BALF | 52.8±31.5 | 50.2±31.0 | 41.7±34.8 |
| Plasma | 52.8±31.5 | 47.3±32.3 | 40.7±35.0 | |
| RAGE | BALF | 14509.2±51201.2 | 1237.2±2958.8 | 451.0±510.7 |
| Plasma | 386.8±630.9 | 170.0±202.4 | 108.4±150.1 | |
| sFasL | BALF | 39.3±2.0 | 40.1±5.7 | 40.1 ±4.7 |
| Plasma | 129.1±381.2 | 60.2±31.1 | 56.2±28.9 | |
| SP-D | BALF | 371.4±414.9 | 312.7±366.6 | 252.7±572.0 |
| Plasma | 186.9±253.9 | 230.4±201.5 | 166.2±124.8 | |
| TNF-α | BALF | 17.3±55.1 | 23.0±80.9 | 14.1±28.6 |
| Plasma | 15.4±2.0 | 15.2±2.8 | 15.0±2.2 | |
| vWF‡ | BALF | 0.8±1.5 | 0.3±0.6 | 0.2±0.4 |
| Plasma | 453.0±1058.2 | 524.3±686.8 | 694.7±881.5 |
Data are presented as mean ± standard deviation in pg/mL, except PMN
data are presented as % of total cells, and vWF
data are presented as % control.
DISCUSSION
This study reports concentrations of BALF and plasma biomarkers of inflammation and injury in a contemporary population of patients with ARDS who were cared for in an era in which low tidal volume ventilation is standard, and other process of care that could reduce inflammation are also common. The data both improve our understanding of the alveolar and circulating inflammatory milieu in today’s patients with ARDS and should also be useful to the scientific community when performing power calculations for future early phase studies of ARDS.
In general, our results indicate decreased baseline levels of alveolar and circulating inflammation when compared to results of prior studies (see comparisons in Table 3), all of which defined ALI according to the 1994 AECC definition (Bernard et al., 1994). BALF concentrations of the early response cytokine IL-1β in our study are approximately 40-fold lower than those measured in a population of ARDS patients from 1988–1991(Goodman et al., 1996). Patients in this prior study by Goodman, et al. were similar to those in our study, as approximately 60% were men, mean age was 45.5 years, APACHE II score was 21.6±7.2, and trauma and sepsis were the ALI risk factor in 39.1% and 34.8%, respectively. BALF IL-1β in our study was also 4-fold lower than values from a 1999 phase II RCT of low tidal volume ventilation (Ranieri et al., 1999), where 61% were men, mean age was 51 years, mean APACHE II Score was 15, and trauma and sepsis were the ALI risk factor in 44.4% and 22.2%, respectively. Alveolar levels of its naturally occurring antagonist, IL-1ra, of which decreased levels are associated with higher mortality, were substantially increased in our study compared with a small cohort of 28 patients with ARDS in the mid-1990s (Donnelly et al., 1996) where mean age was 53 years old and sepsis was the ARDS risk factor in 79% of patients. Our study found concentrations of IL-6, IL-8, and TNF-α in BALF were reduced by several fold compared to levels in both the intervention and control groups in the 1999 phase II RCT of low tidal volume versus conventional mechanical ventilation in 37 patients with ARDS described above.(Ranieri et al., 1999) Plasma concentrations of IL-8 and TNF-α, but not IL-6, were also lower in our trial than in that same 1999 RCT. Plasma IL-6 concentrations in our study, however, were lower than those measured in a more recent study comparing levels of inflammatory mediators in trauma and non-trauma patients with ARDS.(Calfee et al., 2007) This study analyzed samples from two large NIH NHLBI ARDS Network trials (Network, 2000, Brower et al., 2004). Some characteristics of this large cohort were similar to our study including mean age of approximately 50 years, 59% women, and mean APACHE III score of approximately 85. However, only 9.7% of patients in the Calfee et al. 2007 study had trauma as their ALI risk factor, compared with 40% in our study. Our BALF concentrations of LTB4 and TNF-α were also lower than a 1999 phase II RCT (n=146) of an enteral feeding formula containing fish oil versus another high fat enteral formula in patients with ARDS.