Abstract
Background:
Preterm birth is the leading cause of perinatal morbidity and mortality. Preterm prelabor rupture of membranes (pPROM) occurs in 30% of preterm births, and is thus a major contributor to maternal and neonatal morbidity. However, the cellular immune responses in amniotic fluid of women with pPROM have not been investigated.
Methods:
Amniotic fluid samples were obtained from women with pPROM and a positive (n=7) or negative (n=10) microbiological culture. Flow cytometry was performed to evaluate the phenotype and number of amniotic fluid leukocytes. The correlation between amniotic fluid immune cells and interleukin-6 concentration or white blood cell count in amniotic fluid was calculated.
Results:
Women with pPROM and a positive amniotic fluid culture had: 1) a greater number of total leukocytes in amniotic fluid, including neutrophils and monocytes/macrophages, and 2) increased numbers of total T cells in amniotic fluid, namely CD4+ T cells and CD8+ T cells, but not B cells. The numbers of neutrophils and monocytes/macrophages were positively correlated with IL-6 concentrations and WBC counts in amniotic fluid of women with pPROM.
Conclusion:
Women with pPROM and a positive amniotic fluid culture exhibit a more severe cellular immune response than those with a negative culture, which is associated with well-known markers of intra-amniotic inflammation.
Keywords: Acute chorioamnionitis, Clinical chorioamnionitis, Fetal inflammatory response, Funisitis, Innate immune cells, Interleukin-6, Labor, Microbial invasion of the amniotic cavity, Pregnancy, Preterm labor, Prematurity
INTRODUCTION
Preterm birth is the leading cause of perinatal morbidity and mortality worldwide (1–3). Approximately 30% of preterm births are preceded by preterm prelabor rupture of membranes (pPROM) (4–12), defined as the rupture of the chorioamniotic membranes before the onset of preterm labor (i.e. <37 weeks) (4, 6–12), and thus pPROM is a major contributor to maternal and neonatal morbidity (13–31). This clinical condition is considered a great obstetrical syndrome (32–36), which has recently been subcategorized using microbiological techniques coupled with amniotic fluid concentrations of interleukin-6 (IL-6) (37–39). Hence, it is now established that pPROM can occur in the presence of either intra-amniotic infection [detectable microorganisms in amniotic fluid in the presence of elevated concentrations of IL-6 (≥2.6 ng/mL)], sterile intra-amniotic inflammation (elevated concentrations of IL-6 in the absence of detectable microorganisms), or in the absence of intra-amniotic inflammation (IL-6 concentration <2.6 ng/mL) (38). Although the clinical management of pPROM includes antibiotic administration (40–45), it is well documented that the intensity of the intra-amniotic inflammatory response is associated with the severity of fetal inflammatory responses (37, 38, 46–48). Indeed, neonates born to women with pPROM and intra-amniotic infection are at a higher risk of neonatal morbidity than those born to women with intra-amniotic inflammation (37, 38, 47). Therefore, the characterization of the immune response in amniotic fluid of women with pPROM is warranted.
The amniotic fluid contains a diverse array of innate and adaptive immune cells that varies as normal gestation progresses (49). Specifically, neutrophils, monocytes/macrophages, T cells, innate lymphoid cells, natural killer (NK) cells, and B cells are present in the amniotic cavity of women with a normal pregnancy (49–51). These cellular immune responses are augmented in women with pregnancy complications such as preterm labor with intact membranes (52), clinical chorioamnionitis at term (53), and preterm clinical chorioamnionitis (54). However, the cellular immune responses in amniotic fluid of women with pPROM have not been investigated.
Herein, we utilized multi-color flow cytometry to investigate the cellular composition of amniotic fluid from women who underwent pPROM with or without positive microbiological cultures. Moreover, we correlated the number of amniotic fluid immune cells with well-known markers of intra-amniotic inflammation (white blood cell count and IL-6 concentration).
METHODS
Study design and population:
This retrospective cross-sectional study was conducted by searching our clinical database and bank of biological samples. The collection of samples was approved by the Institutional Review Boards of the Detroit Medical Center (Detroit, MI, USA), Wayne State University, and the Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services. All women provided written informed consent prior to the collection of amniotic fluid.
This study included 17 amniotic fluid samples collected from patients with pPROM, and a reachable amniotic fluid pocket, and either a positive amniotic fluid microbiological culture (n=7) or a negative amniotic fluid microbiological culture (n=10) (see clinical definitions and amniotic fluid sample collection below) (Table 1). For all patients, the amniocentesis was performed after the diagnosis of pPROM and the time between the collection of the amniotic fluid sample and rupture of membranes was ≤2 days (this criterion was used to preserve a meaningful relationship between amniotic fluid studies and pPROM). The demographic and clinical characteristics of the study population are shown in Table 1. Multiple pregnancies, fetal malformations and genetic disorders were excluded from this study.
Table 1.
Clinical and Demographic characteristics of women with pPROM.
| Negative culture (n=10) | Positive culture (n=7) | p-value | |
|---|---|---|---|
| Maternal age (years; median [IQR])a | 26.5 (25.3–31.3) | 33 (28.5–33.5) | 0.46 |
| Body mass index (kg/m2; median [IQR])a | 29.1 (23.8–33.7) | 32 (24.6–36.6)c | 0.85 |
| Primiparityb | 30% (3/10) | 0% (0/7) | 0.23 |
| Race/Ethnicityb | 0.74 | ||
| African-American | 80% (8/10) | 71.4% (5/7) | |
| White | 10% (1/10) | 28.6% (2/7) | |
| Other | 10% (1/10) | 0% (0/7) | |
| Gestational age at amniocentesis (weeks; median [IQR])a | 30.6 (26–33) | 25.6 (22.6–31.9) | 0.65 |
| IL-6 (ng/mL; median [IQR])a | 0.2 (0.1–0.4) | 102.8 (22–125.3) | 0.003 |
| Amniotic Fluid Glucose (mg/dl; median [IQR])a | 33.5 (23–41) | 10 (1–15) | 0.003 |
| Amniotic Fluid WBC (cells/mm3; median [IQR])a | 1.5 (0–3.5) | 60 (7.5–319.5) | 0.007 |
| Gestational age at membrane rupture (weeks; median [IQR])a | 30.5 (26–32.9) | 25.4 (22.5–31.9) | 0.67 |
| Gestational age at delivery (weeks; median [IQR])a | 33 (28.4–33.9) | 25.7 (23.1–32.1) | 0.38 |
| Cesarean sectionb | 10% (1/10) | 14.3% (1/7) | 1.0 |
| Birthweight (grams; median [IQR])a | 1725 (1127.5–1850) | 840 (527–2025) | 0.88 |
| Acute maternal inflammatory responseb | |||
| Stage 1 (Early acute subchorionitis or chorionitis) | 40% (4/10) | 0% (0/6)d | 0.23 |
| Stage 2 (Acute chorioamnionitis) | 30% (3/10) | 16.7% (1/6)d | 1.0 |
| Stage 3 (Necrotizing chorioamnionitis) | 0% (0/10) | 50% (3/6)d | 0.036 |
| Acute fetal inflammatory responseb | |||
| Stage 1 (Chorionic vasculitis or umbilical phlebitis) | 20% (2/10) | 50% (3/6)d | 0.3 |
| Stage 2 (Umbilical arteritis) | 10%(1/10) | 16.7 % (1/6)d | 1.0 |
| Stage 3 (Necrotizing funisitis) | 10% (1/10) | 0% (0/6)d | 1.0 |
Data are given as median (interquartile range, IQR) and percentage (n/N).
