Abstract
Sickle cell anaemia (SCA) is a hereditary hemoglobinopathy characterised by extensive vascular dysfunction that stems from inflammation, thrombosis and occlusion of post-capillary venules. Cognitive impairment is a neurological complication of SCA whose pathogenesis is unknown. We hypothesised that cerebral venular abnormalities are linked to cognitive impairment in SCA. Thus, we employed 7T magnetic resonance imaging (MRI) to examine the association between venular density and cognitive function in homozygous SCA. We quantified the density of total, long, and short venules in pre-defined regions of interest between the frontal and occipital cornu on each hemisphere. Cognitive function was assessed using the Hopkins Verbal Learning Test – Revised (HVLT-R) test of learning and memory. Patients (n=11) were compared with race, age and gender-equated controls (n=7). Compared to controls, patients had an overall venular rarefaction, with significantly lower density of long venules and greater density of short venules which was inversely related to HVLT-R performance and haemoglobin. To our knowledge, this is the first 7T MRI study in SCA and first report of associations between cerebral venular patterns and cognitive performance and haemoglobin. Future studies should examine whether these novel neuroimaging markers predict cognitive impairment longitudinally and are mechanistically linked to severity of anaemia.
Keywords: Cognition, Magnetic resonance spectroscopy, Cerebral small vessel diseases
1. Introduction
Sickle cell anaemia (SCA) is a hereditary hemoglobinopathy characterised by endothelial dysfunction, vaso-occlusion and thrombosis of post-capillary venules (Kaul et al., 1989). Neurological complications of SCA include overt and silent cerebrovascular infarction and cognitive impairment (CI) (DeBaun et al., 2012). While CI is associated with cerebrovascular infarction, it also occurs independently from it in SCA (Vichinsky et al., 2010), and is responsible for functional limitations in schooling, occupation and compliance with therapy (Feliu et al., 2011). Our current understanding of the risk factors and pathophysiology of CI in SCA is limited, but based on the pathophysiology of SCA and the evidence in other diseases with CI and dementia (Brown and Thore, 2011; Hunter et al., 2012; Moody et al., 1997; Sinnecker et al., 2013), it is likely that CI stems from small vessel disease. Post-mortem studies have found evidence of small vessel abnormalities in SCA patients, including congested and thrombosed venules (reviewed by Merkel et al., 1978), but did not assess the patients’ cognitive function. Hence, while large vessel stenoses are responsible for overt infarcts in SCA (Switzer et al., 2006), it is not known whether venular abnormalities are associated with CI. Magnetic Resonance Imaging (MRI) is increasingly useful in identifying microvascular abnormalities associated with CI (Rincon and Wright, 2014). Prior MRI studies in adult SCA patients have, however, been limited by the use of lower field 1.5Tesla MRI systems (Alkan et al., 2010; Kugler et al., 1993; Vichinsky et al., 2010), which only provide lower sensitivity markers of small vessel disease, hence hampering detection of microvascular abnormalities. Pre-clinical in vivo and in vitro evidence has shown that the inciting pathogenic event in SCA occurs in post-capillary venules (Kaul et al., 1989; Kaul et al., 1993), including cerebral post-capillary venules of transgenic sickle mice (Wood et al., 2004), and is characterised by increased cellular adhesion, inflammation and endotheliopathy. Thus, we hypothesised that the predominant microvascular abnormality of SCA patients with CI would manifest with markers of venular fragmentation and lower venular density. We applied ultrahigh field, state-of-the-art 7T MRI technology to test this hypothesis by quantifying venular structure in patients with homozygous HbSS SCA. Subjects without SCA underwent the same neuroimaging protocol for comparison. We then explored correlations of venular structure with cognitive function as measured by the Hopkins Verbal Learning Test – Revised (HVLT-R) and with total haemoglobin in the patients.
