Abstract
Pulmonary arterial hypertension (PAH) is characterized by endothelial dysfunction but there are no established methods to study pulmonary artery endothelial cells (PAECs) from living patients. We sought to culture PAECs from pulmonary artery catheter (PAC) balloons used during right heart catheterization (RHC), to make observations about successful culture attempts, and to characterize cultured PAEC behavior.
PAECs were grown in primary culture to confluence and endothelial cell phenotype was confirmed. Standard assays for apoptosis, migration, and tube formation were performed on cells between passage 3 – 8. We collected a total of 49 PAC tips from 45 subjects with successful PAEC culture from 19 (39%) balloons. There were no differences in subject demographic or RHC procedural details in successful versus unsuccessful attempts. There was a higher but nonsignificant proportion of successful (10/19, 53%) vs. unsuccessful (9/30, 30%) attempts from subjects who met hemodynamic criteria for PAH (p = 0.10). Successful culture was more likely in subjects with lower cardiac index (p = 0.03) and higher pulmonary vascular resistance (p = 0.04). Idiopathic PAH PAECs were apoptosis resistant compared to commercial PAECs (p = 0.04) and had reduced migration compared to portopulmonary hypertension, high cardiac output PAECs (p = 0.01). HIV-associated PAH PAECs formed fewer (p = 0.01) and shorter (p = 0.02) branch vessel networks as compared to commercial PAECs.
Sustained culture and characterization of PAECs from RHC balloons is feasible, especially in PAH with high hemodynamic burden. This technique may provide insight into endothelial cell dysfunction during PAH pathogenesis.
Keywords: endothelial cells, cell culture techniques, pulmonary artery, hypertension, pulmonary, apoptosis
INTRODUCTION
Pulmonary arterial hypertension (PAH) is a progressive disease of the pulmonary circulation characterized by endothelial dysfunction. Aberrant pulmonary arterial endothelial cells (PAECs) are a hallmark of disease pathogenesis (1-3), but controversy exists about whether cellular proliferation or apoptosis dominate in the pulmonary vasculature. The balance between neoangiogenesis and vascular rarefaction may shift over a patient’s disease course (4). Current methodologies to study human PAECs include harvested cells from the explanted or post-mortem lungs of PAH patients, commercially available non-diseased PAECs, and the differentiation of endothelial cells from peripheral blood or dermal biopsies (5-7). While these methods have strengths, cells are sourced from end-stage PAH patients, non-diseased humans, or outside of the pulmonary vasculature. There is a critical need for a reliable method to culture and expand PAECs from living patients to examine changes in endothelial cell function over time and in response to current and investigational PAH therapies.
Right heart catheterization (RHC) is the fundamental diagnostic procedure in pulmonary vascular disease evaluation and is often repeated throughout a PAH patient’s disease course to guide therapeutic decisions. This procedure could provide a source of primary cells to be propagated ex vivo and an opportunity to collect PAECs across repetitive samples from patients at variable stages of disease. A single report described the successful collection of cells with endothelial cell phenotype from pulmonary artery catheter (PAC) balloons in 24 patients with PAH for as long as 20 days in culture, however it was unclear if this method could result in enough cells to perform functional experiments without phenotypic transformation (8). We set out to advance this methodology and to determine 1) the features of subjects and procedures in whom this technique most often yielded robust numbers of viable PAECs, 2) the most successful harvest techniques and culture conditions that resulted in cell survival and propagation, and 3) how these cells performed in standard assessments of endothelial function. We hypothesized that this method would be feasible but not in all subjects and that the behavior and function of these isolated cells could be further characterized. For this initial study, it was not our intent to link PAH disease state or subtype to PAEC phenotype.
METHODS
Study Sample
All patients referred to the Rhode Island Hospital Pulmonary Hypertension Center undergoing RHC for the purposes of pulmonary vascular disease evaluation and management were eligible. We included all individuals undergoing RHC irrespective of their hemodynamics and clinical background in an effort to gain knowledge about patient- and procedure-related characteristics more or less likely to result in successful PAEC harvest and sustained culture. Subjects with serial catheterizations during the study period were also included. The RHC procedure was not altered for the purposes of the study, nor was the number of times the catheter was manipulated or “wedged”. All procedures were performed from the internal jugular position. Provocative maneuvers during RHC such as testing for vasoresponsiveness, exercise or fluid challenge were left to the discretion of the treating pulmonary hypertension (PH) clinician (CEV, CJM, JRK). In our center we typically conduct vasoreactivity testing in all patients with suspected or confirmed pre-capillary PH on both index and follow-up RHC. The study was approved by the Lifespan Institutional Review Board (IRB #016311) and informed consent was obtained from all participants. Medical records or our research registry were reviewed for clinical data which was collected at the time of or as close as possible to (within six months) RHC.
