Abstract
Introduction:
Intra-amniotic inflammation, which is associated with adverse pregnancy outcomes, can occur in the presence or absence of detectable microorganisms, and involves activation of the inflammasome. Intra-amniotic inflammasome activation has been reported in clinical chorioamnionitis at term and preterm labor with intact membranes, but it has not yet been investigated in women with preterm prelabor rupture of membranes (preterm PROM) in the presence/absence of detectable microorganisms.
Aim:
To determine whether, among women with preterm PROM, there is an association between detectable microorganisms in amniotic fluid and intra-amniotic inflammation, and whether intra-amniotic inflammasome activation correlates with microbial burden.
Methodology:
Amniotic fluids from 59 cases of preterm PROM were examined for the presence/absence of microorganisms through culture and 16S rRNA gene qPCR, and concentrations of interleukin 6 (IL-6) and ASC (apoptosis-associated spec-like protein containing a CARD), an indicator of inflammasome activation, were determined.
Results:
qPCR identified more microbe-positive amniotic fluids than culture. Greater than 50% of patients with a negative culture and high IL-6 concentration in amniotic fluid yielded a positive qPCR signal. ASC concentrations were greatest in patients with high qPCR signals and elevated IL-6 concentrations in amniotic fluid (i.e. intra-amniotic infection), and ASC concentrations tended to increase in patients without detectable microorganisms but yet with elevated IL-6 concentrations (i.e. sterile intra-amniotic inflammation).
Conclusion:
qPCR is a valuable complement to microbiological culture for the detection of microorganisms in the amniotic cavity in women with preterm PROM, and microbial burden is associated with the severity of intra-amniotic inflammatory response, including inflammasome activation.
Keywords: PPROM, microbial invasion of the amniotic cavity, sterile intra-amniotic inflammation, culture, quantitative real-time PCR (qPCR)
INTRODUCTION
Microbial invasion of the amniotic cavity can lead to intra-amniotic inflammation (i.e. intra-amniotic infection) (1–25) when bacteria from the lower genital tract gain access to this compartment (6, 26–28). Yet, in some cases, intra-amniotic inflammation can occur in the absence of detectable microorganisms, a clinical condition referred to as sterile intra-amniotic inflammation (23, 24, 29–32). Importantly, intra-amniotic infection is more common than sterile intra-amniotic inflammation in women with clinical chorioamnionitis at term (24) and preterm prelabor rupture of membranes (preterm PROM) (31, 32). These clinical conditions are associated with adverse maternal outcomes and increased risk for neonatal sequelae (16, 33–70).
Preterm PROM occurs in approximately 30% of all preterm deliveries (53, 71) and thus represents a major contributing factor to adverse perinatal outcomes associated with prematurity (16, 33–35, 55, 56, 60, 72). Given that the prevalence of intra-amniotic infection is increased in laboring women with preterm PROM (32, 73), it is tempting to suggest that the process of labor facilitates the ascension of microorganisms into the amniotic cavity (18, 74–77). In line with this concept, several reports have shown that approximately 40% of women with preterm PROM have intra-amniotic infection (32, 62, 78–80). Importantly, molecular microbiology is capable of detecting 50% more cases of microbial invasion of the amniotic cavity than conventional microbiological cultures (32). These results suggest that the syndrome of preterm PROM is a heterogeneous condition that requires further investigation.
The mechanisms that lead to preterm birth following intra-amniotic infection involve a localized inflammatory response, which is partially mediated by the NLRP3 [also known as cryopyrin or NLR (nucleotide-binding domain and leucine-rich repeat) family pyrin domain-containing protein 3] inflammasome (81–85). Inflammasomes are cytoplasmic multiprotein complexes composed of a sensor molecule, the adapter protein ASC (apoptosis-associated spec-like protein containing a CARD), and inactive caspase-1 (86–101). The assembly of inflammasomes promotes the activation of caspase-1, which subsequently cleaves the immature forms of the pro-inflammatory cytokines IL-1β and IL-18 into their bioactive forms (102–111). Upon inflammasome activation, ASC proteins are released into the extracellular space where they can serve as a readout of inflammasome activation in vivo (112–114). Indeed, we have previously shown that increased concentrations of extracellular ASC are observed in amniotic fluid from women with spontaneous labor at term (115) and those with clinical chorioamnionitis at term (116) or with preterm labor with intact membranes (82). However, whether amniotic fluid concentrations of extracellular ASC can provide a readout of the intra-amniotic inflammatory response in women with preterm PROM has not been investigated.
Herein, in women with preterm PROM, we investigated: 1) the relationship between conventional microbiological cultures and 16S rRNA gene quantitative real-time PCR (qPCR) signals in amniotic fluid, 2) the association between detection of microbes in amniotic fluid by microbiological cultures and/or 16S rRNA gene qPCR and intra-amniotic inflammation [interleukin 6 > 2.6 ng/mL (14)], and 3) whether intra-amniotic inflammasome activation (amniotic fluid concentrations of extracellular ASC) correlates with microbial burden (defined as positive microbiological culture and/or positive 16S rRNA gene signal).
METHODS
Study design and population
This retrospective cross-sectional study was conducted by searching our clinical database and bank of biological samples. The collection of samples was approved by the Institutional Review Boards of the Detroit Medical Center (Detroit, MI, USA), Wayne State University, and the Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services. All women provided written informed consent prior to the collection of amniotic fluid.
This study included 59 amniotic fluid samples collected from patients with preterm PROM that were initially classified into the following groups (Table 1): i) preterm PROM with a negative microbiological culture and low amniotic fluid concentrations of IL-6 (≤ 2.6 ng/mL), ii) preterm PROM with a negative microbiological culture and high amniotic fluid IL-6 (> 2.6 ng/mL), iii) preterm PROM with a positive microbiological culture and high amniotic fluid IL-6, and iv) preterm PROM with a positive microbiological culture and low amniotic fluid concentrations of IL-6 (see clinical definitions below).
Table 1.
Clinical and demographic characteristics of patients with preterm PROM.
| Negative culture with low IL-6 (n=18) | Negative culture with high IL-6 (n=19) | Positive culture with high IL-6 (n=8) | Positive culture with low IL-6 (n=14) | p-value | |
|---|---|---|---|---|---|
| Maternal age (years; median [IQR])a | 25.5 (22–31) | 29 (22.5–32) | 21.5 (20.8–26.8) | 26.5 (23.3–32) | 0.5 |
| Body mass index (kg/m2; median [IQR])a | 23.6 (21.4–29)c | 24 (21–28) | 21.8 (19.4–30.9)c | 21.1 (18.8–25.7) | 0.6 |
| Primiparityb | 22.2% (4/18) | 15.8% (3/19) | 12.5% (1/8) | 14.3% (2/14) | 0.9 |
| Race/Ethnicityb | 0.5 | ||||
| African-American | 100% (18/18) | 84.2% (16/19) | 87.5% (7/8) | 85.7% (12/14) | |
| White | 0% (0/18) | 10.5% (2/19) | 12.5% (1/8) | 7.1% (1/14) | |
| Hispanic | 0% (0/18) | 0% (0/19) | 0% (0/8) | 7.1% (1/14) | |
| Other | 0% (0/18) | 5.3% (1/19) | 0% (0/8) | 0% (0/14) | |
| Gestational age at membrane rupture (weeks; median [IQR])a | 31.9 (29.2–32.6) | 27.8 (22.8–31.2)c | 29.5 (27.6–30.4) | 31.6 (29.5–33.3) | 0.01 |
| Gestational age at amniocentesis (weeks; median [IQR])a | 32.2 (29.4–32.6) | 27.7 (22.4–30.8) | 29.5 (27.6–30.4) | 31.6 (29.5–33.3) | 0.007 |
| IL-6 (ng/mL; median [IQR])a | 0.9 (0.5–1.2) | 34.1 (6–161.3) | 33.3 (19.9–46) | 1 (0.4–1.9) | <0.001 |
| Gestational age at delivery (weeks; median [IQR])a | 33 (31.1–33.7) | 28.7 (23.1–32.6) | 30.2 (29–30.9) | 31.9 (29.7–33.6) | 0.02 |
| Cesarean sectionb | 11.1% (2/18) | 26.3% (5/19) | 25% (2/8) | 28.6% (4/14) | 0.5 |
| Birthweight (grams)a | 1902.5 (1756.3–2115) | 1185 (490–1927.5) | 1257.5 (1135–1506.3) | 1767.5 (1402.5–2095) | 0.02 |
| Acute maternal inflammatory response | |||||
| Stage 1 (Early acute subchorionitis or chorionitis)b | 17.6% (3/17)c | 6.3% (1/16)e | 0% (0/8) | 7.1% (1/14) | 0.6 |
| Stage 2 (Acute chorioamnionitis)b | 23.5% (4/17)c | 25% (4/16)e | 87.5% (7/8) | 42.9% (6/14) | 0.01 |
| Stage 3 (Necrotizing chorioamnionitis)b | 0% (0/17)c | 37.5% (6/16)e | 12.5% (1/8) | 14.3% (2/14) | 0.02 |
| Acute fetal inflammatory response | |||||
| Stage 1 (Chronic vasculitis or umbilical phlebitis)b | 23.5% (4/17)c | 18.8% (3/16)e | 25% (2/8) | 14.3% (2/14) | 0.9 |
| Stage 2 (Umbilical arteritis)b | 17.6% (3/17)c | 18.8% (3/16)e | 62.5% (5/8) | 28.6% (4/14) | 0.1 |
| Stage 3 (Necrotizing funisitis)b | 0% (0/17)c | 18.8% (3/16)e | 0% (0/8) | 14.3% (2/14) | 0.1 |
Data are given as median (interquartile range, IQR) and percentage (n/N).
Kruskal-Wallis test.
Fisher’s exact test.
One missing datum.
Two missing data.
Three missing data.
Clinical definitions
Gestational age was determined by the date of the last menstrual period and confirmed by ultrasound examination. The gestational age derived from sonographic fetal biometry was used if the estimation was inconsistent with menstrual dating. Preterm PROM was defined as amniorrhexis confirmed by vaginal pooling, ferning, or a positive nitrazine test prior to the onset of labor before 37 weeks of gestation (117–120). Intra-amniotic inflammation was defined using an established cutoff for amniotic fluid concentrations of IL-6 (14), where concentrations > 2.6 ng/mL indicate intra-amniotic inflammation and concentrations ≤ 2.6 ng/mL are considered as no inflammation.
Amniotic fluid sample collection
Amniotic fluid samples were obtained by transabdominal amniocentesis under antiseptic conditions and ultrasound guidance to evaluate the microbial and inflammatory status of the amniotic cavity. Samples of amniotic fluid were transported to the laboratory in a sterile capped syringe. Clinical tests included culture of aerobic/anaerobic bacteria and genital mycoplasmas (7, 121), white blood cell count (122), Gram stain (123), glucose concentration (124), and IL-6 concentration (14). The rest of the sample was utilized for research purposes.
Determination of IL-6 concentration in amniotic fluid
Amniotic fluid concentrations of IL-6 were determined as previously established (14) using a sensitive and specific enzyme immunoassay obtained from R&D systems (Minneapolis, MN, USA). The IL-6 concentrations were determined by interpolation from the standard curves. The inter- and intra-assay coefficients of variation for IL-6 were 8.7% and 4.6%, respectively. The sensitivity of the IL-6 assay was 0.09 pg/mL. The IL-6 concentrations in amniotic fluid were determined for clinical purposes.
Placental histopathological examination
Sampling of the placentas was conducted according to protocols established by the Perinatology Research Branch. A minimum of 5 full-thickness sections of chorionic plate, 3 sections of umbilical cord, and 3 chorioamniotic membrane rolls from each case were examined by placental pathologists who were blinded to the clinical histories and additional testing results. Acute inflammatory lesions of the placenta (maternal inflammatory response and fetal inflammatory response) were diagnosed according to established criteria, including staging and grading (125).
DNA extraction from amniotic fluid
Samples of amniotic fluid (250 μL) were processed inside a biological safety cabinet by personnel equipped with sterile surgical gowns, hoods, surgical masks, and powder-free exam gloves (Kimberly-Clark; Roswell, GA, USA). DNA was extracted using the DNeasy PowerLyzer PowerSoil Kit (Cat# 12855, Qiagen, Germantown, MD, USA) with minor modifications to the manufacturer’s protocol. Briefly, amniotic fluid samples were mixed with 400 μL of bead solution and 200 μL of phenol:chloroform:isoamyl alcohol (pH 7–8) in the supplied bead tube. Next, 60 μL of Solution C1 were added, and microbial cells were lysed by mechanical disruption using a bead beater (BioSpec, Bartlesville, OK, USA) for 30 s. Afterwards, the bead tubes were centrifuged at 10,000 × g for 1 min and the resulting supernatants were transferred to new tubes. Next, 100 μL of solution C2, 100 μL of solution C3, and 1 μL of RNase A enzyme were added to the sample tubes and incubated at 4°C for 5 min. Steps involving solutions C2 and C3 were combined to maximize DNA yield. The sample tubes were centrifuged at 10,000 × g for 1 min and the supernatants were transferred to new tubes containing 650 μL of solution C4 and 650 μL of 100% ethanol. Each amniotic fluid lysate was then loaded onto a filter column, centrifuged at 10,000 × g for 1 min, and the flow-through was discarded. Next, 500 μL of solution C5 were added to the filter columns and centrifuged at 10,000 × g for 1 min, after which the flow-through was discarded and the tube was centrifuged again for an additional 3 min as a dry-spin. Finally, 60 μL of solution C6 were placed on the filter column and incubated for five minutes before centrifuging at 10,000 × g for 30 seconds to elute the extracted DNA. For each set of extractions, at least one blank DNA extraction kit was processed as a background negative control (n = 8). Positive amniotic fluid control samples (n = 6) included DNA extractions performed on amniotic fluid supernatants from 6 different patients whose amniotic fluid yielded a bacterial isolate by culture. Negative amniotic fluid control samples (n = 7) included 7 separate DNA extractions performed on an amniotic fluid sample from a patient previously determined not to have intra-amniotic infection by qPCR. These technical replicates are provided only for perspective and were not included in any statistical analyses in this study. Purified DNA was stored at −80°C.
