Abstract
Objective:
Preterm birth is the leading cause of neonatal morbidity and mortality worldwide. Some preterm births are associated with clinical chorioamnionitis; yet, this condition has been poorly investigated. Herein, we characterized the amniotic fluid cellular immune responses in women with preterm clinical chorioamnionitis.
Methods and subjects:
Amniotic fluid samples were obtained from women with preterm clinical chorioamnionitis and a positive or negative microbiological culture (n=17). The cellular composition of amniotic fluid was evaluated using fluorescence microscopy, scanning and transmission electron microscopy, and flow cytometry. Women without preterm clinical chorioamnionitis were also examined (n=10).
Results:
Amniotic fluid from women with preterm clinical chorioamnionitis and a positive culture had: 1) abundant neutrophils associated with viable and non-viable bacteria, 2) neutrophils performing phagocytosis, 3) neutrophils forming NETs, 4) increased numbers of neutrophils, monocytes/macrophages, and CD4+ T cells, and 5) high expression of IL-1β by neutrophils and monocytes/macrophages. Amniotic fluid from women with preterm clinical chorioamnionitis and proven infection tended to have fewer monocytes/macrophages and CD4+ T cells compared to those without chorioamnionitis.
Conclusion:
We provide the first morphologic and phenotypic characterization of the cellular immune responses in the amniotic cavity of women with preterm clinical chorioamnionitis, a condition associated with adverse neonatal outcomes.
Keywords: Preterm, clinical chorioamnionitis, immunology, neutrophils, amniotic fluid, T cells
INTRODUCTION
Preterm birth is the leading cause of neonatal morbidity and mortality [1] and is preceded by preterm labor in approximately 70% of cases [2]. However, one third of preterm births are medically indicated (i.e. iatrogenic) due to complications such as hypertensive disorders (e.g. preeclampsia), fetal growth restriction, hemorrhage, fetal compromise, and clinical chorioamnionitis, among others [3]. Preterm clinical chorioamnionitis can also occur in 20% of women with preterm prelabor rupture of membranes (PPROM) [4] and in 10% of women with preterm labor and intact membranes [5]. Thus, preterm clinical chorioamnionitis can occur in the presence or absence of spontaneous preterm labor and birth [2]. Preterm clinical chorioamnionitis increases the risk of adverse maternal outcomes [6, 7], and more importantly is associated with neonatal complications such as congenital sepsis [8, 9] and neurodevelopmental disorders including cerebral palsy [10, 11]. Despite its clinical relevance, there is a paucity of studies focused on preterm clinical chorioamnionitis.
Preterm clinical chorioamnionitis is typically thought to occur as a result of microbial invasion of the amniotic cavity, which can elicit systemic and local inflammatory responses [12–17]. Yet, current studies have shown that only 76% of patients with the diagnosis of preterm clinical chorioamnionitis have proven intra-amniotic infection or inflammation, whereas the remaining patients have neither culturable microorganisms nor inflammation in amniotic fluid [18]. A similar heterogeneity is observed in women diagnosed with clinical chorioamnionitis at term [19]. The systemic and local (i.e. the amniotic cavity) immune responses in clinical chorioamnionitis at term have been well investigated [14–16, 20, 21]; yet, the immunobiology of preterm clinical chorioamnionitis is poorly understood.
Herein, we investigate the cellular composition of amniotic fluid from women with preterm clinical chorioamnionitis with and without culture-proven intra-amniotic infection using fluorescence microscopy, scanning and transmission electron microscopy, and multi-color flow cytometry.
METHODS
Study population and characteristics
This cross-sectional study included patients who underwent amniocentesis due to clinical indications. The collection of samples was approved by the Institutional Review Boards of the Detroit Medical Center (Detroit, MI, USA), Wayne State University, and the Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS). All women provided written informed consent prior to the collection of amniotic fluid. This study included 17 amniotic fluid samples from women with preterm clinical chorioamnionitis and either a negative amniotic fluid culture (n=8) or a positive amniotic fluid culture (n=9) (see clinical definitions and amniotic fluid sample collection below). For all patients, the time between the collection of the amniotic fluid sample and delivery was ≤2 days (this criterion was used to preserve a meaningful relationship between amniotic fluid studies and clinical chorioamnionitis). The demographic and clinical characteristics of the study population are shown in Table 1. Placentas from each patient were examined histologically according to standardized Perinatology Research Branch protocols [22]. A second group of women with preterm labor/birth and a positive culture but without clinical chorioamnionitis (n=10) was included in the last set of experiments (Table 2).
Table 1.
Clinical and demographic characteristics of women with preterm clinical chorioamnionitis
| Preterm birth and clinical chorioamnionitis with negative culture (n=8) |
Preterm birth and clinical chorioamnionitis with positive culture (n=9) |
p-value | |
|---|---|---|---|
| Maternal age (years; median [IQR])a | 31 (26.8–36.3) | 24 (22–26) | 0.004 |
| Body mass index (kg/m2; median [IQR])a | 34.9 (31.4–42.3) | 26.9 (22.1–31) | 0.08 |
| Primiparityb | 12.5% (1/8) | 33.3% (3/9) | 0.5 |
| Raceb | 1 | ||
| African-American | 87.5% (7/8) | 88.9% (8/9) | |
| Caucasian | 0% (0/8) | 11.1% (1/9) | |
| Other | 12.5% (1/8) | 0% (0/9) | |
| Gestational age at amniocentesis (weeks; median [IQR])a | 27.9 (22.6–32.8) | 22.7 (20.7–27) | 0.3 |
| IL-6 (ng/mL; median [IQR])a | 11.1 (1.3–94.2) | 122.1 (70.6–266.1) | 0.1 |
| White blood cell count, cells/mm3,a | 1 (0–59.3) | 310 (20–609) | 0.03 |
| Amniotic fluid glucose, mg/dLa | 11.5 (5.5–37.8) | 1 (1–17) | 0.2 |
| Gestational age at delivery (weeks; median [IQR])a | 29.1 (24.4–33.4) | 23.3 (20.7–27.1) | 0.2 |
| Spontaneous preterm laborb | 37.5% (3/8) | 55.5% (5/9) | 0.6 |
| Preterm premature rupture of membranesb | 37.5% (3/8) | 22.2% (2/9) | 0.6 |
| Cesarean sectionb | 0% (0/8) | 22.2% (2/9) | 0.4 |
| Birthweight (grams)a | 1300 (700–1971.3) | 550 (296–901) | 0.1 |
| Acute maternal inflammatory response | |||
| Stage 1 (acute subchorionitis)b | 0% (0/8) | 0% (0/9) | 1 |
| Stage 2 (acute chorioamnionitis)b | 37.5% (3/8) | 11.1% (1/9) | 0.2 |
| Stage 3 (acute necrotizing chorioamnionitis)b | 50% (4/8) | 77.8% (7/9) | 0.3 |
| Acute fetal inflammatory response | |||
| Stage 1 (acute phlebitis/chronic vasculitis)b | 0% (0/8) | 11.1% (1/9) | 1 |
| Stage 2 (acute arteritis)b | 50% (4/8) | 44.4% (4/9) | 1 |
| Stage 3 (necrotizing funisitis)b | 25% (2/8) | 22.2% (2/9) | 1 |
Data are given as median (interquartile range, IQR) and percentage (n/N).
