Abstract
Sexual differentiation of the brain during early development likely underlies the strong sex biases prevalent in many neurological conditions. Mounting evidence indicates that microglia, the innate immune cells of the central nervous system, are intricately involved in these sex-specific processes of differentiation. In this review, we synthesize literature demonstrating sex differences in microglial number, morphology, transcriptional state, and functionality throughout spatio-temporal development as well as highlight current literature regarding ontogeny of microglia. Along with vanRyzin et al in this issue, we explore the idea that differences in microglia imparted by chromosomal or ontogeny-related programming can influence microglial-driven sexual differentiation of the brain, as well as the idea that extrinsic differences in the male and female brain microenvironment may in turn impart sex differences in microglia.
Sexual differentiation of the brain: How understanding intrinsic and extrinsic developmental influences can sculpt the future of microglial sex differences research
Sexual differentiation of the brain is the process in which sex-linked neural and behavioral phenotypes are coordinated with the gonadal sex of the organism. In the brain, sexual differentiation is primarily influenced by the sex chromosome complement of the animal as well as gonadal sex hormones. For instance, male sex behavior and physiology are heavily influenced by a surge of androgens and their metabolites that occurs during a critical period surrounding birth (Konkle and McCarthy 2011; McCarthy 2008; Zuloaga et al. 2008). Importantly, it is increasingly recognized that microglia play an important role in the processes of brain sexual differentiation (Lenz et al. 2013, 2018; VanRyzin, Pickett, and McCarthy 2018; McCarthy et al. 2015; Kopec et al. 2018). Notably, many early-onset neurodevelopmental disorders show a strong sex-bias toward males (Thibaut 2016) while adult-onset neurological disorders are female-biased (Zagni, Simoni, and Colombo 2016). Thus, it is critical to understand how and when sexual differentiation of the brain occurs as this allows for the study of where, when, and how sex differences in neural phenotypes arise, as well as what machinery could be dysfunctional when these processes go awry.
In this issue of Glia, we present coordinated reviews on sex differences in microglial function. The purpose of these joint reviews is to identify the emerging questions surrounding microglia, sex differences, and brain development. VanRyzin et al focus on the role of extrinsic factors influencing microglial function and identity (i.e. hormonal fluxes and microglial communication with microenvironmental factors). We focus on factors that may program intrinsic microglial identity and function such as ontogeny and metabolic programming. Of course, differentiating between intrinsic and extrinsic influences is a blurred line, as extrinsic factors may alter the intrinsic nature of an individual cell, and vice versa. For example, any programming imparted upon cells during their developmental journey from their place of origin to place of residence could be considered as either an extrinsic factor (programmed after the birth of the cell) or an intrinsic factor (programmed prior to its arrival in its tissue or place of residence). On the other hand, an individual cell’s transcriptome or proteome (intrinsic factor) could influence how that cell responds to extrinsic factors. For purposes of this review, we will broadly consider ontogeny as an intrinsic factor that guides cellular functions. It is our goal to move the field of microglial sex differences forward through identification of these major questions from both an intrinsic and extrinsic point of view.
Microglia are unique immune cells of the central nervous system
Microglia, the primary innate immune cells of the central nervous system (CNS), comprise ~10% of the total cellular population in the adult human brain (Lyck et al. 2009; Pelvig et al. 2008). Traditionally, microglia were often referred to as ‘activated’ or ‘quiescent’ macrophages, but this terminology is a massive oversimplification (Ransohoff 2016). These small cells play an outsized role in maintaining tissue homeostasis, responding to CNS perturbations through rapid protrusion into the site of insult (Davalos et al. 2005; Nimmerjahn, Kirchhoff, and Helmchen 2005), induction of phagocytic activity, and release of neuroprotective or cytotoxic signaling factors (Hanisch and Kettenmann 2007). While early microglia research focused on these cells’ roles in the innate immune response, microglia are increasingly recognized for their importance in shaping early brain development. For example, they are heavily involved in the sculpting of neural circuitry in the developing visual system through the refinement of projections from the retina to the LGN in activity- and complement-dependent manners (Schafer et al. 2012; Schafer, Lehrman, and Stevens 2013), in the laminar positioning of interneurons in upper layers of the cortex (Squarzoni et al. 2014), and play an important role in the organization of social behavior (Kopec et al. 2018; VanRyzin et al. 2019). Recent studies show robust sex differences in microglial morphology, maturation, and function (Schwarz, Sholar, and Bilbo 2012; Villa et al. 2018; Hanamsagar et al. 2017; Thion et al. 2018). Many behaviors differ between males and females early in development, and microglia are intimately involved in sculpting many of these behaviors. This suggests that microglia - and their intrinsic sex - may be intimately involved in sculpting sex-specific behaviors, or even behavior writ large.
What makes a microglia male or female?
Male and female microglia are differentially influenced by gonadal hormones and early postnatal environment (reviewed in VanRyzin et al, this issue) as well as the chromosomal sex of the cell. The X chromosome contains the largest number of immune-related genes in the human genome (Fish 2008). X chromosome inactivation serves to match levels of X chromosome gene expression in males and females by silencing one set of X chromosome genes in females. In placental mammals, the paternal X-chromosome is inactivated in extra-embryonic tissues as early as the 8-cell stage (Heard and Disteche 2006). By embryonic day 6.5 in the mouse, random X chromosome inactivation occurs in embryonic tissues (Cheng and Disteche 2004). However, recent studies have suggested that this inactivation is not random at all. Examination of neonatal brains revealed that the paternal X chromosome is consistently inactivated (X. Wang, Soloway, and Clark 2010). The male X chromosome is always maternal, suggesting that in both the yolk sac and the brain, where microglia eventually reside, the maternal X chromosome has elevated expression. These studies raise two interesting considerations: Firstly, if the brain preferentially silences the paternal X chromosome, do other tissues also preferentially silence one chromosome over the other? Secondly, considering that microglia progenitors travel through different tissues, would preferential silencing of the maternal X chromosome in these tissues, such as the vasculature, impact microglial trajectory differentially in males and females? The X chromosome deserves further attention due to the fact that some X chromosome genes, particularly immune-related genes such as toll-like receptor 7 (Tlr7), escape this inactivation (Souyris et al. 2018). Microglial TNF-α release can be stimulated by the miRNA let-7 in a Tlr7-dependent manner (Lehmann et al. 6/2012), and thus biallelic expression of Tlr7 in female microglia may contribute to intrinsic differences in immune response between males and females. The human X chromosome also contains ~10% of all miRNAs (Pinheiro, Dejager, and Libert 2011), and a variety of X-linked miRNAs have been shown to play a role in immune function. One of the more well characterized miRNAs found on the X chromosome is miR-223, which is upregulated in immune cells, including microglia, from human multiple sclerosis (MS) patients. Overexpression of miR-223 diminishes LPS-dependent pro-inflammatory responses (Galloway et al. 2018). Although limited information is available about miR-223 expression in males and females, MS predominantly affects females (Harbo, Gold, and Tintoré 2013), and it stands to reason that X-chromosome miRNAs contribute to intrinsic sex differences in microglia and phenotype/disease state. Furthermore, polymorphisms in genes on the Y chromosome have been associated with differing susceptibility to infection or autoimmune disease (Case et al. 2013; Teuscher et al. 2006; Spach et al. 2009). Together these recent studies highlight the importance of chromosomal genotype on immune function. Studies have additionally highlighted the impact of transgenerational influences on microglia development, suggesting that epigenetic changes may also contribute to sex differences in microglia (see van Ryzin et al, this issue). Despite evidence delineating the intrinsic sex of microglia, more research in this area is needed to understand how “maleness” and “femaleness” are programmed and what factors influence the identity of a male vs. a female microglia.
Sex influences the developmental trajectory of microglia
Emerging literature suggests robust sex differences in microglial developmental trajectories and function early in life. There are significant sex differences in microglial morphology from in vitro cultures, as male-derived cells show more amoeboid-like morphologies compared to female-derived microglia (Villa et al. 2018), indicating inherent differences in male and female microglia where male microglia may be more reactive in culture. In rats there are no sex differences in microglial number at embryonic day 17 (E17) in the hippocampus, parietal cortex, amygdala, or paraventricular nucleus of the hypothalamus, though in general embryonic microglia are more amoeboid and stout rather than ramified (Schwarz, Sholar, and Bilbo 2012). In early postnatal stages (PN2–4), males have more microglia than females in the highly sexually dimorphic preoptic area (Lenz et al. 2013) as well as in the parietal cortex, hippocampus, and amygdala (Schwarz, Sholar, and Bilbo 2012). Male microglia were generally more amoeboid than female microglia at these times (Schwarz, Sholar, and Bilbo 2012), and the number of phagocytic microglia was higher in the early postnatal male amygdala (VanRyzin et al. 2019). However, the number of phagocytic microglia was higher in the female hippocampus than in males in PN3 rat pups and PN8 mice (Nelson, Warden, and Lenz 2017; Weinhard et al. 2018), suggesting sex differences in microglial functionality during early development. Interestingly, the sex difference in microglial morphology evident at early postnatal stages was reversed by PN30, when females were observed to have more microglia with thick and long processes in the hippocampus, amygdala, and parietal cortex (Schwarz, Sholar, and Bilbo 2012).
Microglia may also play a key and sex-specific role in the shaping of brain development during the highly plastic developmental time period that is adolescence. Microglial and complement-dependent processes mediate elimination of dopamine D1 receptors in adolescent male rats, which shapes social behavior changes occurring during the adolescent period (Kopec et al. 2018). Although D1 receptors are eliminated in females, these changes do not appear to be dependent upon the microglial complement system (Kopec et al. 2018), suggesting a differential role for microglial sculpting of social behavior in developing male vs. female rats. Of course, microglia could still be involved in the organization of female social play behaviors in a manner other than the direct elimination of receptors. For instance, microglia in the preoptic area can impact dendritic spine density through the release of the molecule prostaglandin E2 (PGE2), producing sex-specific brain development of this area (Lenz et al. 2013). It is possible that microglia influence social behavior in females through a similar complement-independent process. These findings demonstrate regional heterogeneity in microglial characteristics as well as a robust sex difference in number and function early in development. The developmental timing of the noted sex differences is concurrent with the well-described male-specific gonadal hormone surge that regulates male sexual differentiation by pairing behavioral and molecular phenotypes to gonadal phenotype (VanRyzin, Pickett, and McCarthy 2018). In rodents, this male-specific surge in testes-produced testosterone begins at ~E16–18, after microglial colonization of the CNS, and lasts until a few hours after birth (Weisz and Ward 1980; Konkle and McCarthy 2011). As the majority of microglial studies have been performed at postnatal stages, more work remains to be done to understand how this sex-specific perinatal hormonal organization both influences and is influenced by microglial function, establishing acute or long-lasting sex differences in microglia themselves (see vanRyzin et al, this issue). Indeed, RNA sequencing (RNA-seq) data of isolated microglia from ovariectomized versus control adult female mice did not show significant transcriptomic differences in NF-kB-driven genes, indicating that long-lasting organizational effects and not acute sex hormone circulation primarily influence microglial gene expression (Villa et al. 2018). Additional evidence for early-life sex-specific organization of the brain heavily influencing microglial function comes from the same study in which microglia were depleted using PLX3397, an antagonist of the colony-stimulating factor 1 receptor (CSF1R), which can deplete ~99% of microglia from the adult rodent brain (Elmore et al. 2015). Microglia from 12 week old female mice were transplanted into male brains that had been depleted of microglia, and instead of taking on a male-like transcriptional profile, these female-derived microglia kept a gene expression profile similar to that of microglia from control females (Villa et al. 2018), suggestive of lasting intrinsic differences between male and female microglia. To determine whether this sex-specific differentiation remained under pathological states, the authors utilized permanent middle cerebral artery occlusion (pMCAO), a rodent model of ischemic stroke (McBride and Zhang 2017) in which males tend to have worse neurological outcomes than females. Male or female microglia were transplanted into male mice that had undergone pMCAO. Transplantation of female microglia into the brains of pMCAO-injured males resulted in a significantly lessened injury progression compared to pMCAO-injured males that received transplantation of microglia from other males (Villa et al. 2018), suggesting that microglia can retain sex-specific differentiation even in response to injury.
