Abstract
A classical view of T cell lineages consists two major clades of T cells expressing either the αβ or γδ T cell receptor (TCR). However, genome-wide assessments indicate molecular clusters segregating T cell subsets that are preprogrammed for effector function (innate) from those that mediate conventional adaptive response, regardless of the TCR types. Within this paradigm, γδ T cells remain the prototypic innate-like lymphocytes, many subsets of which are programmed during intrathymic development for committed peripheral tissue localization and effector responses. Emerging evidence for innate γδ T cell lineage choice dictated by developmental gene programs rather than the sensory TCR is discussed in this review.
Introduction
In theory, different T cell subsets can be made from one type of multi-lineage potential mother cell that can integrate distinct lineage specifying cell-extrinsic signals (instructive) or from distinct mother cell types with skewed probabilities to develop into one T cell type over another (stochastic). Historically, γδ T versus γδ T cell lineage commitment models considered the instructive TCR signal versus the stochastic, TCR signal-independent processes [1]. For the latter, the term “stochastic” is imprecise and encompasses any deterministic events prior to and independent of the TCR signaling event in the progenitor. If the stochastic model is correct, precision in terminology was expected to come from the identification of the actual deterministic molecular processes, which may be probabilistic or directed. To what extent TCR type or different strength of TCR signaling dictates T cell lineage specification in otherwise homogeneous progenitors continues to be debated [2,3]. But this question is predominantly considered from a framework where data from TCR signaling studies have been interpreted to demonstrate a deterministic role in γδ versus αβ T cell lineage commitment and effector subset specification. To date, the alternative model of precommitted progenitors to specific T cell types is based on indirect data, relying on the ancestry of genes expressed in a cell type-specific manner [4], or biases in progenies generated from different precursor subsets that have yet to receive antigen receptor signals [5]. Thus, T cell lineage commitment in thymic progenitors is mostly understood as a TCR signal-instructed process. However, this consensus is being challenged by the only manner in which precommitted progenitors can be described with conviction, at the single cell resolution, embedded with predicted gene networks associated with specific T cell lineage.
γδ T subsets as the prototypic, preprogrammed innate lymphocyte
γδ T cells were discovered when the second TCR composed of γδ heterodimeric chains were identified after the αβTCRs were cloned in the early 1980’s [6]. Combined with the identification of step-wise developmental intermediates in the thymus that can generate both αβ and γδ T cells [7], there was a natural tendency to focus on the role of TCR signals to specify cell lineage fate. There were however observations that suggested more complexity. Murine γδ T cells expressing an invariant TCR (Vγ3TCR+, Garman nomenclature [8]) were shown to be the first T cell subset to arise at embryonic day 15.5 (E15.5), whereas mature αβ T cells are not observed in large numbers until after birth [9]. Vγ3+ T cells home to the epidermis and are termed dendritic epidermal T cells (DETCs, Fig. 1). They arise exclusively from FL stem cell or progenitors and not from the adult BM cells and require fetal thymic environment to develop [10–12]. Once in the fetal skin, DETCs can maintain their population size well into adulthood. These properties suggested a unique origin of DETCs compared to conventional T cells. Like other hematopoietic cells that are generated in the embryos and neonates, the consensus was that DETCs were a product of FL HSCs that have been discovered to have distinct transcriptomes than adult BM HSCs [13,14]. Critically, the fetal molecular programs are geared to generate immunocytes with innate-like, preprogrammed effector functions, supporting the theoretical construct of the mammalian immune system into two subroutines: an early developing fast responders, mostly populating tissues for barrier defense, and a later arising conventional slow responders largely designated for recall responses to recurring pathogens in a given habitat. This concept of “layered” immunity was articulated by Herzenbergs in the late 1980’s [15] and has gained firmer traction with an increasing appreciation for lymphocytes with innate-like functions, further catalyzed by the discovery of innate lymphoid cells (ILCs), tissue resident T cells and homeostatic (rather than pathogen defense) functions of lymphocytes in tissues. Innate-like lymphocyte subsets include DETCs, IL-17 producing γδ T (Tγδ17) cells; intestinal intraepithelial lymphocytes (iIELs) and NKT cells expressing either αβTCR (most with the invariant Vα14TCR) or γδTCR, MAITs and B1 B cells. Here, these cells are referred to as innate T or B cells. For the most part, one recurring development feature of these cells is their preferential development in fetal and/or neonatal stages in mice. With improved molecular resolution of diverse early rising innate lymphocytes, embryonic or neonatal hematopoiesis represents the best opportunity to reassess whether specific T cell types originate from dedicated progenitors, independent of TCR. If so, this would further cement the distinctions of early versus late lymphopoiesis, the dominance of “innate” development gene programs versus the fine tuning of development by “adaptive” antigen receptors.