(Gadek et al., 1999), where the n=98 participants had a mean age of 51 years, 47% were women, and 23.5% had trauma as their ALI risk factor. Increased plasma IL-10 is associated with greater mortality, and our plasma levels were lower than a 1997 cohort of 77 ARDS patients (Parsons et al., 1997), who were approximately 40 years old, had APACHE II of 11.0, and 31.6% had trauma as their risk factor. Plasma SP-D levels in our study are also several fold lower than the recent Calfee, et al. 2007 study described above that analyzed samples from two large ARDS Network RCTs of patients with ARDS conducted between 1996 and 2002.(Network, 2000, Brower et al., 2004, Calfee et al., 2007) Similarly, we found that concentrations of plasma G-CSF were four- to five-fold lower than values in another recent study that measured G-CSF in samples from the same two large RCTs (Suratt et al., 2009); participants in this study were a mean of 53 years old, 40% women, 31% sepsis as ALI risk factor, and had mean APACHE III score of 76. Baseline levels of plasma RAGE in our study were more than 10-fold lower than levels obtained from participants in the NIH ARDS Network RCT of low tidal volume ventilation who were randomized to the 6cc/kg group.(Network, 2000, Calfee et al., 2008) Finally, compared with a 1999 study analyzing plasma sFasL in patients with ARDS (mean age 42.7 years, 68.8% men, 37.8% trauma, mean APACHE II score 22.4), our values were approximately 30% lower.(Matute-Bello et al., 1999) Interestingly, our concentrations of plasma vWF in our study were slightly higher than in 2 prior studies in the literature.(Ware et al., 2001, Calfee et al., 2007) The Calfee 2007 study has been described above. The Ware 2001 study included 51 patients with ALI who were 51% men, mean age 48 years, and had a SAPS II score of 49±15 (Le Gall et al., 1993); none of the participants in this study had trauma as their ALI risk factor. Taken as a whole, the baseline characteristics of historical controls from many of these studies are quite similar to those in our study, although some cohorts had varying gender and ALI risk factor proportions.
Table 3.
Comparison of Our Baseline BALF and Plasma Biomarker Concentrations with Prior Studies
| Biomarker* | Site | Baseline concentrations from prior studies§ | Mean Baseline Concentration in Our Study mean±SD, n=89 | Median Baseline Concentration in Our Study Median (IQR), n=89 | |
|---|---|---|---|---|---|
| Author | Concentration | ||||
| GCSF | Plasma | Suratt (2009) | Survivors: 188(80–540) Non-survivors: 207 (74–887) |
2213.6±14658.4 | 46.5 (20.9–122.7) |
| IL-1β | BALF | Park (2001) | 9.8 (4.4–18.9) | 238.5±1370.4 | 13.8 (2.6–104.9) |
| Goodman (1996) | Mean ~ 10,000 | ||||
| Ranieri (1999) | Mean ~900 | ||||
| IL-1ra | BALF | Park (2001) | 1829.2 (1124.3–6868.3) | 3905.6±7336.4 | 1729.9 (593.6–3214.0) |
| Donnelly (1996) | 820 (0–18,900) | ||||
| Ranieri (1999) | 16,503±14,935 | ||||
| Plasma | Parsons (1997) | ~15,000 (1,000–38,000) | 6727.0±17427.5 | 2380.4 (1313.4–4754.6) | |
| Ranieri (1999) | 4,218±3,645 | ||||
| IL-6 | BALF | Park (2001) | 1229.5 (263.3–3232.6) | 796.0±2320.5 | 145.3 (33.5–455.9) |
| Gadek (1999) | 282±145 | ||||
| Ranieri (1999) | Mean ~9,000 | ||||
| Plasma | Calfee (2007) | Trauma: 300 (128–674) Non-trauma: 259 (100–850) |
403.5±1829.8 | 81.7 (40.9–172.0) | |
| Ranieri (1999) | Mean −200 | ||||
| IL-8 | BALF | Goodman (1996) | Mean ~ 400 | 2407.6±8870.3 | 486.4 (253.5–1390.6) |
| Gadek (1999) | 2849±1458 | ||||
| Ranieri (1999) | Mean ~10,000 | ||||
| Plasma | Calfee (2007) | Trauma: 21 (20–59) Non-trauma: 44 (20–103) |
86.1±419.2 | 19.9 (19.8–35.2) | |
| Ranieri (1999) | Mean ~100 | ||||
| IL-10 | BALF | Park (2001) | 4.8 (1.5–9.3) | 7.4±13.4 | 3.6 (3.6–3.6)ll |
| Donnelly (1996) | 100 (0–1600) | ||||
