Abstract
Acute myeloid leukemia (AML) therapies are rapidly evolving with novel targeted therapies showing high-level responses in a notoriously difficult to treat group of patients – the elderly and unfit. This review will examine the outcomes of older AML patients (>60 years old) with conventional induction strategies, and published literature on risks of pursuit of induction. Low-intensity combination therapy response rates appear to be approaching that of induction regimens, and with lower toxicity, low-intensity therapy likely represents the future standard approach in this age group. Lastly, allogeneic transplant appears to have a role in increasing durable remissions regardless of age and should be considered in patients with limited comorbidities.
Keywords: AML, therapy, elderly
Introduction
Acute myeloid leukemia (AML) will be diagnosed in 21,380 patients in 2017 but is much more common in elderly patients with 69.3% of AML-related deaths in the USA occurring in patients ≥65 years old [1,2] (see Figure 1 for incidence and mortality rates). The prognosis of elderly AML patients is inferior to younger patients with a 5-year overall survival (OS) of <25% among patients aged 60–65 and <10% among patients ≥70 years old as opposed to a 5-year survival of ~50% among those <50 years old [3]. Many patients and disease-specific factors contribute to the poorer outcomes among elderly patients including poorer performance status (PS) at diagnosis; lower complete remission (CR) rates with intensive chemotherapy; increased early death rates with intensive chemotherapy; an increased incidence of unfavorable cytogenetics; and an increased incidence of secondary AML (sAML), defined as AML arising from an antecedent hematologic disorder such as myelodysplastic syndrome (MDS) or attributable to prior chemotherapy or radiation [3,4]. These factors lead many clinicians to choose less intensive treatment strategies, which have historically proven less effective at inducing remissions. Similar concerns limit the use of the most effective consolidation strategies when patients do achieve a CR.
Figure 1.
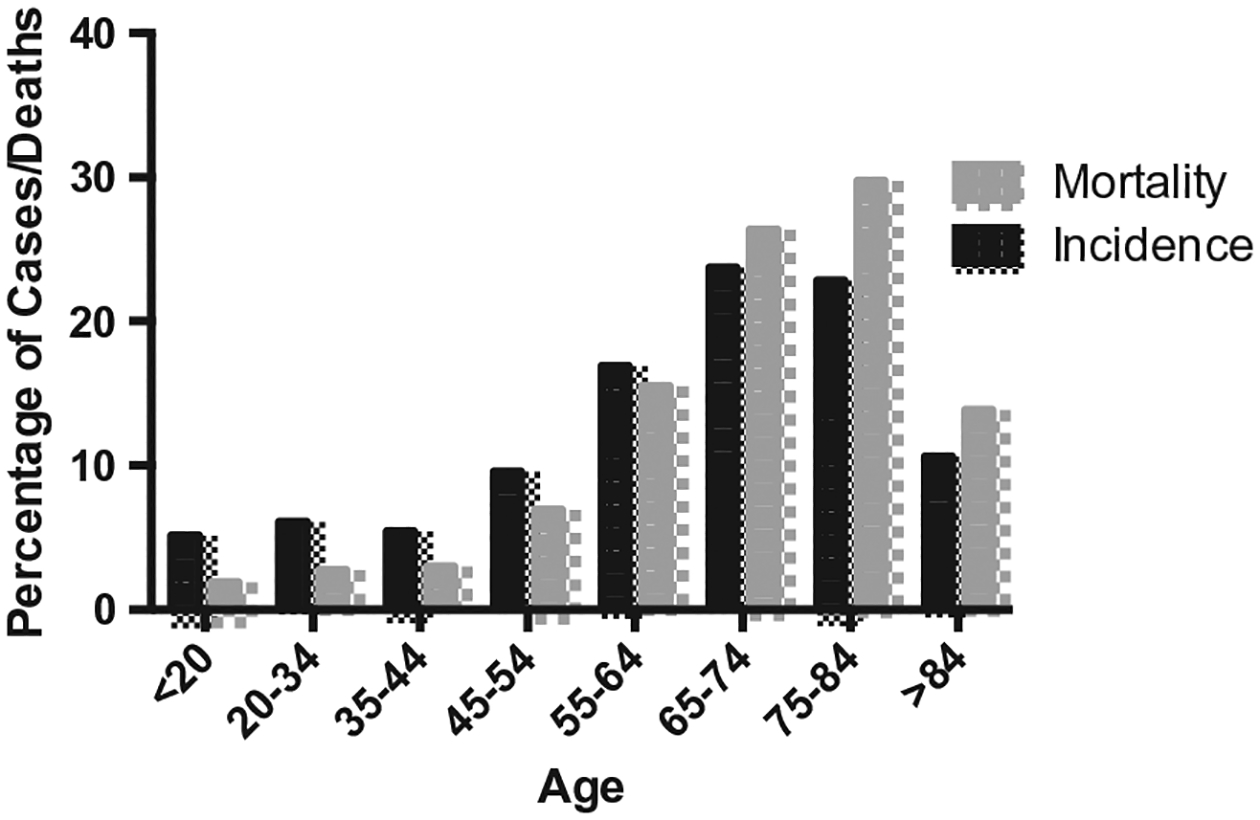
Distribution of AML incidence and deaths by age in the USA 2010–2014. Adapted from SEER database [2].
As the prognosis for elderly patients with acute promyelocytic leukemia (APL) is excellent with long-term survival now in excess of 75% [5], this review will focus on non-APL AML in the elderly with an emphasis on emerging treatment strategies for intensive induction chemotherapy, low-intensity chemotherapy, and consolidation therapy. In particular, we anticipate a broadening array of treatment options which will make the selection of the optimal treatment strategy for each patient more difficult. Most leukemia trials in the elderly enroll patients beginning at age 60 or 65, so we have chosen to use the more inclusive definition for this review and consider any patient age 60 or older with a new diagnosis of AML to be suitable for consideration of treatment using an elderly paradigm.
Fit vs. Unfit
The first step in assessing elderly leukemia patients is to determine their fitness for intensive induction chemotherapy based on age, PS, and comorbidities. The risk of 30-day mortality with induction chemotherapy is 31% for patients aged 66–75 with an ECOG PS of 2 and rises with increasing age or decreasing PS [4]. Clearly, the risk of death with induction chemotherapy is too great for most elderly patients with ECOG PS ≥2, and these patients should be directed toward less intensive treatment options at diagnosis. Similarly, a retrospective analysis of elderly patients receiving intensive induction at MD Anderson demonstrated a 28-day mortality of 29% and a CR rate of just 42% for patients with a score of 3 or greater on the hematopoietic stem cell transplant comorbidity index (HCT-CI, see Table 1) compared to a 28-day mortality of just 11% for patients with HCT-CI scores of 1 or 2 [6]. While it is impossible to prevent all induction-related mortality even among relatively healthy elderly patients, it is clear that the risk of mortality outweighs the benefit of induction for elderly patients with significant comorbidities. Other strategies to gauge fitness for induction include those of Klepin who has proposed incorporating a number of brief tests that can easily be administered in clinic to ascertain the fitness of elderly individuals including the geriatric assessment (GA); short physical performance battery (SPPB); or modified mini-mental state exam (3MS), although none of these have as yet been evaluated in prospective trials [7]. Thus, a number of different factors can be used to select patients who are likely to be harmed by induction chemotherapy, so that they can be directed toward less intensive therapies. However, these factors are not as helpful in determining who is likely to benefit from induction.
Table 1.