Retention of PAC Balloon Tips and Primary Culture
At the end of the RHC procedure, the PAC was retracted into the catheter sheath and both catheter and sheath were removed from the patient. The tip of the catheter was then advanced out of the sheath and placed directly into warm media (37°C) (EndoGRO; Millipore Sigma, Billerica, MA) with the balloon deflated. The rest of the PAC was then cut away and the tips in media were transported directly to the laboratory initially at ambient temperature. Over the course of the study we noted that transport time and temperature change may have been factors in successful culture and began placing the warm media containing the balloon tip immediately into a heater (Therapak Duramark Thermoelectric Warmer Part No. 365163, 18 quart/12-volt powered compact warmer custom preset to 37 °C; Claremont, CA) for travel to the laboratory. The PAC tip with balloon was placed directly into one well of a 24 well plate with Attachment Factor Solution (Cell Applications, Inc; San Diego, CA) and media (EndoGRO-vascular endothelial growth factor [VEGF] complete media kit; Millipore Sigma, Billerica, MA). The PAC tip was washed with fresh media every two days as cells attached to the cell culture dish and previous media from the tip well was moved into a new well in the 24 well plate. Cells were not treated with trypsin. Cells were grown in primary culture to confluence over two to three weeks. Cells were then seeded onto T-25 and T-75 flasks until passaged cells reached confluence over the next four to five days. After the third passage, cells were either directly characterized or frozen prior to further analysis.
Only the PH clinician conducting the RHC was aware of subject and procedural characteristics. Once the balloon tips were transferred to the laboratory, all study staff involved in further experiments (primary PAEC culture and conduct of the characterization assays) remained blinded to subject clinical data, RHC procedural details, and hemodynamic values.
Confirmation of PAEC Phenotype and Characterization Assays
Primary PAECs obtained from catheter tips were screened for the expression of endothelial markers. Flow cytometry and fluorescence-activated cell sorting was utilized as a first step to confirm endothelial cell surface receptor pattern (CD146+/CD31+/CD45−) (data not shown). Once culture with cell confluence was achieved on recurrent PAC tip samples, we used a routine screening panel including von Willebrand factor (vWF) (Dako; Carpinteria, CA), vascular endothelial (VE)-cadherin (Santa Cruz Biotechnologies; Santa Cruz, CA) staining and acetylated low density lipoprotein (AcLDL)(ThermoFisher Scientific; Waltham, MA) uptake to confirm PAEC phenotype. The cells were also stained for alpha-smooth muscle cell actin (α-SMA) (Abcam; Cambridge, MA) as a negative control. Additional negative controls included staining human lung smooth muscle cells (Lonza; Pearland, TX) and normal human lung fibroblasts (Lonza, Pearland, TX).
We considered cells to be endothelial in origin if all cells expressed all three endothelial markers (vWF, VE-cadherin, AcLDL), all cells were negative for α-SMA and were able to generate Matrigel networks in vitro, as below. An attempt to culture PAECs from a balloon tip was considered successful if 1) cells grew to confluence, 2) remained viable in primary culture for at least two to three weeks, and 3) viable cells at two to three weeks exhibited an endothelial phenotype and expressed endothelial cell markers as described above and in the Online Supplementary Material. All studies of endothelial phenotype and function were done with PAECs between passages 3 – 8. Phenotypic drift was noted beyond passage nine with subjective changes in PAEC morphology via phase microscopy, immunofluorescence staining of endothelial cell markers, and/or rates of proliferation. Technical replicates were performed such that each experiment included PAECs derived from a different passage of cells from a single balloon tipped catheter from one RHC procedure for each subject.
Commercially available PAECs were purchased from Lonza Biologics, Inc (Basel, Switzerland) or PromoCell (Heidelberg, Germany). These cells were maintained in the same manner as outgrown PAECs from PAC balloons and used as ‘normal’ control PAECs for all experiments.
Standard TUNEL, scratch migration and Matrigel tube formation assays were used to assess apoptosis, migration, and tube formation, respectively (9-11). Detailed methods are included in the Online Supplementary Material.
Statistical Analysis
Continuous data are presented as median (interquartile range [IQR]) and categorical data are presented as frequency (percentage). Wilcoxon-Mann-Whitney tests were used to compare continuous variables and chi-square or Fisher’s exact tests were used to compare categorical variables, as appropriate. Characterization assays were compared with two-way analysis of variance (ANOVAs) with correction for multiple comparisons. All hypotheses were tested using two-tailed tests. Alpha was established at the 0.05 level.
RESULTS
Subject and Procedural Factors and Culture Success
A total of 49 PAC tips were collected from 45 subjects with successful culture of PAECs from 19 (39%) balloons using our current protocol. Table 1 describes subject and procedural characteristics by PAEC culture success. Subject demographics and anthropometrics were similar in successful versus unsuccessful attempts. There were no differences in the number of clinical PAH diagnoses (i.e., previous World Health Organization [WHO] Group 1 PAH designation by a PH specialist), WHO functional class, or targeted PAH therapy use at the time of catheterization. Shorter six-minute walk distance (6MWD) may have been associated with culture success (355 m [122 – 410 m] in successful attempts vs. 375 m [295 – 462 m] in unsuccessful attempts; p = 0.09). There was a higher proportion of classical hemodynamic PAH (i.e., mPAP ≥ 25 mm Hg, mean pulmonary capillary wedge pressure [PCWP] ≤ 15 mm Hg, and pulmonary vascular resistance [PVR] > 3 Wood units) among successful (10/19, 53%) versus unsuccessful (9/30, 30%) attempts, although this difference was not statistically significant (p = 0.10). Results were unchanged when the mPAP cut-off was lowered to 20 mm Hg (12). Successful culture of PAECs from a balloon tipped PAC was more likely in subjects who had more severe hemodynamic impairment at the time of RHC as evidenced by significantly lower cardiac output (p = 0.04), cardiac index (p = 0.03), and higher PVR (p = 0.04). Procedural details such as the number of times the PAC was “wedged”, the specific operator (i.e., the clinician with the most experience performing RHC), and the transit time ex vivo to the culture dish did not influence success. We used a heater for transport of six balloons toward the end of the study, which may have increased success rate (p = 0.19).