The use of RNA sequencing technologies has vastly improved our understanding of microglial developmental trajectories. A foundational study demonstrated that there are at least three distinct temporal developmental stages in microglial development that are characterized by discrete transcriptional patterns referred to as early (~E10.5-E14), pre-microglia (E14-PN9), and adult microglia (4 weeks of age and older) (Matcovitch-Natan et al. 2016). Interestingly, the pre-microglia category encompasses a wide time frame including the gonadal sex hormone surge and concurrent critical period surrounding birth for sexual differentiation of the brain. A more recent study identified two distinct groups of transcriptional patterns within this time, grouping microglia into an embryonic phase 1 (E12.5-E14.5) and a phase 2 (E16.5-birth) (Thion et al. 2018). This suggests that the perinatal hormone surge may influence the microglial transcriptome, and a deeper temporal investigation may reveal additional distinct transcriptional stages of microglial development. Further studies using bulk RNA-seq of purified hippocampal microglia assessed the role of sex in microglial developmental trajectories. Intriguingly, microglia isolated from males showed a developmental delay when compared to females. In response to an acute immune challenge, male microglia demonstrated a female-like developmental trajectory. Notably, immune stimulation did not impact microglia developmental trajectories in females (Hanamsagar et al. 2017). One interpretation of these findings is that female microglia are more resilient than male microglia in the context of an immune challenge. Alternatively, male microglia may be more adaptive than female microglia, and the adoption of the ‘female-like’ trajectory in response to an immune challenge could represent a protective response.
Microglia continually survey their environment and expand or retract various processes in a time scale of minutes (Nimmerjahn, Kirchhoff, and Helmchen 2005; Davalos et al. 2005). Such highly dynamic cells are also likely to be highly heterogeneous, even within canonically-defined brain regions. Recent advances using single-cell RNA sequencing (scRNA-seq) allow for individual cell analysis of heterogeneous microglial populations within a single brain or brain region. An exhaustive scRNA-seq study examining whole brain microglia isolated from mice at E14.5, PN4–5, PN30, PN100, and PN540 revealed nine unique clusters of microglial states across all ages and conditions (Hammond et al. 2019). The authors found higher microglial diversity at early ages (E14.5 and PN5) than in older mice, suggestive of increased microglial transcriptomic heterogeneity early in development (Hammond et al. 2019), a finding echoed by another scRNA-seq study showing greater transcriptomic heterogeneity earlier in microglial development (Li et al. 2019). Surprisingly, (Hammond et al. 2019) did not find any sex differences in the transcriptomes of these microglia, while (Li et al. 2019) only studied microglia isolated from male mice. That (Hammond et al. 2019) did not find any differences in clustering between males and females is in distinct opposition to the bulk RNA-seq and morphological/cytokine findings of several other studies (Hanamsagar et al. 2017; Guneykaya et al. 2018; Villa et al. 2018). It may be that gene expression differences between males and females are not detected by the relatively shallow sequencing depth (40,000–60,000 reads per sample in (Hammond et al. 2019) compared to ~2 million reads/sample in (Hanamsagar et al. 2017), highlighting the need for deep scRNA-seq (on the scale of 1 million reads/cell as in (Li et al. 2019)) of both male and female microglia across development.
While the recent rise of single cell technologies in microglial research is highly promising, one concern stems from the method of isolation of cells to be analyzed. For example, the majority of scRNA-seq studies in microglia have used FACS sorting of various myeloid cell markers prior to sequencing (Hammond et al. 2019; Li et al. 2019). A comparison of microglial transcriptome analyses using Ribo-Tag and FACS techniques found that the isolation procedures used for FACS sorting resulted in transcriptional artifacts such as an upregulation of pathways involved in nitric oxide and reactive oxygen signaling, as well as phagocytosis and TLR signaling (Haimon et al. 2018). Other studies have also found that the isolation protocols used during FACS can induce upregulation of immediate early gene expression (van den Brink et al. 2017; Ayata et al. 2018). Indeed, using FACS sorting on Tmem119+ cells, (Li et al. 2019) found significant expression of immediate early genes in sequencing experiments that were not found in vivo by immunohistochemistry, highlighting an important caveat in interpretation of single cell sequencing data. Importantly, given that immune activation accelerates the maturation of microglia only in males (Hanamsagar et al. 2017), essentially making the male cells appear transcriptionally more “female-like”, the isolation techniques used may be masking baseline sex differences in scRNAseq studies. Advances in technologies such as antibody-dependent bead isolations are promising as they are faster and involve fewer mechanical stressors, though a comprehensive comparison of activation states in response to this technique has not been reported. Overall, current technical limitations require compromise between obtaining single-cell specificity and maintaining in vivo conditions, and specific experimental questions should instruct the optimal technique.
Taken together, sex differences in microglial number, morphology, transcriptome, and function have been reported in various brain regions throughout early brain development when sexual differentiation of the brain is occurring. Whether these sex differences in microglial functionality drive how the brain develops or whether microglia are simply responding to differences in the male vs. female brain remains an open question.
Sex differences in their developmental journey may program sex differences in microglial behavior
Fate mapping studies demonstrate that microglia are derived from fetal yolk-sac myeloid progenitors (Ginhoux et al. 2010; Hoeffel and Ginhoux 2015). An initial wave of primitive hematopoiesis in mice is observed ~E7.5 with the generation of macrophages and nucleated red blood cells that will colonize the embryo (Thion, Ginhoux, and Garel 2018). These yolk-sac-derived macrophage precursors colonize the central nervous system (CNS) in sequential waves via the vasculature beginning at ~E9, prior to the closure of the blood brain barrier (BBB), an event which occurs ~E13-E14.5 in mice (Ginhoux et al. 2010; Hoeffel and Ginhoux 2015). Correspondingly in humans, microglia arise early in the processes of neural development, first appearing in the forebrain ~4.5 weeks into gestation with early routes of entry thought to be through the choroid plexus, ventricles, and meninges (Verney et al. 2010). A second wave of microglia was observed in the white matter ~10–13 weeks into gestation (Verney et al. 2010). Indeed, microglial brain colonization during embryonic development is conserved across vertebrate species (Schlegelmilch, Henke, and Peri 2011; Herbomel, Thisse, and Thisse 2001; Hanisch and Kettenmann 2007; Swinnen et al. 2013). As neural progenitors at this stage are primarily differentiating into neurons and do not generate astrocytes/oligodendrocytes until later in embryonic life, microglia comprise the majority of the fetal glial population (Thion, Ginhoux, and Garel 2018).This suggests that microglia can play diverse roles in promoting the organization of neural circuitry. Further, these roles are likely to be sex-specific as male and female microglia follow temporally distinct developmental trajectories (Hanamsagar et al, 2017). Although often referred to simply as the macrophages of the CNS, the distinct ontogeny of yolk-sac derived microglia is likely to influence their development and function. For example, the adult CNS is unable to induce full microglia-like differentiation of bone marrow-derived monocytes that were injected into the brain (Cronk et al. 2018), suggesting that simply any background of macrophage will not be as fully differentiated as yolk sac-derived microglial cells. It must be noted that traditional techniques used to distinguish microglia from peripheral macrophages utilized expression of the antigen CD45, with microglia generally thought to have lower CD45 expression than peripheral macrophages (Ford et al. 1995). However, brain-resident microglia can upregulate CD45 expression in response to pathological insults (Sedgwick et al. 1991), indicating difficulty in the use of canonical markers to delineate microglia versus microglia-like cells. However, a recent study identified Ms4a3 as a marker specific for granulocyte-monocyte progenitors, and generated a Ms4a3Cre-RosaTdT fate mapper mouse to specifically follow monocytes and their progeny (Liu et al. 2019). These mice could be used in parallel with newly created Tmem119-eGFP and Tmem119-CreERT2 transgenic mice (Kaiser and Feng 2019) to faithfully distinguish between processes carried out by brain-resident microglia and infiltrating monocytes. That bone marrow-derived monocytes do not fully differentiate in the brain indicates that some intrinsic programming that microglia possess, but bone marrow-derived monocytes do not possess, is important for their interactions with and integration into the brain microenvironment. As there are known sex differences in both the developmental trajectories and adult behaviors of microglia, it is possible that sex differences may arise as early as hematopoiesis in the embryonic yolk-sac, or during the process of CNS colonization that occurs during early embryonic development. While here we highlight the colonization of microglia in the brain, it is also likely that other factors such as proliferative capacity and/or apoptosis influence microglial number and location in the developing brain. However, investigations into microglial proliferation in the PN2 hippocampus (Nelson, Warden, and Lenz 2017) and at 3 months (Villa et al. 2018) found no basal sex differences. Given the variation in overall brain maturational trajectory in males and females (Giedd 2004) it may be that embryonic microglia colonize the CNS at a different rate in males and females, either due to intrinsic sex differences or due to differential extrinsic signals stemming from regional differences in brain maturation. For instance, neural progenitor cells are known to influence microglial colonization (Arnò et al. 2014). Therefore, differing maturational states of neural progenitor cells between males and females may signal for microglial colonization at different time points or to a different degree (VanRyzin, Pickett, and McCarthy 2018). A recent report using intravital microscopy of CX3CR1GFP/+ mice to trace pre-macrophage trafficking from the fetal yolk sac to the embryo found that CX3CR1+ pre-macrophages primarily entered the yolk sac vasculature and trafficked towards the embryo, and that the principal target of these yolk sac-derived pre-macrophages was the developing brain (Stremmel et al. 2018). However, the use of CX3CR1 to track pre-macrophages may not be completely reflective of microglial colonization, as CX3CR1 is not expressed until ~E9.5, two days after yolk-sac progenitors arise (Ginhoux et al. 2010; Hoeffel and Ginhoux 2015). Other markers such as Runx1 or Csf1r are expressed earlier (Samokhvalov, Samokhvalova, and Nishikawa 2007; Qian et al. 2011). This could indicate a change in transcriptional profile between the appearance of yolk-sac progenitors and the start of trafficking, or it could be indicative of different populations of progenitor cells. Lineage tracing studies using intersectional genetic approaches would help address this issue. Recently, Tmem119-EGFP and Tmem119-CreERT2 mouse models have been described that would help address this question (Kaiser and Feng 2019). However, the transcriptomic and proteomic expression patterns of Tmem119 are conflicting. RNAseq has demonstrated that Tmem119 is expressed early in embryonic yolk-sac progenitors, while protein reactivity is only seen postnatally (Matcovitch-Natan et al. 2016; Bennett et al. 2016). In support of the idea of distinct progenitor lineages, Tmem119 reporter animals showed expression in neonatal vasculature in addition to microglia, while Runx1 lineage progenitors did not contribute to early embryonic vasculature (Samokhvalov, Samokhvalova, and Nishikawa 2007; Kaiser and Feng 2019). While the molecular calling cards responsible for pre-macrophage migration from the yolk sac to the brain are still unknown, the time-frame of pre-macrophage migration to the developing brain is similar to the early establishment of a vascular network in the brain (Stremmel et al. 2018). In NCX1 knockout mice lacking a heartbeat and functional blood circulation, microglia do not reach the brain (Ginhoux et al. 2010), suggesting that microglia migrate from the yolk sac to the fetal brain through the vasculature. As such, any sex differences in fetal vasculature may impart sex-specific programming onto these trafficking cells. Indeed, differences in how and where microglia enter the brain from the vasculature may differ between males and females, potentially imparting lasting sex differences in their functionality. While little has been determined regarding sex differences in cerebral vasculature or blood flow during fetal development, several studies in adult humans have determined that cerebral blood flow is higher in females than males throughout much of life (Aanerud et al. 2017; Rodriguez et al. 1988; R. E. Gur and Gur 1990; Amen et al. 2017; Esposito et al. 1996), although the sex difference diminishes by age 60 (Aanerud et al. 2017). A study focusing on sex differences in earlier human life found that females had lower cerebral blood flow than males prior to age 13, which began to change during mid-adolescence, and that by late adolescence female cerebral blood flow was greater than that seen in males (Satterthwaite et al. 2014), indicating a fluidity in sex-specific vasculature function. Differences in the rate at which microglia traffic through the vasculature from the fetal yolk sac to the brain could impact the timing of their arrival in the brain. Given the ability of neural progenitors to influence microglial colonization (Arnò et al. 2014), a slight change in the timing of microglial arrival to certain brain regions could lead to microglia interacting with progenitors at different stages of either microglial or neural progenitor differentiation, resulting in varied microglial outcomes. What could underlie these sex differences in the vasculature? While outside of the scope of this review, yolk sac vasculature formation/remodeling is dependent upon VEGF signaling, of which there are known sex differences early in development, and is a possible explanation for sex differences in vasculature functionality.