Mann-Whitney test.
Fisher’s exact test
Two missing data.
One missing data.
Abbreviations: pPROM, preterm prelabor rupture of membranes; WBC, white blood cells
Clinical definitions
Gestational age was determined by the date of the last menstrual period and confirmed by ultrasound examination. The gestational age derived from sonographic fetal biometry was used if the estimation was inconsistent with menstrual dating. Preterm PROM was defined as amniorrhexis confirmed by vaginal pooling, ferning, or a positive nitrazine test prior to the onset of labor before 37 weeks of gestation (55–57).
Placental histopathological examination
Placentas were examined histologically by perinatal pathologists blinded to clinical diagnoses and obstetrical outcomes according to standardized Perinatology Research Branch protocols (58, 59). Briefly, three to nine sections of the placenta were examined, and at least one full-thickness section was taken from the center of the placenta; others were taken randomly from the placental disc. Acute inflammatory lesions of the placenta (maternal inflammatory response and fetal inflammatory response) were diagnosed according to established criteria, including staging and grading (58, 60). The proportions of patients whose placentas presented acute maternal and/or fetal inflammatory responses are displayed in Table 1.
Amniotic fluid sample collection
Amniotic fluid samples were obtained by transabdominal amniocentesis under antiseptic conditions and monitored by ultrasound in order to detect intra-amniotic inflammation and/or infection in patients with pPROM. Samples of amniotic fluid were transported to the laboratory in a sterile, capped syringe and immunophenotyping was performed immediately. The rest of the sample was centrifuged at 1300 x g for 10 minutes at 4°C, and the supernatant was stored at −80°C until use. Additionally, an aliquot of amniotic fluid was transported to the clinical laboratory for culture of aerobic/anaerobic bacteria and genital mycoplasmas. The clinical laboratory also performed tests to determine an amniotic fluid white blood cell (WBC) count (61), a Gram stain examination (62), and a glucose concentration (63).
Determination of IL-6 concentration in amniotic fluid
Amniotic fluid concentrations of IL-6 were determined, as previously established (64) using a sensitive and specific enzyme immunoassay obtained from R&D systems (Minneapolis, MN, USA). The IL-6 concentrations were determined by interpolation from the standard curves. The inter- and intra-assay coefficients of variation for IL-6 were 8.7% and 4.6%, respectively. The sensitivity of the IL-6 assay was 0.09 pg/mL.
Immunophenotyping by flow cytometry
Amniotic fluid samples (0.5–1 mL) were centrifuged at 300 x g for 5 minutes at room temperature. The resulting amniotic fluid pellet was resuspended in 1 mL of 1X phosphate-buffered saline (PBS) (Life Technologies, Grand Island, NY, USA) and stained with BD Horizon Fixable Viability Stain 510 dye (BD Biosciences, San Jose, CA, USA). Cells were washed in 1X PBS and incubated with 20 μL of human FcR blocking reagent (Miltenyi Biotec, San Diego, CA, USA) in 80 μL of stain buffer (BD Biosciences) for 10 minutes at 4°C. Next, cells were incubated with extracellular fluorochrome-conjugated anti-human monoclonal antibodies for 30 minutes at 4°C in the dark (Supplementary Table 1). Stained cells were then washed with 1X PBS, resuspended in 0.5 mL of stain buffer, and acquired using the BD LSR II or LSRFortessa Flow Cytometer (BD Bioscience) and BD FACSDiva 6.0 software (BD Bioscience). The analysis was performed, and the figures were generated using the FlowJo version 10 software (FlowJo, Ashland, OR, USA). The absolute number of cells was determined using CountBright absolute counting beads (Molecular Probes, Eugene, OR, USA).
Statistical analysis
Statistical analyses were conducted using GraphPad Prism version 8.0.1 for Windows (GraphPad Software, San Diego, California, USA, www.graphpad.com). For patient demographics, the Mann-Whitney U-test was used to compare continuous variables and the Fisher’s exact test was used for nominal variables. The Mann-Whitney U-test was performed to compare the number of amniotic fluid immune cells between the two study groups: two-tailed p-values are reported. The Spearman correlation between amniotic fluid immune cells and IL-6 concentration or white blood cell count in amniotic fluid was calculated. A p-value <0.05 was considered statistically significant for all tests.
RESULTS
Characteristics of the study population
The clinical and demographic characteristics of the study population are shown in Table 1. A total of 17 amniotic fluid samples were collected from women with pPROM. Ten women had amniotic fluid microbiological cultures that were negative for bacterial growth and seven women had positive amniotic fluid microbiological cultures. Women with pPROM and positive cultures had significantly higher amniotic fluid concentrations of IL-6 and white blood cells, and lower glucose concentrations, compared to those with negative cultures (Table 1). There were no significant differences in maternal age, primiparity, pre-pregnancy body mass index, race, gestational age at amniocentesis and delivery, or birthweight between the two study groups, which may be due to the number of samples included in this study (Table 1). The most common microorganism found in amniotic fluid from women with pPROM and a positive microbiological culture was Ureaplasma urealyticum. Out of the seven patients with a positive microbial culture: three patients had only Ureaplasma spp; one patient had only Actinomyces odontoliticus; one patient had Bacteroides, Gardnerella vaginalis, and Ureaplasma urealyticum; one patient had Candida spp., Group B Streptococcus, and Ureaplasma urealyticum; and one patient had both Mycoplasma spp. and Ureaplasma spp. Patients with a positive culture had a greater prevalence of necrotizing chorioamnionitis compared to those with a negative culture (Table 1).
Immune cell populations in amniotic fluid of women with pPROM
A representative image of the flow cytometry gating strategy used to detect leukocytes in amniotic fluid from women with pPROM is shown in Figure 1A. Briefly, viable cells were gated within the single cell population (i.e. singlets), and then further identified as total leukocytes (CD45+ cells), neutrophils (CD45+CD15+CD14− cells), monocytes/macrophages (CD45+CD15−CD14+ cells), B cells (CD45+CD15−CD14−CD3−CD19+ cells), and T cells (CD45+CD15−CD14−CD3+CD19− cells) (Figure 1A). T cells were further subdivided into CD4+ T cells (CD3+CD4+CD8− cells) and CD8+ T cells (CD3+CD4−CD8+ cells) (Figure 1A).
