Abstract
Objective
We reviewed our experience with pediatric chest wall tumors (CWTs) to identify variables associated with survival, scoliosis development, and need for corrective scoliosis surgery.
Background
Chest wall neoplasms in children or adolescents are rare. Consequently, there are few large series that detail survival or quality of life indicators, like scoliosis.
Methods
Medical records were reviewed for all chest wall resections for primary and metastatic CWT performed from 10/1/1986–09/30/2016 on patients ≤21 years of age at diagnosis. Kaplan-Meier distributions were compared using the log-rank test. Variables correlated with survival, scoliosis development, or need for corrective surgeries were analyzed using competing-risk analysis.
Results
Seventy-six cases (57 [75%] primary, 19 [25%] metastatic) were identified. Median age at diagnosis was 15.6 years (range: 0.5–21y). Tumor types were Ewing sarcoma family tumors (54%), other soft tissue sarcomas (21%), osteosarcoma (11%), rhabdomyosarcoma (7%), and other (8%). A median of 3 (range: 1–5) contiguous ribs were resected. Surgical reconstruction included composite Marlex® mesh and methyl-methacrylate, Gore-Tex®, or primary closure in 57%, 28%, and 14% of procedures, respectively. Overall 5-year survival was 61% (95% CI: 50–75%). Scoliosis developed in 19 (25%) patients; 6 patients required corrective surgery. Variables associated with overall survival were the presence of metastatic disease at diagnosis, and whether the chest tumor itself was a primary or metastatic lesion. Younger age at chest wall resection was associated with the need for corrective surgery in patients who developed scoliosis.
Conclusions
Among pediatric and adolescent patients with CWTs, survival depends primarily on the presence of metastases. Age, type of chest wall reconstruction, and tumor size are not associated with scoliosis development. Among patients who develop scoliosis, younger patients are more likely to require corrective surgery.
Keywords: Chest wall tumors, pediatric surgery, Ewing sarcoma, osteosarcoma, rhabdomyosarcoma, pediatric, scoliosis
Introduction
Chest wall tumors (CWTs) are rare in children and adolescents, accounting for less than 2% of solid tumors in childhood [1, 2], and although they can be benign, most are malignant [1, 3]. These neoplasms are heterogeneous in origin, developing in the bones or soft tissue of the chest wall, or as metastasis from a distant primary malignancy. The Ewing sarcoma family neoplasms (e.g. Ewing sarcoma, peripheral primitive neuroectodermal tumor, Askin tumor) are the most commonly encountered primary tumors [2, 4–7]. Osteosarcoma tumors are less frequent, while rhabdomyosarcoma and other sarcomas are rare [3, 5, 8]. Scoliosis has been noted as an important potential complication of chest wall resection associated with treatment of these tumors [5, 9, 10], but risk factors associated with the postoperative development of this skeletal disorder are not well studied
The management of malignant CWTs has evolved over the past two decades. A multidisciplinary approach including surgical resection and, depending on the underlying malignancy, a combination of chemotherapy and radiotherapy has yielded improved survival [3, 5, 11–13]. However, due to the infrequency of these neoplasms, there is a scarcity of analyses regarding predictors of survival and other long-term outcomes including scoliosis [5–8, 11]. Furthermore, existing studies are limited by small sample size, incomplete cohorts, and limited follow-up [1]. Consequently, the major challenges in treating pediatric CWTs include determining ideal surgical management and providing patients and parents with appropriate counseling on expected oncological and functional outcomes.
We reviewed our institutional experience of pediatric patients with CWTs to identify factors associated with survival as well as development of scoliosis and the subsequent need for corrective surgery.
Patients and Methods
The current study was approved by the Institutional Review Board of Memorial Sloan Kettering Cancer Center (IRB #16–1328).
Patients
We reviewed the medical records of all patients 21 years of age and under who underwent chest wall resections for CWTs at our institution between October 1986 and September 2016. All patients were treated according to institutional protocols that included tumor resection, multi-agent chemotherapy, and/or radiation therapy as appropriate.
Patient records were reviewed for demographic data, disease characteristics, surgical outcomes, recurrence, and survival. Data collected included: age at diagnosis, type of biopsy performed, histology of tumor, grade of tumor, ribs involved, ribs resected, surgical margins, type of reconstruction, postoperative complications, local and/or metastatic recurrence, incidence and management of scoliosis, and overall and progression-free survivals. Duration of follow-up was defined from the time of diagnosis to the most recent available follow-up. Local recurrence at the primary tumor site was confirmed by cross-sectional imaging or biopsy, and metastatic recurrence was defined as new onset of distant disease also confirmed by imaging.
A pediatric radiologist (APP), blinded to the clinical course and type of surgical reconstruction used, evaluated the change of spine curvature on chest or upright scoliosis films or scout CT scans during the follow-up to determine the Cobb angles for each patient. Scoliosis was defined as a Cobb angle greater than10% [14].
Management
Details of our management of pediatric CTWs have been described elsewhere [3, 6, 15]. Briefly, after performing a careful history and physical examination, adequate imaging studies are performed to delineate the primary tumor, loco-regional involvement, and identity possible sites of dissemination (Figure 1). A biopsy is done to establish histologic diagnosis and a multimodal care plan is developed. Chemotherapy is utilized in most patients with chest wall tumors, especially those with Ewing’s family tumors and osteosarcoma, who are treated with both neoadjuvant and adjuvant chemotherapy [16,17]. Patients with benign lesions, and those with low-grade soft tissue sarcomas, including chondrosarcoma, fibrosarcoma, liposarcoma, and low-risk synovial sarcoma do not require chemotherapy, as these tumors rarely respond to chemotherapy and have good outcomes with surgical resection alone [18–20]. Children’s Oncology Group recommends no additional treatment for these lesions outside complete resection [21].
Figure 1.