The somewhat controversial idea that microglia may traffic via alternative routes from the fetal yolk sac to the brain gained support from the finding that a proportion of microglia in the brain are derived from fetal yolk sac cells expressing the homeobox 8 (Hoxb8) gene (Greer and Capecchi 2002; Chen et al. 2010). These Hoxb8-lineage microglial progenitors are generated during the second wave of fetal yolk-sac hematopoiesis and are not detected in the brain until ~E12.5, with strong expression apparent in the brain from E14.5 onward (De et al. 2018). Evidence suggesting that these Hoxb8-lineage microglia do not migrate directly from their fetal yolk-sac origin to the brain (Fig. 1) came as Hoxb8-lineage cells were observed in the fetal liver and aorto-gonad-mesonephros (AGM) prior to infiltration of the brain at E12.5 (De et al. 2018). Of note, Hoxb8-labeled hematopoietic progenitor cells isolated from the E12.5 fetal liver were capable of giving rise to mature Tmem119-expressing parenchymal microglia when transplanted into the mouse brain, supporting the idea that Hoxb8-lineage cells destined to become brain microglia may traffic through the fetal liver (De et al. 2018). Intriguingly, Hoxb8-lineage microglia showed a higher proliferation rate than canonical (non-Hoxb8) microglia soon after birth (De et al. 2018). That certain subpopulations of microglia are highly proliferative during the end of the critical period in which there is an organizational male gonadal hormone surge is of interest. It may be that the extrinsic impact of gonadal hormones in males differentially affects the highly proliferative microglial population to a different degree than the less-proliferative microglial population. Any differential programming that male or female microglia acquired during their trafficking through the fetal liver and AGM (or other as-of-yet undefined routes) could also result in different responses to the gonadal hormone surge. Although the potential for microglia to migrate from the fetal yolk sac to the brain via different routes remains open ended, the findings of a possible second source of brain microglia that traffic through the fetal liver and AGM on their developmental route from the fetal yolk-sac to the brain may provide numerous chances for the acquisition of novel programming that may result in altered functionality of these alternatively-trafficked microglia. For example, significant sex differences were found in the expression of various genes within the human fetal liver (O’Shaughnessy et al. 2011). One gene that was differentially expressed in male and female fetal liver was the aryl hydrocarbon receptor (Ahr), which limits microglial inflammatory response via SOCS2-dependent inhibition of NF-kB (Rothhammer et al. 2018). Differential exposure to Ahr within the male or female fetal liver could program Hoxb8-lineage microglial reactivity to immune stimulators of NF-kB later in life. Controversy remains however over the early trafficking routes of microglia from the fetal yolk sac to the embryonic brain, and greater characterization of the developmental journeys of these fascinating cells is likely necessary to understand the potential programming that they experience during their migration into the brain.
Figure 1. Ontogeny-specific programming may lead to sex-specific differences in microglial development.
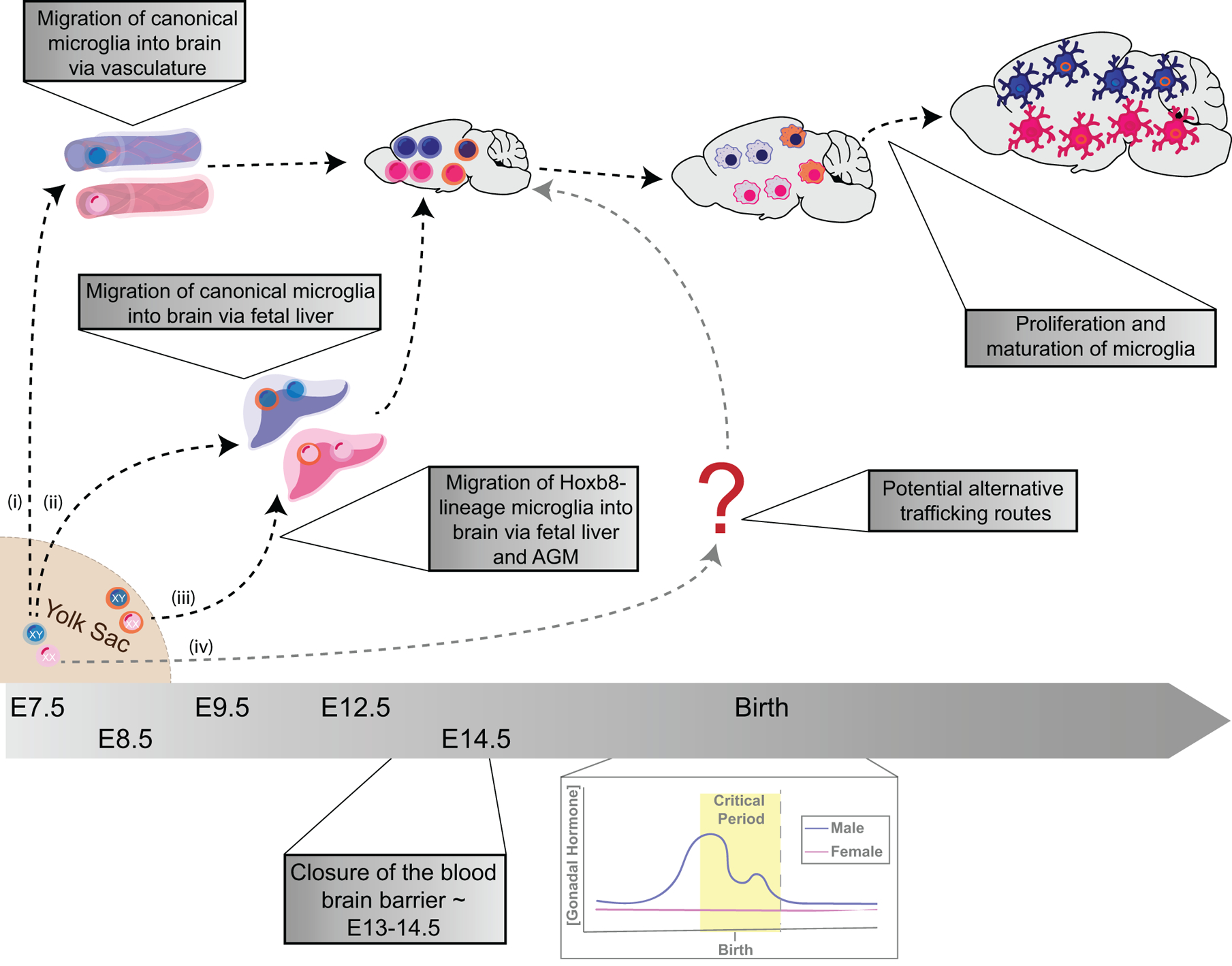
Canonical microglia appear in the fetal yolk sac ~E7.5 in rodents, whereas Hoxb8-lineage microglia appear in the fetal yolk sac ~E8.5. (i) Canonical microglia traffic through the vasculature or (ii) through the fetal liver and aorto-gonad mesonephros, whereas (iii) Hoxb8-lineage microglia traffic through the fetal liver and aorto-gonad mesonephros on the way to the brain. (iv) There is a potential for alternative routes for microglia to traffic from the fetal yolk sac to the brain. The blood brain barrier closes around E13-E14.5, cutting off further migration of monocytes into the healthy developing brain. Around birth, during the time frame of the male-specific gonadal sex hormone surge, there are more canonical microglia in the brain than Hoxb8-lineage microglia. While both subsets of microglia proliferate and mature following birth, the first postnatal week is characterized by a higher rate of proliferation of Hoxb8-lineage microglia than is observed in canonical microglia.
This figure is based on data presented in (Ginhoux et al. 2010; Chen et al. 2010; De et al. 2018; VanRyzin, Pickett, and McCarthy 2018; Hoeffel and Ginhoux 2015)
As mentioned briefly above, a study exploring intrinsic vs extrinsic factors in the determination of microglial identity depleted microglia in male or female mice with CSF1R antagonist treatment, and then transplanted microglia isolated from either male or female mice into depleted brains. Female microglia from 12 week old mice that were transplanted into depleted male brains retained a gene expression profile similar to that seen in control female mice, suggesting lasting sex-specific transcriptomic identity (Villa et al. 2018). These results presumably suggest the importance of both intrinsic and extrinsic microenvironmental factors in the sex-specific identity of microglia, as it is likely that the microenvironment during development imparts changes that will persist in female or male microglia, but that cannot be changed by later life exposure to different extrinsic environments. The same study suggested that this sex-specific identity may also persist during the injury response. Young adult female rodents sustain a lesser injury than males in response to forebrain or focal ischemia (Smith et al. 2014; Demarest et al. 2016). RNA-seq findings that female microglia had a higher propensity to express genes involved in cellular repair and inflammatory control (Villa et al. 2018), suggesting that these microglial transcriptional differences may underlie female resilience to injury. Regardless of their exact route of trafficking, microglia are likely to be programmed by the various environments to which they are exposed on their developmental journey to the brain. Given the many studies highlighting sex differences in microglial developmental trajectories and later life functions, it is surprising that relatively little attention has been given to whether the complex ontogeny of microglia may be influenced by any sex-specific programming. Future studies examining microglial ontogeny in males and females will likely have a significant impact on the field.
Intrinsic differences between brain regions may impart sex-specific regional heterogeneity in microglial function
That significant sex differences have been found in microglial number and function in different brain regions suggests that there may be sex-dependent spatial heterogeneity in microglial function/activity (Fig. 2). Brain regions are often defined by the primary neuronal subtype contained within that region, as well as to which other regions these neurons connect. However, recent scRNA-seq and proteomics studies have observed a high level of heterogeneity within individual brain regions (Tasic et al. 2016; Zeisel et al. 2015), indicating that in addition to heterogeneity between traditionally-defined brain regions, there may also be significant intra-regional heterogeneity (Silvin and Ginhoux 2018; Ayata et al. 2018). Early flow cytometry-based studies examining the potential for spatial heterogeneity of microglial phenotypes described marked variations of canonical microglial markers from microglia isolated from the hippocampus, spinal cord, cerebellum, cerebral cortex, and striatum of 11–12 week-old male mice (de Haas, Boddeke, and Biber 2008). More recent findings using CD11b+CD45low gating to select for microglia found MHCI expression in cortex and hippocampus to be higher in males than females, while MHCII expression was higher only in the male cortex (Guneykaya et al. 2018). A recent landmark study examined microglial transcriptomic, morphological, and functional heterogeneity within different regions of the basal ganglia and ventral tegmentum (De Biase et al. 2017). Microglial density was found to vary significantly across the different subregions studied. Similar to density measures, significant regional heterogeneity was also found in microglial structural complexity (De Biase et al. 2017). Of course, microglial morphology is not a perfect proxy for the ‘activational state’ of the cell (Ransohoff 2016). Indeed, several reports have noted that microglia show upregulation of both classically pro-inflammatory and anti-inflammatory markers in development (Crain, Nikodemova, and Watters 2013; Lenz et al. 2013), suggesting that an individual cell, which can have only one morphology, can express both types of markers. On a functional level, the same study observed regional differences in microglia-lysosome co-localization and microglial resting potentials (De Biase et al. 2017). At a transcriptomic level, the ventral tegmental area (VTA) was found to have the lowest gene expression overlap compared to other regions, whereas the cortex and nucleus accumbens were once again seen to have the most similarity (De Biase et al. 2017). While no sex-stratified data was reported, the authors of this study stated that no sex differences were observed in any of their measures. However, seemingly disparate results were found in a similar study comparing microglial densities as measured by staining for the microglial marker Iba1 across cortex, hippocampus, amygdala, striatum, and cerebellum at both 3 and 13 weeks of age. Although direct comparisons of densities between regions were not reported, heterogeneity was evident across the studied regions (Guneykaya et al. 2018). Significant sex differences were found in Iba1+ cell density, with males showing increased densities compared to females in the cortex, hippocampus, and amygdala at 13 weeks of age, and varying sex biases in Iba1+ density across regions at 3 weeks (Guneykaya et al. 2018). A comparison of differentially expressed genes in male and female cortex and hippocampus found 1,109 genes that were differentially expressed between the sexes in the hippocampus, 55 genes in the cortex, and 46 genes differentially regulated between the sexes in both brain regions (Guneykaya et al. 2018). The same study performed proteomic analyses on whole-brain microglia and found that 268 proteins were expressed at higher levels in male microglia compared to 96 proteins more expressed in females (Guneykaya et al. 2018). Intriguingly, when this same study compared the transcriptomic and proteomic datasets, the authors reported no strong correlations (Guneykaya et al. 2018). While this may be suggestive of further sex differences in the translation machinery, the lack of similarities may also be explained by the simple fact that transcriptomic datasets were obtained from individual brain regions whereas the proteomic dataset was obtained from whole-brain microglia, and by the differences in sensitivity levels between the two methodologies. The increased cell yield necessary to run proteomic studies compared to transcriptomic studies necessitates either the pooling of brain regions from multiple animals or the study of cell types isolated from whole brain rather than from precise regions, lessening our ability to pull apart complex regional heterogeneities in microglial proteomic profiles. Although not yet at the depth of mass spectrometry-based proteomics, single-cell protein techniques such as mass cytometry (CyTOF) are beginning to enable protein-level analyses of single cells, essential for addressing cell-type specific questions, as the transcriptome is not always reflective of the changes translated to the proteome.