2. Methods
2.1. Subjects
All participants provided informed consent (University of Pittsburgh IRB PRO12040139). We recruited a convenience sample of consecutive patients from the UPMC Adult Sickle Cell Disease Program outpatient clinic. The only inclusion criteria were HbSS disease (homozygous SCA) diagnosed by high-performance liquid chromatography and haemoglobin electrophoresis, and being in steady-state according to published criteria (Ballas, 2012). Exclusion criteria included the following: blood transfusion within the prior 90 days, pregnancy, any contraindication to MRI and a history of traumatic brain injury or diseases known to cause cognitive dysfunction, including diabetes, systemic lupus erythematosus and other causes of cerebral vasculitis. The blood draw was performed during the patients’ routine clinic visit and the HVLT-R and MRI were completed at a separate visit occurring within 2 weeks from the blood draw and in the absence of any intercurrent transfusion or vaso-occlusive episode. Control African American subjects (n=7) with no family history of SCA were recruited from the community through IRB-approved flyers and SCA patient referral. They were equated to the patients for age and gender, and subjected to the same exclusion criteria.
2.2. MRI and cognitive assessment
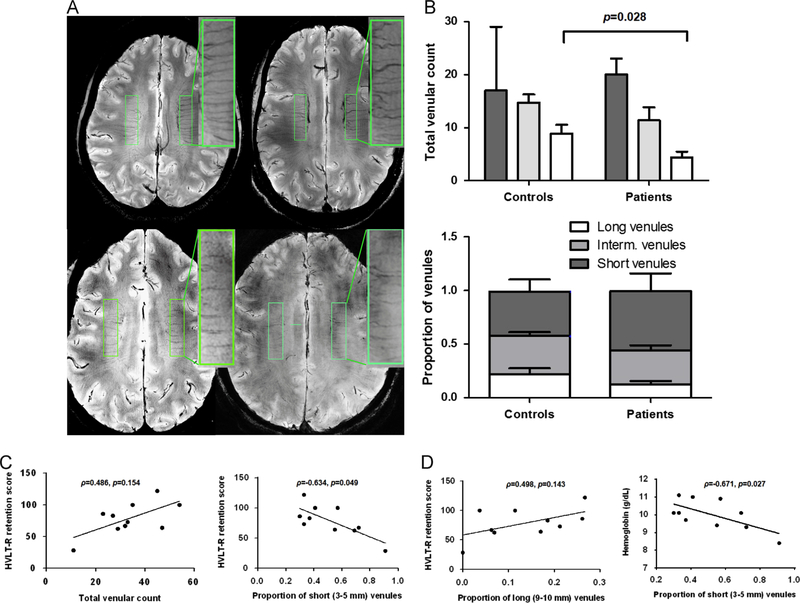
The MRI scans were performed at the University of Pittsburgh using a 7T human MRI system (Magnetom, Siemens Medical Systems, Erlangen, Germany) and utilising an in-house 2-ch transmit and 14-ch receive radiofrequency coil (Ibrahim et al., 2010). Structural image acquisition included a high-resolution Magnetization Prepared Rapid Gradient Echo T1 (MPRAGE-T1WI) image with 3D orientation, 0.6 mm × 0.58 mm × 0.58 mm resolution, TR/TE/TI=3430/3.69/1200 ms, a T2* Susceptibility Weighted Image (SWI) with axial orientation (64 slices), 0.25 mm × 0.25 mm × 1.5 mm resolution, TR/TE/TI=2000/15/NA and a T2* SWI with coronal orientation (50 slices), 0.25 mm × 0.25 mm × 1.5 mm resolution, TR/TE/TI=2000/15/NA. Scans were routinely inspected for stroke and unanticipated findings that warrant a report from the on call neuroradiologist. Small intraparenchymal veins (venules) were quantified from the SWI image within predefined ROIs (width 1 cm, length 4 cm) between the frontal and occipital cornu on each cerebral hemisphere, using a previously published protocol (Sinnecker et al., 2013) with the following modification: short venules were defined as being 3–5 mm in length (dashed arrow), long venules were defined as being 9–10 mm in length (solid arrow) and intermediate venules were defined as being between 6–8 mm in length. The proportion of short or long venules to the total number of venules was also computed for each participant (Fig. 1A).
Fig. 1.