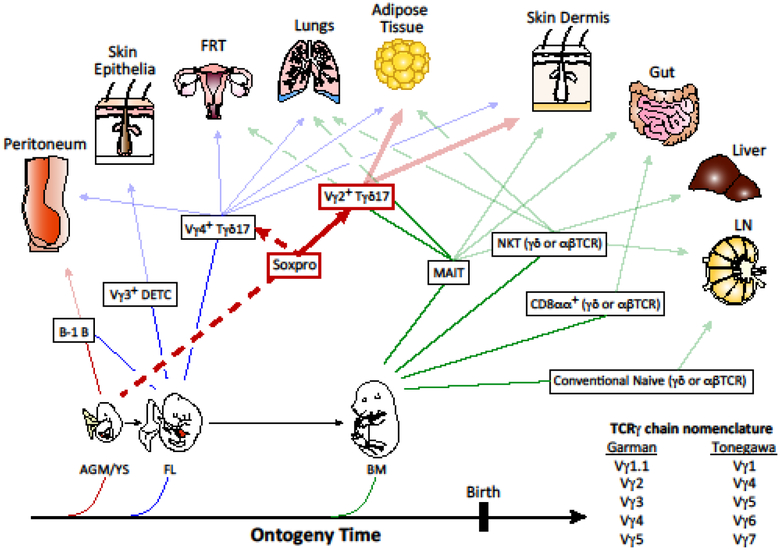
Figure 1. Ontogeny and programmed tissue tropism of innate T and B cells.
Development of innate T and B cells begins with the emergence of hematopoietic progenitors from the aorta-gonad- mesonephros or yolk sac (AGM/YS) regions and the fetal liver (FL). Natural antibody producing B-1 B cells that predominantly home to the peritoneal cavity have been demonstrated to develop from both sites, starting ~E9.5. The first T cell subset observed in mice, Vγ3TCR+ DETC, originates from FL HSCs. Subsequently, two subsets of Tγδ17 cells emerge, expressing either Vγ4TCR and Vγ2TCR, the latter of which are primarily derived from SOX13+ DN1d cells termed Soxpro. Tγδ17 cells are distributed widely in non-lymphoid tissues, but only the primary tissue sites are depicted here. In newborns these cells are required for organogenesis, tissue homeostasis and body thermogenesis, depending on their tissue localization. All innate γδ T cell subsets function and tissue homing property are specified during thymic differentiation. FL HSCs migrate to the fetal bone marrow (BM) to establish long-term residence and sustain adult hematopoietic output. The next phase of innate T cell development also switches to the BM origin, although the initial tissue homing T cells at birth are likely from FL HSCs. MAIT and NKT cells with restricted TCR repertoire (αβTCR or γδTCR) begin to be produced predominantly after birth, some from fetal progenitors with biased innate lymphoid gene programming. Gut homing CD8αα+ intestinal intraepithelial T cells expressing either αβTCR or γδTCR are generated from the neonatal thymus and originate from BM HSCs. Lastly, additional subsets of non-mucosal homing Vγ1.1+ and Vγ2+ cells develop skewed toward IFNγ production as well as “naïve” γδ T cells that acquire effector specificity in peripheral tissues. This phase of lymphatic and blood borne γδ T cell development coincides with the developmental window when conventional naïve γδ T cells begin to be exported from the thymus to populate the secondary lymphoid tissues. Dashed lines indicate developmental pathways awaiting definitive experimental verification. Garman nomenclature for Tcrg genes is used in this figure and throughout this manuscript; the alternative Tonegawa nomenclature is provided for ease of reference. Note that tissues of residence presented here are not exhaustive; for example, Vγ4+ Tγδ17 cells also home to the gut, while Vγ2+ Tγδ17 cells mount responses in the eye and skin- draining LNs. FRT, female reproductive tract; LN, lymph node.
Evidence for distinct progenitors for innate Tγδ17 cells.
Postnatal αβTCR+ innate-like lymphocytes are thought to principally develop from CD4+CD8+ double positive thymocytes that have arranged the Tcr genes and selected on agonist TCR signals [16,17]. Some liver-tropic, IFNγ producing iNKT cells can arise from CD4−CD8− double negative (DN) precursor thymocytes [18], and there exists TCR-unlinked gene circuits centered on counterbalancing E-Id transcription factors (TFs) to control iNKT cell differentiation [19], but whether there are DN precursors geared for iNKT cell production have not been explored. The possibility of a significant thymic origin of ILC2 and ILC3 has also been raised [20,21], although the extent to which the thymic ILCs contribute to the overall ILC pool in the body is unclear. In general therefore, innate αβ T cells produced from the thymus largely track the developmental progression of conventional T cells and are controlled by TCR signaling. However, mixed results from studies of γδ T cell development in TCR signaling defective mice pointed to additional complexity [22,23]. Further, nearly all these studies were performed in juvenile mice or older, uncoupled from the emergence of most of these cells in neonates, and the emerging evidence for primacy of transcription factor (TF) networks of fetal/neonatal T cells was not easily compatible with the TCR- instructive T cell lineage fate commitment.