Establishment of a quantitative real-time PCR for the 16S rRNA gene to determine microbial burden in amniotic fluid
Prior to the performance of qPCR in study samples, a preliminary test was conducted to investigate the existence of DNA amplification inhibition for amniotic fluid (126, 127). For this test, 3 μL of purified DNA from amniotic fluid samples were serially diluted with solution C6 elution buffer by a factor of 1:3 (i.e. 1:0, 1:3, 1:9). Each qPCR reaction was then spiked with 3 μL of purified Escherichia coli ATCC 25922 (GenBank accession: CP009072) genomic DNA (10 pg/μl) containing seven 16S rDNA copies per genome. Genomic DNA was quantified using a Qubit 3.0 fluorometer with a Qubit dsDNA HS Assay kit (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s protocol. Spiked reactions contained approximately 3.989 × 104 E. coli 16S rDNA copies, and there was evidence of DNA amplification inhibition (Fig. S1). All amniotic fluid samples were subsequently diluted with solution C6 elution buffer by a factor of 1:3 prior to further analyses.
Total bacterial DNA abundance within samples was measured via amplification of the V1 - V2 region of the 16S rRNA gene according to the protocol of Dickson et al. (128) with minor modifications as previously described (126, 129). These modifications included the use of a degenerative forward primer (27f-CM: 5’-AGA GTT TGA TCM TGG CTC AG-3’) (130) and a degenerate probe containing locked nucleic acids (+) (BSR65/17: 5’−56FAM-TAA +YA+C ATG +CA+A GT+C GA-BHQ1–3’). Each 20 μL reaction contained 0.6 μM of 27f-CM primer, 0.6 μM of 357R primer (5’-CTG CTG CCT YCC GTA G-3’), 0.25 μM of BSR65/17 probe, 10 μL of 2X TaqMan Environmental Master Mix 2.0 (Life Technologies), and 3 μL of either purified DNA, elution buffer, or nuclease-free water. The total bacterial DNA qPCR was performed using the following conditions: 95° C for 10 min, followed by 45 cycles of 94° C for 30 s, 50° C for 30 s, and 72° C for 30 s. Duplicate reactions were run for all samples. All samples were run across a total of 4 runs. Raw DNA amplification data were normalized to the ROX passive reference dye and analyzed using the on-line platform Thermo Fisher Cloud: Standard Curve (SR) 3.3.0-SR2-build15 using automatic threshold and baseline settings. Cycle of quantification (Cq) values were calculated for each sample based on the mean number of qPCR cycles required to observe an exponential increase in the normalized fluorescence signal.
DNA derived from E. coli ATCC 25922 (described above) and a Ureaplasma parvum isolate previously obtained from an amniotic fluid sample (using the same DNA extraction protocol) was quantified with the use of a Qubit 3.0 fluorometer with a Qubit dsDNA HS Assay kit and used for the generation of standard curves. The E. coli standard curve ranged from 1.99 × 107 - 1.99×101 copies. For the U. parvum isolate, it was estimated that its genome has a mass of 4.78 × 105 kDa and contains two 16S rRNA gene copies. Therefore, the U. parvum standard curve ranged from 3.40 × 106 - 3.40 × 101 copies. Independently diluted standard curves containing 10-fold serial dilutions (3 replicates each) were included in each of the 4 qPCR runs. The standard curves were used to evaluate the performance of the qPCR assay by estimation of its efficiency based on the slope of regression lines (131). Analysis of Cq values generated for the standard curves indicated that the average amplification efficiency of the E. coli and U. parvum assays were 90.32 ± 1.47% (SD) and 91.31 ± 0.93% (SD), respectively, with similar diagnostic sensitivity as previously observed for the qPCR assay (126, 129). The regression curves were linear over the entire range of dilutions for both standard curves (Fig. S2).
Determination of extracellular ASC in amniotic fluid
Concentrations of extracellular ASC in the amniotic fluid were determined as previously established (82, 115, 116) by using a sensitive and specific enzyme-linked immunosorbent assay (ELISA) kit obtained from LifeSpan Biosciences (Seattle, WA, USA). Amniotic fluid concentrations of ASC were obtained by interpolation from the standard curve. The inter- and intra-assay coefficients of variation were 5.0% and 8.6%, respectively. The sensitivity of the ASC assay was 0.131 ng/mL.
Statistical analysis
Differences in cycle of quantification (Cq) values among samples were evaluated using Kruskal-Wallis and Mann-Whitney U tests. Sequential Bonferroni corrections were applied to all post hoc pairwise comparisons. The strength of correlation between ASC concentrations and Cq values in amniotic fluid was evaluated using the Spearman’s rank-order correlation test. Graphical and statistical analyses were performed in PAST v3.25 (https://folk.uio.no/ohammer/past/).
RESULTS
Characteristics of the study population
Clinical and demographic characteristics of the study population (n = 59) are described in Table 1. There were no differences in maternal age, body mass index, rate of primiparity, race, or the rate of cesarean section among the initial study groups (Table 1). Birthweights, as well as gestational ages at membrane rupture, amniocentesis, and delivery, were significantly different (Table 1). Acute maternal inflammatory responses (stage 2 and stage 3), but not fetal inflammatory responses, were significantly different among the study groups.
The relationship between microbiological culture and 16S rRNA gene qPCR signal
Microbial culture is regarded as the gold standard technique to identify microorganisms in amniotic fluid (6, 9, 23, 73, 78, 132–138). Therefore, we first assessed whether microbial burden detected by 16S rRNA gene qPCR was capable of detecting bacterial signals in amniotic fluid samples that had a negative or positive microbiological culture. Microbial burden is reported using cycle of quantification (Cq), which represents the average number of qPCR cycles required to observe an exponential increase in the detected 16S rRNA gene signal. Thus, a lower Cq number is indicative of a greater microbial burden.
Amniotic fluid samples with a positive culture often had a greater microbial burden than those with a negative culture; however, this difference did not reach statistical significance (p = 0.068) (Fig. 1). This result could potentially be explained by the presence of difficult-to-culture (i.e. fastidious) bacteria in amniotic fluid of women with a negative culture (78, 139–154). Nonetheless, amniotic fluid samples, regardless of the microbiological culture result, had higher median 16S rRNA gene signals than the kit/extraction controls (p < 0.0001) (Fig. 1). It is worth mentioning that several samples with a negative culture had similar signals of the 16S rRNA gene compared to kit/extraction controls (samples included in the dotted blue square), indicating that not all samples from cases of preterm PROM have detectable bacteria. Additionally, one sample with a positive amniotic fluid culture had a similar 16S rRNA gene signal compared to kit/extraction controls (sample included in the dotted red square), which could represent a possible laboratory contaminant isolated during microbiological culture (Fig. 1). Taken together, these results indicate that increased microbial burden, as determined by 16S rRNA gene signal, is in general associated with a positive microbiological culture result. Yet, 16S rRNA gene qPCR can detect bacterial signals in samples that did not yield bacterial cultivars, thus demonstrating a higher sensitivity of qPCR than culture for detecting microbial invasion of the amniotic cavity.
Figure 1. Association between microbiological culture and 16s rRNA gene qPCR in amniotic fluid.
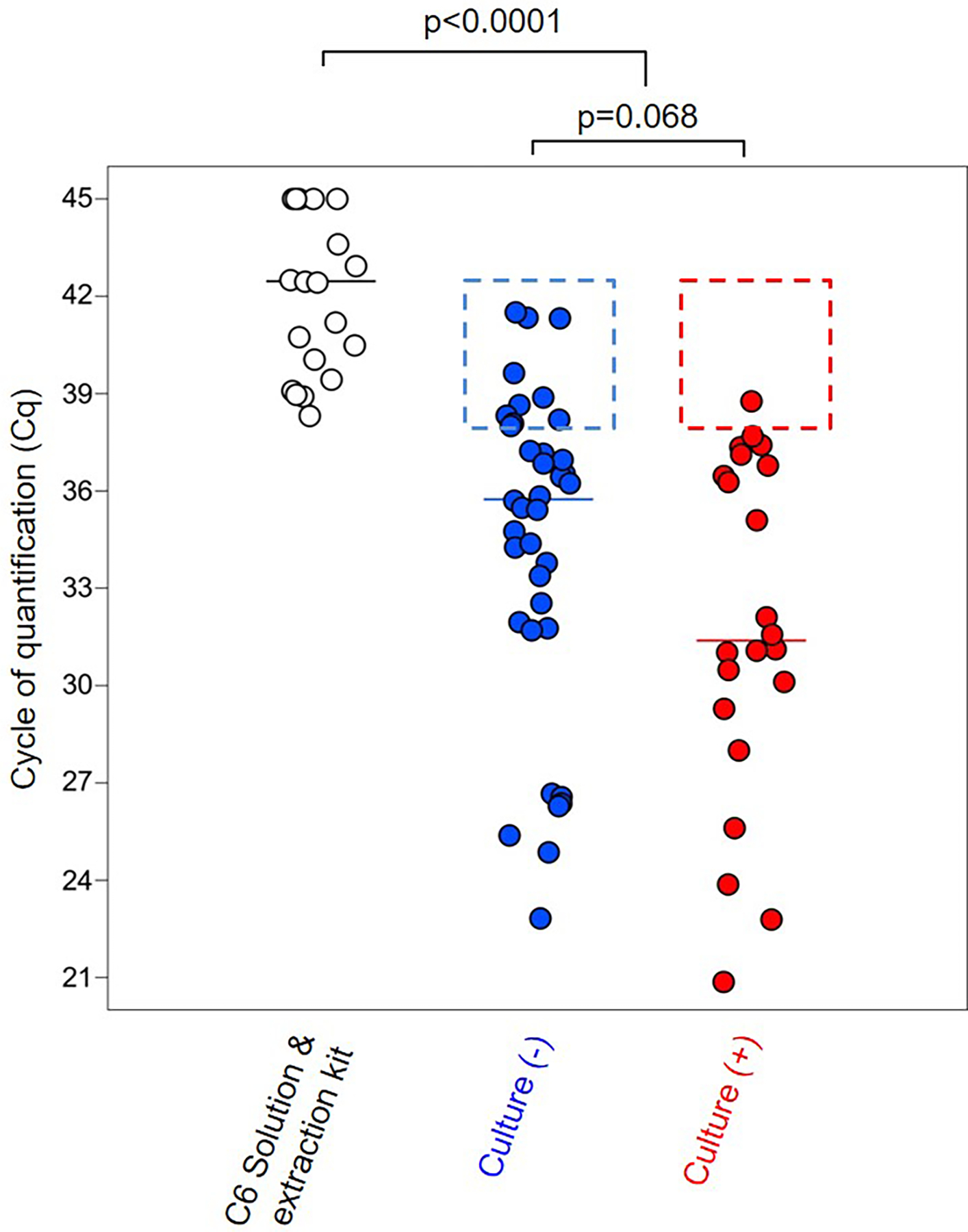
Cycle of quantification (Cq) of background technical controls and amniotic fluid samples from women with preterm PROM based on the presence/absence of positive microbial culture. Median values are indicated. Statistical results are from Mann-Whitney U tests. Dotted squares represent the absence of microbial detection by 16S rRNA gene qPCR in culture positive/negative amniotic fluid samples. N = 20–37 per group.
The association between microbial detection and intra-amniotic inflammation
We next investigated whether the detection of microorganisms is associated with the IL-6 inflammatory response in amniotic fluid. The amniotic fluid concentration of IL-6 was used as the diagnostic criteria for intra-amniotic inflammation as previously described (14, 155–158). According to amniotic fluid culture and IL-6 concentrations, we classified the patients into four groups: 1) negative culture with low IL-6 [culture(−)/IL-6(−)]; 2) negative culture with high IL-6 [culture(−)/IL-6(+)]; 3) positive culture with high IL-6 [culture(+)/IL-6(+)]; and 4) positive culture with low IL-6 [culture(+)/IL-6(−)]. We used amniotic fluid samples from a mid-trimester amniocentesis of a patient without preterm PROM or intra-amniotic inflammation as a negative control in addition to water and kit/extraction controls. Amniotic fluid samples from women with positive microbiological cultures but without preterm PROM were used as positive controls. The cutoff for a positive 16S rRNA gene signal was defined as a Cq value less than 34.66, which was determined based on the lowest Cq value among the water and kit/extraction controls (Fig. 2).