Figure 1. Total leukocytes and innate immune cells in amniotic fluid.

(A) Representative flow cytometry gating strategy showing leukocyte populations in amniotic fluid from women with preterm prelabor rupture of membranes (pPROM). Immune cells were initially gated within the viability gate and CD45+ gate followed by lineage gating for neutrophils (CD45+CD15+CD14−cells), monocytes/macrophages (CD45+CD15−CD14+ cells), T cells (CD45+CD15−CD14−CD3+CD19− cells) and B cells (CD45+CD15−CD14−CD3−CD19+ cells). T cells were subsequently gated for CD4+ T cells (CD3+CD4+CD8− cells) and CD8+ T cells (CD3+CD4−CD8+ cells). Numbers of (B) total leukocytes (CD45+ cells/mL), (C) neutrophils (CD15+ cells/mL), and (D) monocytes/macrophages (CD14+ cells/mL) in amniotic fluid from women with pPROM who had either a negative or positive amniotic fluid culture. N = 7–10 per group. Midlines = median, boxes = interquartile ranges, and whiskers = minimum/maximum ranges. Forward Scatter Height (FSC-H), Forward Scatter Area (FSC-A), and Side Scatter Area (SSC-A).
The overall number of amniotic fluid leukocytes was significantly increased in women with pPROM who had a positive amniotic fluid culture compared to those with a negative culture (Figure 1B). Additionally, quantification of neutrophils and monocytes/macrophages revealed that these innate immune cells were present in higher abundance in amniotic fluid of women with pPROM and a positive culture compared to those with a negative culture (Figure 1C&D).
In addition to innate immune cells, adaptive immune cells (T cells and B cells) are also found in amniotic fluid during normal pregnancy (49). Therefore, we next determined whether the numbers of these adaptive immune cells were altered in amniotic fluid of women from our two study groups. The total T-cell population, CD4+ T cells (i.e. helper T cells), and CD8+ T cells (i.e. cytotoxic T cells) were all significantly increased in amniotic fluid of women with pPROM and a positive culture compared to those with pPROM and a negative culture (Figure 2A–C). The numbers of amniotic fluid B cells in women with pPROM and a positive culture tended to be higher than in women with a negative amniotic fluid culture; however, this increase did not reach statistical significance (Figure 2D).
Figure 2. Adaptive immune cells in amniotic fluid.
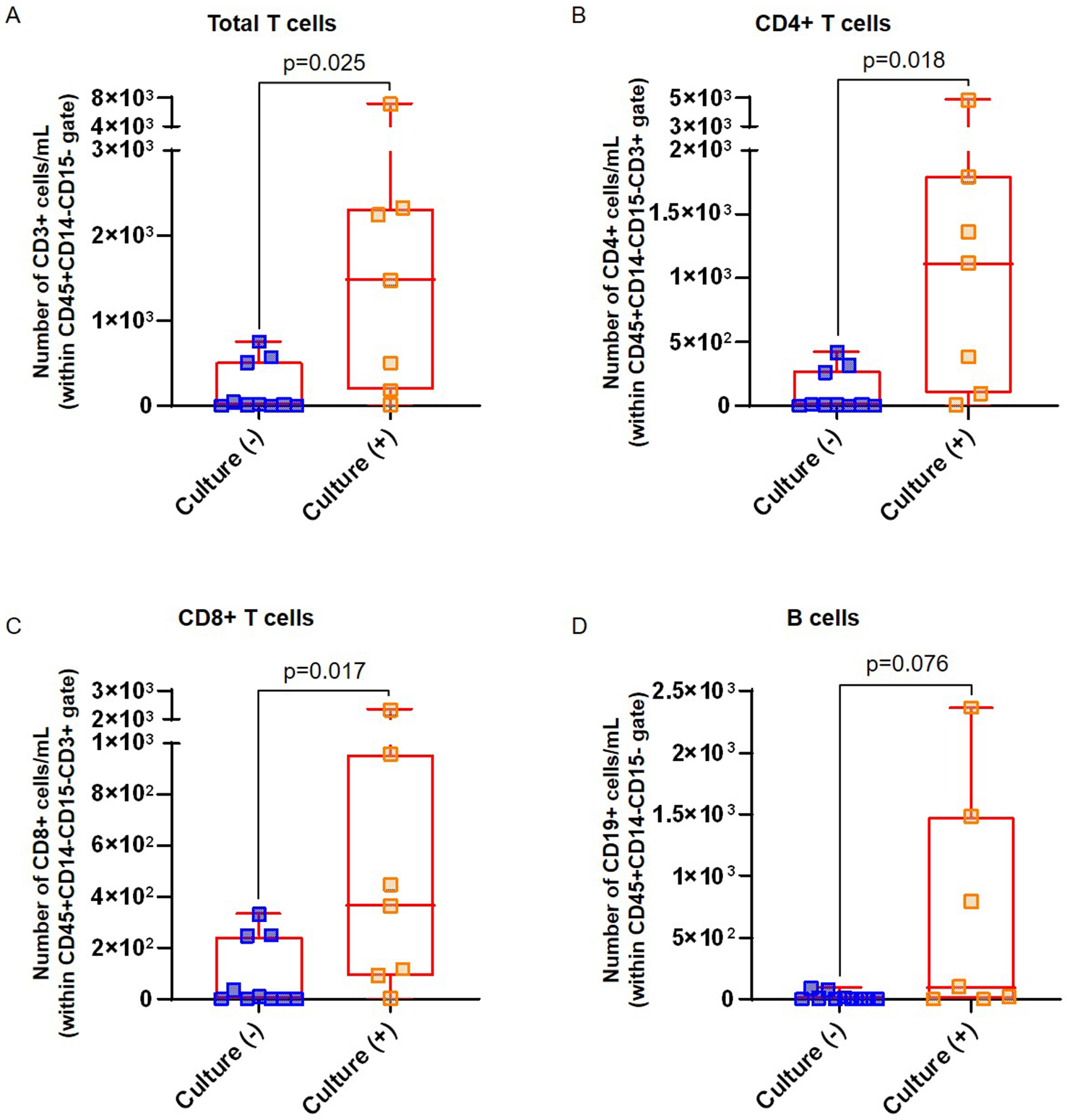
Numbers of (A) total T cells (cells/mL), (B) CD4+ T cells (cells/mL), (C) CD8+ T cells (cells/mL), and (D) B cells (CD19+ cells/mL) in amniotic fluid from women with pPROM who had either a negative or positive amniotic fluid culture. Lymphocyte populations were gated as shown in Figure 1A. N = 7–10 per group. Midlines = median, boxes = interquartile ranges, and whiskers = minimum/maximum ranges.