| Plasma | Parsons (1997) | ~125,000 (50,000–300,000) | 39.6±159.2 | 14.3 (14.3–14.3)†† | |
| LTB4 | BALF | Gadek (1999) | 596±495.2 | 126.9±496.2 | 20.5 (9.3–31.3) |
| MCP-1 | BALF | Goodman (1996) | Mean ~ 1000 | 1122.9±2094.4 | 534.7 (185.2–1318.5) |
| PMNs† | BALF | Ranieri (1999) | Mean ~ 75 | 52.8±31.5 | 54.0 (25.5–82.8) |
| RAGE | Plasma | Calfee (2008) | Survivors: ~3900 (2000–8000) Non-survivors: ~5500 (2500–10,000) |
386.8±630.9 | 261.5 (137.6–438.4) |
| sFasL | BALF | Matute-Bello (1999) | Mean ~ 150 | 39.3±2.0 | 39.0 (39.0–39.0)ll |
| SP-D | Plasma | Calfee (2007) | Trauma: 58,000 (45,000–78,000) Non-trauma: 95,000 (45,000–211,000) |
186.9±253.9 | 122.4 (56.0–205.6) |
| TNF-α | BALF | Ranieri (1999) | Mean~750 | 17.3±55.1 | 3.8 (3.8–7.9)** |
| Plasma | Ranieri (1999) | Mean ~1,000 | 15.4±2.0 | 15.4 (15.4–15.4)†† | |
| vWF‡ | Plasma | Ware (2001) | ~250 (150–450) | 453.0±1058.2 | 248.4 (155.5–544.2) |
| Calfee (2007) | Trauma: 226 (142–331) Non-trauma: 355 (215–551) |
||||
Data are presented as pg/mL, except PMN
data are presented as % of total cells, and vWF
data are presented as % control.
Data presented as mean±SD or median (IQR), as available in prior literature. Use of ‘~’ notes that values are approximated from graphs and charts within that particular prior study. Many prior studies noted means in graphs but did not specifically define SD; this is noted by use of ‘mean~’.
BALF concentrations of IL-10 and sFasL in 75% of participants in our study were at or below the detection limit or our assay.
BALF concentrations of TNF-α in at least 50% of participants in our study were at or below the detection limit or our assay.
Plasma concentrations of IL-10 and TNF-α in 75% of participants in our study were at or below the detection limit or our assay.
There are several possible explanations as to why our results indicate that alveolar and circulating concentrations of mediators of inflammation and injury in contemporary ARDS patients are generally decreased, in some cases several fold, compared with historical studies. Perhaps most notable is that the parent RCT occurred in an era of relatively improved care for ARDS patients. Evidence suggests that survival in ARDS patients has increased over the past 30 years.(Stapleton et al., 2005, Erickson et al., 2009, Phua et al., 2009) One reason for this improved mortality is early delivery of lung protective ventilation, an intervention know to decrease both lung and systemic inflammation as well as mortality.(Ranieri et al., 1999, Network, 2000, Parsons et al., 2005) Additional reasons for the increased survival are not entirely clear, but there has been speculation that other processes of care including early fluid resuscitation and antibiotic administration, prophylaxis against deep venous thrombosis and stress ulcer bleeding, and prevention of nosocomial infections may have lead to reduced lung and systemic inflammation in patients with ARDS.(Hudson, 1995, Cook et al., 1996, Rivers et al., 2001, Pronovost et al., 2006, Cook et al., 2011)
Other explanations for our findings include different populations or assay methods between studies. While most of our measurements were performed with Luminex bead-based immunoassays (R&D Systems), a more recent technology where multiple biomarkers can be measured simultaneously, prior studies used enzyme-linked immunosorbent assays or other methods.(Haegens et al., 2007) However, results from bead-based immunoassays have been shown to correlate well with results from current ELISAs.(Kofoed et al., 2006, Codorean et al., 2010, Wang et al., 2012) ELISAs used in the past, though, may have had different sensitivities and detection limits. Indeed, a few investigations do suggest that cytokine concentrations obtained with two different techniques, while proportionally equivalent, may not necessarily be interchangeable due to differences in the concentration levels.