Figure 2. Sex- and spatial- heterogeneity of microglia early in development.
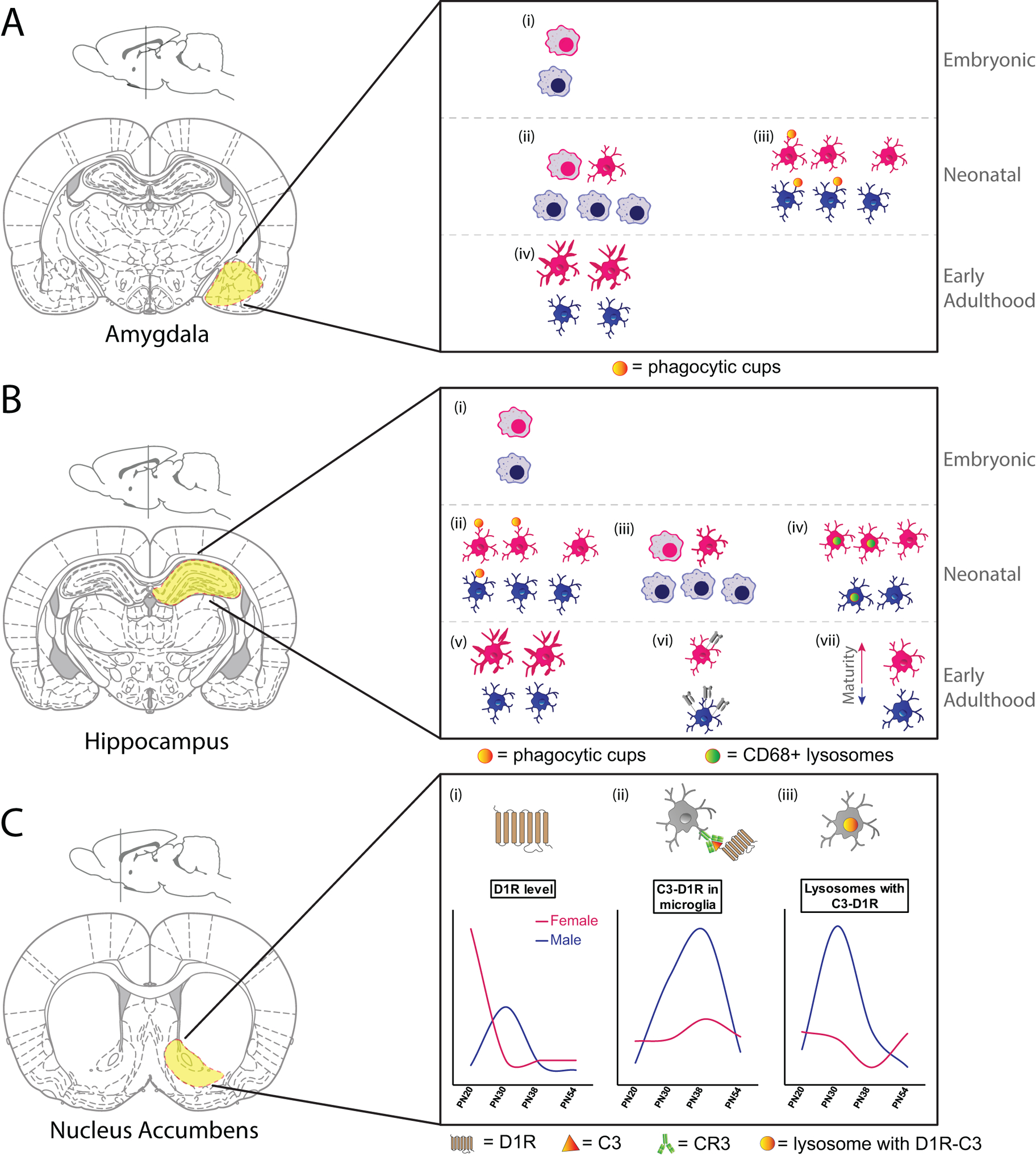
A. (i) There are no sex differences in microglial number or morphology in the E17 amygdala, with the majority of both male and female microglia showing amoeboid morphology. (ii) By PN4 male amygdala had more microglia and a higher proportion of microglia with amoeboid morphology than females. (iii) On PN2 and PN4, male microglia within the amygdala had more microglia with phagocytic cups than did females. (iv) By PN30, microglial morphology within the amygdala had shifted towards a more ramified/complex state. At this time females had more microglia with long, thick processes than males.
This figure is based on data presented in (Schwarz, Sholar, and Bilbo 2012; VanRyzin et al. 2019)
B. (i) There are no sex differences in microglial number or morphology in CA1 or CA3 regions of the hippocampus at E17, with the majority of both male and female microglia showing amoeboid morphology. (ii) At PN3, female rat hippocampus had more microglia with phagocytic cups than male hippocampus. (iii) By PN4 male hippocampus had more microglia and a higher proportion of microglia with amoeboid morphology than females. (iv) Females in PN8 hippocampus had more microglia and more CD68 intensity within microglia than males. (v) By PN30, microglial morphology within the hippocampus had shifted towards a more ramified/complex state. At this time females had more microglia with long, thick processes than males. (vi) 3-week old male mice had more MHC I expression on microglia (CD11b+CD45high cells) isolated from hippocampus than females. (vii) Microglial maturation (determined by microglial developmental index) was lower in microglia isolated from the hippocampus of a PN60 male mouse than from a female mouse.
This figure is based on data presented in (Schwarz, Sholar, and Bilbo 2012; Hanamsagar et al. 2017; Nelson, Warden, and Lenz 2017; Weinhard et al. 2018; Guneykaya et al. 2018)
C. (i) Dopamine D1 receptor levels in the nucleus accumbens peak at PN20 in females and are downregulated by PN30, whereas D1R levels peak at PN30 in males, and are then downregulated. (ii) Co-localization of microglial C3 and D1R peak in males at PN30 and PN38, with only a small upregulation seen by PN38 in females. (iii) CD68+ microglial lysosomes containing co-localized C3 and D1R peak in males at PN30, with a minor dip seen in females at PN38.
This figure is based on data presented in (Kopec et al. 2018).
Although mass cytometry is currently slower and less efficient than traditional flow cytometry, it does offer compelling benefits. For one, the discrete nature of elemental isotopes removes inherent variability in terms of both probe sensitivity and autofluorescence issues common with fluorescent probes (Bendall et al. 2012). The use of elemental isotopes also increases the number of available parameters, with over 40 unique probes being used at the single cell level in practice, and over 100 available in theory (Bendall et al. 2012). Further, combinatorial approaches to investigate protein and transcript levels simultaneously have also been developed, and allow for a unique breadth and depth of characterization of cell function (Frei et al. 2016). Mass cytometry has recently been effectively used in investigations into the heterogeneity of microglia in both rodents and humans (Böttcher et al. 2019; Mrdjen et al. 2018; Ajami et al. 2018), with particular attention being paid to how subpopulations of microglia respond differently in disease state. Of note, subsets of microglia respond to disparate insults such as aging or immune challenge differently. For example, disease associated microglia with a unique transcriptional pattern were first described using an Alzheimer’s disease mouse model (Keren-Shaul et al. 2017), and other single-cell level analyses have revealed distinct microglial subsets in varying conditions (Mrdjen et al. 2018). These studies are a strong first step investigating potential sex differences in unique microglial subtypes such as disease-associated microglia, and highlight the practical usage of new single-cell techniques. Interestingly, a model of neuroinflammation revealed combinatorial cytokine secretion, specifically of TNF-ɑ and GM-CSF, whereas microglia isolated from models of neurodegeneration maintained monosecretion (Ajami et al. 2018). Unfortunately, these current rodent studies utilizing CyTOF have either used one sex or do not report on the sex of the animals used. In total, CyTOF is proving useful in delineating spatially and temporally controlled microglial responses in disease states, though many questions remain, especially in the field of sex differences.
One interesting possible explanation for the widespread spatial heterogeneity observed in microglial profiles may stem from the distribution of canonical and Hoxb8-lineage microglia, as Hoxb8-lineage microglia are more abundant in the cortex and dorsal striatum than in regions such as the substantia nigra and ventral pallidum (De et al. 2018). Considering the potential difference in lineage between Hoxb8 and canonical microglia, we speculate that exposure of similar progenitors to disparate developmental environments may differentially impact their final colonization and/or function.
Intrinsic immunometabolic programming may impart sex differences in microglial innate immune function
As described previously, (De Biase et al. 2017) found that gene expression of many classical microglial expression programs were relatively conserved across regions whereas many genes involved in mitochondrial function, reactive oxygen species homeostasis and oxidative signaling, as well as general cell metabolism, were differentially expressed across brain regions. Notably, microglia within the VTA were observed to show low expression of glycolytic and mitochondrial oxidative phosphorylation genes (De Biase et al. 2017). Although they did not note any sex differences in their findings, these findings suggest significant regional heterogeneity in gene expression of mitochondria, an organelle that is primarily maternally inherited (Sato and Sato 2013; Camus, Clancy, and Dowling 2012). Such a sex-biased inheritance pattern is likely to lead to sex-biased functions as well, alluding to the possibility of sex differences in microglial mitochondrial metabolism.
While there are few studies into mitochondrial metabolism within microglia themselves, there are many examples of sex differences in overall metabolism (reviewed by (Widdowson 1976)) and total brain metabolic outcomes. For example, women have ~20% higher brain glucose metabolic rates as measured by positron emission tomography (PET) than men (Baxter et al. 1987). Another study in adult humans found that males had higher glucose metabolism in lateral and ventro-medial aspects of the temporal lobe regions and the cerebellum, whereas women had higher metabolism in middle and posterior cingulate gyrus (R. C. Gur et al. 1995). Similarly, a more recent study found that women had higher overall cerebral glucose metabolism than males, but lower metabolism in the cerebellum and bilateral inferior temporal gyri (Yoshizawa et al. 2014). Isolated brain mitochondria from 3-month-old female mice were shown to have a higher NADH-like respiratory rate than males (Gaignard et al. 2015), although no sex differences in this measure have been reported in 3- or 6-month-old Wistar rats (Moreira et al. 2013; Guevara, Gianotti, Oliver, et al. 2011; Guevara, Gianotti, Roca, et al. 2011). Intriguingly, ovariectomy dropped the female respiratory rate to that of males (Gaignard et al. 2015), suggesting an impact of extrinsic circulating sex hormones on whole brain mitochondrial metabolism. In addition to sex differences in basal brain metabolism, overall brain mitochondrial bioenergetics are altered differently in males and females in response to perturbations. Following cerebral neonatal hypoxia ischemia in rats, brain mitochondrial oxidative phosphorylation-dependent respiration was more impaired in males than in females (Demarest et al. 2016). The field of microglial immunometabolism is still widely unexplored, particularly when it comes to sex differences, but whole brain sex differences and known changes in microglial metabolic flux in response to immune challenges suggest that there may be sex differences in microglial bioenergetic function.
