The transcription initiation signal elicited by the binding of ferric citrate to the outer membrane FecA protein is transmitted by the FecR protein across the cytoplasmic membrane to the FecI extracytoplasmic function (ECF) sigma factor. In this issue of Journal of Bacteriology, I. J. Passmore, J. M. Dow, F. Coll, J. Cuccui, et al. (J Bacteriol 202:e00541-19, 2020, https://doi.org/10.1128/JB.00541-19) report that the FecR sequence contains both the twin-arginine signal motif and the secretory (Sec) avoidance motif typical of proteins secreted by the twin-arginine translocation (TAT) system.
KEYWORDS: iron regulation, protein secretion, transport
ABSTRACT
The transcription initiation signal elicited by the binding of ferric citrate to the outer membrane FecA protein is transmitted by the FecR protein across the cytoplasmic membrane to the FecI extracytoplasmic function (ECF) sigma factor. In this issue of Journal of Bacteriology, I. J. Passmore, J. M. Dow, F. Coll, J. Cuccui, et al. (J Bacteriol 202:e00541-19, 2020, https://doi.org/10.1128/JB.00541-19) report that the FecR sequence contains both the twin-arginine signal motif and the secretory (Sec) avoidance motif typical of proteins secreted by the twin-arginine translocation (TAT) system. The same study shows that FecR is indeed secreted by Tat and represents a new class of bitopic Tat-dependent membrane proteins.
TEXT
In their paper in this issue of Journal of Bacteriology, Passmore et al. (1) describe the novel twin-arginine translocation (Tat)-dependent translocation of a bitopic cytoplasmic membrane protein with functional domains located in the periplasm and in the cytoplasm. The protein receives a transcriptional regulatory signal in the periplasm and transmits it across the cytoplasmic membrane to the cytoplasm, where it activates an extracytoplasmic function (ECF) sigma factor. In Escherichia coli, a sophisticated system regulates the transport of iron bound to citrate (Fig. 1). The dimeric iron carrier, (Fe3+ citrate)2, is recognized at the cell surface by the outer membrane FecA protein, which serves as both a transport protein and a regulatory protein (2). The binding of (Fe3+ citrate)2 to FecA causes massive long-range structural transitions in FecA. These include the translation of surface loops 7 and 8 by as much as 11 Å and 15 Å, respectively, such that they cover the entry of the surface cavity and thereby prevent the escape of (Fe3+ citrate)2 back to the external milieu (3, 4). Unlike other outer membrane proteins with transport but not regulatory functions, FecA contains an N-terminal extension, recognized as a signaling sequence that is required for regulation but not for transport. Moreover, the regulatory activity of FecA can be uncoupled from its transport activity by point mutations in the fecA gene, such that the mutant proteins are capable of regulation but not transport (5). In addition, the proteins TonB, ExbB, and ExbD, which form a complex in the cytoplasmic membrane (6–8), are needed for the regulation of fec transcription and for FecA transport activity. In a fecA mutant with an additional tonB mutation, FecA is synthesized without the need for an inducer, but the transport function of the protein is abolished. In the absence of TonB, the mutated FecA assumes an induction-active but transport-inactive conformation. These unusual findings indicate a link between the transport and transcription regulation activities of ferric citrate from the cell surface into the cytoplasm.
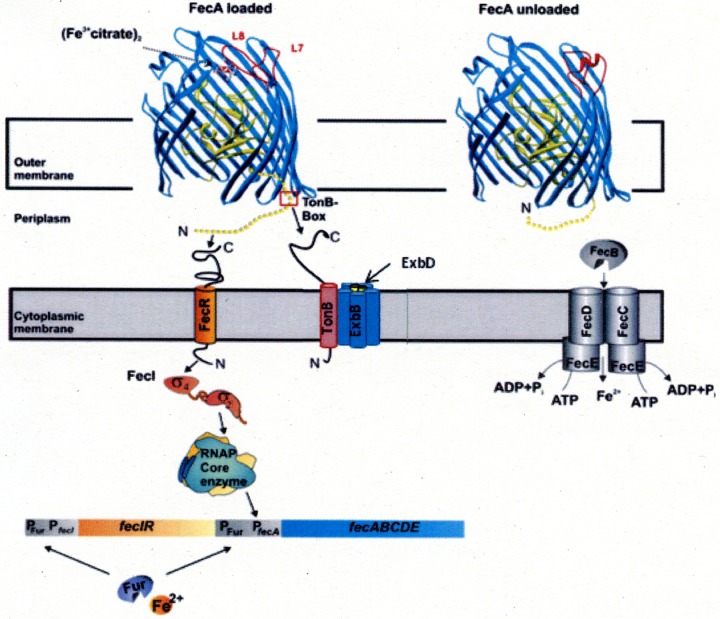
FIG 1.