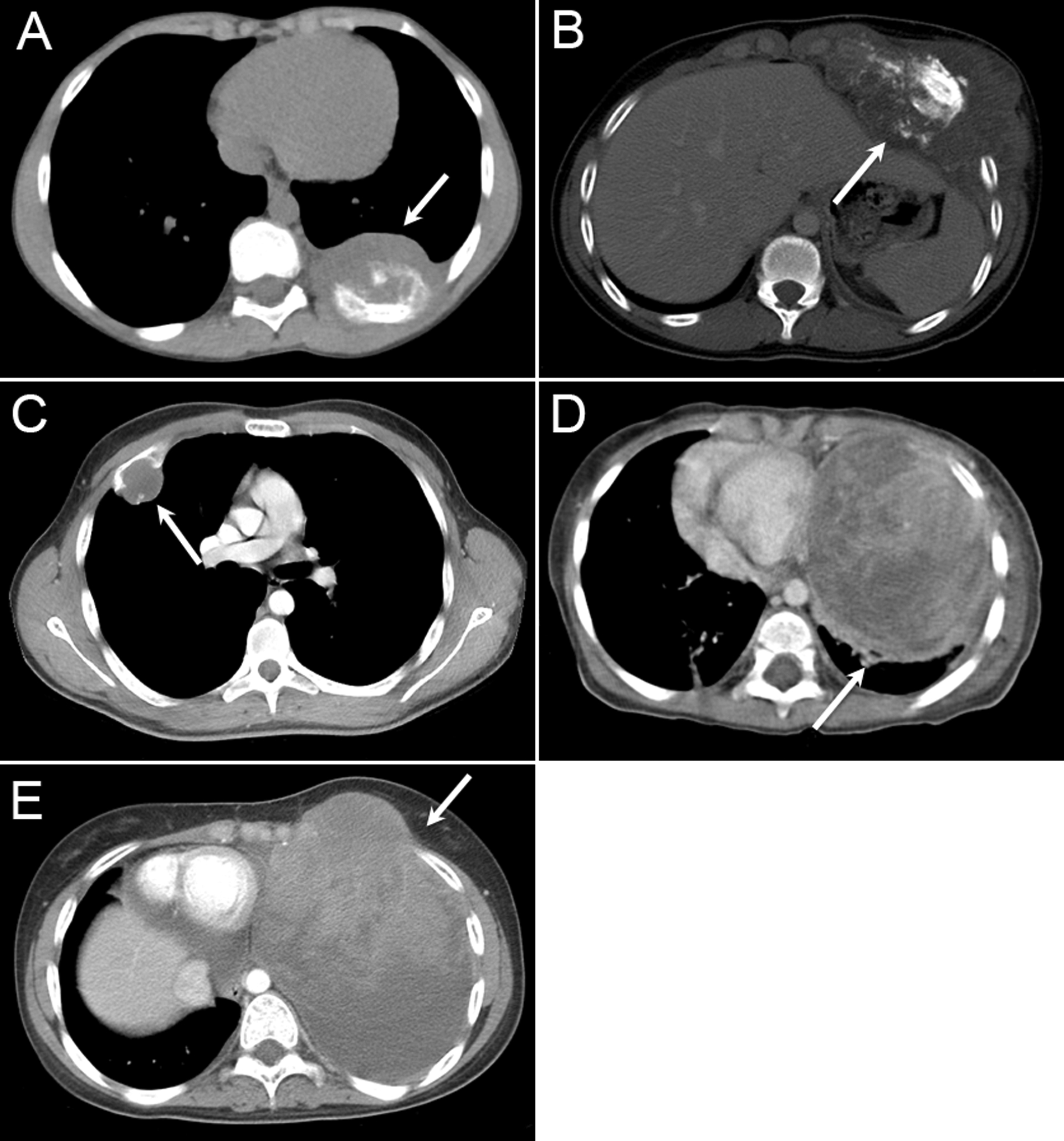
Representative axial CT scans of common chest wall malignancies at diagnosis (white arrow). (A) Ewing sarcoma, (B) Osteosarcoma, (C) Chondrosarcoma, (D) Germ Cell tumor, (E) Rhabdomyosarcoma.
Radiotherapy is used in patients with high-grade radiosensitive sarcomas, including Ewing’s sarcoma, rhabdomyosarcoma, and synovial sarcoma. In our study, consolidative external beam radiation treatment was selectively employed in some patients with close but negative margins despite an R0 resection on final pathology. Radiotherapy was not used in radioresistant tumors, such as osteosarcoma and chondrosarcoma.
In some cases, chest wall tumors may represent metastatic lesions from primary tumors at other sites or may be metastatic to other sites. In these cases, metastasectomy is performed for tumors with histologies that are refractory to chemotherapy and radiation such as osteosarcoma and non-rhabdomyosarcoma soft tissue sarcomas, as it has been shown to result in long-term survival [22].
Surgical resection and chest wall reconstruction are performed during the same procedure. Correct localization of the lesion is paramount and usually achieved by combination of techniques including radiographic needle localization (Figure 2) and opening the chest two interspaces higher or lower from the primary rib involvement, allowing palpation of the inside of the chest wall. We subsequently proceed with the chest wall resection with complete removal of the affected area. The primarily affected rib or ribs are resected along with at least partial resection of the adjacent superior and inferior ribs. Resection of adjacent ribs is frequently necessary to ensure negative microscopic margins. This is especially important in malignant tumors, such as osteosarcoma and non-rhabdomyosarcoma soft tissue sarcomas, that do not respond to adjuvant therapy, making their prognosis highly dependent on completeness of resection. Resection of surrounding structures may also be necessary to achieve complete resection. After resection is complete, chest wall reconstruction is performed. Our preferred method of reconstruction for large chest wall defects in patients of all ages utilizes methylmethacrylate cement spread between two layers of polyprolene mesh. This technique allows for a semi-rigid repair that is customizable in shape and size. Depending on the overall size of the defect and location on the chest wall, other options for reconstruction include Gore-Tex® patch, muscle flap, or primary closure. Primary closure is occasionally possible for small defects that can be closed without chest wall deformity. Muscle flaps and Gore-Tex® patch can be used for small to medium defects that are supported by overlying structures and not at risk for paradoxical motion.