Ontogenic clock is the central feature of γδ T cell subset development, which set the precedent for all other innate-like lymphocytes. Successive “waves” of intrathymic progenitors from fetus to adults generate γδ T cell subtypes with distinct function and tissue homing capacity [12,24]. As alluded to above, the first wave generates Vγ3+ DETC, followed by the second wave that has been proposed to spawn all other fetal/neonatal γδ T cells, Vγ4+ (fetal) and Vγ2+ (neonatal) Tγδ17 cells [24], and Vγ1.1+Vδ6.3+ γδNKT cells [25]. The process is then completed by the third wave that primarily generates IFNγ-producing and “naïve” γδ cells that may have “on-demand” developmental potential into specific effector subtypes based on activation milieu [26–28](Fig. 1). BM HSC can generate IFNγ+ γδ T cells, including γδNKT cells but they are not capable of reconstituting DETCs or Vγ4+ Tγδ17 cells. Vγ2+ Tγδ17 cells by cell lineage tracing in vivo appear to originate from the fetal/neonatal stage [24], and while they can also be variably generated in radiation BM chimeras [29–31], whether this activity is a transplantation artifact, and the relationship of the Vγ2+ cells arising in BM chimeras to “natural programmed” versus “naïve, inducible” Tγδ17 cells, has not been determined. As to the NKT cell types, both γδ and αβ NKT cells can derive from BM cells, although there is more biased production from fetal progenitors and/or fetal niche [25] for the former. Extensive transcriptomic overlap between γδNKT and αβ iNKT cells, despite the non-similarity and even opposing qualities of TCR signaling directing each subset, have raised a possibility that the NKT cell program is in part regulated by a TF network that is not wholly controlled by TCR signaling. While suggestive findings involving the quartet of HMG TFs Sox4, Sox13, Lef1 and Tcf7 in controlling iNKT cells have emerged [32,33], there is so far no data indicating distinct progenitors that selectively express the TFs to direct NKT cell development. However, tracking the same quartet of HMG TFs, in particular Sox13, the first dedicated progenitor to an innate-like T cell lineage has been discovered [34]. Specifically, neonatal Vγ2+ Tγδ17 (nTγδ17) cells are shown to originate from fetal Sox13+ progenitors that are independent of γδTCR.
At the outset, a putative dedicated Tγδ17 cell lineage committed progenitor must exhibit three experimentally demonstrable features: 1) a cell-intrinsic potential to preferentially generate Tγδ17 cells in the fetal thymus; 2) an imprinted gene expression patterns overlapping with developing Tγδ17 cells; and 3) independency from γδTCR signaling. A Cre-fluorescent fusion protein whose expression was driven by Sox13 transcriptional regulatory elements was used as the molecular beacon to track in vivo cells capable of expressing Sox13, the only known γδ lineage-biased TF [35]. Among developing γδTCR+ thymocytes those destined to become Tγδ17 cells (immature Vγ2+ thymocytes also expressing the Tγδ17 specific marker Scart2 (5830411N06Rik) [36]) express the highest amounts of Sox13. Using SOX13 reporter mice, we recently demonstrated that thymic c-Kit− CD24+CD44+ DN precursor cells (termed DN1d cells by the Petrie lab [37], with DN1 referring to CD44+CD25− DN cells that include the earliest T cell progenitors, ETPs) are the only thymic precursors to express the SOX13 reporter and retain the prerequisite features of preprogrammed progenitors. These intrathymic SOX13 reporter+ progenitors were termed Soxpro (Fig. 2). Given the requirement for normal thymic epithelial architecture for Tγδ17 cell development [38,39] cell-intrinsic potential of Soxpro cells to differentiate into γδ T cell subsets was assayed in hanging drop fetal thymus organ culture (hFTOC). Among thymic precursor subsets that do not express RAG1/2 proteins required to generate TCRs, Soxpro or DN1d cells were the primary generator of nTγδ17 cells. Another DN1 subset called DN1e (c-Kit−CD44− DN1 [37]) is also capable of generating Tγδ17 cells and may represent an immediate progeny of DN1d cells (Fig. 2), but they do not reconstitute hFTOCs effectively, precluding a definite conclusion as to their developmental potential. Fetal LMPPs or CLPs that are known as the hematopoietic developmental progenitors towards all lymphocytes can generate some fetal Vγ4+ Tγδ17 cells (found in most mucosal tissues with homeostatic and body temperature control function in the adipose tissue [40] and gut associated mucosal tissues), but they were not able to generate nTγδ17 cells in hFTOC, strongly suggesting that the generation of Soxpro does not follow the conventional lymphopoietic pathway. In the widely used Notch ligand-OP9 BM stromal culture system, progeny outputs largely replicated the hFTOC assay but there was extensive variability in the cell numbers generated from cKitneg thymic DN1 subsets. γδ T cell development may require intermittent Notch signaling [41,42] and the pervasive version of it in the in vitro assay is likely to skew the assay to support those T cell subsets and their precursors that are tolerant of continuous Notch signaling during development [43].
Figure 2. A model of neonatal Vγ2+ Tγδ17 cell differentiation from dedicated progenitors.