The various processes associated with microglial functionality require precise regulation of cellular energy homeostasis. As the major regulators of cellular energy metabolism, cell death, and the maintenance of calcium and redox homeostasis, mitochondria are uniquely positioned to modulate the innate immune response (Mills, Kelly, and O’Neill 2017). Indeed, emerging literature suggests an intimate link between the metabolic state of immune cells and their differentiation and activation (Olenchock, Rathmell, and Vander Heiden 2017). While research into immunometabolic states of microglia is still in its infancy, characterization of closely-related macrophages has recently begun to flourish. Macrophages activated with the classical inflammatory factors LPS and IFN-ɣ quickly enhance glycolytic metabolism and downregulate oxidative phosphorylation mechanisms (Pearce and Pearce 2013; O’Neill, Kishton, and Rathmell 2016; Galván-Peña and O’Neill 2014; Haschemi et al. 2012; L. Wang et al. 2019). LPS + IFN-ɣ-activated macrophages use this enhanced glycolytic metabolism to both produce cellular energy in the form of ATP as well as to feed the pentose phosphate pathway, generating amino acids necessary for the synthesis of inflammatory proteins, and NADPH for reactive oxygen species production (Van den Bossche, O’Neill, and Menon 2017; Nagy and Haschemi 2015). For an excellent review on the non-metabolic role of glycolytic enzymes in immunity, see (Seki and Gaultier 2017).
While the majority of metabolic research has been performed in peripheral macrophages, recent in vitro studies using cell lines or primary cultures of microglia have found similar results, with a shift from mitochondrial respiration to glycolysis and the pentose phosphate pathway following stimulation with LPS or LPS+IFN-ɣ (Orihuela, McPherson, and Harry 2016; Jaber et al. 2017; Voloboueva et al. 2013; L. Wang et al. 2019). Whereas classically-defined anti-inflammatory macrophages activated by IL-4 upregulate fatty acid metabolism, glucose uptake, and mitochondrial biogenesis (Vats et al. 2006), mouse microglia in vitro showed decreased lactate production and glucose consumption in response to IL-4 (Orihuela, McPherson, and Harry 2016), highlighting an important difference between the immunometabolic response of microglial cells and peripheral macrophages. Although many immunometabolic studies lack sex-specific clarification, a recent manuscript using neonatally-derived primary cultures of rat cortical microglia found no sex difference in microglial mitochondrial respiration in response to LPS+IFN-ɣ (Jaber et al. 2017). In addition to being activated by direct immune challenges, microglia have also been shown to be particularly responsive to diet, specifically saturated fatty acids from a high fat diet (HFD). Interestingly, HFD resulted in diminished CX3CL1/CX3CR1 signaling specifically in microglia of male mice, which corresponded with increased hypothalamic inflammation and glucose intolerance in these mice. Knockout of CX3CR1 in females resulted in a similar male-like phenotype (Dorfman et al. 2017). The female-specific protections were not altered by acute estradiol injection, suggesting intrinsic differences in hypothalamic male and female microglia in response to HFD that impact whole body glucose homeostasis. Although this particular study found no effect of estrogens, circulating and local hormone levels certainly play a role in promoting sex-specific responses (see van Ryzin et al, this issue). The majority of studies that focus on microglia or macrophage metabolism investigate changes in the context of a single immune challenge, a simplistic approach that is not necessarily representative of an in vivo state. There is a need to expand these studies to account for situations, such as chronic inflammation, where microglia have long been in a metabolically active state. It is likely that chronic inflammation alters the metabolic state of microglia, and that the metabolic and inflammatory response of chronically activated microglia to another immune challenge would be different than the response of naive microglia.
Although the field of microglia bioenergetics is still relatively young, extrapolating information from sex differences in whole brain glycolytic flux and in vitro studies of macrophages suggests that further investigations into microglial immunometabolism will yield fascinating sex differences. Microglia respond/migrate in response to insult (Davalos et al. 2005; Nimmerjahn, Kirchhoff, and Helmchen 2005), and these challenges surely require significant alterations in energy production and usage. It is also likely that microglial metabolism contributes to whole body metabolic homeostasis, as evidenced by changes in microglial metabolism in response to diet changes and resulting blood glucose homeostasis changes (Dorfman et al. 2017). Microglial metabolism is fluid; microglia display energetic shifts in response to pro- and anti-inflammatory stimuli as well as in a spatiotemporal manner. This corresponds with their known roles in promoting brain development such as axon guidance (Squarzoni et al. 2014), production of growth factors (Lenz et al. 2013, 2018; VanRyzin, Pickett, and McCarthy 2018; McCarthy et al. 2015; Kopec et al. 2018; Parkhurst et al. 2013), neurite outgrowth (Pont-Lezica et al. 2014), synaptic organization (Paolicelli et al. 2011), and elimination of apoptotic neurons (Cunningham, Martínez-Cerdeño, and Noctor 2013) among many other functions. While we have highlighted several intrinsic sex differences in microglial metabolism and function, the question of to what degree of influence a male versus female microenvironment contributes to intrinsic sex differences still lingers.
Conclusions and future directions
It is evident that microglia play an intimate role in the sexual differentiation and overall development of the brain, and that the functions of microglia themselves may be sexually dimorphic in some instances. Here we have focused on the intrinsic factors that may program male or female microglia during their developmental journeys, while vanRyzin et al in this issue have focused upon extrinsic factors that likewise influence microglia. While we briefly alluded to possible interactions between these intrinsic and extrinsic factors throughout the scope of this review, a greater focus must be placed on this interplay if we are to better understand the unique role of sex differences in microglia in sculpting the developing brain. For instance, the ability of gonadal sex hormones or endocannabinoids (extrinsic factors) to modify microglia is likely to be influenced by microglial chromosomal complement or programming during fetal ontogeny (intrinsic factors). The field of sex differences in microglial development and function is wide open. In this review and the parallel review by vanRyzin et al in this issue we have speculated on sex differences in microglia in developmental trafficking, metabolic flux, and contributions to behaviors, as well as highlighted sex-biases in spatiotemporal microglia density, phagocytic capacity, and responses to stress or stimuli. The degree to which these sex differences are influenced by intrinsic or extrinsic factors remains to be fully disseminated.
Figure 3. Effects of microglial activation on metabolism.
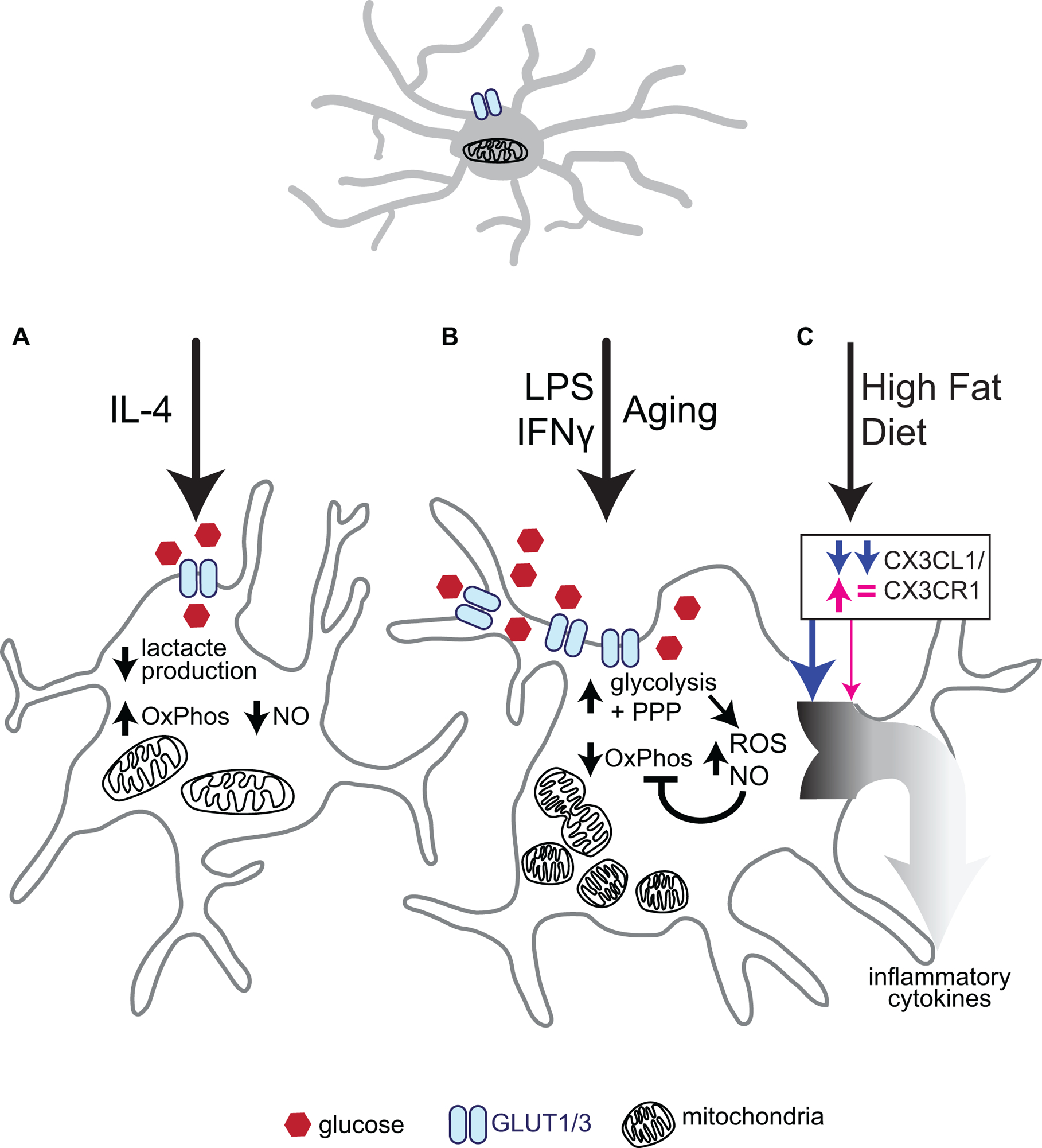
(A) Stimulation with the classic anti-inflammatory interleukin, IL-4, decreases glucose uptake and lactate production. IL-4 stimulated microglia also downregulate Nitric Oxide (NO) and TNF-ɑ production as glucose is preferentially metabolised through oxidative phosphorylation (OxPhos) to increase ATP production. (B) The microglial inflammatory response is stimulated in a number of ways, such as infection (classically simulated with LPS and/or IFNɣ) or naturally through aging. These stressors have been shown to increase levels of the glucose transporter GLUT1 and thereby increase glycolytic and pentose phosphate pathway (PPP) flux, resulting in increased NADPH and ribose 5-phosphate availability to favor nucleotide synthesis versus ATP production. This push towards glycolysis and PPP is coupled with a decrease in mitochondrial respiration (OxPhos) and increased mitochondrial fission, resulting in increased mitochondrial number, but decreased mitochondrial size. Reactive oxygen species (ROS) production through NOX2 and Nitric Oxide (NO) production are increased in a manner dependent on glucose uptake. Prolonged increases in NO can inhibit oxidative phosphorylation further. (C) Interestingly, the inflammatory response is also activated following High Fat Diet (HFD) in hypothalamic microglia only in males. HFD in males diminished CX3CR1 signaling and led to an enhanced inflammatory response, while in females CXCL1 protein was increased, but overall CXC3R1 signaling remained relatively unaffected, and no increased inflammation was observed.