Figure 2. Microbial burden and intra-amniotic inflammation.
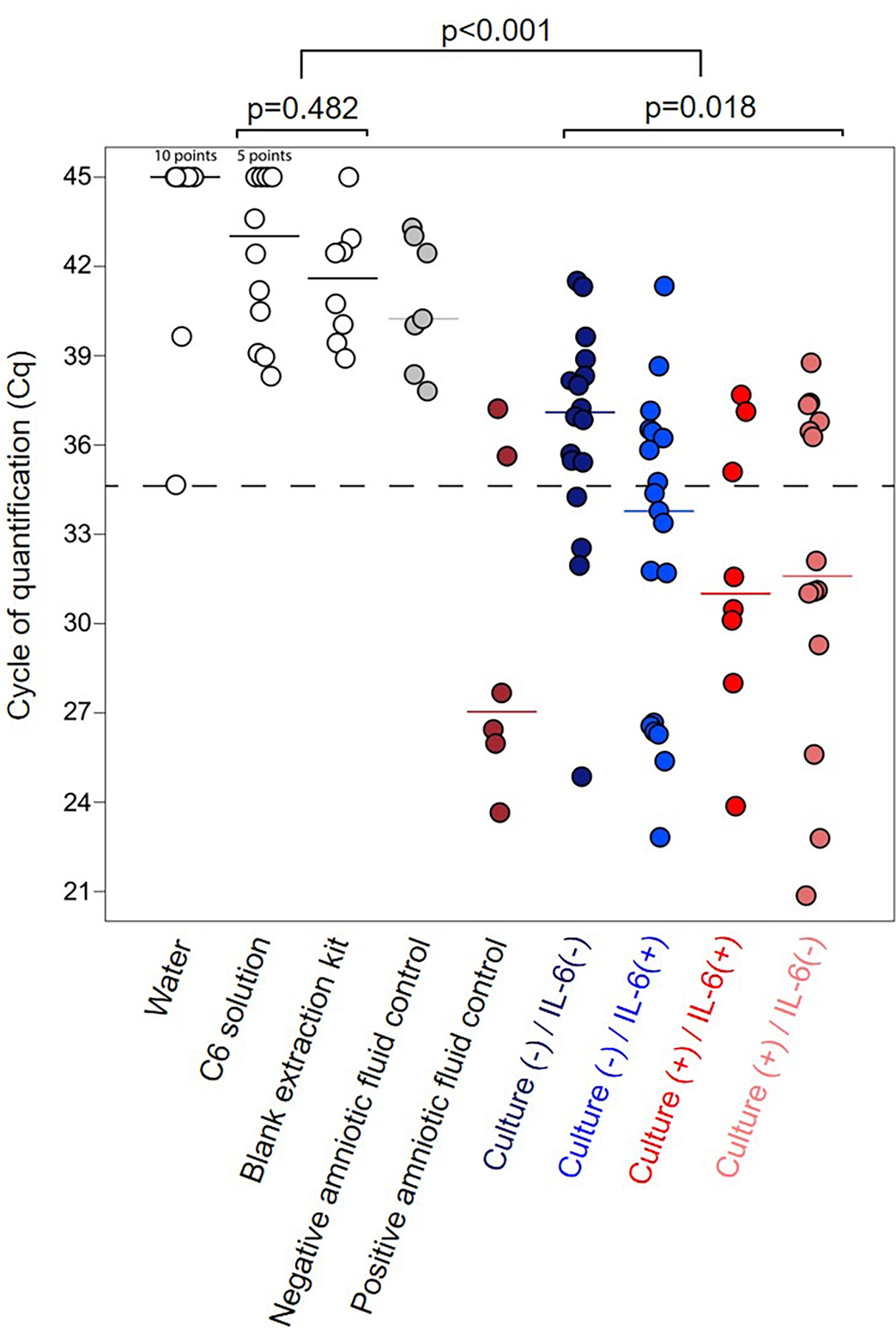
Cycle of quantification (Cq) of background technical controls and amniotic fluid samples based on the presence/absence of positive microbiological culture and intra-amniotic inflammation (IL-6 concentrations > 2.6 ng/mL). Median values are indicated. Statistical results are from Mann-Whitney U and Kruskal-Wallis tests. Sequential Bonferroni corrections were applied to all post hoc pairwise comparisons. The dashed line indicates the lowest Cq value of any background technical control, which was used to define the cutoff for a positive 16S signal (Cq value < 34.66 cycles). N = 12–19 per group.
As described above, the overall 16S rRNA gene signal in preterm PROM patients was significantly higher than in kit/extraction controls (p < 0.001) (Fig. 2). Most of the culture(−)/IL-6(−) patients had a negative (Cq value > 34.66) 16S rRNA gene signal (14/18: 77.8%). More than half of the culture(−)/IL-6(+) patients had a positive (Cq value < 34.66) 16S rRNA gene signal (11/19: 57.9%) (Fig. 2), indicating that non-culturable or fastidious microorganisms may induce an inflammatory response in the amniotic cavity. Notably, nearly 60% of the patients with a positive culture also had a positive (Cq value < 34.66) 16S rRNA gene signal, regardless of the presence of intra-amniotic inflammation [culture(+)/IL-6(+): 5/8, 62.5%; culture(+)/IL-6(−): 8/14, 57.1%] (Fig. 2). Indeed, the median 16S rRNA gene signal between patients with a positive culture, regardless of the presence of intra-amniotic inflammation, was similar (Fig. 2). These results show that high levels of IL-6 (> 2.6 ng/mL) are associated with the detection of microbes by culture or 16S rRNA gene qPCR; however, a subset of preterm PROM patients with detectable microorganisms do not present with intra-amniotic inflammation.
Based on the results of 16S rRNA gene qPCR, we re-stratified our patients into new study groups (Table 2). The first group included cases with both negative amniotic fluid culture and negative 16S rRNA gene qPCR as well as low IL-6 (n = 14/59, 23.7%), and represent preterm PROM patients with neither detectable bacteria nor intra-amniotic inflammation. The second group included patients with both a negative amniotic fluid culture and negative 16S rRNA gene signal but high IL-6 (n = 8/59, 13.6%), a condition which has been termed sterile intra-amniotic inflammation (23, 29, 30, 32). The third group included all patients with a positive amniotic fluid culture and/or positive 16S rRNA gene signal together with high IL-6 (n = 19/59, 32.2%), referred to as microbial-associated intra-amniotic inflammation or intra-amniotic infection (23, 29, 30, 32). Finally, the fourth group included those patients with a positive amniotic fluid culture and/or positive 16S rRNA gene signal with low IL-6 (n = 18/59, 30.5%). These reassigned patient groups are utilized in Figures 3 & 4. These results confirm that preterm PROM is a heterogeneous condition that includes different subsets of patients (31, 32).
Table 2. Amniotic fluid sample categorization.
Categorization of amniotic fluid samples based on the presence/absence of positive microbiological culture, positive bacterial 16S rRNA gene quantitative real-time PCR (qPCR), and intra-amniotic inflammation (IL-6 concentrations > 2.6 ng/mL). A positive bacterial qPCR result was defined as having a Cq value < 34.66 cycles (the lowest Cq value among the negative technical controls).
| Negative microbial detection and low IL-6 | Negative | Negative | Negative | 14 | 23.7 % |
| Negative microbial detection and high IL-6 | Negative | Negative | Positive | 8 | 13.6 % |
| Positive microbial detection and high IL-6 | Positive | Negative | Positive | 3 | 32.2 % |
| Negative | Positive | Positive | 11 | ||
| Positive | Positive | Positive | 5 | ||
| Positive microbial detection and low IL-6 | Positive | Negative | Negative | 6 | 30.5 % |
| Negative | Positive | Negative | 4 | ||
| Positive | Positive | Negative | 8 |
Figure 3. Correlation between microbial burden and extracellular ASC in amniotic fluid.
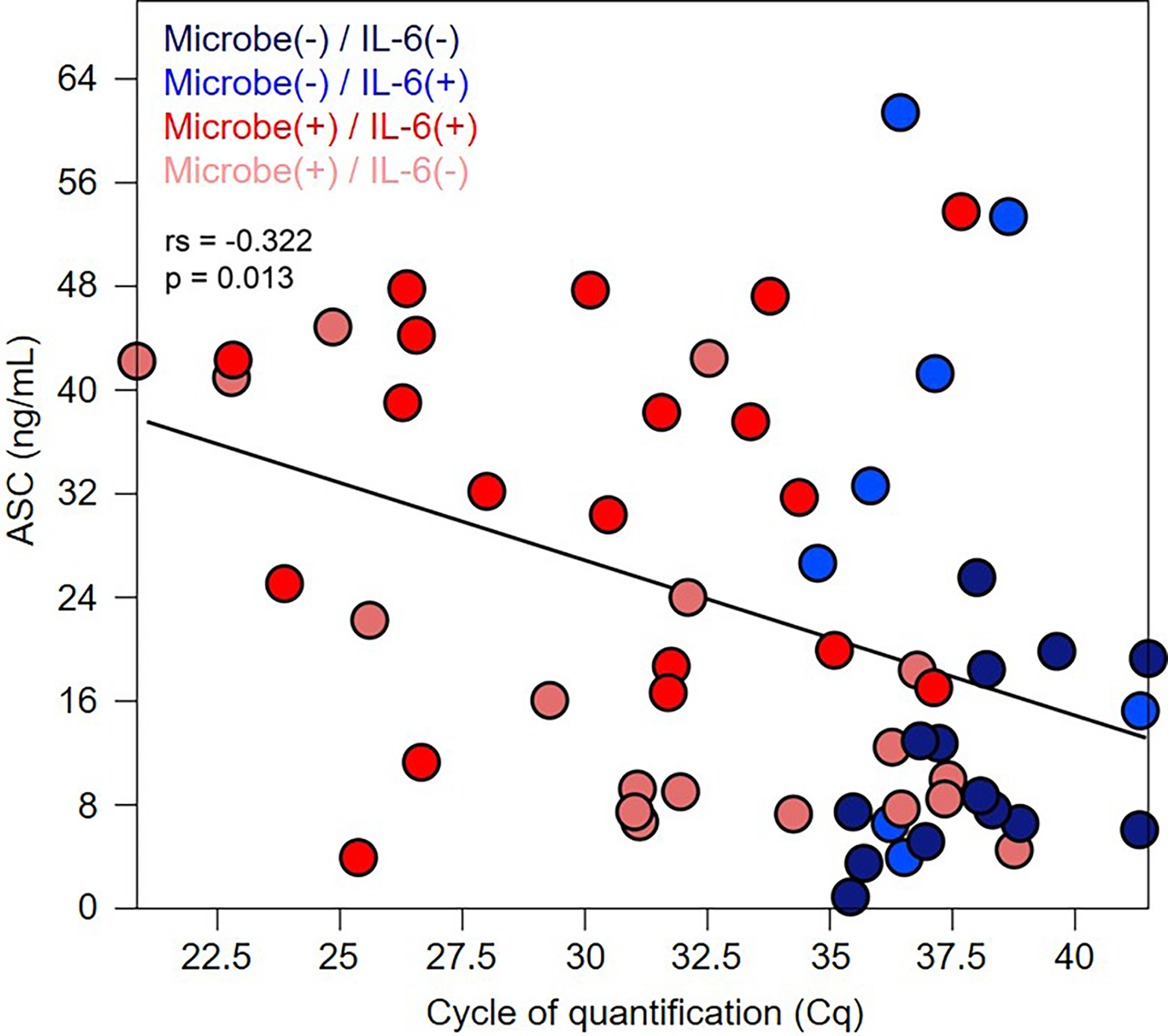
Extracellular ASC concentration in relation to the cycle of quantification (Cq) of amniotic fluid samples. Categorization of amniotic fluid samples is based on the presence/absence of positive microbiological culture and/or positive bacterial 16S rRNA gene qPCR, and intra-amniotic inflammation (IL-6 concentrations > 2.6 ng/mL). The statistical result is from a Spearman’s rank-order correlation test. The regression line is indicated. N = 8–19 per group.
Figure 4. Extracellular ASC concentrations in amniotic fluid of women with preterm PROM in the presence/absence of positive microbial detection and intra-amniotic inflammation.
