A phosphate-anion pillared metal-organic framework can separate ethylene and ethane through synergism of equilibrium and kinetics.
Abstract
Physisorption is a promising technology to cut cost for separating ethylene (C2H4) from ethane (C2H6), the most energy-intensive separation process in the petrochemical industry. However, traditional thermodynamically selective adsorbents exhibit limited C2H4/C2H6 selectivity due to their similar physiochemical properties, and the performance enhancement is typically at the expense of elevated adsorption heat. Here, we report highly-efficient C2H4/C2H6 adsorption separation in a phosphate-anion pillared metal-organic framework ZnAtzPO4 exploiting the equilibrium-kinetic synergetic effect. The periodically expanded and contracted aperture decorated with electronegative groups within ZnAtzPO4 enables effective trapping of C2H4 and impedes the diffusion of C2H6, offering an extraordinary equilibrium-kinetic combined selectivity of 32.4. The adsorption heat of C2H4 on ZnAtzPO4 (17.3 to 30.0 kJ mol−1) is substantially lower than many thermodynamically selective adsorbents because its separation capability only partially relies on thermodynamics. The separation mechanism was explored by computational simulations, and breakthrough experiments confirmed the excellent C2H4/C2H6 separation performance of ZnAtzPO4.
INTRODUCTION
As an important feedstock in petrochemical industries, ethylene (C2H4) is one of the highest-yield chemicals in the world with a global production capacity of more than 170 million tons in 2016 (1). C2H4 is usually manufactured by steam cracking and thermal decomposition of naphtha or ethane (C2H6), and the product inevitably contains a certain amount of C2H6 impurity. To obtain polymer-grade C2H4 as the raw material for downstream high value-added products, it is necessary to remove the residual C2H6, which is a challenging task due to the very close molecular size of C2H6 (3.81 Å × 4.08 Å × 4.82 Å) and C2H4 (3.28 Å × 4.18 Å × 4.84 Å) and their similar physical properties (2, 3). Industrial separation of C2H4 and C2H6 is generally realized by energy-intensive cryogenic distillation that requires very harsh operation conditions (4), typically at temperature as low as −90° to −15°C and pressure up to 23 bar under a high reflux ratio in distillation towers installed with more than 150 trays (5). The energy used for C2H4/C2H6 and C3H6/C3H8 separation accounts for more than 0.3% of the global energy consumption; therefore, developing energy-efficient methods for C2H4/C2H6 separation is highly demanded and is recognized as one of the most important industrial tasks to change the world’s energy footprint (6).
Adsorption separation enabling efficient gas purification under mild conditions is an energy-saving alternative technology to cryogenic distillation (7–13), and the key lies in developing advanced porous materials (14–16). With regard to C2H4/C2H6 separation, introducing transition-metal ions and unsaturated metal sites into the adsorbents has been widely accepted as a feasible approach (5, 17–21), because they can selectively interact with π-electrons of C2H4 molecules. However, the π-complexation–related materials generally present limited stability, especially in the presence of moisture and sulfides (22), and demand high energy cost for the adsorbents’ regeneration because of their strong affinity to C2H4 (23). Porous materials that are amenable to achieve complete molecular sieving are ideal for C2H4/C2H6 separation (24). However, to precisely control aperture size to a critical range necessary to exclude C2H6 is very challenging, and pores with such limited size typically lead to low diffusion rate and gas uptake. Considering that a practical separation process is simultaneously controlled by effects that arise from thermodynamics and kinetics, adsorbents that can exploit both equilibrium and kinetic selectivity are appealing for gas capture and purification (25, 26) but intractable to design and prepare. Previously, most porous materials reported for C2H4/C2H6 separation are principally based on their discrepant thermodynamic affinity for the guests (27–30). Only a few of zeolites and coordination polymer have been described as suitable substances for kinetic separation (31–33), largely because the analogous dimensions of C2H4 and C2H6 bring critical difficulty to fabricate pores with appropriate sizes that permit the passing of C2H4 while limiting the diffusion of slightly bulkier C2H6 molecules. Moreover, the ultimate efficiencies of reported kinetically selective adsorbents are basically confined by disadvantageous thermodynamic effects, in which case the capacity of C2H6 at the equilibrium state is quite close to or even exceeds that of C2H4 under a wide pressure range. These observations suggest that there is still a broad space to further enhance the separation of C2H4 and C2H6 by developing porous materials with optimal adsorption thermodynamics and kinetics.
