Abstract
Background:
Knowledge of HIV status is the entry point for linkage to prevention, care, and treatment, and the first step towards achieving the UNAIDS 90–90-90 targets. Most countries rely on proxies for estimating testing saturation, including periodic population-based sampling and yield (number positive among those tested). We conducted a community-based “Hybrid” HIV testing services (HTS) program to identify persons unaware of their HIV-positive status.
Setting:
Homa Bay County, Kenya; July through September 2016
Methods:
We conducted community mapping, household census, multi-disease community health campaigns (CHCs), and home-based tracking. HIV testing eligibility was based on 2015 national guidelines. The previously-unidentified fraction (PUF) was defined as the proportion of newly-identified persons living with HIV (PLWH) out of all previously-identified and newly-identified PLWH.
Results:
The Hybrid HTS program reached 28,885 persons in total: 25,340 residents and 3,545 non-residents. There were 19,288 persons reached through CHCs and tracking. Of 11,316 individuals eligible for HIV testing, 9,463 (83%) accepted testing, including 1,230 (13%) first-time testers. There were 115 newly-identified PLWH out of 1,589 total HIV-positive persons, representing a 7.2% PUF. Of 93 newly-identified PLWH at the CHCs, 68% initiated same-day antiretroviral therapy.
Conclusion:
The Hybrid HTS program identified persons previously unaware of their HIV-positive status, thereby enabling linkage to care and same-day treatment and reducing onward transmission risk. An approach focused on identifying persons unaware of their HIV-positive status in combination with ascertaining the PUF has the potential to better target testing strategies to identify >90% of PLWH in a community.
Keywords: HIV testing services, antiretroviral therapy, community health, Kenya
INTRODUCTION
Knowledge of HIV status is the entry point for linkage to prevention, care, and treatment, and the first step towards achieving the UNAIDS 90–90-90 targets.1 However, few programs accurately determine the denominator of people eligible for testing. Thus, the HIV testing approaches in most countries rely on proxies for testing saturation, including periodic population-based sampling and yield (i.e., number testing positive among those tested).2–5
UNAIDS and the Kenya National AIDS Strategic Plan apply the metric of yield, defined as the percent of tested individuals who are identified as HIV-positive, as a yard stick towards attaining the first ‘90’. However, in an era when many countries are attempting to reach the first ‘90’, the emphasis on yield may be missing the mark. As programs approach HIV testing saturation in the population, initiatives targeting high-risk populations to find the few remaining persons with undiagnosed infections may also begin to observe a drop in the overall yield similar to the programs offering testing to the broader general population. Therefore, in a dynamic epidemic as a result of rapid scale-up of testing, care and treatment, which is the current scenario in many countries in sub-Saharan Africa, yield may be a poor estimate of testing saturation and following yield as a marker may not help programs to rapidly adjust approaches to identify >90% of persons living with HIV (PLWH) in a community.
In addition, the Office of the U.S. Global AIDS Coordinator (OGAC) and other bi- and multi-lateral donors have focused on yield without considering the true population prevalence. Few programs have successfully ascertained the denominator to compare the impact of various testing strategies. UNAIDS recently convened an expert panel to discuss which indicators can be used to assess when HIV epidemic control has been reached. The panel concluded that using the incidence/prevalence ratio (IPR) provides the most compelling evidence of epidemic control.6
Kenya is one of the four Africa countries considered to have a high HIV burden, with approximately 1.5 million people living with HIV infection.3 Annually, it is estimated there are over 70,000 new infections among adults and over 6,500 new infections among children.3 In the 2014 Kenya Demographic and Health Survey, the percentage of women and men aged 15–49 who had ever been tested was 85% and 72%, respectively.4 Testing coverage was lower among men than women and lower among adolescents than older adults, a pattern observed in other sub-Saharan African countries.3,4,7–12
While Kenya has a generalized HIV epidemic, the burden is greatest in Nairobi and the four counties surrounding Lake Victoria including Siaya, Kisumu, Homa Bay, and Migori counties.3 Homa Bay County, which is located in western Kenya, has a population of 1,101,901 people.13 HIV prevalence was 26% in 2015, which was 4.5 times higher than the national prevalence.14 Prevalence was higher among women (27.8%) then among men (24.0%).3 There were 158,077 PLWH in Homa Bay County at the end of 2015, which accounted for 10.4% of the total PLWH in Kenya, the second highest total nationally.3 In 2015, new HIV infections in Homa Bay County accounted for 15.1% of total new infections among children and 13.6% of total new infections among adults in Kenya.3 In an effort to increase HIV testing coverage, ensure linkage, and increase ART coverage among the residents of Homa Bay County, Family AIDS Care & Education Services (FACES) initiated a community-based “Hybrid” HIV testing services (HTS) program.