Model of (Fe3+ citrate)2 transport and regulation. (Fe3+ citrate)2 is transported by FecA into the periplasm, or its binding to FecA initiates a signal that is transmitted via FecA across the outer membrane to FecR, which channels the signal across the cytoplasmic membrane to the FecI extracytoplasmic function (ECF) sigma factor. fecIR transcription is regulated not by (Fe3+ citrate)2 but by the level of intracellular Fe2+ via the Fur repressor. The ABC transporter is shown on the right. The arrows indicate sites of protein interactions. RNAP, RNA polymerase.
Transcription of the transport genes fecABCDE is regulated by the proximally located fecIR genes. FecI is an ECF sigma factor and was the first ECF to be sequenced (9). A predicted helix-turn-helix motif close to the C-terminal end is typical for DNA-binding regulatory proteins, and the phenotypes of FecI mutant proteins support a function of FecI in the activation of fec transport gene transcription. Standard similarity searching methods available in 1990 did not reveal homology to known sequences, but in 1994, a detailed analysis of sigma E, an RNA polymerase sigma factor of Streptomyces coelicolor, revealed its sequence homology with the sequences of seven other proteins, including FecI of E. coli (10). These proteins were grouped within the σ70 family. Since they regulate both gene transcription in response to stimuli that originate outside the cytoplasm and the synthesis of periplasmic and outer membrane components, they were designated ECF sigma factors. A second gene, fecR, is located downstream of fecI. The induction of iron transport by citrate, and specifically by (Fe3+ citrate)2, requires fecIR and fecA. FecR crosses the cytoplasmic membrane by a hydrophobic sequence from residues 85 to 100 (11). The C terminus resides in the periplasm and interacts with the signaling sequence of FecA. The N terminus resides in the cytoplasm and interacts with FecI (12). FecR is proteolytically cleaved after residue 181. Inactive FecR mutants are not cleaved (13). Cleavage is supported by the cleavage of FecR-BlaMB hybrid proteins which were used for determination of the FecR transmembrane arrangement (11). The hybrid proteins are cut to the same 15-kDa fragment as FecR. N-terminal fragments of FecR (residues 1 to 85, 1 to 58, or 9 to 85) induce fec transcription constitutively in the absence of (Fe3+ citrate)2 (14). The fragments enhance the binding of FecI to the β′ subunit of RNA polymerase. It is conceivable that such C-terminal fragments are formed upon transcription initiation by (Fe3+ citrate)2. Mutants in rseP, which encodes a membrane-bound protease, contain a strongly reduced amount of the FecA protein (15). RseP is involved in the ECF σE signaling pathway that regulates stress response (16). Fec signaling could result in a conformational change of FecR which makes it prone to proteolysis. FecR is not an anti-sigma factor since FecI is inactive in the absence of FecR (12, 17). We propose to name FecR a pro-sigma factor to describe its activity. Extensive genetic and biochemical studies have revealed the entire signaling pathway of fec transport regulation. Thus, (Fe3+ citrate)2 induces signaling by binding to FecA on the cell surface, which causes a large conformational change in FecA that is transmitted across the outer membrane to the FecA signaling sequence. The latter interacts with the FecR C terminus, which via its hydrophobic segment transmits the signal across the cytoplasmic membrane into the cytoplasm, where the FecR N terminus activates FecI. FecI-activated RNA polymerase binds to the promoter upstream of fecA, resulting in the transcription of fecABCDE (17).