The nature of the cell types and factors involved in directing Tγδ17 cell programming in progenitors and fostering a supportive niche for Tγδ17 development remains incompletely resolved. We propose that the initial specification of Tγδ17 progenitor called Soxpro occurs independent of TCR signaling and takes place during development transitions of YS progenitors as they migrate to the fetal thymus. Whether this transition involves trafficking to FL is currently unknown, but conventional FL lymphoid progenitors (LMPPs and CLPs) do not appear to be a major source of neonatal Tγδ17 cells. In the thymus Soxpro is contained within the DN1d subset (cKit−CD24+ DN1 precursors) and some may transit to the DN1e stage (cKit-CD24- DN1, which has dramatically diminished Sox13 transcript amounts) before expressing γδTCR on the cell surface. Sox13 expression is dynamic (as depicted by the light bulb), as it is turned on (or maintained) in immature (CD24+) γδTCR+ thymocytes and turned down in mature (CD24−) counterparts. TCR signaling likely act as a permissive developmental checkpoint, at the transition from DN1 subsets, which include Soxpro, to immature CD24+Vγ2TCR+ thymocytes. In perinatal mice with genetically attenuated TCR signaling, alterations in the Tγδ17 gene signature are noted prior to the expression CD73, a marker induced by TCR signaling. Using Il17a reporter mice, we also observed that effector function is acquired prior to the expression of CD73. These results suggest a two-step model of TCR signaling requirement for Tγδ17 cell maturation, before CD73 expression and in the TCR-dependent acquisition of CD73, which is associated with the loss of CD24 and final maturation. Roles for cell- extrinsic thymic Notch, WNT and TGFβ in Tγδ17 cell development have been reported in genetic models, while IL-1β, IL-21, IL-23 have also been proposed to be involved based on data from in vitro assays (not depicted in the Figure). While the precise identity and supportive thymic niche for Tγδ17 cell differentiation is unknown, mouse models with compromised thymic architecture have implicated a role for cortical epithelial cells (cTEC), although involvements of medullary epithelial cells (mTEC) have also been suggested. One emerging model of unique cTEC-Soxpro-mTEC triad to maintain and facilitate Tγδ17 differentiation is currently under investigation. These studies also emphasize that caution is necessary in interpreting γδ T cell developmental assays employing non- thymic stromal cells.
TCR independence of Tγδ17 cell progenitors
Single cell transcriptomic and protein analyses showed that Soxpro cells were already prewired for the gene network associated with developing Tγδ17 cells [34], including the expression of signature genes Rorc that turns on Il17 transcription, Sox4, Tcf7, Tcf12 (HEB), Maf, Etv5, Runx1, Il7r, Blk and Scart2, in addition to Sox13. Analysis of mice deficient in Tcf7, Sox13 and Cbfb2 (an obligate partner of RUNX proteins) showed defects in DN1d cell generation in a gene dose-dependent manner ([34] and unpublished). Most strikingly, the Tγδ17 transcriptome program in DN1d and Soxpro is largely preserved in Tcrd−/− and Rag1−/− mice, unequivocally demonstrating that γδTCR-dependent signals were not required for Tγδ17 transcriptional programming in Soxpro cells..
As expected of precursors, Soxpro cells do not express TCRs, and are independent of γδTCR signaling for their initial formation as they are generated in mice deficient for TCR, including in Tcrd−/− mice. However, some Soxpro cells from WT and Tcrd−/− mice have rearranged Tcrvg2 genes, but not significant rearrangements involving other Vγ genes tested, and negligible rearranged Tcrd transcripts in WT progenitors. This result suggests that Soxpro originated from precursors that transiently expressed RAG proteins and that the biased rearrangement of Vg2 gene segment in Tcrd−/− mice is a consequence of programmed V-J TCRγ recombination, not TCR-mediated selection. There are lymphopoietic progenitors that express Rag1/2 genes in embryonic YS or fetal liver before E12.5 [44](see below), pointing to one possible source of Soxpro. Data correlating precocious activation of Tcrg loci as a hallmark of other innate lymphocytes such as NK cells and ILCs [45,46]raises an important implication of shared progenitors and/or molecular programs in the construction of the innate lymphoid system.
Potential embryonic sources of innate T lymphocytes
Several studies established that definitive hematopoietic progenitors with multilineage potential encompassing both the myeloid and lymphoid progeny are present in the extraembryonic YS and embryonic AGM before the emergence of the first FL HSC (~E10.5). The earliest of these are erythromyeloid progenitors (EMPs) that give rise to definitive erythrocytes and myeloid cells, including tissue resident macrophages and mast cells starting at ~E8. By ~E10, pre-HSCs are detectable in the YS and AGM, some of which are thought to migrate to FL, become HSCs and then transit to fetal BM, to build the postnatal hematopoietic system [47,48]. Around the same ontogenic timeframe lymphopoietic progenitors (Rag1/2+, Flt3+, IL-7R+) are also detectable, primarily in the YS [44], but also distributed throughout embryonic hematopoietic tissues. E9 YS hemogenic endothelial cells are biased to generate innate-like B1 B cells [49]. They have also been associated with broad T cell subset generative potential (including γδ T cells and iNKT cells) that can be revealed in the OP9-DL1 BM stromal culture, as well as in transplantation models using immunodeficient recipients [50]. Studies to date of developmental potential of embryonic hematopoietic tissue progenitors are low resolution and not geared to test specific innate lymphocyte subset progenitor-progeny relationships.