Here, we reveal the high-efficient separation of C2H4 and C2H6 in a phosphate-anion (PO43−) pillared metal-organic framework (MOF) {Zn3(Atz)3(PO4)}∞ (ZnAtzPO4; Atz = 3-amino-1,2,4-triazole) by exploiting synergetic effect of equilibrium and kinetics. The material features periodically expanded and contracted pore decorated by electronegative groups, which provides sufficient binding sites for C2H4 and effectively impedes the diffusion of C2H6, inducing an outstanding recognition ability to C2H4 over C2H6. The equilibrium-kinetic combined selectivity (32.4), as well as C2H4 capacity of ZnAtzPO4, outperforms those of the state-of-the-art materials. The extraordinary performance was achieved along with ultralow adsorption heat (17.3 kJ mol−1 for C2H4 at zero loading), and gas molecules adsorbed on the material can be easily removed at ambient temperature, indicating the promising prospect of the material for industrial application. DFT-D (dispersion-corrected density functional theory) calculations and molecular dynamics (MD) simulations were used to give insights into the unique separation mechanism, and breakthrough experiments for the C2H4/C2H6 (50:50, v/v) mixture were carried out to confirm the excellent performance of ZnAtzPO4.
RESULTS
Pore structure and C2H4/C2H6 adsorption property
ZnAtzPO4 was prepared by hydrothermal reaction of phosphoric acid, zinc carbonate basic, and Atz in a mixture of water, methanol, and aqueous ammonia under 180°C for 2 days (34). The structure of ZnAtzPO4 contains two-dimensional cationic layers (fig. S1), which are fabricated by Zn2+ cations and deprotonated Atz ligands in both tridentate and bidentate coordination modes. The organic ligands only connect to Zn2+ cations via nitrogen atoms contained in the triazole rings, and the amino groups are all free of coordination. The layers are further pillared by PO43− anions, resulting in the final porous framework of ZnAtzPO4. As shown in Fig. 1A and fig. S1, the channel of ZnAtzPO4 is decorated by intruding amino groups from bidentate-coordinated Atz ligands (highlighted by rose), which are arranged in an antiparallel manner along a, and abundant electronegative oxygen atoms from PO43− anion pillars alongside the channel. The pillars adopt a staggered fashion, periodically contracting and expanding the cross section of the channel (Fig. 1B); therefore, the channel can be vividly described as iterant pocket-like space interconnected by narrow bottleneck structure. The pocket-like space each contains two symmetric passages (Fig. 1C) with the same size of 4.94 Å, which is enough to accommodate both C2H4 and C2H6 guests. In contrast, the neck is much narrower (3.82 Å, distance between N…N; Fig. 1D), which is quite close to the minimum dimension of C2H6 (3.81 Å) but apparently larger than that of C2H4 (3.28 Å). We anticipated that this delicate pocket-like structure would facilitate C2H4 trapping, but the narrow bottleneck would probably set a barrier for C2H6 to diffuse in the channel of ZnAtzPO4.
Fig. 1. Schematic illustration of the structure of ZnAtzPO4.
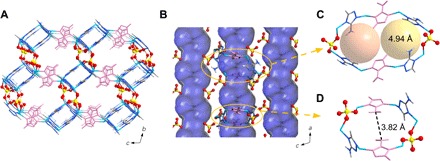
(A) Three-dimensional structure of ZnAtzPO4 viewed from a. (B) Connolly surface indicating periodically expanded and contracted cross-section area of ZnAtzPO4. (C) Schematic representation of the pocket-like space containing two symmetric passages. (D) Local environment and aperture size of the narrow bottleneck structure. [Color mode: C, gray (40%); H, gray (25%); N, light blue; Zn, sky blue; P, yellow; O, red. Bidentate-coordinated Atz ligands are highlighted by rose.]
Inspired by the eligible structure of ZnAtzPO4, we further analyzed its thermodynamic and kinetic adsorption characteristics for C2H4 and C2H6 by measuring single-component adsorption isotherms and time-dependent gas uptake profiles. From the adsorption isotherms shown in Fig. 2A, ZnAtzPO4 has a C2H4 uptake of 1.92 mmol g−1 at 298 K and 1 bar, equivalent to 3.04 mmol cm−3 (table S1). The adsorption capacity of C2H4 is higher than C2H6 in the whole pressure range, indicative of its preferable thermodynamic affinity for C2H4. The C2H4/C2H6 uptake ratio reached 1.85 at 1 bar, exceeding that of some excellently performing equilibrium-based porous materials for C2H4/C2H6 separation, such as HKUST-1 (1.19) (35), zeolite 5A (1.42) (36), and PAF-1-SO3Ag (1.82) (5). As the temperature reduced to 273 K, the gas uptake ratio further increased to 2.04, and the amount of C2H4 captured attained 2.41 mmol g−1. Meanwhile, kinetic studies suggested that the material exhibited much faster adsorption rate for C2H4 than C2H6. As shown in Fig. 2B, the adsorption of C2H4 reached equilibrium within about 40 min at 298 K and about 45 min at 273 K, with the capacities consistent to those taken from adsorption isotherms. After a continuous recording for 150 min, the amount of C2H6 adsorbed on the sample still has not reached that under the equilibrium state and persists climbing up gradually. Fitting the data with micropore diffusion model gave a kinetic selectivity of 36.6 at 298 K and up to 140.7 at 273 K (table S1), highlighting the potential of ZnAtzPO4 to separate the C2H4/C2H6 mixture through a unique equilibrium-kinetic synergetic effect, which has rarely been observed in MOFs (37). To objectively compare the separation performance of ZnAtzPO4 with other adsorbents ever reported for C2H4/C2H6 separation, we further calculated the equilibrium-kinetic combined selectivity (38) based on their diffusivities and Henry’s constants for C2H4 and C2H6 gases. As shown in Fig. 2C and table S1, ZnAtzPO4 displays a dramatic combined selectivity reaching up to 32.4 at 273 K. At ambient temperature, the selectivity is calculated as 12.4, higher than those of other kinetically selective adsorbents measured under similar conditions, like ITQ-29 (~1.31), Si-CHA (~1.31), and ITQ-55 (~6.4) (31, 32).