Taken together, the results of the flow cytometric analysis reveal that total leukocytes, and more specific leukocyte subsets such as neutrophils, monocytes/macrophages, and T cells (CD4+ and CD8+ T cells), are present in higher abundance in amniotic fluid of women with pPROM and a positive amniotic fluid culture compared to those with a negative culture.
Correlation between the number of innate immune cells and clinical inflammatory markers in amniotic fluid of women with pPROM
Next, we determined whether the numbers of the most abundant subsets of amniotic fluid leukocytes (neutrophils and monocytes/macrophages) in women with pPROM were correlated with the IL-6 concentrations and WBC counts measured in amniotic fluid. We found that increasing concentrations of the pro-inflammatory cytokine IL-6 were positively correlated with increasing abundance of neutrophils in amniotic fluid (Figure 3A, r=0.7, p=0.002). Similarly, increasing IL-6 concentrations were positively correlated with the presence of elevated numbers of amniotic fluid monocytes/macrophages, though this relationship was slightly less strong than that with neutrophils (Figure 3B, r=0.63, p=0.007). Positive correlations between WBC counts and neutrophils (Figure 3C, r=0.66, p=0.005) and monocytes/macrophages (Figure 3D, r=0.68, p=0.003) were also found in amniotic fluid. These results showed that the number of innate immune cells in amniotic fluid positively correlate with well-known markers of intra-amniotic inflammation in women with pPROM.
Figure 3. Correlation between neutrophils or monocytes/macrophages and IL-6 concentrations or white blood cell counts in amniotic fluid.

The relationship between amniotic fluid concentrations of IL-6 and the number of neutrophils (CD15+ cells) (A) or monocytes/macrophages (CD14+ cells) (B) in amniotic fluid of women with pPROM. The correlation between amniotic fluid white blood cell counts obtained by hemocytometer and the number of neutrophils (CD15+ cells) (C) or monocytes/macrophages (CD14+ cells) (D) in amniotic fluid of women with pPROM. Correlations were assessed using a Spearman’s test. Correlation coefficients and p values are shown for each plot. The regression line is indicated. N = 17.
DISCUSSION
Principle Findings
Herein, we report that women with pPROM and a positive amniotic fluid culture had: 1) a greater number of total leukocytes in amniotic fluid including neutrophils and monocytes/macrophages, and 2) increased numbers of total T cells in amniotic fluid, namely CD4+ T cells and CD8+ T cells, but not B cells. Further, we report that the number of neutrophils and monocytes/macrophages were positively correlated with IL-6 concentrations and WBC counts in amniotic fluid of women with pPROM. Collectively, these findings indicate that women with pPROM and a positive amniotic fluid culture exhibit a more severe cellular immune response than those with a negative culture, which is associated with well-known markers of intra-amniotic inflammation.
Amniotic fluid neutrophils in women with pPROM
It is well established that neutrophils are the most abundant immune cell type in the amniotic cavity of women with intra-amniotic infection (52–54, 61, 65, 66). These innate immune cells can be predominantly of fetal origin in preterm gestations and of maternal origin at term (66). However, whether the number of amniotic fluid neutrophils differs in the context of pPROM with and without culturable microorganisms had not yet been shown. In the current study, we showed that the number of neutrophils in amniotic fluid is significantly higher in women with pPROM and a positive microbiological culture than in those without culturable microorganisms. Studies investigating the functions of amniotic fluid neutrophils have shown that these innate immune cells can phagocytize bacteria invading the amniotic cavity (67), form neutrophil extracellular traps (NETs) (68) and may degranulate, releasing anti-microbial molecules such as myeloperoxidase (69–71), alpha-defensins (70, 72–74), elastase (70, 75, 76), cathepsin G (70, 77), lactoferrin (78), pentraxin-3 (79), and cathelicidin (69, 70) as well as reactive oxygen species (80) into the amniotic cavity. In addition to participating in the host defense response to microbes, neutrophils can also release pro-inflammatory cytokines such as IL-8, TNF-α, MIP-1α, MIP-1β, IL-1α, and IL-1β, which are implicated in the mechanisms leading to premature labor in the context of intra-amniotic infection (52, 53, 81–94). Furthermore, amniotic fluid neutrophils may also participate in the pathogenesis of pPROM by releasing neutrophil elastase and metalloproteinases (76, 95–102); yet, this hypothesis has not been mechanistically investigated.
It is worth mentioning that amniotic fluid neutrophils are increased in all reported pregnancy complications associated with intra-amniotic inflammation (52–54), suggesting that these innate immune cells participate in the common pathway of parturition and host defense mechanisms taking place in the amniotic cavity regardless of the obstetrical syndrome. Further studies may be required to investigate whether amniotic fluid neutrophils diverge in signaling pathways among the different pregnancy complications associated with prematurity and/or adverse neonatal outcomes.
Amniotic fluid monocytes/macrophages in women with pPROM
In the current study, flow cytometric analysis also revealed that monocyte/macrophages, which are the second most abundant leukocyte population in amniotic fluid (49, 52–54, 61), are increased in women with pPROM and a positive amniotic fluid culture. This is in line with previous studies showing that amniotic fluid monocytes/macrophages are elevated in women with preterm labor and intact membranes (52), clinical chorioamnionitis at term (53), and preterm clinical chorioamnionitis (54) with a positive microbiological culture.
Recently, the origin of the amniotic fluid monocytes/macrophages in women with intra-amniotic inflammation or infection was described (103). Specifically, amniotic fluid monocytes/macrophages were found to be predominantly of maternal origin in women with intra-amniotic inflammation/infection who delivered late preterm or term neonates, whereas most samples with predominantly fetal monocytes/macrophages in amniotic fluid were from women who delivered early preterm neonates (103).
The classical function of monocytes is to produce and release pro-inflammatory mediators such as cytokines (104); however, their functions can vary according to the microenvironment (104–107). Indeed, a recent study demonstrated that placental macrophages can respond to microbes by releasing extracellular traps (METs) (108), providing further evidence that these cells can have additional functions in the amniotic cavity beyond cytokine release. Yet, we have shown that monocytes/macrophages release different cytokines than those released by neutrophils, indicating that both cells have distinct and specific functions in the amniotic cavity (52, 53). For example, we previously showed that IL-1β is expressed in greater amounts by monocytes/macrophages than by neutrophils in women with clinical chorioamnionitis at term and a positive microbiological culture (52, 53). Nonetheless, additional research is required to investigate the diverse signaling pathways between neutrophils and monocytes in the context of intra-amniotic inflammation and/or infection.