Table 1.
Subject and procedural characteristics by pulmonary artery endothelial cell culture success
| Total Sample | Success | No Success | P value | |
|---|---|---|---|---|
| Balloon tips, n (%) | 49 | 19 (39) | 30 (61) | |
| Subject-level characteristics | ||||
| Age, yr | 62 (53 – 71) | 69 (54 – 78) | 61 (52 – 67) | 0.16 |
| Male sex, n (%) | 17 (35) | 8 (42) | 9 (30) | 0.29 |
| Race, n (%) | 0.86 | |||
| White | 34 (69) | 13 (68) | 21 (70) | |
| Black | 11 (22) | 4 (21) | 7 (23) | |
| Other | 3 (6) | 2 (11) | 1 (3) | |
| Body mass index, kg/m2 | 30 (25 – 33) | 32 (27 – 34) | 29 (24 – 33) | 0.25 |
| Clinical PAH diagnosis | 22 (45) | 8 (42) | 14 (47) | 0.49 |
| PAH treatment, n (%) | 19 (39) | 6 (32) | 13 (43) | 0.30 |
| Six-minute walk distance, m | 365 (182 – 425) | 355 (122 – 410) | 375 (295 – 462) | 0.09 |
| Functional class III/IV, n (%) | 15 (47) | 6 (46) | 9 (47) | 1.00 |
| No. available | 32 | 13 | 19 | |
| Brain natriuretic peptide, pg/mL | 80 (23 – 390) | 76 (32 – 413) | 80 (22 – 184) | 0.71 |
| No. available | 42 | 26 | 16 | |
| Hemodynamic PH, n (%) | 39 (80) | 16 (84) | 23 (77) | 0.40 |
| Hemodynamic PAH, n (%) | 19 (39) | 10 (53) | 9 (30) | 0.10 |
| Hemodynamics | ||||
| RAP, mm Hg | 9 (7 – 12) | 10(7 – 14) | 9 (6 – 11) | 0.40 |
| mPAP, mm Hg | 37 (25 – 46) | 38 (26 – 48) | 36 (25 – 42) | 0.34 |
| PCWP, mm Hg | 12 (10 – 16) | 13 (9 – 15) | 12 (10 – 16) | 0.77 |
| CO, L/min | 5.5 (4.4 – 7.7) | 4.7 (4.0 – 5.6) | 6.4 (4.7 – 8.1) | 0.04 |
| CI, L/min/m2 | 2.3 (2.1 – 3.5) | 2.3 (2.1 – 3.5) | 3.2 (2.8 – 4.0) | 0.03 |
| PVR, Wood units | 3.6 (1.9 – 6.5) | 5.1 (2.2 – 8.3) | 2.4 (1.5 – 6.2) | 0.04 |
| Procedural-level characteristics | ||||
| Provocative testing*, n (%) | 33 (67) | 14 (74) | 19 (63) | 0.64 |
| Most experienced operator, n (%) | 19 (39) | 7 (37) | 12 (40) | 0.77 |
| Morning collection†, n (%) | 33 (67) | 14 (74) | 19 (63) | 0.54 |
| Transit time‡, minutes | 28 (20 – 40) | 28 (25 – 40) | 28 (19 – 49) | 0.77 |
| Transport conditions, n (%) | ||||
| Media 37°C, no heater, n (%) | 43 (88) | 15 (35) | 28 (65) | 0.19 |
| Media 37°C, heater 37°C, n (%) | 6 (12) | 4 (67) | 2 (33) | |
Data represented as n (%) or median (IQR). Wilcoxon-Mann-Whitney tests were used to compare continuous variables and chi-square or Fisher’s exact tests were used to compare categorical variables, as appropriate. hemodynamic values taken from baseline assessment. All catheterizations were performed from the internal jugular position.
Maneuver such as vasoreactivity or exercise testing requiring >1 “wedging” of the catheter.
Pulmonary artery catheter tip collected before 12 pm.
Ex vivo to culture dish. PH=pulmonary hypertension, defined as mean pulmonary artery pressure (mPAP) ≥ 25 mmHg; PAH=pulmonary arterial hypertension, defined as mPAP ≥ 25 mmHg, pulmonary capillary wedge pressure (PCWP) ≤ 15 mmHg and pulmonary vascular resistance (PVR) > 3 Wood units. RAP=right atrial pressure; CO=cardiac output; CI=cardiac index.
Detailed characteristics of the subjects with PAEC culture success are provided in Table 2. Three subjects had more than one RHC during the study period. Subject 7 in Table 2 (a 56-year-old woman with portopulmonary hypertension) had three catheterizations, one of which yielded successfully cultured PAECs. We cultured PAECs from only one of two PAC tips from subject 10 (a 58-year-old man with human immunodeficiency virus [HIV] infection and chronic obstructive pulmonary disease). In both cases, there were no changes to the study protocol between procedures and PAH medications and hemodynamics were unchanged and similar, respectively, across procedures. Results were unchanged when these subjects were excluded from analyses. A third subject with systemic sclerosis associated-PAH (data not shown) had two RHCs but we were unsuccessful in culturing PAECs from both balloons.