Mann-Whitney U-test.
Fisher’s exact test.
Table 2.
Clinical and demographic characteristics of women with preterm birth and a positive amniotic fluid culture with or without clinical chorioamnionitis
| Preterm birth and positive culture without clinical chorioamnionitis (n=10) |
Preterm birth and positive culture with clinical chorioamnionitis (n=9) |
p-value | |
|---|---|---|---|
| Maternal age (years; median [IQR])a | 27.5 (22.8–35.8) | 24 (22–26) | 0.1 |
| Body mass index (kg/m2; median [IQR])a | 28.3 (27–36.5)c | 26.9 (22.1–31) | 0.3 |
| Primiparityb | 0% (0/10) | 33.3% (3/9) | 0.08 |
| Raceb | 1 | ||
| African-American | 80% (8/10) | 88.9% (8/9) | |
| White | 20% (2/10) | 11.1% (1/9) | |
| Gestational age at amniocentesis (weeks; median [IQR])a | 25.9 (22.5–29.8) | 22.7 (20.7–27) | 0.5 |
| IL-6 (ng/mL; median [IQR])a | 128.7 (78–186.2) | 122.1 (70.6–266.1) | 0.7 |
| White blood cell count, cells/mm3,a | 307 (89–1254.5) | 310 (20–609) | 0.8 |
| Amniotic fluid glucose, mg/dLa | 3.5 (1–9.8) | 1 (1–17) | 0.9 |
| Gestational age at delivery (weeks; median [IQR])a | 25.9 (23–29.9) | 23.3 (20.7–27.1) | 0.5 |
| Spontaneous preterm laborb | 60% (6/10) | 55.5% (5/9) | 1 |
| Preterm premature rupture of membranesb | 20% (2/10) | 22.2% (2/9) | 1 |
| Cesarean sectionb | 0% (0/10) | 22.2% (2/9) | 0.2 |
| Birthweight (grams)a | 935 (533.3–1428.8) | 550 (296–901) | 0.3 |
| Acute maternal inflammatory response | |||
| Stage 1 (acute subchorionitis)b | 0% (0/8)d | 0% (0/9) | 1 |
| Stage 2 (acute chorioamnionitis)b | 37.5% (3/8)d | 11.1% (1/9) | 0.2 |
| Stage 3 (acute necrotizing chorioamnionitis)b | 50% (4/8)d | 77.8% (7/9) | 0.3 |
| Acute fetal inflammatory response | |||
| Stage 1 (acute phlebitis/chronic vasculitis)b | 12.5% (1/8)d | 11.1% (1/9) | 1 |
| Stage 2 (acute arteritis)b | 75% (6/8)d | 44.4% (4/9) | 0.3 |
| Stage 3 (necrotizing funisitis)b | 0% (0/8)d | 22.2% (2/9) | 0.4 |
Data are given as median (interquartile range, IQR) and percentage (n/N).
Mann-Whitney U-test.
Fisher’s exact test.
Three missing data
Two missing data
Clinical definitions
Gestational age was determined by the date of the last menstrual period and confirmed by ultrasound examination. The gestational age derived from sonographic fetal biometry was used if the estimation was inconsistent with menstrual dating. Preterm birth was defined as delivery <37 weeks of gestation. Clinical chorioamnionitis was diagnosed by the presence of maternal fever (temperature >37.8°C) accompanied by two or more of the following criteria: (1) uterine tenderness, (2) foul-smelling amniotic fluid, (3) fetal tachycardia (heart rate >160 beats/min), (4) maternal tachycardia (heart rate >100 beats/min), and (5) maternal leukocytosis (leukocyte count >15,000 cells/mm3) [23–25]. Intra-amniotic inflammation was detected by elevated amniotic fluid IL-6 concentrations, as previously reported [26].
Amniotic fluid sample collection
Samples of amniotic fluid were transported to the laboratory in a sterile capped syringe. Clinical tests included culture of aerobic/anaerobic bacteria and genital mycoplasmas, white blood cell count, Gram stain, glucose concentration, and IL-6 concentration. The rest of the sample was utilized for research purposes including live/dead staining, scanning and transmission electron microscopy, and immunophenotyping.
Detection of bacteria using fluorescence microscopy
The presence of bacteria in the amniotic fluid was evaluated as previously described [27] using the LIVE/DEAD BacLight™ Bacterial Viability Kit (Cat# L7007, Life Technologies). Briefly, 100 μL of amniotic fluid were mixed with 900 μL of sterile 1X phosphate-buffered saline (PBS). Three microliters of the dye mix (Component A and B mixed at a 1:1 ratio) were added to the cell suspension, which was then incubated for 15 min at room temperature in the dark. Next, the cells were centrifuged at 10,000 × g for 5 min and the supernatant was discarded. The cell pellet was then re-suspended in 5 μL of 1X PBS, and a slide smear was prepared and air-dried. Lastly, the slide was gently rinsed with 1X PBS and mounted with ProLong Diamond Antifade Mountant with 4′,6-diamidino-2-phenylindole (DAPI) (Life Technologies). The presence of bacteria was evaluated using an Olympus BX60 fluorescence microscope with an Olympus DP71 camera and DP Controller Software (Olympus Corporation, Tokyo, Japan).