This figure is based on data presented in (Orihuela, McPherson, and Harry 2016; Jaber et al. 2017; Voloboueva et al. 2013; L. Wang et al. 2019; Dorfman et al. 2017)
References
- Aanerud Joel, Borghammer Per, Rodell Anders, Jónsdottir Kristjana Y., and Gjedde Albert. 2017. “Sex Differences of Human Cortical Blood Flow and Energy Metabolism.” Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism 37 (7): 2433–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajami Bahareh, Samusik Nikolay, Wieghofer Peter, Ho Peggy P., Crotti Andrea, Bjornson Zach, Prinz Marco, Fantl Wendy J., Nolan Garry P., and Steinman Lawrence. 2018. “Single-Cell Mass Cytometry Reveals Distinct Populations of Brain Myeloid Cells in Mouse Neuroinflammation and Neurodegeneration Models.” Nature Neuroscience 21 (4): 541–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amen Daniel G., Trujillo Manuel, Keator David, Taylor Derek V., Willeumier Kristen, Meysami Somayeh, and Raji Cyrus A.. 2017. “Gender-Based Cerebral Perfusion Differences in 46,034 Functional Neuroimaging Scans.” Journal of Alzheimer’s Disease: JAD 60 (2): 605–14. [DOI] [PubMed] [Google Scholar]
- Arnò Benedetta, Grassivaro Francesca, Rossi Chiara, Bergamaschi Andrea, Castiglioni Valentina, Furlan Roberto, Greter Melanie, et al. 2014. “Neural Progenitor Cells Orchestrate Microglia Migration and Positioning into the Developing Cortex.” Nature Communications 5 (November): 5611. [DOI] [PubMed] [Google Scholar]
- Ayata Pinar, Badimon Ana, Strasburger Hayley J., Mary Kaye Duff Sarah E. Montgomery, Loh Yong-Hwee E., Ebert Anja, et al. 2018. “Epigenetic Regulation of Brain Region-Specific Microglia Clearance Activity.” Nature Neuroscience 21 (8): 1049–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter LR Jr, Mazziotta JC, Phelps ME, Selin CE, Guze BH, and Fairbanks L. 1987. “Cerebral Glucose Metabolic Rates in Normal Human Females versus Normal Males.” Psychiatry Research 21 (3): 237–45. [DOI] [PubMed] [Google Scholar]
- Bendall Sean C., Nolan Garry P., Roederer Mario, and Chattopadhyay Pratip K.. 2012. “A Deep Profiler’s Guide to Cytometry.” Trends in Immunology 33 (7): 323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett Mariko L., Bennett F. Chris, Liddelow Shane A., Ajami Bahareh, Zamanian Jennifer L., Fernhoff Nathaniel B., Mulinyawe Sara B., et al. 2016. “New Tools for Studying Microglia in the Mouse and Human CNS.” Proceedings of the National Academy of Sciences of the United States of America 113 (12): E1738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher Chotima, Schlickeiser Stephan, Sneeboer Marjolein A. M., Kunkel Desiree, Knop Anniki, Paza Evdokia, Fidzinski Pawel, et al. 2019. “Human Microglia Regional Heterogeneity and Phenotypes Determined by Multiplexed Single-Cell Mass Cytometry.” Nature Neuroscience 22 (1): 78–90. [DOI] [PubMed] [Google Scholar]
- Brink, van den Susanne C., Sage Fanny, Vértesy Ábel, Spanjaard Bastiaan, Peterson-Maduro Josi, Baron Chloé S., Robin Catherine, and van Oudenaarden Alexander. 2017. “Single-Cell Sequencing Reveals Dissociation-Induced Gene Expression in Tissue Subpopulations.” Nature Methods 14 (10): 935–36. [DOI] [PubMed] [Google Scholar]
- Camus M. Florencia, Clancy David J., and Dowling Damian K.. 2012. “Mitochondria, Maternal Inheritance, and Male Aging.” Current Biology: CB 22 (18): 1717–21. [DOI] [PubMed] [Google Scholar]
- Case Laure K., Wall Emma H., Dragon Julie A., Saligrama Naresha, Krementsov Dimitry N., Moussawi Mohamad, Zachary James F., Huber Sally A., Blankenhorn Elizabeth P., and Teuscher Cory. 2013. “The Y Chromosome as a Regulatory Element Shaping Immune Cell Transcriptomes and Susceptibility to Autoimmune Disease.” Genome Research 23 (9): 1474–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Mimi K., and Disteche Christine M.. 2004. “Silence of the Fathers: Early X Inactivation.” BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology 26 (8): 821–24. [DOI] [PubMed] [Google Scholar]
- Chen Shau-Kwaun, Tvrdik Petr, Peden Erik, Cho Scott, Wu Sen, Spangrude Gerald, and Capecchi Mario R.. 2010. “Hematopoietic Origin of Pathological Grooming in Hoxb8 Mutant Mice.” Cell 141 (5): 775–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain Jessica M., Nikodemova Maria, and Watters Jyoti J.. 2013. “Microglia Express Distinct M1 and M2 Phenotypic Markers in the Postnatal and Adult Central Nervous System in Male and Female Mice.” Journal of Neuroscience Research 91 (9): 1143–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronk James C., Filiano Anthony J., Louveau Antoine, Marin Ioana, Marsh Rachel, Ji Emily, Goldman Dylan H., et al. 2018. “Peripherally Derived Macrophages Can Engraft the Brain Independent of Irradiation and Maintain an Identity Distinct from Microglia.” The Journal of Experimental Medicine 215 (6): 1627–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham Christopher L., Martínez-Cerdeño Verónica, and Noctor Stephen C.. 2013. “Microglia Regulate the Number of Neural Precursor Cells in the Developing Cerebral Cortex.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 33 (10): 4216–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos Dimitrios, Grutzendler Jaime, Yang Guang, Kim Jiyun V., Zuo Yi, Jung Steffen, Littman Dan R., Dustin Michael L., and Gan Wen-Biao. 2005. “ATP Mediates Rapid Microglial Response to Local Brain Injury in Vivo.” Nature Neuroscience 8 (6): 752–58. [DOI] [PubMed] [Google Scholar]
- Biase De, Lindsay M, Schuebel Kornel E., Fusfeld Zachary H., Jair Kamwing, Hawes Isobel A., Cimbro Raffaello, Zhang Hai-Ying, et al. 2017. “Local Cues Establish and Maintain Region-Specific Phenotypes of Basal Ganglia Microglia.” Neuron 95 (2): 341–56.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarest Tyler G., Schuh Rosemary A., Waddell Jaylyn, McKenna Mary C., and Fiskum Gary. 2016. “Sex-Dependent Mitochondrial Respiratory Impairment and Oxidative Stress in a Rat Model of Neonatal Hypoxic-Ischemic Encephalopathy.” Journal of Neurochemistry 137 (5): 714–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Shrutokirti, Donn Van Deren Eric Peden, Hockin Matt, Boulet Anne, Titen Simon, and Capecchi Mario R.. 2018. “Two Distinct Ontogenies Confer Heterogeneity to Mouse Brain Microglia.” Development 145 (13). 10.1242/dev.152306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman Mauricio D., Krull Jordan E., Douglass John D., Fasnacht Rachael, Fernando Lara-Lince Thomas H. Meek, Shi Xiaogang, et al. 2017. “Sex Differences in Microglial CX3CR1 Signalling Determine Obesity Susceptibility in Mice.” Nature Communications 8 (February): 14556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore Monica R. P., Lee Rafael J., West Brian L., and Green Kim N.. 2015. “Characterizing Newly Repopulated Microglia in the Adult Mouse: Impacts on Animal Behavior, Cell Morphology, and Neuroinflammation.” PloS One 10 (4): e0122912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito G, Van Horn JD, Weinberger DR, and Berman KF. 1996. “Gender Differences in Cerebral Blood Flow as a Function of Cognitive State with PET.” Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine 37 (4): 559–64. [PubMed] [Google Scholar]
- Fish Eleanor N. 2008. “The X-Files in Immunity: Sex-Based Differences Predispose Immune Responses.” Nature Reviews. Immunology 8 (9): 737–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford AL, Goodsall AL, Hickey WF, and Sedgwick JD. 1995. “Normal Adult Ramified Microglia Separated from Other Central Nervous System Macrophages by Flow Cytometric Sorting. Phenotypic Differences Defined and Direct Ex Vivo Antigen Presentation to Myelin Basic Protein-Reactive CD4+ T Cells Compared.” Journal of Immunology 154 (9): 4309–21. [PubMed] [Google Scholar]
- Frei Andreas P., Bava Felice-Alessio, Zunder Eli R., Hsieh Elena W. Y., Chen Shih-Yu, Nolan Garry P., and Federico Gherardini Pier. 2016. “Highly Multiplexed Simultaneous Detection of RNAs and Proteins in Single Cells.” Nature Methods 13 (3): 269–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaignard Pauline, Savouroux Stéphane, Liere Philippe, Pianos Antoine, Patrice Thérond Michael Schumacher, Slama Abdelhamid, and Guennoun Rachida. 2015. “Effect of Sex Differences on Brain Mitochondrial Function and Its Suppression by Ovariectomy and in Aged Mice.” Endocrinology 156 (8): 2893–2904. [DOI] [PubMed] [Google Scholar]
- Galloway Dylan A., Blandford Stephanie N., Berry Tangyne, Williams John B., Stefanelli Mark, Ploughman Michelle, and Moore Craig S.. 2018. “miR‐223 Promotes Regenerative Myeloid Cell Phenotype and Function in the Demyelinated Central Nervous System.” Glia, December 10.1002/glia.23576. [DOI] [PubMed] [Google Scholar]
- Galván-Peña Silvia, and O’Neill Luke A. J.. 2014. “Metabolic Reprograming in Macrophage Polarization.” Frontiers in Immunology 5 (September): 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd Jay N. 2004. “Structural Magnetic Resonance Imaging of the Adolescent Brain.” Annals of the New York Academy of Sciences 1021 (June): 77–85. [DOI] [PubMed] [Google Scholar]
- Ginhoux Florent, Greter Melanie, Leboeuf Marylene, Nandi Sayan, See Peter, Gokhan Solen, Mehler Mark F., et al. 2010. “Fate Mapping Analysis Reveals That Adult Microglia Derive from Primitive Macrophages.” Science 330 (6005): 841–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer Joy M., and Capecchi Mario R.. 2002. “Hoxb8 Is Required for Normal Grooming Behavior in Mice.” Neuron 33 (1): 23–34. [DOI] [PubMed] [Google Scholar]
- Guevara Rocío, Gianotti Magdalena, Oliver Jordi, and Roca Pilar. 2011. “Age and Sex-Related Changes in Rat Brain Mitochondrial Oxidative Status.” Experimental Gerontology 46 (11): 923–28. [DOI] [PubMed] [Google Scholar]
- Guevara Rocío, Gianotti Magdalena, Roca Pilar, and Oliver Jordi. 2011. “Age and Sex-Related Changes in Rat Brain Mitochondrial Function.” Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology 27 (3–4): 201–6. [DOI] [PubMed] [Google Scholar]
- Guneykaya Dilansu, Ivanov Andranik, Daniel Perez Hernandez Verena Haage, Wojtas Bartosz, Meyer Niklas, Maricos Meron, et al. 2018. “Transcriptional and Translational Differences of Microglia from Male and Female Brains.” Cell Reports 24 (10): 2773–83.e6. [DOI] [PubMed] [Google Scholar]
- Gur RC, Mozley LH, Mozley PD, Resnick SM, Karp JS, Alavi A, Arnold SE, and Gur RE. 1995. “Sex Differences in Regional Cerebral Glucose Metabolism during a Resting State.” Science 267 (5197): 528–31. [DOI] [PubMed] [Google Scholar]
- Gur RE, and Gur RC. 1990. “Gender Differences in Regional Cerebral Blood Flow.” Schizophrenia Bulletin 16 (2): 247–54. [DOI] [PubMed] [Google Scholar]
- Haas, de Alexander H., Boddeke Hendrikus W. G. M., and Biber Knut. 2008. “Region-Specific Expression of Immunoregulatory Proteins on Microglia in the Healthy CNS.” Glia 56 (8): 888–94. [DOI] [PubMed] [Google Scholar]