Abnormal venular pattern in SCA is associated with worse cognitive function and anaemia. (A) A rectangular region of interest (ROI, width 1 cm, length 4 cm) was defined between the frontal and occipital cornu on each cerebral hemisphere. Small parenchymal veins (venules) were identified as linear structures of intensity darker than the surrounding voxels, and length ≥3 mm. Venules were counted within the ROIs by consensus reading of two trained and blinded investigators with intraclass correlation coefficients of 0.817 (0.409, 0.952) for the rating of the long venules. Short venules were defined as being 3–5 mm in length, long venules were defined as being 9–10 mm in length and intermediate venules were defined as being between 6 and 8 mm in length. The proportion of short or long venules to the total number of venules was also computed for each participant. The two upper scans are from two representative control subjects and the two lower scans are from patient ID 11 (left) and ID 05 (right). As shown in the inlet with the magnified ROIs, patients had venular loss as compared to control subjects. (B) The density (upper graph) and proportion (lower graph) of long venules was decreased in SCA patients (p=0.028 and p=0.127, respectively). There was a parallel increase in the proportion of short venules in SCA patients (lower graph). The groups were compared by two-sided Mann–Whitney U (SPSS 20.0), and the results are shown as median (interquartile range). (C) The proportion of short venules was inversely correlated with the HLVT-R retention score (ρ=−0.634, p=0.024). Correlations with the total number of venules (ρ=0.486, p=0.154), and (D) the proportion of long venules (ρ=0.498, p=0.143) were similar albeit less strong. (D) The proportion of short venules was inversely correlated with the haemoglobin level (ρ=−0.671, p=0.027). Two-sided Spearman’s correlations were computed on SPSS 20.0.
Patients were administered the HVLT-R based on its brief testing time and sensitivity to the key domains of learning and memory in brain-disordered populations (Lacritz et al., 2001; Shapiro et al., 1999).
3. Results
3.1. Patients
Patient characteristics and laboratory values are shown in Table 1. Eight of eleven patients were receiving hydroxyurea at the time of our study and had relatively high levels of foetal (HbF) and total haemoglobin. In spite of these findings, they had a history of multiple SCA-related complications as outlined in Table 1. Haemoglobin A1 was not detected in any of the patients.
Table 1.
Characteristics of the patients.
| Patient ID | Demographics |
Laboratory/Clinical |
Venular density n./8 cm2 |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Sex | Educationa | Haemoglobin, g/dL | HbS, % | HbF, % | HU | MRI findings^ | Clinical complications | HVLT-Rb, % | Total | Short (3–5 mm) | Long (9–10 mm) | |
| 01 | 31 | F | 4 | 11.0 | 89.7 | 5.2 | Yes | BGpv, BGc | AVNc, retinopathy, ACSd | 100 | 54 | 22 | 2 |
| 02 | 22 | M | 2 | 11.1 | 78.1 | 6.7 | No | HC, BGpv | ACS, aplastic crisis, splenic sequestration | 122 | 45 | 15 | 12 |
| 03 | 40 | M | 2 | 8.4 | 86.8 | 8.0 | Yes | HC, BGpv | PHe, leg ulcers, priapism, retinopathy | 29 | 11 | 10 | 0 |
| 04 | 20 | M | 1 | 10.1 | 86.1 | 11.3 | Yes | HC | AVN | 62 | 29 | 20 | 2 |
| 05 | 39 | F | 4 | 10.9 | 71.4 | 26.8 | Yes | ACS, PH | 100 | 35 | 20 | 4 | |
| 06 | 34 | F | 2 | 6.8 | 70.4 | 27.6 | Yes | SCI | Right occipital silent infarct, ACS, bone infarctions, PH | NAf | 28 | 27 | 0 |
| 07 | 21 | F | 1 | 9.3 | NA | NA | No | HC, BGpv | ACS, AVN, PH | 67 | 32 | 23 | 2 |
| 08 | 35 | F | 4 | 9.4 | 77.1 | 7.5 | Yes | BGc | Retinopathy | 64 | 47 | 26 | 8 |
| 09 | 22 | F | 3 | 10.1 | 59.9 | 20.4 | Yes | HC, BGpv | Retinopathy | 73 | 33 | 11 | 7 |
| 10 | 46 | F | 2 | 9.7 | 58.1 | 24.8 | Yes | HC, BGpv | Leg ulcers, PH | 83 | 27 | 10 | 5 |
| 11 | 24 | F | 3 | 10.1 | 87.8 | 8.9 | No | BGc | ACS | 86 | 23 | 7 | 6 |
| Median rangeg | 31.0 | 2.0 | 10.1 | 77.6 | 10.1 | 78.0 | 32.0 | 20.0 | 4.0 | ||||
| 22–39 | 2–4 | 9–11 | 68–87 | 7–25 | 63–100 | 27–45 | 10–23 | 2–7 | |||||
1 = less than high school, 2 = high school, 3 = some college, 4 = college completed.