Scanning and transmission electron microscopy
Amniotic fluid samples were centrifuged at 2,300 × g for 5 min at room temperature and the supernatant was discarded. Electron microscopy fixative [2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4 (Cat# 16537–05, Electron Microscopy Science; Hatfield, PA, USA)] was carefully added to the cell pellet. Following fixation for 2 h at 4°C, the cell pellet was washed with 1X electron microscopy wash buffer [Sorensen’s phosphate buffer 0.2 M, pH 7.4 (Cat# 11601–10, Electron Microscopy Science)] and gently resuspended in 1 mL of the same buffer. Cell pellets were transported to the Microscopy & Image Analysis Laboratory at the University of Michigan. Images were obtained using an AMRAY 1910 Field Emission Scanning Electron Microscope (SEMTechSolutions; North Billerica, MA, USA) and a JSM-1400 Transmission Electron Microscope (JEOL USA, Inc.; Peabody, MA, USA).
Immunophenotyping by flow cytometry
Amniotic fluid samples (0.5–1 mL) were centrifuged at 300 × g for 5 min at room temperature. The resulting amniotic fluid pellet was re-suspended in 1 mL of 1X PBS (Life Technologies, Grand Island, NY, USA) and stained with the BD Horizon Fixable Viability Stain 510 dye (BD Biosciences, San Jose, CA, USA). Cells were washed in 1X PBS and incubated with 20 μL of human FcR blocking reagent (Miltenyi Biotec, San Diego, CA, USA) in 80 μL of stain buffer (BD Biosciences) for 10 min at 4°C. Next, cells were incubated with extracellular fluorochrome-conjugated anti-human monoclonal antibodies for 30 min at 4°C in the dark (Supplemental Table S1). To determine cytokine expression, after extracellular staining the cells were fixed and permeabilized using the BD Cytofix/Cytoperm fixation/permeabilization kit (BD Biosciences) prior to incubation with intracellular antibodies (Supplemental Table S1). Stained cells were then washed and re-suspended in 0.5 mL of stain buffer, and acquired using the BD LSR II or LSRFortessa Flow Cytometer (BD Bioscience) and BD FACSDiva 6.0 software (BD Bioscience). The analysis was performed and the figures were generated using the FlowJo version 10 software (FlowJo, Ashland, OR, USA). The absolute number of cells was determined using CountBright absolute counting beads (Molecular Probes, Eugene, OR, USA). The mean fluorescence intensity (MFI) of each cytokine was calculated by subtracting the MFI of the isotype control from the MFI of the antibody-stained sample.
Statistical analysis
Statistical analyses were conducted using GraphPad Prism version 8.0.1 for Windows (GraphPad Software, San Diego, California, USA, www.graphpad.com). For patient demographics, the Mann-Whitney U-test was used to compare continuous variables and the Fisher’s exact test was used for nominal variables. The Mann-Whitney U-test was performed when comparing non-normally distributed data between study groups. Two-tailed p-values are reported. A p-value <0.05 was considered statistically significant for all tests.
RESULTS
Characteristics of the study population
The demographic and clinical characteristics of the study population used in Figures 1–5 are shown in Table 1. A total of 17 amniotic fluid samples were collected from women with preterm clinical chorioamnionitis. Eight women had amniotic fluid cultures that were negative for bacteria and nine women had positive cultures (Supplemental Figure S1). The majority of the patients included in this study were diagnosed with intra-amniotic inflammation based on the IL-6 concentration (≥2.6 ng/mL) [26] (Supplemental Figure S1). No differences were observed between these two study groups except for the amniotic fluid white blood cell count, which was higher in women with preterm clinical chorioamnionitis and positive cultures compared to those with negative cultures (Table 1). The microorganisms present in women with a positive culture included Ureaplasma urealyticum, Streptococcus agalactiae, and Mycoplasma hominis, among others (Supplemental Table S2).
Fig. 1. Detection of live and dead bacteria in amniotic fluid.
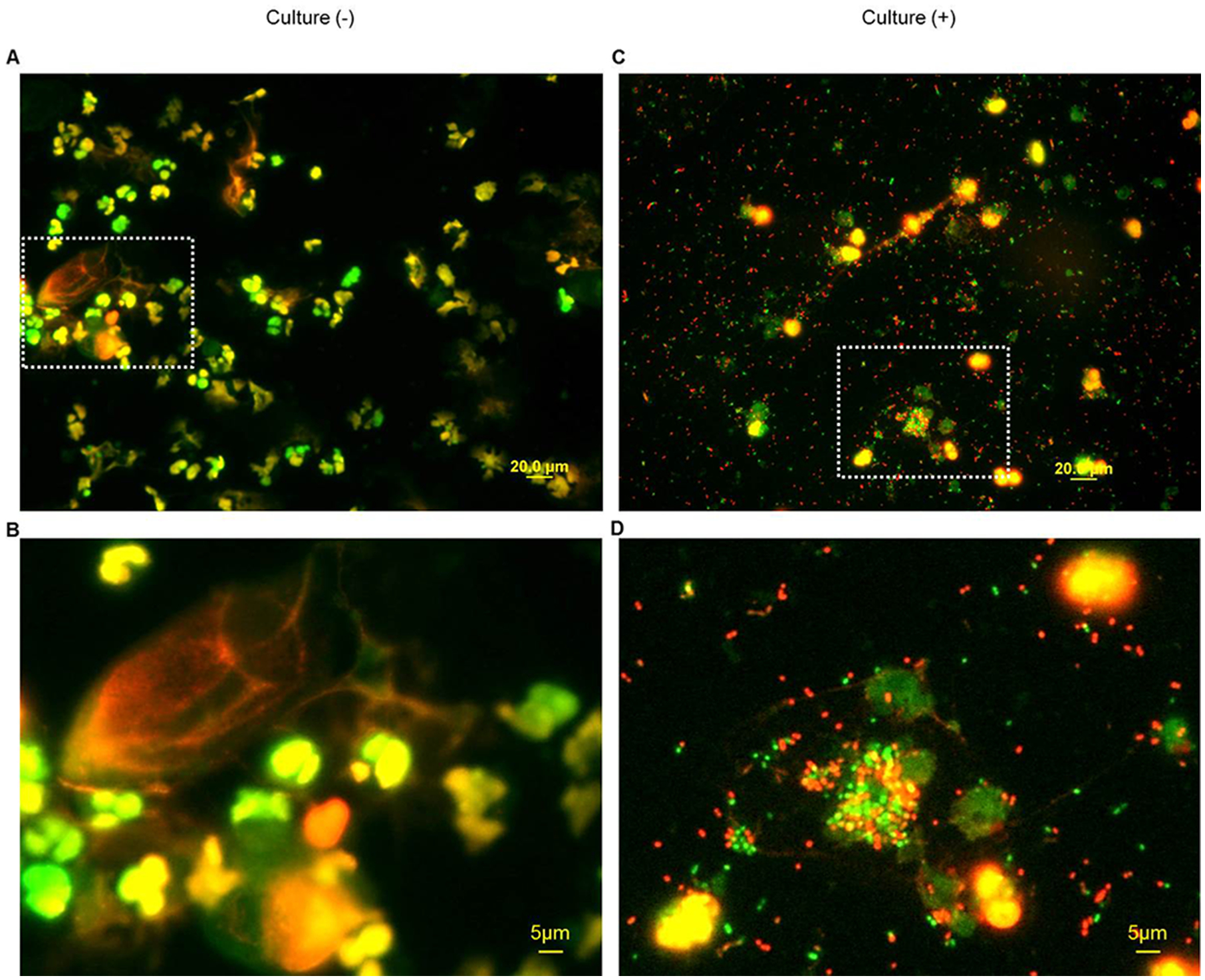
Representative bacterial live/dead staining of amniotic fluid from patients with preterm clinical chorioamnionitis and (A) a negative amniotic fluid bacterial culture, with corresponding enlarged image (B), or (C) a positive amniotic fluid bacterial culture, with corresponding enlarged image (D). Green = SYTO 9 stain, red = propidium iodide stain. Magnification = 400X
Fig. 5. Flow cytometric analysis of cytokine expression by innate immune cells in amniotic fluid.