METHODS
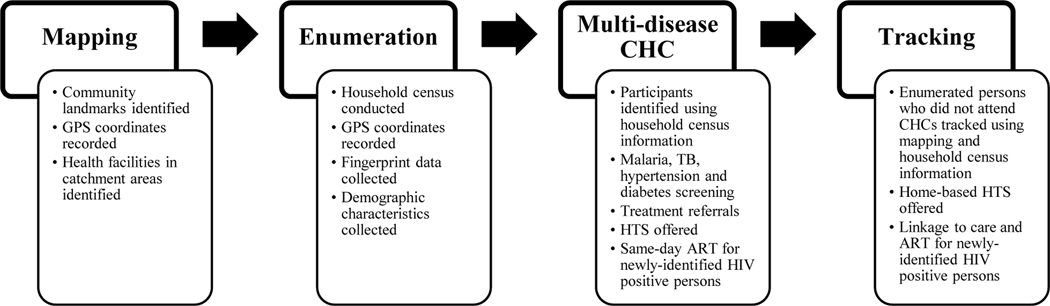
The Hybrid HTS program was implemented by FACES in the Rusinga and Lambwe wards of Homa Bay County between July and September 2016.15 The program entailed community mobilization and sensitization, community mapping, household census, multi-disease community health campaigns (CHCs), and tracking activities to offer home-based HTS, as presented in Figure 1. For the community mapping process, community landmarks and health facilities were identified and their GPS coordinates recorded in order to inform the subsequent census, CHCs, and tracking activities. The mapping team worked with local administrative and community leaders to conduct the community mapping exercise. A household census was conducted to enumerate and obtain demographic and fingerprint data from all persons residing in the program coverage area during the time when the census and CHCs were conducted. Enumeration also took place at the CHCs for those residents who were not located during the census. For each household, GPS coordinates were recorded and locator information (e.g., landmarks) were noted to facilitate the ensuing tracking process. Multi-disease CHCs were held throughout the program coverage area and their locations were determined using the household distribution data gathered during the census. Health services offered at the CHCs in addition to HTS included screening and referrals for tuberculosis (TB), malaria, hypertension, and diabetes, and were available to both residents and non-residents in the coverage area. Residents enumerated during the census who did not attend a CHC were tracked using the GPS data in order to offer home-based HTS. Up to a maximum of three attempts were made to reach each individual during the tracking process. Verbal consent was obtained during the census by program staff who explained that the demographic and fingerprint data collected during the census would be used to confirm an individual’s identity when that person attended a CHC. Verbal consent was also obtained for the collection of demographic and fingerprint data from non-residents who attended the CHCs and residents who were not previously enumerated during the census.
Figure 1:
Components of the Community-Based Hybrid HIV Testing Services Program in Homa Bay County, Kenya; July-September 2016
HIV testing eligibility criteria for the CHCs and tracking activities were based on the 2015 national guidelines in Kenya.16 Persons age ≥15 years who were not previously-identified PLWH were eligible for HIV testing. Children <15 years who reported being sexually active or for whom testing was requested by a parent or guardian, and persons who had been tested within the past 3 months but who reported a recent risk were also eligible for testing. Newly-identified PLWH at the CHCs and through tracking activities were provided with referrals for linkage to care at the health facility of their choosing. Linkage to same-day antiretroviral therapy (ART) initiation was offered to newly-identified PLWH at the CHCs.