Table 2.
Detailed characteristics of subjects with pulmonary arterial endothelial cell culture success
| No. | Age | Sex | Diagnosis | RAP, mm Hg |
mPAP, mm Hg |
PCWP, mm Hg |
CO, L/min |
PVR, Wood units |
PAH treatment, time of procedure |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 75 | F | PAPVR | 12 | 26 | 14 | 9.9 | 1.2 | ERA |
| 2 | 70 | M | Systemic sclerosis | 17 | 47 | 8 | 4.7 | 8.3 | None |
| 3 | 79 | F | CPC-PH | 13 | 41 | 22 | 3.8 | 5.0 | None |
| 4 | 78 | M | Anti-synthetase syndrome, ILD | 14 | 53 | 15 | 3.9 | 9.7 | None |
| 5 | 37 | F | Idiopathic PAH, vasoresponder | 7 | 25 | 9 | 7.4 | 2.2 | PDE5i, ERA, CCB |
| 6 | 69 | M | Congenital heart disease | 18 | 48 | 16 | 5.1 | 6.3 | PDE5 |
| 7* | 56 | F | Portopulmonary hypertension | 4 | 34 | 12 | 12.9 | 1.7 | PDE5i, ERA, PA |
| 8 | 84 | F | Systemic sclerosis, HFpEF | 15 | 60 | 22 | 3.3 | 11.5 | None |
| 9 | 59 | F | Systemic sclerosis, exercise PH | 10 | 21 | 13 | 7.7 | 1.0 | None |
| 10* | 58 | M | HIV, COPD | 10 | 29 | 16 | 5.0 | 2.6 | ERA |
| 11 | 44 | M | Sarcoid | 24 | 50 | 6 | 4.5 | 9.8 | None |
| 12 | 84 | F | Idiopathic PAH | 10 | 43 | 13 | 4.5 | 6.7 | None |
| 13 | 52 | F | HIV | 14 | 57 | 11 | 4.9 | 9.4 | PDE5i, PA |
| 14 | 72 | M | Unexplained dyspnea | 7 | 20 | 11 | 4.1 | 2.2 | None |
| 15 | 81 | F | COPD, HFpEF, OSA | 8 | 35 | 13 | 4.3 | 5.1 | None |
| 16 | 68 | F | Sjogren’s syndrome | 7 | 24 | 9 | 3.8 | 3.9 | None |
| 17 | 54 | M | ESRD, OSA, ILD | 8 | 46 | 15 | 4.8 | 6.5 | None |
| 18 | 43 | F | Idiopathic PAH | 4 | 33 | 6 | 5.6 | 4.8 | PDE5i, ERA |
| 19 | 76 | M | COPD | 2 | 38 | 7 | 3.9 | 7.9 | None |
Subject 7 culture success from 1/3 balloons; subject 10 culture success from 1/2 balloons. RAP=right atrial pressure; mPAP=mean pulmonary artery pressure; PCWP=pulmonary capillary wedge pressure; CO=cardiac output; PVR=pulmonary vascular resistance; PAH=pulmonary arterial hypertension; PAPVR=partial anomalous pulmonary venous return; CPC-PH=combined pre- and post-capillary pulmonary hypertension; ILD=interstitial lung disease; HFpEF=heart failure with preserved ejection fraction; HIV=human immunodeficiency virus; COPD=chronic obstructive pulmonary disease; OSA=obstructive sleep apnea; ESRD=end-stage renal disease; PDE5i=phosphodiesterase type 5 inhibitor; ERA=endothelin receptor antagonist; CCB=calcium channel blocker; PA=prostacyclin analogue
PAEC Characterization
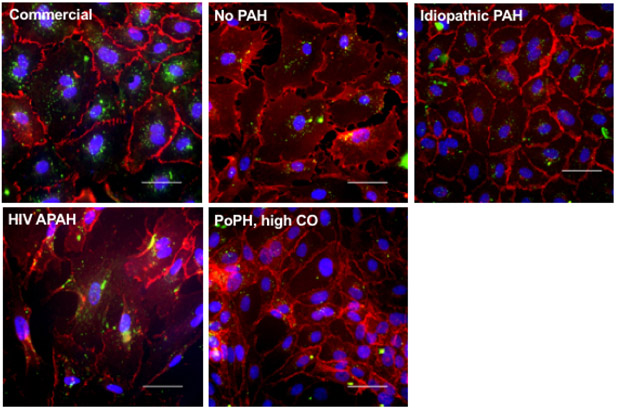
Cells demonstrated marked variability in the ability and speed at which they grew to confluence. Outgrowth of cells from the balloon tip could be observed within the first several days in culture, but confluence in the 24 well plate varied from six days to four weeks. In early experiments, we performed manual cell counts using trypan blue (ThermoFisher Scientific; Waltham, MA) and these ranged from 12.5K – 968K; routine cell counts were not part of our protocol for the data presented herein. We confirmed PAEC phenotype in primary culture and during subsequent passages and then, when yield allowed, conducted characterization assays. Figure 1 and Table 3 demonstrate confirmation of PAEC phenotype in both primary and passaged cells on representative samples for the following subjects, selected because of sufficient yield and because they illustrated a range of clinical and cellular phenotypes:
Figure 1.