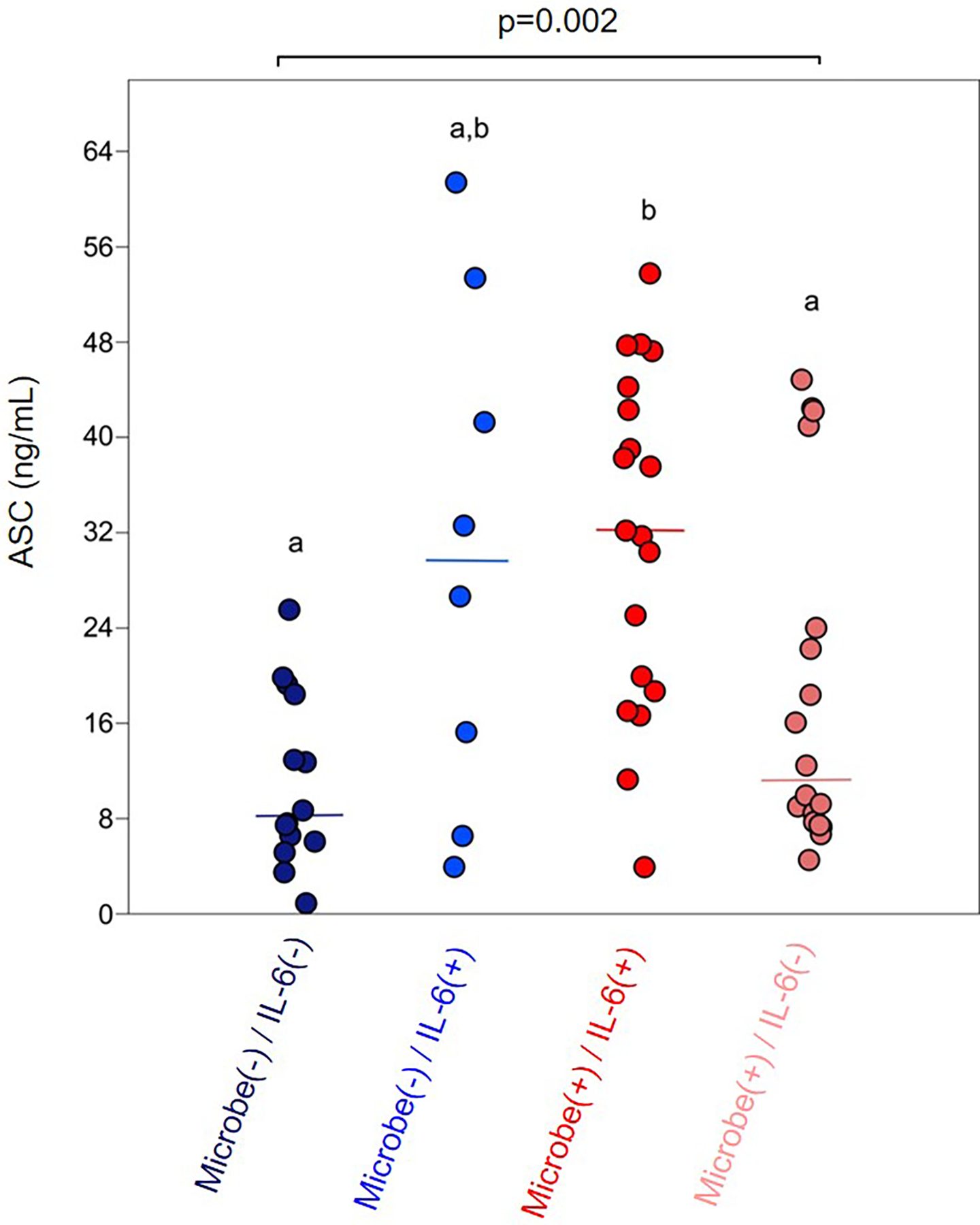
Extracellular ASC concentrations of amniotic fluid samples based on the presence/absence of positive microbial culture and/or positive bacterial 16S rRNA gene qPCR, and intra-amniotic inflammation (IL-6 concentrations > 2.6 ng/mL). Median values are indicated. Statistical results are from Kruskal-Wallis and Mann-Whitney U tests. Amniotic fluid categories marked by different letters were statistically different after sequential Bonferroni corrections were applied. N = 8–19 per group.
The correlation between microbial burden and extracellular ASC concentrations in amniotic fluid (i.e. intra-amniotic inflammasome activation)
Subsequently, we investigated the intra-amniotic inflammatory response in women with preterm PROM by measuring amniotic fluid concentrations of extracellular ASC (i.e. intra-amniotic inflammasome activation). A significant correlation was observed between amniotic fluid ASC concentrations and microbial burden (i.e. low 16S rRNA gene Cq values (p = 0.013, ρ = −0.322) (Fig. 3). Hence, most of the samples with detectable microorganisms (red dots) had elevated concentrations of extracellular ASC, whereas most of the samples without detectable microorganisms (blue dots) had low concentrations of extracellular ASC (Fig. 3).
The correlation between microbial burden and extracellular ASC (Fig. 3) appeared to be related to the severity of the intra-amniotic inflammatory response, as amniotic fluid ASC concentrations were significantly elevated in patients with intra-amniotic infection [microbe(+)/IL-6(+)] compared to those with a positive bacterial culture/16S rRNA gene signal without intra-amniotic inflammation [microbe(+)/IL-6(−)] or those with neither detectable microorganisms nor intra-amniotic inflammation [microbe(−)/IL-6(−)] (Fig. 4). This association was partially independent of the detection of microorganisms given that patients with sterile intra-amniotic inflammation [microbe(−)/IL-6(+)] tended to display higher ASC concentrations in amniotic fluid compared to those with detectable microorganisms without intra-amniotic inflammation [microbe(+)/IL-6(−), non-significant] or those with neither detectable microorganisms nor intra-amniotic inflammation [microbe(−)/IL-6(−), non-significant] (Fig. 4). These data suggest that the intra-amniotic inflammatory response in patients with preterm PROM involves inflammasome activation, which partially depends on the detection of microbes in the amniotic cavity.
DISCUSSION
Principal Findings
In the current study, we report that in patients with preterm PROM: 1) a positive amniotic fluid microbiological culture result was associated with high 16S rRNA gene signal; 2) 16S rRNA gene qPCR can identify a greater number of microbe-positive amniotic fluids than conventional culture; 3) over 50% of patients with a negative culture and high IL-6 in amniotic fluid yielded a positive 16S rRNA gene signal; 4) 16S rRNA gene signal was positively correlated with amniotic fluid concentrations of extracellular ASC; 5) ASC concentrations were greatest in patients with a high positive 16S rRNA gene signal and elevated IL-6 concentrations in amniotic fluid (i.e. intra-amniotic infection); and 6) ASC concentrations tended to increase in patients without detectable microorganisms but yet with elevated IL-6 concentrations in amniotic fluid (i.e. sterile intra-amniotic inflammation). Collectively, these results indicate that 16S rRNA gene qPCR can be an effective and valuable complement to microbiological culture for the detection of microbial invasion of the amniotic cavity in women with preterm PROM, and that microbial burden is associated with intra-amniotic inflammation, including the activation of the inflammasome.
Detection of microbial burden in amniotic fluid by 16S rRNA gene qPCR compared to conventional microbiological cultures
Conventional microbiological culture has been widely used to diagnose microbial invasion of the amniotic cavity (2, 3, 5, 6, 9, 73, 122, 123, 132–138, 145, 146, 159–165). However, this method has several limitations, most notably the length of time required to obtain results and the variety of microorganisms that can be detected (23, 24, 78, 152, 166, 167). Clinically, this delay in obtaining culture results has led to the standard practice of administering broad-spectrum antibiotics to patients presenting with inflammation without knowing the specific microorganisms present (168–171). Recently, the use of advanced molecular microbiological PCR-based techniques was proposed as a solution to these problems (23), since such methods can identify a greater number of microorganisms, including those which may be difficult to culture (78, 139–145, 147–154, 172–174), and the results can be rapidly obtained (23, 175–181). Molecular microbiological techniques can also rule out false positives obtained by conventional culture likely due to contamination (126). In line with these previous studies, we report that patients with preterm PROM and a positive amniotic fluid culture have a higher 16S rRNA gene signal than those with a negative culture. More importantly, several of the patients with a negative culture also displayed a high 16S rRNA gene signal, providing further confirmation that molecular microbiological techniques can detect microorganisms in amniotic fluid that are not found using conventional clinical methods.
Microbial detection and intra-amniotic infection
In the current study, we found that preterm PROM patients with a positive culture and intra-amniotic inflammation (diagnosed as the elevated amniotic fluid concentration of IL-6 > 2.6 ng/ml) have elevated bacterial burden using 16S rRNA gene qPCR. These results are consistent with previous studies in which women with preterm labor (29, 82), clinical chorioamnionitis at term (116) or preterm PROM (31, 32) and proven intra-amniotic infection display higher levels of IL-6 than those with intra-amniotic inflammation without detectable microorganisms. Patients with a positive culture do not seem to display differences in the intensity of the intra-amniotic inflammatory response, as evidenced by amniotic fluid IL6 concentrations. This suggests that further investigation of the identities of the different cultured, as well as uncultured, microorganisms in these amniotic fluids, using deep sequencing, is warranted.
In the current study, we also found that some patients with a negative culture had an elevated amniotic fluid IL-6 concentration and detectable 16S rRNA gene signal, suggesting that microorganisms that were not cultured from amniotic fluid, yet still present, may also initiate an intra-amniotic inflammatory response. There are several microorganisms associated with intra-amniotic infection that are difficult to culture in a clinical laboratory setting, namely mycoplasmas (e.g. Ureaplasma urealyticum) (23, 32, 66, 79, 143, 182–184), Sneathia spp. (23, 32, 66, 152, 184), Neisseria spp. (32, 152), and Fusobacterium nucleatum (23, 66, 184, 185). In addition, there are several fastidious species that are known to exist in a viable but non-culturable state (186). These non-culturable microorganisms can be notably different from their viable counterparts with respect to their metabolic, adhesive, and virulence capacities, as well the biochemical composition of their cell walls and membranes (187–209). Therefore, it is likely that both the viability and culturable state of the microorganisms in amniotic fluid may affect the severity of the inflammatory response.
The mechanisms whereby microbes invading the amniotic cavity induce high concentrations of IL-6 involve the activation of the NF-κB pathway (210–213). Indeed, in vitro studies have shown that incubation of the chorioamniotic membranes with microbial products such as lipopolysaccharide (LPS) trigger the activation of such a pathway (214). Another potential cellular source of IL-6 in amniotic fluid is the immune cells present in this compartment, particularly monocytes/macrophages (215–218). Nonetheless, further research is needed to investigate whether viable yet non-culturable microorganisms are sensed by different pattern recognition receptors than culturable microorganisms, leading to distinct inflammatory responses.
Microbial burden correlated with intra-amniotic inflammasome activation
Herein, we showed that there is significant correlation between 16S rRNA gene signal, microbial burden, and extracellular ASC concentrations in amniotic fluid of patients with preterm PROM. Extracellular ASC has been previously utilized as an in vivo indicator of inflammasome activation in amniotic fluid (82, 115, 116). Indeed, we have recently demonstrated that the concentrations of this protein are increased in the amniotic cavity during the sterile physiological process of spontaneous labor at term (115), as well as in pathological processes such as clinical chorioamnionitis (116) and preterm labor/birth (82). These observations are in line with previous studies showing that inflammasome-related molecules such as caspase-1 (219) and IL-1β are increased in amniotic fluid of women who underwent preterm labor with intact membranes (220–223) or preterm PROM (220). There are several possible sources for extracellular ASC and other inflammasome components in the amniotic cavity. First, the chorioamniotic membranes from women in preterm labor with intra-amniotic inflammation/infection express increased levels of inflammasome sensor molecules and the active forms of both caspase-1 and IL-1β, as well as greater numbers of ASC/caspase-1 protein complexes (i.e. enhanced inflammasome assembly) (81). Second, amniotic fluid of women with intra-amniotic infection contains large numbers of immune cells such as neutrophils and monocytes/macrophages (215, 216, 224), which may undergo inflammasome-mediated inflammatory cell death (i.e. pyroptosis) (225). Together, these data indicate that women with a high microbial burden in the amniotic cavity - intra-amniotic infection - display inflammasome activation. Yet, the sole presence of microorganisms in this compartment may not always result in intra-amniotic inflammasome activation.
In this study, we report that a subset of women with preterm PROM had elevated concentrations of IL-6 and ASC (i.e. intra-amniotic inflammasome activation) in the absence of detectable microorganisms, which has been termed “sterile intra-amniotic inflammation” (23, 29, 30, 32). This is consistent with previous reports showing that there is evidence of in vivo activation of the inflammasome in women with sterile intra-amniotic inflammation and preterm labor/birth (82) or clinical chorioamnionitis at term (116). The mechanisms leading to sterile intra-amniotic inflammation involve the activation of the NLRP3 inflammasome in the chorioamniotic membranes (81, 82, 85). Indeed, animal experimentation has shown that alarmins (molecules that trigger sterile inflammation (226–228)) are capable of activating the NLRP3 inflammasome in the fetal membranes (229). Importantly, these studies have generated promising data showing that, by tackling the activation of the NLRP3 inflammasome, sterile intra-amniotic inflammation can be treated and preterm birth prevented.
It is worth mentioning that, regardless of the nature of the stimuli (microbes and/or alarmins), the single determination of ASC in amniotic fluid does not allow for the identification of the canonical and non-canonical activation of the NLRP3 inflammasome. Yet, in vivo concentrations of extracellular ASC provide an overall readout of inflammasome activation in the amniotic cavity.
Microbial detection in amniotic fluid does not always correspond with intra-amniotic infection
A subset of patients with preterm PROM had detectable microbes either by culture or 16S rRNA gene qPCR but had low concentrations of IL-6 and extracellular ASC. This subset of patients are considered to have microbial invasion of the amniotic cavity in the absence of an inflammatory response (23, 24, 29–32, 230–232). A possible explanation for the lack of inflammation in these patients is that the amniotic fluid sample was collected before the initiation of the inflammatory cascade. However, the detection of microorganisms in the absence of intra-amniotic inflammation may also represent downstream contamination of the amniotic fluid sample. Additional research is required to investigate the clinical significance of the detection of microbes in the amniotic cavity in the absence of an inflammatory response.