FL HSCs have shown to preferentially generate innate-like lymphocytes, such as DETCs and some iNKT cells. Fetal-biased gene circuits centered on Lin28b can direct stem cell differentiation into innate-like lymphocytes by modulating genes such as Zbtb16 (PLZF) that are critical for their differentiation and/or maintenance [51]. In addition, FL HSCs were discriminated based on the Flt3 (FLK2)-driven lineage marking and those “transient” HSCs with history of Flt3 expression showed a lymphopoietic bias with skewed generation of B1 B cells in transplantation models, and of DETCs in FTOCs [52]. For the latter, the noted bias was muted. These studies strongly suggest the existence of lymphopoietic progenitors in YS E8–10 and heterogeneous developmental potential within FL HSCs to generate innate-like lymphocytes. Given the lack of direct embryonic progenitor-progeny demonstration in the innate lymphocyte lineages, these results have been interpreted to indicate that the fetal gene networks in general favor preprogrammed effector function. However, the putative embryonic progenitor of Soxpro may reveal a new architecture. We observed that YS cells from E8.5 to 10.5 retain the Sox13 reporter and can initiate Tγδ17 cell development in hFTOCs. Initial molecular analyses reveal that as yet defined YS progenitors are highly skewed to differentiate into Vγ2+ and Vγ4+ Tγδ17 cells with constrained capacity to generate αβ T cell lineage cells and other γδ T cell subsets, including DETCs. While the definitive proof of the embryonic YS origin of Soxpro independent of the conventional fetal lymphopoietic pathway awaits further studies, the initial forays offer a glimpse of the first building blocks of the innate lymphoid immune system.
Conclusions
Akin to the fetal phase of tissue resident macrophage, mast cell and B1 B cell generation, Tγδ17 cell differentiation likely involves unconventional lineage restricted hematopoietic progenitors, possibly prior to the development of the first FL HSC at E10 that is the source of all HSCs, and therefore all hematopoietic cells, in the adults. Clarification of this possibility, including identification of the unconventional progenitor and detailed mechanistic understanding of the TCR- independent factors driving cell programming, requires new tools to permit cell lineage tracing with a focus on in vivo developmental outcomes. As described in the above studies, high-resolution single-cell genomics methods will likely play a key role in discerning biased progenitors among highly heterogenous and poorly understood cell populations. An obvious question arises as to why Tγδ17 cells may necessarily originate from a specialized embryonic progenitor. Analysis of taxonomically distinct species, most notably the lamprey whose lymphocytes express non-immunoglobulin superfamily variable lymphocyte receptors, suggests establishment of the γδ T cell transcriptional signature, including a potential Sox13 ortholog, before the emergence of RAG-recombined TCRs [53–55]. Further, numerous IL-17 and IL-17R family members are present in the lamprey and sea urchins [56,57], suggesting that IL-17 is a widely conserved mechanism of host defense. Critically, the importance of IL-17 in supporting epithelial tissue integrity [58] raises the possibility that IL-17 pre-programmed cells may primarily serve to maintain tissue homeostasis in early life [40], with IL-17-dependent inflammation only occurring in the context of epithelial barrier breach. This conclusion is further bolstered by findings of highly penetrant spontaneous atopic dermatitis in Sox13-deficient mice (Spidale et al. manuscript in preparation), Thus, understanding the homeostatic function of IL-17 in mucosal tissues will lead to new perspective on organogenesis-centric design principles of the neonatal immune system.
Highlights.
Neonatal IL-17 producing γδ T (Tγδ17) cells depend on SOX13.
SOX13 expressing progenitors, Soxpro, in the thymus generate Tγδ17 cells.
Soxpro generation is not dependent of γδTCR.
Soxpro may not arise from conventional lymphoid progenitors.
Evidence suggests other innate T cells may also originate from preprogrammed progenitors.