Figure 2.
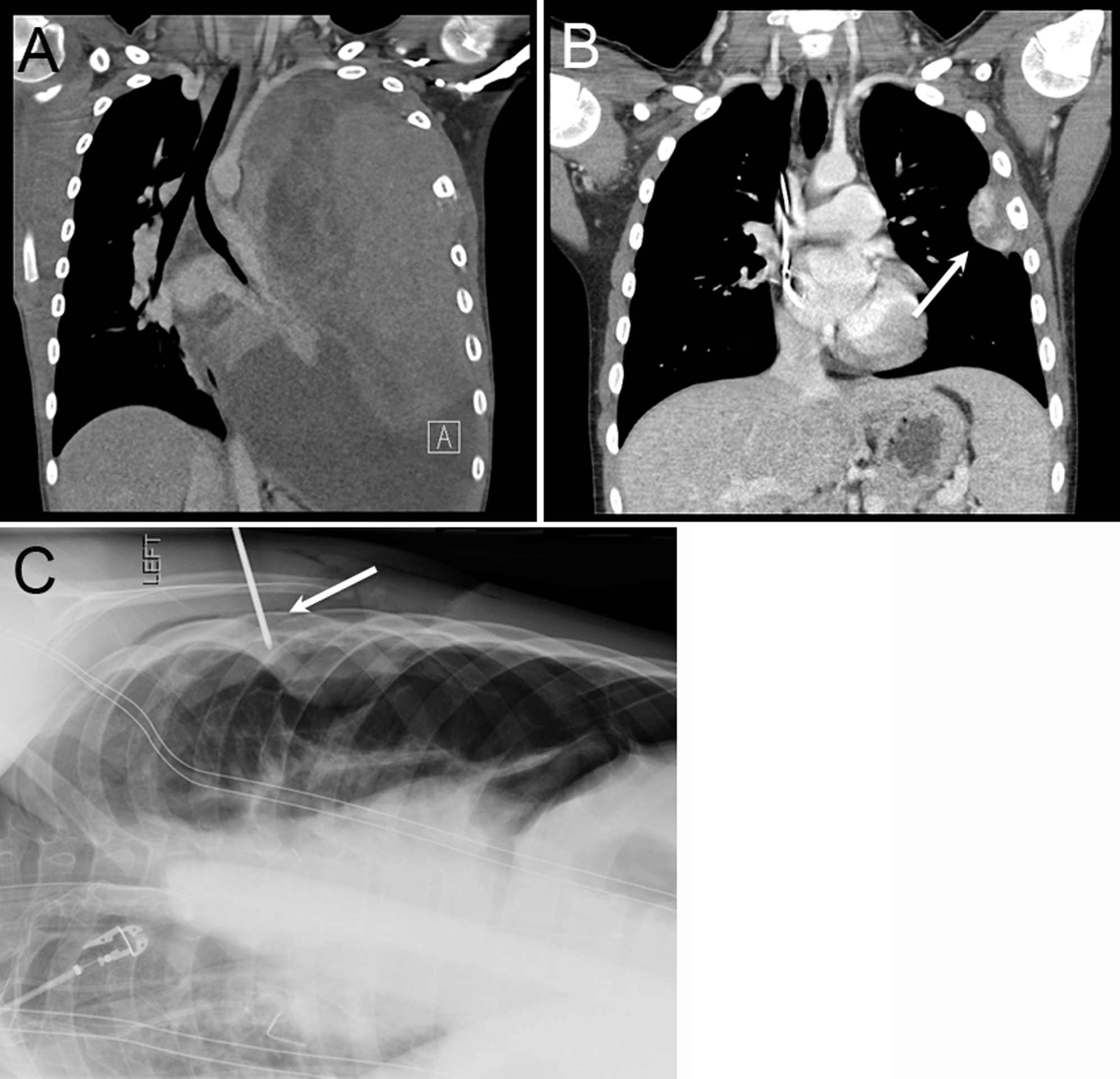
Significant response to neoadjuvant chemotherapy in a 13-year-old female with Ewing sarcoma requiring intraoperative needle localization for correct identification of the primary affected rib. (A) Sagittal chest CT scan of the CWTs at diagnosis, (B) after therapy (white arrow), and (C) intraoperative cross table lateral chest x-ray confirming that the primary involved rib has been correctly identified (white arrow marks the spinal needle in the 4th rib).
Statistical analysis
Nonparametric continuous variables are reported as median values with range and comparisons made using the Wilcoxon signed-rank test. Analyses of categorical variables were completed using Pearson’s chi-square or Fisher’s exact test. Progression was defined as the development of new tumors or increase in tumor size despite therapy. Progression-free survival (PFS) and overall survival (OS) were calculated using Kaplan-Meier estimates and are reported as proportions with 95% confidence interval (CI). Univariate Cox proportional hazards modeling was used to determine factors associated with survival. Competing risks analysis and cumulative incidence curves were used to determine factors associated with the development of scoliosis. All analyses use two-tailed tests with α = 0.05 for significance. Statistical analysis was performed using R version 3.2.4 (The R Foundation for Statistical Computing©, Vienna, Austria; www.r-project.org).