CONCLUSION
In summary, the data presented herein provide evidence that preterm PROM is a heterogeneous condition that can be categorized based on the microbial burden and/or presence of intra-amniotic inflammation. The intra-amniotic inflammatory response is characterized by elevated concentrations of IL-6 as well as enhanced inflammasome activation (i.e. extracellular ASC) in amniotic fluid. These results provide insight into the biological processes occurring in the amniotic cavity of women with preterm PROM, and show that molecular microbiological techniques are valuable complements of conventional microbiological culture for the detection of microbial invasion of the amniotic cavity.
Supplementary Material
Acknowledgements:
We thank the physicians and nurses from the Center for Advanced Obstetrical Care and Research and the Intrapartum Unit for their help in collecting human samples. The authors also thank the staff members of the PRB Clinical Laboratory and the PRB Histology/Pathology Unit for the processing and examination of the pathological sections.
Funding: This research was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C. K. T. and N. G-L. were also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Miller JM Jr., Pupkin MJ, Hill GB. Bacterial colonization of amniotic fluid from intact fetal membranes. Am J Obstet Gynecol. 1980;136(6):796–804. [DOI] [PubMed] [Google Scholar]
- 2.Bobitt JR, Hayslip CC, Damato JD. Amniotic fluid infection as determined by transabdominal amniocentesis in patients with intact membranes in premature labor. Am J Obstet Gynecol. 1981;140(8):947–52. [DOI] [PubMed] [Google Scholar]
- 3.Wallace RL, Herrick CN. Amniocentesis in the evaluation of premature labor. Obstet Gynecol. 1981;57(4):483–6. [PubMed] [Google Scholar]
- 4.Wahbeh CJ, Hill GB, Eden RD, Gall SA. Intra-amniotic bacterial colonization in premature labor. Am J Obstet Gynecol. 1984;148(6):739–43. [DOI] [PubMed] [Google Scholar]
- 5.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, et al. Infection in the pathogenesis of preterm labor. Seminars in perinatology. 1988;12(4):262–79. [PubMed] [Google Scholar]
- 6.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol. 1988;31(3):553–84. [DOI] [PubMed] [Google Scholar]
- 7.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161(3):817–24. [DOI] [PubMed] [Google Scholar]
- 8.Romero R, Avila C, Brekus CA, Morotti R. The role of systemic and intrauterine infection in preterm parturition. Annals of the New York Academy of Sciences. 1991;622:355–75. [DOI] [PubMed] [Google Scholar]
- 9.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166(5):1515–28. [DOI] [PubMed] [Google Scholar]
- 10.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79(3):351–7. [DOI] [PubMed] [Google Scholar]
- 11.Gomez R, Romero R, Edwin SS, David C. Pathogenesis of preterm labor and preterm premature rupture of membranes associated with intraamniotic infection. Infectious disease clinics of North America. 1997;11(1):135–76. [DOI] [PubMed] [Google Scholar]
- 12.Yoon BH, Romero R, Park JS, Chang JW, Kim YA, Kim JC, et al. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998;179(5):1254–60. [DOI] [PubMed] [Google Scholar]
- 13.Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatric and perinatal epidemiology. 2001;15 Suppl 2:41–56. [DOI] [PubMed] [Google Scholar]
- 14.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185(5):1130–6. [DOI] [PubMed] [Google Scholar]
- 15.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Seminars in neonatology : SN. 2002;7(4):259–74. [DOI] [PubMed] [Google Scholar]
- 16.Shim SS, Romero R, Hong JS, Park CW, Jun JK, Kim BI, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2004;191(4):1339–45. [DOI] [PubMed] [Google Scholar]
- 17.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutrition reviews. 2007;65(12 Pt 2):S194–202. [DOI] [PubMed] [Google Scholar]
- 18.Seong HS, Lee SE, Kang JH, Romero R, Yoon BH. The frequency of microbial invasion of the amniotic cavity and histologic chorioamnionitis in women at term with intact membranes in the presence or absence of labor. Am J Obstet Gynecol. 2008;199(4):375 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007;197(3):294 e1–6. [DOI] [PubMed] [Google Scholar]
- 20.Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol. 2008;198(6):633 e1–8. [DOI] [PubMed] [Google Scholar]
- 21.Madan I, Romero R, Kusanovic JP, Mittal P, Chaiworapongsa T, Dong Z, et al. The frequency and clinical significance of intra-amniotic infection and/or inflammation in women with placenta previa and vaginal bleeding: an unexpected observation. Journal of perinatal medicine. 2010;38(3):275–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SM, Romero R, Lee J, Mi Lee S, Park CW, Shin Park J, et al. The frequency and clinical significance of intra-amniotic inflammation in women with preterm uterine contractility but without cervical change: do the diagnostic criteria for preterm labor need to be changed? The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25(8):1212–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. A novel molecular microbiologic technique for the rapid diagnosis of microbial invasion of the amniotic cavity and intra-amniotic infection in preterm labor with intact membranes. American journal of reproductive immunology (New York, NY : 1989). 2014;71(4):330–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Romero R, Miranda J, Kusanovic JP, Chaiworapongsa T, Chaemsaithong P, Martinez A, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. Journal of perinatal medicine. 2015;43(1):19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh KJ, Romero R, Park JY, Hong JS, Yoon BH. The earlier the gestational age, the greater the intensity of the intra-amniotic inflammatory response in women with preterm premature rupture of membranes and amniotic fluid infection by Ureaplasma species. Journal of perinatal medicine. 2019;47(5):516–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. The New England journal of medicine. 2000;342(20):1500–7. [DOI] [PubMed] [Google Scholar]
- 27.Kim MJ, Romero R, Gervasi MT, Kim JS, Yoo W, Lee DC, et al. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab Invest. 2009;89(8):924–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suff N, Karda R, Diaz JA, Ng J, Baruteau J, Perocheau D, et al. Ascending Vaginal Infection Using Bioluminescent Bacteria Evokes Intrauterine Inflammation, Preterm Birth, and Neonatal Brain Injury in Pregnant Mice. Am J Pathol. 2018;188(10):2164–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romero R, Miranda J, Chaiworapongsa T, Korzeniewski SJ, Chaemsaithong P, Gotsch F, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. American journal of reproductive immunology (New York, NY : 1989). 2014;72(5):458–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romero R, Miranda J, Chaiworapongsa T, Chaemsaithong P, Gotsch F, Dong Z, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2014;28:1343–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Musilova I, Kutova R, Pliskova L, Stepan M, Menon R, Jacobsson B, et al. Intraamniotic Inflammation in Women with Preterm Prelabor Rupture of Membranes. PLoS One. 2015;10(7):e0133929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romero R, Miranda J, Chaemsaithong P, Chaiworapongsa T, Kusanovic JP, Dong Z, et al. Sterile and microbial-associated intra-amniotic inflammation in preterm prelabor rupture of membranes. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2015;28(12):1394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson JW, Daikoku NH, Niebyl JR, Johnson TR Jr., Khouzami VA, Witter FR. Premature rupture of the membranes and prolonged latency. Obstet Gynecol. 1981;57(5):547–56. [PubMed] [Google Scholar]
- 34.Daikoku NH, Kaltreider DF, Khouzami VA, Spence M, Johnson JW. Premature rupture of membranes and spontaneous preterm labor: maternal endometritis risks. Obstet Gynecol. 1982;59(1):13–20. [PubMed] [Google Scholar]
- 35.Gibbs RS, Blanco JD. Premature rupture of the membranes. Obstet Gynecol. 1982;60(6):671–9. [PubMed] [Google Scholar]
- 36.Yoder PR, Gibbs RS, Blanco JD, Castaneda YS, St Clair PJ. A prospective, controlled study of maternal and perinatal outcome after intra-amniotic infection at term. Am J Obstet Gynecol. 1983;145(6):695–701. [DOI] [PubMed] [Google Scholar]
- 37.Hauth JC, Gilstrap LC 3rd, Hankins GD, Connor KD. Term maternal and neonatal complications of acute chorioamnionitis. Obstet Gynecol. 1985;66(1):59–62. [PubMed] [Google Scholar]
- 38.Hillier SL, Krohn MA, Kiviat NB, Watts DH, Eschenbach DA. Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am J Obstet Gynecol. 1991;165(4 Pt 1):955–61. [DOI] [PubMed] [Google Scholar]
- 39.McGregor JA. Maternal and fetal infection. Current opinion in obstetrics & gynecology. 1991;3(1):15–23. [PubMed] [Google Scholar]
- 40.Yancey MK, Duff P, Kubilis P, Clark P, Frentzen BH. Risk factors for neonatal sepsis. Obstet Gynecol. 1996;87(2):188–94. [DOI] [PubMed] [Google Scholar]
- 41.Moyo SR, Hagerstrand I, Nystrom L, Tswana SA, Blomberg J, Bergstrom S, et al. Stillbirths and intrauterine infection, histologic chorioamnionitis and microbiological findings. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 1996;54(2):115–23. [DOI] [PubMed] [Google Scholar]
- 42.van Hoeven KH, Anyaegbunam A, Hochster H, Whitty JE, Distant J, Crawford C, et al. Clinical significance of increasing histologic severity of acute inflammation in the fetal membranes and umbilical cord. Pediatric pathology & laboratory medicine : journal of the Society for Pediatric Pathology, affiliated with the International Paediatric Pathology Association. 1996;16(5):731–44. [PubMed] [Google Scholar]
- 43.Grether JK, Nelson KB. Maternal infection and cerebral palsy in infants of normal birth weight. Jama. 1997;278(3):207–11. [PubMed] [Google Scholar]
- 44.Ladfors L, Tessin I, Mattsson LA, Eriksson M, Seeberg S, Fall O. Risk factors for neonatal sepsis in offspring of women with prelabor rupture of the membranes at 34–42 weeks. Journal of perinatal medicine. 1998;26(2):94–101. [DOI] [PubMed] [Google Scholar]
- 45.Mark SP, Croughan-Minihane MS, Kilpatrick SJ. Chorioamnionitis and uterine function. Obstet Gynecol. 2000;95(6 Pt 1):909–12. [PubMed] [Google Scholar]
- 46.Wu YW, Colford JM Jr. Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. Jama. 2000;284(11):1417–24. [DOI] [PubMed] [Google Scholar]
- 47.Rao S, Pavlova Z, Incerpi MH, Ramanathan R. Meconium-stained amniotic fluid and neonatal morbidity in near-term and term deliveries with acute histologic chorioamnionitis and/or funisitis. Journal of perinatology : official journal of the California Perinatal Association. 2001;21(8):537–40. [DOI] [PubMed] [Google Scholar]
- 48.Impey L, Greenwood C, MacQuillan K, Reynolds M, Sheil O. Fever in labour and neonatal encephalopathy: a prospective cohort study. BJOG. 2001;108(6):594–7. [DOI] [PubMed] [Google Scholar]
- 49.Hagberg H, Wennerholm UB, Savman K. Sequelae of chorioamnionitis. Current opinion in infectious diseases. 2002;15(3):301–6. [DOI] [PubMed] [Google Scholar]
- 50.Nelson KB. The epidemiology of cerebral palsy in term infants. Mental retardation and developmental disabilities research reviews. 2002;8(3):146–50. [DOI] [PubMed] [Google Scholar]
- 51.Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. Jama. 2003;290(20):2677–84. [DOI] [PubMed] [Google Scholar]
- 52.Rouse DJ, Landon M, Leveno KJ, Leindecker S, Varner MW, Caritis SN, et al. The Maternal-Fetal Medicine Units cesarean registry: chorioamnionitis at term and its duration-relationship to outcomes. Am J Obstet Gynecol. 2004;191(1):211–6. [DOI] [PubMed] [Google Scholar]
- 53.Mercer BM. Preterm premature rupture of the membranes: current approaches to evaluation and management. Obstet Gynecol Clin North Am. 2005;32(3):411–28. [DOI] [PubMed] [Google Scholar]
- 54.Blume HK, Li CI, Loch CM, Koepsell TD. Intrapartum fever and chorioamnionitis as risks for encephalopathy in term newborns: a case-control study. Developmental medicine and child neurology. 2008;50(1):19–24. [DOI] [PubMed] [Google Scholar]
- 55.Iams JD, Romero R, Culhane JF, Goldenberg RL. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet (London, England). 2008;371(9607):164–75. [DOI] [PubMed] [Google Scholar]
- 56.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet (London, England). 2008;371(9608):261–9. [DOI] [PubMed] [Google Scholar]
- 57.Soraisham AS, Singhal N, McMillan DD, Sauve RS, Lee SK, Canadian Neonatal N. A multicenter study on the clinical outcome of chorioamnionitis in preterm infants. Am J Obstet Gynecol. 2009;200(4):372 e1–6. [DOI] [PubMed] [Google Scholar]