HCT-CI [79].
| Comorbidity | Definition in HCT-CI | HCT-CI score |
|---|---|---|
| Arrhythmia | Atrial fibrillation or flutter, sick sinus syndrome, or ventricular arrhythmias | 1 |
| Cardiac | Coronary artery disease, congestive heart failure, myocardial infarction, or EF ≤ 50% | 1 |
| Gastrointestinal | Crohn’s disease or ulcerative colitis | 1 |
| Diabetes | Requiring insulin or oral hypoglycemic | 1 |
| Cerebrovascular disease | Transient ischemic attack (TIA) or cerebrovascular accident (CVA) | 1 |
| Psychiatric | Depression or anxiety requiring psychiatric consult or treatment | 1 |
| Hepatic, mild | Chronic hepatitis, bilirubin > ULN to 1.5× ULN, or AST/ALT> ULN to 2.5× ULN | 1 |
| Obesity | Patients with a body mass index (BMI) > 35 kg/m2 | 1 |
| Infection | Requiring continuation of antimicrobial treatment after day 0 | 1 |
| Rheumatologic | SLE, RA, polymyositis, mixed CTD, or PMR | 2 |
| Peptic ulcer | Requiring treatment | 2 |
| Moderate/severe renal | Serum creatinine >2 mg/dL, on dialysis, or prior renal transplantation | 2 |
| Moderate pulmonary | DLCO and/or FEV1 66–88% of dyspnea on slight activity | 2 |
| Prior solid tumor | Treated at any time point in the patient’s past history, excluding non-melanoma skin cancer | 3 |
| Heart valve disease | Except mitral valve disease | 3 |
| Severe pulmonary | DLCO and/or FEV1 ≤ 65% or dyspnea at rest or requiring oxygen | 3 |
| Moderate/severe hepatic | Liver cirrhosis, bilirubin >1.5× ULN, or AST/ALT >2.5× ULN | 3 |
Likelihood of benefit from induction
To better assess the likelihood of an elderly patient benefitting from intensive induction chemotherapy, a number of prognostic models have been developed that incorporate additional data beyond age, PS, and comorbidities. Specifically, adverse cytogenetics and sAML are important predictors of both response to treatment and OS with consistently poor outcomes among patients with these risk factors [8–12]. Thus, the use of induction chemotherapy should be carefully considered among healthy elderly patients if they are known to have adverse cytogenetics or sAML. Given the prognostic significance of cytogenetics, it is often reasonable to defer the initiation of therapy until these results are known and can be used to inform the subsequent treatment strategy.
Induction approaches
The utility of intensive induction chemotherapy for fit elderly patients was first established in a phase III study run by the EORTC comparing a three-drug induction regimen of daunorubicin, vincristine, and cytarabine (DOAc) versus best supportive care (BSC) including cytoreductive treatment with hydroxyurea or low-dose cytarabine (LDAC) in newly diagnosed AML patients over age 65. This trial demonstrated a CR rate of 58% in the induction arm compared to 0% in the BSC arm, which translated into improved survival at 2.5 years in the induction arm (17% vs. 0%) [13]. Notably, the median percentage of days hospitalized were similar between the two arms, suggesting that a palliative approach was ineffective at limiting resource utilization in addition to yielding poorer outcomes.
Most subsequent studies of intensive induction chemotherapy in the elderly have been designed to determine the optimal dosing of a standard two-drug regimen comprised of an anthracycline and cytarabine (Table 2). In 2009, Lowenberg and colleagues reported that doubling the dose of daunorubicin to 90 mg/m2 daily in combination with cytarabine yielded an increase in CR rate from 54% to 64% (p = .002) in elderly AML patients, which led to a non-significant improvement in OS at 2 years (26% vs. 31%) [14]. However, when a daunorubicin dose of 60 mg/m2, which is commonly used in practice, was compared to a daunorubicin dose of 90mg/m2 in the NCRI AML17 (a study which included younger AML patients), the CR rates (75% vs. 73%), and 2-year OS (60% vs. 59%) were similar. In particular, no benefit to the escalated daunorubicin dose was seen in any age group, including patients aged 60–65 who had previously shown a survival benefit with an escalated anthracycline dose [14,15]. The NCRI AML17 trial did differ from that of Lowenberg and colleagues in that it used a double induction strategy, so results between the two trials are difficult to compare. Similarly, a number of trials in elderly AML patients have investigated the utility of dose-dense induction regimens with escalated cytarabine doses such as IMA and TAD-HAM/HAM-HAM (see Table 2 for regimen description) but have shown no OS benefit with these regimens [16,17]. Based on these results, an induction regimen of infusional cytarabine for 7 days with daunorubicin 60mg/m2 daily on days 1–3, commonly referred to as ‘7 + 3’, has become standard in the USA for fit, newly diagnosed AML patients age 60 and above.
Table 2.
Induction approaches.
| Trial | Population | Regimens | Responses | Survival | Comments |
|---|---|---|---|---|---|
| EORTC [13] | Newly diagnosed AML, Age >65 | DOAc vs. BSC | CR = 58% vs. 0% | OS at 2.5 years = 17% vs. 0% | Median nights of hospitalization were roughly equal |
| HOVON/SAKK [14] | Newly diagnosed AML or high-risk MDS, Age ≥60 | 7 + 3 (daunorubicin 45 mg/m2/day vs. 90 mg/m2/day) | CR = 54% vs. 64% (p = .002) | OS at 2 years = 26% vs. 31% (p = . 16) | Subsequent studies with daunorubicin 60 show comparable or improved survival vs. daunorubicin 90 |
| E2906 [23] | Newly diagnosed AML, Age ≥60 | 7 + 3 (daunorubicin 60mg/m2/day) vs. clofarabine 30 mg/m2 IV on days 1–5 | CR + CRi = 43.8% vs. 42.8% (p = .87) | OS favored 7 + 3 with HR 1.41 (95% Cl 1.12–1.78) | Induction and consolidation toxicities with clofarabine were reduced compared to 7 + 3 |
| Phase lib: CPX-351 [25] | Newly diagnosed AML, age 60–75 | 7 + 3 (daunorubicin 60mg/m2/day) vs. CPX-351 100U/m2 on days 1, 3, and 5 | CR + CRi = 51.2% vs. 66.7% (p = .07) | Median OS 12.9 months vs. 14.7 months | 60-day mortality was lower with CPX-351 but count recovery was slower |
| Phase III: CPX-351 [26] | Newly diagnosed sAML, age 60–75 | 7 + 3 (daunorubicin 60mg/m2/day) vs. CPX-351 100U/m2 on days 1, 3, and 5 | CR + CRi = 33.3% vs. 47.7% (p = .016) | Median OS 5.95 months vs. 9.56 months (p = .005) | Count recovery was delayed with CPX-351 |
| German AML Intergroup Study [17] | Newly diagnosed AML, age ≥60 | 7 + 3 (daunorubicin 60mg/m2/day) vs. TAD-HAM OR HAM-HAM vs. IMA | CR at 90 days = 51 % vs. 50% vs. 48% (p = n.s.) | 3-year EFS = 12.4% vs. 15.6% vs. 11.4% | Groups were not balanced with the 7 + 3 group being younger with more sAML |
| SAL [16] | AML patients, age >60 | 7 + 3 (daunorubicin 45 mg/m2/day) vs. IMA | CR = 39% vs. 55% (p = .001) | 3-year RFS favored 7 + 3 (29% vs. 14%, p = .042). 3-year OS was similar | Different consolidation strategies used in each arm |
DOAc: daunorubicin 30 mg/m2/day IV on days 1–3, vincristine 1 mg/m2 IV on day 2, and cytarabine 100 mg/m2/day continuous IV plus 50 mg/m2 IV bolus q12hrs on days 1–7.
7 + 3: cytarabine 100 mg/m2/day continuous IV on days 1–7 and daunorubicin at varying doses IV as above on days 1–3.
TAD: cytarabine 100 mg/m2/day IV on days 1–2 and BID on days 3–8, daunorubicin 60 mg/m2/day on days 3–5, and 6-thioguanine 100 mg/m2 PO BID on days 3–9.
HAM: cytarabine 1 g/m2 IV BID on days 1–3 and mitoxantrone 10 mg/m2/day IV on days 3–5.
IMA: cytarabine 1 g/m2 IV BID on days 1, 3, 5, and 7 and mitoxantrone 10 mg/m2/day IV on days 1–3.
Two additional trials have randomized elderly AML patients to 7 + 3 or similar regimens with or without the addition of a third agent (azacitidine or gemtuzumab ozogamicin [GO]). These prospective trials failed to demonstrate an improvement in CR rates with an additional agent, although 3-year RFS was improved with gemtuzumab, but a recent meta-analysis suggested that the addition of gemtuzumab to standard induction provided a survival benefit in patients with favorable or intermediate risk cytogenetics [18–20]. Unfortunately, these findings have not been consistent in other studies combining gemtuzumab with standard induction [21], and concerns about toxicity have led to the withdrawal of gemtuzumab from the US market. Lastly, the Cancer and Leukemia Group B (CALGB) trial 10502 assessed the addition of bortezomib to 7 + 3 for untreated AML patients age ≥60 and demonstrated a CR rate of 65% with an additional 4% achieving a CR with incomplete platelet recovery (CRi) [22]. While the response rates in this elderly population appear higher than with 7 + 3 alone, the two regimens have not been compared directly. Thus, manipulations of conventional 7 + 3 chemotherapy in fit, elderly AML patients have not conclusively shown a benefit from the addition of a third agent in prospective trials.