Results
Study population
A total of 76 consecutive pediatric or adolescent patients with primary or secondary CWTs underwent chest wall resection and reconstruction. Forty (53%) males and 36 (47%) females were identified. The median length of follow-up time for survivors was 6.6 years from diagnosis (range: 3 months to 21 years) and 5.9 years from the time of surgery (range: 1 month to 20.8 years). The presenting symptom was pain in 37 (49%) patients, soft tissue swelling in 14 (18%), recurrent upper respiratory infections or pneumonia in 8 (11%), dyspnea or shortness of breath in 5 (7%), and incidental in 1 case (1%). Tumors were primary in 57 patients (75%) and metastatic lesions from other site primary tumors in 19 (25%). In 9 (12%) patients the diagnosis was made as part of surveillance imaging for their non-thoracic malignancies. Tumors were evenly distributed between the left and right side of the chest (n=39, 51% right, and n=35, 46% left), with a small minority involving the sternum only (n=2, 3%). A total of 36 (47%) patients had anterior CWTs and 40 (53%) had posterior CWTs. The upper (ribs 1–3), middle (ribs 4–8), and lower (ribs 9–12) thoracic levels were affected equally in 23 (30%), 25 (33%), and 28 (37%) subjects, respectively. The median maximum diameter of the CWTs at diagnosis was 7cm (range 1–19 cm). Open biopsy of the CWTs was performed in 37 patients (48%), while 19 (25%) underwent core needle biopsy, and 5 (7%) had image-guided biopsy. In 5 patients (7%) a thoracoscopic approach was used to biopsy the tumor, and 10 (13%) patients underwent initial diagnostic and therapeutic resection.
Malignant neoplasms were seen in 73 (96%) study subjects. The frequency of tumor types were Ewing sarcoma family tumors (n=41, 54%), non-rhabdosarcomas (n=16, 21%), osteosarcoma (n=8, 12%), rhabdomyosarcoma (n=5, 7%), and others (n=6, 8%) (Table 1). Three patients (4%) had benign lesions, including one case each of angiomatoid fibrous histiocytoma, chondroma, and an unidentified benign fibrous tumor. A total of 60 (82%) patients with malignant neoplasms received neoadjuvant chemotherapy and 66 (90%) received adjuvant chemotherapy. Thirty-five patients (48%) received radiotherapy, of whom 30 received external beam radiation (XRT) alone, 3 received XRT and intraoperative radiation (IORT), and 2 received IORT alone. The median XRT dose was 4820 cGy (range: 1500–6540 cGy).
Table 1.
Demographics, tumor characteristics, and treatment characteristics of 76 pediatric patients with chest wall tumors included in the study cohort.
| Characteristic | N (%) |
|---|---|
| Median Age (range) | 15.6 years (0.5 – 21) |
| Gender | |
| Male | 40 (52.6) |
| Female | 36 (47.4) |
| Histology | |
| Ewing Sarcoma/PNET | 41 (53.9) |
| Osteosarcoma | 8 (10.5) |
| Rhabdomyosarcoma | 5 (6.6) |
| Other soft tissue sarcoma | 16 (21.1) |
| Other malignant tumors | 3 (3.9) |
| Benign tumors | 3 (3.9) |
| Number of ribs resected | |
| 1 | 16 (21.1) |
| 2 | 17 (22.4) |
| 3 | 29 (38.2) |
| 4 | 11 (14.5) |
| 5 | 3 (3.9) |
| Tumor position | |
| Anterior | 36 (47.4) |
| Posterior | 40 (52.6) |
| Thoracic level | |
| Upper (ribs 1–3) | 23 (30.3) |
| Middle (ribs 4–8) | 25 (32.9) |
| Lower (ribs 9–12) | 28 (26.8) |
| Surgical Margins | |
| Microscopically negative (R0) | 74 (97.4) |
| Microscopically positive (R1) | 2 (2.6) |
| Reconstruction material | |
| Marlex and methylmethacrylate | 43 (56.6) |
| Gore-Tex® patch | 21 (27.6) |
| Primary closure | 12 (15.8) |
| Scoliosis | |
| Developed scoliosis | 19 (25.0) |
| Required scoliosis surgery | 6 (7.9) |
Median age at definitive resection and reconstruction was 16 years (range, 2–21), with 13 (17%) patients undergoing surgery before age 10, 19 (25%) between age 10 and 16, and 44 (58%) at age 16 years or older. A median of 3 (range, 1–5) contiguous ribs were resected. Concomitant resection of pulmonary parenchyma, diaphragm, or pericardium in locally advanced malignancies was required in 32 patients (42%, lung, n=17; diaphragm, n=13; pericardium, n=2). Surgical reconstruction included composite Marlex® mesh and methyl-methacrylate, Gore-Tex®, primary closure, or muscle flaps in 43 (57%), 21 (28%), 10 (14%) and 1 (1%) of procedures, respectively.
Perioperative outcome
There were no perioperative mortalities. Prolonged ventilator support was not required in any patient, and all patients were extubated at the end of the procedure. Median estimated blood loss during the procedure was 275 ml (range, 25–5000 ml). Median length of hospital stay postoperatively was 6 days (range, 2–20). Three patients (4%) developed wound and soft tissue infection within one year of the initial surgery, requiring removal of reconstructive material and placement of a latissimus dorsi muscle flap. None of the patients developed paradoxical chest wall movements or chest wall instability during the follow-up period.
Oncological outcome
Overall 5-year survival was 61% (95% CI: 50–75%) and the 5-year progression-free survival for the entire cohort was 55% (95% CI: 44–69%) (Figure 3A). Currently, 2 patients with Ewing sarcoma, complicated by multiple local and distant recurrences, are alive with disease at 2 and 6 years after the initial surgical resection. One patient with Ewing sarcoma died secondary to acetaminophen overdose induced fulminant hepatic failure 8 years after complete resection and had no evidence of disease at follow-up. Five-year overall survival (OS) for patients with Ewing sarcoma family tumors (70%, 95% CI: 56–87%), non-rhabdosarcomas (70%, 95% CI: 49–100%), osteosarcoma (43%, 95% CI: 18–100%), rhabdomyosarcoma (40%, 95% CI: 14–100%) are shown in Figure 4. Of note, 4 (50%) of patients with osteosarcoma developed local (n=1), distant (n=2) or local and distant (n=2) recurrences.