(Loo et al., 2011) If this is the case, there remains an argument to power future ARDS trials where cytokine concentrations is an outcome using data obtained utilizing the same assay methodology that will be used in the planned future trial. Additionally, as described above, our study population contained a higher proportion of patients with trauma as their risk factor for ARDS than in other recent RCTs.(Network, 2000, Brower et al., 2004, Rice et al., 2012) Clinical outcomes in patients with trauma-associated ARDS are better than in patients with sepsis or another non-trauma ARDS risk factor, and evidence suggests that this may be partially explained by less severe endothelial and alveolar epithelial lung injury.(Calfee et al., 2007) Therefore, a higher proportion of trauma patients in our cohort could result in lower levels of inflammatory mediators. However, levels of plasma IL-6, a key inflammatory mediator in ARDS, in our study were notably decreased compared to values obtained in both trauma and non-trauma patients with ARDS from 1996–2002.(Calfee et al., 2007)
This study does have some limitations. First, generalizability of our results may be limited due to the population of patients enrolled in the parent RCT.(Stapleton et al., 2011) Additionally, the sample size was modest (n=90).
The fact that our study demonstrates reduced baseline concentrations of biomarkers of alveolar and systemic inflammation and injury in patients with ARDS, compared to prior studies, indicates that we must be very careful when using data from historical controls to perform power calculations for clinical trials. Our results reveal the importance of using biomarker data from a population of patients not only temporally close to the proposed clinical trial, but also similar in terms of ARDS risk factor and receipt of ICU processes of care.
CONCLUSION
In conclusion, this study has reported BALF and plasma levels of many biomarkers of inflammation and injury in a contemporary population of patients with ARDS that can be used to perform accurate power calculations for future early phase clinical trials with biologic endpoints. Furthermore, we have also demonstrated that concentrations of these biomarkers in our population are generally decreased compared with prior studies. If alveolar and systemic inflammation is indeed decreased in today’s ARDS patients, conducting phase II clinical trials of novel therapies with primary biologic endpoints then becomes more difficult as much larger sample sizes are required.
CLINICAL SIGNIFICANCE:
Bronchoalveolar fluid (BALF) and plasma biomarkers are frequent endpoints in studies of human acute respiratory distress syndrome (ARDS)
Data from contemporary ARDS patients are not available, thus making power calculations for future studies difficult
ARDS mortality has decreased over time
We therefore measured baseline biomarkers in 90 ARDS patients by analyzing BALF and blood samples from a recent RCT and compared these values to historical controls
Compared to historical measurements, our biomarker values were generally substantially lower
These findings may result from changes in ICU processes of care over time
Proper design of contemporary ARDS studies is now improved with these newer data
ACKNOWLEDGEMENTS
The authors wish to thank Thomas R. Martin, MD for his helpful input into this study and assistance with manuscript preparation.
Funding:
This research was supported under grant American Thoracic Society/Acute Respiratory Distress Syndrome Foundation Award; under grant American Society for Parenteral and Enteral Nutrition Rhoads Research Foundation Award; and under grants National Institutes of Health P50HL073996, K12RR023265, P20RR015557.
Footnotes
Disclosure Statement: The authors report no conflicts of interest.