(A) Representative gating strategy for determining the mean fluorescence intensity of IL-1β, IL-8, TNFα, IL-1α, MIP-1α, IL-6, and MIP-1β expressed by amniotic fluid neutrophils and monocytes/macrophages. Mean fluorescence intensity of IL-1β, IL-8, TNFα, IL-1α, MIP-1α, IL-6, and MIP-1β expressed by (B) neutrophils and (C) monocytes/macrophage in amniotic fluid from women with preterm clinical chorioamnionitis who had either a negative amniotic fluid culture (blue bar plots) or a positive amniotic fluid culture (red bar plots). (N=6–7).
Visualization of bacteria and leukocytes in amniotic fluid
We first visualized the amniotic fluid exudate using fluorescence microscopy. Figure 1A is a photomicrograph of an amniotic fluid sample from a patient with preterm clinical chorioamnionitis and a negative culture. This image shows abundant polymorphonuclear leukocytes in green or yellow, which is the result of the staining with SYTO 9 (cell-membrane permeable green dye) or the merging of SYTO 9 and propidium iodide (cell-membrane impermeable red dye), respectively. A magnification of this image is shown below, including viable leukocytes in green or yellow as well as a large non-viable epithelial cell and a non-viable leukocyte in red (Figure 1B). Figure 1C is a photomicrograph of amniotic fluid from a patient with preterm clinical chorioamnionitis and a positive culture. In this image, numerous bacteria stained with either SYTO 9 (viable bacteria) or propidium iodide (non-viable bacteria) are observed together with surrounding polymorphonuclear leukocytes, which is in line with the positive culture results for this sample. A magnification of this image is shown in Figure 1D, displaying live and dead bacteria and viable leukocytes engulfing bacteria, a process that has been previously documented in amniotic fluid [28].
We next performed scanning and transmission electron microscopy of amniotic fluid exudates in order to further visualize the immune cells and bacteria present in our samples. Amniotic fluid samples from women with preterm clinical chorioamnionitis and a negative culture contained neutrophils that displayed a classic round morphology that is typical of a resting state [29, 27], and no bacteria were observed (Figure 2A). In contrast, amniotic fluid from women with preterm clinical chorioamnionitis and a positive culture contained neutrophils that appeared active, as indicated by the presence of web-like structures (possibly neutrophil extracellular traps or NETs [30, 31]) (Figure 2B). Moreover, numerous bacteria were observed surrounding these amniotic fluid cells (Figure 2B). Transmission electron microscopy revealed phagocytosed bacteria inside of the polymorphonuclear cells present in amniotic fluid from women with preterm clinical chorioamnionitis and a positive culture, which was not observed in patients with a negative culture (Figure 2C&D).
Fig. 2. Electron microscopy of amniotic fluid neutrophils.

Representative scanning electron microscopy images of amniotic fluid neutrophils from (A) a woman with a negative amniotic fluid microbial culture and (B) a woman with a positive amniotic fluid microbial culture. Representative transmission electron microscopy images of amniotic fluid neutrophils from (C) a woman with a negative amniotic fluid microbial culture and (D) a woman with a positive amniotic fluid microbial culture. Magnifications: (A) Top row: 4000X (left), 2940X (right); bottom row: 10000X. (B) 7000X. (C) 12000X (left), 15000X (right). (D) 7000X
These morphological data indicate that women with preterm clinical chorioamnionitis have abundant amniotic fluid leukocytes in the absence of a positive culture, and when bacteria are present neutrophils perform phagocytosis or NET formation.
Leukocyte populations in amniotic fluid
A representative image of the flow cytometry gating strategy used to detect leukocytes in amniotic fluid from women with preterm clinical chorioamnionitis is shown in Figure 3A. Briefly, viable cells were gated within the single cell population (i.e. singlets) that were further identified as total leukocytes (CD45+ cells), neutrophils (CD45+CD15+CD14- cells), monocytes/macrophages (CD45+CD14+CD15- cells), B cells (CD45+CD19+CD15-CD14-CD3- cells), and T cells (CD45+CD3+CD15-CD14-CD19- cells). T cells were further subdivided into CD4+ T cells (CD3+CD4+CD8- cells) and CD8+ T cells (CD3+CD8+CD4- cells). Other immune cells present in amniotic fluid such as innate lymphoid cells and NK cells were also detected as previously shown [32], yet their full phenotype was not confirmed in this study.
Fig. 3. Flow cytometric analysis of leukocyte populations and innate immune cells in amniotic fluid.