HU: Hydroxyurea
All patients displayed perivascular spaces in the frontal white matter and varying levels of iron deposition. BGpv: perivascular spaces in the basal ganglia; HC: hippocampal cysts; BGc: basal ganglia cysts HVLT-R = Hopkins Verbal Learning Test – Revised. Results are expressed as % retention (delayed recall score / highest correct trial score).
AVN = avascular necrosis.
ACS = acute chest syndrome.
PH = pulmonary hypertension by echocardiographic criteria (tricuspid regurgitant jet velocity > 3.0 m/s).
NA = not available because the patient left the facility before HVLT-R testing.
Denotes interquartile range.
3.2. SCA patients have an abnormal venular pattern by 7T MRI
We employed SWI contrast, which is particularly sensitive to the microvasculature at the 7T field strength, to directly probe the integrity of small vessels in patients and controls (Ding et al., 2008). Total venular density was lower in patients compared with control subjects (33.1±12.0 vs. 42.3±8.6 venules/8 cm2, p=0.103) with the density of long venules being significantly lower (4.4±3.7 vs. 8.8±4.4 venules/8 cm2, p=0.028, Fig.1A). As a result of preferential loss of long venules, patients had a higher proportion of short venules compared to controls (Fig. 1B).
3.3. Venular pattern by 7T MRI correlates with cognitive function and anaemia in SCA patients
There was a non significant positive correlation between total venular count and HVLT-R retention score, a measure of episodic memory (rho symbol = 0.486, p = 0.154, Fig. 1C). Higher proportion of short venules was inversely correlated with HVLT-R retention score (ρ=−0.634, p=0.049, Fig. 1C) and with haemoglobin (ρ=−0.671, p=0.027, Fig. 1D). Higher proportion of long venules was directly correlated with haemoglobin (ρ=0.586, p=0.070, data not shown) and with HVLT-R retention score (ρ=0.498, p=0.143 Fig. 1D), although associations were more modest. The HVLT-R score was also correlated with haemoglobin (ρ=0.632, p=0.027, data not shown).
4. Discussion
To our knowledge, this is the first 7T MRI study in SCA and first report of a cerebral venular pattern associated with worse cognitive performance in SCA. Similar to the results of a published landmark study (Vichinsky et al., 2010), we have also found that lower haemoglobin is positively correlated with worse cognitive function.
Cerebral small vessel disease may arise from venular damage (Rincon and Wright, 2014). For instance, stenosis and occlusion of deep cerebral venules has been associated with leukoaraiosis (Moody et al., 1995). Our finding of venular rarefaction is particularly intriguing, as SCA preferentially targets post-capillary venules (Kaul et al., 1989). Small vessel density also decreases with age in normal subjects and has been observed in Alzheimer’s disease (Brown and Thore, 2011). Our vascular findings parallel those observed in patients with Alzheimer’s disease, ischaemic brain damage and age-dependent leukoaraiosis, where small vessel degeneration leads to the formation of characteristic “string” vessels resulting from the destruction of longer vascular structures, and associated with poorer cognitive function (Brown, 2010; Hunter et al., 2012). Patients with Alzheimer’s disease were also found to have structural changes in retinal venular networks, including narrower and tortuous venules as compared with healthy controls (Cheung et al., 2014), suggesting that alterations in venular anatomy may be linked to decreased cognitive function across a spectrum of neurodegenerative diseases. This novel finding in SCA may, therefore, represent a candidate quantifiable radiological marker of cognitive decline. In keeping with a recent report by Mackin et al. (2014), we did not detect microhemorrhages, another recognised MRI marker of small vessel disease in other conditions (Gouw et al., 2011). This discrepancy between SCA and other vascular diseases may reflect the peculiar nature of SCA neuropathology. While in SCA relative hypertension is associated with stroke (Pegelow et al., 1997), elevated systolic blood pressure (Pegelow et al., 1997) and classical atherosclerosis rarely occur (Mansi and Rosner, 2002).