Representative images confirming endothelial cell phenotype. Top left) commercial human pulmonary artery endothelial cells; passage 6. Top middle) No PAH = subject 14; passage 4. Top right) idiopathic PAH = subject 18; passage 3. Bottom left) HIV APAH = subject 13; passage 4. Bottom right) PoPH, high CO = subject 7; passage All images: VE-cadherin staining (red), acetylated low density lipoprotein uptake (green), images at 40X magnification. Scale bars = 50 μm. PAH = pulmonary arterial hypertension; HIV = human immunodeficiency virus; APAH = associated pulmonary arterial hypertension; PoPH = portopulmonary hypertension; CO = cardiac output.
Table 3.
Confirmation of pulmonary artery endothelial cell phenotype from representative samples
| Subject No. | Source | CD-31 | vWF | VE-cadherin | AcLDL uptake | α-SMA |
|---|---|---|---|---|---|---|
| 14 | Control | + | + | + | + | − |
| 18 | Idiopathic PAH, controlled hemodynamics | + | + | + | + | − |
| 13 | HIV associated PAH, high PVR | + | + | + | + | − |
| 7 | Portopulmonary hypertension, high CO, low PVR | + | + | + | + | − |
vWF=von Willebrand factor; VE=vascular endothelial; AcLDL=acetylated low density lipoprotein; α-SMA=alpha-smooth muscle cell actin; HPAECs=human pulmonary artery endothelial cells; ND=not done; PAH=pulmonary arterial hypertension; HIV=human immunodeficiency virus; PVR=pulmonary vascular resistance; CO=cardiac output. + indicates that 100% of the cells stained positive for antibody or took up AcLDL; − indicates that all cells were negative for antibody staining.
A man with a mPAP of 20 mmHg, referred for unexplained dyspnea but without documented cardiopulmonary disease (subject 14 in Table 2) whose cells had slow proliferation qualitatively.
A woman with idiopathic PAH on a phosphodiesterase type 5 inhibitor (PDE5i) and an endothelin receptor antagonist (ERA) who had improvement in pulmonary hemodynamics in response to these medications and a moderately elevated PVR (subject 18 in Table 2).
A woman with HIV-associated PAH on a PDE5i and prostacyclin analogue (PA) with severe hemodynamic compromise and high PVR (subject 13 in Table 2).
A woman with portopulmonary hypertension on triple PAH therapy (PA, PDE5i, ERA) with a high-output, low PVR state at the time of cardiac catheterization (subject 7 in Table 2) whose cells were rapidly proliferative.
As noted by the immunofluorescence staining in Figure 1 and Table 3, cells stained positive for VE-cadherin, vWF, and uptake of AcLDL and were negative for α-SMA. To confirm the specificity of the endothelial cell markers used, further testing demonstrated primary cultures of human lung smooth muscle cells and lung fibroblasts did not stain for VE-cadherin, CD31, or vWF nor take up AcLDL (See Online Supplementary Material; Table E1 and Figure E1).
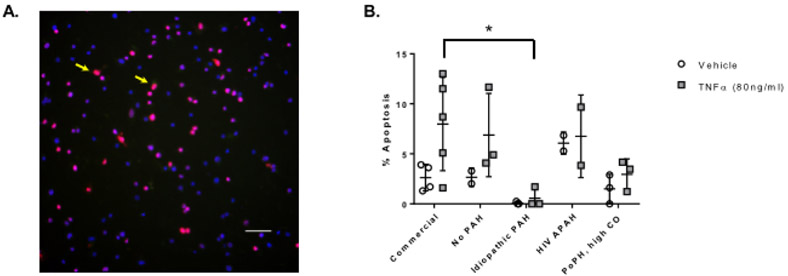
Apoptosis in PAH PAECs
Treatment with TNF-α induced apoptosis detected via TUNEL assay in commercial PAECs, in cells from the subject without PAH (subject 14), and to a lesser extent in cells from the subject with portopulmonary HTN with high cardiac output (subject 7) (Figure 2). PAECs from the subject with idiopathic PAH (subject 18) were highly resistant to apoptosis in response to TNF-α treatment and had significantly lower rates of apoptosis than commercial PAECs (corrected p for multiple comparisons = 0.042). We were limited in our ability to characterize apoptosis in PAECs from the subject with HIV-associated PAH and high PVR (subject 13) due to a low yield of cells (n = 2); apoptotic response trends were similar to commercial PAECs.
Figure 2.
Apoptosis assessed by TUNEL assay following treatment with vehicle and TNF-α. Panel A) Representative image of PAECs exposed to tert-butyl hydroperoxide for 6 hours TUNEL stained for apoptosis and counterstained with 4’,6-diamidino-2-phenylindole (DAPI). TUNEL positive cells are indicated by yellow arrow. Image at 20X magnification. Scale bar = 50 μm. Panel B) PAECs were exposed to vehicle or TNF-α for six hours. Data are presented as mean ± SD. n = 2 – 5; each n is derived from a different passage of cells from a single balloon tipped catheter from one right heart catheterization procedure for a single subject. *p < 0.05. X axis: No PAH = subject 14; idiopathic PAH = subject 18; HIV APAH = subject 13; PoPH, high CO = subject 7. PAEC = pulmonary artery endothelial cell; PAH = pulmonary arterial hypertension; PoPH = portopulmonary hypertension; CO = cardiac output.