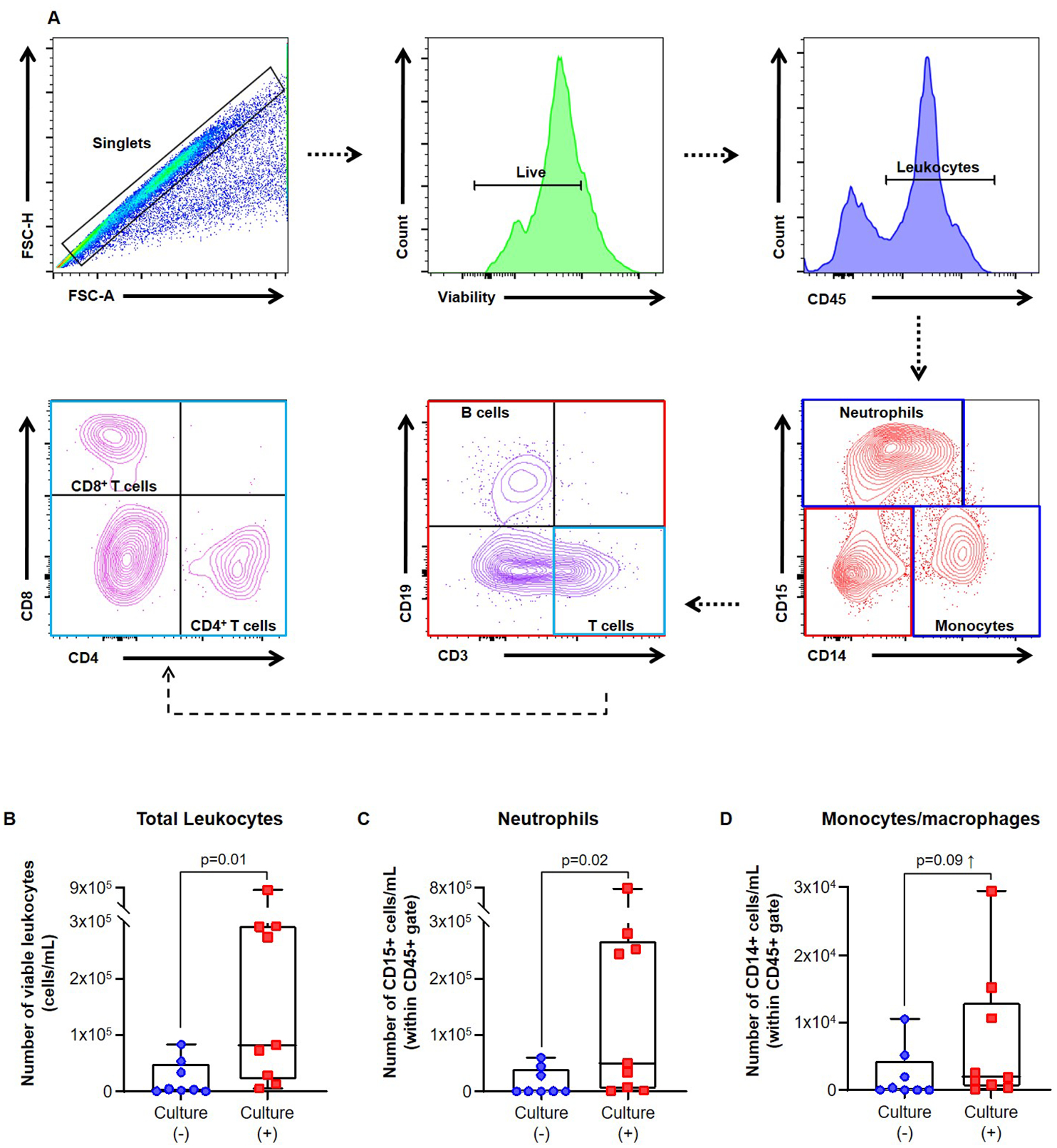
(A) Representative flow cytometry gating strategies showing leukocyte populations in amniotic fluid from women with preterm clinical chorioamnionitis. Immune cells were initially gated within the viability gate and CD45+ gate followed by lineage gating for neutrophils (CD45+CD15+CD14- cells), monocytes/macrophages (CD45+CD14+CD15- cells), T cells (CD45+CD3+CD15-CD14-CD19- cells) and B cells (CD45+CD19+CD15-CD14-CD3- cells). T cells were subsequently gated for CD4+ T cells (CD3+CD4+CD8- cells) and CD8+ T cells (CD3+CD8+CD4- cells). Numbers of (B) total leukocytes (CD45+ cells/mL), (C) neutrophils (CD15+ cells/mL), and (D) monocytes/macrophages (CD14+ cells/mL) in amniotic fluid from women with preterm clinical chorioamnionitis who had either a negative or positive amniotic fluid culture. N = 8–9 per group. Midlines = median, boxes = interquartile ranges, and whiskers = minimum/maximum ranges
The overall number of amniotic fluid leukocytes was significantly increased in patients with preterm clinical chorioamnionitis and a positive culture compared to those with a negative culture (Figure 3B). This increase was likely due to the enhanced numbers of neutrophils observed in amniotic fluid from patients with preterm clinical chorioamnionitis and a positive culture (Figure 3C). Amniotic fluid monocytes/macrophages were also increased in women with a positive culture; however, this rise did not reach statistical significance (Figure 3D).
We then determined the numbers of adaptive immune cells (T cells and B cells) in amniotic fluid from our study groups. The overall number of T cells tended to be greater in women with preterm clinical chorioamnionitis and a positive culture compared to those with a negative culture (Figure 4A). This is likely due to the increased numbers of CD4+ T cells in patients with preterm clinical chorioamnionitis and a positive culture (Figure 4B). This increase was not observed for CD8+ T cells (Figure 4C), nor for B cells (Figure 4D).
Fig. 4. Flow cytometric analysis of adaptive immune cells in amniotic fluid.

Numbers of (A) total T cells (cells/mL), (B) CD4+ T cells (cells/mL), (C) CD8+ T cells (cells/mL), and (D) B cells (cells/mL) in amniotic fluid from women with preterm clinical chorioamnionitis who had either a negative or positive amniotic fluid culture. Lymphocyte populations were gated as shown in Figure 3A. N = 8–9 per group. Midlines = median, boxes = interquartile ranges, and whiskers = minimum/maximum ranges
Collectively, this flow cytometric analysis revealed that women with preterm clinical chorioamnionitis and a positive culture display elevated numbers of leukocytes including neutrophils, monocytes/macrophages, and CD4+ T cells in the amniotic cavity.
Cytokine expression by amniotic fluid neutrophils and monocytes/macrophages
Since neutrophils and monocytes/macrophages were the predominant cells types found in our study groups, we investigated the cytokine expression profiles of these cells. Representative histograms for the expression of IL-1β, IL-8, TNFα, IL-1α, MIP-1α, IL-6, and MIP-1β by amniotic fluid neutrophils and monocytes/macrophages are shown in Figure 5A. The mean fluorescence intensity (MFI) of IL-1β expression was significantly greater on amniotic fluid neutrophils from patients with preterm clinical chorioamnionitis and a positive culture compared to those with a negative culture (Figure 5B). The MFI of IL-8 tended to increase as well, although this did not reach statistical significance (Figure 5B). Amniotic fluid monocytes/macrophages also displayed a higher MFI of IL-1β expression in patients with preterm clinical chorioamnionitis and a positive culture compared to those with a negative culture (Figure 5C). These data show that both neutrophils and monocytes/macrophages in amniotic fluid express high levels of IL-1β in women with preterm clinical chorioamnionitis and a positive culture.