Fig. 2. Single-component gas adsorption properties.
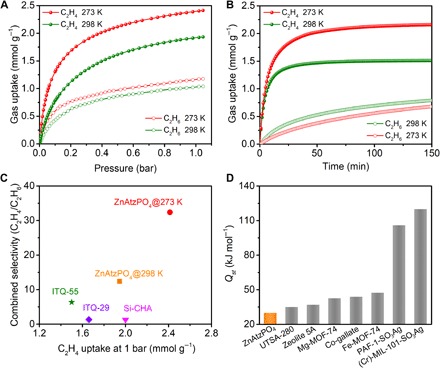
(A) Single-component adsorption isotherms of C2H4 and C2H6 on ZnAtzPO4 under 298 and 273 K. (B) Time-dependent gas uptake profiles of C2H4 and C2H6 at 0.4 bar and different temperatures. (C) Equilibrium-kinetic combined selectivity (C2H4/C2H6) and C2H4 uptake of ZnAtzPO4, ITQ-29, Si-CHA, and ITQ-55. Data for ITQ-55 were collected at 303 K, and those for ITQ-29 and Si-CHA were collected at 301 K. (D) Highest value of Qst calculated for C2H4 on ZnAtzPO4, UTSA-280, zeolite 5A, Mg-MOF-74, Co-gallate, Fe-MOF-74, PAF-1-SO3Ag, and (Cr)-MIL-101-SO3Ag.
To evaluate the strength of interactions between ZnAtzPO4 and C2H4/C2H6 gases, the isosteric heat of adsorption (Qst) was calculated for C2H4 and C2H6 based on their adsorption isotherms under three different temperatures using the Clausius-Clapeyron equation (fig. S2). The material exhibits moderate Qst for C2H4, with the value varying from 17.31 to 29.98 kJ mol−1. It is worth mentioning that the maximum value of Qst for C2H4 is remarkably lower than those of UTSA-280 (35.0 kJ mol−1) (24), zeolite 5A (37 kJ mol−1) (36), Co-gallate (44 kJ mol−1) (3), and many other adsorbents containing transition-metal ions and unsaturated metal sites, such as Mg-MOF-74 (42.6 kJ mol−1) (39), Fe-MOF-74 (47.5 kJ mol−1) (20), PAF-1-SO3Ag (106 kJ mol−1) (5), and (Cr)-MIL-101-SO3Ag (120 kJ mol−1) (21) (Fig. 2D). The moderate heat of adsorption reveals the possibility to regenerate this material under mild conditions; thereby, oligomerization of C2H4 that may happen under the catalysis of open metal sites could be avoided. For equilibrium-based adsorbents, the effective adsorption separation of C2H4 and C2H6 principally relies on the discrepancy between the affinities of the gases to the adsorbent; here, usually high isosteric heat is required for the preferentially adsorbed guest to achieve desirable selectivity. In the case of ZnAtzPO4, the excellent selectivity achieved under the modest Qst largely owns its way to exploit the synergetic effect of equilibrium and kinetics, which means that the separation capability of ZnAtzPO4 is only partially reliant on thermodynamics. The kinetic characteristics of ZnAtzPO4 also contribute to maximize the purification performance, without affecting the adsorption heat.
Besides, ZnAtzPO4 presents great stability to air and moisture. After the sample was exposed to humid atmosphere (298 K, 70% humidity) for 4 weeks, no obvious change in its powder x-ray diffractometry (PXRD) pattern could be observed as compared to the as-synthesized sample (fig. S3). C2H4/C2H6 adsorption isotherms and time-dependent gas uptakes measured on the sample revealed that, after such treatment, their sorption properties still remained almost unchanged (fig. S3). Furthermore, thermal gravimetric analysis (TGA) indicates that this material exhibits excellent thermal stability, with the decomposition temperature approaching 420°C (fig. S4). These results qualify ZnAtzPO4 as a promising candidate for industrial separation of C2H4 and C2H6.