Three recent randomized, controlled trials have compared novel agents with 7 + 3 in newly diagnosed AML patients who are ≥60 years old. In E2906, single agent clofarabine demonstrated a similar response rate (CR + CRi) to 7 + 3 (42.8% vs. 43.8%), but the trial was stopped early due significantly worse OS in the clofarabine arm [23]. Subsequent analysis revealed that quality of response appeared to be associated with poorer outcomes over time in the clofarabine arm [24]. A similar phase IIb trial randomized patients to CPX-351, a liposomal formulation of cytarabine and daunorubicin designed to deliver the optimized 5:1 molar ratio of the drugs, or 7 + 3 and showed an improved response rate (CR + CRi) with CPX-351 (66.7% vs. 51.2%, p = .07) [25]. While CPX-351 improved response rates in patients who traditionally fare poorly with 7 + 3 including those with adverse cytogenetics (77.3% vs. 38.5%, p = .03) and sAML (57.6% vs. 31.6%, p = .06), median OS between the two arms was comparable (8.5 months vs. 6.3 months, p = .19). A subsequent phase III trial of CPX-351 vs. 7 + 3 in newly diagnosed, elderly sAML patients demonstrated similar improvements in response rate (47.7% vs. 33.3%, p = .016) leading to a significant improvement in median OS (9.56 months vs. 5.95 months, p = .005) and survival at 2 years (31.1% vs. 12.3%) [26]. In addition to highlighting the efficacy of CPX-351, these trials also clearly demonstrate the dismal outcomes of elderly patients with adverse cytogenetics or sAML who are treated with standard 7 + 3.
Single agent low-intensity approaches
Over the past 15 years, a significant number of trials have attempted to address the need for effective treatment of AML in elderly patients who are considered unfit for induction chemotherapy. The first trial to show a conclusive benefit of a less intensive chemotherapy regimen was the Medical Research Council’s AML14 study, which compared LDAC with BSC for patients with untreated AML or high-risk MDS. This study demonstrated an improved CR rate among patients receiving LDAC (18% vs. 1%, p < .00006) leading to improved survival (OR 0.60, p = .0009) [27]. Notably, no survival benefit was seen among patients who failed to achieve a CR, further validating CR as a clinically meaningful endpoint even in the absence of traditional induction chemotherapy. The modest success of LDAC in improving survival in unfit, elderly AML patients established a standard-of-care for this population against which future treatments could be judged.
More recently, a number of large studies have assessed the efficacy of hypomethylating agents (HMAs) as front-line treatment for AML in the elderly (Table 3). In the AZA-AML-001 study, patients were randomized to treatment with azacitidine for 7 days every 4 weeks or conventional care (CCR) including supportive care; LDAC; or induction chemotherapy, which was selected prior to randomization and used as a stratification factor for randomization. The response rate (CR + CRi) was similar between the two arms (27.8% for azacitidine vs. 25.1% for CCR, p = .5348), but median OS was improved in the azacitidine arm when censoring for subsequent therapy (12.1 months vs. 6.9 months, p = .019) [28]. Interestingly, among patients who failed to achieve a CR, this study found an improvement in survival in the azacitidine arm, suggesting a benefit to HMAs in the absence of achieving a CR. Among all patients, the largest gains in survival were seen in those who were pre-selected to receive supportive care but randomized to azacitidine, while survival was comparable among patients pre-selected to receive induction chemotherapy regardless of subsequent randomization, consistent with a retrospective study that demonstrated similar survival between elderly AML patients treated with intensive induction chemotherapy versus a HMA [29]. As AZA-AML-001 only enrolled patients with a white blood cell count of ≤15,000/mm3, this finding may not be generalizable to all elderly AML patients who are fit for induction. The DACO-016 study similarly randomized patients between 5 days of decitabine every 4 weeks or treatment choice (TC), which included LDAC or supportive care. This trial showed an improvement in the CR rate with decitabine (17.8% vs. 7.8%, p = .001) and a modest improvement in OS (7.7 months vs. 5.0 months, p = .037) [30]. The vast majority (87%) of the patient’s randomized to TC received LDAC, suggesting that the low remission rate seen in that arm likely could not be accounted for by the choice of subsequent treatment. Thus, based on the survival benefit seen in these two large studies, HMAs have now been widely adopted as a standard option for elderly AML patients who are unfit for conventional induction chemotherapy.
Table 3.
Low-intensity single agent approaches.
| Trial | Population | Regimen(s) | Response(s) | Survival | Comments |
|---|---|---|---|---|---|
| AML14 MRC [27] | Untreated AML or high-risk MDS unfit for intensive therapy | LDAC vs. hydroxyurea | CR = 18% vs. 1% (p < .00006) | OR for survival was 0.60 favoring LDAC (p = .0009) | There was no benefit among patients who did not achieve CR Survival was much improved with AZA for those treated with supportive care or LDAC but similar for those who received induction |
| AZA-AML-001 [28] | Newly diagnosed AML, age ≥65, WBC ≤15K, blasts >30%, ECOG ≤2, not eligible for HSCT with int/adverse cytogenetics | AZA 75 mg/m2 for 7 days every 28 days vs. CCR (SC, LDAC, or induction) | CR + CRi = 27.8% vs. 25.1% | Median OS favored AZA when censoring for subsequent therapy at 12.1 months vs. 6.9 months (p = .0190) | |
| DACO-OI6 [30] | Newly diagnosed AML with int/adverse cytogenetics, age ≥65, WBC ≤40 K, ECOG ≤2 | DEC 20 mg/m2 for 5 days every 28 days vs. TC (SC or LDAC) | CR + CRp = 17.8% vs. 7.8% (p = .001) | Median OS favored DEC 7.7 months vs. 5.0 months (log rank p = .037) | There were more serious AEs with DEC than TC |
| Ohio State [32] | Previously untreated AML, age ≥60, no WBC limit | DEC 20 mg/m2 IV for 10 days every 28 days | CR = 47% CRi = 17% ORR = 64% | Median OS = 55 weeks | Response rate was 50% for WBC ≥50 K, 74% for sAML, 75% for complex karyotype |
| SGI-110–01 [39] | Treatment naive AML not candidates for intensive induction due to age ≥65, ECOG 2, comorbidities or poor-risk cytogenetics | Guadecitabine 60–90 mg/m2 IV for 5 days every 28 days vs. guadecitabine 60 mg/m IV on days 1 −5 and 8–12 every 28 days | ORR (CR + CRp + CRi) = 57% vs. 48% (p = .43) | Median OS 10.5 months vs. 8.7 months (p = .89) | Toxicities were similar. CR and CRp were 40–45% for both arms |
| AML16 [40] | Untreated AML or high-risk MDS unfit for intensive therapy, age ≥60 | LDAC vs. clofarabine 20 mg/m2 IV for 5 days every 4–6 weeks | ORR (CR + CRi) = 19% vs. 38% (p < .001) | 2-year OS was 12% vs. 13% | Greatly increased toxicity in the clofarabine arm led to similar survival, clofarabine required significantly more supportive care including hospitalization |
| AML16 and LI-1 [42] | Untreated AML or high-risk MDS unfit for intensive therapy, age ≥60 | LDAC vs. sapacitabine 300 mg PO BID on days 1–3 and 8–10 every 28 days | ORR (CR + CRi) = 27% vs. 16% (p = .09) | 2-year survival 12% vs. 11% (p = .2) | Toxicities were similar with more grade 3/4 diarrhea with sapacitabine |
| SGN33A-001 [43] | CD33+, previously untreated AML ineligible for or declined conventional treatment | Vadastuximab 40mcg/kg q3wks ×2 cycles | CR + CRi = 54% | Survival data not yet mature | Trials subsequently halted due to increased VOD with HSCT |
LDAC: cytarabine 20 mg SubQ BID for 10 days every 28–42 days.
SC: supportive care.
AZA: azacitidine (Vidaza).
DEC: decitabine (Dacogen).
Given the modest efficacy of HMAs in producing remissions and extending survival, recent studies have evaluated the optimal dosing schedule in newly diagnosed elderly AML or high-risk MDS patients. Three separate groups have reported response rates (CR, CRi, or CRm) of 40.4–64% by doubling the duration of decitabine treatment to 10 days including CR rates of 33.3–50% among patients with adverse cytogenetics [31–33]. These response rates may represent an improvement over a 5-day schedule of decitabine, which are associated with response rates of just 25–27.7% in prior trials [30,34]. Of particular note, the survival among patients with adverse cytogenetics is comparable to survival of those with intermediate-risk cytogenetics using extended decitabine dosing, whereas adverse cytogenetics is associated with worse survival with traditional induction [4]. Similarly, standard doses of HMAs lead to improved survival among patients with high-risk MDS or AML and abnormalities of chromosome 5 or 7 compared to standard induction [35]. Additionally, extended decitabine dosing led to a 100% overall response rate (CR with count recovery in 19%) among patients with TP53 mutations, while the CR rate following induction chemotherapy in patients with TP53 mutations is just 28% [33,36,37]. While these trials suggest the benefit of extended decitabine dosing, especially among patients with poor risk features, a recent randomized phase II trial comparing a 5-day versus 10-day course of decitabine demonstrated similar complete response rates (CR + CRi + CRp) of 50% and 42%, respectively, with comparable OS [38]. Thus, the seemingly encouraging results seen with extended decitabine dosing in multiple trials await confirmation in larger trials.