Correlation between flow cytometric analysis of amniotic fluid and classical biomarkers of intra-amniotic inflammation
The traditional biomarker of intra-amniotic inflammation has been the amniotic fluid WBC count (27, 61, 65, 109, 110). We have previously shown that amniotic fluid WBC counts obtained using the traditional method (hemocytometer) correlate well with counts obtained using flow cytometry in women with clinical chorioamnionitis at term (53). In line with this finding, we report herein that a significant correlation exists between the numbers of amniotic fluid neutrophils or monocytes/macrophages acquired by flow cytometry and WBC counts obtained by hemocytometer in patients with pPROM.
More recently, the concentration of IL-6 in amniotic fluid was demonstrated to be a reliable biomarker for intra-amniotic inflammation (64, 111). Amniotic fluid IL-6 concentrations were also shown to correlate with the numbers of total leukocytes, neutrophils, or monocytes/macrophages obtained using flow cytometry in women with clinical chorioamnionitis at term (53). Similarly, in the current study we show that IL-6 concentrations are significantly correlated with the numbers of neutrophils or monocytes/macrophages in amniotic fluid of women with pPROM.
Together, these results indicate that flow cytometric analysis of amniotic fluid immune cell composition can provide a reliable overview of the intra-amniotic inflammatory response in women with pPROM.
Conclusion
In the current study, we show that women with pPROM and a positive amniotic fluid microbiological culture have increased numbers of neutrophils, monocytes/macrophages, and T cells (CD4+ and CD8+ T cells) compared to patients with a negative culture. Such an increase was correlated with the concomitant rise in amniotic fluid IL-6 concentrations, and immune cell numbers obtained by flow cytometry were also correlated with traditional WBC counts. These results indicate that the intra-amniotic cellular immune response in patients with pPROM is more severe in the presence of invading microorganisms, and that such a response is primarily driven by neutrophils, monocytes/macrophages, and T cells. These data provide insights into the cellular immune responses taking place in the amniotic cavity of women with pPROM.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported, in part, by the Perinatology Research Branch (PRB), Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U. S. Department of Health and Human Services (NICHD/NIH/DHHS), and, in part, with federal funds from the NICHD/NIH/DHHS under Contract No. HHSN275201300006C. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health. We gratefully acknowledge the physicians and nurses from the Center for Advanced Obstetrical Care and Research and the Intrapartum Unit for their help in collecting human samples. The authors also thank the staff members of the PRB Clinical Laboratory and the PRB Histology/Pathology Unit for the processing and examination of the pathological sections.
Footnotes
Declaration of Interests Statement
We have no conflicts of interest to declare.
REFERENCES
- 1.Blencowe H, Cousens S, Oestergaard MZ, Chou D, Moller AB, Narwal R, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–72. [DOI] [PubMed] [Google Scholar]
- 2.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–40. [DOI] [PubMed] [Google Scholar]
- 3.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. The Lancet Global health. 2019;7:e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Athayde N, Maymon E, Pacora P, Romero R. Premature rupture of fetal membranes In: Ransom S, McNeeley G, Munkarah A, Dumbrowski M, Moghissi K, editors. Practical Strategies in Obstetrics and Gynecology. Philadelphia, PA: Lippincott; 1998. p. 249–56. [Google Scholar]
- 5.Parry S, Strauss JF 3rd. Premature rupture of the fetal membranes. The New England journal of medicine. 1998;338:663–70. [DOI] [PubMed] [Google Scholar]
- 6.Romero R, Athayde N, Maymon E, Pacora P, Bahado-Singh R. Premature rupture of the membranes In: Reece E, Hobbins J, editors. Medicine of the Fetus and Mother. Philadelphia, PA: JB Lippincott; 1999. p. 1581–625. [Google Scholar]
- 7.Mercer BM. Preterm premature rupture of the membranes. Obstetrics and gynecology. 2003;101:178–93. [DOI] [PubMed] [Google Scholar]
- 8.Mercer BM. Preterm premature rupture of the membranes: current approaches to evaluation and management. Obstetrics and gynecology clinics of North America. 2005;32:411–28. [DOI] [PubMed] [Google Scholar]
- 9.Romero R, Goncalves L, Chaiworapongsa T, Kusanovic J, Espinoza J. Mechanisms of preterm labor and preterm premature rupture of the membranes In: Kurjak A, Chervenak F, editors. Textbook of Perinatal Medicine, 2nd Edition London: UK: Informa UK Ltd; 2006. p. 1379–93. [Google Scholar]
- 10.Santolaya-Forgas J, Romero R, Espinoza J, Erez O, Friel L, Kusanovic J, et al. Preterm prelabor rupture of the membranes In: Reece E, Hobbins J, editors. Clinical Obstetrics: The Fetus and Mother, 3rd Edition Oxford, UK: Blackwell Publishing; 2007. p. 1130–88. [Google Scholar]
- 11.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim C, Romero R, Hassan S. The placenta in preterm prelabor rupture of membranes and preterm labor In: Kay H, Nelson M, Wang Y, editors. The Placenta: from Development to Disease, 1st Edition Oxford, UK: Wiley-Blackwell; 2011. p. 222–7. [Google Scholar]
- 13.Johnson JW, Daikoku NH, Niebyl JR, Johnson TR Jr., Khouzami VA, Witter FR. Premature rupture of the membranes and prolonged latency. Obstetrics and gynecology. 1981;57:547–56. [PubMed] [Google Scholar]
- 14.Gibbs RS, Blanco JD. Premature rupture of the membranes. Obstetrics and gynecology. 1982;60:671–9. [PubMed] [Google Scholar]
- 15.Averbuch B, Mazor M, Shoham-Vardi I, Chaim W, Vardi H, Horowitz S, et al. Intra-uterine infection in women with preterm premature rupture of membranes: maternal and neonatal characteristics. Eur J Obstet Gynecol Reprod Biol. 1995;62:25–9. [DOI] [PubMed] [Google Scholar]
- 16.Ladfors L, Tessin I, Mattsson LA, Eriksson M, Seeberg S, Fall O. Risk factors for neonatal sepsis in offspring of women with prelabor rupture of the membranes at 34–42 weeks. J Perinat Med. 1998;26:94–101. [DOI] [PubMed] [Google Scholar]
- 17.Ghezzi F, Gomez R, Romero R, Yoon BH, Edwin SS, David C, et al. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol. 1998;78:5–10. [DOI] [PubMed] [Google Scholar]
- 18.Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS, et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000;183:1130–7. [DOI] [PubMed] [Google Scholar]