Migration and Tube Formation by PAH Phenotype
We successfully characterized migration in PAECs from three of the four subjects using a standard scratch migration assay (Figure 3). The subject with portopulmonary hypertension and high cardiac output (subject 7) had higher rates of migration as compared to the subject with idiopathic PAH (subject 18) in low serum (corrected p for multiple comparisons = 0.039) and in response to complete medium (corrected p for multiple comparisons = 0.006) (Figure 3). The rate of migration in low serum was also higher in the subject with portopulmonary hypertension as compared to the subject with no PAH (subject 14) (p = 0.025). We were able to fully assess tube formation by Matrigel assay in PAECs from all four subjects. Cells from the subject with HIV-associated PAH and high PVR (subject 13) were less likely to form branching vessels (corrected p for multiple comparisons = 0.014) and branching vessels were shorter in length (corrected p for multiple comparisons = 0.022) as compared to commercial PAECs exposed to VEGF (Figure 4). There were no significant differences in mesh size, number or length of segments, or number of mesh rings (data not shown) across PAECs from subjects as compared to commercial PAECs.
Figure 3.
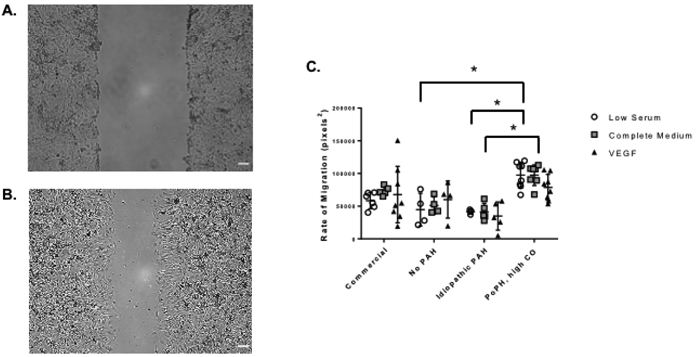
Migration assessed by scratch assay under low, enriched, and VEGF in low serum conditions. Representative phase image of PAECs at 0 (Panel A) and 6 hours (Panel B) after scratch. Images at 4X magnification. Scale bar = 100 μm. Panel C) Data are presented as mean ± SD. n = 3 – 8; each n is derived from a different passage of cells from a single balloon tipped catheter from one right heart catheterization procedure for a single subject. *p < 0.05. X axis: No PAH = subject 14; idiopathic PAH = subject 18; PoPH, high CO = subject 7. VEGF = vascular endothelial growth factor; PAEC = pulmonary artery endothelial cells; PAH = pulmonary arterial hypertension; PoPH=portopulmonary hypertension; CO=cardiac output.
Figure 4.
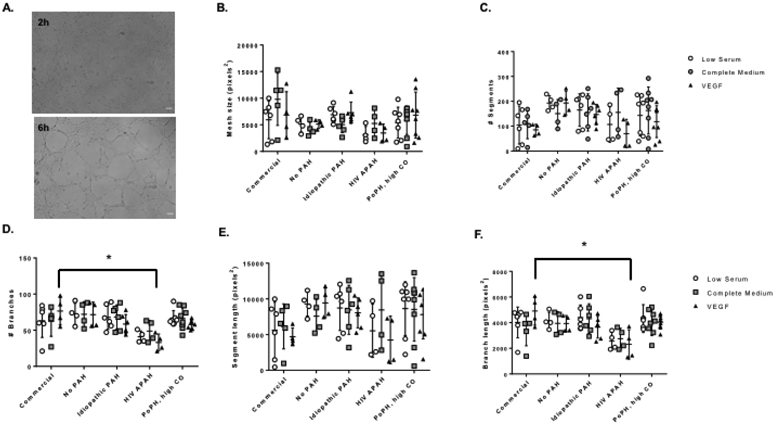
Tube formation following Matrigel treatment as quantified by AngioTool. Panel A) Representative phase images of tube formation at 2 and 6 hours respectively following seeding of PAECs on Matrigel. Images at 4X magnification. Scale bar = 100 μm. Panel B) number of segments, Panel C) number of branches, Panel D) mesh size (pixels2), Panel E) segment length (pixels2), and Panel F) branch length (pixels2). Data are presented as mean ± SD. n = 4 – 8; each n is derived from a different passage of cells from a single balloon tipped catheter from one right heart catheterization procedure for a single subject. *p < 0.05. X axis: No PAH = subject 14; idiopathic PAH = subject 18; HIV APAH = subject 13; PoPH, high CO = subject 7. PAH = pulmonary arterial hypertension; HIV = human immunodeficiency virus; APAH = associated pulmonary arterial hypertension; PoPH = portopulmonary hypertension; CO = cardiac output.