- 58.Han YW, Fardini Y, Chen C, Iacampo KG, Peraino VA, Shamonki JM, et al. Term stillbirth caused by oral Fusobacterium nucleatum. Obstet Gynecol. 2010;115(2 Pt 2):442–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greenwell EA, Wyshak G, Ringer SA, Johnson LC, Rivkin MJ, Lieberman E. Intrapartum temperature elevation, epidural use, and adverse outcome in term infants. Pediatrics. 2012;129(2):e447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsiartas P, Kacerovsky M, Musilova I, Hornychova H, Cobo T, Savman K, et al. The association between histological chorioamnionitis, funisitis and neonatal outcome in women with preterm prelabor rupture of membranes. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2013;26(13):1332–6. [DOI] [PubMed] [Google Scholar]
- 61.Rojas-Suarez J, Paternina-Caicedo AJ, Miranda J, Mendoza R, Duenas-Castel C, Bourjeily G. Comparison of severity-of-illness scores in critically ill obstetric patients: a 6-year retrospective cohort. Crit Care Med. 2014;42(5):1047–54. [DOI] [PubMed] [Google Scholar]
- 62.Kacerovsky M, Musilova I, Andrys C, Hornychova H, Pliskova L, Kostal M, et al. Prelabor rupture of membranes between 34 and 37 weeks: the intraamniotic inflammatory response and neonatal outcomes. Am J Obstet Gynecol. 2014;210(4):325 e1–e10. [DOI] [PubMed] [Google Scholar]
- 63.Malloy MH. Chorioamnionitis: epidemiology of newborn management and outcome United States 2008. Journal of perinatology : official journal of the California Perinatal Association. 2014;34(8):611–5. [DOI] [PubMed] [Google Scholar]
- 64.Viscardi RM, Kallapur SG. Role of Ureaplasma Respiratory Tract Colonization in Bronchopulmonary Dysplasia Pathogenesis: Current Concepts and Update. Clin Perinatol. 2015;42(4):719–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee J, Romero R, Lee KA, Kim EN, Korzeniewski SJ, Chaemsaithong P, et al. Meconium aspiration syndrome: a role for fetal systemic inflammation. Am J Obstet Gynecol. 2016;214(3):366 e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Musilova I, Kacerovsky M, Stepan M, Bestvina T, Pliskova L, Zednikova B, et al. Maternal serum C-reactive protein concentration and intra-amniotic inflammation in women with preterm prelabor rupture of membranes. PLoS One. 2017;12(8):e0182731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Towers CV, Yates A, Zite N, Smith C, Chernicky L, Howard B. Incidence of fever in labor and risk of neonatal sepsis. Am J Obstet Gynecol. 2017;216(6):596 e1–e5. [DOI] [PubMed] [Google Scholar]
- 68.Brabbing-Goldstein D, Nir D, Cohen D, Many A, Maslovitz S. Preterm meconium-stained amniotic fluid is an ominous sign for the development of chorioamnionitis and for in utero cord compression. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2017;30(17):2042–45. [DOI] [PubMed] [Google Scholar]
- 69.Musilova I, Andrys C, Drahosova M, Zednikova B, Hornychova H, Pliskova L, et al. Late preterm prelabor rupture of fetal membranes: fetal inflammatory response and neonatal outcome. Pediatric research. 2018;83(3):630–7. [DOI] [PubMed] [Google Scholar]
- 70.Randis TM, Rice MM, Myatt L, Tita ATN, Leveno KJ, Reddy UM, et al. Incidence of early-onset sepsis in infants born to women with clinical chorioamnionitis. Journal of perinatal medicine. 2018;46:926–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet (London, England). 2008;371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kiver V, Boos V, Thomas A, Henrich W, Weichert A. Perinatal outcomes after previable preterm premature rupture of membranes before 24 weeks of gestation. Journal of perinatal medicine. 2018;46(5):555–65. [DOI] [PubMed] [Google Scholar]
- 73.Romero R, Quintero R, Oyarzun E, Wu YK, Sabo V, Mazor M, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol. 1988;159(3):661–6. [DOI] [PubMed] [Google Scholar]
- 74.Zervomanolakis I, Ott HW, Hadziomerovic D, Mattle V, Seeber BE, Virgolini I, et al. Physiology of upward transport in the human female genital tract. Annals of the New York Academy of Sciences. 2007;1101:1–20. [DOI] [PubMed] [Google Scholar]
- 75.Park HS, Romero R, Lee SM, Park CW, Jun JK, Yoon BH. Histologic chorioamnionitis is more common after spontaneous labor than after induced labor at term. Placenta. 2010;31(9):792–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee SM, Lee KA, Kim SM, Park CW, Yoon BH. The risk of intra-amniotic infection, inflammation and histologic chorioamnionitis in term pregnant women with intact membranes and labor. Placenta. 2011;32(7):516–21. [DOI] [PubMed] [Google Scholar]
- 77.Musilova I, Andrys C, Hornychova H, Pliskova L, Drahosova M, Zednikova B, et al. Gastric fluid used to assess changes during the latency period in preterm prelabor rupture of membranes. Pediatric research. 2018;84(2):240–7. [DOI] [PubMed] [Google Scholar]
- 78.DiGiulio DB, Romero R, Kusanovic JP, Gomez R, Kim CJ, Seok KS, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. American journal of reproductive immunology (New York, NY : 1989). 2010;64(1):38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kacerovsky M, Pliskova L, Bolehovska R, Musilova I, Hornychova H, Tambor V, et al. The microbial load with genital mycoplasmas correlates with the degree of histologic chorioamnionitis in preterm PROM. Am J Obstet Gynecol. 2011;205(3):213 e1–7. [DOI] [PubMed] [Google Scholar]
- 80.Park H, Park KH, Kim YM, Kook SY, Jeon SJ, Yoo HN. Plasma inflammatory and immune proteins as predictors of intra-amniotic infection and spontaneous preterm delivery in women with preterm labor: a retrospective study. BMC Pregnancy Childbirth. 2018;18(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Leng Y, et al. A Role for the Inflammasome in Spontaneous Preterm Labor With Acute Histologic Chorioamnionitis. Reproductive sciences (Thousand Oaks, Calif). 2017;24(10):1382–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gomez-Lopez N, Romero R, Panaitescu B, Leng Y, Xu Y, Tarca AL, et al. Inflammasome activation during spontaneous preterm labor with intra-amniotic infection or sterile intra-amniotic inflammation. American journal of reproductive immunology (New York, NY : 1989). 2018;80(5):e13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Strauss JF 3rd, Romero R, Gomez-Lopez N, Haymond-Thornburg H, Modi BP, Teves ME, et al. Spontaneous preterm birth: advances toward the discovery of genetic predisposition. Am J Obstet Gynecol. 2018;218(3):294–314 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Faro J, Romero R, Schwenkel G, Garcia-Flores V, Arenas-Hernandez M, Leng Y, et al. Intra-amniotic inflammation induces preterm birth by activating the NLRP3 inflammasomedagger. Biol Reprod. 2019;100(5):1290–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gomez-Lopez N, Motomura K, Miller D, Garcia-Flores V, Galaz J, Romero R. Inflammasomes: Their Role in Normal and Complicated Pregnancies. J Immunol. 2019;203(11):2757–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sutterwala FS, Ogura Y, Flavell RA. The inflammasome in pathogen recognition and inflammation. Journal of leukocyte biology. 2007;82(2):259–64. [DOI] [PubMed] [Google Scholar]
- 87.Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nature reviews Immunology. 2007;7(1):31–40. [DOI] [PubMed] [Google Scholar]
- 88.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nature immunology. 2009;10(3):241–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jha S, Ting JP. Inflammasome-associated nucleotide-binding domain, leucine-rich repeat proteins and inflammatory diseases. J Immunol. 2009;183(12):7623–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Latz E The inflammasomes: mechanisms of activation and function. Current opinion in immunology. 2010;22(1):28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–32. [DOI] [PubMed] [Google Scholar]
- 92.Franchi L, Munoz-Planillo R, Reimer T, Eigenbrod T, Nunez G. Inflammasomes as microbial sensors. European journal of immunology. 2010;40(3):611–5. [DOI] [PubMed] [Google Scholar]
- 93.Lamkanfi M, Dixit VM. Modulation of inflammasome pathways by bacterial and viral pathogens. J Immunol. 2011;187(2):597–602. [DOI] [PubMed] [Google Scholar]
- 94.Horvath GL, Schrum JE, De Nardo CM, Latz E. Intracellular sensing of microbes and danger signals by the inflammasomes. Immunological reviews. 2011;243(1):119–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nature immunology. 2012;13(4):325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rathinam VA, Vanaja SK, Fitzgerald KA. Regulation of inflammasome signaling. Nature immunology. 2012;13(4):333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Franchi L, Nunez G. Immunology. Orchestrating inflammasomes. Science. 2012;337(6100):1299–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nature reviews Immunology. 2013;13(6):397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vanaja SK, Rathinam VA, Fitzgerald KA. Mechanisms of inflammasome activation: recent advances and novel insights. Trends in cell biology. 2015;25(5):308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nature medicine. 2015;21(7):677–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nature reviews Immunology. 2019;19(8):477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Black RA, Kronheim SR, Merriam JE, March CJ, Hopp TP. A pre-aspartate-specific protease from human leukocytes that cleaves pro-interleukin-1 beta. The Journal of biological chemistry. 1989;264(10):5323–6. [PubMed] [Google Scholar]
- 103.Kostura MJ, Tocci MJ, Limjuco G, Chin J, Cameron P, Hillman AG, et al. Identification of a monocyte specific pre-interleukin 1 beta convertase activity. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(14):5227–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thornberry NA, Bull HG, Calaycay JR, Chapman KT, Howard AD, Kostura MJ, et al. A novel heterodimeric cysteine protease is required for interleukin-1 beta processing in monocytes. Nature. 1992;356(6372):768–74. [DOI] [PubMed] [Google Scholar]
- 105.Cerretti DP, Kozlosky CJ, Mosley B, Nelson N, Van Ness K, Greenstreet TA, et al. Molecular cloning of the interleukin-1 beta converting enzyme. Science. 1992;256(5053):97–100. [DOI] [PubMed] [Google Scholar]
- 106.Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, et al. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997;275(5297):206–9. [DOI] [PubMed] [Google Scholar]
- 107.Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, et al. Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production. Nature. 1997;386(6625):619–23. [DOI] [PubMed] [Google Scholar]
- 108.Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Annals of the New York Academy of Sciences. 1998;856:1–11. [DOI] [PubMed] [Google Scholar]
- 109.Fantuzzi G, Dinarello CA. Interleukin-18 and interleukin-1 beta: two cytokine substrates for ICE (caspase-1). Journal of clinical immunology. 1999;19(1):1–11. [DOI] [PubMed] [Google Scholar]
- 110.Sansonetti PJ, Phalipon A, Arondel J, Thirumalai K, Banerjee S, Akira S, et al. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity. 2000;12(5):581–90. [DOI] [PubMed] [Google Scholar]
- 111.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappaB-driven protein synthesis. J Immunol. 2005;175(11):7611–22. [DOI] [PubMed] [Google Scholar]
- 112.Balci-Peynircioglu B, Waite AL, Schaner P, Taskiran ZE, Richards N, Orhan D, et al. Expression of ASC in renal tissues of familial mediterranean fever patients with amyloidosis: postulating a role for ASC in AA type amyloid deposition. Experimental biology and medicine (Maywood, NJ). 2008;233(11):1324–33. [DOI] [PubMed] [Google Scholar]
- 113.Franklin BS, Bossaller L, De Nardo D, Ratter JM, Stutz A, Engels G, et al. The adaptor ASC has extracellular and ‘prionoid’ activities that propagate inflammation. Nature immunology. 2014;15(8):727–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Franklin BS, Latz E, Schmidt FI. The intra- and extracellular functions of ASC specks. Immunological reviews. 2018;281(1):74–87. [DOI] [PubMed] [Google Scholar]
- 115.Panaitescu B, Romero R, Gomez-Lopez N, Xu Y, Leng Y, Maymon E, et al. In vivo evidence of inflammasome activation during spontaneous labor at term. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2019;32(12):1978–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gomez-Lopez N, Romero R, Maymon E, Kusanovic JP, Panaitescu B, Miller D, et al. Clinical chorioamnionitis at term IX: in vivo evidence of intra-amniotic inflammasome activation. Journal of perinatal medicine. 2019;47(3):276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tricomi V, Hall JE, Bittar A, Chambers D. Arborization test for the detection of ruptured fetal membranes. Clinical evaluation. Obstet Gynecol 1966;27(2):275–9. [PubMed] [Google Scholar]
- 118.Friedman ML, McElin TW. Diagnosis of ruptured fetal membranes. Clinical study and review of the literature. Am J Obstet Gynecol. 1969;104(4):544–50. [DOI] [PubMed] [Google Scholar]
- 119.Bennett SL, Cullen JB, Sherer DM, Woods JR Jr. The ferning and nitrazine tests of amniotic fluid between 12 and 41 weeks gestation. Am J Perinatol. 1993;10(2):101–4. [DOI] [PubMed] [Google Scholar]