- 19.Tsiartas P, Kacerovsky M, Musilova I, Hornychova H, Cobo T, Savman K, et al. The association between histological chorioamnionitis, funisitis and neonatal outcome in women with preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2013;26:1332–6. [DOI] [PubMed] [Google Scholar]
- 20.Kacerovsky M, Musilova I, Andrys C, Hornychova H, Pliskova L, Kostal M, et al. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am J Obstet Gynecol. 2014;210:325 e1–e10. [DOI] [PubMed] [Google Scholar]
- 21.Tchirikov M, Schlabritz-Loutsevitch N, Maher J, Buchmann J, Naberezhnev Y, Winarno AS, et al. Mid-trimester preterm premature rupture of membranes (PPROM): etiology, diagnosis, classification, international recommendations of treatment options and outcome. J Perinat Med. 2018;46:465–88. [DOI] [PubMed] [Google Scholar]
- 22.Kiver V, Boos V, Thomas A, Henrich W, Weichert A. Perinatal outcomes after previable preterm premature rupture of membranes before 24 weeks of gestation. J Perinat Med. 2018;46:555–65. [DOI] [PubMed] [Google Scholar]
- 23.Sim WH, Ng H, Sheehan P. Maternal and neonatal outcomes following expectant management of preterm prelabor rupture of membranes before viability. J Matern Fetal Neonatal Med. 2018:1–9. [DOI] [PubMed] [Google Scholar]
- 24.Muller H, Storbeck T, Katzer D, Bruns N, Wossner-Stegmann G, Ai M, et al. Neurological outcome at 24 months corrected age of prematurely born infants after preterm premature rupture of membranes (PPROM) of at least 7 days: a two-center experience in Germany. J Matern Fetal Neonatal Med. 2018:1–6. [DOI] [PubMed] [Google Scholar]
- 25.Lorthe E, Torchin H, Delorme P, Ancel PY, Marchand-Martin L, Foix-L’Helias L, et al. Preterm premature rupture of membranes at 22–25 weeks’ gestation: perinatal and 2-year outcomes within a national population-based study (EPIPAGE-2). Am J Obstet Gynecol. 2018;219:298 e1–e14. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez-Trujillo A, Rios J, Angeles MA, Posadas DE, Murillo C, Rueda C, et al. Influence of perinatal inflammation on the neurodevelopmental outcome of premature infants. J Matern Fetal Neonatal Med. 2019;32:1069–77. [DOI] [PubMed] [Google Scholar]
- 27.Oh KJ, Romero R, Park JY, Hong JS, Yoon BH. The earlier the gestational age, the greater the intensity of the intra-amniotic inflammatory response in women with preterm premature rupture of membranes and amniotic fluid infection by Ureaplasma species. J Perinat Med. 2019;47:516–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinto S, Malheiro MF, Vaz A, Rodrigues T, Montenegro N, Guimaraes H. Neonatal outcome in preterm deliveries before 34-week gestation - the influence of the mechanism of labor onset. J Matern Fetal Neonatal Med. 2019;32:3655–61. [DOI] [PubMed] [Google Scholar]
- 29.Skupski D Preterm premature rupture of membranes (PPROM). J Perinat Med. 2019;47:491–2. [DOI] [PubMed] [Google Scholar]
- 30.Kachikis A, Walker CL, McAdams RM, Gyamfi-Bannerman C, Adams Waldorf KM. Phenotypic overlap in neonatal respiratory morbidity following preterm premature rupture of membranes versus spontaneous preterm labor. J Matern Fetal Neonatal Med. 2019:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gultekin-Elbir EE, Ford C, Genc MR. The value of amniotic fluid analysis in patients with suspected clinical chorioamnionitis. J Perinat Med. 2019;47:493–9. [DOI] [PubMed] [Google Scholar]
- 32.Romero R The child is the father of the man. Prenat Neonat Med. 1996;1:8–11. [Google Scholar]
- 33.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG. 2006;113 Suppl 3:17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Renzo GC. The great obstetrical syndromes. J Matern Fetal Neonatal Med. 2009;22:633–5. [DOI] [PubMed] [Google Scholar]
- 35.Romero R Prenatal medicine: the child is the father of the man. 1996. J Matern Fetal Neonatal Med. 2009;22:636–9. [DOI] [PubMed] [Google Scholar]
- 36.Brosens I, Pijnenborg R, Vercruysse L, Romero R. The “Great Obstetrical Syndromes” are associated with disorders of deep placentation. Am J Obstet Gynecol. 2011;204:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2010;64:38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2015;28:1394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Musilova I, Kutova R, Pliskova L, Stepan M, Menon R, Jacobsson B, et al. Intraamniotic Inflammation in Women with Preterm Prelabor Rupture of Membranes. PLoS One. 2015;10:e0133929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mercer BM, Arheart KL. Antimicrobial therapy in expectant management of preterm premature rupture of the membranes. Lancet. 1995;346:1271–9. [DOI] [PubMed] [Google Scholar]
- 41.Kenyon SL, Taylor DJ, Tarnow-Mordi W, Group OC. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. ORACLE Collaborative Group. Lancet. 2001;357:979–88. [DOI] [PubMed] [Google Scholar]
- 42.Kenyon S, Boulvain M, Neilson JP. Antibiotics for preterm rupture of membranes. Cochrane Database Syst Rev. 2013:CD001058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Committee on Practice B-O. ACOG Practice Bulletin No. 188: Prelabor Rupture of Membranes. Obstetrics and gynecology. 2018;131:e1–e14. [DOI] [PubMed] [Google Scholar]
- 44.Bouchet N, Joal A, Gayet-Ageron A, Areta ML, Martinez de Tejada B. Impact of the new guidelines on the management of premature rupture of membranes for the prevention of late preterm birth: an 11-year retrospective study. J Perinat Med. 2019;47:341–6. [DOI] [PubMed] [Google Scholar]
- 45.Navathe R, Schoen CN, Heidari P, Bachilova S, Ward A, Tepper J, et al. Azithromycin vs erythromycin for the management of preterm premature rupture of membranes. Am J Obstet Gynecol. 2019;221:144 e1–e8. [DOI] [PubMed] [Google Scholar]
- 46.Kacerovsky M, Cobo T, Andrys C, Musilova I, Drahosova M, Hornychova H, et al. The fetal inflammatory response in subgroups of women with preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med. 2013;26:795–801. [DOI] [PubMed] [Google Scholar]
- 47.Musilova I, Andrys C, Drahosova M, Zednikova B, Hornychova H, Pliskova L, et al. Late preterm prelabor rupture of fetal membranes: fetal inflammatory response and neonatal outcome. Pediatr Res. 2018;83:630–7. [DOI] [PubMed] [Google Scholar]