Is clinical chorioamnionitis associated with a different leukocyte repertoire in amniotic fluid in preterm gestations?
Our previous studies have shown that women with preterm labor/birth and intra-amniotic infection (a positive amniotic fluid culture and elevated IL-6 concentrations) without clinical chorioamnionitis display a leukocyte repertoire in amniotic fluid [33] similar to that observed herein in women with preterm clinical chorioamnionitis and a positive culture. Therefore, we last sought to investigate whether these two subsets of women delivering preterm displayed differences in their amniotic fluid leukocyte repertoire. Overall, women with preterm clinical chorioamnionitis and a positive culture had fewer amniotic fluid leukocytes compared to those without this clinical condition; however, these differences did not reach statistical significance (Figure 6A–G). Yet, the numbers of monocytes/macrophages and CD4+ T cells were marginally reduced compared to those without clinical chorioamnionitis (Figure 6C&F). These data indicate that preterm clinical chorioamnionitis does not drastically alter the amniotic fluid cellular responses in the presence of culturable bacteria, suggesting that this maternal clinical diagnosis does not reflect the immunobiology of the amniotic cavity (i.e. fetal environment).
Fig. 6. Flow cytometric analysis of amniotic fluid from women with or without preterm clinical chorioamnionitis.

Numbers of (A) total leukocytes (cells/mL), (B) neutrophils (cells/mL), (C) monocytes/macrophages (cells/mL), (D) B cells (cells/mL), (E) total T cells (cells/mL), (F) CD4+ T cells (cells/mL), and (G) CD8+ T cells (cells/mL) in amniotic fluid from women with and without preterm clinical chorioamnionitis who had a positive microbiological culture. Leukocyte populations were gated as shown in Figure 3A. N = 9–10 per group. Midlines = median, boxes = interquartile ranges, and whiskers = minimum/maximum ranges
DISCUSSION
Women with intra-amniotic infection have abundant neutrophils in the amniotic cavity [34, 20, 35, 33], which are mostly of fetal origin in preterm gestations or of maternal origin at term [35]. Indeed, the number of white blood cells, mainly comprised of neutrophils, is used to diagnose intra-amniotic inflammation in preterm [34] and term [36] gestations. Subsequent flow cytometric studies reported that women with clinical chorioamnionitis at term and a positive amniotic fluid culture have increased numbers of neutrophils in the amniotic cavity compared to those with a negative culture [20]. The functions of amniotic fluid neutrophils have been evaluated using ex vivo assays showing that these cells are capable of performing phagocytosis of bacteria invading the amniotic cavity [28]. Neutrophils in the amniotic cavity also form NETs [27] and may degranulate, releasing anti-microbial products [37–39] as well as reactive oxygen species [40] into the amniotic cavity. Herein, we describe that amniotic fluid neutrophils from women with preterm clinical chorioamnionitis display some of the abovementioned functions: phagocytose bacteria and form NETs. The formation of NETs, however, was predominantly observed in cases in which bacteria were detected using cultivation techniques, indicating that amniotic fluid NETs participate in host defense against viable microbes. We have previously hypothesized that NET formation may occur in the absence of detectable microorganisms in the amniotic cavity, i.e. sterile intra-amniotic inflammation [27]; yet, further studies are required to test this hypothesis. Altogether, these findings represent evidence that amniotic fluid neutrophils actively participate in the host response mechanisms against microbial invasion of the amniotic cavity in women with preterm clinical chorioamnionitis.
Neutrophils from women with preterm clinical chorioamnionitis and a positive culture displayed significantly higher expression of IL-1β than those without culturable bacteria. The primary mechanism of IL-1β release is inflammasome-mediated pyroptosis (i.e. inflammatory cell death), which does not typically occur in neutrophils [41]. However, neutrophils produce numerous anti-microbial enzymes, such as neutrophil elastase and cathepsins, which have been shown to directly cleave immature IL-1β into its bioactive form [42]. Hence, besides participating in the host defense mechanisms against infection, neutrophils contribute to the local pro-inflammatory milieu taking place in the amniotic cavity of women with preterm clinical chorioamnionitis.
Monocytes/macrophages were the second most abundant leukocyte population in amniotic fluid of women with preterm clinical chorioamnionitis and a positive culture. This is consistent with previous reports showing that women with clinical chorioamnionitis at term and a positive culture had elevated numbers of monocytes in amniotic fluid [20]. Further, women with preterm labor/birth and intra-amniotic infection, but without clinical chorioamnionitis, showed an increased number of monocytes/macrophages in the amniotic cavity compared to those with intra-amniotic inflammation without culturable microorganisms [33]. Interestingly, women without preterm clinical chorioamnionitis tended to have greater numbers of amniotic fluid monocytes/macrophages than those with this clinical condition. These data suggest that when the mother presents a systemic inflammatory response in preterm gestations, fewer monocytes/macrophages may migrate from the fetal and maternal vasculature into the amniotic cavity. This hypothesis, however, requires additional experimentation that will complement the maternal and fetal origin of amniotic fluid monocytes recently reported [43].
A primary function of monocytes/macrophages is to produce and release cytokines [44]. Indeed, monocytes/macrophages express greater amounts of the pro-inflammatory cytokines IL-1α and IL-1β than neutrophils in amniotic fluid of women with clinical chorioamnionitis at term [20]. Consistently, we found that monocytes/macrophages expressed higher levels of IL-1β than neutrophils in the amniotic cavity of women with preterm clinical chorioamnionitis, regardless of the presence of culturable bacteria. The mechanisms whereby monocytes/macrophages release IL-1β primarily involve the activation of inflammasomes [45], which are cytoplasmic multi-protein complexes implicated in the pathophysiology of preterm labor and birth in the context of intra-amniotic infection [46–49] or sterile intra-amniotic inflammation [50, 47, 51, 49].