- 120.Tchirikov M, Schlabritz-Loutsevitch N, Maher J, Buchmann J, Naberezhnev Y, Winarno AS, et al. Mid-trimester preterm premature rupture of membranes (PPROM): etiology, diagnosis, classification, international recommendations of treatment options and outcome. Journal of perinatal medicine. 2018;46(5):465–88. [DOI] [PubMed] [Google Scholar]
- 121.Oh KJ, Kim SM, Hong JS, Maymon E, Erez O, Panaitescu B, et al. Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation. Am J Obstet Gynecol. 2017;216(6):604 e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, et al. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165(4 Pt 1):821–30. [DOI] [PubMed] [Google Scholar]
- 123.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, et al. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159(1):114–9. [DOI] [PubMed] [Google Scholar]
- 124.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, et al. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163(3):968–74. [DOI] [PubMed] [Google Scholar]
- 125.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol. 2015;213(4 Suppl):S29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Theis KR, Romero R, Winters AD, Greenberg JM, Gomez-Lopez N, Alhousseini A, et al. Does the human placenta delivered at term have a microbiota? Results of cultivation, quantitative real-time PCR, 16S rRNA gene sequencing, and metagenomics. Am J Obstet Gynecol. 2019;220(3):267 e1–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kuperman AA, Zimmerman A, Hamadia S, Ziv O, Gurevich V, Fichtman B, et al. Deep microbial analysis of multiple placentas shows no evidence for a placental microbiome. BJOG. 2019. [DOI] [PubMed] [Google Scholar]
- 128.Dickson RP, Erb-Downward JR, Freeman CM, Walker N, Scales BS, Beck JM, et al. Changes in the lung microbiome following lung transplantation include the emergence of two distinct Pseudomonas species with distinct clinical associations. PLoS One. 2014;9(5):e97214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Winters AD, Romero R, Gervasi MT, Gomez-Lopez N, Tran MR, Garcia-Flores V, et al. Does the endometrial cavity have a molecular microbial signature? Scientific reports. 2019;9(1):9905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Frank JA, Reich CI, Sharma S, Weisbaum JS, Wilson BA, Olsen GJ. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl Environ Microbiol. 2008;74(8):2461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Svec D, Tichopad A, Novosadova V, Pfaffl MW, Kubista M. How good is a PCR efficiency estimate: Recommendations for precise and robust qPCR efficiency assessments. Biomol Detect Quantif. 2015;3:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Asrat T, Nageotte MP, Garite TJ, Gocke SE, Dorchester W. Gram stain results from amniocentesis in patients with preterm premature rupture of membranes--comparison of maternal and fetal characteristics. Am J Obstet Gynecol. 1990;163(3):887–9. [DOI] [PubMed] [Google Scholar]
- 133.Dudley J, Malcolm G, Ellwood D. Amniocentesis in the management of preterm premature rupture of the membranes. Aust N Z J Obstet Gynaecol. 1991;31(4):331–6. [DOI] [PubMed] [Google Scholar]
- 134.Romero R, Ghidini A, Mazor M, Behnke E. Microbial invasion of the amniotic cavity in premature rupture of membranes. Clin Obstet Gynecol. 1991;34(4):769–78. [DOI] [PubMed] [Google Scholar]
- 135.Romero R, Mazor M, Morrotti R, Avila C, Oyarzun E, Insunza A, et al. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol 1992;166(1 Pt 1):129–33. [DOI] [PubMed] [Google Scholar]
- 136.Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169(4):839–51. [DOI] [PubMed] [Google Scholar]
- 137.Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 1993;169(4):805–16. [DOI] [PubMed] [Google Scholar]
- 138.Romero R, Nores J, Mazor M, Sepulveda W, Oyarzun E, Parra M, et al. Microbial invasion of the amniotic cavity during term labor. Prevalence and clinical significance. J Reprod Med. 1993;38(7):543–8. [PubMed] [Google Scholar]
- 139.Blanchard A, Hentschel J, Duffy L, Baldus K, Cassell GH. Detection of Ureaplasma urealyticum by polymerase chain reaction in the urogenital tract of adults, in amniotic fluid, and in the respiratory tract of newborns. Clin Infect Dis. 1993;17 Suppl 1:S148–53. [DOI] [PubMed] [Google Scholar]
- 140.Jalava J, Mantymaa ML, Ekblad U, Toivanen P, Skurnik M, Lassila O, et al. Bacterial 16S rDNA polymerase chain reaction in the detection of intra-amniotic infection. Br J Obstet Gynaecol. 1996;103(7):664–9. [DOI] [PubMed] [Google Scholar]
- 141.Hitti J, Riley DE, Krohn MA, Hillier SL, Agnew KJ, Krieger JN, et al. Broad-spectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin Infect Dis. 1997;24(6):1228–32. [DOI] [PubMed] [Google Scholar]
- 142.Oyarzun E, Yamamoto M, Kato S, Gomez R, Lizama L, Moenne A. Specific detection of 16 micro-organisms in amniotic fluid by polymerase chain reaction and its correlation with preterm delivery occurrence. Am J Obstet Gynecol. 1998;179(5):1115–9. [DOI] [PubMed] [Google Scholar]
- 143.Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS, et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000;183(5):1130–7. [DOI] [PubMed] [Google Scholar]
- 144.Bearfield C, Davenport ES, Sivapathasundaram V, Allaker RP. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. BJOG. 2002;109(5):527–33. [DOI] [PubMed] [Google Scholar]
- 145.Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokstrom H, Holst RM, Wennerholm UB, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta Obstet Gynecol Scand. 2003;82(2):120–8. [DOI] [PubMed] [Google Scholar]
- 146.Jacobsson B, Mattsby-Baltzer I, Andersch B, Bokstrom H, Holst RM, Nikolaitchouk N, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women with preterm prelabor rupture of membranes. Acta Obstet Gynecol Scand. 2003;82(5):423–31. [DOI] [PubMed] [Google Scholar]
- 147.Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187(3):518–21. [DOI] [PubMed] [Google Scholar]
- 148.Yoon BH, Romero R, Lim JH, Shim SS, Hong JS, Shim JY, et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol. 2003;189(4):919–24. [DOI] [PubMed] [Google Scholar]
- 149.Perni SC, Vardhana S, Korneeva I, Tuttle SL, Paraskevas LR, Chasen ST, et al. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004;191(4):1382–6. [DOI] [PubMed] [Google Scholar]
- 150.Yi J, Yoon BH, Kim EC. Detection and biovar discrimination of Ureaplasma urealyticum by real-time PCR. Mol Cell Probes. 2005;19(4):255–60. [DOI] [PubMed] [Google Scholar]
- 151.Leon R, Silva N, Ovalle A, Chaparro A, Ahumada A, Gajardo M, et al. Detection of Porphyromonas gingivalis in the amniotic fluid in pregnant women with a diagnosis of threatened premature labor. J Periodontol. 2007;78(7):1249–55. [DOI] [PubMed] [Google Scholar]
- 152.DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3(8):e3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Han YW, Shen T, Chung P, Buhimschi IA, Buhimschi CS. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J Clin Microbiol. 2009;47(1):38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rodriguez N, Fernandez C, Zamora Y, Berdasquera D, Rivera JA. Detection of Ureaplasma urealyticum and Ureaplasma parvum in amniotic fluid: association with pregnancy outcomes. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2011;24(1):47–50. [DOI] [PubMed] [Google Scholar]
- 155.Chaemsaithong P, Romero R, Korzeniewski SJ, Martinez-Varea A, Dong Z, Yoon BH, et al. A point of care test for interleukin-6 in amniotic fluid in preterm prelabor rupture of membranes: a step toward the early treatment of acute intra-amniotic inflammation/infection. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2016;29(3):360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Romero R, Chaemsaithong P, Chaiyasit N, Docheva N, Dong Z, Kim CJ, et al. CXCL10 and IL-6: Markers of two different forms of intra-amniotic inflammation in preterm labor. American journal of reproductive immunology (New York, NY : 1989). 2017;78(1):e12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Oh KJ, Romero R, Park JY, Lee J, Conde-Agudelo A, Hong JS, et al. Evidence that antibiotic administration is effective in the treatment of a subset of patients with intra-amniotic infection/inflammation presenting with cervical insufficiency. Am J Obstet Gynecol. 2019;221(2):140 e1–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yoon BH, Romero R, Park JY, Oh KJ, Lee J, Conde-Agudelo A, et al. Antibiotic administration can eradicate intra-amniotic infection or intra-amniotic inflammation in a subset of patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2019;221(2):142 e1–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Garite TJ, Freeman RK, Linzey EM, Braly P. The use of amniocentesis in patients with premature rupture of membranes. Obstet Gynecol. 1979;54(2):226–30. [PubMed] [Google Scholar]
- 160.Minkoff H Prematurity: infection as an etiologic factor. Obstet Gynecol. 1983;62(2):137–44. [PubMed] [Google Scholar]
- 161.Cotton DB, Hill LM, Strassner HT, Platt LD, Ledger WJ. Use of amniocentesis in preterm gestation with ruptured membranes. Obstet Gynecol. 1984;63(1):38–43. [PubMed] [Google Scholar]
- 162.Broekhuizen FF, Gilman M, Hamilton PR. Amniocentesis for gram stain and culture in preterm premature rupture of the membranes. Obstet Gynecol. 1985;66(3):316–21. [PubMed] [Google Scholar]
- 163.Leigh J, Garite TJ. Amniocentesis and the management of premature labor. Obstet Gynecol. 1986;67(4):500–6. [PubMed] [Google Scholar]
- 164.Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. American journal of reproductive immunology (New York, NY : 1989). 1994;32(3):200–10. [DOI] [PubMed] [Google Scholar]
- 165.Blackwell SC, Berry SM. Role of amniocentesis for the diagnosis of subclinical intra-amniotic infection in preterm premature rupture of the membranes. Current opinion in obstetrics & gynecology. 1999;11(6):541–7. [DOI] [PubMed] [Google Scholar]
- 166.Onderdonk AB, Hecht JL, McElrath TF, Delaney ML, Allred EN, Leviton A, et al. Colonization of second-trimester placenta parenchyma. Am J Obstet Gynecol. 2008;199(1):52 e1–e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.DiGiulio DB. Diversity of microbes in amniotic fluid. Semin Fetal Neonatal Med. 2012;17(1):2–11. [DOI] [PubMed] [Google Scholar]
- 168.Wing DA, Park AS, Debuque L, Millar LK. Limited clinical utility of blood and urine cultures in the treatment of acute pyelonephritis during pregnancy. Am J Obstet Gynecol. 2000;182(6):1437–40. [DOI] [PubMed] [Google Scholar]
- 169.Gilstrap LC 3rd, Ramin SM. Urinary tract infections during pregnancy. Obstet Gynecol Clin North Am. 2001;28(3):581–91. [DOI] [PubMed] [Google Scholar]
- 170.Wing DA. Pyelonephritis in pregnancy: treatment options for optimal outcomes. Drugs. 2001;61(14):2087–96. [DOI] [PubMed] [Google Scholar]
- 171.Jolley JA, Wing DA. Pyelonephritis in pregnancy: an update on treatment options for optimal outcomes. Drugs. 2010;70(13):1643–55. [DOI] [PubMed] [Google Scholar]
- 172.Relman DA, Falkow S. Identification of uncultured microorganisms: expanding the spectrum of characterized microbial pathogens. Infect Agents Dis. 1992;1(5):245–53. [PubMed] [Google Scholar]
- 173.Relman DA. Emerging infections and newly-recognised pathogens. Neth J Med. 1997;50(5):216–20. [DOI] [PubMed] [Google Scholar]
- 174.Oh KJ, Lee KA, Sohn YK, Park CW, Hong JS, Romero R, et al. Intraamniotic infection with genital mycoplasmas exhibits a more intense inflammatory response than intraamniotic infection with other microorganisms in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 2010;203(3):211 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Harder TC, Hufnagel M, Zahn K, Beutel K, Schmitt HJ, Ullmann U, et al. New LightCycler PCR for rapid and sensitive quantification of parvovirus B19 DNA guides therapeutic decision-making in relapsing infections. J Clin Microbiol. 2001;39(12):4413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cheng VC, Yam WC, Hung IF, Woo PC, Lau SK, Tang BS, et al. Clinical evaluation of the polymerase chain reaction for the rapid diagnosis of tuberculosis. J Clin Pathol. 2004;57(3):281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yang S, Rothman RE. PCR-based diagnostics for infectious diseases: uses, limitations, and future applications in acute-care settings. Lancet Infect Dis. 2004;4(6):337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gardella C, Huang ML, Wald A, Magaret A, Selke S, Morrow R, et al. Rapid polymerase chain reaction assay to detect herpes simplex virus in the genital tract of women in labor. Obstet Gynecol. 2010;115(6):1209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Alfa MJ, Sepehri S, De Gagne P, Helawa M, Sandhu G, Harding GK. Real-time PCR assay provides reliable assessment of intrapartum carriage of group B Streptococcus. J Clin Microbiol. 2010;48(9):3095–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Church DL, Baxter H, Lloyd T, Larios O, Gregson DB. Evaluation of StrepBSelect Chromogenic Medium and the Fast-Track Diagnostics Group B Streptococcus (GBS) Real-Time PCR Assay Compared to Routine Culture for Detection of GBS during Antepartum Screening. J Clin Microbiol. 2017;55(7):2137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rosa-Fraile M, Spellerberg B. Reliable Detection of Group B Streptococcus in the Clinical Laboratory. J Clin Microbiol. 2017;55(9):2590–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kacerovsky M, Pliskova L, Bolehovska R, Skogstrand K, Hougaard DM, Tsiartas P, et al. The impact of the microbial load of genital mycoplasmas and gestational age on the intensity of intraamniotic inflammation. Am J Obstet Gynecol. 2012;206(4):342.e1–8. [DOI] [PubMed] [Google Scholar]