- 48.Oh KJ, Park JY, Lee J, Hong JS, Romero R, Yoon BH. The combined exposure to intra-amniotic inflammation and neonatal respiratory distress syndrome increases the risk of intraventricular hemorrhage in preterm neonates. J Perinat Med. 2018;46:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gomez-Lopez N, Romero R, Xu Y, Miller D, Leng Y, Panaitescu B, et al. The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. Am J Reprod Immunol. 2018;79:e12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller D, Motomura K, Garcia-Flores V, Romero R, Gomez-Lopez N. Innate Lymphoid Cells in the Maternal and Fetal Compartments. Front Immunol. 2018;9:2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gomez-Lopez N, Romero R, Xu Y, Miller D, Arenas-Hernandez M, Garcia-Flores V, et al. Fetal T Cell Activation in the Amniotic Cavity during Preterm Labor: A Potential Mechanism for a Subset of Idiopathic Preterm Birth. J Immunol. 2019;203:1793–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gomez-Lopez N, Romero R, Galaz J, Xu Y, Panaitescu B, Slutsky R, et al. Cellular immune responses in amniotic fluid of women with preterm labor and intra-amniotic infection or intra-amniotic inflammation. Am J Reprod Immunol. 2019:e13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez-Varea A, Romero R, Xu Y, Miller D, Ahmed AI, Chaemsaithong P, et al. Clinical chorioamnionitis at term VII: the amniotic fluid cellular immune response. J Perinat Med. 2017;45:523–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galaz J, Romero R, Xu Y, Miller D, Slutsky R, Gingell L, et al. Cellular immune responses in amniotic fluid of women with preterm clinical chorioamnionitis. Submitted, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tricomi V, Hall JE, Bittar A, Chambers D. Arborization test for the detection of ruptured fetal membranes. Clinical evaluation. Obstetrics and gynecology. 1966;27:275–9. [PubMed] [Google Scholar]
- 56.Friedman ML, McElin TW. Diagnosis of ruptured fetal membranes. Clinical study and review of the literature. Am J Obstet Gynecol. 1969;104:544–50. [DOI] [PubMed] [Google Scholar]
- 57.Bennett SL, Cullen JB, Sherer DM, Woods JR Jr. The ferning and nitrazine tests of amniotic fluid between 12 and 41 weeks gestation. Am J Perinatol. 1993;10:101–4. [DOI] [PubMed] [Google Scholar]
- 58.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213:S29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romero R, Kim YM, Pacora P, Kim CJ, Benshalom-Tirosh N, Jaiman S, et al. The frequency and type of placental histologic lesions in term pregnancies with normal outcome. Journal of perinatal medicine. 2018;46:613–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Redline RW. Classification of placental lesions. American journal of obstetrics and gynecology. 2015;213:S21–8. [DOI] [PubMed] [Google Scholar]
- 61.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165:821–30. [DOI] [PubMed] [Google Scholar]
- 62.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159:114–9. [DOI] [PubMed] [Google Scholar]
- 63.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163:968–74. [DOI] [PubMed] [Google Scholar]
- 64.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–6. [DOI] [PubMed] [Google Scholar]
- 65.Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169:839–51. [DOI] [PubMed] [Google Scholar]
- 66.Gomez-Lopez N, Romero R, Xu Y, Leng Y, Garcia-Flores V, Miller D, et al. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol. 2017;217:693 e1–e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gomez-Lopez N, Romero R, Garcia-Flores V, Xu Y, Leng Y, Alhousseini A, et al. Amniotic fluid neutrophils can phagocytize bacteria: A mechanism for microbial killing in the amniotic cavity. Am J Reprod Immunol. 2017;78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gomez-Lopez N, Romero R, Xu Y, Miller D, Unkel R, Shaman M, et al. Neutrophil Extracellular Traps in the Amniotic Cavity of Women with Intra-Amniotic Infection: A New Mechanism of Host Defense. Reprod Sci. 2017;24:1139–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gravett MG, Novy MJ, Rosenfeld RG, Reddy AP, Jacob T, Turner M, et al. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA. 2004;292:462–9. [DOI] [PubMed] [Google Scholar]
- 70.Romero R, Kusanovic JP, Gotsch F, Erez O, Vaisbuch E, Mazaki-Tovi S, et al. Isobaric labeling and tandem mass spectrometry: a novel approach for profiling and quantifying proteins differentially expressed in amniotic fluid in preterm labor with and without intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2010;23:261–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Myntti T, Rahkonen L, Nupponen I, Patari-Sampo A, Tikkanen M, Sorsa T, et al. Amniotic Fluid Infection in Preterm Pregnancies with Intact Membranes. Dis Markers. 2017;2017:8167276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heine RP, Wiesenfeld H, Mortimer L, Greig PC. Amniotic fluid defensins: potential markers of subclinical intrauterine infection. Clin Infect Dis. 1998;27:513–8. [DOI] [PubMed] [Google Scholar]
- 73.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13:2–21. [DOI] [PubMed] [Google Scholar]
- 74.Akinbi HT, Narendran V, Pass AK, Markart P, Hoath SB. Host defense proteins in vernix caseosa and amniotic fluid. Am J Obstet Gynecol. 2004;191:2090–6. [DOI] [PubMed] [Google Scholar]
- 75.Rivero-Marcotegui A, Larranaga-Azcarate C, Ceres-Ruiz R, Garcia-Merlo S. Polymorphonuclear elastase and interleukin-6 in amniotic fluid in preterm labor. Clin Chem. 1997;43:857–9. [PubMed] [Google Scholar]
- 76.Helmig BR, Romero R, Espinoza J, Chaiworapongsa T, Bujold E, Gomez R, et al. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med. 2002;12:237–46. [DOI] [PubMed] [Google Scholar]
- 77.Musilova I, Andrys C, Drahosova M, Soucek O, Stepan M, Bestvina T, et al. Intraamniotic inflammation and umbilical cord blood interleukin-6 concentrations in pregnancies complicated by preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2017;30:900–10. [DOI] [PubMed] [Google Scholar]
- 78.Pacora P, Maymon E, Gervasi MT, Gomez R, Edwin SS, Yoon BH, et al. Lactoferrin in intrauterine infection, human parturition, and rupture of fetal membranes. Am J Obstet Gynecol. 2000;183:904–10. [DOI] [PubMed] [Google Scholar]
- 79.Musilova I, Andrys C, Krejsek J, Drahosova M, Zednikova B, Pliskova L, et al. Amniotic fluid pentraxins: Potential early markers for identifying intra-amniotic inflammatory complications in preterm pre-labor rupture of membranes. Am J Reprod Immunol. 2018;79:e12789. [DOI] [PubMed] [Google Scholar]
- 80.Novakovic TR, Dolicanin ZC, Djordjevic NZ. Effects of maternal subclinical hypothyroidism on amniotic fluid cells oxidative status. Reprod Toxicol. 2018;78:97–101. [DOI] [PubMed] [Google Scholar]
- 81.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–23. [DOI] [PubMed] [Google Scholar]