Decreased venular conspicuity in SCA may stem from multiple mechanisms, complicating the interpretation of this finding. The visibility of venules depends on the effect of deoxygenated haemoglobin and decreased oxygen saturation and their repercussions on blood flow on the SWI/BOLD (Blood Oxygenation Level Dependence) signal. Thus, any condition that alters these parameters may influence venular conspicuity. Specifically, the impact of increased blood flow and decreased haemoglobin on SWI in SCA is unknown. A recent study found a global decrease in venous conspicuity by 3T in paediatric SCA patients as compared to controls, but no correlation was found between haemoglobin and visible venous volume, while cognitive function was not tested (Winchell et al., 2014). Similarly, in another study assessing the impact of visual stimulation on cerebral blood flow and BOLD signal in children by 1.5T functional MRI there was overall decreased BOLD in SCA children as compared to controls, but no correlation between haemoglobin and BOLD within the SCA group (Zou et al., 2011). One possible interpretation of these and our findings is that increased cerebral blood flow (CBF) and decreased concentration of deoxyhemoglobin in SCA might have been at the base of the diminished SWI contrast and venular conspicuity in SCA patients. Prior research, however, shows that although the oxygen affinity of haemoglobin S is decreased in its polymerised state and in hypoxic conditions, it is near normal in its tetrameric state and in patients in normoxia (Abdu et al., 2008; Fabry et al., 2001), like the steady state SCA patients in our study. In children, lower peripheral oxygen saturation was positively correlated with cerebral desaturation (Quinn and Dowling, 2012). In adults, however, when the oxygen saturation of venous blood of HbSS patients was directly tested, it was found to be similar to that of HbAA controls, probably because increased blood flow in SCA leads to relatively lower oxygen extraction (Gladwin et al., 1999). This phenomenon likely also occurs in the cerebral vasculature of SCA patients, where CBF is generally increased (Strouse et al., 2006). Finally, when the effects of changes in oxygen saturation on SWI where directly assessed in healthy controls, they were found to be modest (with apnea decreasing the mean venous blood voxel number by 1.6%, and hyperventilation increasing it by 2.6% (Chang et al., 2014)) or absent in the white matter (Rauscher et al., 2005). We did not directly assess oxygen saturation or pCO2 in our patients, however, since we observed a difference of total venular density of 10% between SCA patients and controls, with the difference in long and short venules being equal or close to 50%, respectively, the lower visibility of venules due to the effect of deoxyhemoglobin in SCA may contribute to explain some but not all of the between-group differences. As to the effect of CBF on the SWI sequence in our group, we would expect it to be mitigated by our using flow compensation parameter, which minimises the potential flow effect on the visualisation of vessels. Thus, based on these considerations, the BOLD signal may not have been significantly affected by the SCA-specific haematological characteristics of our patients. Thus, as an alternative explanation, the possibility of direct venular destruction by a neuroinflammatory process leading to anatomical loss of venules needs to be considered. This latter hypothesis is particularly intriguing as post-capillary venules are the inciting site of vaso-occlusion and ischaemia in SCA (Kaul et al.,1989) and may be preferentially targeted by the SCA cerebral vasculopathy. It is, therefore, conceivable that the proinflammatory milieu of SCA, characterised by increased cellular adhesion (Hebbel et al.,1980), generation of reactive oxygen species (Chirico and Pialoux, 2012) and nitric oxide dysfunction (Reiter and Gladwin, 2003), results in direct cerebral venular injury, similar to what observed in transgenic mice (Wood et al., 2004).
Our observations indicate that episodic memory is a sensitive cognitive indicator of microvascular disease in SCA. Given the small sample size, however, these associations will need to be confirmed in larger studies that include testing the CBF and its effect on SWI signal. Histopathological correlation and autopsy studies, ideally in combination with post-mortem MRI, will also be important to confirm our observations.