Statistical analysis was performed using SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA). Frequencies and proportions were used to describe demographic characteristics of persons reached by the program, HIV yield, and previously-unidentified fraction (PUF). The PUF was defined as the proportion of newly-identified PLWH out of all previously-identified and newly-identified PLWH. The PUF is a metric that attempts to quantify the few remaining undiagnosed persons within the context of the first ‘90’ target, which is to reduce the number of persons unaware of their HIV-positive status. Demographic factors of newly-identified PLWH were assessed with Fisher’s Exact Test or the Chi-square test. Differences by HIV testing status (first-time vs. repeat testers) were assessed by bivariate logistic regression.
RESULTS
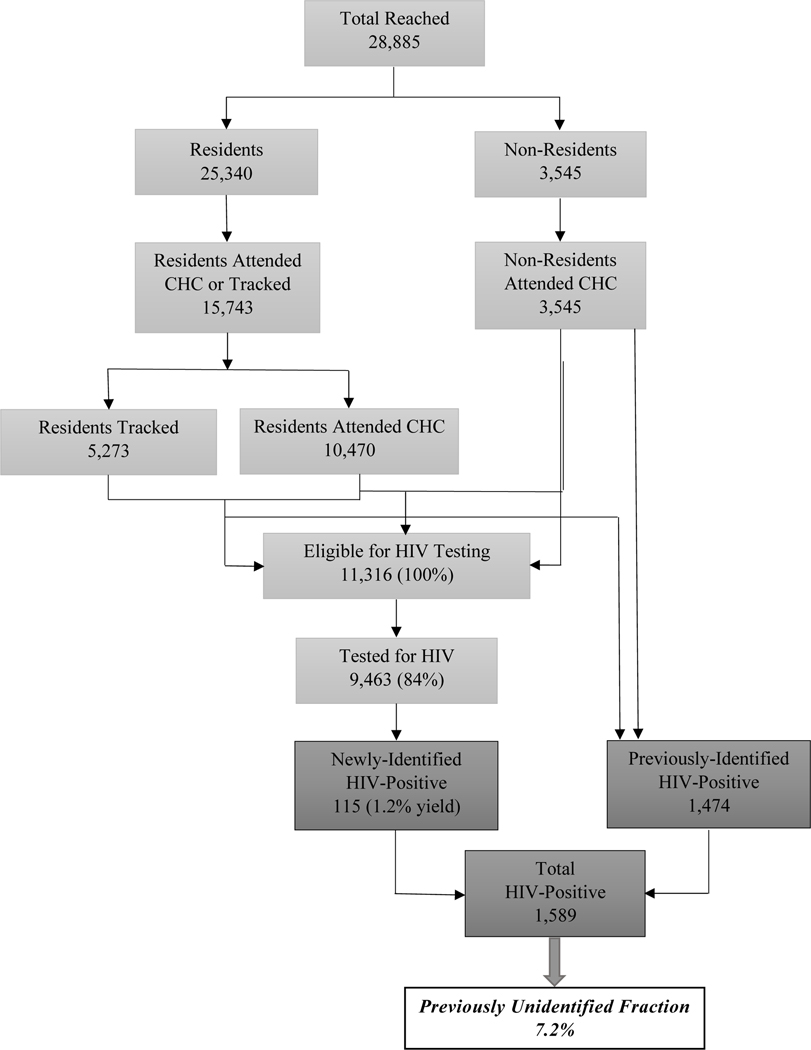
The community-based Hybrid HTS program reached a total of 28,885 persons: 87.7% were enumerated Rusinga and Lambwe residents, and 12.3% were non-residents, as presented in Figure 2. There were 19,288 residents and non-residents reached through the CHCs and tracking activities. The remaining 9,567 persons were enumerated but did not attend a CHC and could not be reached through tracking. Of the 11,316 HTS-eligible individuals, 9,463 (83.6%) accepted testing (6,838 at CHCs and 2,625 during tracking). Of those tested, 55.6% were women and 26.1% were 15–24 years of age, as detailed in Table 1. Individuals ineligible for testing included 1,474 previously-identified PLWH, as well as children under 15 years of age who did not have parental/guardian consent to be tested.