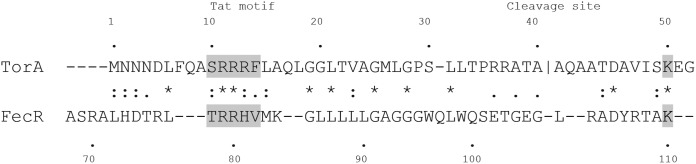
While FecR signaling has been described in detail, little is known about the mechanism of FecR insertion into the cytoplasmic membrane. Although it was assumed that insertion is accomplished by the Sec secretion system, FecR does not contain a standard signal peptide cleavage site, and the final protein is the same size as the original translation product. A recent study pointed out that the FecR N-proximal sequence shares features with proteins of the Tat secretion system, i.e., a basic n-region with a conserved S/T-R-R-X-F-L-K twin-arginine sequence, followed by a hydrophobic h-region, a polar c-region, and a Sec avoidance motif (Fig. 2) (1). However, whereas most Tat proteins contain the Tat motif close to the N terminus, the FecR Tat motif is located deeper inside the sequence (residues 68 to 109). The Tat secretion system is composed of a protein complex consisting of TatB and TatC proteins and various amounts of TatA protein (18). After the substrate has been translocated across the cytoplasmic membrane, the signal peptide is cleaved by signal peptidase I, and the mature protein is released into the periplasm.
FIG 2.
Sequence comparison of the proposed FecR Tat motif with the Tor Tat motif (modified from reference 1). The twin-arginine motif of proteins secreted by Tat and the K residue involved in Sec avoidance are shaded. The signal peptide cleavage site of TorA is indicated. Identical residues are marked by asterisks, strongly similar residues are marked by colons, and weakly similar residues are marked by periods. The numbers show the amino acid residues from the N-terminal end.
FecR secretion by Tat was convincingly demonstrated in a straightforward experimental approach by Passmore et al. (1). Residues 1 to 115 of FecR were fused to the Bla β-lactamase, and the secretion of the construct was then tested based on the ampicillin resistance conferred by Bla in the periplasm. The importance of the arginine pair of FecR was examined by its substitution with twin alanines or lysines, and Tat dependence was examined by using a Tat-deficient mutant. The results showed that the Bla activity of the Tat wild type was abolished in the Tat mutants. Moreover, quantitative PCR showed the downregulation of the fecABCDE genes in the Tat arginine-to-lysine and arginine-to-alanine mutants. In a Tat mutant, FecR localized to the membrane but did not insert into the membrane.
The authors’ conclusion, that FecR constitutes a new class of bitopic Tat-dependent membrane proteins with an internal, uncleaved twin-arginine signal sequence that separates two globular domains, is fully supported by their data. Why FecR prefers the Tat secretion system, which secretes folded proteins, over the Sec secretion system, which secretes unfolded proteins, is not known. Since signaling across the cytoplasmic membrane requires structural interactions of the FecR N-proximal and C-proximal portions, it can be assumed that folding begins at the FecR N terminus as it leaves the ribosome, which initiates folding of the C-portion. This immediate folding of FecR prevents its recognition by Sec. The much longer n-region of FecR than of other Tat signal peptides may play a role in the rapid folding of the protein and in Sec avoidance. Because the structure of FecR is relatively simple, the protein can be used in further studies of Tat secretion by analysis of mutants which can readily be selected and their activities easily quantitated.