Acknowledgements
We are grateful for the members of the Kang laboratory who have contributed to the discovery of Soxpro cells over the last decade. We thank Andrea Reboldi for critical reading of the review. This work was supported by the National Institute of Health (AI07551, AI101301 and AR071269 to JK) and the Charles H. Hood Foundation Child Health Research Award (to MF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Narayan K, Kang J: Disorderly conduct in gammadelta versus alphabeta T cell lineage commitment. Semin Immunol 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munoz-Ruiz M, Sumaria N, Pennington DJ, Silva-Santos B: Thymic Determinants of gammadelta T Cell Differentiation. Trends Immunol 2017, 38:336–344. [DOI] [PubMed] [Google Scholar]
- 3.Zarin P, Chen EL, In TS, Anderson MK, Zuniga-Pflucker JC: Gamma delta T-cell differentiation and effector function programming, TCR signal strength, when and how much? Cell Immunol 2015, 296:70–75. [DOI] [PubMed] [Google Scholar]
- 4.Narayan K, Sylvia KE, Malhotra N, Yin CC, Martens G, Vallerskog T, Kornfeld H, Xiong N, Cohen NR, Brenner MB, et al. : Intrathymic programming of effector fates in three molecularly distinct gammadelta T cell subtypes. Nat Immunol 2012, 13:511–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang J, Volkmann A, Raulet DH: Evidence that gammadelta versus alphabeta T cell fate determination is initiated independently of T cell receptor signaling. J Exp Med 2001, 193:689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Born WK, O’Brien RL: Discovery of the gammadelta TCR: Act II. J Immunol 2016, 196:3507–3508. [DOI] [PubMed] [Google Scholar]
- 7.Godfrey DI, Kennedy J, Suda T, Zlotnik A: A developmental pathway involving four phenotypically and functionally distinct subsets of CD3−CD4−CD8− triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J. Immunol 1993, 150:4244–4252. [PubMed] [Google Scholar]
- 8.Garman RD, Doherty PJ, and Raulet DH: Diversity, rearrangement, and expression of murine T cell gamma genes. Cell 1986, 45:733–742. [DOI] [PubMed] [Google Scholar]
- 9.Havran W, Allison JP: Developmentally ordered appearance of thymocytes expressing different T cell antigen receptors. Nature 1988, 335:443–445. [DOI] [PubMed] [Google Scholar]
- 10.Havran WL, Allison JP: Origin of Thy-1+ dendritic epidermal cells of adult mice from fetal thymic precursors. Nature 1990, 344:68–70. [DOI] [PubMed] [Google Scholar]
- 11.Ikuta K, Kina T, MacNeil I, Uchida N, Peault B, Chien Y-h, Weissman IL: A developmental switch in thymic lymphocyte maturation potential occurs at the level of hematopoietic stem cells. Cell 1990, 62:863–874. [DOI] [PubMed] [Google Scholar]
- 12.Ramond C, Berthault C, Burlen-Defranoux O, de Sousa AP, Guy-Grand D, Vieira P, Pereira P, Cumano A: Two waves of distinct hematopoietic progenitor cells colonize the fetal thymus. Nat Immunol 2014, 15:27–35. [DOI] [PubMed] [Google Scholar]
- 13.Kim I, Saunders TL, Morrison SJ: Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell 2007, 130:470–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA: Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science 2012, 335:1195–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herzenberg LA, Herzenberg LA: Toward a layered immune system. Cell 1989, 59:953–954. [DOI] [PubMed] [Google Scholar]
- 16.Golec DP, Hoeppli RE, Henao Caviedes LM, McCann J, Levings MK, Baldwin TA: Thymic progenitors of TCRalphabeta(+) CD8alphaalpha intestinal intraepithelial lymphocytes require RasGRP1 for development. J Exp Med 2017, 214:2421–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruscher R, Kummer RL, Lee YJ, Jameson SC, Hogquist KA: CD8alphaalpha intraepithelial lymphocytes arise from two main thymic precursors. Nat Immunol 2017, 18:771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dashtsoodol N, Shigeura T, Aihara M, Ozawa R, Kojo S, Harada M, Endo TA, Watanabe T, Ohara O, Taniguchi M: Alternative pathway for the development of Valpha14(+) NKT cells directly from CD4(−)CD8(−) thymocytes that bypasses the CD4(+)CD8(+) stage. Nat Immunol 2017, 18:274–282. [DOI] [PubMed] [Google Scholar]
- 19.Roy S, Moore AJ, Love C, Reddy A, Rajagopalan D, Dave SS, Li L, Murre C, Zhuang Y: Id Proteins Suppress E2A-Driven Invariant Natural Killer T Cell Development prior to TCR Selection. Front Immunol 2018, 9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyazaki M, Miyazaki K, Chen K, Jin Y, Turner J, Moore AJ, Saito R, Yoshida K, Ogawa S, Rodewald HR, et al. : The E-Id Protein Axis Specifies Adaptive Lymphoid Cell Identity and Suppresses Thymic Innate Lymphoid Cell Development. Immunity 2017, 46:818–834 e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones R, Cosway EJ, Willis C, White AJ, Jenkinson WE, Fehling HJ, Anderson G, Withers DR: Dynamic changes in intrathymic ILC populations during murine neonatal development. Eur J Immunol 2018, 48:1481–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wencker M, Turchinovich G, Di Marco Barros R, Deban L, Jandke A, Cope A, Hayday AC: Innate-like T cells straddle innate and adaptive immunity by altering antigen- receptor responsiveness. Nat Immunol 2014, 15:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munoz-Ruiz M, Ribot JC, Grosso AR, Goncalves-Sousa N, Pamplona A, Pennington DJ, Regueiro JR, Fernandez-Malave E, Silva-Santos B: TCR signal strength controls thymic differentiation of discrete proinflammatory gammadelta T cell subsets. Nat Immunol 2016, 17:721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas JD, Ravens S, Duber S, Sandrock I, Oberdorfer L, Kashani E, Chennupati V, Fohse L, Naumann R, Weiss S, et al. : Development of interleukin-17-producing gammadelta T cells is restricted to a functional embryonic wave. Immunity 2012, 37:48–59. [DOI] [PubMed] [Google Scholar]
- 25.Grigoriadou K, Boucontet L, Pereira P: Most IL-4-producing gamma delta thymocytes of adult mice originate from fetal precursors. J Immunol 2003, 171:2413–2420. [DOI] [PubMed] [Google Scholar]
- 26.Buus TB, Odum N, Geisler C, Lauritsen JPH: Three distinct developmental pathways for adaptive and two IFN-gamma-producing gammadelta T subsets in adult thymus. Nat Commun 2017, 8:1911. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Using single-cell transcriptomics, this study profiles subsets of γδ T cells in the adult mouse thymus, identifying novel developmental intermediates in γδ T cell subset development and providing new insights into developmental origin of programmed vs programmable γδ T cells.