Figure 2:
Organogram of the Community-Based Hybrid HIV Testing Services Program in Homa Bay County, Kenya; July-September 2016
Table 1:
Demographic characteristics of persons receiving HIV testing, newly-identified and previously-identified as HIV-positive; Hybrid HIV testing services program; Homa Bay County, Kenya; July through September 2016
| Characteristics | Testers (N=9,463) | Newly-Identified HIV-Positive (N=115) | Previously-Identified HIV-Positive (N=1,474) |
|---|---|---|---|
| Sex | |||
| Male | 4,200 (44.4%) | 39 (33.9%) | 406 (27.5%) |
| Female | 5,263 (55.6%) | 76 (66.1%) | 1,068 (72.5%) |
| Age (years) | |||
| 0–9 | 2,036 (21.5%) | 6 (5.2%) | 96 (6.5%) |
| 10–14 | 1,453 (15.4%) | 4 (3.5%) | 47 (3.2%) |
| 15–19 | 1,498 (15.8%) | 10 (8.7%) | 31 (2.1%) |
| 20–24 | 974 (10.3%) | 19 (16.5%) | 90 (6.1%) |
| 25–34 | 1,356 (14.3%) | 38 (33.0%) | 427 (29.0%) |
| 34–49 | 877 (9.3%) | 27 (23.5%) | 514 (34.9%) |
| ≥50 | 1,269 (13.4%) | 11 (9.6%) | 269 (18.2%) |
Of the 1,853 HTS-eligible individuals who were not tested, 1,023 (55.2%) were female, 830 (44.8%) were male, 488 (26.3%) were 15–19 years old, 302 (16.3%) were 20–24 years old, 439 (23.7%) were 25–34 years old, 261 (14.1%) were 35–49 years old, and 363 (19.6%) were ≥50 years old. HTS-eligible individuals who were not tested were more likely to be male (p<0.01) and adolescents and young people 15–24 years old (p<0.01) compared to persons who accepted testing.
Among persons tested, there were 115 newly-identified PLWH, for an HIV yield of 1.2%. Of persons newly-identified as HIV-positive, 66.1% were women and 33.0% were 25–34 years of age. Women who accepted testing were more likely to be newly-identified as HIV-positive than men who accepted testing (1.4% vs. 0.9%; p=0.02). Prevalence of newly-identified HIV infections increased by age group (p<0.01), from 0.3% among infants and children 0–14 years, 0.7% among persons 15–19 years, 2.0% among 20–24 years, 2.8% among 25–34 years, and 3.1% among 35–49 years, before declining to 0.9% among persons 50 years or older. There were 1,474 previously-identified PLWH, of which 72.5% were women and 34.9% were 35–49 years of age.
Overall, of the 9,463 individuals who accepted testing, 1,230 (13.0%) were first-time testers and 8,233 (87.0%) were repeat testers; 0.98% of first-time testers (12 persons) were newly-identified as HIV-positive compared to 1.25% of repeat testers (103 persons). Among the first-time testers, 408 (33.2%) were ≥15 years old and 822 (66.8%) were <15 years old. Table 2 presents the demographic characteristics of first-time testers who were ≥15 years old. Of the 408 first-time testers ≥15 years old, 222 (54.4%) were male and 166 (40.7%) were adolescents aged 15–19 years, including 107 adolescent males. First-time testers ≥15 years old were more likely to be male (p<0.01) and younger (p<0.01) than repeat testers.