Guadecitabine (SGI-110), a decitabine prodrug, was studied in a phase II study evaluating two different dosing schedules which demonstrated response rates (CR + CRi) of 57% with a 5-day versus 48% with a 10-day dosing scheduling, with comparable survival (OS 10.5 months vs. 8.7 months, p = n.s.) [39]. Based on these promising results, a phase III study randomizing elderly patients unfit for induction to guadecitabine versus treatment choice (LDAC, decitabine, or azacitidine) has enrolled 815 patients worldwide (NCT02348489).
A variety of other agents have been used as monotherapy for elderly patients with newly diagnosed AML with varying degrees of success. Randomized trials have compared LDAC with clofarabine; vosaroxin, a quinolone-derived intercalating agent; and sapacitabine, a novel nucleoside analog. Clofarabine did yield an improved response rate (38% vs. 19%, p < .001) but comparable survival at 2 years [40], while vosaroxin and sapacitabine failed to improve response rate or survival [41,42]. The disappointing long-term results with clofarabine were due to increased toxicities and illustrate the need to balance toxicities with response in this vulnerable patient population.
More recently, the novel CD33-targeted drug-anti-body conjugate vadastuximab talirine (SGN-CD33A) produced a response rate (CR + CRi) of 54% with an additional 19% of patients achieving a morphologic leukemia-free state. The promising results included a response rate of 50% among patients with underlying MDS [43]. Combinations of HMAs and vadastuximab have also been evaluated yielding a 73% composite complete response (CR + CRi) rate with 47% of responding patients achieving an MRD-negative remission by flow cytometry [44]. Notably, the frequency of MRD-negative remissions in this trial was similar to that seen in the AML16 trial using a more intensive approach where 51% of patients in remission were MRD negative after one cycle, and MRD negativity correlated with decreased relapse risk and increased long-term survival [45]. A randomized phase III trial comparing vadastuximab + decitabine/azacitidine with placebo + decitabine/azacitidine is ongoing (NCT02785900) to determine if the promising response rates will translate into a survival benefit. Of note, the FDA recently halted a number of such studies due to concerns about vadastuximab potentiating veno-occlusive disease (VOD) in allogeneic transplant recipients, but the hold has been removed following additional data from the sponsor. Thus, there is not currently sufficient evidence to recommend low-intensity approaches aside from LDAC and HMAs in elderly AML patients.
Novel targeted agents
IDH1/2
Specific activating mutations in isocitrate dehydrogenase (IDH) 1 & 2 are present in a subset of AML cases (16%) and can be more frequent in older patients with AML and low WBC at presentation [46]. These mutations lead to changes in epigenetic regulation of oncogenes and are currently a focus of novel therapies. AG-120, an inhibitor of IDH1 with an activating mutation, was evaluated in a phase I study of advanced IDH1 mutation-positive hematologic malignancies and was associated with an ORR of 36% and CR rate of 18% [47]. Development is ongoing as both a single agent in phase I (NCT02074839) and in combination with induction and consolidation (NCT02632708).
FLT3
For untreated, elderly patients with FLT3-ITD AML, the combination of azacitidine with the tyrosine kinase inhibitor sorafenib led to a composite complete response rate (CR + CRi + CRp) of 77% with a median response duration of 14.5 months [48]. This response rate seems relatively consistent with the response rates among patients with FLT3-ITD AML treated with multiple different front-line regimens including induction in recent years [49]. Given the efficacy of induction chemotherapy in FLT3-ITD AML in achieving a remission, less intensive strategies should currently be reserved for patients who are unable to withstand intensive chemotherapy, although future studies comparing these strategies may be warranted.
Combination low-intensity approaches
Given the proven efficacy and manageable toxicities of both LDAC and HMAs, other trials have focused on combination approaches incorporating a novel agent into one of these standard treatments (Table 4). The MRC’s AML16 trial included randomized comparisons of LDAC with LDAC + tipifarnib, a farnesyl transferase inhibitor; LDAC + arsenic trioxide (ATO); and LDAC + GO. The combination of LDAC and GO led to better responses when compared to LDAC (CR + CRi = 30% vs. 17%, p = .006) but survival was similar, while LDAC in combination with tipifarnib or ATO failed to improve the response rate or survival vis-à-vis LDAC alone [50–52]. Notably, there was scant prior clinical experience to support the combinations of LDAC with tipifarnib or GO aside from the modest single agent activity of each in the treatment of elderly leukemia [53–55], while a prior study of the LDAC + ATO combination showed a 34% response rate [56], better than most trials of LDAC alone. Thus, the latter example highlights the need to confirm promising results with randomized trials.
Table 4.
Low-intensity combination approaches.
| Trial | Population | Regimen(s) | Response(s) | Survival | Comments |
|---|---|---|---|---|---|
| Ml 4–358 [58] | Treatment-naïve AML, age ≥65, not eligible for standard induction | AZA 75 mg/m2 for 7 days or decitabine 20 mg/m2 for 5 days every 28 days + venetoclax daily (dose escalation) | CR + CRi = 71% | Response rate with poor-risk cytogenetics was 88% | |
| SGN33A-001 [44] | CD33+, previously untreated AML ineligible for or declined conventional treatment | AZA 75 mg/m2 for 7 days or DEC 20 mg/m2 for 5 days + vadastuximab l0 mcg/kg q4wks | CR + CRi = 74% | Median RFS 9.1 months | Responses were 77% in patients with sAML and 83% among those with adverse cytogenetics |
| 2014–0076 [48] | Untreated FLT3-ITD AML, age ≥60, and ECOG ≤2 | AZA 75 mg/m2 for 7 days + sorafenib 400 mg PO BID every 28 days | CR + CRi = 73% | Median OS 8.8 months | |
| 2013–0099 [59] | AML or high-risk MDS, age ≥60, and ECOG ≤2 | DEC 20 mg/m2 for 5 days + vosaroxin 70–90 mg/m2 on days 1 and 4 every 4–5 weeks | CR + CRp + CRi = 77% | Median OS 8.3 months | Survival was improved with a reduced dose of vosaroxin |
| Ml 4–387 [57] | AML, age ≥65, unfit for intensive induction | LDAC + venetoclax 600 mg PO for 28 days | CR + CRi = 70% | 1-year survival 74.7% | No TLS. Median time to response was 30 days |
| AML14 and AML16 [52] | AML or high-risk MDS with age ≥60 or unfit for induction | LDAC vs. LDAC + gemtuzumab ozogamicin 5 mg on day 1 every 6 weeks | CR + CRi = 17% vs. 30% (p = .006) | 1-year OS 25% vs. 27% without improvement in RFS | Increased toxicity and resource usage seen with the combined approach |
In contrast to the limited efficacy of prior combination trials with LDAC, the BCL-2 inhibitor, venetoclax (ABT-199), has shown promising results in recent early phase clinical trials in combination with both LDAC and HMAs in elderly AML patients. A recent phase I/II study demonstrated a 70% response rate (CR + CRi) when combining LDAC with venetoclax at the recommended phase II dose. This study reported a 1-year estimated survival of 74.7% [57]. Similarly, a phase Ib study investigating the combination of either decitabine or azacitidine with venetoclax in elderly AML patients demonstrated a composite complete response rate (CR + CRi) of 71% including a response rate of 88% among patients with adverse risk cytogenetics and 82% in patients with IDH1/2 mutations [58]. While preliminary survival data have not yet been reported, a randomized phase III trial comparing this combination with azacitidine alone is already ongoing (NCT02993523). If confirmed in randomized, phase III trials, the response rates and survival seen with venetoclax combinations represent a significant improvement over standard low-intensity treatment approaches.