ACKNOWLEDGMENT
V. Braun thanks Andrei Lupas for generous support in his department.
The views expressed in this article do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1.Passmore IJ, Coll F, Dow JM, Palmer T, Cuccui J, Wren BW. 2020. Ferric citrate regulator FecR is translocated across the bacterial inner membrane via a unique twin-arginine transport-dependent mechanism. J Bacteriol 202:e00541-19. doi: 10.1128/JB.00541-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun V, Mahren S, Ogierman M. 2003. Regulation of the FecI-type ECF sigma factor by transmembrane signalling. Curr Opin Microbiol 6:173–180. doi: 10.1016/s1369-5274(03)00022-5. [DOI] [PubMed] [Google Scholar]
- 3.Ferguson AD, Chakraborty R, Smith BS, Esser L, van der Helm D, Deisenhofer J. 2002. Structural basis of gating by the outer membrane transporter FecA. Science 295:1715–1719. doi: 10.1126/science.1067313. [DOI] [PubMed] [Google Scholar]
- 4.Yue WW, Grizot S, Buchanan SK. 2003. Structural evidence for iron-free citrate and ferric citrate binding to the TonB-dependent outer membrane transporter FecA. J Mol Biol 332:353–368. doi: 10.1016/s0022-2836(03)00855-6. [DOI] [PubMed] [Google Scholar]
- 5.Härle C, Kim I, Angerer A, Braun V. 1995. Signal transfer through three compartments: transcription initiation of the Escherichia coli ferric citrate transport system from the cell surface. EMBO J 14:1430–1438. doi: 10.1002/j.1460-2075.1995.tb07129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pramanik A, Hauf W, Hoffmann J, Cernescu M, Brutschy B, Braun V. 2011. Oligomeric structure of ExbB and ExbB-ExbD isolated from Escherichia coli as revealed by LILBID mass spectrometry. Biochemistry 50:8950–8956. doi: 10.1021/bi2008195. [DOI] [PubMed] [Google Scholar]
- 7.Celia H, Noinaj N, Zakharov SD, Bordignon E, Botos I, Santamaria M, Barnard TJ, Cramer WA, Lloubes R, Buchanan SK. 2016. Structural insight into the role of the Ton complex in energy transduction. Nature 538:60–65. doi: 10.1038/nature19757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Celia H, Botos I, Ni X, Fox T, De Val N, Lloubes R, Jiang J, Buchanan SK. 2019. Cryo-EM structure of the bacterial Ton motor subcomplex ExbB-ExbD provides information on structure and stoichiometry. Commun Biol 2:358. doi: 10.1038/s42003-019-0604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Hove B, Staudenmaier H, Braun V. 1990. Novel two-component transmembrane transcription control: regulation of iron dicitrate transport in Escherichia coli K-12. J Bacteriol 172:6749–6758. doi: 10.1128/jb.172.12.6749-6758.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lonetto MA, Brown KL, Rudd KE, Buttner MJ. 1994. Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial RNA polymerase sigma factors involved in the regulation of extracytoplasmic functions. Proc Natl Acad Sci U S A 91:7573–7577. doi: 10.1073/pnas.91.16.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welz D, Braun V. 1998. Ferric citrate transport of Escherichia coli: functional regions of the FecR transmembrane regulatory protein. J Bacteriol 180:2387–2394. doi: 10.1128/JB.180.9.2387-2394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun V, Mahren S. 2005. Transmembrane transcriptional control (surface signalling) of the Escherichia coli Fec type. FEMS Microbiol Rev 29:673–684. doi: 10.1016/j.femsre.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Wriedt K, Angerer A, Braun V. 1995. Transcription regulation from the cell surface: confomational change in the transmembrane protein FecR lead to altered transcription of the ferric citrate genes in Escherichia coli. J Bacteriol 177:3320–3322. doi: 10.1128/jb.177.11.3320-3322.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ochs M, Veitinger S, Kim I, Welz D, Angerer A, Braun V. 1995. Regulation of citrate-dependent iron transport of Escherichia coli: fecR is required for transcription activation by FecI. Mol Microbiol 15:119–132. doi: 10.1111/j.1365-2958.1995.tb02226.x. [DOI] [PubMed] [Google Scholar]
- 15.Braun V, Mahren S, Sauter A. 2005. Gene regulation by transmembrane signaling. Biometals 18:507–517. doi: 10.1007/s10534-005-3497-0. [DOI] [PubMed] [Google Scholar]
- 16.Brooks BE, Buchanan SK. 2008. Signaling mechanisms for activation of extracytoplasmic function (ECF) sigma factors. Biochim Biophys Acta 1778:1930–1945. doi: 10.1016/j.bbamem.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Braun V. 2010. Outer membrane signaling in Gram-negative bacteria, p 117–133. In Krämer R, Jung K (ed), Bacterial signaling. Wiley-Blackwell, Weinheim, Germany. [Google Scholar]
- 18.Natale P, Bruser T, Driessen AJ. 2008. Sec- and Tat-mediated protein secretion across the bacterial cytoplasmic membrane—distinct translocases and mechanisms. Biochim Biophys Acta 1778:1735–1756. doi: 10.1016/j.bbamem.2007.07.015. [DOI] [PubMed] [Google Scholar]