Table 2:
Demographic characteristics of persons 15 years and older receiving HIV testing stratified by first-time and repeat testing status (N=5,974); Hybrid HIV testing services program; Homa Bay County, Kenya; July through September 2016
| Characteristics | First-Time Testers (N=408) | Repeat Testers (N=5,566) | Odds Ratio (95% CI) | p-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 222 (54.4%) | 2,236 (40.2%) | 1.77 (1.44, 2.18) | <0.01 |
| Female | 186 (45.6%) | 3,330 (59.8%) | ref | |
| Age (years) | ||||
| 15–19 | 166 (40.7%) | 1,332 (23.9%) | ref | ref |
| 20–24 | 40 (9.8%) | 934 (16.8%) | 0.36 (0.25, 0.52) | <0.01 |
| 25–34 | 49 (12.0%) | 1,307 (23.5%) | 0.31 (0.22, 0.43) | <0.01 |
| 34–49 | 31 (7.6%) | 846 (15.2%) | 0.30 (0.20, 0.45) | <0.01 |
| ≥50 | 122 (29.9%) | 1,147 (20.6%) | 0.93 (0.72, 1.19) | 0.55 |
Among newly-identified PLWH, 93 persons (80.9%) participated in the CHCs and 22 persons (19.1%) were identified through tracking activities. Thus, HIV yield was higher at CHCs than through tracking activities (1.4% vs. 0.8%; p=0.04). Of the 93 newly-identified PLWH at the CHCs, 63 (67.7%) initiated ART the same day as part of the campaign.
Newly-identified PLWH represent a PUF of 7.2% of the total 1,589 PLWH, which is comprised of the 1,474 (7.6%) previously-identified PLWH plus the 115 newly-identified PLWH during the CHCs and tracking activities. The PUF was higher among men than women (8.8% vs. 6.6%; p=0.03). Adolescents and young people ages 15–24 years old had a PUF of 19.3%, which was higher than the 6.5% among infants and children <15 years of age and 5.9% among persons 25 years and older (p<0.01). The PUF was higher at CHCs compared to tracking activities but the difference was not significant (7.7% vs. 5.8%; p=0.22). Overall, the PUF was higher among men, adolescents and young people, and CHC attendees.
Stratified by ward, there were 16,601 persons reached in Rusinga and 12,284 persons reached in Lambwe. In Rusinga, 74 of the 4,531 persons tested were newly-identified PLWH and 777 were previously-identified PLWH. In Lambwe, 41 of the 4,891 persons tested were newly-identified PLWH and 697 were previously-identified PLWH. HIV yield was higher in Rusinga than in Lambwe (1.6% vs 0.8%; p<0.01). The PUF was also higher in Rusinga than in Lambwe (8.7% vs. 5.6%; p=0.02).
Of the 14,015 individuals who attended CHCs, 10,470 were residents and 3,545 were non-residents. The proportion of attendees who were ≥15 years old compared to <15 years old was lower among residents than non-residents (43.9% vs. 46.3%; p=0.01). The proportion of attendees who were female compared to male was higher among residents than non-residents (56.9% vs. 53.2%, p<0.01). With respect to HIV testing eligibility, 6,225 residents (59.5%) and 1,939 non-residents (54.7%) were eligible. The proportion of eligible attendees who were ≥15 years old compared to <15 years old was lower among residents than non-residents (61.2% vs. 73.9%; p<0.01). The proportion of eligible attendees who were female compared to male was higher among residents than non-residents (57.7% vs. 52.7%; p<0.01). Of those who were eligible for HIV testing, 5,293 resident (85.0%) and 1,545 non-residents (79.7%) were tested. The proportion of persons tested who were ≥15 years old compared to <15 years old was lower among residents than non-residents (54.4% vs. 67.2%; p<0.01). The proportion of persons tested who were female compared to male was higher among residents compared to non-residents (57.3% vs. 52.4%; p<0.01). Overall, female residents and non-residents 15 years and older were more likely to attend the CHCs, be eligible for HIV testing and accept testing.
Among residents attending the CHCs there were 887 previously-identified PLWH and 65 newly-identified PLWH; 44 of the newly-identified PLWH initiated same-day ART. Among non-residents, there were 231 previously-identified PLWH and 28 newly-identified PLWH; 19 of newly-identified PLWH initiated same-day ART. There were no significant differences by age or gender between residents and non-residents among previously-identified PLWH, newly-identified PLWH, and newly-identified PLWH who initiated same-day ART. HIV yield was slightly higher among non-residents than residents but the difference was not significant (1.8% vs 1.2%; p=0.08). The PUF higher among non-residents than residents (10.8% vs. 6.8%; p=03).