Our study has several limitations. We hypothesised that the disease-mitigating effect of chronic transfusion may blunt neuropathology in SCA by improving anaemia, a known CI risk factor (Vichinsky et al., 2010). Thus, we did not enrol patients receiving chronic transfusions. This strategy may have, however, excluded the sickest patients, who are chronically transfused for secondary prevention of severe complications. Another potential limitation is that most of our patients were receiving hydroxyurea, which although inferior to chronic transfusion for the secondary prevention of paediatric SCA strokes (Ware et al., 2012), may have beneficial effects on cognitive function (Puffer et al., 2007). A larger study, fully reflective of the phenotypic heterogeneity of SCA may help account for all potential confounders. Finally, it is not possible to infer causation between radiological findings and CI from a cross-sectional study.
We hope that our findings will spur further longitudinal studies to determine whether MRI-detectable venular density may be a useful, proximate biomarker able to identify SCA patients at risk of CI early, so that they can receive targeted preventive interventions.
Acknowledgement
This work was supported by a University of Pittsburgh Department of Radiology and Magnetic Resonance Research Center-sponsored Pilot Funding Project, the University of Pittsburgh Vascular Medicine Institute and the Department of Epidemiology eBRAIN Project. We are particularly grateful to Dr. Mark Gladwin for his support and intellectual insight, to Suchitra Barge for help recruiting the participants and to Hyogin Park for assistance with image processing.
Footnotes
The authors have no relevant financial conflict of interest, regardless of amount or value.
References
- Abdu A, Gomez-Marquez J, Aldrich TK, 2008. The oxygen affinity of sickle hemoglobin. Respiratory Physiology & Neurobiology 161, 92–94. [DOI] [PubMed] [Google Scholar]
- Alkan O, Kizilkilic E, Kizilkilic O, Yildirim T, Karaca S, Yeral M, Kasar M, Ozdogu H, 2010. Cranial involvement in sickle cell disease. European Journal of Radiology 76, 151–156. [DOI] [PubMed] [Google Scholar]
- Ballas SK, 2012. More definitions in sickle cell disease: steady state v base line data. American Journal of Hematology 87, 338. [DOI] [PubMed] [Google Scholar]
- Brown WR, 2010. A review of string vessels or collapsed, empty basement membrane tubes. Journal of Alzheimer’s Disease: JAD 21, 725–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WR, Thore CR, 2011. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathology and Applied Neurobiology 37, 56–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K, Barnes S, Haacke EM, Grossman RI, Ge Y, 2014. Imaging the effects of oxygen saturation changes in voluntary apnea and hyperventilation on susceptibility-weighted imaging. AJNR. American Journal of Neuroradiology 35, 1091–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CY, Ong YT, Ikram MK, Ong SY, Li X, Hilal S, Catindig JA, Venketasubramanian N, Yap P, Seow D, Chen CP, Wong TY, 2014. Microvascular network alterations in the retina of patients with Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association 10, 135–142. [DOI] [PubMed] [Google Scholar]
- Chirico EN, Pialoux V, 2012. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life 64, 72–80. [DOI] [PubMed] [Google Scholar]
- DeBaun MR, Armstrong FD, McKinstry RC, Ware RE, Vichinsky E, Kirkham FJ, 2012. Silent cerebral infarcts: a review on a prevalent and progressive cause of neurologic injury in sickle cell anemia. Blood 119, 4587–4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G, Jiang Q, Li L, Zhang L, Zhang ZG, Ledbetter KA, Gollapalli L, Panda S, Li Q, Ewing JR, Chopp M, 2008. Angiogenesis detected after embolic stroke in rat brain using magnetic resonance T2nWI. Stroke; A Journal of Cerebral Circulation 39, 1563–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry ME, Desrosiers L, Suzuka SM, 2001. Direct intracellular measurement of deoxygenated hemoglobin S solubility. Blood 98, 883–884. [DOI] [PubMed] [Google Scholar]