REFERENCES
- Aisiku IP, Yamal JM, Doshi P, Benoit JS, Gopinath S, Goodman JC & Robertson CS, 2016. Plasma cytokines IL-6, IL-8, and IL-10 are associated with the development of acute respiratory distress syndrome in patients with severe traumatic brain injury. Crit Care, 20, 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen TC & Kurdowska A, 2014. Interleukin 8 and acute lung injury. Arch Pathol Lab Med, 138, 266–9. [DOI] [PubMed] [Google Scholar]
- Bautista E, Arcos M, Jimenez-Alvarez L, Garcia-Sancho MC, Vazquez ME, Pena E, Higuera A, Ramirez G, Fernandez-Plata R, Cruz-Lagunas A, Garcia-Moreno SA, Urrea F, Ramirez R, Correa-Rotter R, Perez-Padilla JR & Zuniga J, 2013. Angiogenic and inflammatory markers in acute respiratory distress syndrome and renal injury associated to A/H1N1 virus infection. Exp Mol Pathol, 94, 486–92. [DOI] [PubMed] [Google Scholar]
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A & Spragg R, 1994. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med, 149, 818–24. [DOI] [PubMed] [Google Scholar]
- Bhatia M & Moochhala S, 2004. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol, 202, 145–56. [DOI] [PubMed] [Google Scholar]
- Brower RG, Lanken PN, Macintyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D & Thompson BT, 2004. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med, 351, 327–36. [DOI] [PubMed] [Google Scholar]
- Calfee CS, Eisner MD, Ware LB, Thompson BT, Parsons PE, Wheeler AP, Korpak A & Matthay MA, 2007. Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med, 35, 2243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calfee CS, Ware LB, Eisner MD, Parsons PE, Thompson BT, Wickersham N & Matthay MA, 2008. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax, 63, 1083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codorean E, Nichita C, Albulescu L, Raducan E, Popescu ID, Lonita AC & Albulescu R, 2010. Correlation of XMAP and ELISA cytokine profiles; development and validation for immunotoxicological studies in vitro. Roum Arch Microbiol Immunol, 69, 13–9. [PubMed] [Google Scholar]
- Cook D, Meade M, Guyatt G, Walter S, Heels-Ansdell D, Warkentin TE, Zytaruk N, Crowther M, Geerts W, Cooper DJ, Vallance S, Qushmaq I, Rocha M, Berwanger O & Vlahakis NE, 2011. Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med, 364, 1305–14. [DOI] [PubMed] [Google Scholar]
- Cook DJ, Reeve BK, Guyatt GH, Heyland DK, Griffith LE, Buckingham L & Tryba M, 1996. Stress ulcer prophylaxis in critically ill patients. Resolving discordant metaanalyses. Jama, 275, 308–14. [PubMed] [Google Scholar]
- Debley JS, Hallstrand TS, Monge T, Ohanian A, Redding GJ & Zimmerman J, 2007. Methods to improve measurement of cysteinyl leukotrienes in exhaled breath condensate from subjects with asthma and healthy controls. J Allergy Clin Immunol, 120, 1216–7. [DOI] [PubMed] [Google Scholar]
- Donnelly SC, Strieter RM, Reid PT, Kunkel SL, Burdick MD, Armstrong I, Mackenzie A & Haslett C, 1996. The association between mortality rates and decreased concentrations of interleukin-10 and interleukin-1 receptor antagonist in the lung fluids of patients with the adult respiratory distress syndrome. Ann Intern Med, 125, 191–6. [DOI] [PubMed] [Google Scholar]
- Eisner MD, Parsons P, Matthay MA, Ware L & Greene K, 2003. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax, 58, 983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson SE, Martin GS, Davis JL, Matthay MA & Eisner MD, 2009. Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med, 37, 1574–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadek JE, Demichele SJ, Karlstad MD, Pacht ER, Donahoe M, Albertson TE, Van Hoozen C, Wennberg AK, Nelson JL & Noursalehi M, 1999. Effect of enteral feeding with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants in patients with acute respiratory distress syndrome. Enteral Nutrition in ARDS Study Group. Crit Care Med, 27, 1409–20. [DOI] [PubMed] [Google Scholar]