DISCUSSION
We have demonstrated that PAECs can be harvested and sustained in culture from PAC balloon tips used during routine hemodynamic evaluation. Primary cells were maintained in culture out to four weeks and were confirmed to have endothelial cell phenotype and to express endothelial cell markers through several passages. Cells isolated by this technique demonstrated functional characteristics that were generally similar to commercially available human PAECs. Our findings suggest that this approach could be harnessed and further developed to identify and characterize abnormalities in endothelial cell function in patients with pulmonary vascular disease.
The ability to successfully culture PAECs and variability in the harvested cell phenotype may be related to an individual’s genetic background, treatment, environmental exposures, epigenetic and pharmacogenomic changes, and/or the procedure itself. The earlier report by Pollett et al (8) commented that successful culture was influenced by the clinician performing the RHC and the hydration status of the patient but did not quantitate these findings. We found that our success rate was not associated with operator experience or most subject characteristics. Protocol details for PAC tip processing and PAEC harvest differed substantially from the prior report, which may have increased our yield. We did observe that the degree of hemodynamic compromise contributed to the success of PAEC culture. We were more likely to obtain viable PAECs from PAH patients with more severe disease (as evidenced by hemodynamics and possibly shorter 6MWD), which in part may be related to our observation that PAH PAECS were more apoptosis resistant compared to commercial PAECs. Altered mechanical properties (i.e., hyperpermeability) of PAH PAECs may have lead to increased transfer from the endothelium to the PAC balloon, as has been shown in experimental PH (13). These observations are preliminary and require confirmation. Distal PAECs from explanted PAH lungs have increased proliferation and decreased apoptosis as compared to control lung PAECs (14). This mirrors our in vitro qualitative and (to a limited extent) quantitative observations, in which cells from subjects with PAH and higher PVR tended to be rapidly proliferative and apoptosis resistant. Others have shown that PAH is characterized by accelerated endothelial cell apoptosis with loss of vasculature and impaired migration (15). While we demonstrated some differences in migration and network formation depending on PAH sub-type, whether or not these patterns are typical will require a larger number of subjects.
Functional abnormalities of PAECs from a second or third order pulmonary artery may not represent those from the distal resistance vessels implicated in PAH. In healthy rats, lung microvascular endothelial cells have higher proliferative potential than proximal PAECs but also significant microheterogeneity in replication competency that depends on the parent cell population (16). It is possible that similar microheterogeneity exists in human proximal PAECs (or local progenitors) and that single cell cloning could reduce the variability we observed. There is also a rationale for studying proximal PAECs, given wall stress changes in proximal pulmonary arteries contribute to compliance and coupling in PAH (17).
This preliminary report has limitations. Cultured endothelial cells could have derived from the central veins, right heart or from circulating blood outgrowth endothelial cells (BOECs). Only the balloon and catheter tip are collected which contains 17–33-fold less blood (at most 2.4 mL, typically < 1 mL) than the 40–80 mL required for isolation of BOECs (7). It is unlikely that sufficient numbers of endothelial cells from elsewhere could attach to the balloon during incidental contact en route to the wedge position. No distinct -omic signature is able to differentiate PAECs from other locations, and we contend that the source of cells is most likely the pulmonary artery where the balloon has by far the greatest surface-to-surface contact. As a proof-of-concept, in two subjects who recently underwent RHC we advanced a PAC to the right ventricle, but no further, and did not inflate the balloon. Neither of these PAC tips yielded endothelial cells. In both procedures, a second catheter was then used to float to the PA and then into wedge position with the balloon inflated to complete the procedure. In the patient with PAH (the second had PH/pulmonary venous hypertension), we successfully cultured PAECs from this catheter tip. The experiments conducted represent technical, not biological, replicates. We were unable to culture cells from serial catheterizations of several subjects performed during the study period despite the same protocol and in some cases cell yield limited the number of experiments for a given assay (e.g., characterization of apoptosis in HIV-associated PAH). After we introduced the heater for sample transport, the subject with portopulmonary hypertension (subject 7) had three subsequent catheterizations from which all three balloons yielded PAECs (a total within subject success rate of 4/6, 67%)(data not shown). Characterization of PAEC behavior in these biological replicates and banked PAECs from all successful attempts is the subject of ongoing work. Finally, we do not know the bone morphogenetic receptor type 2 (BMPR2) mutation status of the subjects although none of the participants had heritable disease. PAECs from patients with BMPR2 mutations may be pro-proliferative as compared to other forms of PAH and may have contributed to our higher success rates in patients with more severe PAH (18). A larger sample size is needed to draw conclusions about the functional differences seen here. It is our goal to publish these early observations so that the rigor of the method can be improved as a necessary first step. Finally, we were encouraged that we had greater success culturing PAECs from subjects with the disease of interest (i.e., hemodynamic PAH, higher PVR states) in which this technique may prove the most scientifically fruitful.
Conclusions
Routine RHC for pulmonary vascular disease evaluation may represent a novel opportunity for successful harvest and culture of PAECs from PAC balloon tips, especially in individuals with greater hemodynamic compromise.
Supplementary Material
Take Home Message:
Pulmonary artery endothelial cells (PAECs) from pulmonary artery catheter balloons used during routine right heart catheterization can be cultured and sustained to study endothelial cell dysfunction at various stages of pulmonary hypertension.
Acknowledgments
Sources of Support: This work was completed with support from an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences (P20 GM103652) and National Institutes of Health R01-HL141268.