An increased number of CD4+ T cells, but not CD8+ T cells, was observed in the amniotic cavity of women with preterm clinical chorioamnionitis. This is in line with recent reports showing that fetal CD4+ T cells play a central role in the inflammatory processes driven by microbes invading the amniotic cavity in women without clinical chorioamnionitis [33, 52]. A central question that arose from these studies is: What is the function of fetal CD4+ T cells in intra-amniotic infection? Recent studies have shown that amniotic fluid fetal T cells can undergo ex vivo activation [52]. Yet, the functionality of these adaptive immune cells needs additional experimentation.
It is worth mentioning that women diagnosed with preterm clinical chorioamnionitis but without intra-amniotic infection (a negative amniotic fluid culture and low intra-amniotic inflammation) have similar numbers of amniotic fluid leukocytes to those with normal pregnancy [32]. This observation suggests that some women diagnosed with preterm clinical chorioamnionitis are not undergoing microbial invasion of the amniotic cavity and thus are not at risk of delivering a preterm neonate. These data also support the call for reexamination of the criteria used to diagnose preterm clinical chorioamnionitis as was recently suggested using samples collected from a South Korean population [18].
In summary, we found that neutrophils, monocytes/macrophages, and CD4+ T cells are increased in the amniotic cavity of women with preterm clinical chorioamnionitis and culturable bacteria. Neutrophils mainly performed phagocytosis and NET formation in the presence of bacteria, whereas monocytes/macrophages released pro-inflammatory cytokines such as IL-1β in amniotic fluid. Interestingly, women with clinical chorioamnionitis who delivered preterm presented mildly distinct cellular responses in amniotic fluid compared to those without this clinical condition. Taken together, these data provide the first morphologic and phenotypic characterization of the cellular immune responses in the amniotic cavity of women with preterm clinical chorioamnionitis.
Supplementary Material
Acknowledgements:
We gratefully acknowledge Yaozhu Leng, MSc, and Valeria Garcia-Flores, PhD, for their help performing some of the bacterial staining included in this study, and Kenichiro Motomura for providing helpful discussion of the findings. We thank the physicians and nurses from the Center for Advanced Obstetrical Care and Research and the Intrapartum Unit for their help in collecting human samples. The authors also thank the staff members of the PRB Clinical Laboratory and the PRB Histology/Pathology Unit for the processing and examination of the pathological sections.
Funding: This research was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C. N. G.-L. was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health.
Footnotes
Disclosure Statement: The authors have no financial conflicts of interest.
REFERENCES
- 1.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385(9966):430–40. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371(9606):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iams JD, Romero R, Culhane JF, Goldenberg RL. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet. 2008;371(9607):164–75. doi: 10.1016/S0140-6736(08)60108-7. [DOI] [PubMed] [Google Scholar]
- 4.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191(4):1339–45. doi: 10.1016/j.ajog.2004.06.085. [DOI] [PubMed] [Google Scholar]
- 5.Sung JH, Choi SJ, Oh SY, Roh CR, Kim JH. Revisiting the diagnostic criteria of clinical chorioamnionitis in preterm birth. BJOG. 2017;124(5):775–83. doi: 10.1111/1471-0528.14176. [DOI] [PubMed] [Google Scholar]
- 6.Casey BM, Cox SM. Chorioamnionitis and endometritis. Infect Dis Clin North Am. 1997;11(1):203–22. [DOI] [PubMed] [Google Scholar]
- 7.Romero R, Kadar N, Vaisbuch E, Hassan SS. Maternal death following cardiopulmonary collapse after delivery: amniotic fluid embolism or septic shock due to intrauterine infection? Am J Reprod Immunol. 2010;64(2):113–25. doi: 10.1111/j.1600-0897.2010.00823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pappas A, Kendrick DE, Shankaran S, Stoll BJ, Bell EF, Laptook AR et al. Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. JAMA Pediatr. 2014;168(2):137–47. doi: 10.1001/jamapediatrics.2013.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Randis TM, Rice MM, Myatt L, Tita ATN, Leveno KJ, Reddy UM et al. Incidence of early-onset sepsis in infants born to women with clinical chorioamnionitis. J Perinat Med. 2018;46(8):926–33. doi: 10.1515/jpm-2017-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu YW, Colford JM, Jr. Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA. 2000;284(11):1417–24. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 11.Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA. 2003;290(20):2677–84. doi: 10.1001/jama.290.20.2677. [DOI] [PubMed] [Google Scholar]
- 12.Lee SE, Romero R, Kim CJ, Shim SS, Yoon BH. Funisitis in term pregnancy is associated with microbial invasion of the amniotic cavity and intra-amniotic inflammation. J Matern Fetal Neonatal Med. 2006;19(11):693–7. doi: 10.1080/14767050600927353. [DOI] [PubMed] [Google Scholar]
- 13.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50(3):652–83. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 14.Romero R, Chaemsaithong P, Korzeniewski SJ, Tarca AL, Bhatti G, Xu Z et al. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med. 2016;44(1):5–22. doi: 10.1515/jpm-2015-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Tarca AL, Bhatti G et al. Clinical chorioamnionitis at term IV: the maternal plasma cytokine profile. J Perinat Med. 2016;44(1):77–98. doi: 10.1515/jpm-2015-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Tarca AL, Bhatti G et al. Clinical chorioamnionitis at term V: umbilical cord plasma cytokine profile in the context of a systemic maternal inflammatory response. J Perinat Med. 2016;44(1):53–76. doi: 10.1515/jpm-2015-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Romero R, Chaemsaithong P, Docheva N, Korzeniewski SJ, Kusanovic JP, Yoon BH et al. Clinical chorioamnionitis at term VI: acute chorioamnionitis and funisitis according to the presence or absence of microorganisms and inflammation in the amniotic cavity. J Perinat Med. 2016;44(1):33–51. doi: 10.1515/jpm-2015-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oh KJ, Kim SM, Hong JS, Maymon E, Erez O, Panaitescu B et al. Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation. Am J Obstet Gynecol. 2017;216(6):604 e1–e11. doi: 10.1016/j.ajog.2017.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med. 2015;43(1):19–36. doi: 10.1515/jpm-2014-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Varea A, Romero R, Xu Y, Miller D, Ahmed AI, Chaemsaithong P et al. Clinical chorioamnionitis at term VII: the amniotic fluid cellular immune response. J Perinat Med. 2017;45(5):523–38. doi: 10.1515/jpm-2016-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomez-Lopez N, Romero R, Maymon E, Kusanovic JP, Panaitescu B, Miller D et al. Clinical chorioamnionitis at term IX: in vivo evidence of intra-amniotic inflammasome activation. J Perinat Med. 2019;47(3):276–87. doi: 10.1515/jpm-2018-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S29–52. doi: 10.1016/j.ajog.2015.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gibbs RS. Diagnosis of intra-amniotic infection. Semin Perinatol. 1977;1(1):71–7. [PubMed] [Google Scholar]