Figure 3.
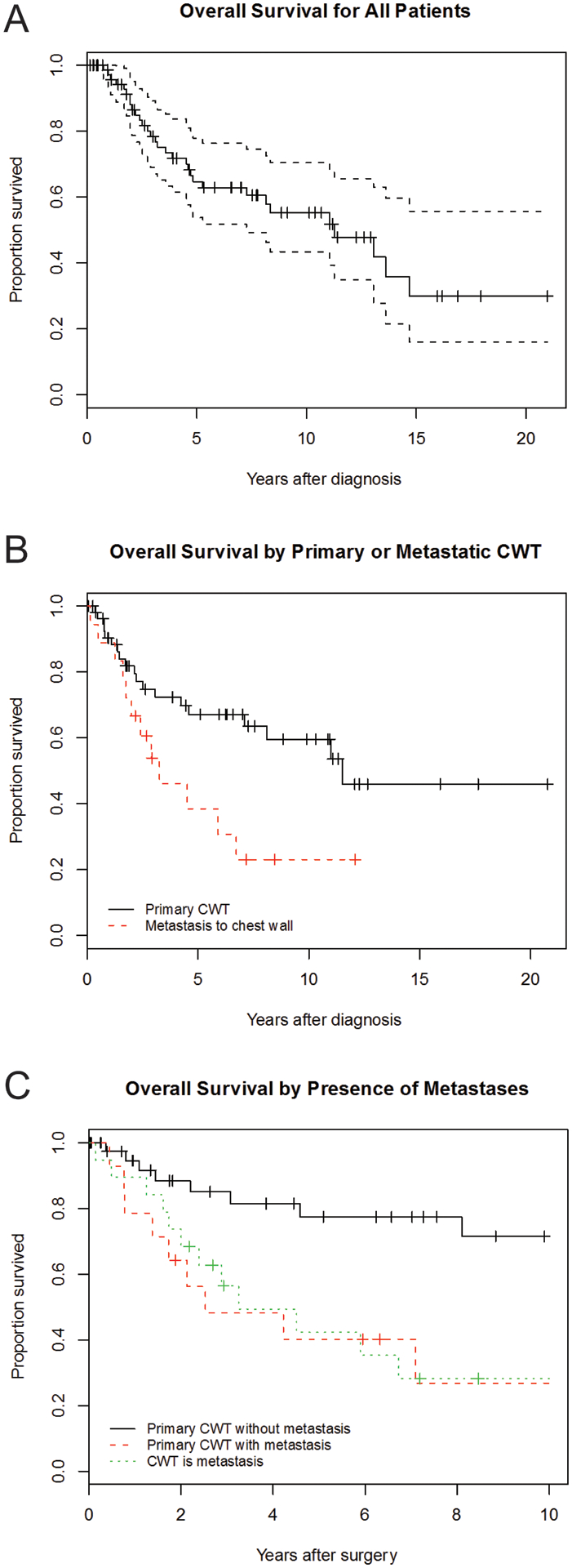
Overall survival for the entire cohort (A) and by whether CWT is a primary neoplasm versus a metastasis form a distant primary (B). (C) Overall survival for primary CWT with and without metastases, as well as CWT that are metastatic lesions.
Figure 4.
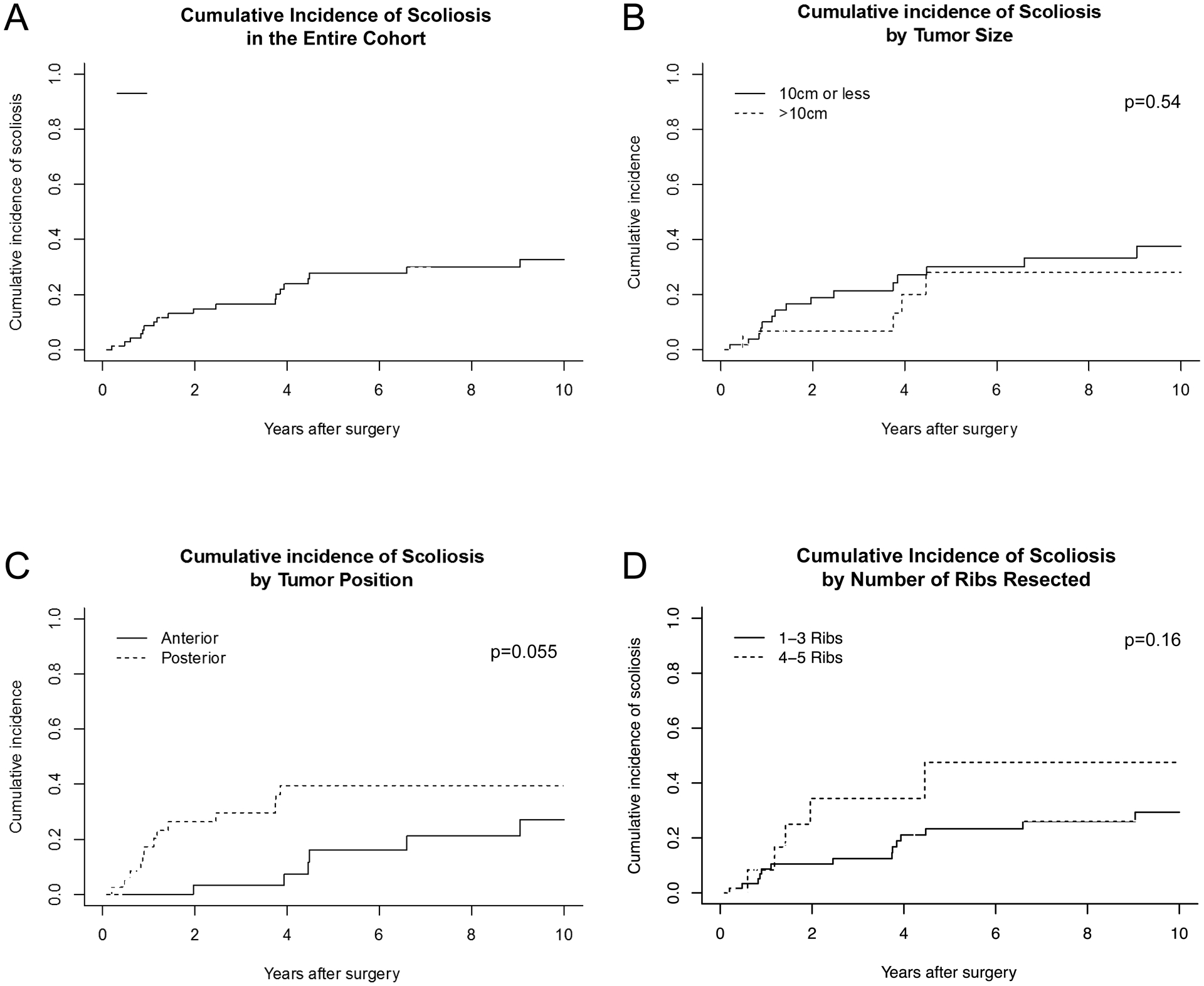
Cumulative incidence of scoliosis for the entire cohort (A) and stratified by tumor size (B), tumor position (C), and number of ribs resected (D). P values are for modified χ2 statistic between groups.
A complete resection (R0) was achieved in 74 (97%) patients and R1 in 2 (3%). Both patients with R1 resection underwent re-resection at our institution after the initial surgery was performed at outside institutions. Overall, eight patients (11%) developed local recurrence disease and 24 patients (32%) developed distant recurrences, including 2 (3%) patients that developed both, local and distant recurrence. The histologies of tumors with local recurrence were embryonal rhabdomyosarcoma (2), osteosarcoma (2), Ewing family sarcoma (3), and leiomyosarcoma (1). The median time to recurrence was 1.3 years for distant disease (range: 0.1–6.1), and 1.6 years for local recurrence (range 0.5– 2.6).
Univariate survival analysis
Whether tumors were primary or metastatic lesions was associated with overall survival (HR 2.3 for metastatic lesions, 95% CI: 1.1 – 4.7, p=0.03) (Figure 3B). For patients with primary CWT, the presence of synchronous metastatic lesions was predictive of overall survival (HR 3.05, 95% CI: 1.24 – 7.54, p=0.02) (Figure 3C). Tumor size approached statistical significance with tumors >10cm in diameter associated with decreased survival (HR 2.2, 95% CI: 1.0 – 5.1, p= 0.057).
Survival differences among the histologic tumor groups did not meet statistical significance (p=0.57). The position of the tumor (anterior/posterior and thoracic level) and whether the tumor was locally advanced (e.g. required contiguous lung or diaphragm or pericardial resection in addition to the chest wall resection) also were not associated with improved survival (p=0.87 and p=0.71, respectively). Moreover, the method of reconstruction was not correlated with survival (p=0.31). Small sample size likely limited our ability to detect meaningful survival differences related to these factors.
Scoliosis