A similarly impressive composite complete response (CR + CRi + CRp) rate of 77% was previously reported for the combination of decitabine and vosaroxin in patients age 60 and older with AML or high-risk MDS, but median OS was 8.3 months [59]. While the response rate in this trial was impressive, there was concern that the toxicity of vosaroxin may have limited its survival benefit, and the combination is not currently being pursued in further trials in treatment-naïve elderly patients given the recent proliferation of alternative options. Other trials investigating the addition of HDAC inhibitors (pracinostat and vorinostat), a NEDD8-activating enzyme inhibitor (pevonedistat), or the immunomodulatory agent revlimid to azacitidine for unfit, elderly AML patients have shown composite complete response rates (CR + CRi) of 28–46% [60–63]. While the response rates with these combinations were less impressive than recently reported results with HMAs and venetoclax, the reported 2-year survival of 45% following azacitidine and pracinostat warrants further investigation in the form of a phase III trial [62]. Thus, numerous combinations of HMAs with other agents have recently been investigated and many seem to improve on response rates to HMAs alone, but a survival benefit with these combination approaches has yet to be conclusively proven and awaits larger randomized trials.
In addition to the above, novel immunotherapeutic agents may also play a role in the treatment of elderly AML. The combination of azacytidine and nivolumab, a monoclonal antibody targeting the immune checkpoint PD-1, led to a complete response rate (CR + CRi) of 18% in patients with relapsed/refractory AML, who were primarily elderly, and OS in this trial compared favorably with historical controls treated with azacytidine-based salvage regimens [64]. While the response rates in this trial were less impressive than other combination strategies highlighted above, the median OS of 9.3 months in relapsed/refractory patients is promising and necessitates further investigation.
Treatment beyond remission
In younger patients who achieve a CR, there is clear evidence for a survival benefit from consolidation therapy including high-dose cytarabine (HIDAC) for those with core-binding factor leukemias, or allogeneic hematopoietic stem cell transplant (alloHSCT) for those with intermediate or poor-risk cytogenetics in first remission (Table 5) [65,66]. Unfortunately, there is a paucity of trials that specifically address the question of the optimal consolidation therapy for elderly AML patients. Consolidation chemotherapy with HIDAC has proven to be toxic for most elderly patients with only 29% completing four cycles due in part to increased neurotoxicity among elderly patients and no clear survival benefit compared to less intensive regimens [65]. A less intensive regimen using LDAC demonstrated a very modest benefit in 5-year DFS (13% vs. 7%, p = .006) compared to no consolidation therapy, but no benefit in 5-year OS (18% vs. 15%, p = .29). A nonrandomized study using intermediate-dose cytarabine 1 g/m2 intravenously every 12 h on days 1, 3, and 5 (IDAC) in elderly patients demonstrated a 5-year OS of 34% with 53% of patients completing all four cycles of treatment [67]. While subsequent randomized trials of this regimen are lacking, given its tolerability, IDAC is a reasonable consolidation regimen for elderly AML patients for whom alloHSCT is not indicated or a suitable donor cannot be found.
Table 5.
Treatment beyond remission.
| Trial/institution | Population | Regimen(s) | Survival | Comments |
|---|---|---|---|---|
| CALGB 8525 [65] | AML patients in CR following induction | HIDAC vs. Ara-C 100 mg/m2 CIV ×5 days vs. Ara-C 400 mg/m2 CIV × 5 days. All for four courses every 28 days | OS at 4 years = 46% vs. 35% vs. 31 % (p = .04). However, this benefit was not seen for patients age >60 | Only 29% of patients age 60+ could tolerate 4 courses of HIDAC, possibly due to significant neurotoxicity |
| Vienna [67] | AML patients age ≥60 in CR following induction | IDAC: Ara-C 1 g/m2 intravenously every 12 h on days 1, 3, and 5 | Median OS 31.8 months with 5-year OS of 34% | 53% of patients received all 4 cycles and an additional 19% received 3 cycles |
| HOVON AML-9 [80] | AML patients age >60 in CR following induction | Ara-C 10 mg/m2 subQ q12hrs on days 1–12 every 42 days ×8 cycles vs. no further therapy | 5-year DFS 13% vs. 7% (p = .006). 5-year OS 18% vs. 15% (p = .29) | No overall survival benefit |
| AML2004 from OSHO [69] | AML patients age 60–75 in CR following induction | Assigned to consolidation chemotherapy vs. HLA-matched allogeneic transplant vs. HLA-mismatched allogeneic transplant depending on donor availability | Leukemia-free survival at 9 years was 25% for those who underwent transplant vs. 14% for those who underwent consolidation (p < .001) | NRM was higher among those who underwent transplant. Relapse was significantly lower among those who underwent transplant (42% vs. 78%, p < .0001) |
| Hopkins [71] | Patients aged 50–75 who underwent allogeneic transplant | Nonmyeloablative T-cell replete haploidentical transplants with post-transplant cyclophosphamide | 3-year OS was similar by decade of life: 48% for patients in their 50s vs. 45% for patients in their 60s vs. 44% for patients in their 70s | 3-year DFS in AML for patients age ≥60 was 31% and OS was 38%. Age did not lead to more NRM |
| Fred Hutchinson [72] | Patients with AML who underwent allogeneic transplant | Nonmyeloablative conditioning with fludarabine and total body irradiation (TBI) followed by related or unrelated transplant | 5-year OS = 33% | Age did not have a statistically significant impact on relapse/progression or NRM |
| ALWP of EBMT [73] | Patients age >50 with AML who underwent allogeneic transplant | Reduced intensity conditioning followed by a HLA-matched unrelated or HLA partially mismatched unrelated transplant | 2-year LFS was 43.3% and 2-year OS was 48.4% | Age at transplant was a significant risk factor for NRM, LFS, and OS |
HIDAC: cytarabine 3 g/m2 q12hrs on days 1, 3, and 5.
IDAC: cytarabine 1 g/m2 q12hrs on days 1, 3, and 5.
While alloHSCT has proven beneficial for consolidation in younger AML patients with intermediate and poor-risk cytogenetics in first remission, this conclusion was drawn from studies that exclusively enrolled younger patients who could tolerate myeloablative conditioning [66]. Subsequent studies in middle-aged patients (40–60 years old) have shown that OS following transplantation is similar regardless of whether myeloablative or reduced intensity conditioning (RIC) is used [68]. These RIC regimens, which significantly reduce non-relapse mortality (NRM) at the expense of increased relapse risk, make transplant a viable option for elderly patients. The East German Study Group has shown that transplant in remission leads to significant improvements in leukemia-free survival (LFS) when compared to consolidation chemotherapy in patients aged 60–75 (25% vs. 14% at 9 years, p < .001) [69]. CALGB trial 100103 investigating RIC allogeneic transplantation in elderly patients with AML also demonstrated a promising 2-year DFS of 42% and OS of 48% [70]. Interestingly, separate retrospective analyses at Johns Hopkins Hospital and Fred Hutchinson Cancer Center demonstrated the feasibility of transplant in elderly patients and suggested that age was not a significant risk factor for NRM [71,72]. However, other studies have suggested that age is a risk factor for both NRM and poorer LFS [73]. These contradictory findings may be a consequence of differences in the preparatory and immunosuppressive regimens used in each study, but there is clearly ongoing debate about whether chronological age alone should be used to exclude patients from alloHSCT.
In addition to alloHSCT, a number of other immunotherapeutic approaches have been explored to prolong remissions including some studies focusing specifically on elderly AML patients. Rosenblatt et al. recently reported promising results from a study of a personalized cancer vaccine fusing patient-derived AML cells with autologous dendritic cells, which led to a marked, durable increase in circulating T cells that recognize whole AML cells and AML-specific antigens with limited toxic effects beyond injection site reactions. Seventeen patients received vaccination in combination with standard consolidation therapy, including 8 patients with European Leukemia Network-defined favorable risk disease and 3 patients with adverse risk disease, and 12 patients (71%) remained in remission at a median follow-up of 57 months [74]. Given the tolerability of this treatment and promising initial results, it may have implications for the treatment of elderly patients in the future. Similarly, a phase I trial of the anti-KIR monoclonal antibody IPH2101, which is designed to enhance natural kill cell cytotoxicity, in elderly AML patients in CR demonstrated an impressive 29.7-month median OS in patients treated at the two highest dose levels compared to just 11.8 months among those treated at lower dose levels [75]. As this treatment was also well tolerated, it may be an ideal agent for use in the elderly population. A similar anti-KIR antibody, lirilumab, is currently being investigated in combination with azacytidine in relapsed/refractory AML (NCT02399917).