- 82.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol. 1989;161:336–41. [DOI] [PubMed] [Google Scholar]
- 83.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol. 1991;165:813–20. [DOI] [PubMed] [Google Scholar]
- 84.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol. 1992;27:117–23. [DOI] [PubMed] [Google Scholar]
- 85.Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol. 1992;166:1576–87. [DOI] [PubMed] [Google Scholar]
- 86.Cherouny PH, Pankuch GA, Romero R, Botti JJ, Kuhn DC, Demers LM, et al. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol. 1993;169:1299–303. [DOI] [PubMed] [Google Scholar]
- 87.Romero R, Gomez R, Galasso M, Munoz H, Acosta L, Yoon BH, et al. Macrophage inflammatory protein-1 alpha in term and preterm parturition: effect of microbial invasion of the amniotic cavity. Am J Reprod Immunol. 1994;32:108–13. [DOI] [PubMed] [Google Scholar]
- 88.Osmers RG, Blaser J, Kuhn W, Tschesche H. Interleukin-8 synthesis and the onset of labor. Obstetrics and gynecology. 1995;86:223–9. [DOI] [PubMed] [Google Scholar]
- 89.Dudley DJ, Hunter C, Mitchell MD, Varner MW. Elevations of amniotic fluid macrophage inflammatory protein-1 alpha concentrations in women during term and preterm labor. Obstetrics and gynecology. 1996;87:94–8. [DOI] [PubMed] [Google Scholar]
- 90.Elliott CL, Loudon JA, Brown N, Slater DM, Bennett PR, Sullivan MH. IL-1beta and IL-8 in human fetal membranes: changes with gestational age, labor, and culture conditions. Am J Reprod Immunol. 2001;46:260–7. [DOI] [PubMed] [Google Scholar]
- 91.Thomakos N, Daskalakis G, Papapanagiotou A, Papantoniou N, Mesogitis S, Antsaklis A. Amniotic fluid interleukin-6 and tumor necrosis factor-alpha at mid-trimester genetic amniocentesis: relationship to intra-amniotic microbial invasion and preterm delivery. Eur J Obstet Gynecol Reprod Biol. 2010;148:147–51. [DOI] [PubMed] [Google Scholar]
- 92.Kacerovsky M, Celec P, Vlkova B, Skogstrand K, Hougaard DM, Cobo T, et al. Amniotic fluid protein profiles of intraamniotic inflammatory response to Ureaplasma spp. and other bacteria. PLoS One. 2013;8:e60399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gomez-Lopez N, Tong WC, Arenas-Hernandez M, Tanaka S, Hajar O, Olson DM, et al. Chemotactic activity of gestational tissues through late pregnancy, term labor, and RU486-induced preterm labor in Guinea pigs. Am J Reprod Immunol. 2015;73:341–52. [DOI] [PubMed] [Google Scholar]
- 94.Romero R, Grivel JC, Tarca AL, Chaemsaithong P, Xu Z, Fitzgerald W, et al. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol. 2015;213:836 e1–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vadillo-Ortega F, Hernandez A, Gonzalez-Avila G, Bermejo L, Iwata K, Strauss JF 3rd. Increased matrix metalloproteinase activity and reduced tissue inhibitor of metalloproteinases-1 levels in amniotic fluids from pregnancies complicated by premature rupture of membranes. Am J Obstet Gynecol. 1996;174:1371–6. [DOI] [PubMed] [Google Scholar]
- 96.Fortunato SJ, Menon R, Lombardi SJ. MMP/TIMP imbalance in amniotic fluid during PROM: an indirect support for endogenous pathway to membrane rupture. J Perinat Med. 1999;27:362–8. [DOI] [PubMed] [Google Scholar]
- 97.Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183:94–9. [DOI] [PubMed] [Google Scholar]
- 98.Maymon E, Romero R, Pacora P, Gervasi MT, Gomez R, Edwin SS, et al. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. Am J Obstet Gynecol. 2000;183:887–94. [DOI] [PubMed] [Google Scholar]
- 99.Maymon E, Romero R, Pacora P, Gervasi MT, Bianco K, Ghezzi F, et al. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol. 2000;183:914–20. [DOI] [PubMed] [Google Scholar]
- 100.Fortunato SJ, Menon R. Distinct molecular events suggest different pathways for preterm labor and premature rupture of membranes. Am J Obstet Gynecol. 2001;184:1399–405; discussion 405–6. [DOI] [PubMed] [Google Scholar]
- 101.Maymon E, Romero R, Pacora P, Gomez R, Mazor M, Edwin S, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med. 2001;29:308–16. [DOI] [PubMed] [Google Scholar]
- 102.Tromp G, Kuivaniemi H, Romero R, Chaiworapongsa T, Kim YM, Kim MR, et al. Genome-wide expression profiling of fetal membranes reveals a deficient expression of proteinase inhibitor 3 in premature rupture of membranes. Am J Obstet Gynecol. 2004;191:1331–8. [DOI] [PubMed] [Google Scholar]
- 103.Gomez-Lopez N, Romero R, Leng Y, Xu Y, Slutsky R, Levenson D, et al. The origin of amniotic fluid monocytes/macrophages in women with intra-amniotic inflammation or infection. J Perinat Med. 2019;47:822–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. [DOI] [PubMed] [Google Scholar]
- 105.Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40:274–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu Y, Romero R, Miller D, Kadam L, Mial TN, Plazyo O, et al. An M1-like Macrophage Polarization in Decidual Tissue during Spontaneous Preterm Labor That Is Attenuated by Rosiglitazone Treatment. J Immunol. 2016;196:2476–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rogers LM, Serezani CH, Eastman AJ, Hasty AH, Englund-Ogge LE, Jacobsson B, et al. Palmitate induces apoptotic cell death and inflammasome activation in human placental macrophages. bioRxiv. 2019:799718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Doster RS, Sutton JA, Rogers LM, Aronoff DM, Gaddy JA. Streptococcus agalactiae Induces Placental Macrophages To Release Extracellular Traps Loaded with Tissue Remodeling Enzymes via an Oxidative Burst-Dependent Mechanism. MBio. 2018;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Oh KJ, Romero R, Park JY, Lee J, Conde-Agudelo A, Hong JS, et al. Evidence that antibiotic administration is effective in the treatment of a subset of patients with intra-amniotic infection/inflammation presenting with cervical insufficiency. Am J Obstet Gynecol. 2019;221:140 e1–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yoon BH, Romero R, Park JY, Oh KJ, Lee J, Conde-Agudelo A, et al. Antibiotic administration can eradicate intra-amniotic infection or intra-amniotic inflammation in a subset of patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2019;221:142 e1–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Musilova I, Andrys C, Holeckova M, Kolarova V, Pliskova L, Drahosova M, et al. Interleukin-6 measured using the automated electrochemiluminescence immunoassay method for the identification of intra-amniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2018:1–131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