- 183.Kacerovsky M, Celec P, Vlkova B, Skogstrand K, Hougaard DM, Cobo T, et al. Amniotic fluid protein profiles of intraamniotic inflammatory response to Ureaplasma spp. and other bacteria. PLoS One. 2013;8(3):e60399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Musilova I, Pliskova L, Gerychova R, Janku P, Simetka O, Matlak P, et al. Maternal white blood cell count cannot identify the presence of microbial invasion of the amniotic cavity or intra-amniotic inflammation in women with preterm prelabor rupture of membranes. PLoS One. 2017;12(12):e0189394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gardella C, Riley DE, Hitti J, Agnew K, Krieger JN, Eschenbach D. Identification and sequencing of bacterial rDNAs in culture-negative amniotic fluid from women in premature labor. Am J Perinatol. 2004;21(6):319–23. [DOI] [PubMed] [Google Scholar]
- 186.Xu HS, Roberts N, Singleton FL, Attwell RW, Grimes DJ, Colwell RR. Survival and viability of nonculturableEscherichia coli andVibrio cholerae in the estuarine and marine environment. Microb Ecol. 1982;8(4):313–23. [DOI] [PubMed] [Google Scholar]
- 187.Rahman I, Shahamat M, Kirchman PA, Russek-Cohen E, Colwell RR. Methionine uptake and cytopathogenicity of viable but nonculturable Shigella dysenteriae type 1. Appl Environ Microbiol. 1994;60(10):3573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Oliver JD, Bockian R. In vivo resuscitation, and virulence towards mice, of viable but nonculturable cells of Vibrio vulnificus. Appl Environ Microbiol. 1995;61(7):2620–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Weichart D, Kjelleberg S. Stress resistance and recovery potential of culturable and viable but nonculturable cells of Vibrio vulnificus. Microbiology. 1996;142:845–53. [DOI] [PubMed] [Google Scholar]
- 190.Signoretto C, Lleo MM, Tafi MC, Canepari P. Cell wall chemical composition of Enterococcus faecalis in the viable but nonculturable state. Appl Environ Microbiol. 2000;66(5):1953–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Adams BL, Bates TC, Oliver JD. Survival of Helicobacter pylori in a natural freshwater environment. Appl Environ Microbiol. 2003;69(12):7462–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pruzzo C, Tarsi R, Lleo MM, Signoretto C, Zampini M, Pane L, et al. Persistence of adhesive properties in Vibrio cholerae after long-term exposure to sea water. Environ Microbiol. 2003;5(10):850–8. [DOI] [PubMed] [Google Scholar]
- 193.Day AP, Oliver JD. Changes in membrane fatty acid composition during entry of Vibrio vulnificus into the viable but nonculturable state. J Microbiol. 2004;42(2):69–73. [PubMed] [Google Scholar]
- 194.Shleeva M, Mukamolova GV, Young M, Williams HD, Kaprelyants AS. Formation of ‘non-culturable’ cells of Mycobacterium smegmatis in stationary phase in response to growth under suboptimal conditions and their Rpf-mediated resuscitation. Microbiology. 2004;150(Pt 6):1687–97. [DOI] [PubMed] [Google Scholar]
- 195.Wong HC, Wang P. Induction of viable but nonculturable state in Vibrio parahaemolyticus and its susceptibility to environmental stresses. J Appl Microbiol. 2004;96(2):359–66. [DOI] [PubMed] [Google Scholar]
- 196.Cappelier JM, Besnard V, Roche S, Garrec N, Zundel E, Velge P, et al. Avirulence of viable but non-culturable Listeria monocytogenes cells demonstrated by in vitro and in vivo models. Vet Res. 2005;36(4):589–99. [DOI] [PubMed] [Google Scholar]
- 197.Gonzalez-Escalona N, Fey A, Hofle MG, Espejo RT, C AG. Quantitative reverse transcription polymerase chain reaction analysis of Vibrio cholerae cells entering the viable but non-culturable state and starvation in response to cold shock. Environ Microbiol. 2006;8(4):658–66. [DOI] [PubMed] [Google Scholar]
- 198.Du M, Chen J, Zhang X, Li A, Li Y, Wang Y. Retention of virulence in a viable but nonculturable Edwardsiella tarda isolate. Appl Environ Microbiol. 2007;73(4):1349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cook KL, Bolster CH. Survival of Campylobacter jejuni and Escherichia coli in groundwater during prolonged starvation at low temperatures. J Appl Microbiol. 2007;103(3):573–83. [DOI] [PubMed] [Google Scholar]
- 200.Asakura H, Ishiwa A, Arakawa E, Makino S, Okada Y, Yamamoto S, et al. Gene expression profile of Vibrio cholerae in the cold stress-induced viable but non-culturable state. Environ Microbiol. 2007;9(4):869–79. [DOI] [PubMed] [Google Scholar]
- 201.Lleo MM, Benedetti D, Tafi MC, Signoretto C, Canepari P. Inhibition of the resuscitation from the viable but non-culturable state in Enterococcus faecalis. Environ Microbiol. 2007;9(9):2313–20. [DOI] [PubMed] [Google Scholar]
- 202.Lleo M, Bonato B, Tafi MC, Caburlotto G, Benedetti D, Canepari P. Adhesion to medical device materials and biofilm formation capability of some species of enterococci in different physiological states. FEMS Microbiol Lett. 2007;274(2):232–7. [DOI] [PubMed] [Google Scholar]
- 203.Muela A, Seco C, Camafeita E, Arana I, Orruno M, Lopez JA, et al. Changes in Escherichia coli outer membrane subproteome under environmental conditions inducing the viable but nonculturable state. FEMS Microbiol Ecol. 2008;64(1):28–36. [DOI] [PubMed] [Google Scholar]
- 204.Asakura H, Kawamoto K, Haishima Y, Igimi S, Yamamoto S, Makino S. Differential expression of the outer membrane protein W (OmpW) stress response in enterohemorrhagic Escherichia coli O157:H7 corresponds to the viable but non-culturable state. Res Microbiol. 2008;159(9–10):709–17. [DOI] [PubMed] [Google Scholar]
- 205.Duffy LL, Dykes GA. The ability of Campylobacter jejuni cells to attach to stainless steel does not change as they become nonculturable. Foodborne Pathog Dis. 2009;6(5):631–4. [DOI] [PubMed] [Google Scholar]
- 206.Anuchin AM, Mulyukin AL, Suzina NE, Duda VI, El-Registan GI, Kaprelyants AS. Dormant forms of Mycobacterium smegmatis with distinct morphology. Microbiology. 2009;155(Pt 4):1071–9. [DOI] [PubMed] [Google Scholar]
- 207.Lindback T, Rottenberg ME, Roche SM, Rorvik LM. The ability to enter into an avirulent viable but non-culturable (VBNC) form is widespread among Listeria monocytogenes isolates from salmon, patients and environment. Vet Res. 2010;41(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Nowakowska J, Oliver JD. Resistance to environmental stresses by Vibrio vulnificus in the viable but nonculturable state. FEMS Microbiol Ecol. 2013;84(1):213–22. [DOI] [PubMed] [Google Scholar]
- 209.Li L, Mendis N, Trigui H, Oliver JD, Faucher SP. The importance of the viable but non-culturable state in human bacterial pathogens. Front Microbiol. 2014;5:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Elliott CL, Allport VC, Loudon JA, Wu GD, Bennett PR. Nuclear factor-kappa B is essential for up-regulation of interleukin-8 expression in human amnion and cervical epithelial cells. Molecular human reproduction. 2001;7(8):787–90. [DOI] [PubMed] [Google Scholar]
- 211.Lindstrom TM, Bennett PR. The role of nuclear factor kappa B in human labour. Reproduction (Cambridge, England). 2005;130(5):569–81. [DOI] [PubMed] [Google Scholar]
- 212.Vora S, Abbas A, Kim CJ, Summerfield TL, Kusanovic JP, Iams JD, et al. Nuclear factor-kappa B localization and function within intrauterine tissues from term and preterm labor and cultured fetal membranes. Reproductive biology and endocrinology : RB&E. 2010;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.MacIntyre DA, Sykes L, Teoh TG, Bennett PR. Prevention of preterm labour via the modulation of inflammatory pathways. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25 Suppl 1:17–20. [DOI] [PubMed] [Google Scholar]
- 214.Lappas M, Permezel M, Georgiou HM, Rice GE. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol Reprod. 2002;67(2):668–73. [DOI] [PubMed] [Google Scholar]
- 215.Martinez-Varea A, Romero R, Xu Y, Miller D, Ahmed AI, Chaemsaithong P, et al. Clinical chorioamnionitis at term VII: the amniotic fluid cellular immune response. Journal of perinatal medicine. 2017;45(5):523–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Gomez-Lopez N, Romero R, Xu Y, Miller D, Leng Y, Panaitescu B, et al. The immunophenotype of amniotic fluid leukocytes in normal and complicated pregnancies. American journal of reproductive immunology (New York, NY : 1989). 2018;79(4):e12827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Gomez-Lopez N, Roberto R, Galaz J, Xu Y, Panaitescu B, Slutsky R, et al. Cellular immune responses in amniotic fluid of women with preterm labor and intra-amniotic infection or intra-amniotic inflammation. American journal of reproductive immunology (New York, NY : 1989). 2019: e13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Gomez-Lopez N, Romero R, Leng Y, Xu Y, Slutsky R, Levenson D, et al. The origin of amniotic fluid monocytes/macrophages in women with intra-amniotic inflammation or infection. Journal of perinatal medicine. 2019;47(8):822–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2008;21(9):605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. American journal of reproductive immunology (New York, NY : 1989). 1992;27(3–4):117–23. [DOI] [PubMed] [Google Scholar]
- 221.Keelan JA, Marvin KW, Sato TA, Coleman M, McCowan LM, Mitchell MD. Cytokine abundance in placental tissues: evidence of inflammatory activation in gestational membranes with term and preterm parturition. Am J Obstet Gynecol. 1999;181(6):1530–6. [DOI] [PubMed] [Google Scholar]
- 222.Marconi C, de Andrade Ramos BR, Peracoli JC, Donders GG, da Silva MG. Amniotic fluid interleukin-1 beta and interleukin-6, but not interleukin-8 correlate with microbial invasion of the amniotic cavity in preterm labor. American journal of reproductive immunology (New York, NY : 1989). 2011;65(6):549–56. [DOI] [PubMed] [Google Scholar]
- 223.Romero R, Grivel JC, Tarca AL, Chaemsaithong P, Xu Z, Fitzgerald W, et al. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol. 2015;213(6):836 e1–e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Gomez-Lopez N, Roberto R, Galaz J, Xu Y, Panaitescu B, Slutsky R, et al. Cellular immune responses in amniotic fluid of women with preterm labor and intra-amniotic infection or intra-amniotic inflammation. Am J Reprod Immunol. 2019:e13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Gomez-Lopez N, Romero R, Tarca AL, Miller D, Panaitescu B, Schwenkel G, et al. Gasdermin D: Evidence of Pyroptosis in Spontaneous Preterm Labor with Sterile Intra-amniotic Inflammation or Intra-amniotic Infection. American journal of reproductive immunology (New York, NY : 1989). 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Current opinion in immunology. 2005;17(4):359–65. [DOI] [PubMed] [Google Scholar]
- 227.Rubartelli A, Lotze MT. Inside, outside, upside down: damage-associated molecular-pattern molecules (DAMPs) and redox. Trends Immunol. 2007;28(10):429–36. [DOI] [PubMed] [Google Scholar]
- 228.Lotze MT, Zeh HJ, Rubartelli A, Sparvero LJ, Amoscato AA, Washburn NR, et al. The grateful dead: damage-associated molecular pattern molecules and reduction/oxidation regulate immunity. Immunological reviews. 2007;220:60–81. [DOI] [PubMed] [Google Scholar]
- 229.Gomez-Lopez N, Romero R, Garcia-Flores V, Leng Y, Miller D, Hassan SS, et al. Inhibition of the NLRP3 inflammasome can prevent sterile intra-amniotic inflammation, preterm labor/birth, and adverse neonatal outcomesdagger. Biol Reprod. 2019;100(5):1306–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cobo T, Kacerovsky M, Jacobsson B. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;211(6):708. [DOI] [PubMed] [Google Scholar]
- 231.Combs CA, Gravett M, Garite TJ, Hickok DE, Lapidus J, Porreco R, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol. 2014;210(2):125 e1–15. [DOI] [PubMed] [Google Scholar]
- 232.Musilova I, Bestvina T, Hudeckova M, Michalec I, Cobo T, Jacobsson B, et al. Vaginal fluid interleukin-6 concentrations as a point-of-care test is of value in women with preterm prelabor rupture of membranes. Am J Obstet Gynecol. 2016;215(5):619.e1–.e12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