- Feliu MH, Crawford RD, Edwards L, Wellington C, Wood M, Whitfield KE, Edwards CL, 2011. Neurocognitive testing and functioning in adults sickle cell disease. Hemoglobin 35, 476–484. [DOI] [PubMed] [Google Scholar]
- Gladwin MT, Schechter AN, Shelhamer JH, Pannell LK, Conway DA, Hrinczenko BW, Nichols JS, Pease-Fye ME, Noguchi CT, Rodgers GP, Ognibene FP, 1999. Inhaled nitric oxide augments nitric oxide transport on sickle cell hemoglobin without affecting oxygen affinity. The Journal of Clinical Investigation 104, 937–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouw AA, Seewann A, van der Flier WM, Barkhof F, Rozemuller AM, Scheltens P, Geurts JJ, 2011. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. Journal of Neurology, Neurosurgery, and Psychiatry 82, 126–135. [DOI] [PubMed] [Google Scholar]
- Hebbel RP, Boogaerts MA, Eaton JW, Steinberg MH, 1980. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. The New England Journal of Medicine 302, 992–995. [DOI] [PubMed] [Google Scholar]
- Hunter JM, Kwan J, Malek-Ahmadi M, Maarouf CL, Kokjohn TA, Belden C, Sabbagh MN, Beach TG, Roher AE, 2012. Morphological and pathological evolution of the brain microcirculation in aging and Alzheimer’s disease. PloS One 7, e36893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim TS, Zhao T, Boada FE, 2010. 7 Tesla 16-element TEM Tx Coil with dedicated 14-channel receive-only array. In: Proceedings of the 18th International Society of Magnetic Resonance in Medicine Annual Meeting, Stockholm, Sweden, p. 3832. [PMC free article] [PubMed] [Google Scholar]
- Kaul DK, Fabry ME, Nagel RL, 1989. Microvascular sites and characteristics of sickle cell adhesion to vascular endothelium in shear flow conditions: pathophysiological implications. Proceedings of the National Academy of Sciences of the United States of America 86, 3356–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul DK, Nagel RL, Chen D, Tsai HM, 1993. Sickle erythrocyte-endothelial interactions in microcirculation: the role of von Willebrand factor and implications for vasoocclusion. Blood 81, 2429–2438. [PubMed] [Google Scholar]
- Kugler S, Anderson B, Cross D, Sharif Z, Sano M, Haggerty R, Prohovnik I, Hurlet-Jensen A, Hilal S, Mohr JP, et al. , 1993. Abnormal cranial magnetic resonance imaging scans in sickle-cell disease. Neurological correlates and clinical implications. Archives of Neurology 50, 629–635. [DOI] [PubMed] [Google Scholar]
- Lacritz LH, Cullum CM, Weiner MF, Rosenberg RN, 2001. Comparison of the hopkins verbal learning test-revised to the California verbal learning test in Alzheimer’s disease. Applied Neuropsychology 8, 180–184. [DOI] [PubMed] [Google Scholar]
- Mackin RS, Insel P, Truran D, Vichinsky EP, Neumayr LD, Armstrong FD, Gold JI, Kesler K, Brewer J, Weiner MW, 2014. Neuroimaging abnormalities in adults with sickle cell anemia: associations with cognition. Neurology 82, 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansi IA, Rosner F, 2002. Myocardial infarction in sickle cell disease. Journal of the National Medical Association 94, 448–452. [PMC free article] [PubMed] [Google Scholar]
- Merkel KH, Ginsberg PL, Parker JC Jr., Post MJ, 1978. Cerebrovascular disease in sickle cell anemia: a clinical, pathological and radiological correlation. Stroke; A Journal of Cerebral Circulation 9, 45–52. [DOI] [PubMed] [Google Scholar]
- Moody DM, Brown WR, Challa VR, Anderson RL, 1995. Periventricular venous collagenosis: association with leukoaraiosis. Radiology 194, 469–476. [DOI] [PubMed] [Google Scholar]
- Moody DM, Brown WR, Challa VR, Ghazi-Birry HS, Reboussin DM, 1997. Cerebral microvascular alterations in aging, leukoaraiosis, and Alzheimer’s disease. Annals of the New York Academy of Sciences 826, 103–116. [DOI] [PubMed] [Google Scholar]