Discussion/conclusions
The general approach to elderly patients with AML (Figure 2) begins with an assessment of their fitness for intensive induction chemotherapy based on PS, comorbidities, and age. Therapy in the form of a clinical trial is highly recommended when available. If a patient is deemed fit for intensive induction, then additional assessment for poor prognostic factors such as adverse cytogenetics; prior treatment with chemotherapy or radiation; or an antecedent hematologic disorder is merited, as these factors portend a poor response to conventional induction chemotherapy. Fit patients without poor prognostic factors should receive induction chemotherapy, while unfit patients should be considered for less intensive therapies. Fit patients with secondary or therapy-related AML derive significantly more benefit from induction with CPX-351 than conventional regimens such as 7 + 3. Thus, CPX-351 will become the preferred agent for induction in this patient population if/when it is approved. At the moment, there is a lack of Level I evidence to support approaches other than induction in fit patients with poor prognostic factors, although there are retrospective data that suggest that HMAs may have an important role [33,35].
Figure 2.
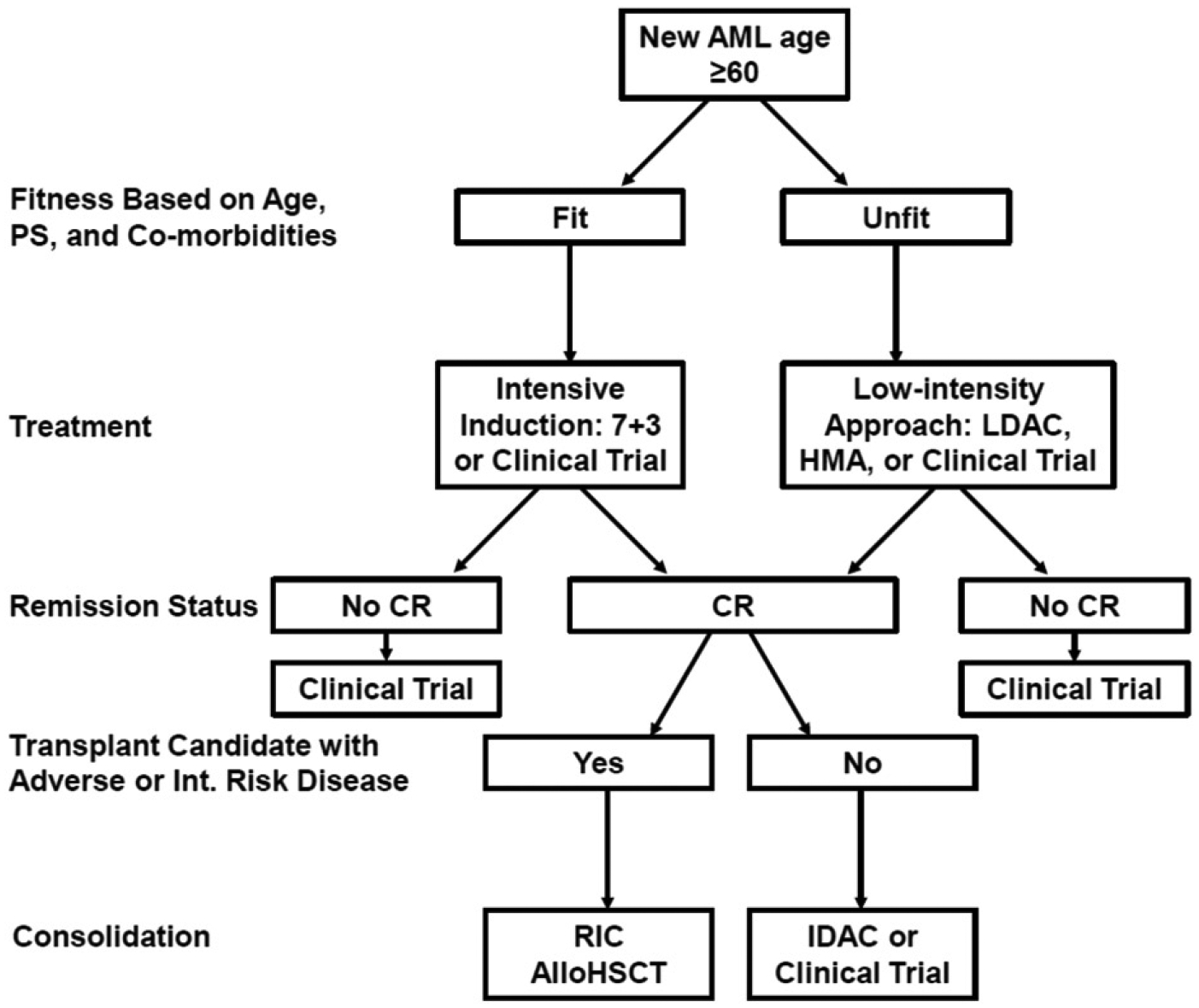
Approach to elderly AML.
Among unfit elderly AML patients, the two standard-of-care choices for less intensive treatment are LDAC and HMAs. HMAs generally lead to more remissions compared to LDAC and also confer a survival benefit for the majority of patients who fail to achieve remission. Thus, HMAs are often preferable to LDAC. Numerous recent trials have suggested the greatly increased efficacy of LDAC and HMAs when combined with other agents such as venetoclax or vadastuximab talirine. Larger, randomized trials are needed to verify these results before they become standard in this patient population. Furthermore, the impressive initial efficacy results with these approaches, especially among patients with poor prognostic factors, suggest that they may eventually replace conventional induction in certain populations of fit, elderly patients. This would represent a paradigm shift in the treatment of elderly AML, where the inability to increase treatment intensity has long been felt to be a major obstacle to achieving better outcomes.
The primary goal of therapy in fit elderly patients is to achieve a complete pathologic remission, and if a patient is able to achieve remission, then subsequent consolidation is indicated. AlloHSCT is the standard approach for younger patients with intermediate- or poor-risk cytogenetics, and the safety and efficacy of RIC transplants allow this same paradigm to be applied in certain elderly patients. For fit, elderly patients with core-binding factor leukemias and those who lack a suitable donor, consolidation chemotherapy with IDAC should be considered. A number of trials have also investigated the utility of HMAs as maintenance therapies for patients in remission including those who have completed consolidation, but these studies have failed to demonstrate a clear benefit to further treatment [76,77]. While each of these consolidation approaches have been used in patients in complete pathologic remission, there is now clear evidence that the depth of response to initial induction, as measured by assays for MRD, correlates with the durability of response and long-term survival [45]. Thus, numerous ongoing trials are investigating the utility of MRD measurement to better tailor post-remission consolidation therapies.
Long-term survival in elderly AML has improved markedly over the last 4 decades from just 1–2% in the 1970s and early 1980s to nearly 20% today [78]. This improvement can be attributed to many different factors including an increased willingness to use intensive induction in this patient population, better low-intensity therapies for unfit patients, improved consolidation approaches including increased use of RIC alloHSCT, and better supportive care. Unfortunately, the vast majority of elderly AML will still die of their disease, so there are opportunities for further improvement. Specifically, recently reported trials of novel low-intensity treatment combinations using HMAs or LDAC in combination with venetoclax or vadastuximab show great promise, while CPX-351 leads to improved outcomes with intensive induction for certain high-risk patients.
Supplementary Material
Funding
KWP’s effort on these studies was supported as a co-investigator for translational science team at Johns Hopkins (UM1 CA186691) and cancer center support grant (P30 CA006973).
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article online at https://doi.org/10.1080/10428194.2017.1330956.