Exploring separation mechanism by computational method
To gain insights into the unusual equilibrium-kinetic synergetic effect of ZnAtzPO4 to effectively adsorb C2H4, first-principles DFT-D calculations were conducted to explore the preferential binding sites. The computational results demonstrate that ZnAtzPO4 provides two distinct binding sites for C2H4 (Fig. 3, A and B). The capacious pocket-like space each contains two symmetric apertures (Fig. 1C), and they offer identical binding site (site I) to the olefin and can be reckoned as molecule traps. In this site, C2H4 molecule interacts with the channel mainly through weak hydrogen bonding interactions (Fig. 3A). It locates close to the pillaring PO43− anions and bonds to the surrounding oxygen atoms from three different pillars via C─H…O hydrogen bonds. The shortest C─H…O bond has a length of 2.65 Å, while the others are longer and range from 2.90 to 3.16 Å. In addition, the C2H4 guest also strengthens its interaction with ZnAtzPO4 by forming C─H…N hydrogen bonds with nitrogen atom coming from both amino group and triazole ring of the Atz ligands, with lengths of 2.54 to 3.16 Å. The second binding site (site II, Fig. 3B) for C2H4 is near the center of the bottleneck structure, which joins two adjacent pockets. The mechanism for C2H4 to interact with the adsorbent in this site is quite different from that in site I (Fig. 3A), where the molecular interactions are mainly dominated by hydrogen bonds. In site II, there is only one quite weak C─H…O bond formed between C2H4 and the PO43− pillar, with the length being 3.05 Å. Because of the specific chemical environment, the adsorbate is mainly stabilized by supermolecular N─H…C interactions with hydrogen atoms of the intruding amino groups from the bidentate-coordinated Atz ligands, and their lengths are in the range of 2.94 to 3.18 Å. The fact that there is a lack of strong hydrogen bond (C─H…O/N < 2.3 Å) (2) in sites I and II proves that the adsorbate merely interacts with the pore through weak intermolecular interactions, consistent to the quite modest value of Qst as has been calculated. Furthermore, the DFT-D study on C2H6 reveals that unlike C2H4, the C2H6 molecule can only approach the binding site in the capacious pocket-like space (fig. S5). The molecular dimension of C2H6 is comparable to the size of the bottleneck structure, and it induces a steric hindrance to prevent the bottleneck to expose the second binding site to C2H6. The distinct modes between C2H4 and C2H6 to interact with the pore may be the reason that endows ZnAtzPO4 with the excellent thermodynamic selectivity.
Fig. 3. DFT-D–calculated preferable binding sites for C2H4 in ZnAtzPO4 and distribution of the aperture size of the bottleneck calculated from MD simulations.
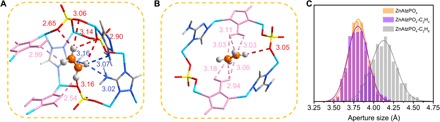
(A) Site I. (B) Site II. [Color mode: C, gray (40%); H, gray (25%); N, light blue; Zn, sky blue; P, yellow; O, red. Bidentate-coordinated Atz ligands are highlighted by rose. Broken lines refer to C─H…N/O hydrogen bonds and supermolecular N─H…C interactions. All interatomic distances are in angstroms.] (C) Distribution of the aperture size of the bottleneck for empty structure of ZnAtzPO4 (orange), ZnAtzPO4 with C2H4 molecules (violet), and ZnAtzPO4 with C2H6 molecules (gray) at 298 K.
The diffusion behaviors of C2H4 and C2H6 in the pore of ZnAtzPO4 were further investigated by performing MD simulations to reveal the kinetic characteristics. The simulations demonstrate that, when each cell of ZnAtzPO4 contains two gas molecules, the diffusivities of single-component C2H4 and C2H6 are 2.07 × 10−10 m2 s−1 and 7.17 × 10−12 m2 s−1 under 298 K. The ideal kinetic selectivity is calculated as 29 and agrees well with the experimental measurement (~36). For the equimolar C2H4/C2H6 gas mixture, the diffusivities of C2H4 and C2H6 are 1.12 × 10−10 m2 s−1 and 7.47 × 10−12 m2 s−1, respectively. Although the adsorption kinetics of C2H4 in the mixture declines as compared to its single component maybe due to the steric effect of slow-diffusing C2H6, the material still exhibits a prominent kinetic selectivity of 15. The results also indicate that after introducing C2H6 molecules to the channel of ZnAtzPO4, the bottleneck exhibits obvious transient structural transformations to adapt to the sluggish passing of C2H6, and the aperture size prominently increases to 4.1 Å, about 0.3 Å larger than that without gas molecules (Fig. 3C). Snapshots (fig. S6) show that the expansion of the bottleneck-like window is mainly facilitated by slight rotation of the bidentate-coordinated Atz ligands that intrude amino groups to the channel. On the contrary, study on the diffusion of C2H4 reveals that after inserting the gas molecule to the channel, no obvious change in the aperture size of the bottleneck was observed, and its probability distribution is quite consistent to that of an empty host (Fig. 3C). These results suggest that the diffusion of C2H6 in the channel strongly relies on the flexibility of ZnAtzPO4, considering that the size of the bottleneck (3.82 Å) at the ground state is so close to the extreme size (3.81 Å) to allow the passing of C2H6, setting a barrier for its penetration. The much smaller dimension of C2H4 (3.28 Å) allows it to travel along the pore more freely, without the necessity to expand the narrow windows, which thereby induces the marked kinetic selectivity of ZnAtzPO4.