During the follow-up period, 19 (25%) patients developed scoliosis, defined as a Cobb angle of greater than 10%. Scoliosis developed at median of 2.0 years (range, 0.2 to 9) after the initial chest wall resection and reconstructive surgery. No factors achieved statistical significance in predicting the incidence of scoliosis in competing risks analysis. Although not statistically significant, there was a trend toward increasing incidence of scoliosis in patients with greater numbers of ribs removed (4 or 5 ribs vs. 1–3, p=.17), and an increased incidence in patients with posterior compared to anterior resections (p=0.055) (Figure 4C). Patient age, gender, tumor size, radiation treatment, reconstruction method, and resection of other surrounding tissues were not associated with the development of scoliosis.
Six patients (32% of those with scoliosis, 8% of total) underwent corrective surgery for their scoliosis. In all cases, the corrective surgery was performed due to progressively worsening scoliosis with physical deformity. One patient also complained of musculoskeletal pain. Pre-operative Cobb angles were available for three patients who underwent scoliosis surgery and were 51, 58, and 61 degrees. Although also not statistically significant (p=0.15), children in our cohort who required corrective spine surgery tended to develop scoliosis sooner following chest wall reconstruction compared to those patients who did not require scoliosis surgery (mean time to scoliosis diagnosis = 1.7 years in the group requiring surgery, and 3.2 years in the group not requiring surgery). Younger age at reconstruction (under 13 years at time of surgery) was the only clinical factor correlated with the need for scoliosis surgery (odds ratio, 16.7, 95% CI: 1.4 – 204.0, p=0.03).
Discussion
We present our experience evaluating the outcome of pediatric and adolescent patients who required chest wall reconstruction for primary and metastatic CWT. Previous studies of chest wall tumors in pediatric patients have established scoliosis as a potential long-term complication of chest wall resection and reconstruction, but few studies have had sufficient follow-up time to evaluate long-term outcomes. The current study adds to this literature by describing the long-term survival and scoliosis outcomes in a relatively large cohort of pediatric patients with chest wall tumors.