- Goodman RB, Pugin J, Lee JS & Matthay MA, 2003. Cytokine-mediated inflammation in acute lung injury. Cytokine Growth Factor Rev, 14, 523–35. [DOI] [PubMed] [Google Scholar]
- Goodman RB, Strieter RM, Martin DP, Steinberg KP, Milberg JA, Maunder RJ, Kunkel SL, Walz A, Hudson LD & Martin TR, 1996. Inflammatory cytokines in patients with persistence of the acute respiratory distress syndrome. Am J Respir Crit Care Med, 154, 602–11. [DOI] [PubMed] [Google Scholar]
- Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L & Group PS, 2013. Prone positioning in severe acute respiratory distress syndrome. NEngl JMed, 368, 2159–68. [DOI] [PubMed] [Google Scholar]
- Haegens A, Barrett TF, Gell J, Shukla A, Macpherson M, Vacek P, Poynter ME, Butnor KJ, Janssen-Heininger YM, Steele C & Mossman BT, 2007. Airway epithelial NF-kappaB activation modulates asbestos-induced inflammation and mucin production in vivo. J Immunol, 178, 1800–8. [DOI] [PubMed] [Google Scholar]
- Hudson LD, 1995. New therapies for ARDS. Chest, 108, 79S–91S. [DOI] [PubMed] [Google Scholar]
- Knaus WA, Draper EA, Wagner DP & Zimmerman JE, 1985. APACHE II: a severity of disease classification system. Crit Care Med, 13, 818–29. [PubMed] [Google Scholar]
- Kofoed K, Schneider UV, Scheel T, Andersen O & Eugen-Olsen J, 2006. Development and validation of a multiplex add-on assay for sepsis biomarkers using xMAP technology. Clin Chem, 52, 1284–93. [DOI] [PubMed] [Google Scholar]
- Krupa A, Kato H, Matthay MA & Kurdowska AK, 2004. Proinflammatory activity of anti-IL-8 autoantibody:IL-8 complexes in alveolar edema fluid from patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol, 286, L1105–13. [DOI] [PubMed] [Google Scholar]
- Le Gall JR, Lemeshow S & Saulnier F, 1993. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. Jama, 270, 2957–63. [DOI] [PubMed] [Google Scholar]
- Lee KS, Choi YH, Kim YS, Baik SH, Oh YJ, Sheen SS, Park JH, Hwang SC & Park KJ, 2008. Evaluation of bronchoalveolar lavage fluid from ARDS patients with regard to apoptosis. Respir Med, 102, 464–9. [DOI] [PubMed] [Google Scholar]
- Loo BM, Marniemi J & Jula A, 2011. Evaluation of multiplex immunoassays, used for determination of adiponectin, resistin, leptin, and ghrelin from human blood samples, in comparison to ELISA assays. Scand J Clin Lab Invest, 71, 221–6. [DOI] [PubMed] [Google Scholar]
- Maca J, Jor O, Holub M, Sklienka P, Bursa F, Burda M, Janout V & Sevcik P, 2017. Past and Present ARDS Mortality Rates: A Systematic Review. Respir Care, 62, 113–122. [DOI] [PubMed] [Google Scholar]
- Martin TR, Pistorese BP, Chi EY, Goodman RB & Matthay MA, 1989. Effects of leukotriene B4 in the human lung. Recruitment of neutrophils into the alveolar spaces without a change in protein permeability. J Clin Invest, 84, 1609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthay MA & Zemans RL, 2011. The acute respiratory distress syndrome: pathogenesis and treatment. Annu Rev Pathol, 6, 147–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute-Bello G, Liles WC, Steinberg KP, Kiener PA, Mongovin S, Chi EY, Jonas M & Martin TR, 1999. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS). J Immunol, 163, 2217–25. [PubMed] [Google Scholar]
- Mukhopadhyay S, Hoidal JR & Mukherjee TK, 2006. Role of TNFalpha in pulmonary pathophysiology. Respir Res, 7, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JF, Matthay MA, Luce JM & Flick MR, 1988. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis, 138, 720–3. [DOI] [PubMed] [Google Scholar]
- Network, T.a.R.D.S., 2000. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. . N Engl J Med, 342, 1301–8. [DOI] [PubMed] [Google Scholar]
- Oppenheim JJ, Zachariae CO, Mukaida N & Matsushima K, 1991. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol, 9, 617–48. [DOI] [PubMed] [Google Scholar]