Breakthrough experiments
It is worth emphasizing that removal of C2H6 from C2H4 is yet a technical challenge in industry. To further probe the validity of the equilibrium-kinetic synergetic effect for C2H4/C2H6 separation, breakthrough experiments for a C2H4/C2H6 (50:50, v/v) gas mixture were performed on a stainless column packed with the ZnAtzPO4 material at 273 K and 1 bar. As shown in Fig. 4A, the component of C2H6 broke through the column quickly after 26 min, whereas C2H4 was retained in the adsorption bed for nearly 70 min. The retention time for C2H4 is two times more than that of C2H6. Moreover, the elution of C2H4 was accompanied by a remarkable roll-up phenomenon of C2H6, meaning that the C2H6 molecules that have already been adsorbed can be largely displaced by the olefin, indicative of the excellent competition ability of C2H4 over C2H6 on the binding sites of ZnAtzPO4. After the concentration of eluting gas remained unchanged, the amount of C2H4 adsorbed into the column reached 1.80 mmol g−1, equivalent to 6.7 times that of C2H6 (0.27 mmol g−1) (fig. S7), highlighting the great efficiency of ZnAtzPO4 for actual C2H4/C2H6 adsorption separation. Besides, recycling measurements reveal that ZnAtzPO4 can retain its separation capability, with the breakthrough time being almost unchanged within five cycles (Fig. 4B and fig. S7). After simple desorption procedure manipulated by purging the material with inert gas (He) at ambient temperature, the column was well regenerated and reserved similar breakthrough curve (fig. S7), benefitting from the very modest adsorption heat as has been calculated. This further presents ZnAtzPO4 as a brilliant microporous material for industrial separation of C2H4 and C2H6. By exploiting the equilibrium-kinetic synergetic effect, we successfully achieved the high-efficient C2H4/C2H6 adsorption separation with ZnAtzPO4. With the solid empirical evidence, we believe that it would bring a new train of thoughts and tactics for the splitting of close-boiling light hydrocarbons.
Fig. 4. Breakthrough curve and recycling tests for C2H4/C2H6 gas mixture on ZnAtzPO4.
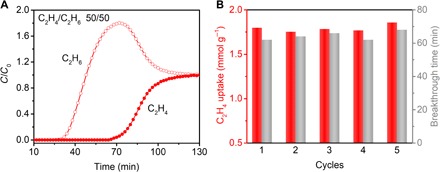
(A) Breakthrough curve of ZnAtzPO4 for C2H4/C2H6 gas mixture (50:50, v/v) at 273 K and 1 bar with a flow rate of 0.75 ml/min. (B) Recycling breakthrough tests for C2H4/C2H6 (50:50, v/v) separation with ZnAtzPO4.
DISCUSSION
In summary, we reported the separation of C2H4/C2H6 in a phosphate-anion pillared microporous MOF, which exhibits a unique equilibrium-kinetic combined selectivity to the olefin and is attractive for practical separation. The equilibrium-kinetic synergetic effect of ZnAtzPO4 mainly originates from its delicate pore structure, featuring periodically expanded and contracted cross section. Its minimum aperture size approaches the limit for C2H6 to pass through, and the diffusion of C2H6 depends on transient structural transformations of the neck-like structure, which seriously slows down the diffusion rate of the paraffin. On the other hand, the pore of ZnAtzPO4 is decorated with abundant electronegative functional groups that are amenable to construct stable interaction network with C2H4 and trap the olefin efficiently. Computational methods were applied to explore the binding sites of the guest molecules and further verify the faster adsorption kinetics of C2H4. Moreover, recycling breakthrough experiment for the C2H4/C2H6 gas mixture (50:50, v/v) was carried out, and it confirmed the outstanding capability of ZnAtzPO4 for C2H4/C2H6 separation.
As most porous materials ever reported for C2H4/C2H6 separation are typically based on single mechanism (either thermodynamic or kinetic), examples to explore synergetic effect of equilibrium and kinetics in this regard are rare. The unprecedented selectivity achieved on ZnAtzPO4 proves that the strategy conveyed by this work is feasible and practical and highlights a broad space to further boost the adsorption separation of C2H4 and C2H6 in a newfangled way. Taking advantage of such synergetic effect allows highly efficient selective adsorption of C2H4 under modest Qst, because the separation process only partially relies on thermodynamics. As a glaring merit, the material can be regenerated under mild conditions with less energy consumption. In brief, this work not only provides a porous material with impressive C2H4 purification performance but also brings a new strategy for developing the next-generation materials for energy-efficient gas separation.