Our results demonstrate that major chest wall reconstruction in the pediatric population can safely be performed with no perioperative mortality and minimal perioperative morbidity. Overall 5-year survival (61%) and progression-free survival (55%) in this study was similar to the previously reported survival rate for chest wall malignancies in children [2–4, 6–8, 11, 12, 15, 23]. Our data also corroborate previous findings in smaller cohorts that suggest complete resection, and absence of metastatic disease at diagnosis are the most important predictors of prolonged survival. Other series have contributed valuable information supporting the importance of surgical resection; however, many lacked the distinction between gross resection and microscopically negative margins. This may explain why Hayes-Jordan and colleagues [8] and Lopez at al. [5] did not find an association between margin status and survival rate in their recent studies. We were not able to perform statistical analysis on tumor resection margin status, as only two of the 76 patients in the cohort had positive R1 resection. Although Lopez and colleagues found that position on the chest wall influenced patient survival, this factor had no significant effect in our study [5].
In addition to oncological outcome, the development of scoliosis following major chest wall reconstructive surgery is also important to consider in patients with CWT. However, given the small sample size, incomplete follow-up, and a wide age range at the time of resection and reconstructive surgery, it remains challenging to accurately estimate the prevalence of scoliosis and other functional problems after chest wall resection [4, 9, 12, 13]. In the present study, 25% of patients (n = 19) developed scoliosis during follow-up, 6 of whom (32%) subsequently required surgical correction of their scoliosis. This rate is higher than previously reported in some studies [2, 5, 8, 12], but lower than the rate reported in others [9, 23]. The discrepancies in outcome between studies might be in part explained by relative short follow-up of some reports. Glotzbecker at al. [9], followed 21 children who were operated on between ages 8 to 17 years for more than 10 years and found progressive worsening of the spine curvature in 11 patients (52%), of whom 3 required spinal surgery. In our cohort, patients continued to develop new-onset scoliosis at over 9 years after chest wall surgery. These findings combined suggest that scoliosis surveillance in patients undergoing resection of CWT should continue long-term. Additionally, patients and their families should be made aware of the risk of developing scoliosis before undergoing chest wall resection.
Although previous studies have suggested that the age of the patient may influence the development of scoliosis [10, 24, 25], we did not observe this finding in our cohort. The effect of age was also not observed in some other previous studies [5, 9]. We acknowledge that there is a theoretical increase in the risk of scoliosis resulting from chest wall resection in younger patients who have greater growth potential and suggest that this issue requires further investigation. Our data do suggest that patients who undergo a chest wall resection at a younger age are at increased risk of requiring corrective surgery if they develop scoliosis. Additionally, although we did not observe a statistically significant association between anterior/posterior tumor position, and number of ribs resected, these factors approached significance and should be investigated further, especially since they have found to be predictive of developing scoliosis in previous studies [5, 10]. In addition to number of ribs, another measure for further study may be the total area and dimensions of chest wall resected, as larger defects may have a greater impact on development of scoliosis than smaller defects even when the same number of ribs are resected.
Although several investigators suggest that non-rigid reconstruction using GoreTex® or biologic mesh improves functional outcomes [12, 26], we found no effect of reconstruction method on the development of scoliosis. It has been postulated that chest wall reconstructions with softer prosthetics change the chest wall contour and compliance more than reconstruction with rigid materials and, in turn, may increase the risk of scoliosis [9]. Well-designed experiments with standardized defect size, long-term follow-up, and direct comparisons between the available soft and rigid prosthetics are necessary to further examine this issue.
There are limitations to this study. This analysis is retrospective in nature, and although it represents one of the largest cohorts in evaluating the management and outcomes of pediatric CWT, the small number of patients in each tumor group limited our ability to perform multivariable analysis to further evaluate significant predictors of adverse outcomes. Also, our extensive study period accounted for 30 years of experience, during which some elements of treatment of these tumors has changed. Further, dedicated upright scoliosis films were not available for all patients during the follow-up. Using supine scout CT for analysis may underestimate the scoliosis, compared to upright imaging [9]. Hence, the current findings should be verified by a prospective and cooperative approach among institutions involved in the care and treatment of patients diagnosed with CWTs.
In summary, this report represents one of the largest single-institution studies describing the oncological and functional outcomes for patients who require chest wall resection and reconstruction for CWTs. These tumors include a diverse group of neoplasms in the pediatric population. Resection of the chest wall with subsequent reconstruction is safe and effective in children and adolescents, and various combinations of reconstructive materials can be used. Risk of scoliosis is relatively high in these patients; therefore, they should undergo regular orthopedic surveillance postoperatively. This study illustrates the strong association between the presence of metastatic disease and overall survival among patients with CWT. Still, larger multi-institutional studies are needed to allow further investigation into the impact of other factors, including tumor type and size on overall survival.
Funding:
Research at Memorial Sloan Kettering is supported in part by a grant from the National Institutes of Health/National Cancer Institute (#P30 CA008748).
Footnotes
Conflict of Interest Disclosure: The authors declare that they have no financial conflicts of interest.