DISCUSSION
The Hybrid HTS program identified PLWH in Homa Bay County who were previously unaware of their status, thereby enabling linkage to care and same-day treatment and reducing onward transmission risk. The program was also able to reach many first-time testers, more than half of whom were men and nearly half were adolescents. Engaging men to uptake HIV testing continues to be an ongoing challenge.3,4 A recent systematic review and meta-analysis of community and facility-based HTS approaches in sub-Saharan Africa found that testing coverage was particularly low among men and young adults and recommended expanding home and mobile HTS as a way to increase uptake among men and young adults.17
The number of newly-identified PLWH, HIV yield and the PUF were higher in Rusinga compared to Lambwe. The observed differences between the two communities are likely attributable to the fact that Rusinga is a fishing community whose residents are considerably more mobile than those residing in the farming community of Lambwe. Studies have shown that residents whose mobility is related to their work are often at higher risk for HIV, such as residents of fishing communities.18,19 HIV yield and the PUF were higher at the CHCs than through tracking activities. These findings could reflect the fact that persons who perceived themselves to be at higher risk for HIV infection may have actively sought out testing at the CHCs, whereas those who perceived themselves to be at lower risk for HIV were tested during the tracking activities; and that the CHCs may be more efficient at reaching persons who are unaware of their HIV infection status. The observation that the PUF was higher among CHC attendees who were non-residents than residents may be attributable in part to the fact that a higher proportion of non-residents who attended the CHCs were ≥15 years old, an age group at higher risk for HIV infection compared to persons <15 years old.
While overall HIV yield was 1.2%, the PUF of 7.2% overall and 13.3% among youth are important findings since individuals unaware of their infection status pose a tremendous risk of sexual and vertical HIV transmission. Our approach of identifying persons unaware of their HIV-positive status in combination with ascertaining the PUF aligns with the UNAIDS concept of IPR and therefore has the potential to help target HIV testing approaches to reduce the number of persons unaware of their HIV-positive status.
We recognize several limitations with the Hybrid HTS program. The program was conceived as a last-minute addition to the final year of a PEPFAR/CDC cooperative agreement, and thus only had funding available for three months of implementation. This brief time frame limited the ability to conduct extensive community sensitization and mobilization activities, the number of CHCs, duration of tracking activities, and follow-up efforts to facilitate linkage to care and treatment. The programmatic data were collected using standardized HIV reporting forms issued by the Ministry of Health, therefore not allowing for additional variables to be added. In addition, we were unable to calculate a comprehensive cascade for all persons reached by the program since many non-residents attended the CHCs and we were unable to determine a denominator for non-residents. Same-day start of ART upon diagnosis became standard of care in Kenya in the same month that we began implementing the CHCs, so it is possible that some newly-identified PLWH were hesitant about initiating treatment right away.20,21 Since the Hybrid HTS program was among the first programs in Kenya to implement new national treatment guidelines, we anticipate that the proportion of individuals accepting same-day ART start will increase over time as the information about the new treatment guideline disseminates within communities.
Increasing uptake of HIV testing among individuals who never tested or remain at higher risk for incident infection remains challenging. Lessons learned from the evaluation of this program, however, will inform future implementation of community-based HTS approaches in sub-Saharan Africa. Our approach showed that offering HTS at CHCs in combination with follow-up home visits is an effective strategy for reaching first-time testers, particularly men and adolescents. Innovative approaches that make HIV testing more accessible and acceptable to the community, such as HIV testing as part of a package of health services, may be critical for reaching populations that might otherwise be reticent to take up standard facility-based testing services. In addition, as countries adopt Universal Health Care, CHCs may serve as a useful approach to identify persons previously unaware of their HIV-positive status while also screening for other common illnesses with referral for further evaluation and treatment. Future evaluations should consider including the PUF as a metric to monitor and adjust testing strategies as communities move towards epidemic control.
Acknowledgments
Funding Support: This work was supported by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) through the U.S. Centers for Disease Control and Prevention (CDC) under the terms of U2GPS001913
Footnotes
Disclaimer: The findings and conclusions in this paper are those of the author(s) and do not necessarily represent the official position of the funding agencies.
REFERENCES
- 1.UNAIDS. 90–90-90 – An ambitious treatment target to help end the AIDS epidemic. Available at: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf.