MATERIALS AND METHODS
Experimental design
Chemicals
All the chemicals were obtained from commercial resources and used as received without any further purification. Methanol (anhydrous, 99%) was purchased from Sigma-Aldrich. Phosphoric acid [85 weight % (wt %)], ammonium hydroxide (30 wt %), and 3-amino-1,2,4-triazole (96%) were purchased from Macklin. 3Zn(OH)2·2ZnCO3 was purchased from Strem Chemicals.
Synthesis of {Zn3(Atz)3(PO4)}∞ (ZnAtzPO4)
Samples of ZnAtzPO4 were synthesized according to the literature report (34) with minor modifications. A mixture containing 0.035 g of phosphoric acid, 0.1 g of 3Zn(OH)2·2ZnCO3, 0.4 g of 3-amino-1,2,4-triazole, 2 ml of H2O, and 2 ml of methanol was added to a Teflon tube, then sealed and placed in an oven with a temperature of 180°C for 48 hours, and cooled to room temperature naturally. The colorless precipitation was collected by filtration, then washed with methanol, and dried in air. Last, the product was heated at 60°C under high vacuum for 2 hours and then at 100°C for another 12 hours to obtain the activated sample of ZnAtzPO4.
Characterization methods
PXRD data were collected on a SHIMADZU XRD-600 diffractometer (Cu Kαλ = 1.540598 Å) with an operating power of 40 kV, 30 mA, and a scan speed of 4.0°/min. The range of 2θ was from 5° to 50°. TGA data for activated sample of ZnAtzPO4 were recorded on an apparatus of TGA Q500 V20.13 Build 39, from room temperature to 800°C, with a ramp of 10°/min under N2 atmosphere.
Kinetic adsorption measurement
The time-dependent adsorption profiles of C2H4 and C2H6 were measured on Intelligent Gravimetric Analyzer (IGA-100, HIDEN). About 100 mg of ZnAtzPO4 was first loaded to the sample chamber and activated at 100°C under high vacuum for 4 hours. After being cooled to specific temperature, the chamber was backfilled with He, until the pressure reached 0.4 bar. Upon the analysis started, a single-component gas of C2H4 or C2H6 was introduced into the chamber at a rate of 35 ml/min. The mass of the sample loaded with gas molecules was continuously recorded for 150 min.
Statistical analysis
The equilibrium-kinetic combined selectivity (Sij) is defined as (38)
| (1) |
In Eq. 1, αij represents the separation selectivity based on thermodynamic equilibrium alone and can be calculated from the ratio of Henry’s constants (Eq. 2). βij represents the kinetic selectivity based on diffusion rates of the gas molecules and can be obtained from the ratio of diffusion time constants (Eq. 3)
| (2) |
| (3) |
In Eq. 3, D′ (Dc/rc2) can be further derived from the following micropore diffusion model (Eq. 4), where mt is the gas uptake at time t, m∞ is the gas uptake at equilibrium, Dc is the intracrystalline diffusivity of gas molecules in porous media, and rc is the radius of the equivalent spherical particle. D′ can be obtained from the square of the slope ( plotted against ) multiplied by π/36
| (4) |
Supplementary Material
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (nos. 21938011, 21725603, and U1862110), the Zhejiang Provincial Natural Science Foundation of China (no. LZ18B060001), and the National Supercomputing Center in Shenzhen. Author contributions: Q.D. carried out the experimental work on synthesis, adsorption isotherm measurements, and breakthrough tests and performed the computational simulations. Z.Z. and C.Y. took part in synthesis and recycling tests. P.Z., J.W., and S.D. measured time-dependent gas uptake profiles of C2H4 and C2H6. X.C. and C.-H.H. took part in modeling studies. H.X. and Q.D. conceived the idea and analyzed the results. All authors contributed to the final version of the manuscript. Competing interests: H.X., X.C., and Q.D. are inventors on a patent related to this work filed by Zhejiang University (no. 201910084873.8, filed on 29 January 2019). The authors declare no other competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/15/eaaz4322/DC1
REFERENCES AND NOTES
- 1.Li L., Lin R.-B., Krishna R., Li H., Xiang S., Wu H., Li J., Zhou W., Chen B., Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites. Science 362, 443–446 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Liao P.-Q., Zhang W.-X., Zhang J.-P., Chen X.-M., Efficient purification of ethene by an ethane-trapping metal-organic framework. Nat. Commun. 6, 8697 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bao Z., Wang J., Zhang Z., Xing H., Yang Q., Yang Y., Wu H., Krishna R., Zhou W., Chen B., Ren Q., Molecular sieving of ethane from ethylene through the minimum molecular dimension differentiation in gallate-based metal-organic frameworks. Angew. Chem. Int. Ed. Engl. 57, 16020–16025 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rungta M., Zhang C., Koros W. J., Xu L., Membrane-based ethylene/ethane separation: The upper bound and beyond. AIChE J. 59, 3475–3489 (2013). [Google Scholar]