References
- [1].American Cancer Society. Cancer facts and figures 2017 [Internet]. Atlanta, GA: American Cancer Society; 2016. [Google Scholar]
- [2].SEER cancer stat facts: acute myeloid leukemia [Internet]. Bethesda, MD: National Cancer Institute; 2017. [updated April 2017; cited May 2, 2017]. Available from: http://seer.cancer.gov/statfacts/html/amyl.html [Google Scholar]
- [3].Juliusson G, Antunovic P, Derolf A, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179–4187. [DOI] [PubMed] [Google Scholar]
- [4].Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Burnett AK, Russell NH, Hills RK, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16:1295–1305. [DOI] [PubMed] [Google Scholar]
- [6].Giles FJ, Borthakur G, Ravandi F, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007;136:624–627. [DOI] [PubMed] [Google Scholar]
- [7].Klepin HD. Elderly acute myeloid leukemia: assessing risk. Curr Hematol Malig Rep. 2015;10:118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kantarjian H, O’Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–1098. [DOI] [PubMed] [Google Scholar]
- [9].Wheatley K, Brookes CL, Howman AJ, et al. Prognostic factor analysis of the survival of elderly patients with AML in the MRC AML11 and LRF AML14 trials. Br J Haematol. 2009;145:598–605. [DOI] [PubMed] [Google Scholar]
- [10].Malfuson JV, Etienne A, Turlure P, et al. Risk factors and decision criteria for intensive chemotherapy in older patients with acute myeloid leukemia. Haematologica. 2008;93:1806–1813. [DOI] [PubMed] [Google Scholar]
- [11].Krug U, Rollig C, Koschmieder A, et al. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: a web-based application for prediction of outcomes. Lancet. 2010;376:2000–2008. [DOI] [PubMed] [Google Scholar]
- [12].Rollig C, Thiede C, Gramatzki M, et al. A novel prognostic model in elderly patients with acute myeloid leukemia: results of 909 patients entered into the prospective AML96 trial. Blood. 2010;116:971–978. [DOI] [PubMed] [Google Scholar]
- [13].Lowenberg B, Zittoun R, Kerkhofs H, et al. On the value of intensive remission-induction chemotherapy in elderly patients of 65+ years with acute myeloid leukemia: a randomized phase III study of the European Organization for Research and Treatment of Cancer Leukemia Group. J Clin Oncol. 1989;7: 1268–1274. [DOI] [PubMed] [Google Scholar]
- [14].Lowenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361:1235–1248. [DOI] [PubMed] [Google Scholar]
- [15].Burnett AK, Russell NH, Hills RK, et al. A randomized comparison of daunorubicin 90mg/m2 vs 60mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood. 2015;125:3878–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Röllig C, Kramer M, Gabrecht M, et al. Randomized comparison of intermediate-dose cytarabine plus mitoxantrone (IMA) versus standard-dose cytarabine plus daunorubicin (DA) for induction therapy in AML patients >60 years. Results from the SAL 60+ Trial. Blood. 2015;126:222.26031918 [Google Scholar]
- [17].Niederwieser D, Hoffmann VS, Pfirrmann M, et al. Comparison of treatment strategies in patients over 60 years with AML: final analysis of a prospective randomized German AML Intergroup Study. Blood. 2016;128:1066. [Google Scholar]
- [18].Muller-Tidow C, Tschanter P, Rollig C, et al. Azacitidine in combination with intensive induction chemotherapy in older patients with acute myeloid leukemia: the AML-AZA trial of the Study Alliance Leukemia. Leukemia. 2016;30:555–561. [DOI] [PubMed] [Google Scholar]
- [19].Burnett AK, Russell NH, Hills RK, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. JCO. 2012;30:3924–3931. [DOI] [PubMed] [Google Scholar]
- [20].Hills RK, Castaigne S, Appelbaum FR, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15: 986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Petersdorf SH, Kopecky KJ, Slovak M, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013; 121: 4854–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Attar EC, Johnson JL, Amrein PC, et al. Bortezomib added to daunorubicin and cytarabine during induction therapy and to intermediate-dose cytarabine for consolidation in patients with previously untreated acute myeloid leukemia age 60 to 75 years: CALGB (Alliance) study 10502. JCO. 2013;31:923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Foran JM, Sun Z, Claxton DF, et al. North American Leukemia, Intergroup Phase III Randomized Trial of single agent clofarabine as induction and post-remission therapy, and decitabine as maintenance therapy in newly-diagnosed acute myeloid leukemia in older adults (age ≥60 years): a trial of the ECOG-ACRIN Cancer Research Group (E2906). Blood. 2015; 126:217. [Google Scholar]
- [24].Foran JM, Sun Z, Claxton DF, et al. Importance of achieving complete remission (CR) after intensive therapy for acute myeloid leukemia (AML) in older adults age ≥60 years: analysis of risk factors for early mortality and re-induction, and impact of quality of response on overall survival (OS) in the ECOG-ACRIN E2906 Randomized Trial. Blood. 2016;128:339. [Google Scholar]
- [25].Lancet JE, Cortes JE, Hogge DE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014;123:3239–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Lancet JE, Uy GL, Cortes JE, et al. Final results of a phase III randomized trial of CPX-351 versus 7 + 3 in older patients with newly diagnosed high risk (secondary) AML. J Clin Oncol. 2016;34:Abstract 7000. [Google Scholar]
- [27].Burnett AK, Milligan D, Prentice AG, et al. A comparison of low-dose cytarabine and hydroxyurea with or without all-trans retinoic acid for acute myeloid leukemia and high-risk myelodysplastic syndrome in patients not considered fit for intensive treatment. Cancer. 2007;109:1114–1124. [DOI] [PubMed] [Google Scholar]
- [28].Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126:291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Quintas-Cardama A, Ravandi F, Liu-Dumlao T, et al. Epigenetic therapy is associated with similar survival compared with intensive chemotherapy in older patients with newly diagnosed acute myeloid leukemia. Blood. 2012;120:4840–4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30:2670–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ritchie EK, Feldman EJ, Christos PJ, et al. Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leuk Lymphoma. 2013;54: 2003–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Blum W, Garzon R, Klisovic RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci USA. 2010;107:7473–7478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Welch JS, Petti AA, Miller CA, et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med. 2016;375:2023–2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Cashen AF, Schiller GJ, O’Donnell MR, et al. Multicenter, phase II study of decitabine for the first-line treatment of older patients with acute myeloid leukemia. JCO. 2010;28:556–561. [DOI] [PubMed] [Google Scholar]
- [35].Ravandi F, Issa JP, Garcia-Manero G, et al. Superior outcome with hypomethylating therapy in patients with acute myeloid leukemia and high-risk myelodysplastic syndrome and chromosome 5 and 7 abnormalities. Cancer. 2009;115:5746–5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Rucker FG, Schlenk RF, Bullinger L, et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood. 2012;119:2114–2121. [DOI] [PubMed] [Google Scholar]
- [37].Bowen D, Groves MJ, Burnett AK, et al. TP53 gene mutation is frequent in patients with acute myeloid leukemia and complex karyotype, and is associated with very poor prognosis. Leukemia. 2009;23:203–206. [DOI] [PubMed] [Google Scholar]
- [38].Khan M, Kantarjian HM, Garcia-Manero G, et al. Randomized phase II trial of two schedules of decitabine as frontline therapy in elderly patients with acute myeloid leukemia ineligible for standard cytotoxic induction regimens. Blood. 2016;128:1612. [Google Scholar]
- [39].Kantarjian HM, Roboz GJ, Kropf PL, et al. Comparison of efficacy and safety results in 103 treatment-naïve acute myeloid leukemia (TN-AML) patients not candidates for intensive chemotherapy using 5-day and 10-day regimens of guadecitabine (SGI-110), a novel hypomethylating agent (HMA). Blood 2015;126:458. [Google Scholar]
- [40].Burnett AK, Russell NH, Hunter AE, et al. Clofarabine doubles the response rate in older patients with acute myeloid leukemia but does not improve survival. Blood. 2013;122:1384–1394. [DOI] [PubMed] [Google Scholar]
- [41].Dennis M, Russell N, Hills RK, et al. Vosaroxin and vosaroxin plus low-dose Ara-C (LDAC) vs low-dose Ara-C alone in older patients with acute myeloid leukemia. Blood. 2015;125:2923–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Burnett AK, Russell N, Hills RK, et al. A randomised comparison of the novel nucleoside analogue sapacitabine with low-dose cytarabine in older patients with acute myeloid leukaemia. Leukemia. 2015;29: 1312–1319. [DOI] [PubMed] [Google Scholar]
- [43].Bixby DL, Stein AS, Fathi AT, et al. Vadastuximab talirine monotherapy in older patients with treatment naive CD33-positive acute myeloid leukemia (AML). Blood. 2016;128:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fathi AT, Erba HP, Lancet JE, et al. Vadastuximab talirine plus hypomethylating agents: a well-tolerated regimen with high remission rate in frontline older patients with acute myeloid leukemia (AML). Blood. 2016;128:591. [Google Scholar]