- 24.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. The Journal of infectious diseases. 1982;145(1):1–8. doi: 10.1093/infdis/145.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Romero R, Chaemsaithong P, Korzeniewski SJ, Kusanovic JP, Docheva N, Martinez-Varea A et al. Clinical chorioamnionitis at term III: how well do clinical criteria perform in the identification of proven intra-amniotic infection? J Perinat Med. 2016;44(1):23–32. doi: 10.1515/jpm-2015-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185(5):1130–6. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 27.Gomez-Lopez N, Romero R, Xu Y, Miller D, Unkel R, Shaman M et al. Neutrophil Extracellular Traps in the Amniotic Cavity of Women with Intra-Amniotic Infection: A New Mechanism of Host Defense. Reprod Sci. 2017;24(8):1139–53. doi: 10.1177/1933719116678690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Lopez N, Romero R, Garcia-Flores V, Xu Y, Leng Y, Alhousseini A et al. Amniotic fluid neutrophils can phagocytize bacteria: A mechanism for microbial killing in the amniotic cavity. Am J Reprod Immunol. 2017;78(4). doi: 10.1111/aji.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCarthy DA, Rampton DS, Liu YC. Peripheral blood neutrophils in inflammatory bowel disease: morphological evidence of in vivo activation in active disease. Clinical and experimental immunology. 1991;86(3):489–93. doi: 10.1111/j.1365-2249.1991.tb02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–5. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 31.Sil P, Wicklum H, Surell C, Rada B. Macrophage-derived IL-1beta enhances monosodium urate crystal-triggered NET formation. Inflamm Res. 2017;66(3):227–37. doi: 10.1007/s00011-016-1008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez-Lopez N, Romero R, Xu Y, Miller D, Leng Y, Panaitescu B et al. The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. Am J Reprod Immunol. 2018;79(4):e12827. doi: 10.1111/aji.12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez-Lopez N, Roberto R, Galaz J, Xu Y, Panaitescu B, Slutsky R et al. Cellular immune responses in amniotic fluid of women with preterm labor and intra-amniotic infection or intra-amniotic inflammation. Am J Reprod Immunol. 2019:e13171. doi: 10.1111/aji.13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165(4 Pt 1):821–30. [DOI] [PubMed] [Google Scholar]
- 35.Gomez-Lopez N, Romero R, Xu Y, Leng Y, Garcia-Flores V, Miller D et al. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol. 2017;217(6):693 e1–e16. doi: 10.1016/j.ajog.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol. 1994;32(3):200–10. [DOI] [PubMed] [Google Scholar]
- 37.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13(1):2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 38.Gravett MG, Novy MJ, Rosenfeld RG, Reddy AP, Jacob T, Turner M et al. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA. 2004;292(4):462–9. doi: 10.1001/jama.292.4.462. [DOI] [PubMed] [Google Scholar]
- 39.Romero R, Kusanovic JP, Gotsch F, Erez O, Vaisbuch E, Mazaki-Tovi S et al. Isobaric labeling and tandem mass spectrometry: a novel approach for profiling and quantifying proteins differentially expressed in amniotic fluid in preterm labor with and without intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2010;23(4):261–80. doi: 10.3109/14767050903067386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novakovic TR, Dolicanin ZC, Djordjevic NZ. Effects of maternal subclinical hypothyroidism on amniotic fluid cells oxidative status. Reprod Toxicol. 2018;78:97–101. doi: 10.1016/j.reprotox.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 41.Chen KW, Gross CJ, Sotomayor FV, Stacey KJ, Tschopp J, Sweet MJ et al. The neutrophil NLRC4 inflammasome selectively promotes IL-1beta maturation without pyroptosis during acute Salmonella challenge. Cell Rep. 2014;8(2):570–82. doi: 10.1016/j.celrep.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 42.Kono H, Orlowski GM, Patel Z, Rock KL. The IL-1-dependent sterile inflammatory response has a substantial caspase-1-independent component that requires cathepsin C. J Immunol. 2012;189(7):3734–40. doi: 10.4049/jimmunol.1200136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomez-Lopez N, Romero R, Leng Y, Xu Y, Slutsky R, Levenson D et al. The Origin of Amniotic Fluid Monocytes/Macrophages in Women with Intra-Amniotic Inflammation and/or Infection. J Perinat Med. 2019;47(8):822–40. doi: 10.1515/jpm-2019-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 45.Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Molecular cell. 2002;10(2):417–26. [DOI] [PubMed] [Google Scholar]
- 46.Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Leng Y et al. A Role for the Inflammasome in Spontaneous Preterm Labor With Acute Histologic Chorioamnionitis. Reprod Sci. 2017;24(10):1382–401. doi: 10.1177/1933719116687656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomez-Lopez N, Romero R, Panaitescu B, Leng Y, Xu Y, Tarca AL et al. Inflammasome activation during spontaneous preterm labor with intra-amniotic infection or sterile intra-amniotic inflammation. Am J Reprod Immunol. 2018;80(5):e13049. doi: 10.1111/aji.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faro J, Romero R, Schwenkel G, Garcia-Flores V, Arenas-Hernandez M, Leng Y et al. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasomedagger. Biol Reprod. 2019;100(5):1290–305. doi: 10.1093/biolre/ioy261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomez-Lopez N, Motomura K, Miller D, Garcia-Flores V, Galaz J, Romero R. Inflammasomes: Their role in normal and complicated pregnancies. J Immunol. In Press, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Plazyo O, Romero R, Unkel R, Balancio A, Mial TN, Xu Y et al. HMGB1 Induces an Inflammatory Response in the Chorioamniotic Membranes That Is Partially Mediated by the Inflammasome. Biol Reprod. 2016;95(6):130. doi: 10.1095/biolreprod.116.144139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomez-Lopez N, Romero R, Garcia-Flores V, Leng Y, Miller D, Hassan SS et al. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomesdagger. Biol Reprod. 2019;100(5):1306–18. doi: 10.1093/biolre/ioy264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gomez-Lopez N, Romero R, Xu Y, Miller D, Arenas-Hernandez M, Garcia-Flores V et al. Fetal T-cell activation in the amniotic cavity during preterm labor: A potential etiology for a subset of idiopathic preterm birth. J Immunol. 2019;203(7):1793–807. doi: 10.4049/jimmunol.1900621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