- 27.Muschaweckh A, Petermann F, Korn T: IL-1beta and IL-23 Promote Extrathymic Commitment of CD27(+)CD122(−) gammadelta T Cells to gammadeltaT17 Cells. J Immunol 2017, 199:2668–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papotto PH, Goncalves-Sousa N, Schmolka N, Iseppon A, Mensurado S, Stockinger B, Ribot JC, Silva-Santos B: IL-23 drives differentiation of peripheral gammadelta17 T cells from adult bone marrow-derived precursors. EMBO Rep 2017, 18:1957–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray EE, Ramirez-Valle F, Xu Y, Wu S, Wu Z, Karjalainen KE, Cyster JG: Deficiency in IL-17-committed Vgamma4(+) gammadelta T cells in a spontaneous Sox13-mutant CD45.1(+) congenic mouse substrain provides protection from dermatitis. Nat Immunol 2013, 14:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gray EE, Suzuki K, Cyster JG: Cutting edge: Identification of a motile IL-17-producing gammadelta T cell population in the dermis. J Immunol 2011, 186:6091–6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai Y, Xue F, Fleming C, Yang J, Ding C, Ma Y, Liu M, Zhang HG, Zheng J, Xiong N, et al. : Differential developmental requirement and peripheral regulation for dermal Vgamma4 and Vgamma6T17 cells in health and inflammation. Nat Commun 2014, 5:3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malhotra N, Qi Y, Spidale NA, Frascoli M, Miu B, Cho O, Sylvia K, Kang J: SOX4 controls invariant NKT cell differentiation by tuning TCR signaling. J Exp Med 2018. doi: 10.1084/jem.20172021. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engel I, Seumois G, Chavez L, Samaniego-Castruita D, White B, Chawla A, Mock D, Vijayanand P, Kronenberg M: Innate-like functions of natural killer T cell subsets result from highly divergent gene programs. Nat Immunol 2016, 17:728–739. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This study employs high-resolution single cell transciptional profiling to establish molecular distinctions amongst functionally discrete subsets of thymic invariant NKT cells, identifying significant overlaps with transcriptomes of other innate lymphoid subtypes, including γδ T cell subsets.
- 34.Spidale NA, K.; Narayan K; Miu B; Frascoli M; Melichar HJ; Zhihao W; Kisielow J; Palin A; Serwold T; Love P; Kobayashi M; Yoshimoto M; Jain N; Kang J: Interleukin 17 producing γδ T cells originate from SOX13+ progenitors that are independent of γδTCR signaling. Immunity 2018, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; ** Providing the most definitive support for the “stochatic” model of T cell lineage programming to date, this study is the first to identify a lineage-biased progenitor for Tγδ17 cells and demonstrates TCR-independent establishment of the Tγδ7 transcriptional program defined by the TFs SOX13 and TCF1.
- 35.Melichar HJ, Narayan K, Der SD, Hiraoka Y, Gardiol N, Jeannet G, Held W, Chambers CA, Kang J: Regulation of gammadelta versus alphabeta T lymphocyte differentiation by the transcription factor SOX13. Science 2007, 315:230–233. [DOI] [PubMed] [Google Scholar]
- 36.Kisielow J, Kopf M, Karjalainen K: SCART scavenger receptors identify a novel subset of adult gammadelta T cells. J Immunol 2008, 181:1710–1716. [DOI] [PubMed] [Google Scholar]
- 37.Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zuniga-Pflucker JC, Petrie HT: Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity 2004, 20:735–745. [DOI] [PubMed] [Google Scholar]
- 38.Nitta T, Muro R, Shimizu Y, Nitta S, Oda H, Ohte Y, Goto M, Yanobu-Takanashi R, Narita T, Takayanagi H, et al. : The thymic cortical epithelium determines the TCR repertoire of IL-17-producing gammadeltaT cells. EMBO Rep 2015, 16:638–653. [DOI] [PMC free article] [PubMed] [Google Scholar]; *
- 39.Mair F, Joller S, Hoeppli R, Onder L, Hahn M, Ludewig B, Waisman A, Becher B: The NFkappaB-inducing kinase is essential for the developmental programming of skin- resident and IL-17-producing gammadelta T cells. Elife 2015, 4 pii: e10087. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Along with Reference 38, this study identifies a surprising role for a specific subset of thymic epithelial cells in fostering proper development of Tγδ17 cells, raising the possibility for a subanatomical thymic niche necessary to support innate T cell development.