- [45].Freeman SD, Virgo P, Couzens S, et al. Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. JCO. 2013;31: 4123–4131. [DOI] [PubMed] [Google Scholar]
- [46].Koszarska M, Bors A, Feczko A, et al. Type and location of isocitrate dehydrogenase mutations influence clinical characteristics and disease outcome of acute myeloid leukemia. Leuk Lymphoma. 2013;54: 1028–1035. [DOI] [PubMed] [Google Scholar]
- [47].DiNardo C, de Botton S, Pollyea DA, et al. Molecular profiling and relationship with clinical response in patients with IDH1 mutation-positive hematologic malignancies receiving AG-120, a first-in-class potent inhibitor of mutant IDH1, in addition to data from the completed dose escalation portion of the phase 1 study. Blood. 2015;126:1306. [Google Scholar]
- [48].Ohanian M, Garcia-Manero G, Jabbour EJ, et al. Combination of sorafenib and 5-azacytidine in older patients with untreated acute myeloid leukemia with FLT3-ITD mutation. Blood. 2016; 128:1611. [Google Scholar]
- [49].Badar T, Kantarjian HM, Nogueras-Gonzalez GM, et al. Improvement in clinical outcome of FLT3 ITD mutated acute myeloid leukemia patients over the last one and a half decade. Am J Hematol. 2015;90:1065–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Burnett AK, Hills RK, Hunter A, et al. The addition of arsenic trioxide to low-dose Ara-C in older patients with AML does not improve outcome. Leukemia 2011;25:1122–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Burnett AK, Russell NH, Culligan D, et al. The addition of the farnesyl transferase inhibitor, tipifarnib, to low dose cytarabine does not improve outcome for older patients with AML. Br J Haematol. 2012;158:519–522. [DOI] [PubMed] [Google Scholar]
- [52].Burnett AK, Hills RK, Hunter AE, et al. The addition of gemtuzumab ozogamicin to low-dose Ara-C improves remission rate but does not significantly prolong survival in older patients with acute myeloid leukaemia: results from the LRF AML14 and NCRI AML16 pick-a-winner comparison. Leukemia 2013;27:75–81. [DOI] [PubMed] [Google Scholar]
- [53].Lancet JE, Gojo I, Gotlib J, et al. A phase 2 study of the farnesyltransferase inhibitor tipifarnib in poor-risk and elderly patients with previously untreated acute myelogenous leukemia. Blood. 2007;109:1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Harousseau JL, Martinelli G, Jedrzejczak WW, et al. A randomized phase 3 study of tipifarnib compared with best supportive care, including hydroxyurea, in the treatment of newly diagnosed acute myeloid leukemia in patients 70 years or older. Blood. 2009;114:1166–1173. [DOI] [PubMed] [Google Scholar]
- [55].Larson RA, Boogaerts M, Estey E, et al. Antibody-targeted chemotherapy of older patients with acute myeloid leukemia in first relapse using Mylotarg (gemtuzumab ozogamicin). Leukemia. 2002;16: 1627–1636. [DOI] [PubMed] [Google Scholar]
- [56].Roboz GJ, Ritchie EK, Curcio T, et al. Arsenic trioxide and low-dose cytarabine in older patients with untreated acute myeloid leukemia, excluding acute promyelocytic leukemia. Cancer. 2008;113:2504–2511. [DOI] [PubMed] [Google Scholar]
- [57].Wei A, Strickland SA, Roboz GJ, et al. Safety and efficacy of venetoclax plus low-dose cytarabine in treatment-naive patients aged ≥65 years with acute myeloid leukemia. Blood. 2016;128:102. [Google Scholar]
- [58].Pollyea DA, Dinardo CD, Thirman MJ, et al. Results of a phase 1b study of venetoclax plus decitabine or azacitidine in untreated acute myeloid leukemia patients ≥65 years ineligible for standard induction therapy. J Clin Oncol. 2016;128:34. [Google Scholar]
- [59].Daver N, Kantarjian HM, Garcia-Manero G, et al. Phase I/II study of Vosaroxin and decitabine in newly diagnosed older patients (pts) with acute myeloid leukemia (AML) and high risk myelodysplastic syndrome (MDS). Blood. 2015;126:461. [Google Scholar]
- [60].Pollyea DA, Zehnder J, Coutre S, et al. Sequential azacitidine plus lenalidomide combination for elderly patients with untreated acute myeloid leukemia. Haematologica. 2013;98:591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Swords RT, Coutre S, Maris MB, et al. Results of a clinical study of pevonedistat (Pev), a first-in-class NEDD8-activating enzyme (NAE) inhibitor, combined with azacitidine (Aza) in older patients (Pts) with acute myeloid leukemia (AML). Blood. 2016;128:98. [Google Scholar]
- [62].Garcia Manero G, Atallah E, Khaled SK, et al. A phase 2 study of pracinostat and azacitidine in elderly patients with acute myeloid leukemia (AML) not eligible for induction chemotherapy: response and long-term survival benefit. Blood. 2016;128:100. [Google Scholar]
- [63].Craddock C, Houlton AE, Ferguson P, et al. Vorinostat does not improve outcome in patients with acute myeloid leukemia and high risk myelodysplasia treated with azacitidine: results of the UK Trials Acceleration Programme Ravva Trial. Blood. 2016; 128:1065. [Google Scholar]
- [64].Daver N, Basu S, Garcia-Manero G, et al. Phase IB/II study of nivolumab in combination with azacytidine (AZA) in patients (pts) with relapsed acute myeloid leukemia (AML). Blood. 2016;128:763.27354720 [Google Scholar]
- [65].Mayer RJ, Davis RB, Schiffer CA, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. N Engl J Med. 1994;331:896–903. [DOI] [PubMed] [Google Scholar]
- [66].Koreth J, Schlenk R, Kopecky KJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301: 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Sperr WR, Piribauer M, Wimazal F, et al. A novel effective and safe consolidation for patients over 60 years with acute myeloid leukemia: intermediate dose cytarabine (2 × 1 g/m2 on days 1, 3, and 5). Clin Cancer Res. 2004;10:3965–3971. [DOI] [PubMed] [Google Scholar]
- [68].Passweg JR, Labopin M, Cornelissen J, et al. Conditioning intensity in middle-aged patients with AML in first CR: no advantage for myeloablative regimens irrespective of the risk group-an observational analysis by the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2015;50:1063–1068. [DOI] [PubMed] [Google Scholar]
- [69].Niederwieser D, Al-Ali HK, Krahl R, et al. Hematopoietic stem cell transplantation (HSCT) compared to consolidation chemotherapy (CT) to increase leukemia free survival (LFS) in acute myelogenous leukemia (AML) patients between 60 and 75 years irrespective of genetic risk: report from the AML 2004 of the East German Study Group (OSHO). J Clin Oncol. 2016;34:e18501. [Google Scholar]
- [70].Devine SM, Owzar K, Blum W, et al. Phase II study of allogeneic transplantation for older patients with acute myeloid leukemia in first complete remission using a reduced-intensity conditioning regimen: results from Cancer and Leukemia Group B 100103 (Alliance for Clinical Trials in Oncology)/Blood and Marrow Transplant Clinical Trial Network 0502. JCO. 2015;33:4167–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kasamon YL, Bolanos-Meade J, Prince GT, et al. Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose posttransplantation cyclophosphamide in older adults. J Clin Oncol. 2015;33:3152–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gyurkocza B, Storb R, Storer BE, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. JCO. 2010;28:2859–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Rubio MT, Savani BN, Labopin M, et al. The impact of HLA-matching on reduced intensity conditioning regimen unrelated donor allogeneic stem cell transplantation for acute myeloid leukemia in patients above 50 years – a report from the EBMT acute leukemia working party. J Hematol Oncol. 2016;9:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Rosenblatt J, Stone RM, Uhl L, et al. Individualized vaccination of AML patients in remission is associated with induction of antileukemia immunity and prolonged remissions. Sci Transl Med. 2016;8:368ra171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Vey N, Bourhis JH, Boissel N, et al. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012;120:4317–4323. [DOI] [PubMed] [Google Scholar]
- [76].Blum W, Sanford BL, Klisovic R, et al. Maintenance therapy with decitabine in younger adults with acute myeloid leukemia in first remission: a phase 2 Cancer and Leukemia Group B Study (CALGB 10503). Leukemia. 2017;31:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Boumber Y, Kantarjian H, Jorgensen J, et al. A randomized study of decitabine versus conventional care for maintenance therapy in patients with acute myeloid leukemia in complete remission. Leukemia. 2012;26:2428–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Burnett AK. Treatment of acute myeloid leukemia: are we making progress? Hematology Am Soc Hematol Educ Program. 2012;2012:1–6. [DOI] [PubMed] [Google Scholar]
- [79].Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Lowenberg B, Suciu S, Archimbaud E, et al. Mitoxantrone versus daunorubicin in induction-consolidation chemotherapy – the value of low-dose cytarabine for maintenance of remission, and an assessment of prognostic factors in acute myeloid leukemia in the elderly: final report. European Organization for the Research and Treatment of Cancer and the Dutch-Belgian Hemato-Oncology Cooperative Hovon Group. J Clin Oncol. 1998;16:872–881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