- Pegelow CH, Colangelo L, Steinberg M, Wright EC, Smith J, Phillips G, Vichinsky E, 1997. Natural history of blood pressure in sickle cell disease: risks for stroke and death associated with relative hypertension in sickle cell anemia. American Journal of Medicine 102, 171–177. [DOI] [PubMed] [Google Scholar]
- Puffer E, Schatz J, Roberts CW, 2007. The association of oral hydroxyurea therapy with improved cognitive functioning in sickle cell disease. Child Neuropsychology: A Journal on Normal and Abnormal Development in Childhood and Adolescence 13, 142–154. [DOI] [PubMed] [Google Scholar]
- Quinn CT, Dowling MM., 2012. Cerebral tissue hemoglobin saturation in children with sickle cell disease. Pediatr Blood Cancer 59 (5), 881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauscher A, Sedlacik J, Barth M, Haacke EM, Reichenbach JR, 2005. Nonnvasive assessment of vascular architecture and function during modulated blood oxygenation using susceptibility weighted magnetic resonance imaging. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine 54, 87–95. [DOI] [PubMed] [Google Scholar]
- Reiter CD, Gladwin MT, 2003. An emerging role for nitric oxide in sickle cell disease vascular homeostasis and therapy. Current Opinion in Hematology 10, 99–107. [DOI] [PubMed] [Google Scholar]
- Rincon F, Wright CB, 2014. Current pathophysiological concepts in cerebral small vessel disease. Frontiers in Aging Neuroscience 6, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro AM, Benedict RH, Schretlen D, Brandt J, 1999. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clinical Neuropsychology 13, 348–358. [DOI] [PubMed] [Google Scholar]
- Sinnecker T, Bozin I, Dorr J, Pfueller CF, Harms L, Niendorf T, Brandt AU, Paul F, Wuerfel J, 2013. Periventricular venous density in multiple sclerosis is inversely associated with T2 lesion count: a 7 Tesla MRI study. Multiple Sclerosis 19, 316–325. [DOI] [PubMed] [Google Scholar]
- Strouse JJ, Cox CS, Melhem ER, Lu H, Kraut MA, Razumovsky A, Yohay K, van Zijl PC, Casella JF, 2006. Inverse correlation between cerebral blood flow measured by continuous arterial spin-labeling (CASL) MRI and neurocognitive function in children with sickle cell anemia (SCA). Blood 108, 379–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switzer JA, Hess DC, Nichols FT, Adams RJ, 2006. Pathophysiology and treatment of stroke in sickle-cell disease: present and future. Lancet Neurology 5, 501–512. [DOI] [PubMed] [Google Scholar]
- Vichinsky EP, Neumayr LD, Gold JI, Weiner MW, Rule RR, Truran D, Kasten J, Eggleston B, Kesler K, McMahon L, Orringer EP, Harrington T, Kalinyak K, De Castro LM, Kutlar A, Rutherford CJ, Johnson C, Bessman JD, Jordan LB, Armstrong FD, Neuropsychological D, Neuroimaging Adult Sickle Cell Anemia Study, G, 2010. Neuropsychological dysfunction and neuroimaging abnormalities in neurologically intact adults with sickle cell anemia. JAMA: The Journal of the American Medical Association, 303;, pp. 1823–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware RE, Helms RW, Investigators SW, 2012. Stroke With Transfusions Changing to Hydroxyurea (SWiTCH). Blood 119, 3925–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchell AM, Taylor BA, Song R, Loeffler RB, Grundlehner P, Hankins JS, Wang WC, Ogg RJ, Hillenbrand CM, Helton KJ, 2014. Evaluation of SWI in children with sickle cell disease. AJNR. American Journal of Neuroradiology 35, 1016–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KC, Hebbel RP, Granger DN, 2004. Endothelial cell P-selectin mediates a proinflammatory and prothrombogenic phenotype in cerebral venules of sickle cell transgenic mice. American Journal of Physiology. Heart and Circulatory Physiology 286, H1608–H1614. [DOI] [PubMed] [Google Scholar]
- Zou P, Helton KJ, Smeltzer M, Li CS, Conklin HM, Gajjar A, Wang WC, Ware RE, Ogg RJ, 2011. Hemodynamic responses to visual stimulation in children with sickle cell anemia. Brain Imaging and Behavior 5, 295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]