- 5.Li B., Zhang Y., Krishna R., Yao K., Han Y., Wu Z., Ma D., Shi Z., Pham T., Space B., Liu J., Thallapally P. K., Liu J., Chrzanowski M., Ma S., Introduction of π-complexation into porous aromatic framework for highly selective adsorption of ethylene over ethane. J. Am. Chem. Soc. 136, 8654–8660 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Sholl D. S., Lively R. P., Seven chemical separations to change the world. Nature 532, 435–437 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Peng J., Wang H., Olson D. H., Li Z., Li J., Efficient kinetic separation of propene and propane using two microporous metal organic frameworks. Chem. Commun. 53, 9332–9335 (2017). [DOI] [PubMed] [Google Scholar]
- 8.Cadiau A., Adil K., Bhatt P. M., Belmabkhout Y., Eddaoudi M., A metal-organic framework-based splitter for separating propylene from propane. Science 353, 137–140 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Li J.-R., Sculley J., Zhou H.-C., Metal-organic frameworks for separations. Chem. Rev. 112, 869–932 (2012). [DOI] [PubMed] [Google Scholar]
- 10.Chen B., Liang C., Yang J., Contreras D. S., Clancy Y. L., Lobkovsky E. B., Yaghi O. M., Dai S., A microporous metal-organic framework for gas-chromatographic separation of alkanes. Angew. Chem. Int. Ed. Engl. 45, 1390–1393 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Cui X., Chen K., Xing H., Yang Q., Krishna R., Bao Z., Wu H., Zhou W., Dong X., Han Y., Li B., Ren Q., Zaworotko M. J., Chen B., Pore chemistry and size control in hybrid porous materials for acetylene capture from ethylene. Science 353, 141–144 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Yang L., Cui X., Yang Q., Qian S., Wu H., Bao Z., Zhang Z., Ren Q., Zhou W., Chen B., Xing H., A single-molecule propyne trap: Highly efficient removal of propyne from propylene with anion-pillared ultramicroporous materials. Adv. Mater. 30, 1705374 (2018). [DOI] [PubMed] [Google Scholar]
- 13.Zhang Z., Yang Q., Cui X., Yang L., Bao Z., Ren Q., Xing H., Sorting of C4 olefins with interpenetrated hybrid ultramicroporous materials by combining molecular recognition and size-sieving. Angew. Chem. Int. Ed. 129, 16500–16505 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Zhai Q.-G., Bu X., Mao C., Zhao X., Daemen L., Cheng Y., Ramirez-Cuesta A. J., Feng P., An ultra-tunable platform for molecular engineering of high-performance crystalline porous materials. Nat. Commun. 7, 13645 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z.-J., Shi W., Niu Z., Li H.-H., Zhao B., Cheng P., Liao D.-Z., Yan S. P., A new type of polyhedron-based metal-organic frameworks with interpenetrating cationic and anionic nets demonstrating ion exchange, adsorption and luminescent properties. Chem. Commun. 47, 6425–6427 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Bu X.-H., Tong M.-L., Chang H.-C., Kitagawa S., Batten S. R., A neutral 3D copper coordination polymer showing 1D open channels and the first interpenetrating NbO-type network. Angew. Chem. Int. Ed. 43, 192–195 (2004). [DOI] [PubMed] [Google Scholar]
- 17.Aguado S., Bergeret G., Daniel C., Farrusseng D., Absolute molecular sieve separation of ethylene/ethane mixtures with silver zeolite A. J. Am. Chem. Soc. 134, 14635–14637 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Böhme U., Barth B., Paula C., Kuhnt A., Schwieger W., Mundstock A., Caro J., Hartmann M., Ethene/ethane and propene/propane separation via the olefin and paraffin selective metal-organic framework adsorbents CPO-27 and ZIF-8. Langmuir 29, 8592–8600 (2013). [DOI] [PubMed] [Google Scholar]
- 19.Uchida S., Kawamoto R., Tagami H., Nakagawa Y., Mizuno N., Highly selective sorption of small unsaturated hydrocarbons by nonporous flexible framework with silver ion. J. Am. Chem. Soc. 130, 12370–12376 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Bloch E. D., Queen W. L., Krishna R., Zadrozny J. M., Brown C. M., Long J. R., Hydrocarbon separations in a metal-organic framework with open iron(II) coordination sites. Science 335, 1606–1610 (2012). [DOI] [PubMed] [Google Scholar]