- Haimon Zhana, Volaski Alon, Orthgiess Johannes, Sigalit Boura-Halfon Diana Varol, Shemer Anat, Yona Simon, et al. 2018. “Re-Evaluating Microglia Expression Profiles Using RiboTag and Cell Isolation Strategies.” Nature Immunology 19 (6): 636–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond Timothy R., Dufort Connor, Lasse Dissing-Olesen Stefanie Giera, Young Adam, Wysoker Alec, Walker Alec J., et al. 2019. “Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes.” Immunity 50 (1): 253–71.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamsagar Richa, Alter Mark D., Block Carina S., Sullivan Haley, Bolton Jessica L., and Bilbo Staci D.. 2017. “Generation of a Microglial Developmental Index in Mice and in Humans Reveals a Sex Difference in Maturation and Immune Reactivity.” Glia 65 (9): 1504–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanisch Uwe-Karsten, and Kettenmann Helmut. 2007. “Microglia: Active Sensor and Versatile Effector Cells in the Normal and Pathologic Brain.” Nature Neuroscience 10 (11): 1387–94. [DOI] [PubMed] [Google Scholar]
- Harbo Hanne F., Gold Ralf, and Tintoré Mar. 2013. “Sex and Gender Issues in Multiple Sclerosis.” Therapeutic Advances in Neurological Disorders 6 (4): 237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haschemi Arvand, Kosma Paul, Gille Lars, Evans Charles R., Burant Charles F., Starkl Philipp, Knapp Bernhard, et al. 2012. “The Sedoheptulose Kinase CARKL Directs Macrophage Polarization through Control of Glucose Metabolism.” Cell Metabolism 15 (6): 813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard Edith, and Disteche Christine M.. 2006. “Dosage Compensation in Mammals: Fine-Tuning the Expression of the X Chromosome.” Genes & Development 20 (14): 1848–67. [DOI] [PubMed] [Google Scholar]
- Herbomel P, Thisse B, and Thisse C. 2001. “Zebrafish Early Macrophages Colonize Cephalic Mesenchyme and Developing Brain, Retina, and Epidermis through a M-CSF Receptor-Dependent Invasive Process.” Developmental Biology 238 (2): 274–88. [DOI] [PubMed] [Google Scholar]
- Hoeffel Guillaume, and Ginhoux Florent. 2015. “Ontogeny of Tissue-Resident Macrophages.” Frontiers in Immunology 6 (September): 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaber Sausan M., Bordt Evan A., Bhatt Niraj M., Lewis Daniel M., Gerecht Sharon, Fiskum Gary, and Polster Brian M.. 2017. “Sex Differences in the Mitochondrial Bioenergetics of Astrocytes but Not Microglia at a Physiologically Relevant Brain Oxygen Tension.” Neurochemistry International, September 10.1016/j.neuint.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser Tobias, and Feng Guoping. 2019. “Tmem119-EGFP and Tmem119-CreERT2 Transgenic Mice for Labeling and Manipulating Microglia.” eNeuro 6 (4). 10.1523/ENEURO.0448-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren-Shaul Hadas, Spinrad Amit, Weiner Assaf, Orit Matcovitch-Natan Raz Dvir-Szternfeld, Ulland Tyler K., David Eyal, et al. 2017. “A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease.” Cell 169 (7): 1276–90.e17. [DOI] [PubMed] [Google Scholar]
- Konkle Anne T. M., and McCarthy Margaret M.. 2011. “Developmental Time Course of Estradiol, Testosterone, and Dihydrotestosterone Levels in Discrete Regions of Male and Female Rat Brain.” Endocrinology 152 (1): 223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopec Ashley M., Smith Caroline J., Ayre Nathan R., Sweat Sean C., and Bilbo Staci D.. 2018. “Microglial Dopamine Receptor Elimination Defines Sex-Specific Nucleus Accumbens Development and Social Behavior in Adolescent Rats.” Nature Communications 9 (1): 3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann Sabrina M., Christina Krüger Boyoun Park, Derkow Katja, Rosenberger Karen, Baumgart Jan, Trimbuch Thorsten, et al. 6/2012. “An Unconventional Role for miRNA: Let-7 Activates Toll-like Receptor 7 and Causes Neurodegeneration.” Nature Neuroscience 15 (6): 827–35. [DOI] [PubMed] [Google Scholar]
- Lenz Kathryn M., Nugent Bridget M., Haliyur Rachana, and McCarthy Margaret M.. 2013. “Microglia Are Essential to Masculinization of Brain and Behavior.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 33 (7): 2761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz Kathryn M., Pickett Lindsay A., Wright Christopher L., Davis Katherine T., Joshi Aarohi, and McCarthy Margaret M.. 2018. “Mast Cells in the Developing Brain Determine Adult Sexual Behavior.” The Journal of Neuroscience: The Official Journal of the Society for Neuroscience 38 (37): 8044–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Qingyun, Cheng Zuolin, Zhou Lu, Darmanis Spyros, Neff Norma F., Okamoto Jennifer, Gulati Gunsagar, et al. 2019. “Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing.” Neuron 101 (2): 207–23.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Zhaoyuan, Gu Yaqi, Chakarov Svetoslav, Bleriot Camille, Chen Xin, Shin Amanda, Huang Weijie, et al. 2019. “Fate Mapping via Ms4a3 Expression History Traces Monocyte-Derived Cells.” bioRxiv. 10.1101/652032. [DOI] [PubMed] [Google Scholar]
- Lyck Lise, Ishar Dalmau Santamaria Bente Pakkenberg, Chemnitz John, Henrik Daa Schrøder Bente Finsen, and Hans Jørgen G. Gundersen. 2009. “An Empirical Analysis of the Precision of Estimating the Numbers of Neurons and Glia in Human Neocortex Using a Fractionator-Design with Sub-Sampling.” Journal of Neuroscience Methods 182 (2): 143–56. [DOI] [PubMed] [Google Scholar]
- Matcovitch-Natan Orit, Winter Deborah R., Giladi Amir, Stephanie Vargas Aguilar Amit Spinrad, Sarrazin Sandrine, Hila Ben-Yehuda, et al. 2016. “Microglia Development Follows a Stepwise Program to Regulate Brain Homeostasis.” Science 353 (6301): aad8670. [DOI] [PubMed] [Google Scholar]
- McBride Devin W., and Zhang John H.. 2017. “Precision Stroke Animal Models: The Permanent MCAO Model Should Be the Primary Model, Not Transient MCAO.” Translational Stroke Research, July 10.1007/s12975-017-0554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy Margaret M. 2008. “Estradiol and the Developing Brain.” Physiological Reviews 88 (1): 91–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy Margaret M., Pickett Lindsay A., VanRyzin Jonathan W., and Kight Katherine E.. 2015. “Surprising Origins of Sex Differences in the Brain.” Hormones and Behavior 76 (November): 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills Evanna L., Kelly Beth, and O’Neil Luke A. J.. 2017. “Mitochondria Are the Powerhouses of Immunity.” Nature Immunology 18 (5): 488–98. [DOI] [PubMed] [Google Scholar]
- Moreira Ana C., Silva Ana M., Santos Maria S., and Sardão Vilma A.. 2013. “Resveratrol Affects Differently Rat Liver and Brain Mitochondrial Bioenergetics and Oxidative Stress in Vitro: Investigation of the Role of Gender.” Food and Chemical Toxicology: An International Journal Published for the British Industrial Biological Research Association 53 (March): 18–26. [DOI] [PubMed] [Google Scholar]
- Mrdjen Dunja, Pavlovic Anto, Hartmann Felix J., Schreiner Bettina, Utz Sebastian G., Leung Brian P., Lelios Iva, et al. 2018. “High-Dimensional Single-Cell Mapping of Central Nervous System Immune Cells Reveals Distinct Myeloid Subsets in Health, Aging, and Disease.” Immunity 48 (2): 380–95.e6. [DOI] [PubMed] [Google Scholar]
- Nagy Csörsz, and Haschemi Arvand. 2015. “Time and Demand Are Two Critical Dimensions of Immunometabolism: The Process of Macrophage Activation and the Pentose Phosphate Pathway.” Frontiers in Immunology 6 (April): 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson Lars H., Warden Spencer, and Lenz Kathryn M.. 2017. “Sex Differences in Microglial Phagocytosis in the Neonatal Hippocampus.” Brain, Behavior, and Immunity 64 (August): 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn Axel, Kirchhoff Frank, and Helmchen Fritjof. 2005. “Resting Microglial Cells Are Highly Dynamic Surveillants of Brain Parenchyma in Vivo.” Science 308 (5726): 1314–18. [DOI] [PubMed] [Google Scholar]
- Olenchock Benjamin A., Rathmell Jeffrey C., and Vander Heiden. 2017. “Biochemical Underpinnings of Immune Cell Metabolic Phenotypes.” Immunity 46 (5): 703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill Luke A. J., Kishton Rigel J., and Rathmell Jeff. 2016. “A Guide to Immunometabolism for Immunologists.” Nature Reviews. Immunology 16 (9): 553–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orihuela Ruben, McPherson Christopher A., and Jean Harry Gaylia. 2016. “Microglial M1/M2 Polarization and Metabolic States.” British Journal of Pharmacology 173 (4): 649–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shaughnessy Peter J., Monteiro Ana, Bhattacharya Siladitya, and Fowler Paul A.. 2011. “Maternal Smoking and Fetal Sex Significantly Affect Metabolic Enzyme Expression in the Human Fetal Liver.” The Journal of Clinical Endocrinology and Metabolism 96 (9): 2851–60. [DOI] [PubMed] [Google Scholar]
- Paolicelli Rosa C., Bolasco Giulia, Pagani Francesca, Maggi Laura, Scianni Maria, Panzanelli Patrizia, Giustetto Maurizio, et al. 2011. “Synaptic Pruning by Microglia Is Necessary for Normal Brain Development.” Science 333 (6048): 1456–58. [DOI] [PubMed] [Google Scholar]
- Parkhurst Christopher N., Yang Guang, Ninan Ipe, Savas Jeffrey N., Yates John R. 3rd, Lafaille Juan J., Hempstead Barbara L., Littman Dan R., and Gan Wen-Biao. 2013. “Microglia Promote Learning-Dependent Synapse Formation through Brain-Derived Neurotrophic Factor.” Cell 155 (7): 1596–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce Erika L., and Pearce Edward J.. 2013. “Metabolic Pathways in Immune Cell Activation and Quiescence.” Immunity 38 (4): 633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelvig DP, Pakkenberg H, Stark AK, and Pakkenberg B. 2008. “Neocortical Glial Cell Numbers in Human Brains.” Neurobiology of Aging 29 (11): 1754–62. [DOI] [PubMed] [Google Scholar]
- Pinheiro Iris, Dejager Lien, and Libert Claude. 2011. “X-Chromosome-Located microRNAs in Immunity: Might They Explain Male/female Differences? The X Chromosome-Genomic Context May Affect X-Located miRNAs and Downstream Signaling, Thereby Contributing to the Enhanced Immune Response of Females.” BioEssays: News and Reviews in Molecular, Cellular and Developmental Biology 33 (11): 791–802. [DOI] [PubMed] [Google Scholar]
- Pont-Lezica Lorena, Beumer Wouter, Colasse Sabrina, Drexhage Hemmo, Versnel Marjan, and Bessis Alain. 2014. “Microglia Shape Corpus Callosum Axon Tract Fasciculation: Functional Impact of Prenatal Inflammation.” The European Journal of Neuroscience 39 (10): 1551–57. [DOI] [PubMed] [Google Scholar]