Footnotes
DISCLOSURES
CEV: Consultant for Acceleron Pharma, outside of the submitted work. Grant funding to institution from United Therapeutics and Eiger.
JMA: Nothing to disclose.
JB: Nothing to disclose.
HC: Nothing to disclose.
MD: Nothing to disclose.
DM: Nothing to disclose.
CJM: Nothing to disclose.
JN: Nothing to disclose.
MP: Nothing to disclose.
AP: Nothing to disclose.
PJQ: Nothing to disclose.
TW: Nothing to disclose.
MW: Nothing to disclose.
JRK: Nothing to disclose.
EOH: Nothing to disclose.
References
- 1.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation 2004; 109: 159–165. [DOI] [PubMed] [Google Scholar]
- 2.Morrell NW, Adnot S, Archer SL, Dupuis J, Jones PL, MacLean MR, McMurtry IF, Stenmark KR, Thistlethwaite PA, Weissmann N, Yuan JX, Weir EK. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54: S20–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuder RM, Archer SL, Dorfmuller P, Erzurum SC, Guignabert C, Michelakis E, Rabinovitch M, Schermuly R, Stenmark KR, Morrell NW. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humbert M, Guignabert C, Bonnet S, Dorfmuller P, Klinger JR, Nicolls MR, Olschewski AJ, Pullamsetti SS, Schermuly RT, Stenmark KR, Rabinovitch M. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 2019; 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu M, Shao NY, Sa S, Li D, Termglinchan V, Ameen M, Karakikes I, Sosa G, Grubert F, Lee J, Cao A, Taylor S, Ma Y, Zhao Z, Chappell J, Hamid R, Austin ED, Gold JD, Wu JC, Snyder MP, Rabinovitch M. Patient-specific iPSC-derived endothelial cells uncover pathways that protect against pulmonary hypertension in BMPR2 mutation carriers. Cell Stem Cell 2017; 20: 490–504.e495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sa S, Gu M, Chappell J, Shao NY, Ameen M, Elliott KA, Li D, Grubert F, Li CG, Taylor S, Cao A, Ma Y, Fong R, Nguyen L, Wu JC, Snyder MP, Rabinovitch M. Induced pluripotent stem cell model of pulmonary arterial hypertension reveals novel gene expression and patient specificity. Am J Respir Crit Care Med 2017; 195: 930–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geti I, Ormiston ML, Rouhani F, Toshner M, Movassagh M, Nichols J, Mansfield W, Southwood M, Bradley A, Rana AA, Vallier L, Morrell NW. A practical and efficient cellular substrate for the generation of induced pluripotent stem cells from adults: blood-derived endothelial progenitor cells. Stem Cells Transl Med 2012; 1: 855–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollett JB, Benza RL, Murali S, Shields KJ, Passineau MJ. Harvest of pulmonary artery endothelial cells from patients undergoing right heart catheterization. J Heart Lung Transplant 2013; 32: 746–749. [DOI] [PubMed] [Google Scholar]
- 9.Lu Q, Patel B, Harrington EO, Rounds S. Transforming growth factor-beta1 causes pulmonary microvascular endothelial cell apoptosis via ALK5. Am J Physiol Lung Cell Mol Physiol 2009; 296: L825–L838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ottosson M, Jakobsson A, Johansson F. Accelerated wound closure - differently organized nanofibers affect cell migration and hence the closure of artificial wounds in a cell based in vitro model. PLoS One 2017; 12: e0169419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zudaire E, Gambardella L, Kurcz C, Vermeren S. A computational tool for quantitative analysis of vascular networks. PLoS One 2011; 6: e27385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, Williams PG, Souza R. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019; 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou C, Francis CM, Xu N, Stevens T. The role of endothelial leak in pulmonary hypertension (2017 Grover Conference Series). Pulm Circ 2018; 8: 2045894018798569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, Drazba J, Anand-Apte B, Erzurum SC. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2007; 293: L548–554. [DOI] [PubMed] [Google Scholar]
- 15.Nickel NP, Spiekerkoetter E, Gu M, Li CG, Li H, Kaschwich M, Diebold I, Hennigs JK, Kim KY, Miyagawa K, Wang L, Cao A, Sa S, Jiang X, Stockstill RW, Nicolls MR, Zamanian RT, Bland RD, Rabinovitch M. Elafin reverses pulmonary hypertension via caveolin-1-dependent bone morphogenetic protein signaling. Am J Resp Crit Care Med 2015; 191: 1273–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JY, McMurtry SA, Stevens T. Single cell cloning generates lung endothelial colonies with conserved growth, angiogenic, and bioenergetic characteristics. Pulm Circ 2017; 7: 777–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schafer M, Kheyfets VO, Schroeder JD, Dunning J, Shandas R, Buckner JK, Browning J, Hertzberg J, Hunter KS, Fenster BE. Main pulmonary arterial wall shear stress correlates with invasive hemodynamics and stiffness in pulmonary hypertension. Pulm Circ 2016; 6: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferrer E, Dunmore BJ, Hassan D, Ormiston ML, Moore S, Deighton J, Long L, Yang XD, Stewart DJ, Morrell NW. A potential role for exosomal translationally controlled tumor protein export in vascular remodeling in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 2018; 59: 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.