- 21.Chang G., Huang M., Su Y., Xing H., Su B., Zhang Z., Yang Q., Yang Y., Ren Q., Bao Z., Chen B., Immobilization of Ag(I) into a metal-organic framework with -SO3H sites for highly selective olefin-paraffin separation at room temperature. Chem. Commun. 51, 2859–2862 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Cadiau A., Belmabkhout Y., Adil K., Bhatt P. M., Pillai R. S., Shkurenko A., Martineau-Corcos C., Maurin G., Eddaoudi M., Hydrolytically stable fluorinated metal-organic frameworks for energy-efficient dehydration. Science 356, 731–735 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Li L., Lin R.-B., Wang X., Zhou W., Jia L., Li J., Chen B., Kinetic separation of propylene over propane in a microporous metal-organic framework. Chem. Eng. J. 354, 977–982 (2018). [Google Scholar]
- 24.Lin R.-B., Li L., Zhou H.-L., Wu H., He C., Li S., Krishna R., Li J., Zhou W., Chen B., Molecular sieving of ethylene from ethane using a rigid metal–organic framework. Nat. Mater. 17, 1128–1133 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Nugent P., Belmabkhout Y., Burd S. D., Cairns A. J., Luebke R., Forrest K., Pham T., Ma S., Space B., Wojtas L., Eddaoudi M., Zaworotko M. J., Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 495, 80–84 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Wang Y., Huang N.-Y., Zhang X.-W., He H., Huang R.-K., Ye Z.-M., Li Y., Zhou D.-D., Liao P.-Q., Chen X.-M., Zhang J.-P., Selective aerobic oxidation of a metal-organic framework boosts thermodynamic and kinetic propylene/propane selectivity. Angew. Chem. Int. Ed. Engl. 58, 7692–7696 (2019). [DOI] [PubMed] [Google Scholar]
- 27.Lin R.-B., Wu H., Li L., Tang X.-L., Li Z., Gao J., Cui H., Zhou W., Chen B., Boosting ethane/ethylene separation within isoreticular ultramicroporous metal-organic frameworks. J. Am. Chem. Soc. 140, 12940–12946 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Wang Y., Yuan S., Hu Z., Kundu T., Zhang J., Peh S. B., Cheng Y., Dong J., Yuan D., Zhou H.-C., Zhao D., Pore size reduction in zirconium metal–organic fameworks for ethylene/ethane separation. ACS Sustainable Chem. Eng. 7, 7118–7126 (2019). [Google Scholar]
- 29.Bao Z., Chang G., Xing H., Krishna R., Ren Q., Chen B., Potential of microporous metal–organic frameworks for separation of hydrocarbon mixtures. Energ. Environ. Sci. 9, 3612–3641 (2016). [Google Scholar]
- 30.Yang S., Ramirez-Cuesta A. J., Newby R., Garcia-Sakai V., Manuel P., Callear S. K., Campbell S. I., Tang C. C., Schröder M., Supramolecular binding and separation of hydrocarbons within a functionalized porous metal–organic framework. Nat. Chem. 7, 121–129 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Bereciartua P. J., Cantín Á., Corma A., Jordá J. L., Palomino M., Rey F., Valencia S., Corcoran Jr E. W., Kortunov P., Ravikovitch P. I., Burton A., Yoon C., Wang Y., Paur C., Guzman J., Bishop A. R., Casty G. L., Control of zeolite framework flexibility and pore topology for separation of ethane and ethylene. Science 358, 1068–1071 (2017). [DOI] [PubMed] [Google Scholar]
- 32.Hedin N., Demartin G. J., Roth W. J., Strohmaier K. G., Reyes S. C., PFG NMR self-diffusion of small hydrocarbons in high silica DDR, CHA and LTA structures. Microporous Mesoporous Mater. 109, 327–334 (2008). [Google Scholar]
- 33.Gu C., Hosono N., Zheng J.-J., Sato Y., Kusaka S., Sakaki S., Kitagawa S., Design and control of gas diffusion process in a nanoporous soft crystal. Science 363, 387–391 (2019). [DOI] [PubMed] [Google Scholar]
- 34.Vaidhyanathan R., Iremonger S. S., Shimizu G. K. H., Boyd P. G., Alavi S., Woo T. K., Competition and cooperativity in carbon dioxide sorption by amine-functionalized metal-organic frameworks. Angew. Chem. Int. Ed. 51, 1826–1829 (2012). [DOI] [PubMed] [Google Scholar]
- 35.He Y., Krishna R., Chen B., Metal-organic frameworks with potential for energy-efficient adsorptive separation of light hydrocarbons. Energ. Environ. Sci. 5, 9107–9120 (2012). [Google Scholar]
- 36.Mofarahi M., Salehi S. M., Pure and binary adsorption isotherms of ethylene and ethane on zeolite 5A. Adsorption 19, 101–110 (2013). [Google Scholar]
- 37.Bao Z., Alnemrat S., Yu L., Vasiliev I., Ren Q., Lu X., Deng S., Kinetic separation of carbon dioxide and methane on a copper metal-organic framework. J. Colloid Interface Sci. 357, 504–509 (2011). [DOI] [PubMed] [Google Scholar]
- 38.R. T. Yang, Adsorbents: Fundamentals and Applications (Wiley-Interscience, 2003). [Google Scholar]
- 39.Bao Z., Alnemrat S., Yu L., Vasiliev I., Ren Q., Lu X., Deng S., Adsorption of ethane, ethylene, propane, and propylene on a magnesium-based metal-organic framework. Langmuir 27, 13554–13562 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/15/eaaz4322/DC1