- 2.Kenya AIDS Indicator Survey 2012. Available at: http://nacc.or.ke/wp-content/uploads/2015/10/KAIS-2012.pdf. [DOI] [PubMed]
- 3.Kenya HIV County Profiles 2016. Available at: http://nacc.or.ke/wp-content/uploads/2016/12/Kenya-HIV-County-Profiles-2016.pdf.
- 4.Kenya Demographic and Health Survey 2014. Available at: https://dhsprogram.com/pubs/pdf/FR308/FR308.pdf.
- 5.The Demographic and Health Surveys Program. Available at: https://dhsprogram.com/.
- 6.Making the end of AIDS real: consensus building around what we mean by “epidemic control”. Available at: http://www.unaids.org/sites/default/files/media_asset/glion_oct2017_meeting_report_en.pdf.
- 7.Makusha T, Mabaso M, Richter L, Desmond C, Jooste S, Simbayi L. Trends in HIV testing and associated factors among men in South Africa: evidence from 2005, 2008 and 2012 national population-based household surveys. Public Health. 2017; 143:1–7. [DOI] [PubMed] [Google Scholar]
- 8.Takarinda KC, Madyira LK, Mhangara M, et al. Factors associated with ever being HIV-tested in Zimbabwe: an extended analysis of the Zimbabwe Demographic and Health Survey (2010–2011). PLoS One. 2016; 11:e0147828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grobler A, Cawood C, Khanyile D, Puren A, Kharsany AMB. Progress of UNAIDS 90–90-90 targets in a district in KwaZulu-Natal, South Africa, with high HIV burden, in the HIPSS study: a household-based complex multilevel community survey. Lancet HIV. 2017; 4:e505–513. [DOI] [PubMed] [Google Scholar]
- 10.Hayes R, Floyd S, Schaap A, et al. A universal testing and treatment intervention to improve HIV control: one-year results from intervention communities in Zambia in the HPTN 071 (PopART) cluster-randomised trial. PLoS Med. 2017; 14:e1002292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwuji CC, Orne-Gliemann J, Larmarange J, et al. Uptake of home-based HIV testing, linkage to care, and community attitudes about ART in rural KwaZulu-Natal, South Africa: descriptive results from the first phase of the ANRS 12249 TasP cluster-randomized trial. PLoS Med. 2016; 13:e1002107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamie G, Clark TD, Kabamim J, et al. A hybrid mobile approach for population-wide HV testing in rural east Africa: an observational study. Lancet HIV. 2016: 3:e111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.KNBS Population Projections 2015. Available at: https://www.knbs.or.ke/download/population-projections/.
- 14.Kenya HIV Estimates 2015. Available at: nacc.or.ke/wp-content/uploads/2016/12/Kenya-HIV-Estimates-2015.pdf.
- 15.Lewis Kulzer J, Penner JA, Marima R, et al. Family model of HIV care and treatment: a retrospective study in Kenya. J Int AIDS Soc. 2012; 15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The Kenya HIV Testing Services Guidelines. Available at: https://archive.org/details/hts_policy_kenya_2015.
- 17.Sharma M, Ying R, Tarr G, Barnabas R. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature. 2015; 528:S77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwena ZA, Camlin CS, Shisanya CA, Mwanzo I, Bukusi EA. Short-term mobility and the risk of HIV infection among married couples in the fishing communities along Lake Victoria, Kenya. PLoS One. 2013; 8:e54523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Camlin C, Akullian A, Neilands TB, et al. Population mobility associated with higher risk sexual behavior in eastern African communities participating in a universal testing and treatment trial. J Int AIDS Soc. 2018; 21 Suppl 4:e25115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guidelines on use of antiretroviral drugs for treating and preventing HIV infection in Kenya, 2016 edition. Available at: https://aidsfree.usaid.gov/sites/default/files/kenya_art_2016.pdf.
- 21.Historic moment as Kenya launches revolutionary HIV treatment. Available at: http://www.health.go.ke/wp-content/uploads/2016/07/Press-release-HISTORIC-MOMENT-AS-KENYA-LAUNCHES-REVOLUTIONARY-HIV-TREATMENT.pdf.