References
- [1].Incarbone M, Pastorino U. Surgical treatment of chest wall tumors. World J Surg 2001;25(2):218–230. [DOI] [PubMed] [Google Scholar]
- [2].Soyer T, Karnak I, Ciftci AO, Senocak ME, Tanyel FC, Buyukpamukcu N. The results of surgical treatment of chest wall tumors in childhood. Pediatr Surg Int 2006;22(2):135–139. [DOI] [PubMed] [Google Scholar]
- [3].La Quaglia MP. Chest wall tumors in childhood and adolescence. Semin Pediatr Surg 2008;17(3):173–180. [DOI] [PubMed] [Google Scholar]
- [4].Dang NC, Siegel SE, Phillips JD. Malignant chest wall tumors in children and young adults. J Pediatr Surg 1999;34(12):1773–1778. [DOI] [PubMed] [Google Scholar]
- [5].Lopez C, Correa A, Vaporciyan A, Austin M, Rice D, Hayes-Jordan A. Outcomes of chest wall resections in pediatric sarcoma patients. J Pediatr Surg 2017;52(1):109–114. [DOI] [PubMed] [Google Scholar]
- [6].Saenz NC, Hass DJ, Meyers P, Wollner N, Gollamudi S, Bains M, et al. Pediatric chest wall Ewing’s sarcoma. J Pediatr Surg 2000;35(4):550–555. [DOI] [PubMed] [Google Scholar]
- [7].Shamberger RC, LaQuaglia MP, Gebhardt MC, Neff JR, Tarbell NJ, Marcus KC, et al. Ewing sarcoma/primitive neuroectodermal tumor of the chest wall: impact of initial versus delayed resection on tumor margins, survival, and use of radiation therapy. Ann Surg 2003;238(4):563–567; discussion 7–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hayes-Jordan A, Stoner JA, Anderson JR, Rodeberg D, Weiner G, Meyer WH, et al. The impact of surgical excision in chest wall rhabdomyosarcoma: a report from the Children’s Oncology Group. J Pediatr Surg 2008;43(5):831–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Glotzbecker MP, Gold M, Puder M, Hresko MT. Scoliosis after chest wall resection. J Child Orthop 2013;7(4):301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Scalabre A, Parot R, Hameury F, Cunin V, Jouve JL, Chotel F. Prognostic risk factors for the development of scoliosis after chest wall resection for malignant tumors in children. J Bone Joint Surg Am 2014;96(2):e10. [DOI] [PubMed] [Google Scholar]
- [11].Girelli L, Luksch R, Podda MG, Meazza C, Puma N, Scanagatta P, et al. Surgical approach to primary tumors of the chest wall in children and adolescents: 30 years of mono-institutional experience. Tumori 2016;102(1):89–95. [DOI] [PubMed] [Google Scholar]
- [12].Lin SR, Kastenberg ZJ, Bruzoni M, Albanese CT, Dutta S. Chest wall reconstruction using implantable cross-linked porcine dermal collagen matrix (Permacol). J Pediatr Surg 2012;47(7):1472–1475. [DOI] [PubMed] [Google Scholar]
- [13].Athanassiadi K, Kalavrouziotis G, Rondogianni D, Loutsidis A, Hatzimichalis A, Bellenis I. Primary chest wall tumors: early and long-term results of surgical treatment. Eur J Cardiothorac Surg 2001;19(5):589–593. [DOI] [PubMed] [Google Scholar]
- [14].Kim H, Kim HS, Moon ES, Yoon CS, Chung TS, Song HT, et al. Scoliosis imaging: what radiologists should know. Radiographics 2010;30(7):1823–1842. [DOI] [PubMed] [Google Scholar]
- [15].Saenz NC, Ghavimi F, Gerald W, Gollamudi S, LaQuaglia MP. Chest wall rhabdomyosarcoma. Cancer 1997;80(8):1513–1517. [DOI] [PubMed] [Google Scholar]
- [16].Lamplot JD, Denduluri S, Qin J, Li R, Liu X, Zhang H, et al. The current and future therapies for human osteosarcoma. Curr Cancer Ther Rev 2013;9(1):55–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kridis WB, Toumi N, Chaari H, Khanfir A, Ayadi K, Keskes H, et al. A review of Ewing sarcoma treatment: is it still a subject of debate? Rev Recent Clin Trials 2017;12(1):19–23. [DOI] [PubMed] [Google Scholar]
- [18].Ferrari A, De Salvo GL, Brennan B, van Noesel MM, De Paoli A, Casanova M, et al. Synovial sarcoma in children and adolescents: the European Pediatric Soft Tissue Sarcoma Study Group prospective trial (EpSSG NRSTS 2005). Ann Oncol 2015;26(3):567–72. [DOI] [PubMed] [Google Scholar]
- [19].Italiano A, Mir O, Cioffi A, Palmerini E, Piperno-Neumann S, Perrin C, et al. Advanced chondrosarcomas: role of chemotherapy and survival. Ann Oncol 2013;24(11):2916–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ferrari A, Chi YY, De Salvo GL, Orbach D, Brennan B, Randall RL, et al. Surgery alone is sufficient therapy for children and adolescents with low-risk synovial sarcoma: A joint analysis from the European Paediatric Soft Tissue Sarcoma Study Group and the Children’s Oncology Group. Eur J Cancer 2017;78:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Waxweiler TV, Rusthoven CG, Proper MS, Cost CR, Cost NG, Donaldson N, et al. Non-rhabdomyosarcoma soft tissue sarcomas in children: a Surveillance, Epidemiology, and End Results analysis validating COG risk stratifications. Int J Radiat Oncol Biol Phys 2015;92(2):339–348. [DOI] [PubMed] [Google Scholar]
- [22].Croteau NJ, Heaton TE. Pulmonary metastasectomy in pediatric solid tumors. Children (Basel) 2019;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chui CH, Billups CA, Pappo AS, Rao BN, Spunt SL. Predictors of outcome in children and adolescents with rhabdomyosarcoma of the trunk--the St Jude Children’s Research Hospital experience. J Pediatr Surg 2005;40(11):1691–1695. [DOI] [PubMed] [Google Scholar]
- [24].Kawakami N, Winter RB, Lonstein JE, Denis F. Scoliosis secondary to rib resection. J Spinal Disord 1994;7(6):522–527. [PubMed] [Google Scholar]
- [25].DeRosa GP. Progressive scoliosis following chest wall resection in children. Spine (Phila Pa 1976) 1985;10(7):618–622. [DOI] [PubMed] [Google Scholar]
- [26].Guillen G, Garcia L, Marhuenda C, Pellise F, Molino JA, Fontecha CG, et al. Thoracic wall reconstruction with bioabsorbable plates in pediatric malignant thoracic wall tumors. J Pediatr Surg 2017;52(3):377–381. [DOI] [PubMed] [Google Scholar]