- Pacht ER, Demichele SJ, Nelson JL, Hart J, Wennberg AK & Gadek JE, 2003. Enteral nutrition with eicosapentaenoic acid, gamma-linolenic acid, and antioxidants reduces alveolar inflammatory mediators and protein influx in patients with acute respiratory distress syndrome. Crit Care Med, 31, 491–500. [DOI] [PubMed] [Google Scholar]
- Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR & Wheeler AP, 2005. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med, 33, 1–6; discussion 230–2. [DOI] [PubMed] [Google Scholar]
- Parsons PE, Moss M, Vannice JL, Moore EE, Moore FA & Repine JE, 1997. Circulating IL-1ra and IL-10 levels are increased but do not predict the development of acute respiratory distress syndrome in at-risk patients. Am J Respir Crit Care Med, 155, 1469–73. [DOI] [PubMed] [Google Scholar]
- Peters-Golden M & Henderson WR Jr., 2007. Leukotrienes. N Engl J Med, 357, 1841–54. [DOI] [PubMed] [Google Scholar]
- Phua J, Badia JR, Adhikari NK, Friedrich JO, Fowler RA, Singh JM, Scales DC, Stather DR, Li A, Jones A, Gattas DJ, Hallett D, Tomlinson G, Stewart TE & Ferguson ND, 2009. Has mortality from acute respiratory distress syndrome decreased over time? : A systematic review. Am J Respir Crit Care Med, 179, 220–7. [DOI] [PubMed] [Google Scholar]
- Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, Sexton B, Hyzy R, Welsh R, Roth G, Bander J, Kepros J & Goeschel C, 2006. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med, 355, 2725–32. [DOI] [PubMed] [Google Scholar]
- Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F & Slutsky AS, 1999. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. Jama, 282, 54–61. [DOI] [PubMed] [Google Scholar]
- Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, Morris A, Dong N & Rock P, 2012. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. Jama, 307, 795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E & Tomlanovich M, 2001. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med, 345, 1368–77. [DOI] [PubMed] [Google Scholar]
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ & Hudson LD, 2005. Incidence and outcomes of acute lung injury. N Engl J Med, 353, 1685–93. [DOI] [PubMed] [Google Scholar]
- Sittipunt C, Steinberg KP, Ruzinski JT, Myles C, Zhu S, Goodman RB, Hudson LD, Matalon S & Martin TR, 2001. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med, 163, 503–10. [DOI] [PubMed] [Google Scholar]
- Stapleton RD, Martin TR, Weiss NS, Crowley JJ, Gundel SJ, Nathens AB, Akhtar SR, Ruzinski JT, Caldwell E, Curtis JR, Heyland DK, Watkins TR, Parsons PE, Martin JM, Wurfel MM, Hallstrand TS, Sims KA & Neff MJ, 2011. A phase II randomized placebo-controlled trial of omega-3 fatty acids for the treatment of acute lung injury. Crit Care Med, 39, 1655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton RD, Wang BM, Hudson LD, Rubenfeld GD, Caldwell ES & Steinberg KP, 2005. Causes and timing of death in patients with ARDS. Chest, 128, 525–32. [DOI] [PubMed] [Google Scholar]
- Suratt BT, Eisner MD, Calfee CS, Allard JB, Whittaker LA, Engelken DT, Petty JM, Trimarchi T, Gauthier L & Parsons PE, 2009. Plasma granulocyte colony-stimulating factor levels correlate with clinical outcomes in patients with acute lung injury. Crit Care Med, 37, 1322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LS, Leung YY, Chang SK, Leight S, Knapik-Czajka M, Baek Y, Shaw LM, Lee VM, Trojanowski JQ & Clark CM, 2012. Comparison of xMAP and ELISA assays for detecting cerebrospinal fluid biomarkers of Alzheimer’s disease. J Alzheimers Dis, 31, 439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware LB, Conner ER & Matthay MA, 2001. von Willebrand factor antigen is an independent marker of poor outcome in patients with early acute lung injury. Crit Care Med, 29, 2325–31. [DOI] [PubMed] [Google Scholar]
- Ware LB & Matthay MA, 2000. The acute respiratory distress syndrome. N Engl J Med, 342, 1334–49. [DOI] [PubMed] [Google Scholar]