- Qian Bin-Zhi, Li Jiufeng, Zhang Hui, Kitamura Takanori, Zhang Jinghang, Campion Liam R., Kaiser Elizabeth A., Snyder Linda A., and Pollard Jeffrey W.. 2011. “CCL2 Recruits Inflammatory Monocytes to Facilitate Breast-Tumour Metastasis.” Nature 475 (7355): 222–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff Richard M. 2016. “A Polarizing Question: Do M1 and M2 Microglia Exist?” Nature Neuroscience 19 (8): 987–91. [DOI] [PubMed] [Google Scholar]
- Rodriguez G, Warkentin S, Risberg J, and Rosadini G. 1988. “Sex Differences in Regional Cerebral Blood Flow.” Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism 8 (6): 783–89. [DOI] [PubMed] [Google Scholar]
- Rothhammer Veit, Borucki Davis M., Tjon Emily C., Takenaka Maisa C., Chao Chun-Cheih, Ardura-Fabregat Alberto, Alves de Lima Kalil, et al. 2018. “Microglial Control of Astrocytes in Response to Microbial Metabolites.” Nature 557 (7707): 724–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samokhvalov Igor M., Samokhvalova Natalia I., and Nishikawa Shin-Ichi. 2007. “Cell Tracing Shows the Contribution of the Yolk Sac to Adult Haematopoiesis.” Nature 446 (7139): 1056–61. [DOI] [PubMed] [Google Scholar]
- Sato Miyuki, and Sato Ken. 2013. “Maternal Inheritance of Mitochondrial DNA by Diverse Mechanisms to Eliminate Paternal Mitochondrial DNA.” Biochimica et Biophysica Acta 1833 (8): 1979–84. [DOI] [PubMed] [Google Scholar]
- Satterthwaite Theodore D., Shinohara Russell T., Wolf Daniel H., Hopson Ryan D., Elliott Mark A., Vandekar Simon N., Ruparel Kosha, et al. 2014. “Impact of Puberty on the Evolution of Cerebral Perfusion during Adolescence.” Proceedings of the National Academy of Sciences of the United States of America 111 (23): 8643–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer Dorothy P., Lehrman Emily K., Kautzman Amanda G., Koyama Ryuta, Mardinly Alan R., Yamasaki Ryo, Ransohoff Richard M., Greenberg Michael E., Barres Ben A., and Stevens Beth. 2012. “Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner.” Neuron 74 (4): 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer Dorothy P., Lehrman Emily K., and Stevens Beth. 2013. “The ‘Quad-Partite’ Synapse: Microglia-Synapse Interactions in the Developing and Mature CNS.” Glia 61 (1): 24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegelmilch Timm, Henke Katrin, and Peri Francesca. 2011. “Microglia in the Developing Brain: From Immunity to Behaviour.” Current Opinion in Neurobiology 21 (1): 5–10. [DOI] [PubMed] [Google Scholar]
- Schwarz Jaclyn M., Sholar Paige W., and Bilbo Staci D.. 2012. “Sex Differences in Microglial Colonization of the Developing Rat Brain.” Journal of Neurochemistry 120 (6): 948–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick JD, Schwender S, Imrich H, Dörries R, Butcher GW, and ter Meulen V. 1991. “Isolation and Direct Characterization of Resident Microglial Cells from the Normal and Inflamed Central Nervous System.” Proceedings of the National Academy of Sciences of the United States of America 88 (16): 7438–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki Scott M., and Gaultier Alban. 2017. “Exploring Non-Metabolic Functions of Glycolytic Enzymes in Immunity.” Frontiers in Immunology 8 (November): 1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvin Aymeric, and Ginhoux Florent. 2018. “Microglia Heterogeneity along a Spatio-Temporal Axis: More Questions than Answers.” Glia 66 (10): 2045–57. [DOI] [PubMed] [Google Scholar]
- Smith Amanda L., Alexander Michelle, Rosenkrantz Ted S., Lisa Sadek Mona, and Fitch R. Holly. 2014. “Sex Differences in Behavioral Outcome Following Neonatal Hypoxia Ischemia: Insights from a Clinical Meta-Analysis and a Rodent Model of Induced Hypoxic Ischemic Brain Injury.” Experimental Neurology 254 (April): 54–67. [DOI] [PubMed] [Google Scholar]
- Souyris Mélanie, Cenac Claire, Azar Pascal, Daviaud Danièle, Canivet Astrid, Grunenwald Solange, Pienkowski Catherine, Chaumeil Julie, Mejía José E., and Guéry Jean-Charles. 2018. “TLR7 Escapes X Chromosome Inactivation in Immune Cells.” Science Immunology 3 (19): eaap8855. [DOI] [PubMed] [Google Scholar]
- Spach Karen M., Blake Melissa, Bunn Janice Y., Ben McElvany Rajkumar Noubade, Blankenhorn Elizabeth P., and Teuscher Cory. 2009. “Cutting Edge: The Y Chromosome Controls the Age-Dependent Experimental Allergic Encephalomyelitis Sexual Dimorphism in SJL/J Mice.” Journal of Immunology 182 (4): 1789–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squarzoni Paola, Oller Guillaume, Hoeffel Guillaume, Lorena Pont-Lezica Philippe Rostaing, Low Donovan, Bessis Alain, Ginhoux Florent, and Garel Sonia. 2014. “Microglia Modulate Wiring of the Embryonic Forebrain.” Cell Reports 8 (5): 1271–79. [DOI] [PubMed] [Google Scholar]
- Stremmel C, Schuchert R, Wagner F, Thaler R, Weinberger T, Pick R, Mass E, et al. 2018. “Yolk Sac Macrophage Progenitors Traffic to the Embryo during Defined Stages of Development.” Nature Communications 9 (1): 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinnen Nina, Smolders Sophie, Avila Ariel, Notelaers Kristof, Paesen Rik, Ameloot Marcel, Bert Brône Pascal Legendre, and Rigo Jean-Michel. 2013. “Complex Invasion Pattern of the Cerebral Cortex Bymicroglial Cells during Development of the Mouse Embryo.” Glia 61 (2): 150–63. [DOI] [PubMed] [Google Scholar]
- Tasic Bosiljka, Menon Vilas, Thuc Nghi Nguyen Tae Kyung Kim, Jarsky Tim, Yao Zizhen, Levi Boaz, et al. 2016. “Adult Mouse Cortical Cell Taxonomy Revealed by Single Cell Transcriptomics.” Nature Neuroscience 19 (2): 335–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher Cory, Noubade Rajkumar, Spach Karen, Benjamin McElvany Janice Y. Bunn, Fillmore Parley D., Zachary James F., and Blankenhorn Elizabeth P.. 2006. “Evidence That the Y Chromosome Influences Autoimmune Disease in Male and Female Mice.” Proceedings of the National Academy of Sciences of the United States of America 103 (21): 8024–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaut Florence. 2016. “The Role of Sex and Gender in Neuropsychiatric Disorders.” Dialogues in Clinical Neuroscience 18 (4): 351–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thion Morgane S., Ginhoux Florent, and Garel Sonia. 2018. “Microglia and Early Brain Development: An Intimate Journey.” Science 362 (6411): 185–89. [DOI] [PubMed] [Google Scholar]
- Thion Morgane Sonia, Low Donovan, Silvin Aymeric, Chen Jinmiao, Grisel Pauline, Jonas Schulte-Schrepping Ronnie Blecher, et al. 2018. “Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner.” Cell 172 (3): 500–516.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bossche Jan, O’Neill Luke A., and Menon Deepthi. 2017. “Macrophage Immunometabolism: Where Are We (Going)?” Trends in Immunology 38 (6): 395–406. [DOI] [PubMed] [Google Scholar]
- VanRyzin Jonathan W., Marquardt Ashley E., Argue Kathryn J., Vecchiarelli Haley A., Ashton Sydney E., Arambula Sheryl E., Hill Matthew N., and McCarthy Margaret M.. 2019. “Microglial Phagocytosis of Newborn Cells Is Induced by Endocannabinoids and Sculpts Sex Differences in Juvenile Rat Social Play.” Neuron, February 10.1016/j.neuron.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRyzin Jonathan W., Pickett Lindsay A., and McCarthy Margaret M.. 2018. “Microglia: Driving Critical Periods and Sexual Differentiation of the Brain.” Developmental Neurobiology 78 (6): 580–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vats Divya, Mukundan Lata, Odegaard Justin I., Zhang Lina, Smith Kristi L., Morel Christine R., Wagner Roger A., Greaves David R., Murray Peter J., and Chawla Ajay. 2006. “Oxidative Metabolism and PGC-1beta Attenuate Macrophage-Mediated Inflammation.” Cell Metabolism 4 (1): 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verney Catherine, Monier Anne, Fallet-Bianco Catherine, and Gressens Pierre. 2010. “Early Microglial Colonization of the Human Forebrain and Possible Involvement in Periventricular White-Matter Injury of Preterm Infants.” Journal of Anatomy 217 (4): 436–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villa Alessandro, Gelosa Paolo, Castiglioni Laura, Cimino Mauro, Rizzi Nicoletta, Pepe Giovanna, Lolli Federica, et al. 2018. “Sex-Specific Features of Microglia from Adult Mice.” Cell Reports 23 (12): 3501–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voloboueva Ludmila A., Emery John F., Sun Xiaoyun, and Giffard Rona G.. 2013. “Inflammatory Response of Microglial BV-2 Cells Includes a Glycolytic Shift and Is Modulated by Mitochondrial Glucose-Regulated Protein 75/mortalin.” FEBS Letters 587 (6): 756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Luxi, Pavlou Sofia, Du Xuan, Bhuckory Mohajeet, Xu Heping, and Chen Mei. 2019. “Glucose Transporter 1 Critically Controls Microglial Activation through Facilitating Glycolysis.” Molecular Neurodegeneration 14 (1): 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Xu, Soloway Paul D., and Clark Andrew G.. 2010. “Paternally Biased X Inactivation in Mouse Neonatal Brain.” Genome Biology 11 (7): R79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinhard Laetitia, Neniskyte Urte, Vadisiute Auguste, Giulia di Bartolomei Nil Aygün, Riviere Laurie, Zonfrillo Francesca, Dymecki Susan, and Gross Cornelius. 2018. “Sexual Dimorphism of Microglia and Synapses during Mouse Postnatal Development.” Developmental Neurobiology 78 (6): 618–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz J, and Ward IL. 1980. “Plasma Testosterone and Progesterone Titers of Pregnant Rats, Their Male and Female Fetuses, and Neonatal Offspring.” Endocrinology 106 (1): 306–16. [DOI] [PubMed] [Google Scholar]
- Widdowson EM 1976. “The Response of the Sexes to Nutritional Stress.” The Proceedings of the Nutrition Society 35 (2): 175–80. [DOI] [PubMed] [Google Scholar]
- Yoshizawa Hiroshi, Gazes Yunglin, Stern Yaakov, Miyata Yoko, and Uchiyama Shinichiro. 2014. “Characterizing the Normative Profile of 18F-FDG PET Brain Imaging: Sex Difference, Aging Effect, and Cognitive Reserve.” Psychiatry Research 221 (1): 78–85. [DOI] [PubMed] [Google Scholar]
- Zagni Emanuela, Simoni Lucia, and Colombo Delia. 2016. “Sex and Gender Differences in Central Nervous System-Related Disorders.” Neuroscience Journal 2016 (May): 2827090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisel Amit, Muñoz-Manchado Ana B., Codeluppi Simone, Lönnerberg Peter, La Manno Gioele, Juréus Anna, Marques Sueli, et al. 2015. “Brain Structure. Cell Types in the Mouse Cortex and Hippocampus Revealed by Single-Cell RNA-Seq.” Science 347 (6226): 1138–42. [DOI] [PubMed] [Google Scholar]
- Zuloaga Damian G., Puts David A., Jordan Cynthia L., and Breedlove S. Marc. 2008. “The Role of Androgen Receptors in the Masculinization of Brain and Behavior: What We’ve Learned from the Testicular Feminization Mutation.” Hormones and Behavior 53 (5): 613–26. [DOI] [PMC free article] [PubMed] [Google Scholar]