- 40.Kohlgruber AC, Gal-Oz ST, LaMarche NM, Shimazaki M, Duquette D, Nguyen HN, Mina AI, Paras T, Tavakkoli A, von Andrian U, et al. : gammadelta T cells producing interleukin-17A regulate adipose regulatory T cell homeostasis and thermogenesis. Nat Immunol 2018, 19:464–474. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Providing further support for the non-inflammatory role of innate-like tissue-resident T cells, this study identifies a significant role for crosstalk between Tγδ17 cells and adipose tissue regulatory T cells necessary to maintain normal thermogenesis.
- 41.Ciofani M, Knowles GC, Wiest DL, von Boehmer H, Zuniga-Pflucker JC: Stage-specific and differential notch dependency at the alphabeta and gammadelta T lineage bifurcation. Immunity 2006, 25:105–116. [DOI] [PubMed] [Google Scholar]
- 42.Garbe AI, Krueger A, Gounari F, Zuniga-Pflucker JC, von Boehmer H: Differential synergy of Notch and T cell receptor signaling determines alphabeta versus gammadelta lineage fate. J Exp Med 2006, 203:1579–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schmitt TM, Ciofani M, Petrie HT, Zuniga-Pflucker JC: Maintenance of T cell specification and differentiation requires recurrent notch receptor-ligand interactions. J Exp Med 2004, 200:469–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boiers C, Carrelha J, Lutteropp M, Luc S, Green JC, Azzoni E, Woll PS, Mead AJ, Hultquist A, Swiers G, et al. : Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell 2013, 13:535–548. [DOI] [PubMed] [Google Scholar]
- 45.Veinotte LL, Greenwood CP, Mohammadi N, Parachoniak CA, Takei F: Expression of rearranged TCRgamma genes in natural killer cells suggests a minor thymus- dependent pathway of lineage commitment. Blood 2006, 107:2673–2679. [DOI] [PubMed] [Google Scholar]
- 46.Yang Q, Saenz SA, Zlotoff DA, Artis D, Bhandoola A: Cutting edge: Natural helper cells derive from lymphoid progenitors. J Immunol 2011, 187:5505–5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samokhvalov IM: Deconvoluting the ontogeny of hematopoietic stem cells. Cell Mol Life Sci 2014, 71:957–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perdiguero EG, Geissmann F: The development and maintenance of resident macrophages. Nat Immunol 2016, 17:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshimoto M, Montecino-Rodriguez E, Ferkowicz MJ, Porayette P, Shelley WC, Conway SJ, Dorshkind K, Yoder MC: Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci U S A 2011, 108:1468–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshimoto M, Porayette P, Glosson NL, Conway SJ, Carlesso N, Cardoso AA, Kaplan MH, Yoder MC: Autonomous murine T-cell progenitor production in the extra- embryonic yolk sac before HSC emergence. Blood 2012, 119:5706–5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lu Y, Cao X, Zhang X, Kovalovsky D: PLZF Controls the Development of Fetal-Derived IL-17+Vgamma6+ gammadelta T Cells. J Immunol 2015, 195:4273–4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beaudin AE, Boyer SW, Perez-Cunningham J, Hernandez GE, Derderian SC, Jujjavarapu C, Aaserude E, MacKenzie T, Forsberg EC: A Transient Developmental Hematopoietic Stem Cell Gives Rise to Innate-like B and T Cells. Cell Stem Cell 2016, 19:768–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hirano M, Guo P, McCurley N, Schorpp M, Das S, Boehm T, Cooper MD: Evolutionary implications of a third lymphocyte lineage in lampreys. Nature 2013, 501:435–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boehm T, Hirano M, Holland SJ, Das S, Schorpp M, Cooper MD: Evolution of Alternative Adaptive Immune Systems in Vertebrates. Annu Rev Immunol 2018, 36:19–42. [DOI] [PubMed] [Google Scholar]
- 55.Flajnik MF: A Convergent Immunological Holy Trinity of Adaptive Immunity in Lampreys: Discovery of the Variable Lymphocyte Receptors. J Immunol 2018, 201:1331–1335. [DOI] [PubMed] [Google Scholar]
- 56.Han Q, Das S, Hirano M, Holland SJ, McCurley N, Guo P, Rosenberg CS, Boehm T, Cooper MD: Characterization of Lamprey IL-17 Family Members and Their Receptors. J Immunol 2015, 195:5440–5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buckley KM, Ho ECH, Hibino T, Schrankel CS, Schuh NW, Wang G, Rast JP: IL17 factors are early regulators in the gut epithelium during inflammatory response to Vibrio in the sea urchin larva. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee JS, Tato CM, Joyce-Shaikh B, Gulen MF, Cayatte C, Chen Y, Blumenschein WM, Judo M, Ayanoglu G, McClanahan TK, et al. : Interleukin-23-Independent IL-17 Production Regulates Intestinal Epithelial Permeability. Immunity 2015, 43:727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Seeking to understand the disparate effects of therapeutic targeting IL-17/Il-17R versus Il-23/IL- 23R in inflammatory bowel disease, this study identifies an IL-17 dependent mechanism regulating gut epithelial cell homeostasis that is separable from the IL-23 mediated inflammatory pathway.